Abstract
Cyclooxygenase (COX)-2 has been shown to be involved in regulating basal airway function, bacterial LPS-induced airway hyperresponsiveness (AHR) and lung inflammation, and bleomycin-induced lung fibrosis; however, the cellular source of COX-2 that underlies these effects is unknown. We generated mice with alveolar type II (ATII) cell–specific knockdown of COX-2 (AT2CC−/−), to examine the role of ATII cell–derived prostaglandins (PGs) in these processes. Specific knockdown of COX-2 was confirmed by real-time RT-PCR and Western blot analyses. LC/MS/MS analysis showed that ATII cells produced PGs. Basal airway responsiveness of AT2CC−/− mice was decreased compared to that of wild-type (WT) mice. LPS-induced hypothermic response, infiltration of inflammatory cells into the airway, and lung inflammation were enhanced in AT2CC−/− mice relative to WT controls; however, LPS-induced AHR and proinflammatory cytokine and chemokine expression were similar between the genotypes. After 21 d of bleomycin administration, AT2CC−/− mice behaved in a manner similar to WT mice. Thus, ATII cell–derived COX-2 plays an important role in regulating basal airway function and LPS-induced lung inflammation, but does not play a role in bleomycin-induced fibrosis. These findings provide insight into the cellular source of COX-2 related to these lung phenotypes.—Cheng, J., Dackor, R. T., Bradbury, J. A., Li, H., DeGraff, L. M., Hong, L. K., King, D., Lih, F. B., Gruzdev, A., Edin, M. L., Travlos, G. S., Flake, G. P., Tomer, K. B., Zeldin, D. C. Contribution of alveolar type II cell–derived cyclooxygenase-2 to basal airway function, lung inflammation, and lung fibrosis.
Keywords: prostaglandins, airway hyperresponsiveness, lipopolysaccharide, bleomycin
Lung diseases are among the most common medical conditions worldwide. The underlying causes and mechanisms of action that contribute to some of the diseases have been well explored and elucidated. Others remain under investigation in an effort to develop more effective disease therapies. Pulmonary fibrosis is an idiopathic condition that is characterized by the dysregulated formation or development of excess fibrous connective tissue in the lungs (1). In humans, it may be a secondary effect of interstitial lung disease or of environmental pollutants, such as cigarette smoke (2). In animal models, induction of pulmonary fibrosis is achieved by administering agents, such as bleomycin (3) or vanadium pentoxide (4); both agents cause fibrosis within ∼2 wk.
The mechanisms responsible for airway hyperresponsiveness (AHR) in diseases such as asthma and chronic obstructive pulmonary disease are also unclear. AHR is often accompanied by inflammation of the lungs and is a major risk factor for the decline in lung function. Compounds such as bacterial LPS are potent inducers of inflammatory responses in the lungs and have been shown to increase lung resistance and elastance significantly (5).
In recent years, cyclooxygenases (COXs) have been implicated in the pathogenesis of pulmonary fibrosis, inflammation, and AHR. The COX enzymes metabolize arachidonic acid to form prostaglandin (PG)H2, which is further metabolized by specific PG synthases to generate PGs, prostacyclin, and thromboxane. There are 2 isoforms of COX: COX-1 and COX-2. COX-1 is a physiologic housekeeping enzyme that is constitutively expressed in most lung tissues, including the bronchiolar and alveolar epithelia, alveolar macrophages, bronchiolar smooth muscle, and bronchiolar Clara cells (6). COX-2 is the inducible isoform that is normally expressed at very low levels and has been localized to the alveolar epithelia, alveolar macrophages, and bronchial smooth muscle cells (6). It is widely considered to be a proinflammatory enzyme that is induced by various factors, including LPS and oxidative stress (7, 8). Intravascular administration of LPS has been shown to upregulate COX-2 in the alveolar macrophages and endothelial cells of perfused rat lungs (9). However, results from studies conducted in recent years suggest that COX-2 and its metabolites mediate a protective effect in lung fibrosis. In 2002, Bonner et al. (10) demonstrated that global COX-2–null mice treated with vanadium pentoxide exhibit severe lung inflammatory responses and fibrosis, compared with wild-type (WT) mice. This reaction was associated with decreases in the production of PGs such as PGE2. Our laboratory has reported that the lung function of COX-2–null mice was decreased after exposure to bleomycin (11). In addition, administration of PGE2 before bleomycin exposure significantly attenuated lung dysfunction and fibrosis in C57BL/6 mice (12). COX-1–null mice, in contrast, did not have altered lung fibrosis (11).
COX-2 has also been implicated in AHR associated with asthma and LPS-induced lung inflammation. Administration of lumiracoxib, a selective COX-2 inhibitor, to adjuvant-free, ovalbumin-challenged mice enhanced AHR compared with control mice (13). In 2001, Zeldin et al. (14) reported that mice lacking the PTGS-2 (COX-2) gene have dissociated lung inflammation and AHR in response to intratracheally administered LPS. In isolated perfused mouse lungs, LPS-induced increases in AHR response to methacholine were attenuated after administration of NS-398, a specific COX-2 inhibitor (15). Again, COX-1 disruption had minimal effects in this model system (14).
Although it is clear that COX-2 plays a role in the development of airway responsiveness, lung inflammation, and lung fibrosis, the cellular source of COX-2 related to these effects is unclear. Multiple cell types within the lung are capable of producing COX-2 and its metabolites. The alveolar type II (ATII) epithelial cells comprise 15% of all lung cells and are the main sources of pulmonary surfactant (16). They are widely involved in ion transport and alveolar repair in response to injury. Most importantly, they are in direct contact with compounds, such as bleomycin and LPS, or with pathogens that may cause lung injury. Unlike most cells, ATII cells constitutively express significant levels of COX-2. These levels can be further induced by various stimuli (17), suggesting that ATII cell–derived COX-2 plays a crucial role in regulating basal lung function, as well as bleomycin-induced pulmonary fibrosis and LPS-induced airway inflammation and AHR.
In this study, we generated mice with ATII cell-specific knockdown of COX-2 (AT2CC−/− mice) and assessed their basal lung function and lung inflammatory and functional responses to bleomycin and LPS. AT2CC−/− mice had reduced airway responsiveness to methacholine under basal conditions and enhanced lung inflammation under LPS-stimulated conditions. In contrast, ATII cell–derived COX-2 did not play a significant role in bleomycin-induced lung fibrosis. This study is the first investigation of the role of ATII cell–derived COX-2 in the lung under basal conditions or after treatment with agents that induce lung inflammation and fibrosis.
MATERIALS AND METHODS
Generation of AT2CC−/− mice
The Cre-lox-P recombination approach was used to generate mice that lack COX-2 in ATII cells (18). SPC-Cre mice that express Cre-recombinase via the surfactant protein (SP)C promoter solely in ATII cells were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) (19). These mice were bred to homozygous COX-2 floxed mice, which carry a conditional COX-2 allele where exons 4 and 5 of the Ptgs-2 gene are flanked by Lox-P sites (20–22), to produce mice with ATII cell-specific deletion of COX-2 on a pure C57BL/6 genetic background. WT controls contained the floxed locus, but were Cre negative. Mice were given access to food and water ad libitum. All studies were conducted in accordance with principles and procedures outlined in the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the U.S. National Institute of Environmental Health Sciences Animal Care and Use Committee.
