Abstract
Deficiencies of the human cystathionine β-synthase (CBS) enzyme are characterized by a plethora of vascular disorders and hyperhomocysteinemia. However, several clinical trials demonstrated that despite reduction in homocysteine levels, disease outcome remained unaffected, thus the mechanism of endothelial dysfunction is poorly defined. Here, we show that the loss of CBS function in endothelial cells (ECs) leads to a significant down-regulation of cellular hydrogen sulfide (H2S) by 50% and of glutathione (GSH) by 40%. Silencing CBS in ECs compromised phenotypic and signaling responses to the VEGF that were potentiated by decreased transcription of VEGF receptor (VEGFR)-2 and neuropilin (NRP)-1, the primary receptors regulating endothelial function. Transcriptional down-regulation of VEGFR-2 and NRP-1 was mediated by a lack in stability of the transcription factor specificity protein 1 (Sp1), which is a sulfhydration target of H2S at residues Cys68 and Cys755. Reinstating H2S but not GSH in CBS-silenced ECs restored Sp1 levels and its binding to the VEGFR-2 promoter and VEGFR-2, NRP-1 expression, VEGF-dependent proliferation, and migration phenotypes. Thus, our study emphasizes the importance of CBS-mediated protein S-sulfhydration in maintaining vascular health and function.—Saha, S., Chakraborty, P. K., Xiong, X., Dwivedi, S. K. D., Mustafi, S. B., Leigh, N. R., Ramchandran, R., Mukherjee, P., Bhattacharya, R. Cystathionine β-synthase regulates endothelial function via protein S-sulfhydration.
Keywords: angiogenesis, VEGFRs, signal transduction, metabolism
Cystathionine, the precursor for cysteine, is generated from the condensation of serine and homocysteine (Hcy) in the transsulfuration pathway (1–3). Cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) are the predominant enzymes that generate hydrogen sulfide (H2S), the third gasotransmitter, from cysteine that serves as a precursor for glutathione (GSH) biosynthesis (4, 5). There are over 150 mutations, and deficiencies in the human CBS enzyme are characterized by hyperhomocysteinemia (HHcy) (6, 7). Initially elevated Hcy was established as an independent risk factor; however, later it was implicated in cardiovascular diseases, thromboembolism, and stroke—all diseases associated with significant endothelial dysfunction and impaired angiogenesis (8–10). In addition, CBS knockout mice display poor response to vasodilators and impaired angiogenesis that have been largely attributed to redox imbalance and high levels of plasma Hcy (11, 12). However, clinical trials using vitamin supplements lowered Hcy but did not alter disease outcome, suggesting an intricate involvement of metabolites generated through the transsulfuration pathway in causing endothelial dysfunction (13, 14). Furthermore, a number of studies using supraphysiologic concentrations of Hcy on endothelial cells (ECs) have made it extremely difficult to decipher the underlying etiology of endothelial dysfunction (15–19). More recently, H2S, an otherwise toxic gas with the smell of rotten eggs, has attracted considerable attention in vascular biology (20–22). Recent studies have demonstrated that H2S functions by modifying free thiol groups (-SH) in a protein to form persulfides (-SSH), a process known as protein S-sulfhydration (23, 24). A few significant proteins have recently been identified to be targets of protein S-sulfhydration, including glyceraldehyde 3-phosphate dehydrogenase (GAPDH), actin, tubulin, NF-κB, Parkin, and Ca2+-TRP channels (23, 25–27). Thus, we speculate that patients with compromised CBS function, besides accumulating Hcy, more importantly present with deficiency of H2S and the ubiquitous antioxidant GSH, which have remained largely unexplored in the context of endothelial function (9).
The proliferative, migratory, and tube-forming ability of ECs is critical in adult tissues, supporting repair and renewing lining of established blood vessels, in addition to angiogenesis. Arguably, VEGF/VEGF receptor (VEGFR)-2/neuropilin (NRP)-1 (VEGF A, VEGFR-2, NRP-1) signaling is the predominant axis critical in maintaining EC function exemplified by embryonic lethality of the VEGFR-2−/− and NRP1−/− mice (28–30). Surprisingly, this major signaling pathway remains unexplored in the context of endothelial dysfunction in CBS deficiency. Here, we dissect the underlying cause of endothelial dysfunction in CBS deficiency. Using pharmacological and genetic approaches, we show that deregulation of metabolite homeostasis, primarily H2S but not Hcy or GSH, in CBS-silenced ECs results in specificity protein 1 (Sp1)-mediated transcriptional down-regulation of VEGFR-2 and NRP-1 that are required for EC function. We show that endogenous Sp1 is sulfhydrated by H2S produced by the enzymatic action of CBS. Sulfhydrated Sp1 is stable and functionally active in that it can bind promoter regions and drive transcription of responsive genes.
MATERIALS AND METHODS
Reagents, cell lines, and culture
The following antibodies were used for immunoblotting: rabbit polyclonal CSE antibody (12217-1-AP; Proteintech, Chicago, IL, USA), rabbit polyclonal CBS antibody (H-300, sc-67154), rabbit polyclonal to Flk-1 (sc-504), Flt-1 (sc-316), rabbit polyclonal anti-NRP2 (sc-5542) are from Santa Cruz Biotechnology (Dallas, TX, USA). Mouse monoclonal β-Actin antibody (A-2228) and rabbit polyclonal to GAPDH (G9545) are from Sigma-Aldrich (St. Louis, MO, USA). Rabbit polyclonal to Sp1(07-645; Millipore, Temecula, CA, USA). Anti–α-tubulin (ab4074) and anti-Hcy antibody (ab15154) were from Abcam (Cambridge, MA, USA). Small interfering RNA (siRNA) against human CBS was from Qiagen (SI02777159; Valencia, CA, USA) and Sigma-Aldrich (SASI_Hs01_00214623), and scrambled control siRNA (1027280) was from Qiagen. EGM bullet kit, 0.25% trypsin-EDTA, and trypsin neutralizing solution were from Lonza (Basel, Switzerland). MCDB105 and Medium199 were from Sigma-Aldrich. Oligofectamine, Lipofectin, and Optimem-I were from Invitrogen (Carlsbad, CA, USA). HUVECs (CC-2517) from Lonza were cultured in complete endothelial basal medium (EBM) medium (EBM + EGM kit) and all experiments were performed at Passage5. Ovarian surface epithelial cells (OSEs) were grown in MCDB105 medium supplemented with 15% FBS and 1% hygromycin.
Immunohistochemistry
Staining of tissues was performed on the Leica autostainer (Bond III), using bond polymer refine detection kit (catalog #DS9800; Leica Microsystems, Buffalo Grove, IL, USA). Slides were exposed to primary antibody recognizing CBS (1:1000, mouse polyclonal A01; Abnova, Taipei City, Taiwan) (31), after optimizing staining conditions on positive control tissues (granulosa cell tumors). Negative controls included a nonspecific isotype match and negative mouse sera (1:500); conditions were optimized until no background staining was observed without the specific primary antibody. Slides were prepared according to the manufacturer's instructions (Bond III; Leica Microsystems), and digital images were acquired using ×60 objective of Nikon (Tokyo, Japan) Eclipse Ni microscope.
siRNA transfection
siRNA transfection of HUVECs in 100 mm dishes with scrambled siRNA or CBS siRNA (CBS siRNA-1 from Sigma-Aldrich; CBS siRNA-2 from Qiagen) was carried out using Oligofectamine. Briefly, HUVECs at 30–40% confluency were plated in collagen-coated 100 mm dishes and grown overnight in complete EBM medium. Before transfection, cells were washed once with OptiMemI (Thermo Fisher Scientific, Waltham, MA, USA), then 3 ml of OptiMem I was added and kept in CO2 incubator until addition of transfection mixture. Transfection mixture of Oligofectamine (25 µl) and siRNA (25 µl of 10 µM) in 600 µl OptiMem was prepared as per the supplier’s protocol and added to cells, and after 6–7 h, 6.5 ml/plate of complete EBM was added to the plates. The cells were cultured for 48 h before any subsequent analysis.
