Abstract
Activation of the intrarenal renin angiotensin system (RAS) is believed to play an important role in the development of hypertension and cystogenesis in autosomal dominant polycystic kidney disease (ADPKD). Results of clinical studies testing RAS inhibitors in slowing the progression of cystic disease in ADPKD are inconclusive, and we hypothesized that current RAS inhibitors do not adequately suppress intrarenal RAS. For this study, we compared a novel Gen 2 antisense oligonucleotide (ASO) that inhibits angiotensinogen (Agt) synthesis to lisinopril in adult conditional Pkd1 systemic-knockout mice, a model of ADPKD. Six weeks after Pkd1 global gene knockout, the mice were treated with Agt-ASO (66 mg/kg/wk), lisinopril (100 mg/kg/d), PBS (control), or scrambled ASO (66 mg/kg/wk) for 10 wk, followed by tissue collection. Agt ASO resulted in significant reduction in plasma, liver, and kidney Agt, and increased kidney renin compared with control treatments. Kidneys from Agt-ASO-treated mice were not as enlarged and showed reduced cystic volume compared with lisinopril or control treatments. Blood pressure was better controlled with lisinopril than with Agt-ASO. Agt-ASO suppressed cell proliferation in both cystic and noncystic cells compared with lisinopril and control treatments. However, Agt-ASO did not reduce cell proliferation in liver, which indicates that Agt-ASO targets cell signaling pathways that specifically suppresses cystogenesis in the kidney. These data suggest that Agt-ASO effectively attenuates intrarenal RAS and therefore can be a novel and effective agent for treating ADPKD.—Saigusa, T., Dang, Y., Mullick, A. E., Yeh, S. T., Zile, M. R., Baicu, C. F., Bell, P. D. Suppressing angiotensinogen synthesis attenuates kidney cyst formation in a Pkd1 mouse model.
Keywords: renin angiotensin system, antisense oligonucleotide, polycystic kidney disease, hypertension
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic disorder that results in multiple kidney cysts, with greater than 50% of affected patients eventually developing end-stage renal disease (1). Candidate drugs that target the signaling pathways involved in the pathogenesis of ADPKD have been shown to slow cyst progression in PKD murine models. Several of these agents have been tested in humans, demonstrating equivocal effectiveness (2), but at the present time, there are no U.S. Food and Drug Administration (FDA)–approved drugs to treat ADPKD. One of the pathways that stimulate cystic growth is the renin–angiotensin system (RAS), which is activated, in part, by cystic compression of kidney tissue (3, 4). Activation of intrarenal RAS leads to the formation of angiotensin (Ang)-II, which binds to the angiotensin type (AT) 1 receptor, promoting proliferation in both kidney epithelial and interstitial cells and contributing to cyst growth and expansion (5, 6). Ang-converting enzyme (ACE) inhibitors lower blood pressure (BP) and inhibit kidney cyst expansion in rodents (7, 8). In human ADPKD, BP is relatively well controlled with ACE inhibitors, but the effects on slowing kidney cyst formation are inconsistent (9). Lack of a consistent effect of ACE inhibitors could be related to the chymase pathway, which is an alternative AngII-generating pathway that is elevated in advanced ADPKD (10) and may play a role in stimulating cyst growth. Generation of AngII through the chymase pathway is not blocked by ACE inhibitors. In a recent clinical trial, a combination of an ACE inhibitor with an AT1 receptor blocker (telmisartan + lisinopril) failed to alter the time course of changes in total kidney volume and kidney function, compared with lisinopril alone, in both early (11) and late ADPKD (12). One explanation for this finding is that RAS inhibitors adequately suppress systemic RAS and lower BP, but do not effectively block intrarenal RAS in cystic structures and dilated tubules. Studies have shown that renin, AngII, and angiotensinogen (Agt) are produced by some cysts and dilated tubules and are also present in cystic fluid (4, 13, 14). Hence, intrarenal Agt-generated AngII may contribute to increased BP and kidney cyst expansion. This repetitive cycle of cyst expansion–RAS activation, may contribute to a positive feedback cycle that perpetuates cyst growth, thereby leading to eventual renal failure. Therefore, it is essential to find an effective means of targeting components of intrarenal RAS (15).
A novel therapeutic approach to the suppression of critical components of RAS is to use an antisense oligonucleotide (ASO) directed toward Agt. Although present in several locations, Agt is most abundant in liver, renal proximal tubular epithelium, and cystic cells derived from proximal tubules. Agt is the initial substrate of the RAS cascade, and abolishing Agt synthesis may result in an efficient intrarenal RAS blockade. Indeed, an ASO that inhibits Agt demonstrated excellent cellular uptake in kidney from a Pkd2-knockout mouse and significant reduced kidney cyst expansion, compared with vehicle-treated knockout mice (16). Although Agt-ASO therapy produces a global reduction in Agt, it appears to be particularly effective in blocking the intrarenal Agt located in the proximal tubule. Thus, it may be much more effective than a systemic ACE inhibitor or AT1 receptor blocker in specifically inhibiting the intrarenal generation of AngII. We have recently shown in mice that 3 months after global Pkd1 gene knockout, there is hypertension, focal kidney cysts, increased renal Agt, and elevated urinary Agt (17). Agt-ASO has not been tested in the Pkd1 mouse model, which resembles the most prevalent form of human ADPKD. In addition, results of Agt-ASO treatment have not been compared with those obtained with an ACE inhibitor. In the current study, a novel Gen 2 Agt-ASO given to a Pkd1-knockout mouse inhibited Agt synthesis, significantly suppressed intrarenal RAS, and slowed kidney cyst progression, compared with lisinopril.
MATERIALS AND METHODS
Mouse and genotyping
Pkd1 floxed-allele mouse was provided by Dr. Gregory Germino (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, USA). It is also available at The Jackson Laboratory (Bar Harbor, ME, USA; B6.129S4 Pkd1tm2Ggg/J, stock number 010671) (18). Pkd1 floxed allele mice possess loxP (loss of crossover in P1) sites on either side of exons 2–4 of Pkd1. Pkd1 conditional-knockout mice were generated by crossbreeding Pkd1 floxed-allele female mice with male mice that express tamoxifen-inducible site-specific DNA recombinase [Cre (protein)] (CAGG-creER) (19). Deactivation of the Pkd1 gene results in >50% efficiency in knockout. Genotyping was performed by PCR with published primer sequences (18). Mice were maintained in accordance with the Institutional Animal Care and Use Committee regulations at the Medical University of South Carolina.
