Abstract
Ophthalmic changes have occurred in a subset of astronauts on International Space Station missions. Visual deterioration is considered the greatest human health risk of spaceflight. Affected astronauts exhibit higher concentrations of 1-carbon metabolites (e.g., homocysteine) before flight. We hypothesized that genetic variations in 1-carbon metabolism genes contribute to susceptibility to ophthalmic changes in astronauts. We investigated 5 polymorphisms in the methionine synthase reductase (MTRR), methylenetetrahydrofolate reductase (MTHFR), serine hydroxymethyltransferase (SHMT), and cystathionine β-synthase (CBS) genes and their association with ophthalmic changes after flight in 49 astronauts. The number of G alleles of MTRR 66 and C alleles of SHMT1 1420 both contributed to the odds of visual disturbances. Preflight dehydroepiandrosterone was positively associated with cotton wool spots, and serum testosterone response during flight was associated with refractive change. Block regression showed that B-vitamin status and genetics were significant predictors of many of the ophthalmic outcomes that we observed. In one example, genetics trended toward improving (P = 0.10) and B-vitamin status significantly improved (P < 0.001) the predictive model for refractive change after flight. We document an association between MTRR 66 and SHMT1 1420 polymorphisms and spaceflight-induced vision changes. This line of research could lead to therapeutic options for both space travelers and terrestrial patients.—Zwart, S. R., Gregory, J. F., Zeisel, S. H., Gibson, C. R., Mader, T. H., Kinchen, J. M., Ueland, P. M., Ploutz-Snyder, R., Heer, M. A., Smith, S. M. Genotype, B-vitamin status, and androgens affect spaceflight-induced ophthalmic changes.
A subset of astronauts on International Space Station (ISS) missions have adverse ophthalmic outcomes, including disc edema, choroidal folds, globe flattening, cotton wool spots, and hyperopic shifts. A detailed description of these conditions is provided by Mader et al. (1). These findings are hypothesized to occur as a result of increased intracranial pressure, optic nerve sheath compartment syndrome, or some combination thereof. The result of any of the hypotheses is pressure impinging on the optic nerve and eye during spaceflight, secondary to fluid shifts, carbon dioxide exposure, resistance exercise, or another as yet unknown factor (2). The risk of visual deterioration is one of the leading human health concerns for long-duration space missions for the National Aeronautics and Space Administration (NASA).
In 2012, we identified biochemical differences between a group of astronauts who experienced visual alterations and a group of those who did not (3). The differences were in circulating concentrations of total homocysteine, cystathionine, 2-methylcitric acid, and methylmalonic acid, all metabolites in the 1-carbon metabolism system. All levels were higher in affected than in unaffected astronauts. These differences were present before, during, and after flight.
All astronauts on the ISS live and work in the same enclosed environment (microgravity, cabin air, foods, and exercise equipment), but not all experience ophthalmic changes. This, combined with the fact that the biochemical differences in the 1-carbon pathway are present before flight, leads to the hypothesis that polymorphisms in enzymes of 1-carbon metabolism represent a potential genetic difference between those astronauts who experience ophthalmic changes and those who do not. Total homocysteine in affected astronauts was in the clinically normal range, but it was in the range associated with individuals homozygous for variant forms of the methylenetetrahydrofolate reductase (MTHFR) 677C→T polymorphism (4, 5). The MTHFR 677 TT genotype is also associated with lower serum folate concentrations, which were found in affected astronauts, as well (3).
We hypothesized that single-nucleotide polymorphisms (SNPs) in genes involved in 1-carbon metabolism contribute to susceptibility to ophthalmic changes after long-duration spaceflight. The higher concentrations of homocysteine and other 1-carbon metabolites in crewmembers with vision-related problems (3) amount to circumstantial evidence. We sought evidence of a genetic cause of these biochemical differences and ultimately to find the link between genetics and visual changes in astronauts.
Because idiopathic intracranial hypertension (pseudotumor cerebri) in patients on Earth can be associated with hyperandrogenism (6–8), we also sought to determine whether androgen concentrations are related to ophthalmic changes. We also used nontargeted metabolomic analyses to evaluate alterations in related or other biochemical pathways and their association with ophthalmic outcomes.
MATERIALS AND METHODS
Participants
The protocol was approved by the NASA Johnson Space Center Institutional Review Board, and informed consent was obtained from all subjects. The participants (n = 49, 8 women and 41 men) were crewmembers on ISS Expeditions 1–38 (total days in space, 58–382). Their mean (±SD) age at launch was 48 (±4) yr.
Astronaut eye- and vision-related data were obtained from the NASA Lifetime Surveillance of Astronaut Health database. The presence or absence of the dichotomous (present/absent) ocular outcomes (i.e., choroidal folds, globe flattening, disc edema, and cotton wool spots) was established at the person level; left and right eyes were not evaluated separately. In addition, the pre- vs. postflight difference in diopters per eye was available. A continuously scaled outcome was used to characterize changes in diopters after flight in each eye, according to cycloplegic refraction. The number of subjects (and male/female distribution) for each genotype is shown in Table 1, and the number of astronauts with each ophthalmic outcome is listed in Table 2. Additional data were obtained from ISS mission-associated research and nutritional assessments (9).
TABLE 1.
Number of subjects bearing each genotype
| Polymorphism | Homozygous, minor allele | Heterozygous | Homozygous, major allele |
|---|---|---|---|
| MTRR A66G | 10 (1) | 25 (7) | 13 (1) |
| MTHFR A1298C | 6 (1) | 16 (1) | 27 (7) |
| MTHFR C677T | 6 (3) | 19 (3) | 24 (3) |
| SHMT C1420T | 3 (1) | 16 (2) | 29 (6) |
| CBS 844ins68 | 0 | 8 (1) | 41 (8) |
The number of females is in parentheses.
