Abstract
Adenosine (ADO) and nucleotides such as ATP, ADP, and uridine 5′-triphosphate (UTP), among others, may serve as extracellular signaling molecules. These mediators activate specific cell-surface receptors—namely, purinergic 1 and 2 (P1 and P2)—to modulate crucial pathophysiological responses. Regulation of this process is maintained by nucleoside and nucleotide transporters, as well as the ectonucleotidases ectonucleoside triphosphate diphosphohydrolase [ENTPD; cluster of differentiation (CD)39] and ecto-5′-nucleotidase (5′-NT; CD73), among others. Cells involved in tissue repair, healing, and scarring respond to both ADO and ATP. Our recent investigations have shown that modulation of purinergic signaling regulates matrix deposition during tissue repair and fibrosis in several organs. Cells release adenine nucleotides into the extracellular space, where these mediators are converted by CD39 and CD73 into ADO, which is anti-inflammatory in the short term but may also promote dermal, heart, liver, and lung fibrosis with repetitive signaling under defined circumstances. Extracellular ATP stimulates cardiac fibroblast proliferation, lung inflammation, and fibrosis. P2Y2 (UTP/ATP) and P2Y6 [ADP/UTP/uridine 5′-diphosphate (UDP)] have been shown to have profibrotic effects, as well. Modulation of purinergic signaling represents a novel approach to preventing or diminishing fibrosis. We provide an overview of the current understanding of purinergic signaling in scarring and discuss its potential to prevent or decrease fibrosis.—Ferrari, D., Gambari, R., Idzko, M., Müller, T., Albanesi, C., Pastore, S., La Manna, G., Robson, S. C., Cronstein, B. Purinergic signaling in scarring.
Keywords: adenosine, COPD, Crohn’s disease, extracellular nucleotides, fibrosis
Tissue repair and wound healing are complex, dynamic, multistep processes tightly interwoven with the immune defense response. The replacement of devitalized or missing cellular structures and tissues can last for years, and the optimal outcome is the complete re-establishment of pre-existing tissue size and functional homeostasis (1). The reparative process starts at the first (bleeding) phase that occurs as a consequence of injury or trauma. The following (inflammatory) phase is essential to tissue repair. Inflammation occurs early, with a rapid increase in magnitude before a gradual decrease until resolution. During the next (proliferative) phase, generation of the cellular and noncellular repair material takes place.
Proper scar formation is not achieved until late in the overall repair process, and the so-called remodelling phase is responsible for the final organization and functional quality of the scar. The process of tissue repair can go awry, however, with two main consequences (2). If the reparative process is inefficient, the outcome will be insufficient healing, resulting in a hypotrophic or atrophic scar; in contrast, when tissue repair ends with an abundance of fibroblasts and matrix deposition, the improper outcome is a hypertrophic scar (if confined to the wound site) or generalized organ fibrosis, extending beyond the area of the original insult and often heavily impairing organ function. Two additional important aspects of noncanonical matrix deposition are reduced tissue resistance of mechanical stress, often accompanied by pain.
Fibrosis underlies many pathologic states initiated by traumatic insults. Keloids, for example, are markedly hypertrophic scars of the skin, but fibrosis also causes tendon adhesions, reduced or arrested nervous signal transmission after nerve injury, and partial or total organ loss of function, such as kidney or gut fibrosis. Localized or systemic fibrosis is the hallmark of different immune diseases, culminating in systemic sclerosis or scleroderma, with clinically significant vascular occlusion, major organ (lung, gut, and cardiac) dysfunction and diffuse skin fibrosis. Aberrant matrix deposition causing the intestinal fibrosis that results in formation of small intestine and colon stricture is the primary reason for surgical treatment in Crohn’s disease (3). Replacement of damaged liver tissue with fibrotic tissue is the characteristic pathologic feature of liver cirrhosis caused by hepatitis B and C, alcohol abuse, and nonalcoholic fatty liver disease (4). Fibrosis necessitating surgery causes strictures and consequent impaired passage in the esophagus and urethra or formation of capsules surrounding breast implants (5).
Because extracellular matrix (ECM) synthesis and turnover are mainly caused by tissue fibroblasts, investigation of the molecular basis of fibrosis has been concentrated mostly on mediators that modulate fibroblast functions (2). Growth factors, such as angiotensin (ANG) II and TGFβ and its receptor connective tissue growth factor (CTGF), which are known to stimulate fibroblast proliferation, migration, and profibrogenic activity, are at the basis of the development of tissue fibrosis (6–8). Recent data show that the fibrotic process is under control of previously unrecognized cellular molecules. The Nod-like receptor family pyrin domain containing (NLRP)3 inflammasome, as well as Notch signaling, have been linked to fibrosis (9, 10). Compelling evidence points to aberrant expression of peroxisome proliferator–activated receptor-γ during fibrosis (11), and recent data have indicated the participation of a newly identified cell population potentially involved in lung fibrosis. Human adult lung tissue contains a population of perivascular mesenchymal stem cells (ABCG2pos), the precursors of myofibroblasts, which cause the detrimental matrix remodeling that is at the basis of pulmonary fibrosis (12). A recent contribution to a better understanding of fibrogenesis came from the comparison of the microRNA (miR) expression pattern in matched normal and hypertrophic scar samples. In particular, miR-200b appears to be down-regulated by more than 2-fold in hypertrophic scar tissues and human hypertrophic scar fibroblasts (13), by affecting the synthesis of collagen I and III, fibronectin expression, and TGF-β1/α-smooth muscle actin signaling and ultimately modulating proliferation and apoptosis of human hypertrophic scar fibroblasts (13). Another microRNA, miR-21, was shown to control fibroblast cell growth in hypertrophic scars by regulating hTERT expression via the phosphatase and tensin homolog (PTEN)/PI3K/protein kinase B (AKT) signaling pathway. However, despite these recent advances, fibrosis is still a critical pathologic condition, and new pharmacological compounds and strategies aimed to prevent and reverse the process are greatly needed (1, 2).
THE PURINERGIC NETWORK
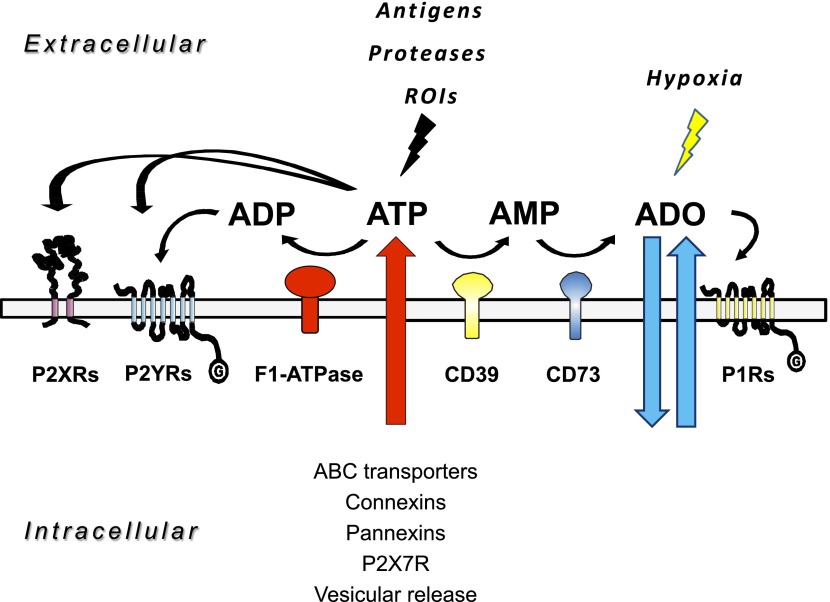
Adenosine (ADO) and nucleotides, such as ATP, ADP, uridine 5′-triphosphate (UTP) and uridine 5′-diphosphate (UDP), are present both inside and outside living cells. In the extracellular fluid, they mediate cell-to-cell communication (14). Receptors for extracellular nucleosides and nucleotides are currently classified as two subgroups: purinergic 1 (P1) receptors activated by extracellular ADO and P2 receptors activated by ATP and other nucleotides (15). The first group includes 4 receptor types: ADO receptor a (Adora)-1 (A1), Adora 2 (A2A), Adora 2B (A2B), and Adora 3 (A3), and the second comprises 7 P2X and 8 P2Y human subtypes. Increased levels of the purinergic receptor agonists ADP, AMP, and ADO can result from the activity of 2 membrane enzymes, the ectonucleoside triphosphate diphosphohydrolase (ENTPD; CD39), which converts ATP/ADP to AMP, and ecto-5′-nucleotidase (CD73), which converts AMP to ADO (16, 17) (Fig. 1). An increase in the extracellular concentration of ATP can be achieved by transport of the nucleotide through the plasma membrane by ATP-binding cassette transporters, connexin hemichannels, and pannexin channels (18–20); vesicular exocytosis; and extracellular synthesis via plasma membrane F(1)/F(0)-ATP synthase. ATP is included in the so-called damage-associated molecular patterns (DAMPs), because it is a normal intracellular constituent that is released into the extracellular milieu as a consequence of tissue damage, cell stress, or cell death. Physiologic release and degradation of ATP and related nucleotides must be tightly regulated to avoid perpetuation of the immune response, even in the absence of microbes (16, 17). A moderate, controlled release of ADO, ATP, or UTP exerts beneficial effects by activating reparative homeostatic responses, whereas the uncontrolled, prolonged release of these mediators induces excessive activation of immune and nonimmune cells, leading to increased, prolonged secretion of the inflammatory mediators [i.e., prostaglandins, leukotrienes, and reactive oxygen intermediates (ROIs)] and proinflammatory cytokines that mediate tissue damage, induce massive recruitment of immune cells, and establish the chronic inflammatory conditions often accompanied by fibrosis.