Experimental protocol
Female and male Pkd1 conditional floxed-allele mice expressing Cre were randomly assigned to Agt-ASO, lisinopril, PBS, or scrambled ASO groups. For induction of Cre, tamoxifen (Sigma-Aldrich, St. Louis, MO, USA) was administered every other day for 3 doses at 8–12 wk of age. Tamoxifen was dissolved in corn oil (5 mg/20 g, i.p. body weight). Six weeks after global Pkd1 gene knockout, the mice were given Agt-ASO or lisinopril (Tokyo Chemical Industry, Tokyo, Japan) in drinking water, and PBS or scrambled ASO intraperitoneally for a total of 10 wk. Both ASOs were administered weekly at 100 mg/kg/wk, i.p. for the first 4 wk and then 50 mg/kg/wk for the remaining 6 wk of treatments. Lisinopril was given 100 mg/kg/d in drinking water. Systolic BP (SBP) was measured by tail cuff (Kent Scientific, Torrington, CT, USA). Tail cuff systolic BP data are the average of 10–15 measurements obtained from each mouse (20).
ASOs
ASOs were obtained from Isis Pharmaceuticals (Carlsbad, CA, USA). Agt-ASOs were 20-mer second generation MOE gapmers of the 5-10-5 design. Specifically, the phosphorothioate oligonucleotides contained 2′-O-(2-methoxyethyl)-modified ribonucleoside (2′-MOE) groups at positions 1–5 and 16–20, with 2′-deoxynucleosides at positions 6–15. The Agt-ASO was chosen after a screening of ∼150 prospective leads in primary murine hepatocytes. The top in vitro leads were then tested in C57BL/6 mice, and the lead Agt-ASO was chosen on the basis of renal activity and tolerability. The scrambled ASO does not hybridize to any known target and was used to control for any ASO-class effects that could affect cystogenesis, kidney function, or both.
Real-time quantitative PCR
Quantitative (q)RT-PCR mRNA analysis was performed with TaqMan primer probes (Thermo Fisher-Applied Biosystems, Foster City, CA, USA). In brief, total RNA was extracted from whole tissue with the RNeasy RNA isolation kit (Qiagen, Valencia, CA, USA). Samples (50 ng) were subjected to qRT-PCR analysis with commercial reagents (Thermo Fisher-Invitrogen, Carlsbad, CA, USA) and analyzed with the ABI StepOne Plus Sequence Detector (Thermo Fisher-Applied Biosystems). TaqMan primers and probe are described in Supplemental Materials. Values were normalized to total RNA via Ribogreen measurement (Thermo Fisher-Invitrogen). The PCR probes were labeled with 5′-FAM (a 6-carboxyfluorescein reporter) and 3′-TAMRA [a 5(6)-carboxytetramethyl rhodamine quencher]. After 40 amplification cycles, absolute values were obtained with SDS analysis software (Thermo Fisher-Applied Biosystems). Values were normalized to total RNA by Ribogreen.
Primers and probe
The following primers were used: Agt (forward: 5′-AGGACCCATGAAGAAACTGCAT-3′, reverse: 5′-CGTCCTTGGAGACCCTTCTG-3′, probe: 5′-CAGCACCATCAACCTCCAAAAGGCC-3′); Agt-ASO: 5′-TCTTCCACCCTGTCACAGCC-3′; and scrambled ASO: 5′-CCTTCCCTGAAGGTTCCTCC-3′.
Pan-ASO antibody
Pan-ASO antibody was used to determine tissue distribution of Agt-ASO. This antibody was developed in a rabbit and is capable of detecting the ASO regardless of the ASO sequence (21). The antibody was generated with keyhole limpet hemocyanin (KLH) conjugation to an ASO. ASOs are haptens that are antigenic when conjugated to a protein; therefore, KLH conjugation makes an ASO antigenic in a rabbit.
Western blot analysis
Mouse kidney and liver were homogenized, and proteins were extracted. Protease and phosphatase inhibitors (Thermo Fisher Scientific, Waltham, MA, USA) were added to each sample, and equal amounts of protein was resolved on a 10–20% SDS polyacrylamide gel and transferred to PVDF membranes (Thermo Fisher, Grand Island, NY, USA). The membrane was blocked with 5% nonfat milk followed by incubation with rabbit anti-Agt antibody (1:1000, 28101A; Immunobiological Laboratories, Minneapolis, MN, USA), overnight. After it was washed, the membrane was incubated with horseradish peroxidase (HRP)–conjugated secondary antibody (EMS-Millipore, Billerica, MA, USA). Bands were visualized with ECL (Amersham International, Little Chalfont, United Kingdom). Data were normalized by GAPDH.
Measurement of creatinine, Agt, and aldosterone
A 24 h urine collection was made on ice via metabolic cages. Mouse plasma was obtained via aortic collection after euthanization. Urine creatinine was measured with a QuantiChrom assay kit (BioAssay Systems, Hayward, CA, USA). Plasma and urine Agt and urine aldosterone were measured with mouse ELISA kits (Agt; Clontech, Mountain View, CA, USA; aldosterone: Enzo Life Sciences, Farmingdale, NY, USA), according to the manufacturers’ instructions.
Histologic analysis
For light microscopy, 4 μm sections were cut from paraffin-embedded kidneys and stained with hematoxylin-eosin (H&E), Sirius red (Sigma-Aldrich), or Agt-ASO (21). After they were deparaffinized, the slides were incubated for 10 min with an endogenous peroxide blocker (S2003; Dako, Carpinteria, CA, USA) and then incubated for 3 min with proteinase K. Next, the were incubated with a protein-blocking solution (Background Buster; Innovex, Richmond, CA, USA) for 30 min before incubation with the Agt-ASO antibody for 1 h at 1:100. Sections were incubated with a donkey anti-rabbit HRP-conjugated Ab for 30 min at 1:200 followed by 3,3′-diaminobenzidine (DAB) chromogen for 5 min. Sections were counterstained (Autostainer; Leica, Wetzlar, Germany), and images were digitally captured (Aperio ScanScope; Leica). Between each step, the sections were washed (two times for 5 min each) with Tris-buffered saline (TBS)–Tween (TBST) buffer.
Quantification of cysts
H&E-stained sections were used to determine cyst volume. To avoid variation in field selection, at least 10–12 different kidney cortex or liver images were taken randomly in increments of 30–45° from the hilum, to cover the entire kidney or liver. Cysts were defined as epithelium lined, hollow structures, and the degree of involvement was calculated as a percentage of total kidney or liver area per image (ImageJ, U.S. National Institutes of Health).