Isolation of ATII cells
AT2CC−/− mice and their WT littermates (8–12 wk old) were killed with Fatal Plus (Virbac AH, Inc., Fort Worth, TX, USA). The lungs were perfused with 1× PBS, inflated with 1.5 ml Dispase (Life Technologies, Grand Island, NY, USA) and 0.5 ml 1% agarose, removed from the mouse, and collected in tubes containing 1 ml Dispase. Tissues were incubated at room temperature for 45 min, after which they were minced in DMEM supplemented with 40 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer and 1% penicillin-streptomycin in the presence of 10,000 U/ml DNase I (Sigma-Aldrich, St. Louis, MO, USA). Cell homogenates were filtered through 70, 40, and 20 µm cell strainers. The resulting cell suspension was centrifuged at 130 g for 8 min. Cell pellets were incubated with red blood cell lysis buffer to remove red blood cells, transferred to antibody plates containing 32 µg anti-CD16/32 and 84 µg anti-CD45 (BD Biosciences, San Jose, CA, USA), and incubated at 37°C for 2 h. After 2 h, medium from the plates was collected and centrifuged at 130 g for 8 min, to yield pellets containing ATII cells. The cells were processed for biochemical analyses. Identification of ATII cells was confirmed by using modified Papanicolaou (PAP) staining (16).
Treatment of mice with bleomycin or LPS
AT2CC−/− mice and their WT littermates (8–12 wk old) were treated with a single dose of saline or bleomycin sulfate (0.5 U/kg body weight; Sigma-Aldrich) via oropharyngeal aspiration while under isoflurane/O2 anesthesia. Body weights were recorded weekly over a 21-d period, after which they were placed on a Flexivent (Scireq, Tempe, AZ, USA) computer-controlled ventilator, to assess lung function. For the LPS (strain 0111:B4) study, AT2CC−/− mice and their WT littermates (8–12 wk old) were treated with saline or LPS (50 µg/mouse; Sigma-Aldrich) via oropharyngeal aspiration while under brief isoflurane/O2 anesthesia. Body temperatures were recorded hourly over a 4-h period. The mice were then placed on the Flexivent, to assess airway responsiveness.
Analysis of lung function and airway responsiveness
Invasive analyses of lung function and airway responsiveness were performed on bleomycin- and LPS-treated mice, respectively, along with saline-treated controls. After treatments with bleomycin (21 d) or LPS (4 h), the mice were anesthetized with urethane (1.5 g/kg i.p.) and paralyzed with pancuronium bromide (0.8 mg/kg i.p.), to prevent the animals from breathing on their own when placed on the ventilator. A tracheostomy was performed, and a 19-gauge stainless-steel cannula was inserted into the trachea. The mouse was placed on a 37°C heating pad, and the cannula was connected to the tubing on the ventilator. Parameters corresponding to lung function were assessed as has been described (12). Parameters corresponding to airway responsiveness were assessed as described by Card et al. (23).
Assessment of cell counts and differentials in bronchoalveolar lavage fluid
Mice were killed by exsanguination after the Flexivent procedures. Bronchoalveolar lavage was performed with two 1 ml aliquots of HBSS and collected into 15 ml conical tubes. The fluid (BALF) was centrifuged at 3000 rpm for 10 min, to separate the cells from the fluid. The cells were counted (Coulter counter; Beckman Coulter, Indianapolis, IN, USA) and were normalized according to the volume of recovered BALF. For differentials, cytospin slides were made and analyzed for the presence of neutrophils, macrophages, lymphocytes, and eosinophils by a hematologist who was blinded to genotype and treatment group assignments.
Lung histopathologic analysis
Lungs from the LPS study were fixed with 4% paraformaldehyde and embedded in paraffin. Five micrometer sections were stained with hematoxylin and eosin and scored for the following characteristics: margination of neutrophils, perivascular neutrophils, peribronchial neutrophils, and perivascular and peribronchial hemorrhage. A total inflammation score (0–16) was determined by a pathologist who was blinded to genotype and treatment group assignments.
Measurement of PG and leukotriene levels in BALF and ATII reaction medium
PG and leukotriene levels in BALF and ATII medium were analyzed by liquid chromatography–tandem mass spectrometry (LC/MS/MS). In brief, 500 µl BALF was spiked with the internal standard, PGE2-d4 (Cayman Chemical, Ann Arbor, MI, USA) and applied to Amprep Octadecyl C18 columns (GE Life Sciences, Pittsburgh, PA, USA), followed by washes with water and hexane. Samples were eluted from the columns with 2 ml ethyl acetate (acidic solvent) into 10 ml collection tubes and dried under nitrogen gas. ATII reaction medium samples were prepared (24), eluted in ethyl acetate and methanol (acidic solvent), and dried under nitrogen gas and then resuspended in 50 µl of 30% ethanol and injected into the 1200 Series capillary HPLC system (Agilent Technologies, Santa Clara, CA, USA) coupled to an API 3000 triple quadrupole mass spectrometer (Sciex; Foster City, CA, USA) with negative-mode electrospray ionization and multiple-reaction monitoring (24). Quantitation of PGs and leukotrienes was performed with Analyst 5.1 software (PE Sciex). Good separations of PGE2 and PGD2 were achieved. The limits of detection ranged from 0.5 to 2 pg/ml.
Collagen assay
Caudal lung lobes were lysed in 625 µl RIPA buffer containing EDTA-free complete protease inhibitor pellet (Roche, Indianapolis, IN, USA), and collagen levels were assessed with the Sircol Collagen Assay kit (Biocolor, Carrickfergus, United Kingdom), according to the manufacturer’s instructions and as published (12).
RNA extraction and real-time RT-PCR analysis
Whole lungs and isolated ATII cells were lysed in lysis buffer provided in the Ambion RNA extraction kit. RNA was extracted according to the manufacturer’s protocol (Life Technologies). The elution volume for each sample was 30–50 µl, and quantitation of samples was performed with a DU 640 Spectrophotometer (Beckman Coulter). RNA was reverse transcribed to cDNA using the High Capacity cDNA Reverse Transcription Kit (Life Technologies-Applied Biosystems, Grand Island, NY, USA), as described (24). Expression of murine Ptgs1, Ptgs2, Sftpc, IL-1b, IL-6, CCL-2, TNF-a, Ptges, Ptgds, aLox5, and Gapdh was quantified by real-time quantitative RT-PCR with Taqman Assays on Demand (Life Technologies-Applied Biosystems). Expression of all genes was normalized to murine GAPDH using the 2−∆Ct method.
Western blot analysis
Whole lungs and isolated ATII cells were lysed in tissue lysis buffer (50 mM Tris-HCl, 1% Triton X-100, 150 mM NaCl, 1 mM EGTA, 0.25% sodium deoxycholate, 1 mM NaF, and 1 mM Na3Vo4), supplemented with EDTA-free complete protease inhibitor pellets (Roche) and 1 mM PMSF. The proteins were loaded onto 12% Tris-glycine gels and run at 125 V for 3 h, after which they were transferred onto 0.45 µm nitrocellulose membranes for 1 h (Life Technologies). Blots were blocked in 10% nonfat dry milk and incubated with primary antibodies against COX-2 (Cayman Chemical) and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by a horseradish peroxidase–conjugated goat anti-rabbit polyclonal antibody (Calbiochem, Billerica, MA, USA) or a horseradish peroxidase–conjugated donkey anti-goat antibody (Santa Cruz Biotechnology).