Figure 1.
Purinergic signaling network. ADO and ATP are released in the extracellular compartment by different means. Other than by lesions of cell membranes with injury, ADO is released by means of equilibrium transporters, whereas ATP escapes via vesicles, connexins, pannexins, ABC transporters, and P2X7 receptors. Both ADO and ATP function as signaling molecules via activation of the P1 receptor (A1, A2A, A2B, and A3) and the P2 receptor (P2X and P2Y) subtypes, respectively. In the extracellular space, ADO is inactivated by ADA to inosine, ultimately to form hypoxanthine, by deribosylation. P1 receptor-mediated signaling is also terminated by cellular uptake of ADO by the equilibrative nucleoside transporters ENT1 and -2. In contrast, ATP is metabolized by enzymatic phosphohydrolysis in a 2-step process via CD39 conversion of ATP (or ADP) to AMP and CD73 phosphohydrolysis of AMP to ADO.
Sequential CD39 and CD73 activity generates ADO in the extracellular milieu. Involvement of these enzymes in modulating tissue function during both physiologic and pathologic conditions has been appreciated in several tissues (18, 19). Recent evidence points to a role for ectonucleotidases in the fibrotic process, by reducing nucleotide concentration, thus preventing or reducing P2 receptor activation, and by generation of ADO, which in turn stimulates different P1 ADO receptor subtypes. The concentration of ADO in the extracellular milieu ranges from 100 to 500 nM and increases to levels in the low micromolar range in response to inflammation, hypoxia, and ischemia (20, 21). The P1 receptors interact with ADO in different manners. The high-affinity receptors A1, A2A, and A3 are consistently activated by low concentrations (>10–50 nM) of extracellular ADO. In contrast, the low-affinity receptor A2B is activated by much higher ADO concentrations, as in cell injury or cell death (>1 μM).
The nucleoside promotes tissue remodelling and repair, but depending on cell type and stimulated receptor subtype, it can favor nonregulated extracellular matrix elaboration, abnormal deposition, and misalignment of collagen (i.e., fibrotic responses in liver, lung, and skin); in contrast, it decreases heart fibrosis (8, 22) (Table 1). Herein, we report on recent advancements in elucidating the contribution of molecular components of the purinergic signaling network to the genesis of fibrosis.
TABLE 1.
Effects of single purinergic receptors or ectonucleotidases on tissue and organ fibrosis
| Receptor | Agonist | Stimulus | Tissue | Profibrotic | Antifibrotic | Species | Reference |
|---|---|---|---|---|---|---|---|
| A2A | ADO | Heart | + | 73 | |||
| UUO | Kidney | + | M | 88 | |||
| CCl4, TAA | Liver | + | M | 100 | |||
| Ethanol | Liver | + | M | 99, 101 | |||
| CG, PDF | Peritoneum | + | M | 109 | |||
| Skin | + | H, M | 62, 71, 73, 111 | ||||
| A2B | ADO | Heart | + | M | 72, 111, 114 | ||
| Kidney | + | M | 90, 91 | ||||
| Liver | + | H | 102, 103 | ||||
| Lung | + | H | 58 | ||||
| A3 | ADO | UUO | Kidney | + | 92 | ||
| P2X7 | ATP | CCl4 | Liver | + | M | 98 | |
| Bleomycin | Lung | + | M | 51 | |||
| BzATP | Kidney | + | R, M | 86, 87 | |||
| P2Y2 | ATP | Lung | + | H | 55 | ||
| P2Y4 | UDP | Lung | + | H | 56 | ||
| CD39 | Bleomycin | Skin | + | M | 71 | ||
| CD73 | CCl4, TAA | Liver | + | M | 104 | ||
| Lung | + | H | 58 | ||||
| Bleomycin | Skin | + | M | 8, 71 |
CG, chlorhexidine gluconate; H, human; M, mouse; PDF, peritoneal dialysis fluid; R, rat; TAA, thioacetamide.
PURINERGIC MODULATION OF THE IMMUNE SYSTEM IN WOUND HEALING AND FIBROSIS
Fibrosis occurs in tissues that have frequent exposure to chemical and biologic insults that provoke chronic stimulation of immune and nonimmune cells of the injured organs. Immune cells participate in a coordinated series of defensive and tissue repair events with the main objective of isolating and destroying nonself molecules and bodies. Innate immune functions, including chemotaxis, phagocytosis, and collagen degradation and remodeling, ultimately leading to neoangiogenesis and organ repair or re-epithelization, are crucial for reaching this goal.
An important ability of immune cells is to detect and discriminate between self and nonself antigens, but also to become activated when normal intracellular constituents are liberated extracellularly as a consequence of cell stress or damage (21). Among the latter, nucleosides and nucleotides are important activators of immune responses, once they reach proper extracellular concentration. Therefore, expression of P1 and P2 receptors by immune cells enables them to detect the release of diverse ADO and ATP concentrations, thus continuously monitoring tissue stress or injury arising from disparate causes (18, 23). Neutrophil extravasation and infiltration of wounded tissues is an early crucial response that protects wounds from invading microbes; however, excessive neutrophil infiltration and the consequent prolonged release of proinflammatory cytokines, proteolytic enzymes, and ROIs leads to abnormal tissue repair and chronic nonhealing wounds. Therefore, the role of neutrophils in wound healing is still debatable and, according to recent data obtained in mice, the cells tend to inhibit tissue repair (24).
Bacterial constituents stimulate ATP release and IL-8 production from neutrophils and lead to further chemoattraction of these cells (24). Neutrophils are also attracted by the ATP and ADP released during platelet clotting (25). Cooperation between P1/P2 receptors and ectonucleotidases (particularly P2Y2, A3 subtypes, and CD39) is involved in sensing and producing receptor ligands, thus modulating the speed of neutrophil migration (26–29). Macrophages are also a prominent cell population in wounds, and besides the important defensive function, they remove apoptotic cells and prompt cell proliferation and matrix elaboration after an injury. Because of their phenotypic plasticity, macrophages have been compared with lymphocytes. They acquire the M1 proinflammatory phenotype to counteract microbe invasion, whereas they become M2 reparative and immune suppressive, to complete the wound-healing process and therefore represent an important target in promoting wound healing and reducing fibrosis (30). Many responses induced by ADO have been shown in macrophages, among them secretion of pro- and anti-inflammatory cytokines and mediators and even modulation of the antigen-presenting function (31). The A2A receptor synergizes with Toll-like receptor-2, -4, -7, and -9 to switch macrophage activity from defensive to proangiogenic and reparative, thus contributing to tissue repair (32, 33). A fibroblast-secreted protein, osteopontin (OPN), has been convincingly shown to act as a potent profibrotic factor. Macrophages and mast cells induce expression of fibroblast OPN by secretion of platelet-derived growth factor (34). The P2X7 subtype is a central proinflammatory macrophage ATP receptor, the triggering of which induces secretion of IL-1β, IL-18, prostaglandin E2 (PGE2), phosphatidylserine, and ROIs by fibroblasts (35). P2X7 activation has been linked to inflammation and defense against microbes and, recently, to fibrosis. Accordingly, bleomycin-treated P2X7-knockout (KO) mice show reduced inflammation and lung fibrosis (36). Activation of this receptor in mice is linked to silica-induced lung inflammatory and fibrotic response. Mice lacking the receptor show lower infiltration of inflammatory cells and collagen deposition in lung parenchyma (37).