TABLE 2.
Number of subjects who had each of the 5 postflight ophthalmic outcomes
| Ophthalmic outcome | Total (n) | Groups [n (%)] |
|
|---|---|---|---|
| + | − | ||
| Cotton wool spots | 39 | 5 (13) | 34 (87) |
| Change in diopters ≥0.5 in at least 1 eye | 32 | 19 (59) | 13 (41) |
| Choroidal folds | 48 | 6 (13) | 42 (88) |
| Optic disc edema | 48 | 9 (19) | 39 (81) |
| Globe flattening | 34 | 22 (65) | 12 (35) |
The total number of subjects analyzed for each of the ophthalmic outcomes is shown. Not all subjects were evaluated for each condition. The percentages after each n represent the portion of subjects with or without each ophthalmic condition based on the total number evaluated for that condition.
Biochemical analyses and genotyping
Fasting (8 h) blood samples were collected. The average time from landing after the 2–6 mo ISS mission to the time of the blood draw was 4.2 ± 3.9 yr. Within 30 min of sample collection, serum and plasma were separated by centrifugation and stored at −80°C until analysis in batches. One EDTA whole-blood tube was frozen immediately and transferred to the University of Florida Clinical and Translational Science Institute’s Genotyping Core (Gainesville, FL, USA). Five SNPs—MTHFR 677C→T, MTHFR 1298 A→C, MTRR 66 A→G, serine hydroxymethyltransferase (SHMT)1 1420 C→T, and cystathionine β-synthase (CBS) 844ins68—were genotyped by TaqMan allelic discrimination (System 7900; Life Technologies-Applied Biosystems, Foster City, CA, USA). Five microliter reactions in 384-well plates were prepared by the epModtion 5070 (Eppendorf North America, Inc., Westbury, NY, USA) robotic liquid-handling and sample-processing system, and the assays were performed and analyzed according to the manufacturer’s recommendations. A separate serum sample, collected at the same time as the blood draw, was sent for untargeted metabolomics analysis (Metabolon, Inc., Durham, NC, USA) by liquid chromatography–tandem mass spectrometry and gas chromatography–tandem mass spectrometry (10).
Landing-day erythrocyte glutathione reductase activation coefficient [riboflavin (B2) status], erythrocyte aspartate aminotransferase (EAST) activation coefficient (vitamin B6 status), serum methylmalonic acid (vitamin B12 status), and red blood cell (RBC) folate were measured, to estimate in-flight B-vitamin status (11). The area under the curve (AUC) for in-flight serum ferritin concentration was determined as an estimate of oxidative stress during flight (12). Preflight dehydroepiandrosterone (DHEA), dehydroepiandrosterone-sulfate (DHEA-S), and AUC for in-flight serum testosterone and 24 h urinary testosterone excretion were also determined (9). Atmospheric CO2 was determined by the ISS cabin gas analyzer, and a mission average was generated from daily averages.
Statistical methods
All statistical analyses were performed with Stata, IC software (version 13.1; StataCorp LP, College Station, TX, USA), with 2-sided α to reject the null hypothesis of no effect set at 0.05.
We determined whether, as a block, several key genetic factors contributed significantly to the prediction of the presence or absence of each dichotomous outcome beyond a simple age and flight-duration model (base model) and whether the addition of several nutritional variables represented an additional improvement over the base + genetics model. B-vitamin status can modify the effect of the SNPs of the 1-carbon pathway on pathway function (4, 13). We ran separate nested logistic regression analyses (1 per outcome) that compared a base model consisting of astronaut age at launch and the number of days in space on prior short-duration missions before launch on the long-duration mission evaluated herein, with a model that included those predictors plus a block of the 5 SNPs, and ultimately to a full model that also included 3 nutritionally relevant predictors (RBC folate, EAST activation coefficient, and erythrocyte glutathione reductase activation coefficient). We report the Wald test statistic for comparing nested models and provide the Akaike information criterion (AIC) as a descriptive measure of relative model fit. Lower AIC values indicate a better fitting model. We used similarly designed nested regression models for our change-in-diopters outcome, except that for this continuously scaled outcome, we compared nested mixed-effects linear regression models, as appropriate for continuously scaled outcomes. For this model, we included a random intercept for person to accommodate the repeated left and right eye observations within subjects.
In exploratory analyses, we used Somers’ D measure of association to evaluate the pair-wise associations between several ophthalmic changes after long-duration spaceflight (choroidal folds, globe flattening, papilledema, and change in diopters) and dietary (RBC folate, B6, riboflavin, B12 status, and ferritin) and environmental (CO2) factors; the number of variant alleles for each genotype (MTHFR 677C→T, MTHFR 1298 A→C, MTRR 66 A →G, SHMT1 1420 C →T, and CBS 844ins68); testosterone concentrations (in serum or urine); and preflight DHEA and DHEA-S concentrations.
RESULTS
The nested logistic regression results are presented in Table 3. In general, the age at launch and the number of days in space before the long-duration mission did not have a strong ability to accurately predict the ophthalmic outcomes, but the addition of genetics and B-vitamin nutritional status indicators often significantly improved model fit. For example, the model to predict choroidal folds was significantly improved with the addition of the 5 SNPs (P = 0.02; AICs = 58.91 vs. 44.52). Choroidal folds were not affected by the inclusion of B-vitamin status indicators. In contrast, the model to predict globe flattening was improved by including B-vitamin status indicators, but not the SNPs (P < 0.05). Change in diopters revealed similar effects, with genetics trending toward improving the model (P = 0.10) and B-vitamin status significantly improving it (P < 0.001).
TABLE 3.