RESULTS
Expression of COX-2 is decreased in ATII cells in AT2CC−/−mice
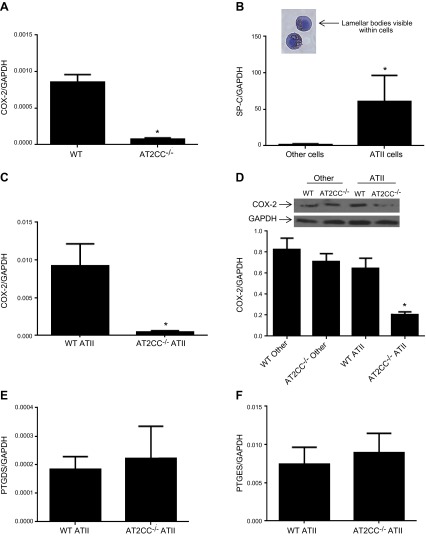
COX-2 is expressed in many cell types of the lung (6). Whole lungs from untreated AT2CC−/− mice contained less COX-2 mRNA than whole lungs from WT mice (Fig. 1A). ATII cells were isolated from WT and AT2CC−/− mice via centrifugation and antibody plating, to assess the COX-2 expression specifically within those cells. Modified PAP staining of the isolated cells was performed to assess the purity of the isolation procedure. ATII cells were identified based on the presence of lamellar bodies within the cells (Fig. 1B) (16). Approximately 80–90% ATII cell purity was achieved in all isolation batches from both WT and AT2CC−/− mice. Real-time RT-PCR analysis revealed that ATII cells also had a significant increase in SPC mRNA levels, compared with those in cells that attached to the antibody plate and thus represent other cells, (Fig. 1B) including macrophages, lymphocytes, and dendritic cells. COX-2 mRNA levels were decreased in ATII cells from AT2CC−/− mice, compared with WT ATII cells (Fig. 1C). It is important to note that endogenous levels of COX-2 in WT ATII cells were quite low (Ct ∼29). In addition, COX-2 protein expression was markedly decreased in ATII cells from AT2CC−/− mice relative to WT. In contrast, COX-2 protein levels were similar between WT and AT2CC−/− other cells (Fig. 1D).
Figure 1.
Characterization of AT2CC−/− mice. A) Whole lungs were isolated from WT and AT2CC−/− mice and COX-2 expression was assessed by real-time RT-PCR. The ratio relative to WT is shown (n = 5). ATII cells were isolated from WT and AT2CC−/− mice, and levels of SPC mRNA (B), COX-2 mRNA (C), COX-2 protein (D), PTGDS mRNA (E), and PTGES mRNA (F) were quantified (n = 8–10). *P < 0.05 vs. WT.
Levels of PGE synthase (Ptges) and PGD synthase (Ptgds) were also assessed in ATII cells from WT and AT2CC−/− mice. Both synthases were present in ATII cells, albeit in low amounts. The Ct values of Ptgds (Fig. 1) and Ptges were ∼33 and 29, respectively. There were no differences in the expression of either synthase between WT and AT2CC−/− mice for Ptgds (Fig. 1E) or Ptges (Fig. 1F).
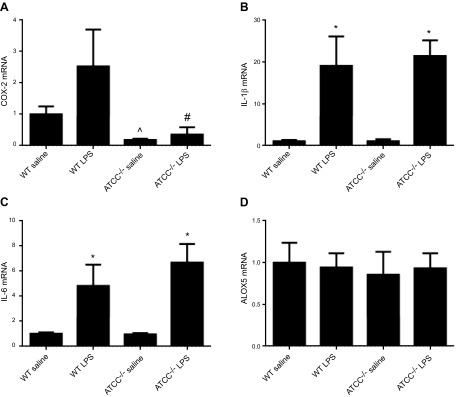
In some experiments, ATII cells were isolated from naive WT and AT2CC−/− mice and treated with LPS for 4 h ex vivo, to assess COX-2 induction and eicosanoid production. COX-2 mRNA levels were increased by ∼2-fold in LPS-treated WT cells; however, no differences in COX-2 mRNA levels were observed in LPS-treated cells from AT2CC−/− mice, further confirming knockdown of COX-2 in ATII cells of those mice (Fig. 2A). Despite clear differences in COX-2 levels between LPS-treated WT and AT2CC−/− cells, there were no changes in the expression of IL-1β or -6 (Fig. 2B, C), suggesting that there may be COX-2–independent pathways within ATII cells that upregulate the expression of proinflammatory molecules. In addition, ALOX5 levels were similar, irrespective of genotype or treatment with LPS (Fig. 2D). In a related set of experiments, ATII cells were isolated from WT and AT2CC−/− mice after in vivo oropharyngeal LPS treatment. Consistent with the ex vivo results, COX-2 mRNA levels were increased in LPS-treated WT cells; however, no differences in COX-2 mRNA levels were observed in LPS-treated cells from AT2CC−/− mice, and there were no changes in the expression of CCL2, IL-1β, IL-6, or TNF-α between the genotypes (Supplemental Fig. 1).
Figure 2.
Effect of LPS on levels of COX-2, proinflammatory molecules, and ALOX5 in ATII cells from WT and AT2CC−/− mice. ATII cells from WT and AT2CC−/− mice were isolated and treated with saline or LPS (1 µg/ml in 1% FBS/DMEM) for 4 h. ATII cells were collected, and mRNA levels of Cox-2 (A), Il-1b (B), Il-6 (C), and Alox5 (D) were determined by real-time RT-PCR (n = 4). *P < 0.05 vs. saline of same genotype; ^P < 0.05 vs. WT saline; #P < 0.05 vs. WT LPS.
Levels of PGs are decreased in ATII cells from LPS-treated AT2CC−/− mice
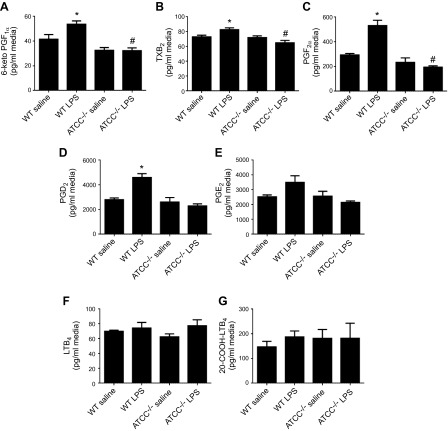
ATII cells from AT2CC−/− mice exhibited lower levels of COX-2 in the presence and absence of LPS compared with ATII cells from WT mice. To investigate whether production of PGs was also affected by knockdown of COX-2, isolated ATII cells from WT and AT2CC−/− mice were treated with either saline or LPS for 4 h in vitro. PGs were extracted from the media and analyzed by LC/MS/MS. As shown in Fig. 3A–E, levels of 6-keto-PGF1α (a stable prostacyclin metabolite), thromboxane B2 (TXB2; a stable thromboxane metabolite), PGF2α, PGD2, and PGE2 were not significantly different between saline-treated WT and AT2CC−/− ATII cells, suggesting that COX-2 is not critical for basal prostanoid production in these cells. In contrast, the LPS-induced increases in 6-keto-PGF1α, TXB2, PGF2α, and PGD2 in WT cells were markedly attenuated in AT2CC−/− cells. LPS did not induce formation of the ALOX5 metabolites LTB4 and 20-COOH-LTB4 (Fig. 3F, G), and there were no differences in the levels of these metabolites by genotype. ATII cells isolated from WT and AT2CC−/− mice after in vivo LPS treatment yielded similar eicosanoid production profiles (Supplemental Fig. 2).