Cells of the adaptive immune response also appear in the wound tissue; however, their role in healing is likely less important. In T-cell population–depletion studies performed in mice, T-helper lymphocytes had no effect on wound healing, whereas activity of T-suppressor and cytotoxic lymphocytes was inhibitory, as depletion of these cells increased collagen synthesis and improved wound resistance (38). Lymphocyte involvement in fibrosis is under investigation. In systemic sclerosis, patients subjected to a therapy based on anifrolumab, a monoclonal antibody targeting IFN-α and -β receptors, undergo suppression of T-lymphocyte activation and a decrease in collagen accumulation (39).
Another example of fibrosis in which T lymphocytes are likely to play an important role is encapsulating peritoneal sclerosis [i.e., an excessive fibrotic response that occurs in the peritoneum after long-term peritoneal dialysis]. In this case, inflammatory T helper 1 cells may play a role (40).
It has been shown recently that stimulation of the A3 receptor in a human mast cell line down-regulates expression of this subtype and heavily affects the gene expression profile of these cells. Both A3 stimulation and knockdown lead to upregulated expression of genes involved in tissue remodeling (i.e., IL-6, IL-8, VEGF, amphiregulin, and OPN) (41). An interesting point that has yet to be addressed is the increased presence of mast cells in some fibrotic tissues and whether their contribution is profibrotic via release of proteases and digestion of wound proteins, with the production of profibrotic peptides. This hypothesis seems to be confirmed by the high chymase gene expression and activity in mast cells that are present in keloids, compared with normal skin, and by the fact that chymase promotes proliferation of fibroblasts and collagen synthesis by activating TGF-β1 in keloids (42). Thus, the type and degree of inflammation has a critical impact on scar formation and fibrosis. Therefore, a deeper understanding of inflammation would be likely to lead to an enhanced capacity to counteract fibrosis.
LUNG SCARRING AND FIBROSIS, CHRONIC OBSTRUCTIVE PULMONARY DISEASE, AND PURINERGIC SIGNALING
Pulmonary fibrosis is a chronic disease of the lung characterized by tissue fibrosis with consequent loss of elasticity, shortness of breath, and the presence of inflammatory infiltrate, which can develop after an increased immune response to inhaled organic molecules, such as those from bacteria, fungi, mites, parasites, or occupational chemicals (43). In contrast, idiopathic pulmonary fibrosis (IPF) is a lung disease of unknown etiology, with an even greater degree of fibrosis, resulting in a very poor prognosis. Though some progress has been made, there are few therapeutic options available at the moment (44–46). Although the persistence of inciting pathologic agents and the consequent chronic inflammatory state have been suggested to be prerequisites to different forms of pulmonary fibrosis, the situation is likely to be more complex for IPF, because of the poor efficacy of standard anti-inflammatory therapies, including corticosteroids (47). In chronic obstructive pulmonary disease (COPD) consisting of chronic inflammation, oxidative stress, and bronchial obstruction, increased ECM deposition underlying the bronchial epithelium produces fibrosis and thickening of the airway wall (48).
Cells of the human respiratory system express receptors for extracellular nucleosides and nucleotides (49). Lung hypoxia causes ATP release, thus enhancing adventitial fibroblast proliferation and transformation to myofibroblasts (50). Increased ATP in bronchoalveolar lavage fluid has been found, both in patients with IPF and in animals with experimental lung fibrosis (51). Similar observations have been made in individuals or animal models of COPD (52, 53). In experimental lung fibrosis induced by the antitumor drug bleomycin, ATP, through the connexin-1/P2X7 receptor axis, induces inflammatory cell recruitment and lung fibrosis by stimulating IL-1β secretion and tissue inhibitor of metalloproteinase (TIMP)-1 production (51). Moreover, release of ATP is paralleled by that of other intracellular molecules. In bleomycin-induced lung fibrosis, uric acid released by damaged dying cells represents an additional DAMP that activates NLRP3 with subsequent IL-1β release, inflammation, and fibrosis (54). That ATP is a crucial trigger of epithelial stress-induced inflammation and fibrosis has been convincingly shown by various means. The ATP-degrading enzyme apyrase greatly reduces bleomycin-induced inflammation, as does the absence of the P2X7 subtype. P2X7-KO mice show much less lung inflammation and fibrosis markers (lung collagen content, and the matrix-remodeling proteins TIMP-1 and matrix metalloproteinase-9) than do wild-type mice (51).
P2Y receptors are involved in the repair of human airway epithelia. The P2Y2 subtype induces TNFα-converting enzyme activation, which then releases the membrane-bound ligands of the epidermal growth factor receptor, thus inducing cell migration and proliferation (49, 55). However, P2Y receptor signaling is most likely also involved in lung fibrosis, as in vitro stimulation of human lung fibroblasts by nucleotides increases the expression of P2Y4, TGF-β, collagen A1, and fibronectin (55). ADO receptors are expressed in the lung, where they regulate inflammation and airway remodeling by favoring differentiation of lung fibroblasts into myofibroblasts that are typically found in fibrotic tissues (56). Subjects with COPD and IPF show an altered ADO metabolism (57). CD73 and A2B receptor expression is increased in surgical lung biopsies of patients with severe COPD or IPF. Moreover, the profibrotic mediators IL-6, IL-8, and OPN are increased in these samples (58, 59). The profibrotic activity of ADO in the lung has been elegantly shown in mice deficient in adenosine deaminase (ADA), the enzyme that transforms ADO to inosine, which is inactive at ADO receptors, thus eliminating the P1 receptor agonist from the extracellular milieu. Hence, the absence of ADA induces ADO-dependent pulmonary fibrosis in these animals (60). Furthermore, a recent study demonstrated that A2B receptor expression on myeloid cells is necessary for the development of pulmonary fibrosis and pulmonary hypertension in response to bleomycin (61). Consistent with these findings, ADO has been shown to mediate many of the anti-inflammatory effects of low-dose, weekly methotrexate treatment, which is used to treat rheumatoid arthritis and psoriasis (62, 63). A feared side effect of this therapy is pulmonary fibrosis, and it is possible that ADO release in the lung plays a role in the pathogenesis of this drug toxicity as well. Therefore, in the respiratory system, dysregulation of purinergic signaling with activation of ATP and ADO receptors appears to contribute substantially to the development of fibrosis.
SKIN REPAIR AND SCARRING ARE MODULATED BY P2 AND P1 RECEPTORS
Keloids are a classic example of the skin fibrosis that occurs during wound healing. They are different from hypertrophic scars, as they do not contain myofibroblasts (64). Fibroblasts that are present in keloids produce greater concentrations of collagen, probably because of increased expression of TGF-β and TGF-β receptor. Skin cells express purinergic receptors (65), and the gene expression profile of ATP-treated keratinocytes reveals marked changes (66). If it is obvious that, upon acute epidermal injury (e.g., lacerations, trauma, burns, or abrasions or after tape stripping), ATP is released from damaged keratinocytes (67), it is less intuitively obvious that ATP is normally released by keratinocytes during epidermal homeostasis and in response to mild mechanical and thermal stimuli. It is even less obvious that air stimulation induces release of ATP from human neonatal keratinocytes in vitro (68). Moreover, opening of pannexin hemichannels in keratinocytes as a consequence of nonmetal hapten stimulation causes release of ATP (69).
The positive effects of ADO on wound healing have been described. The nucleoside promotes wound neovascularization by stimulating the A2A receptor (70). However, excessive ADO generation by CD39 and CD73 promotes dermal fibrosis (8, 71), activating the same receptor that has been recognized as a fine-tuned regulator of the collagen1:collagen3 balance (72). A2A-stimulated collagen secretion by human fibroblasts occurs via at least 2 pathways: a cyclic AMP- and AKT-dependent pathway (73) and a Fli1- and CTGF-mediated mechanism (74). Despite previous failures of A2A receptor agonists to promote wound healing in patients with diabetic foot ulcers (unpublished data), a more recent study indicates that the ADO A2A receptor agonist polydeoxyribonucleotide effectively promotes wound healing of diabetic foot ulcers better than a placebo (75).