Subsequent analyses were carried out with CBS-siRNA-1 referred to as CBS siRNA.
Proliferation assays
Forty-eight hours posttransfection, HUVECs were collected by trypsinization, counted, seeded in collagen-coated 24-well plates (2.5 × 104 per well), and cultured for 24 h. Subsequently, 1 μCi of [3H]thymidine per milliliter of culture medium per well was added; 4 h later, the cells were washed with chilled PBS, fixed with100% cold methanol for 20 min at room temperature, and lysed with 0.1 N NaOH, and radioactivity was estimated using BioSafe II.
VEGF-induced proliferation
Forty-eight hours posttransfection, HUVECs were collected by trypsinization, counted, seeded in collagen-coated 24-well plates (2.5 × 104 per well), and cultured for 8 h in complete medium, followed by overnight starvation in serum-free EBM medium. VEGF A165 (10 ng/ml) added in fresh serum-free medium and allowed to grow for 24 h before ascertaining proliferation using [3H]thymidine as above.
Scratch migration
Transfected HUVECs after 48 h transfection were collected by trypsinization, counted, seeded in collagen-coated 24-well plates (8 × 104 per well), and cultured for 24 h in complete medium to 100% confluency. The cells were washed with serum-free EBM and starved for 8 h. Wounds were made with the tip of a 20 µl pipette tip, washed with PBS, replaced with serum-supplemented EBM medium, and imaged. The closing of the wound was followed every hour, and after 8 h, cells were fixed with 4% paraformaldehyde for 10 min, stained with DAPI nuclear stain, and imaged. The number of migrated cells was counted from 9 different imaged fields.
Tube formation assay
Reduced growth factor Matrigel (BD Biosciences, San Jose, CA, USA) was thawed at 4°C overnight. Forty-eight hours after transfection, HUVECs were serum starved for 12 h. Matrigel was diluted in 1:5 ratio with cold serum-free medium and 50 µl of the diluted matrigel was added per well of a 96 well plate and incubated at 37°C overnight for 3 h. Serum-starved cells were harvested by 4 ml of collagenase solution containing 0.2 mg/ml collagenase, 0.2 mg/ml soybean trypsin inhibitor, 2 mg/ml bovine serum albumin (BSA), and 2 mM EDTA in PBS, counted and 2 × 104 cells in 100 µl medium were plated onto Matrigel (2 mg/ml) under serum-free condition. Tube formation was monitored after 30 min up to 4 h from plating of cells. Images were acquired using Zeiss Apotome microscope (Carl Zeiss GmbH, Jena, Germany) in phase contrast mode.
Boyden chamber migration
Boyden chamber migration assay was carried out with scrambled control siRNA or CBS siRNA-treated HUVECs and 10 ng/ml VEGF A165, as reported previously (32). Briefly, 36 h after transfection with scrambled siRNA or CBS siRNA, HUVECs were serum starved overnight, detached from culture plates using collagenase solution (0.2 mg/ml collagenase type I, 0.2 mg/ml soybean trypsin inhibitor, 1 mg/ml BSA, and 2 mM EDTA) and 5 × 104 cells were plated into 8 µm transwell chamber in 200 µl of serum-free EBM. The lower chambers of the plate were supplied with 650 µl serum-free EBM medium with or without 10 ng/ml VEGFA165. The cells were allowed to migrate for 12 h after which the number of migrated cells were stained with crystal violet and imaged from 5 unique fields and manually counted.
Immunoblotting and rescue experiments
Immunoblotting was performed as reported previously (33). In brief, cells were homogenized in RIPA supplemented with 1× protease-phosphatase mix and centrifuged at 14,000 rpm for 10 min at 4°C, and protein concentration in the supernatant was measured using bicinchoninic acid assay (Pierce, Rockford, IL, USA). A total of 20 µg protein was separated in 4–15% SDS-PAGE gel, transferred to PVDF membrane, blocked using 5% milk in Tris buffered saline with 0.1% Tween 20 and probed with primary antibody and then detected with horseradish peroxidase-conjugated secondary antibodies using enhanced chemiluminescence.
Rescue experiment with MG132, reduced GSH, and sodium hydrosulfide (NaHS) were carried out by supplementing them after 24 h transfection and analyzed 24 h posttreatment. For rescue experiments with chloroquine (200 µM and 500 µM) or Bafilomycin A1 (200 and 500 nM), treatment was carried out for 6 h after 42 h transfection of HUVECs with scrambled control or CBS siRNA.
Real-time PCR
Total RNA was extracted using the RNeasy Plus Mini kit (Qiagen) and retrotranscribed using iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA, USA), and qRT-PCR was performed using iTaq SYBR Green (Bio-Rad) following suppliers’ protocols. The primers for human CBS (PPH13484B-200), VEGF-A (PPH00251B-200) and β-actin (PPH00073G-200 ACTB) were from Qiagen. The comparative Ct method was used to calculate the relative abundance of the mRNA compared with that of β-actin expression. The experiments were performed independently 3 times in triplicate. Custom-designed primer sequences were as follows: CSE, forward: 5′-CAGCAATTACACCAGAAACCAAG-3′; reverse: 5′-CAGCCTTCAATGTCAATCACC-3′; 3-mercaptopyruvate sulfotransferase (3-MST), forward: 5′-CTACGAGGACATCAAGGAGAAC-3′; reverse: 5′-GTTCACGGTACCTGGGATG-3′; VEGFR1, forward: 5′-TCCCTCAACCTACAATCAAGTG-3′; reverse: 5′-GCTCTCAATTCTGTTTCCCATG-3′; VEGFR2, forward: 5′-ATAGAAGGTGCCCAGGAAAAG-3′; reverse: 5′-GTCTTCAGTTCCCCTCCATTG-3′; NRP-1, forward: 5′-CCCCAAACCACTGATAACTCG-3′; reverse: 5′-AGACACCATACCCAACATTCC-3′; NRP-2, forward: 5′-CTACATCAAGTTCACCTCCGAC-3′; reverse: 5′-GGATACTTCTCAGGAAACCCA-3′; and Sp1, forward: 5′-CAGATGCCCAACCCCAAG-3′; reverse: 5′-TGCCATACACTTTCCCACAG-3′.