Immunofluorescence
After paraffin-embedded kidney and liver sections were deparaffinized and rehydrated, they were boiled in Tris-EDTA for 10 min for antigen retrieval. The slides were blocked with 5% bovine serum albumen (BSA) for 1 h at room temperature. The slides were then incubated overnight at 4°C with either rabbit polyclonal anti-renin antibody for kidney sections (1:200, 54371; AnaSpec, Fremont, CA, USA) or antiproliferating cell nuclear antigen (PCNA) antibody for kidney and liver sections (1:200, 18197; Abcam, Cambridge, MA, USA) and then washed 5 times with TBST, followed by incubation with fluorescein labeled Lotus tetragonolobus lectin (LTL) for kidney sections (FL-1321, 10 μg/ml; Vector Laboratories, Burlingame, CA, USA), overnight at 4°C. After the slides were washed with TBS, Hoechst (1:500) was added to the last wash at 1:500, and the sections were mounted with mounting medium. The slides were examined by confocal laser microscopy (Leica). For PCNA+ cell counts, at least 10 kidney images (×20) were taken randomly at each of 90, 180, and 270° from the hilum to avoid variation in field selection. Laser settings were fixed during image capture among all treatment groups. PCNA+ cells per 1000 nuclei were calculated for both cystic and noncystic tubules.
Statistical analysis
Results are shown as means ± sd. The significance of the results was determined by unpaired t tests or 1-way ANOVA, followed by the Holm-Sidak or Dunnett test for post hoc comparison (Prism 6; GraphPad, La Jolla, CA, USA). P < 0.05 denoted statistical significance.
RESULTS
Agt-ASO significantly suppresses Agt synthesis
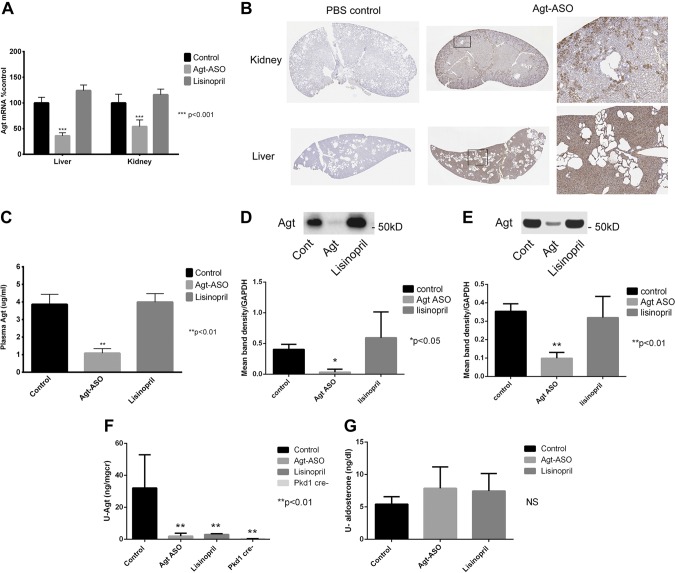
Adult Pkd1 floxed-allele mice were given tamoxifen to induce Cre and knockout (50%) of the Pkd1 protein product, polycystin 1 (18). Six weeks after tamoxifen injection, the mice were given either Agt-ASO (66 mg/kg/wk, i.p; n = 6) or lisinopril (100 mg/kg/d; n = 5) in drinking water or PBS (500 μl/wk, i.p; n = 6) or scrambled ASO (66 mg/kg.wk, i.p.; n = 4) for a total of 10 wk. Because there were no differences in any of the measurements performed between the PBS- and scrambled ASO-treatment groups, these 2 groups were combined and are referred to as the control. At the end of the experimental period, kidney, liver, heart, and plasma were harvested for tissue histology, protein, and mRNA analysis. Whole-body, kidney, and liver weights from each treatment group were measured. As shown in Table 1, the control kidney:BW ratio was higher than that of Agt-ASO mice. However, there were no significant differences in body or liver weights of all treatment groups. All mice were female, with the exception of 4 males in the control group. Figure 1A is a summary of Agt mRNA in the liver and kidney from control, Agt-ASO, and lisinopril groups. After 10 wk of treatment with Agt-ASO, Agt mRNA was significantly suppressed, to 34% baseline in the liver and 57% of baseline in the kidney, compared with that in the control. No Agt mRNA was detected in the heart (data not shown). ASO distribution was assessed with an anti-Agt-ASO antibody in both kidney and liver. Agt-ASO was distributed throughout the kidney, but was predominantly found in cortical proximal tubules. Agt-ASO was uniformly distributed in liver tissue (Fig. 1B). Plasma Agt concentrations after 10 wk of treatment for each group were determined by ELISA (Fig. 1C). Agt-ASO significantly suppressed plasma Agt concentration (1.08 μg/ml vs. control, 3.8 μg/ml, and lisinopril, 3.99 μg/ml). Next, liver and kidney Agt protein was determined by Western blot analysis. Levels in both organs were significantly suppressed by Agt-ASO compared with lisinopril or control (Fig. 1D, E). Twenty-four hour urine collections were performed in each group, and Agt levels were measured to determine the effect of Agt-ASO and lisinopril on intrarenal RAS activity (Fig. 1F). Both Agt-ASO and lisinopril treatments significantly suppressed urinary Agt compared with that in the control. For comparison, urinary Agt was measured in a Cre-negative mouse, which expresses normal levels of polycystin 1 and, as expected, Agt levels were exceedingly low. Twenty-four hour urinary aldosterone measured by ELISA did not reveal significant differences between groups (Fig. 1G). In summary, 10-wk treatment of Agt-ASO significantly decreased liver and kidney Agt mRNA, protein, and plasma Agt concentrations compared with lisinopril and the control. Agt-ASO and lisinopril both significantly suppressed urinary Agt levels vs. control levels.
TABLE 1.
Mouse body, kidney, and liver weight
| Parameter | Agt-ASO | Lisinopril | Control |
|---|---|---|---|
| BW (g) | 25.1 ± 0.9 | 25.2 ± 1.9 | 28.5 ± 2.7 |
| Kidney/BW (%) | 0.90 ± 0.05 | 1.11 ± 0.15 | 1.52 ± 0.20* |
| Liver/BW (%) | 6.8 ± 0.10 | 6.9 ± 0.21 | 6.7 ± 0.12 |
P < 0.05.
Figure 1.