The demonstration that A2A agonists could promote wound healing, including matrix production in the wound, suggests that A2A receptors play a role in fibrosis (73). The pivotal role of ADO and A2A receptor in skin fibrosis has been elegantly shown in ADA-deficient mice. These animals have increased levels of TGF-β1, CTGF, and IL-13, which are accompanied by increased collagen deposition and fibrosis. Accordingly, the A2A receptor antagonist ZM-241385 prevents the development of dermal fibrosis in ADA-KO mice (76). Reported observations show that purinergic signaling modulates skin physiology and fibrotic response, identifying novel opportunities for the development of purinergic-based treatments to prevent or block dermal fibrosis.
KIDNEY TRANSPLANT, FIBROSIS, AND SCARRING: WHAT IS THE ROLE FOR PURINES?
Kidney fibrosis is present in most forms of chronic renal disease, and progressive accumulation of ECM often leads to renal failure, necessitating dialysis or kidney transplantation (77). Major cellular actors in renal fibrosis are kidney fibroblasts and mesangial cells under the control of the renin–angiotensin–aldosterone system or of TGF-β1/bone morphogenic protein-7. Renal injury often starts with monocyte/macrophage and T-lymphocyte infiltration, leading to chronic inflammation and eventually to glomerulosclerosis, tubulointerstitial fibrosis, and loss of renal parenchyma (77, 78). Polarization of macrophages seems to be central in kidney fibrosis in mice. In particular, macrophages polarize to an M2 subtype after renal injury; M2 macrophages secrete high levels of TGF-β1 levels and promote ECM accumulation (79). Subclinical inflammation is also a consistent risk factor for the development of the interstitial fibrosis associated with chronic humoral rejection of kidney grafts (80). Accordingly, initial fibrotic levels in the transplanted kidney (significantly correlating with donor age) strongly affects graft outcome (81, 82). Among the factors contributing to renal fibrosis, nucleotides have been shown to play an important role. Purinergic receptors are expressed in the kidney and are involved in modulating physiologic organ functions, such as vascular tone and glomerular pressure (83). Different stimuli induce ADO and ATP release in the kidney, and the absence of CD73 in mice diminishes renal function and induces autoimmune inflammation (84). Responses induced by ATP, UTP, and the pharmacological ATP analog 2′(3′)-O-(4-benzoylbenzoyl)ADO-5′-triphosphate (BzATP) have been shown in rat mesangial cells. Whereas ATP and UTP induce cell proliferation via P2Y2 and P2Y4 receptors, stimulation of the P2X7 receptor by BzATP causes cell death (85). A role for the P2X7 subtype in kidney fibrosis has also been hypothesized (86). Opposing effects of P2Y and P2X receptors on TGF-β and collagen production have also been reported. ATP and BzATP increase TGF-β and collagen secretion, and UTP decreases them (87). ADO-mediated responses in the kidney are also complex and intriguing because, depending on the type of P1 receptor subtype stimulated, they can induce both beneficial and detrimental effects. Although the nucleoside plays a positive role in protecting the organ tissue during ischemia, the chronic profibrotic effect of ADO has also been shown by different groups by means of the unilateral ureteral obstruction (UUO) model in mice (88). Thus, in this model, there is renal tissue hypoxia and ADO release. Stimulation of A2A receptor reduces renal damage, down-regulates expression of fibrosis markers, and decreases collagen deposition (88). Similarly, activation of the A2A subtype down-modulates macrophage-mediated inflammation, which plays a central role in the pathogenesis of crescentic glomerulonephritis in Wistar-Kyoto rats (89). The role of the A2B receptor in kidney inflammation and fibrosis is opposite that of the A2A receptor, as stimulation of the A2B receptor induces IL-6 secretion (90), and its inhibition during hypoxic conditions reduces proliferation and secretion of profibrotic cytokines (91). The role of the A3 subtype has not been well investigated in kidney fibrosis; so far, its activation has been linked to UUO-induced tubulointerstitial fibrosis in mice (92).
LIVER AND GUT REGENERATION, REMODELING, AND SCARRING ARE MODULATED BY NUCLEOTIDES
Liver fibrosis is caused by the hepatic chronic injury often accompanied by inflammation (93). Among the initial damaging agents are viral infections (hepatitis B or C viruses), alcohol abuse, accumulation of iron in hemochromatosis or copper in Wilson disease, and biliary obstruction (94). Excessive collagen accumulation can lead to portal hypertension and loss of the canonical hepatic architecture (cirrhosis). Advanced cirrhosis causes liver failure, often resulting in liver transplantation.
Long-term liver fibrosis has also been linked to the development of hepatocellular carcinoma (95). Myofibroblasts, portal fibroblasts, and hepatic stellate cells have been identified as major players in abnormal matrix deposition and among the fibrogenic signals, leptin, ANG II, and TGF-1β, are crucial in hepatic fibrosis (96).
Liver cell functions are modulated by nucleotides and nucleosides (97). Involvement of the P2X7 receptor in the pathogenesis of carbon tetrachloride (CCl4)-induced liver fibrosis has been described in mice (98). Subcutaneous injection of this chemical up-regulates P2X7 expression and increases collagen deposition, whereas inhibition of this subtype by the specific antagonist A438079 reduces TGF-β1 secretion and collagen formation (98).
ADO also plays a role in liver fibrosis, as triggering of the A2A receptor induces expression of collagen by stellate cells (99), and inhibition or deletion of this subtype prevents CCl4-, thioacetamide-, and ethanol-induced fibrosis in mice (100, 101). ADO-mediated effects are dependent, at least in part, on A2B activation, as the receptor antagonist MRS1754 reduces liver fibrosis, as well (102, 103). CD73 has been linked to hepatic fibrosis for its ability to produce extracellular ADO (104). However, participation of this ectonucleotidase in healing appears complex, given that the knockdown of CD73 message induces an increase in collagen I and augments cell migration in stellate cells (105). Another interesting observation is that CD73 gene expression increases during myofibroblastic differentiation (106).
Of note are recent epidemiologic observations indicating major protective effects of coffee in the setting of progressive liver fibrosis and cirrhosis (107). Laboratory studies suggest that these salutary effects are mediated by stellate cell expression of A2A, where caffeine may serve as an inhibitor. In a similar vein, low-dose weekly methotrexate has been used for the treatment of psoriasis and rheumatoid arthritis for many years, and prior studies have indicated that ADO accounts for many of the anti-inflammatory effects of this drug (63). One well-described toxicity of methotrexate therapy is hepatic fibrosis; it is possible that ADO mediates this effect as well.
An emerging role for purinergic signaling in the evolution of gut fibrosis and stricturing has also been suggested. P2Y2 receptor is highly expressed by rat intestinal myofibroblasts, and ATP stimulation has been shown to stimulate cellular activation and contraction (108).
MODULATION OF PERITONEAL AND CARDIAC FIBROSIS BY ADO
Peritoneal fibrosis is a common complication of chronic peritoneal dialysis or surgery and may lead to diminished efficacy of peritoneal dialysis, development of intestinal adhesions, and obstruction. Recent work indicates that either pharmacological blockade or deletion of ADO A2A receptors can diminish peritoneal fibrosis (109). In contrast, others have reported that topical application of ADO and phosphorylated ADO derivatives could, by acting via ADO receptors, diminish peritoneal adhesions in a rodent model (110). Recent work suggests an explanation for these diametrically opposed findings with respect to the effects of ADO A2A and A2B receptor-mediated promotion and inhibition of collagen production and fibrosis (111). Of interest, A2A receptors stimulate a modest increase of cAMP, which inhibits collagen production, but that the much higher cAMP levels induced by forskolin (and potentially A2B receptors) suppresses collagen production (72). Thus, chronic A2A stimulation leads to fibrosis, but stimulation of A2B receptors, which requires higher levels of endogenous ADO for stimulation, inhibits collagen production. Moreover, in contrast to other tissues, the preponderance of evidence indicates that the principal P1 receptor subtype in the heart that regulates cardiac fibroblast function is the A2B receptor. In 1986, Dubey et al. (112) first reported that ADO inhibits cardiac fibroblast collagen production via stimulation of A2B receptors. Later work further indicated that maneuvers that increase intracellular cAMP diminish collagen production by cardiac fibroblasts (113, 114). This observation led to the demonstration of the homeostatic role of ATP hydrolysis, by the action of ENTPD (CD39) in diminishing collagen production by increasing extracellular ADO levels, which act at A2B receptors to raise cAMP and diminish fibroblast production of collagen (115). Similarly, hydrolysis of AMP by CD73 on the surface of immune cells protects against cardiac fibrosis (116). These studies further suggest that regional and tissue differences in the expression of A2B receptors can lead to opposing effects of ADO on fibrosis.