Endogenous H2S measurement
H2S concentrations in HUVECs were measured using methylene blue assay as previously reported (22, 33). Briefly, scrambled or CBS siRNA-treated HUVECs 48 h posttransfection were collected by trypsinization and resuspended in a total volume of 200 μl of PBS (pH 7.4), and 100 μl was then transferred directly into a tube containing zinc acetate (1% wt/vol, 187.5 μl) and NaOH (12%, 12.5 μl) to trap the H2S for 20 min at room temperature. The rest of the cell suspension was used to estimate protein concentration by bicinchoninic acid assay. The reaction was terminated by adding 1 ml of H2O (pH 12.8), 200 μl of N,N-dimethyl-p-phenylenediamine sulfate (20 mM in 7.2 M HCl), and 200 μl of FeCl3 (30 mM in 1.2 M HCl). The mixture was incubated at room temperature in darkness for 15 min, and finally 600 μl of the mixture was added to a tube with 150 μl of trichloroacetic acid (10% wt/vol) to precipitate protein. The precipitated protein was removed by centrifugation at 10,000 g for 5 min, and absorbance at 670 nm of the resulting supernatant (200 μl) was determined. The H2S concentration of each sample was calculated against a calibration curve of NaHS. For determination of intracellular H2S concentration after AOAA (amino-oxyacetic acid) or PAG (N-propargylglycine) treatment, 3 × 106 HUVECs in 150 mm dishes were treated with 1 and 2 mM AOAA or PAG for 24 h in complete EBM medium. After 24 h treatment, the cells were collected and processed as mentioned above.
CSE and CBS activity assay
CBS and CSE activity assays were performed using Hcy or cysteine, respectively, as substrates, as reported earlier (27). Briefly, scrambled control siRNA-treated or CBS siRNA-treated or CSE siRNA-treated HUVECs 48 h posttransfection were homogenized in PBS (pH 6.8) containing 1× protease inhibitor cocktail. The enzymatic assay was performed in 250 µl reaction mixture containing the cell homogenate (equal protein from transfected cells), PBS (pH 7.4), 10 mM cysteine (for CSE)/10 mM Hcy (for CBS), and 15 µM pyridoxal-5′-phosphate. The reaction mixture was flushed with nitrogen and sealed, and the reactions were initiated by transferring the vials from ice to a 37°C incubator shaker. After incubating for 60 min, H2S trapping solution (125 µl 1% zinc acetate and 2.5 µl 10 N NaOH) was injected into each reaction vial, sealed, and further incubated and shaken horizontally for 60 min to completely trap liberated H2S. Following this incubation, 0.5 ml H2O was added per reaction vial, followed by 0.1 ml of N,N-dimethyl-p-phenylenediamine sulfate in 7.2 M HCl and 0.1 ml of ferric chloride hexahydrate in 1.2 M HCl. After 20 min of incubation at room temperature, the absorbance of the resulting solution was measured at 670 nm with a spectrophotometer. Negative controls included all components of the reaction mixture except the cell homogenates. H2S concentration of each sample was calculated against a calibration curve of NaHS.
GSH measurement
The assay was performed according to manufacturer's protocol (Cayman Chemicals, Ann Arbor, MI, USA). Briefly, HUVECs were transfected with the scrambled control or CBS siRNA in 100 mm culture plates as mentioned above. After 48 h, the cells were scraped off and collected in 50 mM MES buffer [2-(N-morpholino)ethanesulfonic acid; pH 6.0] and lysed by sonication at 4°C. The lysate was cleared by spinning at 14,000 rpm for 10 min at 4°C and the supernatant was collected. The lysate was deproteinated using triethanolamine reagent and total intracellular GSH determined against a standard curve of GSH by measuring absorbance at 405 nm for 25 min after addition of assay cocktail.
Hcy analysis
Homocysteinylated protein levels were estimated by dot blot analysis (34–36). Protein from siRNA-transfected cells (20 µg) was spotted onto nitrocellulose membranes, blocked (5% milk), incubated with anti-Hcy antibody (1:1000) in 1% BSA, and detected with goat anti-rabbit linked-horseradish peroxidase using chemiluminescence.
Reactive oxygen species assay
The reactive oxygen species (ROS) assay was carried out in HUVECs 48 h posttransfection with scrambled control or CBS siRNA following a previously published procedure with minor modifications (37). In brief, transfected cells were washed twice with prewarmed PBS and incubated with 5 µM carboxy-H2DCFDA [5 (6)-carboxy-2′,7′-dichlorofluorescein diacetate, Invitrogen] in fresh EBM medium for 30 min at 37°C. After the incubation, the excessive probe was washed off with 3 PBS washes and HUVECs were harvested with 0.25% trypsin-EDTA, and transferred to fluorescence-activated cell sorting tubes in 400 µl PBS. Dichlorodihydrofluorescein fluorescence was quantified by flow cytometry (3 × 104 cells) at an excitation wavelength of 488 nm and emission wavelength of 530 nm in a BD FACS Calibur flow cytometer (BD Biosciences).
mRNA stability assay
Forty-eight hours after siRNA transfection, HUVECs were treated with 2.5 μg/ml of actinomycin D (Enzo Life Sciences, Farmingdale, NY, USA), RNA was extracted every 2 h up to 8 h, and quantitative RT-PCR (qRT-PCR) was performed as above with GAPDH as internal control using the literature-reported method (38).
Luciferase assay
HUVECs (2.5 × 104) were plated per well of a collagen-coated 24-well plate and grown overnight. On the day of transfection, the cells were washed once with OptiMem, and then 400 µl of OptiMem was added to each well before adding transfection mixture. Transfection mixture per well was prepared with 5 µl Lipofectin, 2.5 µl of 10 µM siRNA, 1 µg promoter-firefly plasmid, and 100 ng wild-type pRLTK-Renilla plasmid in 100 µl OptiMem following the manufacturer’s protocol. After addition of transfection mixture, HUVECs were kept for 4–5 h in a CO2 incubator before adding 500 µl of serum-supplemented EBM. Luciferase activity was measured using a Promega (Madison, WI, USA) Dual-Glo luciferase kit as per the suppliers’ protocol 48 h posttransfection.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
HUVECs (8 × 104 )were plated onto collagen I-coated 96 well plates and were grown overnight. The following day, the cells were treated with various doses of NaHS or vehicle (PBS) and treatment continued for 24 h. After the treatment period, the medium was discarded and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution in serum-supplemented EBM (final concentration: 1 mg/ml) was added to the cells. After incubating for 3 h, the MTT solution was discarded, and 200 µl of dimethylsulfoxide was added to dissolve the formazan crystals. Absorbance was read at 570 nm, and the cell viability was expressed as a normalized percentage ratio of NaHS-treated cells to vehicle-only treated cells.
Modified biotin switch assay
This assay was carried out as described previously (23, 39). Briefly, cells were homogenized in HEN (Hepes-EDTA-NaOH) buffer [250 mM Hepes-NaOH (pH 7.7), 1 mM EDTA, and 0.1 mM neocuproine] supplemented with 100 μM deferoxamine and centrifuged at 14,000 rpm for 30 min at 4°C to remove cell debris. Cell lysates (500 μg) were added to blocking buffer [HEN buffer supplemented with 2.5% SDS and 20 mM methyl methanethiosulfonate (MMTS)] and incubated at 50°C for 20 min with frequent vortexing. MMTS was then removed after acetone precipitation of the proteins at −20°C for 20 min. After acetone removal, the precipitated proteins were washed twice with chilled acetone, and precipitated proteins were resuspended in HEN buffer supplemented with 1% SDS. To this suspension, 4 mM N-[6-(biotinamido)hexyl]-3′-(2’-pyridyldithio)propionamide in dimethylsulfoxide was added (final concentration: 0.8 mM) and incubated for 3 h at 25°C; finally, biotinylated proteins were precipitated by streptavidin-agarose beads, which were then washed 3 times with HEN buffer supplemented with 1% SDS. As a negative control, biotinylated proteins bound to streptavidin-agarose beads were treated with 2 mM DTT for 30 min at room temperature and washed 3 times with HEN buffer supplemented with 1% SDS to remove unbound proteins. The biotinylated proteins and negative controls were eluted by 2× SDS-PAGE sample buffer and subjected to Western blot analysis.