A) Summary of Agt mRNA from liver and kidneys. Agt-ASO treatment (10 wk) significantly decreased Agt mRNA to 34% in the liver and 57% in the kidney compared with the control (100% ; Ribogreen normalized; n = 5–7). Agt mRNA of both the liver and kidney did not change with lisinopril or control treatment. B) Representative histology image stained with pan–anti-ASO antibody of kidney and liver obtained from Agt-ASO-treated and PBS control mouse. ASO staining (brown) was homogeneously dispersed throughout the hepatic tissue (bottom middle and right). Agt-ASO-stained predominantly in the kidney cortex (proximal tubules) and, to some extent, the medulla (top middle and right). Most cyst-lining cells lacked pan-ASO staining. PBS control kidneys showed no ASO staining (top and bottom left). Magnification: left and middle, ×1; right: ×6. C) Summary of plasma Agt concentration. Agt-ASO significantly suppressed plasma Agt concentration to 1.08 μg/ml, compared with control (3.86 μg/ml) and lisinopril (3.99 μg/ml) (n = 5– 7). D, E) Western blot analysis of liver and kidney Agt protein. Liver and kidney Agt proteins were both significantly suppressed in Agt-ASO-treated mice compared with lisinopril-treated or control mice (n = 4–6). F, G) Urinary Agt and aldosterone level by ELISA. Agt-ASO and lisinopril treatment significantly suppressed urinary Agt compared with control. Control: 32 ± 10 ng/mg; Agt-ASO: 1.9 ± 0.8 ng/mg; lisinopril: 3.0 ± 0.2 ng/mg; and Cre-negative: 0.35 ± 0.05 ng/mg. The 24 h urinary aldosterone level was not significantly different in the treatment groups.
Agt-ASO and lisinopril treatment increases renin in the kidney
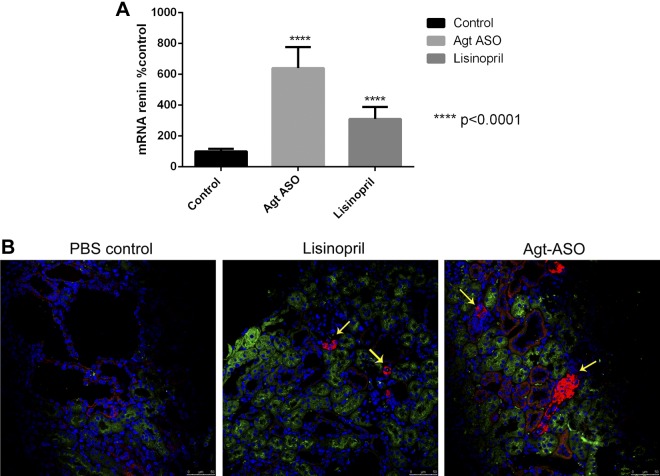
Agt-ASO and lisinopril significantly increased renin mRNA in the kidney by ∼3- to 6-fold compared with levels in the control group (Fig. 2A). Immunofluorescence staining for renin demonstrated significant renin staining in the juxtaglomerular apparatus in both Agt-ASO- and lisinopril-treated kidneys (Fig. 2B). All groups showed renin staining in distal tubules and collecting ducts, which stained negative for the proximal tubule marker LTL.
Figure 2.
A) Summary of renin mRNA from kidneys. There was a significant increase in renin mRNA in the kidneys treated with Agt-ASO (481 ± 92%) and lisinopril (314 ± 58%) compared with control group (4 mo kidneys). B) Representative immunofluorescence image of renin (red), LTL (green), and Hoechst (blue) from 4 mo kidneys. There was intense staining for renin at the juxtaglomerular apparatus (yellow arrow) in Agt-ASO- and lisinopril-treated but not in control kidneys. Magnification ×40.
Effects of suppression of Agt synthesis on BP and kidney cystic disease
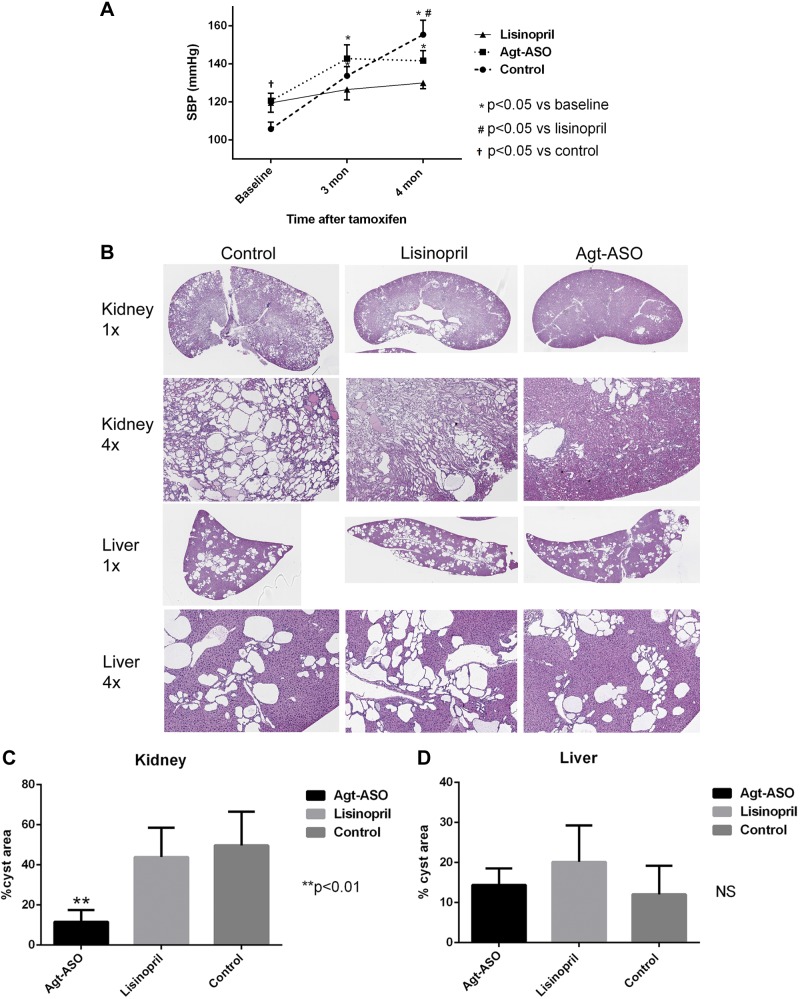
BP was measured by the tail cuff method at baseline (6 wk) and 3 and 4 mo after tamoxifen injection. Figure 3A shows mean SBP data obtained from each treatment group at baseline and at 3 and 4 mo. SBP for Agt-ASO was higher than in the control (PBS and scrambled ASO combined) at baseline. At 3 and 4 mo, there was a significant increase in SBP in both Agt-ASO and control groups compared with baseline. At 4 mo, SBP in the control group was significantly higher than in the lisinopril group, but there were no difference in SBP in the control group compared with the Agt-ASO group. Overall, SBP in the lisinopril group did not change throughout the course of treatment. Despite lower SBP in the lisinopril-treated group, but not in the Agt-ASO-treated group, the development of kidney cysts was significantly attenuated with Agt-ASO treatment; kidney cysts occupied 11% of total kidney area, compared with control (49%) and lisinopril-treated mice (43%) (Fig. 3B, C). That there was no difference in the extent of liver cyst in the Agt-ASO, lisinopril, or control groups was and interesting finding (Fig. 3B, D). These results demonstrate that Agt-ASO treatment compared with lisinopril or control, significantly suppressed kidney, but not liver, cyst formation.