CONCLUSIONS AND FUTURE PERSPECTIVES
Fibrosis underlies different pathologic conditions that, because of disfigurement, may lead to psychologic, social, and economic problems, but can also cause organ failure and increased risk of mortality (117). It is therefore crucial to find new therapeutic solutions, especially for diffuse organ fibrosis and for those fibrotic states, such as IPF, that do not respond to medical therapy.
It has become clear that excessive ligand-mediated activation of specific nucleoside and nucleotide receptor subtypes as a consequence of uncontrolled ligand release gives the tissue microenvironment a surplus of signals, promoting abnormal replication of smooth muscle cells, fibroblasts, and myofibroblasts, with pathologic matrix deposition causing fibrosis. Recent reports demonstrate the significant contribution of extracellular nucleosides and nucleotides in promoting abnormal matrix production (8, 55, 62). However, at present, this field is at an initial stage and many developments have still to be made. Moreover, relevant scientific and clinical questions are awaiting an answer. A central question dogging the field is why the same receptor subtype can have both anti- and profibrotic effects, depending on tissue expression.
Therefore, collaboration between purinologists and clinicians (dermatologists, rheumatologists, gastroenterologists, cardiologists, and pulmonologists) may open unexpected opportunities for deeper investigation of this subject and, more important, for development of treatments of fibrotic diseases.
Acknowledgments
This work was supported by U.S. National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR56672 and AR568593; and New York University–Health and Hospitals Corporation Clinical and Translational Science Institute Grant UL1TR000038.
Glossary
- ADA
adenosine deaminase
- Adora
ADO receptor a
- ANG
angiotensin
- ADO
adenosine
- BzATP
2′(3′)-O-(4-benzoylbenzoyl)adenosine-5′-triphosphate
- CD39
ectonucleoside triphosphate diphosphohydrolase (ENTPD)
- CD73
ecto-5′-nucleotidase (5′-NT)
- CCl4
carbon tetrachloride
- CD
cluster of differentiation
- COPD
chronic obstructive pulmonary disease
- CTGF
connective tissue growth factor
- DAMP
damage-associated molecular pattern
- ECM
extracellular matrix
- IPF
idiopathic pulmonary fibrosis
- KO
knockout
- miR
microRNA
- NLRP3
Nod-like receptor family pyrin domain containing 3
- P1/2
purinergic 1/2
- OPN
osteopontin
- ROI
reactive oxygen intermediate
- TIMP
tissue inhibitor of metalloproteinase
- UUO
unilateral ureteral obstruction
REFERENCES
- 1.Friedman S. L., Sheppard D., Duffield J. S., Violette S. (2013) Therapy for fibrotic diseases: nearing the starting line. Sci. Transl. Med. 5, 167sr1 [DOI] [PubMed] [Google Scholar]
- 2.Diegelmann R. F., Evans M. C. (2004) Wound healing: an overview of acute, fibrotic and delayed healing. Front. Biosci. 9, 283–289 [DOI] [PubMed] [Google Scholar]
- 3.Spinelli A., Correale C., Szabo H., Montorsi M. (2010) Intestinal fibrosis in Crohn’s disease: medical treatment or surgery? Curr. Drug Targets 11, 242–248 [DOI] [PubMed] [Google Scholar]
- 4.Chen R. J., Wu H. H., Wang Y. J. (2015) Strategies to prevent and reverse liver fibrosis in humans and laboratory animals. [E-pub ahead of print] Arch. Toxicol. 10.1007/s00204-015-1525-6 [DOI] [PubMed] [Google Scholar]
- 5.Gsell F. (1984) Preventing and treating capsular fibrosis after breast augmentation: a case report. Aesthetic Plast. Surg. 8, 51–53 [DOI] [PubMed] [Google Scholar]
- 6.Trojanowska M. (2012) Mediators of fibrosis. Open Rheumatol. J. 6, 70–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeisberg M., Kalluri R. (2013) Cellular mechanisms of tissue fibrosis: 1, common and organ-specific mechanisms associated with tissue fibrosis. Am. J. Physiol. Cell Physiol. 304, C216–C225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu D., Insel P. A. (2014) Cellular mechanisms of tissue fibrosis. 6. Purinergic signaling and response in fibroblasts and tissue fibrosis. Am. J. Physiol. Cell Physiol. 306, C779–C788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artlett C. M., Thacker J. D. (2015) Molecular activation of the NLRP3 inflammasome in fibrosis: common threads linking divergent fibrogenic diseases. Antioxid. Redox Signal. 22, 1162–1175 [DOI] [PubMed] [Google Scholar]
- 10.Nemir M., Metrich M., Plaisance I., Lepore M., Cruchet S., Berthonneche C., Sarre A., Radtke F., Pedrazzini T. (2014) The Notch pathway controls fibrotic and regenerative repair in the adult heart. Eur. Heart J. 35, 2174–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei J., Bhattacharyya S., Varga J. (2010) Peroxisome proliferator-activated receptor γ: innate protection from excessive fibrogenesis and potential therapeutic target in systemic sclerosis. Curr. Opin. Rheumatol. 22, 671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marriott S., Baskir R. S., Gaskill C., Menon S., Carrier E. J., Williams J., Talati M., Helm K., Alford C. E., Kropski J. A., Loyd J., Wheeler L., Johnson J., Austin E., Nozik-Grayck E., Meyrick B., West J. D., Klemm D. J., Majka S. M. (2014) ABCG2pos lung mesenchymal stem cells are a novel pericyte subpopulation that contributes to fibrotic remodeling. Am. J. Physiol. Cell Physiol. 307, C684–C698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li P., He Q. Y., Luo C. Q. (2014) Overexpression of miR-200b inhibits the cell proliferation and promotes apoptosis of human hypertrophic scar fibroblasts in vitro. J. Dermatol. 41, 903–911 [DOI] [PubMed] [Google Scholar]
- 14.Burnstock G. (2012) Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. BioEssays 34, 218–225 [DOI] [PubMed] [Google Scholar]
- 15.Fredholm B. B., Abbracchio M. P., Burnstock G., Dubyak G. R., Harden T. K., Jacobson K. A., Schwabe U., Williams M. (1997) Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol. Sci. 18, 79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmermann H., Zebisch M., Sträter N. (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 8, 437–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yegutkin G. G. (2008) Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 1783, 673–694 [DOI] [PubMed] [Google Scholar]
- 18.Eltzschig H. K., Sitkovsky M. V., Robson S. C. (2012) Purinergic signaling during inflammation. N. Engl. J. Med. 367, 2322–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chekeni F. B., Elliott M. R., Sandilos J. K., Walk S. F., Kinchen J. M., Lazarowski E. R., Armstrong A. J., Penuela S., Laird D. W., Salvesen G. S., Isakson B. E., Bayliss D. A., Ravichandran K. S. (2010) Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467, 863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Belle H., Goossens F., Wynants J. (1987) Formation and release of purine catabolites during hypoperfusion, anoxia, and ischemia. Am. J. Physiol. 252, H886–H893 [DOI] [PubMed] [Google Scholar]
- 21.Lohman A. W., Billaud M., Isakson B. E. (2012) Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc. Res. 95, 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cronstein B. N. (2011) Adenosine receptors and fibrosis: a translational review. F1000 Biol. Rep. 3, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacob F., Pérez Novo C., Bachert C., Van Crombruggen K. (2013) Purinergic signaling in inflammatory cells: P2 receptor expression, functional effects, and modulation of inflammatory responses. Purinergic Signal. 9, 285–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dovi J. V., He L. K., DiPietro L. A. (2003) Accelerated wound closure in neutrophil-depleted mice. J. Leukoc. Biol. 73, 448–455 [DOI] [PubMed] [Google Scholar]