Chromatin immunoprecipitation
Transfected HUVECs 48 h after transfection were treated with 18.5% formaldehyde to cross-link proteins to DNA. Chromatin immunoprecipitation (ChIP) assays were performed using EZ-ChIP chromatin immunoprecipitation kit (Millipore; catalog no. 17-371) as per manufacturer's instructions. Briefly, after in vivo cross-linking, cells were lysed and sonicated to shear the chromatin to a manageable size (200–1000 bp). Immunoselections of cross-linked protein-DNA were performed with anti-rabbit IgG (negative control), anti-Sp1 antibody, and protein G-conjugated agarose beads. Protein-DNA complexes were washed, and then protein-DNA cross-links were reversed to free DNAs. The purified DNAs were analyzed by PCR using primers for VEGFR2 promoter as follows: hVEGFR2: 5′-GTCCACTTGTGTGGGGAAAT-3′; hVEGFR2: 5′-GAGCTGGAGCCGAAACTCTA-3′.
Maleimide assay
Maleimide was assayed following a published protocol (27). Briefly, HUVECs were homogenized in lysis buffer (150 mM NaCl; 50 mM Tris, pH 7.5; 1.2% Triton X-100; and 1 mM EDTA), and 1 µg of protein lysate was immunoprecipitated with 10 µg anti-Sp1 antibody using protein A/G agarose beads. The beads were washed 3 times with lysis buffer and then incubated with 5 µM Alexa 680-maleimide (red maleimide) in lysis buffer for 2 h at 4°C with gentle mixing intermittently. The beads were then pelleted, washed with lysis buffer 5 times each for 3 min on a rotating platform, and thereafter split into 2 tubes, one receiving lysis buffer and the other 1 mM DTT. The beads were further incubated for 1 h with occasional gentle mixing, washed with lysis buffer 5 times for 3 min each, and finally boiled with 2× SDS-PAGE buffer for gel electrophoresis. The gel was scanned with the Li-COorOdyssey system (setting 0.5; Li-Cor Biosciences, Lincoln, NE, USA) to detect red maleimide signal. The scanned gel was transferred to a PVDF membrane to detect Sp1 protein loading using immunoblotting with anti-Sp1 antibody. The maleimide signal was finally quantified and normalized against protein load using NIH ImageJ (National Institutes of Health, Bethesda, MD, USA).
Mass spectrometric analysis of Sp1
Purified full-length human Sp1 protein (OriGene Technologies, Rockville, MD, USA) treated with 100 µM NaHS or vehicle (H2O) for 30 min at 37°C was protected with iodoacetamide in the absence of reductant and digested with trypsin. The ensuing peptides were analyzed by LC-MS/MS on a Synapt G2S (Waters, Milford, MA, USA) in data independent mode. The peptides and their modification sites (cysteine carboxymethylation, methionine oxidation and cysteine sulfhydration) were identified using Biopharmalynx 1.3 (Waters).
Statistical analysis
All experiments were performed independently 3 times in triplicate, and statistical analysis was performed using 2-sided Student’s t test or 1-way ANOVA as applicable. P ≤ 0.05 was deemed significant.
RESULTS
CBS is expressed in ECs
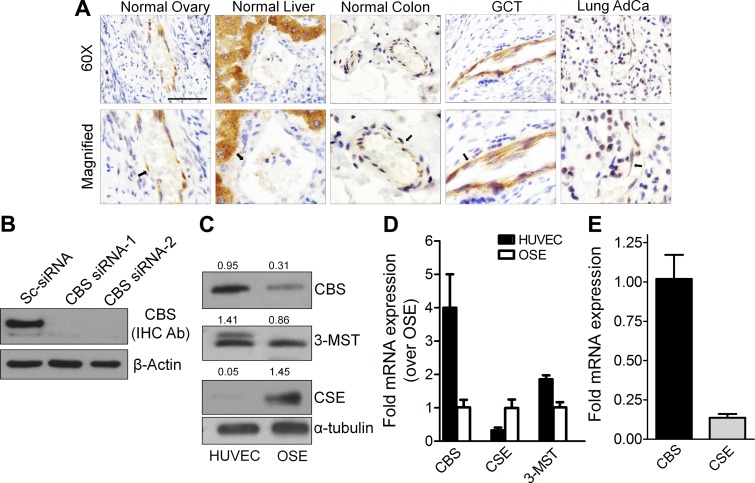
To investigate the expression of CBS in ECs, we performed immunohistochemistry in a number of normal and cancer tissues (Fig. 1A) using an antibody that was validated to detect CBS in HUVECs (Fig. 1B). The blood vessels, easily identified by frequent presence of red blood cells, were lined by CBS stained ECs. Moderate to strong staining of CBS in ECs of the normal ovary, granulosa cell tumors, normal colon, and lung adenocarcinoma were observed. In lung adenocarcinoma, besides ECs, staining for CBS was also observed in the epithelial cells. Although CBS remained undetected in ECs of the normal liver, a strong expression was observed in the hepatocytes (Fig. 1A). Tissue-specific expression of CBS in the brain and the nervous system and for CSE in the vascular smooth muscle and the pancreas has been previously reported (40). These results indicate that ECs from several normal or malignant tissues express CBS.
Figure 1.
Expression of CBS in tissues and primary endothelial and epithelial cells. A) Immunohistochemistry staining for CBS expression in normal ovary, normal liver, normal colon, granulosa cell tumor (GCT) and lung adenocarcinoma tissues. Upper panel: Images are acquired with ×60 objective. Lower panel: Magnified image of the respective upper panels to visualize ECs in the vessels. Arrows indicate the ECs. Scale bar, 100 µm. B) Validation of CBS antibody to identify human CBS used in immunohistochemistry through immunoblotting of scrambled control siRNA-treated or CBS siRNA-1/CBS siRNA-2-treated HUVECs. C) CBS, CSE, and 3-MST protein expression in HUVECs and OSEs determined by immunoblotting. Ratios represent CBS/CSE/3-MST signal to α-tubulin signal. Quantification of the images were performed in NIH ImageJ. D) Relative mRNA expression levels of CBS, CSE and 3-MST in HUVECs (passage 5) and OSEs as determined by qRT-PCR. OSE mRNA levels were set to 1. E) Relative mRNA abundance of CBS and CSE in HUVECs determined by qRT-PCR at passage 5.
To appreciate the role of CBS in endothelial function we first determined the relative expression of the 3 H2S-producing enzymes, CBS, CSE, and 3-MST in HUVECs and in an epithelial, human OSEs using immunoblotting and qRT-PCR. As determined by immunoblotting, compared with OSE, expression of CBS was higher in HUVECs (Fig. 1C). Expression of 3-MST was similar in HUVECs and OSEs and expression of CSE was highest in OSEs and least in HUVECs (Fig. 1C). These results were further supported by qRT-PCR, which largely showed a similar trend between protein and mRNA expression in HUVECs and OSEs (Fig. 1D). Within HUVECs, the expression of CBS mRNA was about 9-fold higher than CSE (Fig. 1E), indicating that CBS was the predominant H2S-producing enzyme in HUVECs and was thus utilized for further studies.