Statistical models predicting the appearance of pathologic changes in the eye after long-duration spaceflight
| Outcome | Test | df | P | AIC |
|---|---|---|---|---|
| Choroidal folds, total n = 48 | Wald χ2 | |||
| Block 1 | 3.97 | 2 | 0.140 | 58.91 |
| Block 2 | 12.90 | 5 | 0.020 | 44.52 |
| Block 3 | 6.18 | 3 | 0.100 | 45.28 |
| Globe flattening, total n = 34 | Wald χ2 | |||
| Block 1 | 5.70 | 2 | 0.060 | 77.03 |
| Block 2 | 7.04 | 5 | 0.220 | 72.42 |
| Block 3 | 8.04 | 3 | 0.050 | 46.49 |
| Disc edema, total n = 48 | Wald χ2 | |||
| Block 1 | 3.79 | 2 | 0.150 | 90.77 |
| Block 2 | 9.51 | 5 | 0.090 | 79.63 |
| Block 3 | 1.93 | 3 | 0.590 | 82.54 |
| Change in diopters, total n = 31 | F | |||
| Block 1 | 3.70 | 2 | 0.040 | 99.06 |
| Block 2 | 2.04 | 5 | 0.100 | 86.88 |
| Block 3 | 13.61 | 3 | 0.001 | 70.38 |
Block 1, base model (days in space, age at launch); block 2, genetics + base vs. base; block 3, nutrition + genetics + base vs. genetics + base. Regression-based statistical methods were used to determine whether, as a block, additional predictors entered in blocks contributed significantly to the prediction of each of the outcomes shown above. See “Statistical methods” for details. The base model (block 1) was compared to a model (block 2) that, in addition to age and flight duration, included a block of key genetic factors, and block 2 was then compared to a final model (block 3) that also included several nutrition variables. The Wald test statistic allows for comparing nested models, and the AIC is provided as a descriptive measure of relative model fit. Lower AIC values indicate a better fitting model.
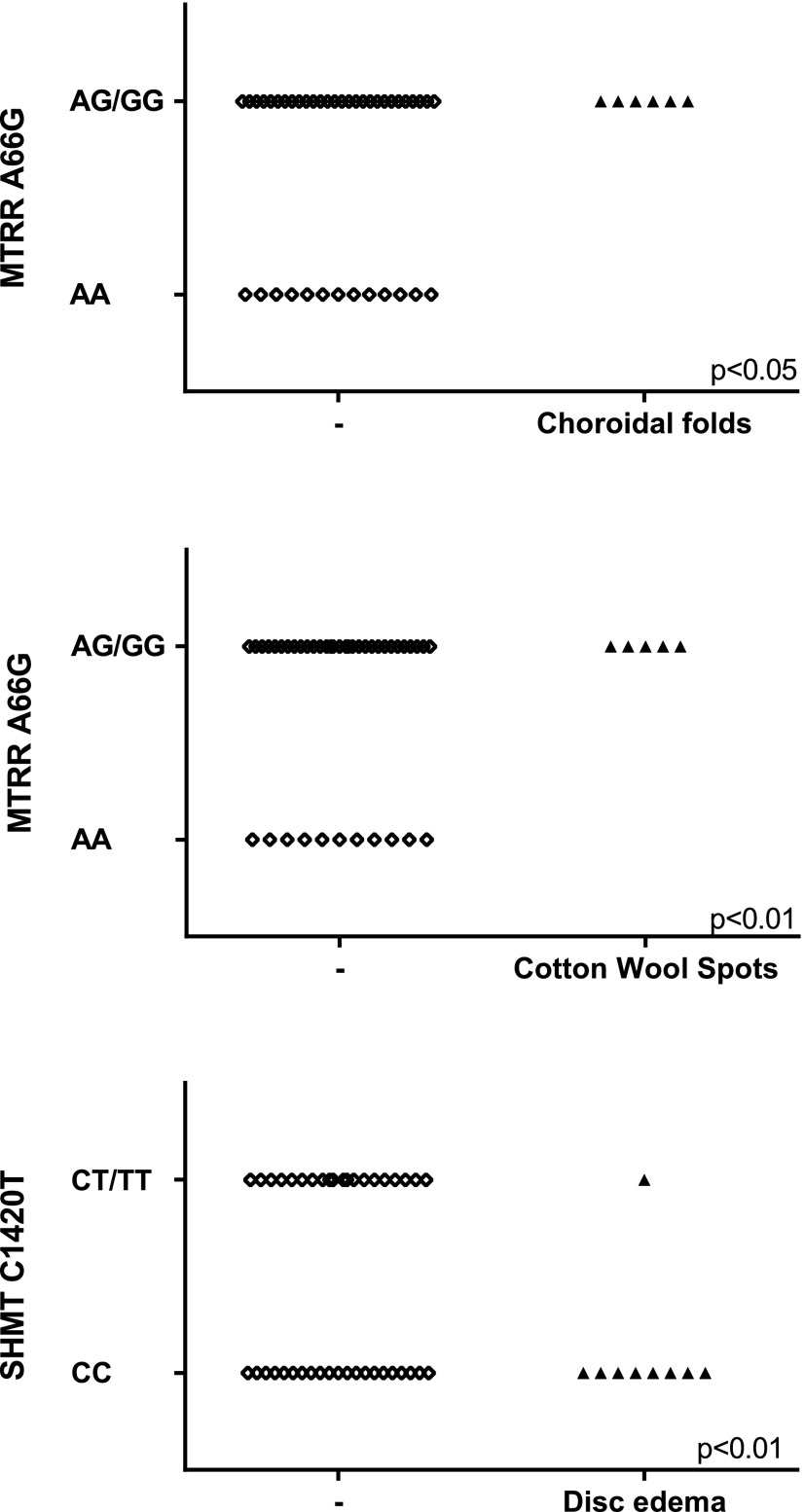
The MTRR 66 G allele was more prevalent among astronauts with choroidal folds (P < 0.05) and cotton wool spots (P < 0.01; Table 4 and Fig. 2). In contrast, the common C allele of SHMT1 1420 was positively associated with disc edema (P < 0.05; Table 4 and Fig. 2). None of the 5 SNPs studied in this protocol were related to globe flattening or to cycloplegic refraction change in diopters after flight.