- 25.Kukulski F., Bahrami F., Ben Yebdri F., Lecka J., Martín-Satué M., Lévesque S. A., Sévigny J. (2011) NTPDase1 controls IL-8 production by human neutrophils. J. Immunol. 187, 644–653 [DOI] [PubMed] [Google Scholar]
- 26.Ward P. A., Cunningham T. W., McCulloch K. K., Phan S. H., Powell J., Johnson K. J. (1988) Platelet enhancement of O2-. responses in stimulated human neutrophils: identification of platelet factor as adenine nucleotide. Lab. Invest. 58, 37–47 [PubMed] [Google Scholar]
- 27.Chen Y., Corriden R., Inoue Y., Yip L., Hashiguchi N., Zinkernagel A., Nizet V., Insel P. A., Junger W. G. (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314, 1792–1795 [DOI] [PubMed] [Google Scholar]
- 28.Dwyer K. M., Deaglio S., Gao W., Friedman D., Strom T. B., Robson S. C. (2007) CD39 and control of cellular immune responses. Purinergic Signal. 3, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corriden R., Insel P. A. (2012) New insights regarding the regulation of chemotaxis by nucleotides, adenosine, and their receptors. Purinergic Signal. 8, 587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills C. D. (2015) Anatomy of a discovery: m1 and m2 macrophages. Front. Immunol. 6, 212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koh T. J., DiPietro L. A. (2011) Inflammation and wound healing: the role of the macrophage. Expert Rev. Mol. Med. 13, e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haskó G., Pacher P. (2012) Regulation of macrophage function by adenosine. Arterioscler. Thromb. Vasc. Biol. 32, 865–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinhal-Enfield G., Ramanathan M., Hasko G., Vogel S. N., Salzman A. L., Boons G. J., Leibovich S. J. (2003) An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. Am. J. Pathol. 163, 711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori R., Shaw T. J., Martin P. (2008) Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J. Exp. Med. 205, 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Idzko M., Ferrari D., Eltzschig H. K. (2014) Nucleotide signalling during inflammation. Nature 509, 310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebeling G., Bläsche R., Hofmann F., Augstein A., Kasper M., Barth K. (2014) Effect of P2X7 receptor knockout on AQP-5 expression of type I alveolar epithelial cells. PLoS One 9, e100282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monção-Ribeiro L. C., Faffe D. S., Santana P. T., Vieira F. S., da Graça C. L., Marques-da-Silva C., Machado M. N., Caruso-Neves C., Zin W. A., Borojevic R., Takiya C. M., Coutinho-Silva R. (2014) P2X7 receptor modulates inflammatory and functional pulmonary changes induced by silica. PLoS One 9, e110185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbul A., Breslin R. J., Woodyard J. P., Wasserkrug H. L., Efron G. (1989) The effect of in vivo T helper and T suppressor lymphocyte depletion on wound healing. Ann. Surg. 209, 479–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo X., Higgs B. W., Bay-Jensen A. C., Karsdal M. A., Yao Y., Roskos L. K., White W. I. (2015) Suppression of T cell activation and collagen accumulation by an anti-type I interferon receptor monoclonal antibody in adult patients with systemic sclerosis. J. Invest. Dermatol. (May):20. [DOI] [PubMed] [Google Scholar]
- 40.Betjes M. G., Habib M. S., Struijk D. G., Lopes Barreto D., Korte M. R., Abrahams A. C., Nagtzaam N. M., Clahsen-van Groningen M. C., Dik W. A., Litjens N. H. (2015) Encapsulating peritoneal sclerosis is associated with T-cell activation. Nephrol. Dial. Transplant. (May):1. [DOI] [PubMed] [Google Scholar]
- 41.Rudich N., Dekel O., Sagi-Eisenberg R. (2015) Down-regulation of the A3 adenosine receptor in human mast cells upregulates mediators of angiogenesis and remodeling. Mol. Immunol. 65, 25–33 [DOI] [PubMed] [Google Scholar]
- 42.Dong X., Zhang C., Ma S., Wen H. (2014) Mast cell chymase in keloid induces profibrotic response via transforming growth factor-β1/Smad activation in keloid fibroblasts. Int. J. Clin. Exp. Pathol. 7, 3596–3607 [PMC free article] [PubMed] [Google Scholar]
- 43.Chambers R. C., Mercer P. F. (2015) Mechanisms of alveolar epithelial injury, repair, and fibrosis. Ann. Am. Thorac. Soc. 12(Suppl 1), S16–S20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gross T. J., Hunninghake G. W. (2001) Idiopathic pulmonary fibrosis. N. Engl. J. Med. 345, 517–525 [DOI] [PubMed] [Google Scholar]
- 45.Lederer D. J., Bradford W. Z., Fagan E. A., Glaspole I., Glassberg M. K., Glasscock K. F., Kardatzke D., King T. E. Jr., Lancaster L. H., Nathan S. D., Pereira C. A., Sahn S. A., Swigris J. J., Noble P. W. (2015) Sensitivity analyses of the change in forced vital capacity in a phase 3 trial of cirfenidone for idiopathic pulmonary fibrosis. Chest 148, 196–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richeldi L., du Bois R. M., Raghu G., Azuma A., Brown K. K., Costabel U., Cottin V., Flaherty K. R., Hansell D. M., Inoue Y., Kim D. S., Kolb M., Nicholson A. G., Noble P. W., Selman M., Taniguchi H., Brun M., Le Maulf F., Girard M., Stowasser S., Schlenker-Herceg R., Disse B., Collard H. R.; INPULSIS Trial Investigators (2014) Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 370, 2071–2082 [DOI] [PubMed] [Google Scholar]
- 47.Demedts M., Behr J., Buhl R., Costabel U., Dekhuijzen R., Jansen H. M., MacNee W., Thomeer M., Wallaert B., Laurent F., Nicholson A. G., Verbeken E. K., Verschakelen J., Flower C. D., Capron F., Petruzzelli S., De Vuyst P., van den Bosch J. M., Rodriguez-Becerra E., Corvasce G., Lankhorst I., Sardina M., Montanari M.; IFIGENIA Study Group (2005) High-dose acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 353, 2229–2242 [DOI] [PubMed] [Google Scholar]
- 48.Gao W., Li L., Wang Y., Zhang S., Adcock I. M., Barnes P. J., Huang M., Yao X. (2015) Bronchial epithelial cells: The key effector cells in the pathogenesis of chronic obstructive pulmonary disease? Respirology 20, 722–729 [DOI] [PubMed] [Google Scholar]
- 49.Burnstock G., Brouns I., Adriaensen D., Timmermans J. P. (2012) Purinergic signaling in the airways. Pharmacol. Rev. 64, 834–868 [DOI] [PubMed] [Google Scholar]
- 50.Gerasimovskaya E. V., Ahmad S., White C. W., Jones P. L., Carpenter T. C., Stenmark K. R. (2002) Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth: signaling through extracellular signal-regulated kinase-1/2 and the Egr-1 transcription factor. J. Biol. Chem. 277, 44638–44650 [DOI] [PubMed] [Google Scholar]
- 51.Riteau N., Gasse P., Fauconnier L., Gombault A., Couegnat M., Fick L., Kanellopoulos J., Quesniaux V. F., Marchand-Adam S., Crestani B., Ryffel B., Couillin I. (2010) Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 182, 774–783 [DOI] [PubMed] [Google Scholar]
- 52.Lommatzsch M., Cicko S., Müller T., Lucattelli M., Bratke K., Stoll P., Grimm M., Dürk T., Zissel G., Ferrari D., Di Virgilio F., Sorichter S., Lungarella G., Virchow J. C., Idzko M. (2010) Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 181, 928–934 [DOI] [PubMed] [Google Scholar]
- 53.Lucattelli M., Cicko S., Müller T., Lommatzsch M., De Cunto G., Cardini S., Sundas W., Grimm M., Zeiser R., Dürk T., Zissel G., Sorichter S., Ferrari D., Di Virgilio F., Virchow J. C., Lungarella G., Idzko M. (2011) P2X7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am. J. Respir. Cell Mol. Biol. 44, 423–429 [DOI] [PubMed] [Google Scholar]