Loss of CBS function deregulates metabolite homeostasis
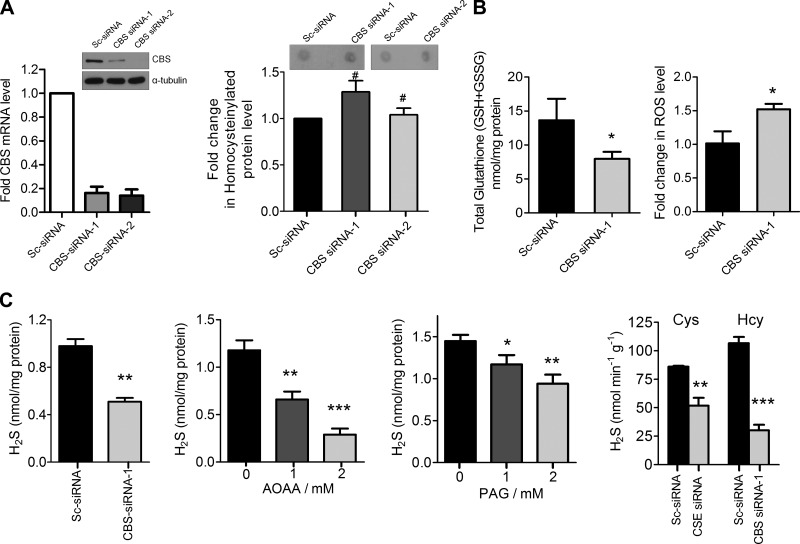
CBS being a metabolic enzyme of the transsulfuration pathway, we first estimated the levels of key relevant metabolites in CBS-silenced HUVECs. Hcy accumulation and protein homocysteinylation have been suggested as possible mechanisms of Hcy toxicity in humans, causing vascular dysfunction (9, 41, 42). Therefore, we estimated the levels of Hcy incorporation into the proteins from the lysates of scrambled-control or CBS siRNA-transfected HUVECs using dot-blot analysis (34–36). An insignificant increase in homocysteinylated proteins was observed upon transfection with 2 different siRNA’s targeting CBS (Fig. 2A), suggesting that transient knockdown of CBS does not lead to significant Hcy accumulation. Significant knockdown of CBS was confirmed by both immunoblotting and qRT-PCR (Fig. 2A). The enzymatic function of CBS leads to synthesis of cystathionine, a precursor of cysteine, required for the biosynthesis of GSH (1). Therefore, we measured total cellular GSH levels from scrambled-control and CBS siRNA-transfected HUVECs. Silencing CBS depleted the intracellular pool of GSH by 40%, which was accompanied by a 1.4-fold increase in ROS levels as estimated by 2′,7′-dichlorofluorescein diacetate-based flow cytometry (Fig. 2B). CBS can primarily generate H2S from Hcy or cysteine and thus concentrations of H2S were determined using a modified methylene blue assay (43). In scrambled-control siRNA-transfected cells, basal H2S concentration was ∼1.1 nmol/mg protein that decreased to ∼0.51 nmol/mg protein in CBS siRNA-transfected cells (Fig. 2C) (44). Treatment with AOAA, a chemical inhibitor of CBS (45), at 2 different concentrations (1 and 2 mM) also resulted in a dose-dependent decrease in total H2S levels to ∼0.6 and ∼0.32 nmol/mg protein, respectively (Fig. 2C). However, the decrease was significantly less pronounced in treatment with PAG, a CSE inhibitor with decreased endogenous H2S levels, compared with AOAA (Fig. 2C). To further confirm this observation, we determined CSE and CBS activity in scrambled control siRNA-, CBS siRNA-, or CSE siRNA-treated HUVECs. Compared with the control, silencing CSE resulted in a ∼40% reduction in H2S generation using cysteine as a substrate; however, H2S generation from Hcy was reduced by 72% when CBS was silenced (Fig. 2C). Therefore, our results indicate that CBS is the primary H2S-generating enzyme in ECs. Together these results indicate an alteration of the redox balance and H2S levels in ECs with transient lack of CBS enzyme function.
Figure 2.
Silencing CBS alters metabolite levels. A) Left panel: ImageJ quantification of the relative levels of Hcy by dot blot analysis for homocysteinylated protein levels in lysates of scrambled siRNA, CBS siRNA-1 and CBS siRNA-2-treated HUVECs after 48 h transfection. A total of 20 µg of lysate was used for dot blot analysis to detect homocysteinylated proteins and the intensities were quantified using NIH ImageJ. Efficiency of CBS knockdown was estimated by immunoblotting (middle panel) and (right panel) qRT-PCR analysis 48 h posttransfection. Data represent means ± sd, n = 3. #P > 0.05 (not significant). B) Change in total glutathione [GSH + glutathione disulfide (GSSG)] level and ROS (dichloro-dihydro-fluorescein diacetate assay-flow cytometry) in CBS siRNA-treated HUVECs compared with scrambled control siRNA-treated cells after 48 h transfection. C) First panel, from left: H2S levels in scrambled and CBS siRNA-treated HUVECs determined by methylene blue assay 48 h posttransfection; second panel, with various doses of AOAA determined by methylene blue assay 24 h posttreatment; third panel, with various doses of PAG determined by methylene blue assay 24 h posttreatment; and fourth panel, CBS and CSE activity assay using Hcy (for CBS) and cysteine (for CSE) as substrates in CBS- and CSE-silenced HUVECs, respectively, determined by methylene blue assay 48 h posttransfection. Data represent means ± SD (n = 3), 1-way ANOVA. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Silencing CBS affects key endothelial phenotypes
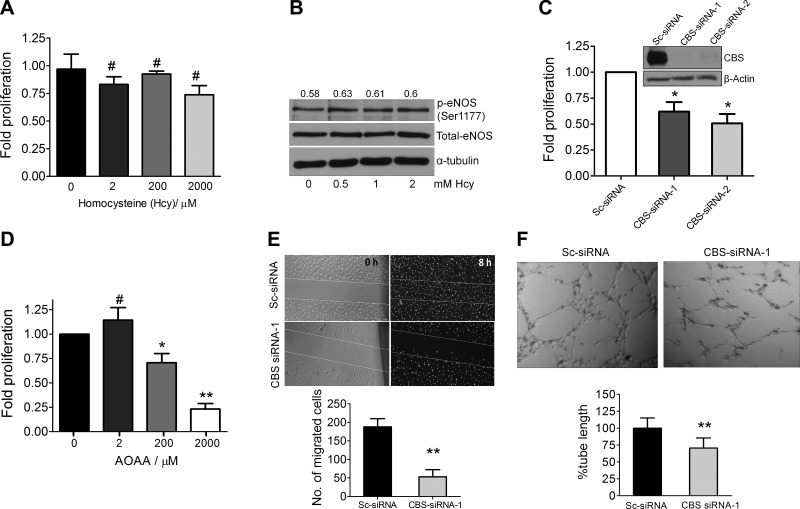
Given the altered metabolite levels in CBS-silenced ECs, we next investigated functional phenotypes. To determine the effect of Hcy on EC proliferation, we supplemented media with increasing concentrations of Hcy for 24 h. This treatment resulted in marginal reduction in HUVEC proliferation (Fig. 3A) that did not reach statistical significance. Surprisingly, Hcy even at supraphysiologic concentrations of up to 2 mM did not impact EC proliferation significantly. Additionally, treatment of HUVEC cells with various doses of L-cystathionine did not affect the proliferation. Because Hcy-mediated eNOS inhibition has been implicated in endothelial dysfunction (15), we determined eNOS activation in HUVECs after treatment with Hcy. Phospho-eNOS (Ser1177) levels remained unchanged and similar to baseline with increasing concentrations of Hcy (Fig. 3B). These results ruled out any direct effect of Hcy in causing endothelial dysfunction in terms of proliferation or eNOS inhibition. In contrast, RNAi-mediated silencing of CBS in HUVECs resulted in a 40–50% decrease in proliferation as determined by the [3H]-thymidine incorporation, 72 h posttransfection (Fig. 3C). Using AOAA, the small molecule inhibitor of CBS, a dose-dependent decrease in HUVEC proliferation was observed (Fig. 3D). Taken together, these results indicate that inhibition of CBS function directly affects proliferation of ECs.