TABLE 4.
Somers’ D associations and risk allele frequency for 2 SNPs and postflight ophthalmic outcomes
| MTRR 66 A→G |
SHMT1 1420 C→T |
|||
|---|---|---|---|---|
| Ophthalmic outcome | Somers’ D | G allele frequency (case/noncase) | Somers’ D | C allele frequency (case/noncase) |
| Optic disc edema | — | −0.36** | 0.94/0.75 | |
| Choroidal folds | 0.37* | 0.67/0.43 | — | |
| Cotton wool spot | 0.53** | 0.80/0.46 | — | |
There were no significant associations between MTHFR 677 C→T, MTHFR 1298 A→C, or CBS 844ins68 and disc edema, choroidal folds, globe flattening, cotton wool spots, or change in diopters. Forty-nine subjects were evaluated for optic disc edema and choroidal folds and 39 for cotton wool spots.
P < 0.05 (unadjusted);
P < 0.01 (unadjusted).
Figure 2.
MTRR and SHMT genotypes of astronauts with choroidal folds, cotton wool spots, and optic disc edema. Each point represents an individual astronaut. Significance of Somers' D association is indicated on each graph. Genotypes were merged (i.e., AG/GG) to ensure that the data are not identifiable.
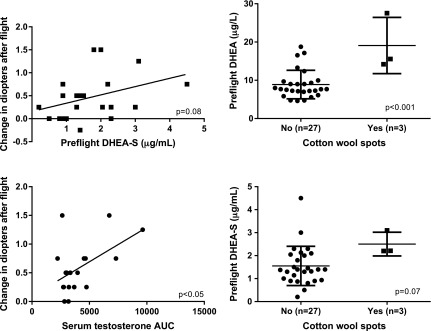
Landing-day RBC folate was negatively associated with globe flattening (P < 0.05), and landing-day EAST activation was positively associated with globe flattening (P < 0.01) and change in diopters (P < 0.05), all indicating that a lower vitamin B6 status and lower folate status were associated with more ophthalmic changes after flight. The AUC of in-flight ferritin and serum and urinary testosterone, as well as preflight DHEA, were higher in individuals with cotton wool spots (Fig. 1 and Table 5). DHEA-S tended to be higher (P = 0.07) in those with cotton wool spots.
Figure 1.
Preflight DHEA-S tended to be associated with change in diopters in the eye with the biggest postflight change (n = 23) and with the incidence of cotton wool spots (P = 0.08 and P = 0.07, respectively). The increase in serum testosterone during flight (area under the curve) was related to the change in diopters after flight (n = 19). P < 0.05. Each symbol represents an individual value, and horizontal lines represent the mean and sd. Unadjusted P values are presented on each graph.
TABLE 5.
Somers’ D associations between ophthalmic outcomes and markers of B-vitamin status, ferritin, androgen status, and atmospheric CO2
| Marker | Choroidal folds | Cotton wool spots | Globe flattening | Optic disc edema | Change in diopters |
|---|---|---|---|---|---|
| Postflight RBC folate | — | — | −0.42* | — | — |
| Postflight EAST | — | — | 0.51** | — | 0.36* |
| Postflight EGR | — | — | — | ||
| Postflight MMA | — | — | — | — | — |
| In-flight ferritin AUC | −0.46* | 0.50* | — | — | — |
| In-flight serum testosterone AUC | — | 0.56* | — | — | — |
| In-flight urine testosterone AUC | — | 0.49* | — | — | — |
| Preflight DHEA | — | 0.85*** | — | — | 0.29* |
| Preflight DHEA-S | — | 0.79*** | — | — | |
| In-flight mission average atm CO2 | — | 0.53** | — | — |
EGR, erythrocyte glutathione reductase; MMA, methylmalonic acid. Postflight refers to landing-day values.
P < 0.05 (unadjusted);
P < 0.01 (unadjusted);
P < 0.001 (unadjusted).
Serum testosterone response during flight (as assessed by the AUC) was positively associated with the change in diopters after flight (Fig. 1; P < 0.05). Preflight DHEA-S tended to be related to the change in diopters after flight (P = 0.08). Average mission atmospheric CO2 was also higher in crewmembers with cotton wool spots (Table 5; P < 0.01).
Untargeted metabolomics analyses suggested that alterations occurred in lipid and carbohydrate metabolism, but the q values, an index that accounts for false discoveries when multiple comparisons are made, were quite large, because of the small sample size. Metabolites that were significantly different between groups (P < 0.05 and q < 0.2) or metabolites with at least 2 other significant metabolites in the same pathway (regardless of q value) are shown in Table 6. Crewmembers with optic disc edema had higher concentrations of metabolites from the methionine pathway than did those without disc edema. Also, several lipid concentrations were higher in crewmembers with disc edema. Those with a change of at least 0.5 diopters in 1 eye had higher concentrations of mannose and galactose metabolites, as well as higher myo-, chiro-, and scyllo-inositol. The ratio of myo-inositol to chiro-inositol was lower in these individuals. Crewmembers with cotton wool spots after flight had higher concentrations of some metabolites involved in steroid metabolism and xanthine metabolism.
TABLE 6.