- 54.Gasse P., Riteau N., Charron S., Girre S., Fick L., Pétrilli V., Tschopp J., Lagente V., Quesniaux V. F., Ryffel B., Couillin I. (2009) Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 179, 903–913 [DOI] [PubMed] [Google Scholar]
- 55.Van der Vliet A., Bove P. F. (2011) Purinergic signaling in wound healing and airway remodeling. Subcell. Biochem. 55, 139–157 [DOI] [PubMed] [Google Scholar]
- 56.Janssen L. J., Farkas L., Rahman T., Kolb M. R. (2009) ATP stimulates Ca(2+)-waves and gene expression in cultured human pulmonary fibroblasts. Int. J. Biochem. Cell Biol. 41, 2477–2484 [DOI] [PubMed] [Google Scholar]
- 57.Della Latta V., Cabiati M., Rocchiccioli S., Del Ry S., Morales M. A. (2013) The role of the adenosinergic system in lung fibrosis. Pharmacol. Res. 76, 182–189 [DOI] [PubMed] [Google Scholar]
- 58.Zhou Y., Murthy J. N., Zeng D., Belardinelli L., Blackburn M. R. (2010) Alterations in adenosine metabolism and signaling in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. PLoS One 5, e9224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider D. J., Lindsay J. C., Zhou Y., Molina J. G., Blackburn M. R. (2010) Adenosine and osteopontin contribute to the development of chronic obstructive pulmonary disease. FASEB J. 24, 70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chunn J. L., Molina J. G., Mi T., Xia Y., Kellems R. E., Blackburn M. R. (2005) Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J. Immunol. 175, 1937–1946 [DOI] [PubMed] [Google Scholar]
- 61.Karmouty-Quintana H., Philip K., Acero L. F., Chen N. Y., Weng T., Molina J. G., Luo F., Davies J., Le N. B., Bunge I., Volcik K. A., Le T. T., Johnston R. A., Xia Y., Eltzschig H. K., Blackburn M. R. (2015) Deletion of ADORA2B from myeloid cells dampens lung fibrosis and pulmonary hypertension. FASEB J. 29, 50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan E. S., Fernandez P., Merchant A. A., Montesinos M. C., Trzaska S., Desai A., Tung C. F., Khoa D. N., Pillinger M. H., Reiss A. B., Tomic-Canic M., Chen J. F., Schwarzschild M. A., Cronstein B. N. (2006) Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum. 54, 2632–2642 [DOI] [PubMed] [Google Scholar]
- 63.Chan E. S., Cronstein B. N. (2010) Methotrexate: how does it really work? Nat. Rev. Rheumatol. 6, 175–178 [DOI] [PubMed] [Google Scholar]
- 64.Jumper N., Paus R., Bayat A. (2015) Functional histopathology of keloid disease. Histol. Histopathol. 30, 1033–1057 [DOI] [PubMed] [Google Scholar]
- 65.Burnstock G., Knight G. E., Greig A. V. (2012) Purinergic signaling in healthy and diseased skin. J. Invest. Dermatol. 132, 526–546 [DOI] [PubMed] [Google Scholar]
- 66.Ohara H., Saito R., Hirakawa S., Shimada M., Mano N., Okuyama R., Aiba S. (2010) Gene expression profiling defines the role of ATP-exposed keratinocytes in skin inflammation. J. Dermatol. Sci. 58, 143–151 [DOI] [PubMed] [Google Scholar]
- 67.Takahashi T., Kimura Y., Niwa K., Ohmiya Y., Fujimura T., Yamasaki K., Aiba S. (2013) In vivo imaging demonstrates ATP release from murine keratinocytes and its involvement in cutaneous inflammation after tape stripping. J. Invest. Dermatol. 133, 2407–2415 [DOI] [PubMed] [Google Scholar]
- 68.Barr T. P., Albrecht P. J., Hou Q., Mongin A. A., Strichartz G. R., Rice F. L. (2013) Air-stimulated ATP release from keratinocytes occurs through connexin hemichannels. PLoS One 8, e56744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Onami K., Kimura Y., Ito Y., Yamauchi T., Yamasaki K., Aiba S. (2014) Nonmetal haptens induce ATP release from keratinocytes through opening of pannexin hemichannels by reactive oxygen species. J. Invest. Dermatol. 134, 1951–1960 [DOI] [PubMed] [Google Scholar]
- 70.Montesinos M. C., Shaw J. P., Yee H., Shamamian P., Cronstein B. N. (2004) Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am. J. Pathol. 164, 1887–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernández P., Perez-Aso M., Smith G., Wilder T., Trzaska S., Chiriboga L., Franks A. Jr., Robson S. C., Cronstein B. N., Chan E. S. (2013) Extracellular generation of adenosine by the ectonucleotidases CD39 and CD73 promotes dermal fibrosis. Am. J. Pathol. 183, 1740–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perez-Aso M., Mediero A., Cronstein B. N. (2013) Adenosine A2A receptor (A2AR) is a fine-tune regulator of the collagen1:collagen3 balance. Purinergic Signal. 9, 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perez-Aso M., Fernandez P., Mediero A., Chan E. S., Cronstein B. N. (2014) Adenosine 2A receptor promotes collagen production by human fibroblasts via pathways involving cyclic AMP and AKT but independent of Smad2/3. FASEB J. 28, 802–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chan E. S., Liu H., Fernandez P., Luna A., Perez-Aso M., Bujor A. M., Trojanowska M., Cronstein B. N. (2013) Adenosine A(2A) receptors promote collagen production by a Fli1- and CTGF-mediated mechanism. Arthritis Res. Ther. 15, R58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Squadrito F., Bitto A., Altavilla D., Arcoraci V., De Caridi G., De Feo M. E., Corrao S., Pallio G., Sterrantino C., Minutoli L., Saitta A., Vaccaro M., Cucinotta D. (2014) The effect of PDRN, an adenosine receptor A2A agonist, on the healing of chronic diabetic foot ulcers: results of a clinical trial. J. Clin. Endocrinol. Metab. 99, E746–E753 [DOI] [PubMed] [Google Scholar]
- 76.Hu X., Ran H., Dechang W., Yibing W., Yongqiang F., Qiang L. (2013) Absence of the adenosine A(2A) receptor attenuates hypertrophic scarring in mice. J. Burn Care Res. 34, e161–e167 [DOI] [PubMed] [Google Scholar]
- 77.Liu Y. (2006) Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 69, 213–217 [DOI] [PubMed] [Google Scholar]
- 78.Torres I. B., Moreso F., Sarró E., Meseguer A., Serón D. (2014) The interplay between inflammation and fibrosis in kidney transplantation. BioMed Res. Int. 2014, 750602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan B., Liu G., Jiang Z., Zheng D. (2015) Regulation of renal fibrosis by macrophage polarization. Cell. Physiol. Biochem. 35, 1062–1069 [DOI] [PubMed] [Google Scholar]
- 80.Park W. D., Griffin M. D., Cornell L. D., Cosio F. G., Stegall M. D. (2010) Fibrosis with inflammation at one year predicts transplant functional decline. J. Am. Soc. Nephrol. 21, 1987–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Azancot M. A., Moreso F., Salcedo M., Cantarell C., Perello M., Torres I. B., Montero A., Trilla E., Sellarés J., Morote J., Seron D. (2014) The reproducibility and predictive value on outcome of renal biopsies from expanded criteria donors. Kidney Int. 85, 1161–1168 [DOI] [PubMed] [Google Scholar]
- 82.De Vusser K., Lerut E., Kuypers D., Vanrenterghem Y., Jochmans I., Monbaliu D., Pirenne J., Naesens M. (2013) The predictive value of kidney allograft baseline biopsies for long-term graft survival. J. Am. Soc. Nephrol. 24, 1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burnstock G., Evans L. C., Bailey M. A. (2014) Purinergic signalling in the kidney in health and disease. Purinergic Signal. 10, 71–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blume C., Felix A., Shushakova N., Gueler F., Falk C. S., Haller H., Schrader J. (2012) Autoimmunity in CD73/Ecto-5′-nucleotidase deficient mice induces renal injury. PLoS One 7, e37100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harada H., Chan C. M., Loesch A., Unwin R., Burnstock G. (2000) Induction of proliferation and apoptotic cell death via P2Y and P2X receptors, respectively, in rat glomerular mesangial cells. Kidney Int. 57, 949–958 [DOI] [PubMed] [Google Scholar]