Figure 3.
Eicosanoid levels in ATII cells isolated from WT and AT2CC−/− mice. ATII cells from WT and AT2CC−/− mice were isolated and treated with saline or LPS (1 µg/ml in 1% FBS/DMEM) for 4 h. The media were collected, and eicosanoids were extracted with C18 columns and quantified by LC/MS/MS. Levels of 6-keto-PGF1α (A), TXB2 (B), PGF2α (C), PGD2 (D), PGE2 (E), LTB4 (F), and 20-COOH-LTB4 (G) were detected (n = 4). *P < 0.05 vs. saline of same genotype; #P < 0.05 vs. WT.
LPS-induced AHR and lung inflammation in AT2CC−/− mice
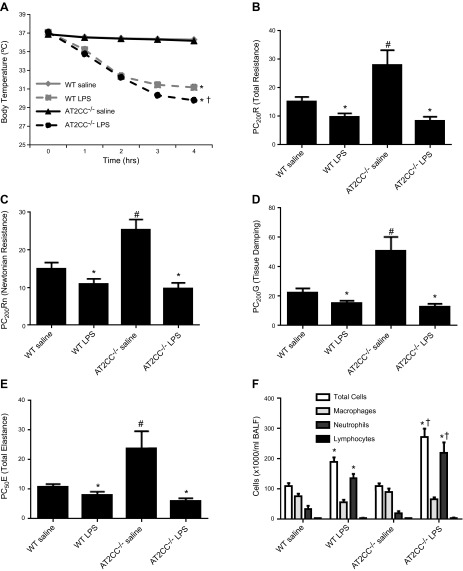
Zeldin et al. (14) reported that mice lacking the Ptgs-2 (COX-2) gene (global knockout mice) exhibit increased airway responsiveness after intranasally administered LPS. Moreover, several published studies have demonstrated an instrumental role for COX-2, but not COX-1, in the development of LPS-induced lung inflammation (15, 25). To investigate whether knockdown of COX-2 in ATII cells affects LPS-induced lung inflammation and AHR, mice were treated with LPS (50 µg/mouse) or saline (75 µl) via oropharyngeal aspiration. Body temperatures were recorded hourly over a period of 4 h until the mice were placed on the Flexivent to measure lung function. WT mice treated with LPS exhibited a significant decrease in temperature over the 4-h period, from 37.10 ± 0.09 to 31.16 ± 0.36°C. At the 4-h time point, the body temperature of LPS-treated AT2CC−/− mice (29.78 ± 0.32°C) was significantly lower than that of WT (31.16 ± 0.36°C) mice, indicating an exaggerated hypothermic response to LPS (Fig. 4A). No differences in the body temperature of saline-treated WT and AT2CC−/− mice were observed.
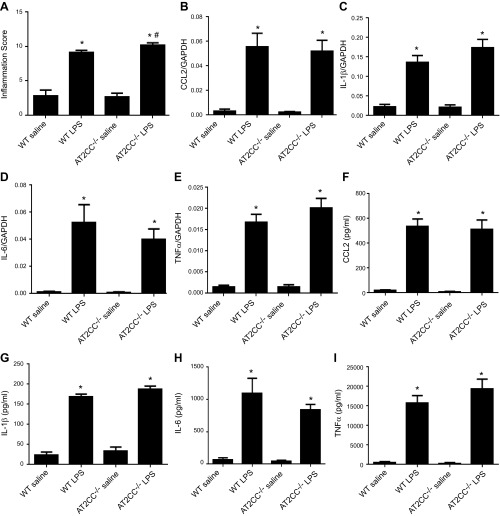
Figure 4.
Effect of LPS on airway responsiveness and BALF cells in WT and AT2CC−/− mice. WT and AT2CC−/− mice were treated with saline or LPS (50 µg via oropharyngeal aspiration) for 4 h. A) Body temperatures were recorded hourly throughout the 4-h period. Mice were then placed on the Flexivent to assess airway responsiveness to increasing doses of aerosolized methacholine (3.125, 6.25, 12.5, and 25 mg/ml). Provocative concentrations for R (B), Rn (C), G (D), and E (E), were calculated by extrapolation of the respective dose–response curves. F) BALF was collected from the mice and the counts of total cells, neutrophils, and macrophages were determined (n = 10–12). *P < 0.05 vs. respective saline; #P < 0.05 vs. WT saline; †P < 0.05 vs. WT LPS.
LPS is a well-known inducer of AHR (5, 15). Four hours after LPS treatment, mice were placed on the Flexivent, and airway responsiveness to increasing doses of methacholine, a bronchoconstrictor agent, was recorded. Total resistance (R), newtonian resistance (Rn), tissue damping (resistance) (G), and total elastance (E) measurements were collected, and provocative concentrations (PCs) were calculated for each parameter, to denote the methacholine concentration at which a 200% increase (denoted as PC200R, PC200Rn, or PC200G) or 50% change (denoted as PC50E) in response was observed. Especially notable was that basal airway responsiveness to methacholine was significantly lower in saline-treated AT2CC−/− mice than in WT, as evidenced by increases in PC200R, PC200Rn, PC200G, and PC50E (Fig. 4B). This result suggests that ATII-derived COX-2 increases airway responsiveness to methacholine in the absence of LPS. After LPS treatment, the PCs of all lung function parameters decreased by 10–15% in WT mice, consistent with LPS-induced AHR. A more pronounced decrease in the PCs for all lung function parameters was observed in AT2CC−/− mice after LPS treatment, relative to saline (Fig. 4B–E). Thus, airway responsiveness to methacholine was comparable in LPS-treated WT and AT2CC−/− mice, despite basal airway hyporesponsiveness in saline-treated AT2CC−/− mice.
LPS induces an inflammatory response that is characterized by increases in neutrophils and expression of proinflammatory cytokines. Saline-treated WT mice had a total of 111 ± 10 × 103 cells/ml BALF. LPS increased the number of total cells to 189 ± 15 × 103 cells/ml BALF (Fig. 4F). The number of total BALF cells in saline-treated AT2CC−/− mice was not different from that in saline-treated WT mice (108 ± 10 × 103 cells/ml); however, LPS-treated AT2CC−/− mice had an increase in total BALF cells (272 ± 27 × 103 cells/ml) compared with LPS-treated WT mice, suggesting an enhanced cellular inflammatory response to LPS in AT2CC−/− mice. A similar pattern was observed with BALF neutrophils, such that LPS-treated AT2CC−/− mice exhibited a greater number of neutrophils compared with LPS-treated WT mice. BALF macrophages were decreased with LPS treatment, but the number did not differ between WT and AT2CC−/− mice. In addition, no differences in the number of BALF lymphocytes were observed.
To further investigate lung inflammation in LPS-treated AT2CC−/− mice, we fixed lung sections and stained them with hematoxylin and eosin, and inflammation scores were quantitatively assessed by a pathologist blinded to study group. There were no differences in inflammation scores between saline-treated WT and AT2CC−/− mice (Fig. 5A). Consistent with BALF cell data, treatment with LPS increased the lung inflammation score in both WT and AT2CC−/− mice; however, the score was significantly (11%) higher in AT2CC−/− lungs relative to WT lungs (10.1 ± 0.4 vs. 9.1 ± 0.3, respectively).
Figure 5.