Fold changes in fasted serum metabolite concentrations in astronauts with and without pathologic optical changes after flight
| Outcome and metabolic pathway | Metabolite | Change |
|---|---|---|
| Disc edema, total n = 48 | ||
| Histidine metabolism | 1-Methylhistidine | 1.30 |
| Imidazole propionate | 1.64 | |
| 1-Methylimidazoleacetate | 1.45 | |
| Methionine metabolism | 2-Aminobutyrate | 1.29 |
| Hypotaurine | 1.80 | |
| N-acetyltaurine | 1.28 | |
| Lipid metabolism | 2-Hydroxyadipate | 1.63 |
| 1-Palmitoylglycerophosphocholine (16:0) | 1.26 | |
| 2-Palmitoylglycerophosphocholine | 1.16 | |
| 1-Stearoylglycerophosphocholine (18:0) | 1.28 | |
| 2-Stearoylglycerophosphocholine | 1.22 | |
| 1-Oleoylglycerophosphocholine (18:1) | 1.39 | |
| 1-Arachidonoylglycerophosphocholine (20:4n6) | 1.27 | |
| 1-Stearoylglycerophosphoethanolamine | 1.30 | |
| 1-Arachidonoylglycerophosphate | 1.28 | |
| Palmitoyl-arachidonoyl-glycerophosphocholine (2) | 1.26 | |
| Pimelate | 1.52 | |
| Change in diopters, total n = 31 | ||
| Carbohydrate metabolism | Mannose | 1.21 |
| Galactonate | 2.41 | |
| Glucuronate | 1.25 | |
| N-acetylneuraminate | 1.41 | |
| Inositol metabolism | Myo-inositol | 1.27 |
| Chiro-inositol | 2.42 | |
| Scyllo-inositol | 1.40 | |
| Myo-inositol:chiro-inositol | 0.52 | |
| Cotton wool spots, total n = 39 | ||
| Steroid metabolism | Epiandrosterone sulfate | 1.15 |
| 5α-Androstan-3β,17β-diol monosulfate (2) | 1.12 | |
| 5α-Androstan-3β,17β-diol disulfate | 1.47 | |
| 5α-Androstan-3α,17β-diol monosulfate (2) | 2.30 | |
| Xanthine metabolism | 3,7-Dimethylurate | 1.49 |
| 3-Methylxanthine | 1.62 | |
| 7-Methylxanthine | 1.39 |
All of the fold changes in this table were significant at q < 0.2, or the metabolite was in a metabolic pathway in which at least 2 other metabolites were significant. P < 0.05.
DISCUSSION
A key finding of our study is the higher frequency of the MTRR 66 G minor allele in astronauts who experienced choroidal fold and cotton wool spots after a long-duration spaceflight. That is, all of the astronauts with the homozygous GG genotype exhibited choroidal folds and cotton wool spots. Furthermore, none of the individuals with the MTRR 66 AA genotype had evidence of choroidal folds or cotton wool spots after ISS missions. A second key finding of this study is the apparent protective nature of the SHMT 1420 T minor allele with regard to optic disc edema. None of the subjects with the SHMT 1420 TT genotype had evidence of disc edema after flight. These data provide initial evidence of a genetic predisposition to ophthalmic changes in astronauts. Although the mechanism of this effect is unknown, the data provide a path toward understanding, and ideally, preventing or treating these conditions. Whether these genotypes are functionally involved in the biochemical and physiologic mechanisms associated with the ophthalmic changes or they constitute genetic markers for other potential genetic associations is presently unclear.
Consistent with these findings, others have shown that the SHMT 1420 TT genotype protects against coronary artery disease, whereas MTRR 66 GG has been found to increase oxidative stress (14). In another study, the prevalence of a homozygous MTHFR 677 TT genotype was higher in patients with idiopathic intracranial hypertension (IIH) (6); elevated intracranial hypertension is thought to be a causative factor in spaceflight-related ophthalmic changes (2). The MTHFR 677 C→T polymorphism was not a significant factor in our analysis.
An interesting finding was that the higher homocysteine, increased androgen response, increased incidence of optic disc edema, inositol metabolism, and previously documented increased retinal nerve fiber layer (1, 15) in astronauts with ocular changes after spaceflight are of a pattern similar to a condition that commonly affects people on Earth—polycystic ovary syndrome (PCOS). PCOS is a condition that affects 8–15% of women and has a broad spectrum of manifestations, including insulin resistance, hyperandrogenism, vascular dysfunction, hyperlipidemia, glucose intolerance, and polycystic ovaries (which occurs in about 80% of patients) (16–20). There are several similarities between crewmembers with ophthalmic changes and individuals with PCOS. First, both have higher concentrations of circulating homocysteine than do unaffected persons. Astronauts with spaceflight-induced ophthalmic changes had higher homocysteine concentrations than those who had no changes (3). Patients with PCOS and their first-degree male and female relatives have higher serum homocysteine concentrations than do controls (21–24). Second, both conditions are associated with optic disc edema and decreased visual acuity (1, 6, 25, 26). Third, patients with PCOS are characterized by androgen excess and insulin resistance. Although crewmembers with ophthalmic changes did not have testosterone concentrations different from those of unaffected crewmembers before or after flight, the AUC for serum (Fig. 1; Table 5) and urinary testosterone during flight was related to the prevalence of cotton wool spots, and the preflight concentration of serum DHEA was higher in individuals with cotton wool spots and in those with a change in diopters. Also, although no data are available for insulin resistance among crewmembers with ophthalmic changes, the metabolomics data provide clues that glucose–mannose metabolism may be altered in those individuals.