Figure 3.
A) Mean SBP measured at baseline, 3 and 4 mo by tail cuff in polycystin 1–knockout mice. Baseline SBP for Agt-ASO was higher compared with control. SBP in lisinopril group remained unchanged. However, SBP was elevated in both the Agt-ASO and control group at 3 and 4 mo compared with baseline (n = 5–8). SBP in the control group was significantly higher than in the lisinopril group at 4 mo. B, C) Representative H&E image of kidney and liver histology at 4 mo. Left: magnifications. Kidney cysts were significantly attenuated in Agt-ASO mice (11%) compared with lisinopril (43%) and control (49%) mice (n = 4–6). An average of 1 normal-appearing kidney from each group was excluded from the analysis. D) There were no significant differences in liver cystic area in the 3 groups.
Agt-ASO treatment decreases cell proliferation in the kidney
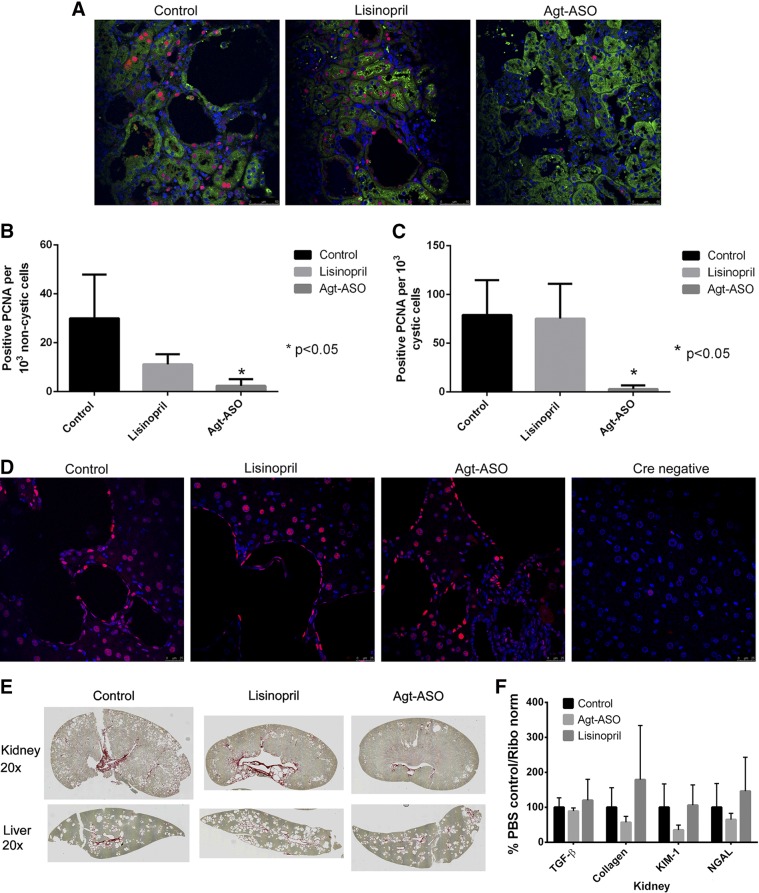
Figure 4A shows representative immunofluorescence images from all 3 groups demonstrating PCNA, a marker for cell proliferation, costained with a proximal tubule marker LTL and nuclear Hoechst stain. Agt-ASO significantly decreased PCNA+ nuclei per 1000 cells in both cystic and noncystic renal tubular areas compared with lisinopril or control kidneys (Fig. 4B, C). However, Agt-ASO, lisinopril, or control treatment did not suppress cell proliferation in the liver (Fig. 4D). For comparison, PCNA was stained in liver tissue from Cre-negative Pkd1 mice, which were negative for cell proliferation. Figure 4E presents representative kidney and liver stained with Sirius red to detect fibrotic tissues. A small amount of fibrosis was detected across all treatment groups that was associated with cystic structures. Similarly, in all 3 groups, there was little evidence of disease-related fibrosis in liver. Overall, fibrosis was minimal in this Pkd1 mouse model at the end of the experiment. There was no difference in renal mRNA levels for TGF-β, collagen, kidney injury molecule (KIM)-1, and neutrophil gelatinase-associated lipocalin (NGAL) in control-, Agt-ASO-, or lisinopril-treated mice (Fig. 4F).
Figure 4.
A) Representative immunofluorescence image of PCNA (red), which is an index of cell proliferation, costained with LTL (green) and Hoechst (blue). Magnification, ×40. Agt-ASO significantly decreased PCNA+ nuclei per 1000 cells in cystic and noncystic renal tubular cells compared with that detected in cells of lisinopril-treated or control kidneys. PCNA+ nuclei per 1000 cells in the Agt-ASO group were quantified for both noncystic (B) and cystic (C) epithelial cells compared with that in cells of lisinopril-treated or control kidneys (n = 4). D) Representative immunofluorescence image of liver stained with PCNA and nuclei. PCNA+ cells were seen in cyst-lining cells and in noncyst hepatic cells, but there were no differences in the Agt-ASO, lisinopril, and control groups. Cre-negative mouse liver, used as a negative control, was PCNA−. E) Representative images of Sirius red staining for fibrosis in kidneys and liver. The staining was predominant in the renal pelvis and along some cysts and was similar across control and treatment groups. In the liver, staining was mostly along the portal vein, biliary structures, and cyst, but the areas of fibrosis were similar in the control and treatment groups. F) Summary of fibrosis and tissue injury marker mRNA from kidneys. RT-PCR of the kidney revealed no difference in TGF-β, collagen, KIM1, and NGAL mRNA levels between PBS control and Agt-ASO- and lisinopril-treated mice.