Figure 3.
Silencing CBS affects proliferation, migration, and vessel formation. A) Effect of various doses of Hcy for 24 h on the rate of proliferation of HUVECs ascertained through [3H]thymidine incorporation assay. Data represent means ± sd (n = 3), 1-way ANOVA. #P > 0.05 (not significant). B) Immunoblot data demonstrating the effect of various doses of Hcy for 24 h on eNOS (Ser1177) phosphorylation in HUVECs grown in serum-supplemented EBM medium. C) Effect of silencing CBS using CBS siRNA-1 and CBS siRNA-2 on the relative rate of proliferation of HUVECs compared with scrambled siRNA-treated cells ascertained through [3H]thymidine incorporation assay after 72 h transfection. Data represent means ± sd (n = 3), 2-tailed Student’s t test. *P ≤ 0.05. D) Effect of various doses of AOAA for 24 h on the rate of proliferation of HUVECs ascertained through 3H-thymidine incorporation assay. Data represent means ± sd (n = 3), 1-way ANOVA. *P ≤ 0.05, **P ≤ 0.01, #P > 0.05. E) Wound healing assay with scrambled control siRNA or CBS siRNA-treated HUVECs after 48 h transfection in serum-supplemented EBM medium to determine the effect of CBS on migration and wound healing. Data represent means ± sd (n = 3), 2-tailed Student’s t test. **P ≤ 0.01. F) Tube formation assay of scrambled control siRNA- or CBS siRNA-treated HUVECs after 72 h transfection on 2 mg/ml growth-factor reduced Matrigel. The images were acquired 4 h after plating serum-starved HUVECs on matrigel in serum-free EBM medium. Data represent means ± sd (n = 3), 2-tailed Student’s t test. **P ≤ 0.01.
Next, using the wound healing assay migratory ability of HUVECs was determined in the scrambled control or CBS siRNA-treated cells. An 8 h stimulation with complete serum-supplemented medium resulted in near-complete wound healing of the scrambled-control siRNA-treated cells (200 cells/field) in contrast to the CBS siRNA-treated HUVECs (40 cells/field) (Fig. 3E). In addition, compared with the scrambled-control siRNA-treated HUVECs, endothelial tube formation on Matrigel also demonstrated a 30% decrease in tube length in CBS-silenced cells (Fig. 3F). These data indicate that loss of CBS function results in a significant loss of key endothelial phenotypes.
CBS regulates VEGF-induced response in ECs
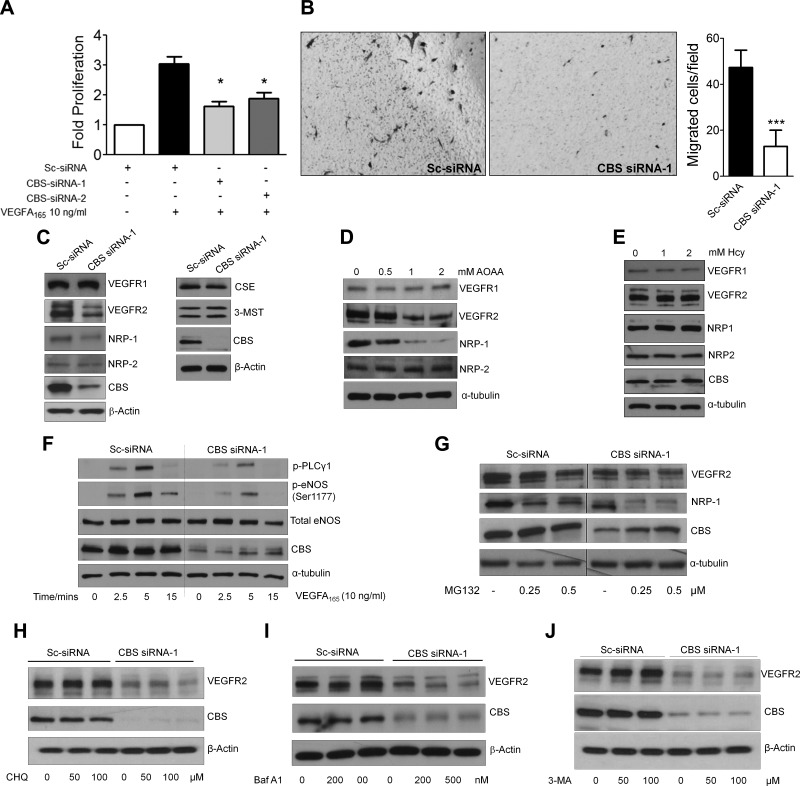
To determine how CBS regulates EC function, we focused on the VEGF/VEGFR-2 signaling axis, arguably the predominant pathway responsible for EC proliferation, migration, and survival (28). NRP-1, by enhancing VEGF binding to VEGFR-2, further potentiates signaling in ECs (46). Therefore, we investigated whether VEGF-induced phenotypes were affected in CBS-silenced HUVECs. To this end, we treated serum-starved scrambled-control and CBS siRNA-transfected HUVECs with 10 ng/ml VEGF for 24 h and determined proliferation using the [3H]-thymidine incorporation assay. Upon VEGF stimulation a ∼3-fold increase in proliferation was observed in the control vs. ∼1.8-fold in the CBS-silenced ECs (Fig. 4A). Next VEGF-induced migration was determined using a Boyden chamber-based chemotaxis assay. A significant inhibition in VEGF-induced migration was observed in CBS-silenced cells (15 cells/field) compared with the scrambled-control-treated HUVECs (45 cells/field) (Fig. 4B). These results demonstrated that VEGF-induced responses were significantly inhibited in CBS-silenced ECs, and prompted us to probe the status of VEGF receptors in these cells. Using immunoblotting, a significant decrease in VEGFR-2 and NRP-1 total protein but not VEGFR-1 or NRP-2 was observed in CBS-silenced ECs (Fig. 4C). Also the levels of the 2 other major H2S-synthesizing enzymes, CSE and 3-MST, remained unaltered upon silencing CBS in HUVECs (Fig. 4C). Similar results were obtained with AOAA (Fig. 4D). In addition, treatment with Hcy (1 and 2 mM) for 24 h did not alter the levels of any endothelial specific proteins in HUVECs (Fig. 4E). In ECs, phosphorylation of phospholipase C γ1 and eNOS is downstream of VEGFR-2 activation. Therefore we evaluated and observed a significant decrease in p-phospholipase C γ1 and p-eNOS in CBS-silenced HUVECs (Fig. 4F), thus confirming inhibition of the VEGFR-2/NRP-1 signaling axis in ECs. Because GSH and H2S can posttranslationally modify proteins that could lead to their potential degradation, we used inhibitors of different degradation and recycling compartments in an effort to rescue VEGFR-2 and NRP-1 from CBS-silenced cells. Surprisingly, lysosomal inhibitors bafilomycin A1, chloroquine, autophagy inhibitor 3-methyl adenine or the proteasomal inhibitor MG132, all failed to rescue VEGFR-2 and NRP-1 levels in CBS-silenced HUVECs (Fig. 4G–J). Together these data indicated that silencing CBS did not affect VEGFR-2 or NRP-1 posttranslationally and prompted us to investigate their transcriptional regulation.