LPS-induced inflammation and cytokine/chemokine response in WT and AT2CC−/− mice. WT and AT2CC−/− mice were treated with saline or LPS (50 μg via oropharyngeal aspiration) for 4 h. A) Lungs were fixed in 4% paraformaldehyde overnight and embedded in paraffin. Sample sections were stained with hematoxylin and eosin, and the degree of inflammation was scored by a pathologist who was blinded to the genotype and treatment group assignment (n = 10–12), Levels of CCL2 (B), IL-1β (C), IL-6 (D), and TNF-α (E) mRNA were determined by real-time RT-PCR in lung tissues. Protein levels of CCL2 (F), IL-1β (G), IL-6 (H), and TNF-α (I) were measured in BALF (n = 10–12). *P < 0.05 vs. saline of same genotype; #P < 0.05 vs. WT LPS.
Proinflammatory chemokines and cytokines, such as IL-6, IL-1β, CCL2, and TNF-α, were also measured in lung tissues and BALF (Bio-Plex; Bio-Rad, Hercules, CA, USA). Despite increases in BALF total cells and neutrophils in LPS-treated AT2CC−/− mice relative to counts in WT, there were no significant differences in expression in the lungs of WT and AT2CC−/− mice of any of these proinflammatory molecules (Fig. 5B–E). As well, there were no significant differences between the genotypes in BALF levels of CCL2, IL-1β, IL-6, and TNF-α (Fig. 5F–I).
COX-1 mRNA levels were unchanged, regardless of treatment or genotype (Fig. 6A). Consistent with the data in Fig. 1, COX-2 mRNA levels were reduced in saline-treated AT2CC−/− lungs relative to WT (Fig. 6B). LPS increased COX-2 mRNA levels in WT lungs; however, the increase in COX-2 mRNA was less pronounced in AT2CC−/− lungs (Fig. 6B). The residual increase in COX-2 mRNA levels in AT2CC−/− lungs was most likely attributable to the induction of COX-2 in other cell types within the lung, such as macrophages and dendritic cells. As demonstrated earlier, these other cell types also expressed COX-2 under unstimulated (basal) conditions (Fig. 1D).
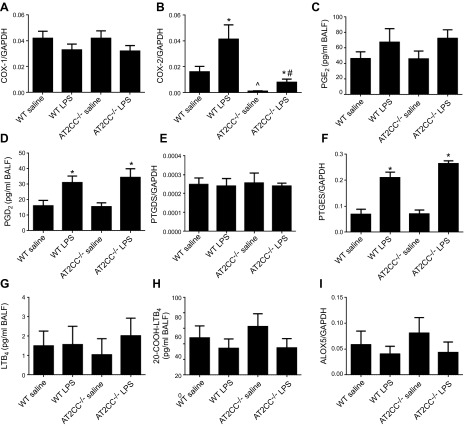
Figure 6.
COX expression and eicosanoid levels in LPS-treated WT and AT2CC−/− mice. WT and AT2CC−/− mice were treated with saline or LPS (50 μg via oropharyngeal aspiration) for 4 h. COX-1 (A) and COX-2 (B) levels were determined in lung tissues by real time RT PCR. BALF levels of PGE2 (C) and PGD2 (D) were measured by LC/MS/MS. PTGDS (E) and PTGES (F) levels were determined in lung tissues by real time RT PCR. BALF levels of LTB4(G) and 20-COOH-LTB4 (H) were measured by LC/MS/MS. I) ALOX5 mRNA levels were determined in lung tissues by real time RT PCR (n = 12–31). *P < 0.05 vs. saline of same genotype; ^P < 0.05 vs. WT saline; #P < 0.05 vs. WT LPS.
We also measured BALF eicosanoid levels by LC/MS/MS in saline- and LPS-treated animals. BALF levels of PGE2 and PGD2 were similar in saline-treated WT and AT2CC−/− mice (Fig. 6C, D). LPS treatment led to an increase in BALF PGE2 and PGD2 levels; however, there were no significant differences between WT and AT2CC−/− mice. This lack of difference was not unexpected, as other cells in the lung are capable of generating COX-2 and producing PGs. In addition, COX-1 likely contributes to increases in PG levels after LPS stimulation and may be directly coupled to PTGDS (26). Levels of Ptgds and Ptges mRNA were similar in lungs from WT and AT2CC−/− mice treated with or without LPS (Fig. 6E, F). LPS increased Ptges expression in both strains; however, no changes in Ptgds expression were observed after LPS. Certain forms of Ptgds have been reported to undergo posttranslational modification, which affects its enzyme function and may explain why LPS increased PGD2 levels in isolated ATII cells and BALF in the absence of changes in expression of lung Ptgds mRNA (27, 28). Lung expression of ALOX5 and BALF levels of LTB4 and COOH-LTB4 was not altered by LPS treatment or COX-2 disruption in ATII cells (Fig. 6G–I).
Together, these results suggest that ATII-derived COX-2 affects basal airway responsiveness to methacholine and alters the hypothermic and lung inflammatory response to LPS, but does not influence LPS-induced AHR or expression of proinflammatory cytokines and chemokines.
Bleomycin-induced lung dysfunction and fibrosis in AT2CC−/− mice
COX-2 has been shown to play a beneficial role in bleomycin-induced lung fibrosis, particularly through the actions of its metabolite PGE2 (12). To assess whether ATII cell–derived COX-2 plays a prominent role in this effect, AT2CC−/− and WT mice were given bleomycin (0.5 U/kg) via oropharyngeal aspiration. COX-1 and -2 mRNA levels were unchanged in the lungs of saline- and bleomycin-treated WT and AT2CC−/− mice (Fig. 7A), whereas COX-2 mRNA levels were decreased in lungs of saline-treated ATCC−/− mice compared with WT (Fig. 7B). Body weights were measured weekly, as bleomycin is known to cause weight loss in the first several days after treatment, because of loss of appetite (12). One week after bleomycin administration, WT mice lost 2.1 ± 0.4 g, whereas those given saline gained weight (1.3 ± 0.3 g) (Fig. 7C). Similarly, bleomycin-treated AT2CC−/− mice lost 2.0 ± 0.4 g and saline-treated AT2CC−/− mice gained 1.0 ± 0.2 g. After 21 d, all mice had recovered and gained similar weight.
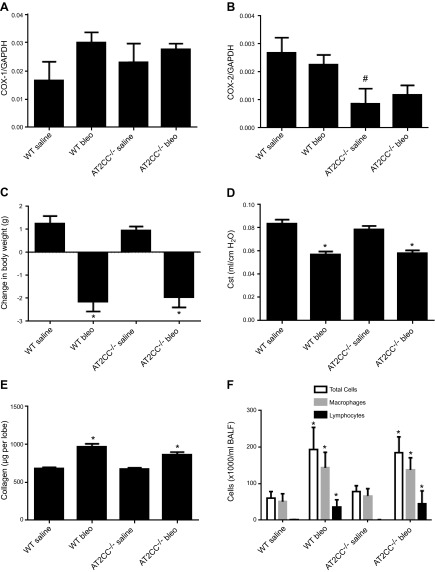
Figure 7.
Bleomycin-induced lung dysfunction and fibrosis in WT and AT2CC−/− mice. WT and AT2CC−/− mice were treated with saline or bleomycin (1 mg/kg body weight) via oropharyngeal aspiration and studied 21 d later. COX-1 (A) and COX-2 (B) mRNA levels in whole lungs were determined by real-time RT-PCR. C) Body weights of mice 1 wk after bleomycin/saline administration. D) Lung function measured as Cst by Flexivent. E) Caudal lung lobes were lysed, and collagen content was measured by colorimetric assay. F) BALF was collected, and the counts of total cells, macrophages, and lymphocytes were assessed (n = 8–10). *P < 0.05 vs. saline of same genotype; #P < 0.05 vs. WT saline.