DISCUSSION
Key RAS components such as renin, Agt, and AngII are known to be present in renal cystic structures and tubules, and it is possible that the intrarenal RAS contributes to hypertension and cystogenesis in PKD (13, 14). We have recently shown that Pkd1 mice have more renal Agt than do control mice, further supporting activated intrarenal RAS in PKD (17). Therefore, the purpose of this study was to determine the effect of depleting Agt by using ASO and to compare the results to those obtained with an ACE inhibitor in a Pkd1 mouse model. ASO-mediated suppression of Agt slowed the progression of kidney cysts compared with the ACE inhibitor lisinopril in Pkd1 conditional-knockout mice. Although, the exact mechanism of how systemic Agt suppression leads to the decrease in progression of kidney cysts is unclear, these results are consistent with those in a recent study in which there was decreased cystic disease with Agt-ASO treatment in a Pkd2-knockout mouse model (16). Because well over 85% ADPKD patients are thought to have mutations in the Pkd1 gene, these results suggest that Agt-ASO therapy is a candidate for treatment of PKD in humans. In addition, Agt-ASO therapy may have advantages over other potential treatments for PKD, such as tolvaptan, which induces increased urine output, leading to excessive thirst, and exhibits potential liver toxicity.
A major site of Agt synthesis is the liver. Agt serves as the substrate for renin and is an essential component in the RAS cascade. In other studies, in a global or liver-specific Agt-knockout mouse, plasma AngII levels were suppressed, resulting in hypotension (22, 23). Thus, suppression of Agt has an effect on BP and may be used as a treatment for hypertension. This possibility has been tested by using early generation of ASOs in the spontaneously hypertensive rat. These ASOs, when administered intravenously, decrease Agt synthesis, resulting in short-term BP reductions (24, 25). The shortcomings of the early generation of ASOs include restricted mode of administration (intravenous), short half-life, poor organ/cellular uptake, and susceptibility to nuclease degradation (26). However, recently developed ASOs are substantially improved, having overcome nearly all of these deficiencies, and are currently used in clinical trials or have been approved for clinical use. For example, an ASO that reduces factor XI has recently been shown to treat deep vein thrombosis effectively, and an ASO that suppresses ApoB-100 has been approved for the treatment of familial hypercholesterolemia (27, 28).
An important finding of our study is that inhibiting Agt with ASO was more effective in slowing the progression of kidney cyst formation than was lisinopril. This result was not necessarily dependent on reductions in BP, because mice treated with lisinopril achieved a lower BP level than did Agt-ASO-treated or control mice. Although Agt-ASO may have stabilized BP from 3 to 4 mo, the attenuation of renal cystogenesis by Agt-ASO, but not lisinopril, treatment was presumably caused by more effective inhibition of intrarenal RAS by the antisense therapy. The proximal tubular epithelium is enriched in Agt, which is derived from both its local synthesis and circulating levels (23, 29, 30).
In pathologic states with high AngII, urinary Agt levels are elevated, leading to the suggestion that urinary Agt reflects intrarenal RAS activity (31, 32). The reason for this suggestion is that high renal AngII concentrations increase renal Agt mRNA and protein levels in proximal tubular cells, thus leading to enhanced urinary excretion of Agt (15). In a study, mostly performed in AngII-infused rats (15), ACE inhibitors decreased urinary Agt levels by suppressing AngII levels. The conundrum in our findings is that Agt-ASO and lisinopril decreased urinary Agt concentrations equally, suggesting similar reduced intrarenal RAS activities, yet only Agt-ASO decreased renal Agt and was more effective in slowing cystic growth. One explanation is that the ACE inhibitors and AngII receptor blockers (ARBs) used in most AngII infusion rat studies inhibit the renal Agt level but do not suppress Agt below the preinfusion baseline level, which may explain why lisinopril did not decrease renal Agt in our study. However, little is known regarding the mechanisms that regulate proximal tubular synthesis and tubular handling of Agt, and the mechanism whereby lisinopril decreases urinary Agt levels is currently unknown. We did not measure renal AngII, and therefore whether Agt-ASO and lisinopril were in fact equally effective in reducing intrarenal AngII levels should be examined further. Studies have demonstrated non-ACE chymase-dependent pathways of AngII formation to be involved in pathogenic states such as heart failure (33, 34). Agt-ASO treatment resulted in a greater induction of renin than did lisinopril, which may have been a consequence of superior intrarenal AngII suppression.
If Agt-ASO is more effective in reducing intrarenal AngII levels, then it may help explain why this agent was more effective in reducing the rate of cystogenesis. On the other hand, if both are equally effective in reducing intrarenal AngII levels, then the effects of ASO may be through an AngII-independent mechanism. Agt is believed to be inactive until cleaved by renin at its N terminus, with the resultant release of the decapeptide AngI. There is evidence that the remaining Agt fragment, referred to as des(AngI)-AGT possesses biologic properties, such as antiangiogenic effects (35, 36). Because angiogenesis involves cell proliferative processes, this notion would be consistent with, but would not clearly prove, a link between des(AngI)-AGT and proliferation of renal epithelial cells. In addition, other AngI metabolites besides AngII may have a role in cystogenesis. The biologic properties of cleaved Agt, chymase, and the noncanonical Ang metabolites require further investigation in the context of renal cystic disease.
In hypertension and chronic diseases, therapeutic efficacy of treatment of ACE inhibitors and AT1 receptor blockers are thought to occur, at least in part, by preventing or reducing fibrosis (37). In this Pkd1 mouse model, there was little evidence of overt fibrosis, nor was there evidence of elevated markers of renal injury. These observations suggest that the effects of Agt inhibition in suppressing cystic expansion did not occur through fibrotic injury pathways. An important finding in our study was that Agt-ASO treatment in Pkd1 mice significantly reduced kidney cell proliferation in both cystic and noncystic areas, but not in the liver. Cell proliferation participates in cyst expansion, and this process may occur, at least in part, through a variety of aberrant gene transcription pathways (38), altered mTOR (39), Wnt signaling (40), or cAMP pathways (41) and possibly through activation of the RAS system. One of the mechanisms of RAS-stimulated cell proliferation is a direct effect of AngII mediated by AT1 receptors which promotes tubular epithelial and interstitial cell proliferation (6, 42). If it is shown that Agt inhibits cystic growth through an AngII-independent pathway, then it would be particularly important to determine whether cleaved Agt exerts biologic effects that stimulate cell proliferation. Nevertheless, these studies suggest an important and specific role for intrarenal RAS in the regulation of cystic development mediated through cell proliferation. Indeed although Agt-ASO suppressed both kidney and liver Agt, there was no effect of Agt-ASO or lisinopril on the severity of liver cystic disease. These results suggest that mechanisms that control or modulate the rate of cystic progression differ between kidney and liver. The limitations of this study include a small sample of animals and the timing of Cre induction, which varied between 8 and 12 wk. We also acknowledge that BP data obtained with the tail cuff method should be assessed and verified with telemetry BP measurements.