Ocular mechanical and structural similarities are also evident. Ocular blood flow velocity of the ophthalmic artery, central retinal artery, and posterior ciliary artery in patients with PCOS is higher, and vascular resistance is decreased (27). Preliminary flight data have shown that central retinal artery blood flow velocity increases during flight (28). Further studies are needed to determine whether individuals with visual changes have greater increases in central retinal artery blood flow velocity than those without changes.
Another similarity between patients with PCOS and astronauts who have spaceflight-induced ophthalmic changes is that both groups have a thicker retinal nerve fiber layer (1, 15). The increased thickness of the retinal nerve fiber layer among patients with PCOS is thought to be secondary to hyperandrogenism, related to the androgen trophic effect on nerves (29). Finally, theca cells of patients with PCOS have lower myo-inositol–to–chiro-inositol (M:C) ratios relative to that of controls, which is consistent with increased insulin sensitivity (30). The metabolomics data from the present study also show a lower M:C ratio in affected individuals.
Despite the similarities between these conditions, there is a major difference: only male astronauts have experienced severe ophthalmic changes after spaceflight (2). Although PCOS obviously affects only women, PCOS-type manifestations are not limited to women. Evidence shows that male relatives of PCOS patients have similar symptoms (24, 31), and some researchers have hypothesized that PCOS occurs in men (32) and that the 1 specific symptom of polycystic ovaries may be a downstream effect of the upstream androgen and insulin pathway disruptions. Furthermore, as mentioned above, not all (80%) women with PCOS actually have polycystic ovaries.
Testosterone treatment can induce IIH, as seen in several case studies of individuals taking testosterone routinely (33, 34), and it is therefore not unreasonable to suggest that the androgen response during flight can affect ophthalmic outcomes. One animal study provided evidence that SHMT1 can be regulated by testosterone (35), which is another potential link between the genetic findings reported herein and androgen response.
This study has some limitations that could prevent finding other more important associations. Although large by spaceflight study standards, the small subject sample is a significant limitation. Nonetheless, with the small number of astronauts flying long-duration space missions, we have included virtually all astronauts for whom data (e.g., detailed ophthalmic examination) were available. Another limiting factor is the small subset of SNPs selected for analysis. The 5 SNPs were selected because they are documented genetic determinants of circulating homocysteine concentrations (36–38), and homocysteine was a metabolite shown to be higher in astronauts with vision changes after long-duration spaceflight (3). Ideally, SNPs of all enzymes of the 1-carbon pathway would have been studied. Another potential limitation is that the genetic relationships observed in this study may be susceptible to population stratification. For example, the differences in the observed allele frequencies of the risk alleles for MTRR and SHMT1 between astronauts with and without ophthalmic changes may be caused by differences in their subregional origins (for example, southern or northern Europe), rather than being tied to those particular genes, and the possible derivation should be considered and evaluated further.
We have found that the minor allele of MTRR 66 and the major allele for SHMT1 are associated with ophthalmic changes and that, when the presence of these alleles is combined with lower B-vitamin status (folate, B6, and riboflavin in particular), the risk of visual deterioration in astronauts is greater. A higher androgen concentration (DHEA) before launch or a larger response (testosterone) during spaceflight may also be involved. Thus, we have identified a relationship between 1-carbon pathway polymorphisms and nutritional, biochemical, and endocrine factors among individuals who experience ophthalmic changes during and after spaceflight. More work is needed to identify specific mechanisms leading to these vision changes. This line of research may lead to methods for treating or preventing these outcomes in at-risk astronauts. There are many metabolic similarities between crewmembers with ophthalmic changes and individuals with the terrestrial condition PCOS. Thus, understanding the relationships between genetics, physiology, and the environment may not only benefit future space exploration missions, but could profoundly affect advances in terrestrial medicine.
Acknowledgments
The authors thank the astronauts for their participation in and support of this study; the staff of the NASA Johnson Space Center Nutritional Biochemistry Laboratory for their assistance in all aspects of the project; the ISS Medical Project, specifically T. Bauer, for help with coordinating the project; the NASA Lifetime Surveillance of Astronaut Health team for their help with eye- and vision-related data; and J. Krauhs for editorial assistance. This project was funded by the NASA Human Research Program’s Human Health and Countermeasures Element, by the German Federal Ministry for Economics and Technology/DLR Forschung unter Weltraumbedingungen Grant 50WB0931 (to M.A.H.), and by the U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK56350 (to S.H.Z.). S.M.S., S.R.Z., and M.A.H. designed the research; S.M.S. and S.R.Z. oversaw data collection and management of the biological sample collections and analyses; C.R.G. and T.H.M. oversaw data collection and management of the vision and related data; J.M.K. conducted metabolomics analyses; and R.P.S. performed statistical analysis; and all authors were involved in interpreting the data and preparing the manuscript. All authors read and approved the final manuscript. S.M.S. had primary responsibility for the content of the final version. The authors declare no conflicts of interest.