Maximal lung fibrotic response to bleomycin occurs 21–28 d after administration (29). We and others (11, 30) used the 21 d time point to assess the effects of bleomycin on lung function and fibrosis. One study showed that bleomycin causes a decrease in lung function, as measured by quasi-static compliance (Cst) via Flexivent (11). As shown in Fig. 7D, bleomycin treatment lowered Cst of WT mice from 0.083 ± 0.003 to 0.057 ± 0.003 ml/cm H2O. The Cst of saline-treated AT2CC−/− mice was no different than that of WT mice (0.079 ± 0.003 ml/cm H2O). Moreover, bleomycin decreased Cst to a comparable degree in AT2CC−/− mice (0.058 ± 0.002 ml/cm H2O).
Bleomycin causes an increase in collagen and extracellular matrix deposition in the lung. To determine lung collagen levels, caudal lobes were isolated, lysed, and processed for collagen content with the Biocolor Sircol Assay. Full caudal lobes were specifically used to minimize potential variations that may be introduced by the use of different lobes. Treatment with bleomycin increased collagen content in WT lungs from 680 ± 16 to 967 ± 39 µg (Fig. 7E). The collagen content of AT2CC−/− lungs was increased to a similar degree from 677 ± 15 to 864 ± 33 µg.
Another feature of bleomycin-induced lung injury is increased inflammatory cells in the BALF. Macrophages and lymphocytes are the most common inflammatory cell types that are observed after bleomycin challenge. Neutrophils may also be present, but in smaller numbers. There was a ∼3–4-fold increase in the number of total BALF cells in WT mice treated with bleomycin, relative to the number in saline-treated WT mice (Fig. 7F). A similar increase in total BALF cells was observed in AT2CC−/− mice. Cell differential analyses revealed that the number of macrophages and lymphocytes was significantly and similarly increased in the BALF of WT and AT2CC−/− mice. No differences in the number of neutrophils were observed 21 d after bleomycin treatment.
Proinflammatory chemokines and cytokines were also measured in the lung tissue and BALF after bleomycin administration. Treatment with bleomycin resulted in a significant increase in lung expression of CCL2, IL-1β, IL-6, and TNF-α (Bio-Plex; Bio-Rad) (Fig. 8A–D). There were no significant differences between WT and AT2CC−/− mice in lung expression of any of these proinflammatory molecules. There were also no significant differences in BALF levels of CCL2 or IL-6 between the genotypes after bleomycin treatment (Fig. 8E, F).
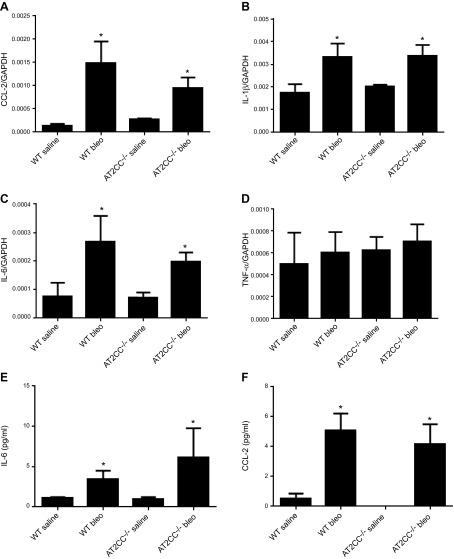
Figure 8.
Bleomycin-induced inflammation in WT and AT2CC−/− mice. WT and AT2CC−/− mice were treated with saline or bleomycin (1 mg/kg body weight) via oropharyngeal aspiration and studied 21 d later. Whole lungs were lysed, and mRNA levels of CCL2 (A), IL-1β (B), IL-6 (C), and TNF-α (D) were determined by real-time RT-PCR. Levels of IL-6 (E), and CCL2 (F) were measured in BALF (n = 8–10); *P < 0.05 vs. saline of same genotype.
Together, these results suggest that ATII-derived COX-2 does not play an essential role in the deleterious effects of bleomycin in the lungs of mice.
DISCUSSION
In this study, we investigated the effect of ATII cell–specific knockdown of COX-2 in mouse models of bleomycin-induced lung fibrosis and LPS-induced lung inflammation and AHR. COX-2 is an inducible enzyme that metabolizes arachidonic acid to PGs, prostacyclin, and thromboxane. It is commonly characterized as a proinflammatory protein and is upregulated in several inflammatory diseases. It is expressed in many cell types of the lung, including epithelial cells and alveolar macrophages (6). Recent studies have shown that disruption or inhibition of COX-2 in mice exacerbates lung injury and inflammation (30, 31), suggesting that COX-2 plays a beneficial role in the pathogenesis of lung diseases. Because most of these studies have used global COX-2–null mice or those given COX-2 inhibitors systemically, the role of COX-2 and its eicosanoid products in specific cell types remains unclear. This study is the first to report the impact of ATII cell–derived COX-2 in regulating basal lung function and in the pathogenesis of bleomycin-induced lung fibrosis and LPS-induced lung inflammation and AHR.
The ATII cells reside on the basement membrane of the alveolar epithelium, comprise 15% of all lung cells, and are involved in the regulation of surfactant metabolism and ion transport. A distinct feature of these cells is the presence of lamellar bodies within the cytoplasm, as well as their ability to secrete multiple SPs: SPA, -B, and -C (16). In addition, ATII cells play a role in alveolar repair and are in direct contact with environmental agents that may cause lung injury (16). Administration of diphtheria toxin to mice expressing the human diphtheria toxin receptor specifically on ATII cells resulted in lung fibrosis (32). Thus, these cells may participate in the development of lung fibrosis and inflammation in response to bleomycin and LPS. That COX-2 is both basally expressed and inducible in ATII cells raises the possibility that ATII cell–derived COX-2 plays a significant role in these effects.
Mice with ATII cell–specific knockdown of COX-2 (AT2CC−/−) were generated by crossing COX-2 floxed null mice with SPC-Cre mice using the Lox-P method (20). Knockdown of COX-2 in ATII cells was confirmed by a variety of methods. It should be noted that basal levels of COX-2 in ATII cells are relatively low compared with those in macrophages or other cell types; however, these cells are more than capable of generating multiple PGs and thromboxane, all of which may contribute to the phenotypes observed in lung diseases. ATII cells from LPS-treated WT mice exhibited significant increases in PGF2α, PGE2, and PGD2. None of these PGs were increased in LPS-treated AT2CC−/− ATII cells. ATII cells from WT and AT2CC−/− mice expressed Ptgds and Ptges mRNA at similar levels. Therefore, decreased PG production was due to COX-2 knockdown, not to alterations in synthase expression.