In conclusion, Agt-ASO treatment resulted in decreased plasma, liver, and kidney Agt levels and significantly reduced cyst expansion in the kidney compared with lisinopril and control treatments. Although the mechanism of how Agt suppression slowed the progression of kidney cyst formation is unclear, it appears to have a specific effect that diminishes cell proliferation in the kidneys. Agt may be a novel and effective therapeutic target for treating ADPKD.
Acknowledgments
This work was supported by U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grants K08DK106465 (to T.S.) and P30 DK074038 (to P.D.B.); U.S. Department of Veterans Affairs Merit Award (to P.D.B.); and a Dialysis Clinic, Inc. (Nashville, TN, USA) grant (to T.S. and P.D.B.). The authors declare no conflicts of interest.
Glossary
- ADPKD
autosomal dominant polycystic kidney disease
- Agt
angiotensinogen
- Ang
angiotensin
- ASO
antisense oligonucleotide
- BP
blood pressure
- BW
body weight
- Cre (protein)
site-specific DNA recombinase
- H&E
hematoxylin and eosin
- HRP
horseradish peroxidase
- KIM
kidney injury molecule
- KLH
keyhole limpet hemocyanin
- LTL
Lotus tetragonolobus lectin
- MOE
methoxyl-ethyl
- NGAL
neutrophil gelatinase-associated lipocalin
- PCNA
proliferating cell nuclear antigen
- qRT-PCR
quantitative RT-PCR
- RAS
renin-angiotensin system
- SBP
systolic BP
- TBS
Tris-buffered saline
- TBST
TBS-Tween 20
REFERENCES
- 1.Torres V. E., Harris P. C., Pirson Y. (2007) Autosomal dominant polycystic kidney disease. Lancet 369, 1287–1301 [DOI] [PubMed] [Google Scholar]
- 2.Saigusa T., Bell P. D. (2015) Molecular pathways and therapies in autosomal-dominant polycystic kidney disease. Physiology (Bethesda) 30, 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman A. B., Johnson A., Gabow P. A., Schrier R. W. (1990) The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N. Engl. J. Med. 323, 1091–1096 [DOI] [PubMed] [Google Scholar]
- 4.Graham P. C., Lindop G. B. (1988) The anatomy of the renin-secreting cell in adult polycystic kidney disease. Kidney Int. 33, 1084–1090 [DOI] [PubMed] [Google Scholar]
- 5.Chapman A. B., Stepniakowski K., Rahbari-Oskoui F. (2010) Hypertension in autosomal dominant polycystic kidney disease. Adv. Chronic Kidney Dis. 17, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Z., Cooper M. E. (2001) Role of angiotensin II in tubulointerstitial injury. Semin. Nephrol. 21, 554–562 [DOI] [PubMed] [Google Scholar]
- 7.Keith D. S., Torres V. E., Johnson C. M., Holley K. E. (1994) Effect of sodium chloride, enalapril, and losartan on the development of polycystic kidney disease in Han:SPRD rats. Am. J. Kidney Dis. 24, 491–498 [DOI] [PubMed] [Google Scholar]
- 8.Zafar I., Tao Y., Falk S., McFann K., Schrier R. W., Edelstein C. L. (2007) Effect of statin and angiotensin-converting enzyme inhibition on structural and hemodynamic alterations in autosomal dominant polycystic kidney disease model. Am. J. Physiol. Renal Physiol. 293, F854–F859 [DOI] [PubMed] [Google Scholar]
- 9.Jafar T. H., Stark P. C., Schmid C. H., Strandgaard S., Kamper A. L., Maschio G., Becker G., Perrone R. D., Levey A. S.; ACE Inhibition in Progressive Renal Disease (AIPRD) Study Group (2005) The effect of angiotensin-converting-enzyme inhibitors on progression of advanced polycystic kidney disease. Kidney Int. 67, 265–271 [DOI] [PubMed] [Google Scholar]
- 10.McPherson E. A., Luo Z., Brown R. A., LeBard L. S., Corless C. C., Speth R. C., Bagby S. P. (2004) Chymase-like angiotensin II-generating activity in end-stage human autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 15, 493–500 [DOI] [PubMed] [Google Scholar]
- 11.Schrier R. W., Abebe K. Z., Perrone R. D., Torres V. E., Braun W. E., Steinman T. I., Winklhofer F. T., Brosnahan G., Czarnecki P. G., Hogan M. C., Miskulin D. C., Rahbari-Oskoui F. F., Grantham J. J., Harris P. C., Flessner M. F., Bae K. T., Moore C. G., Chapman A. B.; HALT-PKD Trial Investigators (2014) Blood pressure in early autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371, 2255–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres V. E., Abebe K. Z., Chapman A. B., Schrier R. W., Braun W. E., Steinman T. I., Winklhofer F. T., Brosnahan G., Czarnecki P. G., Hogan M. C., Miskulin D. C., Rahbari-Oskoui F. F., Grantham J. J., Harris P. C., Flessner M. F., Moore C. G., Perrone R. D.; HALT-PKD Trial Investigators (2014) Angiotensin blockade in late autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371, 2267–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres V. E., Donovan K. A., Scicli G., Holley K. E., Thibodeau S. N., Carretero O. A., Inagami T., McAteer J. A., Johnson C. M. (1992) Synthesis of renin by tubulocystic epithelium in autosomal-dominant polycystic kidney disease. Kidney Int. 42, 364–373 [DOI] [PubMed] [Google Scholar]
- 14.Loghman-Adham M., Soto C. E., Inagami T., Cassis L. (2004) The intrarenal renin-angiotensin system in autosomal dominant polycystic kidney disease. Am. J. Physiol. Renal Physiol. 287, F775–F788 [DOI] [PubMed] [Google Scholar]
- 15.Kobori H., Nangaku M., Navar L. G., Nishiyama A. (2007) The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev. 59, 251–287 [DOI] [PubMed] [Google Scholar]
- 16.Ravichandran, K., Ozkok, A., Wang, Q., Mullick, A. E., and Edelstein, C. L. (2015) Antisense-mediated angiotensinogen inhibition slows polycystic kidney disease in mice with a targeted mutation in Pkd2. Am. J. Physiol. Renal Physiol. 308, F349–F357 [DOI] [PMC free article] [PubMed]