Glossary
- AIC
Akaike information criterion
- AUC
area under the curve
- CBS
cystathionine β-synthase
- DHEA
dehydroepiandrosterone
- DHEA-S
dehydroepiandrosterone sulfate
- EAST
erythrocyte aspartate aminotransferase
- IIH
idiopathic intracranial hypertension
- ISS
International Space Station
- NASA
National Aeronautics and Space Administration
- PCOS
polycystic ovary syndrome
- RBC
red blood cell
- SHMT
serine hydroxymethyltransferase
- SNP
single-nucleotide polymorphism
REFERENCES
- 1.Mader T. H., Gibson C. R., Pass A. F., Kramer L. A., Lee A. G., Fogarty J., Tarver W. J., Dervay J. P., Hamilton D. R., Sargsyan A., Phillips J. L., Tran D., Lipsky W., Choi J., Stern C., Kuyumjian R., Polk J. D. (2011) Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 118, 2058–2069 [DOI] [PubMed] [Google Scholar]
- 2.Marshall-Bowman K., Barratt M. R., Gibson C. R. (2013) Ophthalmic changes and increased intracranial pressure associated with long duration spaceflight: an emerging understanding. Acta Astronaut. 87, 77–87 [Google Scholar]
- 3.Zwart S. R., Gibson C. R., Mader T. H., Ericson K., Ploutz-Snyder R., Heer M., Smith S. M. (2012) Vision changes after spaceflight are related to alterations in folate- and vitamin B-12-dependent one-carbon metabolism. J. Nutr. 142, 427–431 [DOI] [PubMed] [Google Scholar]
- 4.Davis S. R., Quinlivan E. P., Shelnutt K. P., Maneval D. R., Ghandour H., Capdevila A., Coats B. S., Wagner C., Selhub J., Bailey L. B., Shuster J. J., Stacpoole P. W., Gregory J. F. III (2005) The methylenetetrahydrofolate reductase 677C→T polymorphism and dietary folate restriction affect plasma one-carbon metabolites and red blood cell folate concentrations and distribution in women. J. Nutr. 135, 1040–1044 [DOI] [PubMed] [Google Scholar]
- 5.Kluijtmans L. A., Young I. S., Boreham C. A., Murray L., McMaster D., McNulty H., Strain J. J., McPartlin J., Scott J. M., Whitehead A. S. (2003) Genetic and nutritional factors contributing to hyperhomocysteinemia in young adults. Blood 101, 2483–2488 [DOI] [PubMed] [Google Scholar]
- 6.Glueck C. J., Aregawi D., Goldenberg N., Golnik K. C., Sieve L., Wang P. (2005) Idiopathic intracranial hypertension, polycystic-ovary syndrome, and thrombophilia. J. Lab. Clin. Med. 145, 72–82 [DOI] [PubMed] [Google Scholar]
- 7.Finsterer J., Kuntscher D., Brunner S., Krugluger W. (2007) Pseudotumor cerebri from sinus venous thrombosis, associated with polycystic ovary syndrome and hereditary hypercoagulability. Gynecol. Endocrinol. 23, 179–182 [DOI] [PubMed] [Google Scholar]
- 8.Klein A., Stern N., Osher E., Kliper E., Kesler A. (2013) Hyperandrogenism is associated with earlier age of onset of idiopathic intracranial hypertension in women. Curr. Eye Res. 38, 972–976 [DOI] [PubMed] [Google Scholar]
- 9.Smith S. M., Heer M., Wang Z., Huntoon C. L., Zwart S. R. (2012) Long-duration space flight and bed rest effects on testosterone and other steroids. J. Clin. Endocrinol. Metab. 97, 270–278, correction 3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin S. Y., Fauman E. B., Petersen A. K., Krumsiek J., Santos R., Huang J., Arnold M., Erte I., Forgetta V., Yang T. P., Walter K., Menni C., Chen L., Vasquez L., Valdes A. M., Hyde C. L., Wang V., Ziemek D., Roberts P., Xi L., Grundberg E., Waldenberger M., Richards J. B., Mohney R. P., Milburn M. V., John S. L., Trimmer J., Theis F. J., Overington J. P., Suhre K., Brosnan M. J., Gieger C., Kastenmüller G., Spector T. D., Soranzo N., Soranzo N.; Multiple Tissue Human Expression Resource (MuTHER) Consortium (2014) An atlas of genetic influences on human blood metabolites. Nat. Genet. 46, 543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zwart S. R., Crawford G. E., Gillman P. L., Kala G., Rodgers A. S., Rogers A., Inniss A. M., Rice B. L., Ericson K., Coburn S., Bourbeau Y., Hudson E., Mathew G., Dekerlegand D. E., Sams C. F., Heer M. A., Paloski W. H., Smith S. M. (2009) Effects of 21 days of bed rest, with or without artificial gravity, on nutritional status of humans. J. Appl. Physiol. 107, 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zwart S. R., Morgan J. L., Smith S. M. (2013) Iron status and its relations with oxidative damage and bone loss during long-duration space flight on the International Space Station. Am. J. Clin. Nutr. 98, 217–223 [DOI] [PubMed] [Google Scholar]
- 13.García-Minguillán C. J., Fernandez-Ballart J. D., Ceruelo S., Ríos L., Bueno O., Berrocal-Zaragoza M. I., Molloy A. M., Ueland P. M., Meyer K., Murphy M. M. (2014) Riboflavin status modifies the effects of methylenetetrahydrofolate reductase (MTHFR) and methionine synthase reductase (MTRR) polymorphisms on homocysteine. Genes Nutr. 9, 435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vijaya Lakshmi S. V., Naushad S. M., Seshagiri Rao D., Kutala V. K. (2013) Oxidative stress is associated with genetic polymorphisms in one-carbon metabolism in coronary artery disease. Cell Biochem. Biophys. 67, 353–361 [DOI] [PubMed] [Google Scholar]