The role of COX-2 in lung fibrosis has been well characterized in mice. In 2001, Keerthisingam et al. (31) demonstrated that COX-2–null mice exhibited an enhanced response to bleomycin-induced lung fibrosis. This finding was subsequently confirmed by Lovgren et al. (33). The exacerbated response to bleomycin-induced lung fibrosis in COX-2–deficient mice has been shown to be due to a reduction in PGE2 biosynthesis (30). Indeed, our lab has recently shown that prophylactic treatment of bleomycin-exposed mice with PGE2 in osmotic minipumps significantly prevents lung dysfunction and attenuates increases in lung fibrosis (12). In the current study, no differences in fibrotic or inflammatory responses to bleomycin were observed between AT2CC−/− and WT mice. Body weight, Cst, collagen level, and inflammatory index were not significantly different between the genotypes. Bleomycin may have a greater effect on inducing COX-2 in other cell types, such as the lymphocytes and macrophages, thus providing a possible explanation for the absence of fibrotic, lung functional, and inflammatory phenotypes in AT2CC−/− mice. It should be pointed out that bleomycin has been shown to induce ATII cell death in mouse and rat ATII cell lines in vitro (34); whether this occurs in vivo is unknown. Nevertheless, the contribution of ATII cell–derived COX-2 and its eicosanoid products appears to be minimal in this model of bleomycin-induced lung injury and fibrosis.
COX-2 has also been shown to play a role in LPS-induced lung inflammation and AHR. Dissociated lung inflammation and AHR in response to intranasally administered LPS has been observed in COX-2–null mice (14). Moreover, administration of a COX-2 inhibitor attenuated the LPS-induced increase in the AHR response to methacholine in isolated perfused mouse lungs (15). The COX-2 product PGE2 has been shown to protect the lower airways from bronchoconstriction (35). To determine whether ATII cell–derived COX-2 plays a role in LPS-induced inflammation and AHR, we examined whole lungs in vivo and isolated ATII cells in vitro. LPS-induced AHR response to methacholine is associated with increases in R, Rn, G, and E (23). No differences in any of these parameters were observed between LPS-treated WT and AT2CC−/− mice; however, a significant decrease in basal airway responsiveness to methacholine was observed in AT2CC−/− mice.
Although ATII cell–derived COX-2 did not appear to affect LPS-induced AHR, BALF from AT2CC−/− mice had significantly more total cells and neutrophils than did WT mice. Moreover, histopathologic scoring of lung sections showed that AT2CC−/− lungs had increased inflammation compared with WT lungs. No significant differences in the expression of proinflammatory molecules, such as IL-1β, IL-6, CCL2, and TNF-α, were observed between genotypes. COX-2 knockdown also did not affect the levels of these inflammatory molecules in ATII cells. One possible explanation of the increased number of total cells and neutrophils in AT2CC−/− mice may be reduced production of PGE2 by ATII cells after LPS administration. Indeed, PGE2 has been shown to block neutrophil recruitment after short-term LPS inhalation in BALB/c mice (36). Thus, the reduction in ATII PGE2 levels with LPS may be sufficient to affect the number of BALF cells and neutrophil infiltration, but not sufficiently to affect airway responsiveness to methacholine, given that BALF PGE2 levels were unchanged. In addition, it has been suggested that prostanoid generation in BALF is predominantly dependent on COX-1 activity, whereas the number of BALF inflammatory cells depends on COX-2 activity (13). In our study, COX-1 expression was similar in WT and AT2CC−/− mice.
In this study, we explored the possibility of ATII cells as a source of COX-2 in models of lung diseases. We found that ATII cells expressed COX-2 and were capable of PG biosynthesis. Although global COX-2 disruption exacerbates bleomycin-induced injury, we showed that ATII cell–specific COX-2 knockdown played a minimal role in bleomycin-induced lung dysfunction and fibrosis. In contrast, disruption of COX-2 in ATII cells decreased basal airway responsiveness to methacholine and increased LPS-induced total cell and neutrophil infiltration of the BALF. ATII cell–derived COX-2 appeared to play a minimal role in LPS-induced AHR and the production of proinflammatory molecules. Future work is needed to investigate other cellular sources of COX-2 in the lung that may contribute to the pathogenesis of these pulmonary diseases.
Acknowledgments
This work was supported by U.S. National Institutes of Health, National Institute of Environmental Health Sciences, Intramural Research Program Grants Z01 ES050167 (to K.B.T.) and Z01 ES025034 (to D.C.Z.). The authors declare no conflicts of interest.
Glossary
- AHR
airway hyperresponsiveness
- ALOX5
5-lipoxygenase
- ATII
alveolar type II
- BALF
bronchoalveolar lavage fluid
- Cst
quasi-static compliance
- CCL
C-C chemokine ligand
- COX
cyclooxygenase
- E
total elastance
- FBS
fetal bovine serum
- G
tissue damping (resistance)
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- LC/MS/MS
liquid chromatography–tandem mass spectrometry
- LTB4
leukotriene B4
- PG
prostaglandin
- PTGDS
prostaglandin D2 synthase
- PTGES
prostaglandin E2 synthase
- R
total resistance
- Rn
newtonian peripheral resistance
- SP
surfactant protein
- TXB2
stable thromboxane metabolite
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Farkas L., Gauldie J., Voelkel N. F., Kolb M. (2011) Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am. J. Respir. Cell Mol. Biol. 45, 1–15 [DOI] [PubMed] [Google Scholar]
- 2.Galvin J. R., Franks T. J. (2009) Smoking-related lung disease. J. Thorac. Imaging 24, 274–284 [DOI] [PubMed] [Google Scholar]
- 3.Garantziotis S., Steele M. P., Schwartz D. A. (2004) Pulmonary fibrosis: thinking outside of the lung. J. Clin. Invest. 114, 319–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonner J. C., Rice A. B., Moomaw C. R., Morgan D. L. (2000) Airway fibrosis in rats induced by vanadium pentoxide. Am. J. Physiol. Lung Cell. Mol. Physiol. 278, L209–L216 [DOI] [PubMed] [Google Scholar]
- 5.Michel O., Kips J., Duchateau J., Vertongen F., Robert L., Collet H., Pauwels R., Sergysels R. (1996) Severity of asthma is related to endotoxin in house dust. Am. J. Respir. Crit. Care Med. 154, 1641–1646 [DOI] [PubMed] [Google Scholar]
- 6.Bauer A. K., Dwyer-Nield L. D., Malkinson A. M. (2000) High cyclooxygenase 1 (COX-1) and cyclooxygenase 2 (COX-2) contents in mouse lung tumors. Carcinogenesis 21, 543–550 [DOI] [PubMed] [Google Scholar]
- 7.Lee S. H., Soyoola E., Chanmugam P., Hart S., Sun W., Zhong H., Liou S., Simmons D., Hwang D. (1992) Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J. Biol. Chem. 267, 25934–25938 [PubMed] [Google Scholar]
- 8.Nakamura T., Sakamoto K. (2001) Reactive oxygen species up-regulates cyclooxygenase-2, p53, and Bax mRNA expression in bovine luteal cells. Biochem. Biophys. Res. Commun. 284, 203–210 [DOI] [PubMed] [Google Scholar]