- 86.Gonçalves R. G., Gabrich L., Rosário A. Jr., Takiya C. M., Ferreira M. L., Chiarini L. B., Persechini P. M., Coutinho-Silva R., Leite M. Jr (2006) The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int. 70, 1599–1606 [DOI] [PubMed] [Google Scholar]
- 87.Solini A., Iacobini C., Ricci C., Chiozzi P., Amadio L., Pricci F., Di Mario U., Di Virgilio F., Pugliese G. (2005) Purinergic modulation of mesangial extracellular matrix production: role in diabetic and other glomerular diseases. Kidney Int. 67, 875–885 [DOI] [PubMed] [Google Scholar]
- 88.Xiao H., Shen H. Y., Liu W., Xiong R. P., Li P., Meng G., Yang N., Chen X., Si L. Y., Zhou Y. G. (2013) Adenosine A2A receptor: a target for regulating renal interstitial fibrosis in obstructive nephropathy. PLoS One 8, e60173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garcia G. E., Truong L. D., Chen J. F., Johnson R. J., Feng L. (2011) Adenosine A(2A) receptor activation prevents progressive kidney fibrosis in a model of immune-associated chronic inflammation. Kidney Int. 80, 378–388 [DOI] [PubMed] [Google Scholar]
- 90.Dai Y., Zhang W., Wen J., Zhang Y., Kellems R. E., Xia Y. (2011) A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J. Am. Soc. Nephrol. 22, 890–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang J., Jiang X., Zhou Y., Dai Y. (2015) Effects of A2BR on the biological behavior of mouse renal fibroblasts during hypoxia. Mol. Med. Rep. 11, 4397–4402 [DOI] [PubMed] [Google Scholar]
- 92.Lee J., Hwang I., Lee J. H., Lee H. W., Jeong L.-S., Ha H. (2013) The selective A3AR antagonist LJ-1888 ameliorates UUO-induced tubulointerstitial fibrosis. Am. J. Pathol. 183, 1488–1497 [DOI] [PubMed] [Google Scholar]
- 93.Poli G. (2000) Pathogenesis of liver fibrosis: role of oxidative stress. Mol. Aspects Med. 21, 49–98 [DOI] [PubMed] [Google Scholar]
- 94.Alcolado R., Arthur M. J. P., Iredale J. P. (1997) Pathogenesis of liver fibrosis. Clin. Sci. 92, 103–112 [DOI] [PubMed] [Google Scholar]
- 95.Cabibbo G., Maida M., Genco C., Antonucci M., Cammà C. (2012) Causes of and prevention strategies for hepatocellular carcinoma. Semin. Oncol. 39, 374–383 [DOI] [PubMed] [Google Scholar]
- 96.Bataller R., Brenner D. A. (2005) Liver fibrosis. J. Clin. Invest. 115, 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burnstock G., Vaughn B., Robson S. C. (2014) Purinergic signalling in the liver in health and disease. Purinergic Signal. 10, 51–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang C., Yu W., Cui H., Wang Y., Zhang L., Han F., Huang T. (2014) P2X7 blockade attenuates mouse liver fibrosis. Mol. Med. Rep. 9, 57–62 [DOI] [PubMed] [Google Scholar]
- 99.Che J., Chan E. S., Cronstein B. N. (2007) Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway. Mol. Pharmacol. 72, 1626–1636 [DOI] [PubMed] [Google Scholar]
- 100.Chan E. S., Montesinos M. C., Fernandez P., Desai A., Delano D. L., Yee H., Reiss A. B., Pillinger M. H., Chen J. F., Schwarzschild M. A., Friedman S. L., Cronstein B. N. (2006) Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br. J. Pharmacol. 148, 1144–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chiang D. J., Roychowdhury S., Bush K., McMullen M. R., Pisano S., Niese K., Olman M. A., Pritchard M. T., Nagy L. E. (2013) Adenosine 2A receptor antagonist prevented and reversed liver fibrosis in a mouse model of ethanol-exacerbated liver fibrosis. PLoS One 8, e69114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhong H., Yang L., Belardinelli L., Zeng D. (2007) Pro-fibrotic roles of the A2B adenosine receptor in human primary hepatic stellate cells. J. Hepatol. 46 (Suppl 1), S135 [Google Scholar]
- 103.Stoll M., Kim Y. O., Bebich B., Robson S. C., Schuppan D. (2012) The selective adenosine 2B receptor antagonist MRS1754 mitigates hepatic collagen deposition during fibrosis progression and induces mild fibrosis regression. Gastroenterology 142 (Suppl 1), S-974–S-975 [Google Scholar]
- 104.Peng Z., Fernandez P., Wilder T., Yee H., Chiriboga L., Chan E. S., Cronstein B. N. (2008) Ecto-5′-nucleotidase (CD73)-mediated extracellular adenosine production plays a critical role in hepatic fibrosis. FASEB J. 22, 2263–2272 [DOI] [PubMed] [Google Scholar]
- 105.Andrade C. M., Lopez P. L., Noronha B. T., Wink M. R., Borojevic R., Margis R., Lenz G., Battastini A. M., Guma F. C. (2011) Ecto-5′-nucleotidase/CD73 knockdown increases cell migration and mRNA level of collagen I in a hepatic stellate cell line. Cell Tissue Res. 344, 279–286 [DOI] [PubMed] [Google Scholar]
- 106.Klatsky A. L., Morton C., Udaltsova N., Friedman G. D. (2006) Coffee, cirrhosis, and transaminase enzymes. Arch. Intern. Med. 166, 1190–1195 [DOI] [PubMed] [Google Scholar]
- 107.Fausther M., Sheung N., Saiman Y., Bansal M. B., Dranoff J. A. (2012) Activated hepatic stellate cells upregulate transcription of ecto-5′-nucleotidase/CD73 via specific SP1 and SMAD promoter elements. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G904–G914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakamura T., Iwanaga K., Murata T., Hori M., Ozaki H. (2011) ATP induces contraction mediated by the P2Y(2) receptor in rat intestinal subepithelial myofibroblasts. Eur. J. Pharmacol. 657, 152–158 [DOI] [PubMed] [Google Scholar]
- 109.Nakav S., Kachko L., Vorobiov M., Rogachev B., Chaimovitz C., Zlotnik M., Douvdevani A. (2009) Blocking adenosine A2A receptor reduces peritoneal fibrosis in two independent experimental models. Nephrol. Dial. Transplant. 24, 2392–2399 [DOI] [PubMed] [Google Scholar]
- 110.Forman M. B., Gillespie D. G., Cheng D., Jackson E. K. (2014) A novel adenosine precursor 2′,3′-cyclic adenosine monophosphate inhibits formation of post-surgical adhesions. Dig. Dis. Sci. 59, 2118–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perez-Aso M., Chiriboga L., Cronstein B. N. (2012) Pharmacological blockade of adenosine A2A receptors diminishes scarring. FASEB J. 26, 4254–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dubey R. K., Gillespie D. G., Osaka K., Suzuki F., Jackson E. K. (1996) Adenosine inhibits growth of rat aortic smooth muscle cells: possible role of A2b receptor. Hypertension 27, 786–793 [DOI] [PubMed] [Google Scholar]
- 113.Swaney J. S., Roth D. M., Olson E. R., Naugle J. E., Meszaros J. G., Insel P. A. (2005) Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc. Natl. Acad. Sci. USA 102, 437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ryzhov S., Sung B. H., Zhang Q., Weaver A., Gumina R. J., Biaggioni I., Feoktistov I. (2014) Role of adenosine A2B receptor signaling in contribution of cardiac mesenchymal stem-like cells to myocardial scar formation. Purinergic Signal. 10, 477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu D., Insel P. A. (2013) Hydrolysis of extracellular ATP by ectonucleoside triphosphate diphosphohydrolase (ENTPD) establishes the set point for fibrotic activity of cardiac fibroblasts. J. Biol. Chem. 288, 19040–19049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bönner F., Borg N., Jacoby C., Temme S., Ding Z., Flögel U., Schrader J. (2013) Ecto-5′-nucleotidase on immune cells protects from adverse cardiac remodeling. Circ. Res. 113, 301–312 [DOI] [PubMed] [Google Scholar]
- 117.Costabel U. (2012) Idiopathic pulmonary fibrosis: recent milestones in disease management. Eur. Respir. Rev. 21, 140 [DOI] [PMC free article] [PubMed] [Google Scholar]