Figure 4.
Silencing CBS compromises response to VEGFA. A) Effect of VEGF A165 (10 ng/ml) treatment for 24 h on the rate of proliferation of scrambled or CBS siRNA-treated HUVECs ascertained through [3H]thymidine incorporation assay. Data represent means ± sd (n = 3), 2-tailed Student’s t test. *P ≤ 0.05. B) VEGFA165-induced chemotaxis assay to ascertain the effect of silencing CBS on the migratory aptitude of HUVECs. Quantification of number of migrated cells per field of view (n = 3). *P ≤ 0.05. C) Immunoblot analysis to study levels of key endothelial proteins and H2S-synthesizing enzymes in scrambled siRNA- and CBS siRNA-1-treated HUVECs after 48 h transfection. D) Immunoblot analysis to study VEGFR1, NRP-2, VEGFR-2, and NRP-1 levels in HUVECs after 24 h treatment with various doses of AOAA. E) Effect of 1 and 2 mM Hcy (Hcy) treatment for 24 h on key endothelial protein levels in HUVECs ascertained through immunoblotting. F) Time-dependent effect of VEGF A165 (10 ng/ml) on eNOS (Ser1177) phosphorylation in scrambled or CBS siRNA-treated HUVECs after 48 h transfection determined by immunoblotting. G) Effect of various doses of proteasomal inhibitor MG132 for 24 h on scrambled and CBS siRNA-treated HUVECs. Treatment was initiated 24 h posttransfection and continued for 24 h before immunoblot analysis. H) Effect of treatment with 2 different doses of chloroquine (lysosomal inhibitor) for 6 h on scrambled and CBS siRNA-transfected HUVECs, 48 h posttransfection. I) Effect of treatment with 2 different doses of Bafilomycin A1 (lysosomal inhibitor) on scrambled control and CBS siRNA-transfected HUVECs 48 h posttransfection. Treatment was initiated 42 h posttransfection for 6 h and then subjected to immunoblot analysis. J) Effect of treatment with 2 different doses 3-methyl adenine (autophagy inhibitor) on scrambled control and CBS siRNA-transfected HUVECs, 48 h posttransfection. Treatment was initiated 42 h posttransfection for 6 h and then subjected to immunoblot analysis.
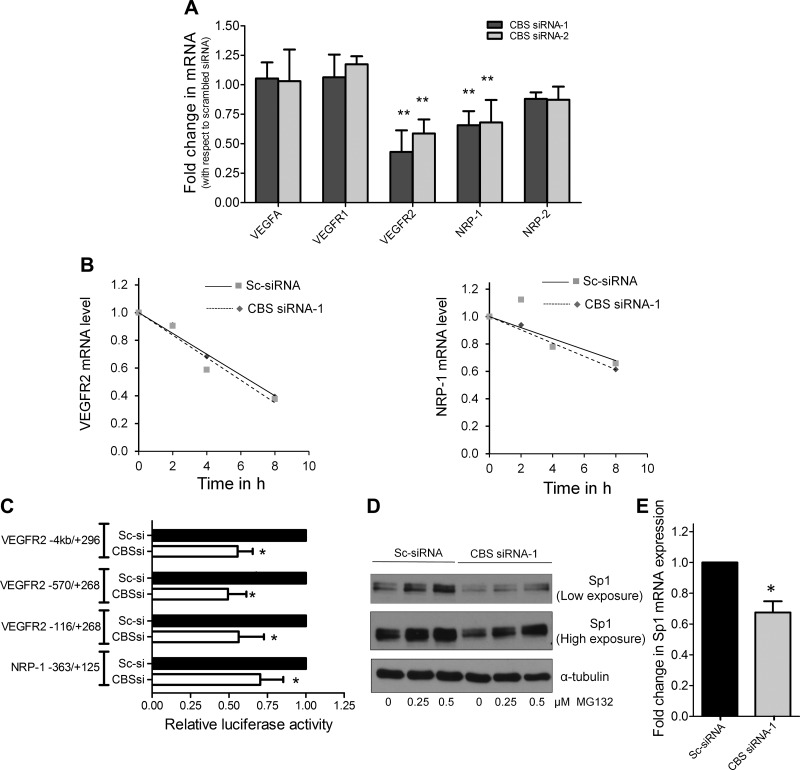
CBS regulates transcription of VEGFR-2 and NRP-1
Scrambled-control and CBS siRNA-transfected HUVECs were analyzed for the expression of VEGFR-1, VEGFR-2, NRP-1, NRP-2, and VEGF by qRT-PCR. Although mRNA levels of VEGF, VEGFR-1, or NRP-2 did not change, VEGFR-2 and NRP-1 were significantly down-regulated in CBS-silenced cells (Fig. 5A). The mRNA stability assay demonstrated that the decrease in VEGFR-2 and NRP-1 mRNA was not due to a lack of stability in CBS-silenced ECs (Fig. 5B). Therefore, we next utilized the VEGFR-2 promoter luciferase assay to sequentially determine a minimal region of the VEGFR-2 promoter that was regulated by CBS. In CBS-silenced HUVECs, a 40–60% inhibition of firefly luciferase activity was observed with all the VEGFR-2 promoters tested (full-length −4/+296, −570/+268, and −116/+268) (Fig. 5C). Similarly, the minimal NRP-1 promoter (−363/+125) also demonstrated a 30% reduction in luciferase activity upon silencing CBS (Fig. 5C). Interestingly, the minimal VEGFR-2 promoter contains 4 and the minimal NRP-1 promoter contains 2 Sp1 binding sites. Therefore, we determined Sp1 protein levels in CBS-silenced ECs. We observed that while silencing CBS decreased, treatment with MG132 significantly restored total Sp1 levels in HUVECs (Fig. 5D). This data suggested that silencing of CBS caused Sp1 to undergo proteasomal degradation, either due to free radical damage or due to a lack in protein stability. It is interesting to note that a ∼30% decrease in Sp1 at the transcript level was also observed in the CBS-silenced ECs (Fig. 5E) likely due to autoregulation as previously reported (47). Together these data suggested that the transcriptional down-regulation of VEGFR-2 and NRP-1 was likely caused by decreased levels of Sp1 in CBS-silenced ECs.
Figure 5.