- 15.Demir M., Guven D., Koc A., Ozdemir S., Can E. (2013) Retinal nerve fiber layer thickness in women with polycystic ovary syndrome. J. Ophthalmol. 2013, 752186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenner M. M., Taylor H. S., Stachenfeld N. S. (2013) Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors. Am. J. Physiol. Endocrinol. Metab. 305, E818–E825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sprung V. S., Cuthbertson D. J., Pugh C. J., Daousi C., Atkinson G., Aziz N. F., Kemp G. J., Green D. J., Cable N. T., Jones H. (2013) Nitric oxide-mediated cutaneous microvascular function is impaired in polycystic ovary sydrome but can be improved by exercise training. J. Physiol. 591, 1475–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Randeva H. S., Tan B. K., Weickert M. O., Lois K., Nestler J. E., Sattar N., Lehnert H. (2012) Cardiometabolic aspects of the polycystic ovary syndrome. Endocr. Rev. 33, 812–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daan N. M., Louwers Y. V., Koster M. P., Eijkemans M. J., de Rijke Y. B., Lentjes E. W., Fauser B. C., Laven J. S. (2014) Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: who is really at risk? Fertil. Steril. 102, 1444–1451.e3 [DOI] [PubMed] [Google Scholar]
- 20.March W. A., Moore V. M., Willson K. J., Phillips D. I., Norman R. J., Davies M. J. (2010) The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 25, 544–551 [DOI] [PubMed] [Google Scholar]
- 21.Maleedhu P., M V., S S B S., Kodumuri P. K., Devi D V. (2014) Status of homocysteine in polycystic ovary syndrome (PCOS). J. Clin. Diagn. Res. 8, 31–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loverro G., Lorusso F., Mei L., Depalo R., Cormio G., Selvaggi L. (2002) The plasma homocysteine levels are increased in polycystic ovary syndrome. Gynecol. Obstet. Invest. 53, 157–162 [DOI] [PubMed] [Google Scholar]
- 23.Grodnitskaya E. E., Kurtser M. A. (2012) Homocysteine metabolism in polycystic ovary syndrome. Gynecol. Endocrinol. 28, 186–189 [DOI] [PubMed] [Google Scholar]
- 24.Yilmaz M., Bukan N., Ersoy R., Karakoç A., Yetkin I., Ayvaz G., Cakir N., Arslan M. (2005) Glucose intolerance, insulin resistance and cardiovascular risk factors in first degree relatives of women with polycystic ovary syndrome. Hum. Reprod. 20, 2414–2420 [DOI] [PubMed] [Google Scholar]
- 25.Shin S. H., Kim Y. M., Kim H. Y., Lee Y. J., Nam S. O. (2014) Idiopathic intracranial hypertension associated with polycystic ovarian syndrome. Pediatr. Int. 56, 411–413 [DOI] [PubMed] [Google Scholar]
- 26.Avisar I., Gaton D. D., Dania H., Stiebel-Kalish H. (2012) The prevalence of polycystic ovary syndrome in women with idiopathic intracranial hypertension. Scientifica (Cairo) 2012, 708042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Örnek N., İnal M., Tulmaç O. B., Özcan-Dağ Z., Örnek K. (2015) Ocular blood flow in polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 41, 1080–1086 [DOI] [PubMed] [Google Scholar]
- 28.Sirek A. S., Garcia K., Foy M., Ebert D., Sargsyan A., Wu J. H., Dulchavsky S. A. (2014) Doppler ultrasound of the central retinal artery in microgravity. Aviat. Space Environ. Med. 85, 3–8 [DOI] [PubMed] [Google Scholar]
- 29.Jones K. J. (1988) Steroid hormones and neurotrophism: relationship to nerve injury. Metab. Brain Dis. 3, 1–18 [DOI] [PubMed] [Google Scholar]
- 30.Heimark D., McAllister J., Larner J. (2014) Decreased myo-inositol to chiro-inositol (M/C) ratios and increased M/C epimerase activity in PCOS theca cells demonstrate increased insulin sensitivity compared to controls. Endocr. J. 61, 111–117 [DOI] [PubMed] [Google Scholar]
- 31.Liu D. M., Torchen L. C., Sung Y., Paparodis R., Legro R. S., Grebe S. K., Singh R. J., Taylor R. L., Dunaif A. (2014) Evidence for gonadotrophin secretory and steroidogenic abnormalities in brothers of women with polycystic ovary syndrome. Hum. Reprod. 29, 2764–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurzrock R., Cohen P. R. (2007) Polycystic ovary syndrome in men: Stein-Leventhal syndrome revisited. Med. Hypotheses 68, 480–483 [DOI] [PubMed] [Google Scholar]
- 33.Kapoor K. G. (2009) Secondary pseudotumour cerebri in a patient undergoing sexual reassignment therapy [comment]. Clin. Exp. Optom. 92, 519–520, author reply 520 [DOI] [PubMed] [Google Scholar]
- 34.Mowl A. D., Grogg J. A., Klein J. (2009) Secondary pseudotumour cerebri in a patient undergoing sexual reassignment therapy. Clin. Exp. Optom. 92, 449–453 [DOI] [PubMed] [Google Scholar]
- 35.Sanborn T. A., Kowle R. L., Sallach H. J. (1975) Regulation of enzymes of serine and one-carbon metabolism by testosterone in rat prostate, liver, and kidney. Endocrinology 97, 1000–1007 [DOI] [PubMed] [Google Scholar]
- 36.Gaughan D. J., Kluijtmans L. A., Barbaux S., McMaster D., Young I. S., Yarnell J. W., Evans A., Whitehead A. S. (2001) The methionine synthase reductase (MTRR) A66G polymorphism is a novel genetic determinant of plasma homocysteine concentrations. Atherosclerosis 157, 451–456 [DOI] [PubMed] [Google Scholar]
- 37.Nilsson T. K., Böttiger A. K., Henríquez P., Serra Majem L. (2014) MTHFR polymorphisms and serum cobalamin affect plasma homocysteine concentrations differentially in females and males. Mol. Med. Rep. 10, 2706–2712 [DOI] [PubMed] [Google Scholar]
- 38.Liang S., Zhou Y., Wang H., Qian Y., Ma D., Tian W., Persaud-Sharma V., Yu C., Ren Y., Zhou S., Li X. (2014) The effect of multiple single nucleotide polymorphisms in the folic acid pathway genes on homocysteine metabolism. BioMed Res. Int. 2014, 560183 [DOI] [PMC free article] [PubMed] [Google Scholar]