- 9.Ermert L., Ermert M., Merkle M., Goppelt-Struebe M., Duncker H. R., Grimminger F., Seeger W. (2000) Rat pulmonary cyclooxygenase-2 expression in response to endotoxin challenge: differential regulation in the various types of cells in the lung. Am. J. Pathol. 156, 1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonner J. C., Rice A. B., Ingram J. L., Moomaw C. R., Nyska A., Bradbury A., Sessoms A. R., Chulada P. C., Morgan D. L., Zeldin D. C., Langenbach R. (2002) Susceptibility of cyclooxygenase-2-deficient mice to pulmonary fibrogenesis. Am. J. Pathol. 161, 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Card J. W., Voltz J. W., Carey M. A., Bradbury J. A., Degraff L. M., Lih F. B., Bonner J. C., Morgan D. L., Flake G. P., Zeldin D. C. (2007) Cyclooxygenase-2 deficiency exacerbates bleomycin-induced lung dysfunction but not fibrosis. Am. J. Respir. Cell Mol. Biol. 37, 300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dackor R. T., Cheng J., Voltz J. W., Card J. W., Ferguson C. D., Garrett R. C., Bradbury J. A., DeGraff L. M., Lih F. B., Tomer K. B., Flake G. P., Travlos G. S., Ramsey R. W. Jr., Edin M. L., Morgan D. L., Zeldin D. C. (2011) Prostaglandin E₂ protects murine lungs from bleomycin-induced pulmonary fibrosis and lung dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L645–L655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swedin L., Ellis R., Neimert-Andersson T., Ryrfeldt A., Nilsson G., Inman M., Dahlén S. E., Adner M. (2010) Prostaglandin modulation of airway inflammation and hyperresponsiveness in mice sensitized without adjuvant. Prostaglandins Other Lipid Mediat. 92, 44–53 [DOI] [PubMed] [Google Scholar]
- 14.Zeldin D. C., Wohlford-Lenane C., Chulada P., Bradbury J. A., Scarborough P. E., Roggli V., Langenbach R., Schwartz D. A. (2001) Airway inflammation and responsiveness in prostaglandin H synthase-deficient mice exposed to bacterial lipopolysaccharide. Am. J. Respir. Cell Mol. Biol. 25, 457–465 [DOI] [PubMed] [Google Scholar]
- 15.Held H. D., Uhlig S. (2000) Mechanisms of endotoxin-induced airway and pulmonary vascular hyperreactivity in mice. Am. J. Respir. Crit. Care Med. 162, 1547–1552 [DOI] [PubMed] [Google Scholar]
- 16.Dobbs L. G. (1990) Isolation and culture of alveolar type II cells. Am. J. Physiol. 258, L134–L147 [DOI] [PubMed] [Google Scholar]
- 17.Marcet B., Libert F., Boeynaems J. M., Communi D. (2007) Extracellular nucleotides induce COX-2 up-regulation and prostaglandin E2 production in human A549 alveolar type II epithelial cells. Eur. J. Pharmacol. 566, 167–171 [DOI] [PubMed] [Google Scholar]
- 18.Kühn R., Torres R. M. (2002) Cre/loxP recombination system and gene targeting. Methods Mol. Biol. 180, 175–204 [DOI] [PubMed] [Google Scholar]
- 19.Okubo T., Knoepfler P. S., Eisenman R. N., Hogan B. L. (2005) Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development 132, 1363–1374 [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa T. O., Oshima M., Herschman H. R. (2011) Cox-2 deletion in myeloid and endothelial cells, but not in epithelial cells, exacerbates murine colitis. Carcinogenesis 32, 417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikawa T. O., Herschman H. R. (2006) Conditional knockout mouse for tissue-specific disruption of the cyclooxygenase-2 (Cox-2) gene. Genesis 44, 143–149 [DOI] [PubMed] [Google Scholar]
- 22.Narasimha A. J., Watanabe J., Ishikawa T. O., Priceman S. J., Wu L., Herschman H. R., Reddy S. T. (2010) Absence of myeloid COX-2 attenuates acute inflammation but does not influence development of atherosclerosis in apolipoprotein E null mice. Arterioscler. Thromb. Vasc. Biol. 30, 260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Card J. W., Carey M. A., Bradbury J. A., DeGraff L. M., Morgan D. L., Moorman M. P., Flake G. P., Zeldin D. C. (2006) Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J. Immunol. 177, 621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee C. R., Imig J. D., Edin M. L., Foley J., DeGraff L. M., Bradbury J. A., Graves J. P., Lih F. B., Clark J., Myers P., Perrow A. L., Lepp A. N., Kannon M. A., Ronnekleiv O. K., Alkayed N. J., Falck J. R., Tomer K. B., Zeldin D. C. (2010) Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 24, 3770–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ejima K., Layne M. D., Carvajal I. M., Kritek P. A., Baron R. M., Chen Y. H., Vom Saal J., Levy B. D., Yet S. F., Perrella M. A. (2003) Cyclooxygenase-2-deficient mice are resistant to endotoxin-induced inflammation and death. FASEB J. 17, 1325–1327 [DOI] [PubMed] [Google Scholar]
- 26.Kihara Y., Gupta S., Maurya M. R., Armando A., Shah I., Quehenberger O., Glass C. K., Dennis E. A., Subramaniam S. (2014) Modeling of eicosanoid fluxes reveals functional coupling between cyclooxygenases and terminal synthases. Biophys. J. 106, 966–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ragolia L., Hall C. E., Palaia T. (2007) Post-translational modification regulates prostaglandin D2 synthase apoptotic activity: characterization by site-directed mutagenesis. Prostaglandins Other Lipid Mediat. 83, 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lescuyer P., Gandini A., Burkhard P. R., Hochstrasser D. F., Sanchez J.-C. (2005) Prostaglandin D2 synthase and its post-translational modifications in neurological disorders. Electrophoresis 26, 4563–4570 [DOI] [PubMed] [Google Scholar]
- 29.Moore B. B., Hogaboam C. M. (2008) Murine models of pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L152–L160 [DOI] [PubMed] [Google Scholar]
- 30.Hodges R. J., Jenkins R. G., Wheeler-Jones C. P., Copeman D. M., Bottoms S. E., Bellingan G. J., Nanthakumar C. B., Laurent G. J., Hart S. L., Foster M. L., McAnulty R. J. (2004) Severity of lung injury in cyclooxygenase-2-deficient mice is dependent on reduced prostaglandin E(2) production. Am. J. Pathol. 165, 1663–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keerthisingam C. B., Jenkins R. G., Harrison N. K., Hernandez-Rodriguez N. A., Booth H., Laurent G. J., Hart S. L., Foster M. L., McAnulty R. J. (2001) Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am. J. Pathol. 158, 1411–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sisson T. H., Mendez M., Choi K., Subbotina N., Courey A., Cunningham A., Dave A., Engelhardt J. F., Liu X., White E. S., Thannickal V. J., Moore B. B., Christensen P. J., Simon R. H. (2010) Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 181, 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovgren A. K., Jania L. A., Hartney J. M., Parsons K. K., Audoly L. P., Fitzgerald G. A., Tilley S. L., Koller B. H. (2006) COX-2-derived prostacyclin protects against bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L144–L156 [DOI] [PubMed] [Google Scholar]
- 34.Lee V. Y., Schroedl C., Brunelle J. K., Buccellato L. J., Akinci O. I., Kaneto H., Snyder C., Eisenbart J., Budinger G. R. S., Chandel N. S. (2005) Bleomycin induces alveolar epithelial cell death through JNK-dependent activation of the mitochondrial death pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L521–L528 [DOI] [PubMed] [Google Scholar]
- 35.Hartney J. M., Coggins K. G., Tilley S. L., Jania L. A., Lovgren A. K., Audoly L. P., Koller B. H. (2006) Prostaglandin E2 protects lower airways against bronchoconstriction. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L105–L113 [DOI] [PubMed] [Google Scholar]
- 36.Gonçalves de Moraes V. L., Boris Vargaftig B., Lefort J., Meager A., Chignard M. (1996) Effect of cyclo-oxygenase inhibitors and modulators of cyclic AMP formation on lipopolysaccharide-induced neutrophil infiltration in mouse lung. Br. J. Pharmacol. 117, 1792–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]