- 17.Saigusa T., Dang Y., Bunni M. A., Amria M. Y., Steele S. L., Fitzgibbon W. R., Bell P. D. (2015) Activation of the intrarenal renin-angiotensin-system in murine polycystic kidney disease. Physiol. Rep. 3, e12405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piontek K. B., Huso D. L., Grinberg A., Liu L., Bedja D., Zhao H., Gabrielson K., Qian F., Mei C., Westphal H., Germino G. G. (2004) A functional floxed allele of Pkd1 that can be conditionally inactivated in vivo. J. Am. Soc. Nephrol. 15, 3035–3043 [DOI] [PubMed] [Google Scholar]
- 19.Hayashi S., McMahon A. P. (2002) Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 244, 305–318 [DOI] [PubMed] [Google Scholar]
- 20.Sas K. M., Yin H., Fitzgibbon W. R., Baicu C. F., Zile M. R., Steele S. L., Amria M., Saigusa T., Funk J., Bunni M. A., Siegal G. P., Siroky B. J., Bissler J. J., Bell P. D. (2015) Hyperglycemia in the absence of cilia accelerates cystogenesis and induces renal damage. Am. J. Physiol. Renal Physiol. 309, F79–F87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kordasiewicz H. B., Stanek L. M., Wancewicz E. V., Mazur C., McAlonis M. M., Pytel K. A., Artates J. W., Weiss A., Cheng S. H., Shihabuddin L. S., Hung G., Bennett C. F., Cleveland D. W. (2012) Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuron 74, 1031–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanimoto K., Sugiyama F., Goto Y., Ishida J., Takimoto E., Yagami K., Fukamizu A., Murakami K. (1994) Angiotensinogen-deficient mice with hypotension. J. Biol. Chem. 269, 31334–31337 [PubMed] [Google Scholar]
- 23.Matsusaka T., Niimura F., Shimizu A., Pastan I., Saito A., Kobori H., Nishiyama A., Ichikawa I. (2012) Liver angiotensinogen is the primary source of renal angiotensin II. J. Am. Soc. Nephrol. 23, 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomita N., Morishita R., Higaki J., Aoki M., Nakamura Y., Mikami H., Fukamizu A., Murakami K., Kaneda Y., Ogihara T. (1995) Transient decrease in high blood pressure by in vivo transfer of antisense oligodeoxynucleotides against rat angiotensinogen. Hypertension 26, 131–136 [DOI] [PubMed] [Google Scholar]
- 25.Makino N., Sugano M., Ohtsuka S., Sawada S. (1998) Intravenous injection with antisense oligodeoxynucleotides against angiotensinogen decreases blood pressure in spontaneously hypertensive rats. Hypertension 31, 1166–1170 [DOI] [PubMed] [Google Scholar]
- 26.Bennett C. F., Swayze E. E. (2010) RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 50, 259–293 [DOI] [PubMed] [Google Scholar]
- 27.Raal F. J., Santos R. D., Blom D. J., Marais A. D., Charng M. J., Cromwell W. C., Lachmann R. H., Gaudet D., Tan J. L., Chasan-Taber S., Tribble D. L., Flaim J. D., Crooke S. T. (2010) Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 375, 998–1006 [DOI] [PubMed] [Google Scholar]
- 28.Büller, H. R., Bethune, C., Bhanot, S., Gailani, D., Monia, B. P., Raskob, G. E., Segers, A., Verhamme, P., and Weitz, J. I., for the FXI-ASO TKA Investigators. (2015) Factor XI antisense oligonucleotide for prevention of venous thrombosis. N. Engl. J. Med. 372, 232–240 [DOI] [PMC free article] [PubMed]
- 29.Pohl M., Kaminski H., Castrop H., Bader M., Himmerkus N., Bleich M., Bachmann S., Theilig F. (2010) Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J. Biol. Chem. 285, 41935–41946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamiyama M., Farragut K. M., Garner M. K., Navar L. G., Kobori H. (2012) Divergent localization of angiotensinogen mRNA and protein in proximal tubule segments of normal rat kidney. J. Hypertens. 30, 2365–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobori H., Urushihara M. (2013) Augmented intrarenal and urinary angiotensinogen in hypertension and chronic kidney disease. Pflugers Arch. 465, 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobori H., Harrison-Bernard L. M., Navar L. G. (2002) Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 61, 579–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad S., Varagic J., Groban L., Dell’Italia L. J., Nagata S., Kon N. D., Ferrario C. M. (2014) Angiotensin-(1-12): a chymase-mediated cellular angiotensin II substrate. Curr. Hypertens. Rep. 16, 429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei C. C., Hase N., Inoue Y., Bradley E. W., Yahiro E., Li M., Naqvi N., Powell P. C., Shi K., Takahashi Y., Saku K., Urata H., Dell’italia L. J., Husain A. (2010) Mast cell chymase limits the cardiac efficacy of Ang I-converting enzyme inhibitor therapy in rodents. J. Clin. Invest. 120, 1229–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Célérier J., Cruz A., Lamandé N., Gasc J. M., Corvol P. (2002) Angiotensinogen and its cleaved derivatives inhibit angiogenesis. Hypertension 39, 224–228 [DOI] [PubMed] [Google Scholar]
- 36.Wu C., Lu H., Cassis L. A., Daugherty A. (2011) Molecular and pathophysiological features of angiotensinogen: a mini review. N. Am. J. Med. Sci. 4, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rockey D. C., Bell P. D., Hill J. A. (2015) Fibrosis: a common pathway to organ injury and failure. N. Engl. J. Med. 372, 1138–1149 [DOI] [PubMed] [Google Scholar]
- 38.Chapin H. C., Caplan M. J. (2010) The cell biology of polycystic kidney disease. J. Cell Biol. 191, 701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres V. E., Boletta A., Chapman A., Gattone V., Pei Y., Qian Q., Wallace D. P., Weimbs T., Wüthrich R. P. (2010) Prospects for mTOR inhibitor use in patients with polycystic kidney disease and hamartomatous diseases. Clin. J. Am. Soc. Nephrol. 5, 1312–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lancaster M. A., Gleeson J. G. (2010) Cystic kidney disease: the role of Wnt signaling. Trends Mol. Med. 16, 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallace D. P. (2011) Cyclic AMP-mediated cyst expansion. Biochim. Biophys. Acta 1812, 1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf G., Neilson E. G. (1993) Angiotensin II as a renal growth factor. J. Am. Soc. Nephrol. 3, 1531–1540 [DOI] [PubMed] [Google Scholar]