VEGFR-2 and NRP-1 are transcriptionally down-regulated in CBS-silenced cells. A) Effect of silencing CBS in HUVECs on the relative mRNA levels of key endothelial genes compared with scrambled siRNA-treated HUVECs determined by qPCR analysis 48 h posttransfection. Actin was used as an internal loading control. Data represent means ± sd (n = 3), 2-tailed Student’s t test. *P ≤ 0.05, **P ≤ 0.01. B) Actinomycin d-based mRNA stability studies to study the effect of silencing CBS on the stability of VEGFR-2 and NRP-1 transcripts by qRT-PCR performed 48 h posttransfection with scrambled control or CBS siRNA. C) Promoter luciferase assay with full-length or minimal VEGFR-2 promoters and minimal NRP-1 promoter to study their transcriptional regulation upon silencing CBS using RNAi. Data represent means ± sd (n = 3), 2-tailed Student’s t test. *P ≤ 0.05, **P ≤ 0.01. D) Effect of RNAi-mediated silencing of CBS in HUVECs and the effect of 24 h MG132 treatment on Sp1 levels. MG132 was added 24 h posttransfection and treatment was carried out for 24 h, after which immunoblotting was performed. α-tubulin was used as the loading control. E) Effect of silencing CBS using CBS siRNA-1 in HUVECs on Sp1 transcript levels relative to scrambled control siRNA-treated HUVECs determined by qRT-PCR.
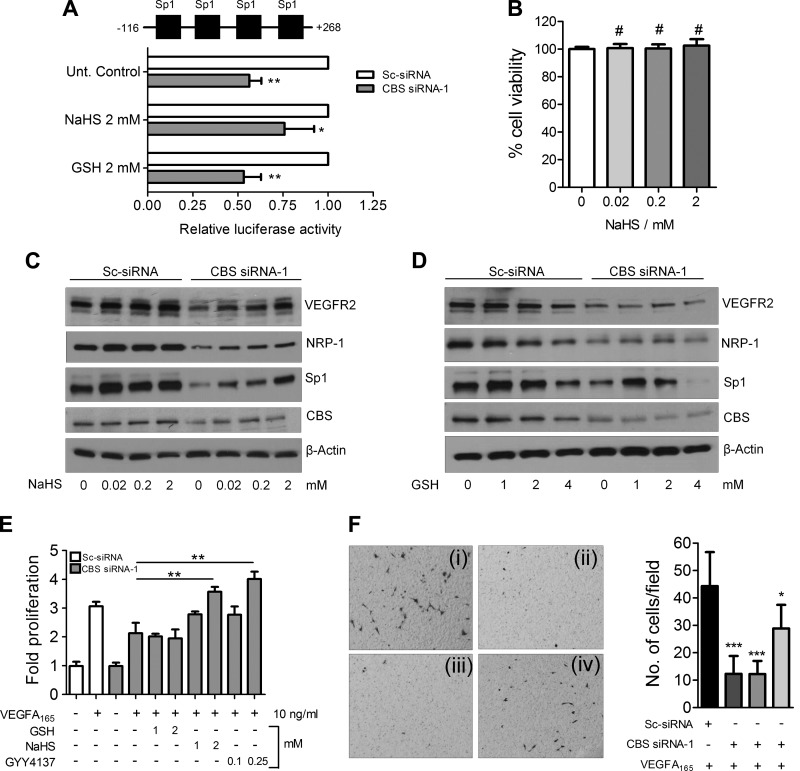
H2S rescues transcription and VEGF-induced responses in ECs
Because Sp1 is known to drive transcription of VEGFR-2 (48) and can potentially be altered by redox imbalance (49), we next determined VEGFR-2 promoter (−116/+268) driven luciferase activity in CBS-silenced ECs upon supplementation with GSH or the H2S donor, NaHS. Although GSH was unable, NaHS at a dose of 2 mM partially rescued VEGFR-2 promoter driven luciferase activity (∼30%) in CBS-silenced HUVECs (Fig. 6A). To rule out any potential toxic effects of NaHS, we treated HUVECs for 24 h with various doses of NaHS and ascertained cell viability by the MTT assay. No adverse effect of NaHS on HUVEC cell viability was noted even at a 2 mM concentration (Fig. 6B). We next immunoblotted for Sp1, VEGFR-2, and NRP-1 in CBS-silenced cells with or without GSH and NaHS supplementation. In CBS-silenced cells, VEGFR-2, NRP-1, and Sp1, all were significantly reduced compared with the scrambled-control siRNA-transfected HUVECs (Fig. 6C, D). Treatment with increasing concentrations of GSH (1–4 mM) had no effect on VEGFR-2 or NRP-1 levels, although some rescue of Sp1 could be observed, especially at the lower concentrations (Fig. 6D). The partially rescued Sp1 with GSH, however, failed to drive expression of VEGFR-2 or NRP-1 (Fig. 6D). In contrast, treatment with increasing concentrations of NaHS (0.02–2 mM) significantly rescued Sp1, VEGFR-2, and NRP-1 levels in CBS-silenced HUVECs (Fig. 6C). To further confirm these results in functional assays, we determined VEGF-induced proliferation in CBS-silenced HUVECs supplemented with GSH, NaHS, or GYY4137, another donor of H2S. VEGF-induced proliferation was completely restored by NaHS and GYY4137 but not by GSH in CBS-silenced HUVECs (Fig. 6E). Similarly compared with the control, a significant increase in VEGF-induced migration was observed in NaHS but not with GSH-treated CBS-silenced HUVECs (Fig. 6F). Together these data confirmed that H2S produced by the action of CBS in ECs is important for the stabilization of Sp1 that then drives transcription of VEGFR-2 and NRP-1.
Figure 6.
Supplementation with H2S rescues transcription of specific genes and endothelial phenotype. A) Effect of GSH and NaHS supplementation on the relative luciferase activity of −116/+268 VEGFR-2 promoter in scrambled or CBS siRNA-treated HUVECs after 48 h transfection with siRNA, −116/+268 VEGFR-2 promoter-firefly luciferase, and wild-type pRLTK-Renilla luciferase. Data represent means ± sd (n = 3), 2-tailed Student’s t test. *P ≤ 0.05, **P ≤ 0.01. B) Effect of increasing dose of NaHS supplementation for 24 h on the cell viability of HUVECs determined by MTT assay. Data represent means ± sd (n = 3), 1-way ANOVA; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, #P > 0.05 (not significant). C) Immunoblot analysis of VEGFR-2, NRP-1, and Sp1 protein levels in scrambled and CBS siRNA-treated HUVECs after treatment with various doses of NaHS for 24 h. NaHS treatment was initiated 24 h posttransfection and continued for 24 h after which after immunoblotting was performed. D) Immunoblot analysis of VEGFR-2, NRP-1, and Sp1 protein levels in scrambled and CBS siRNA-treated HUVECs after treatment with various doses of reduced GSH. NaHS treatment was initiated 24 h posttransfection and continued for 24 h after which immunoblotting was performed. E) Rescue of VEGF A165-induced HUVEC proliferation in CBS-silenced HUVECs with various doses of GSH, NaHS or GYY4137. Statistics analysis was performed between VEGF165-treated CBS-silenced HUVECs with metabolite (GSH/NaHS/GYY4137) supplemented VEGF165-treated CBS-silenced HUVECs. Data represent means ± sd (n = 3), 1-way ANOVA; *P ≤ 0.05, **P ≤ 0.01. F) Rescue of VEGF A165-induced HUVEC chemotaxis in CBS-silenced HUVECs with or without treatment with 2 mM doses of GSH or NaHS. GSH or NaHS treatment was initiated 24 h posttransfection and treatment carried out for another 24 h before performing the chemotaxis assay. i) Scrambled control siRNA + VEGF A165 (10 ng/ml). ii) CBS siRNA + VEGF A165 (10 ng/ml). iii) CBS siRNA + GSH (2 mM) + VEGF A165 (10 ng/ml). iv) CBS siRNA + VEGF A165 (10 ng/ml). Data represent means ± sd (n = 3), 1-way ANOVA. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, #P > 0.05.
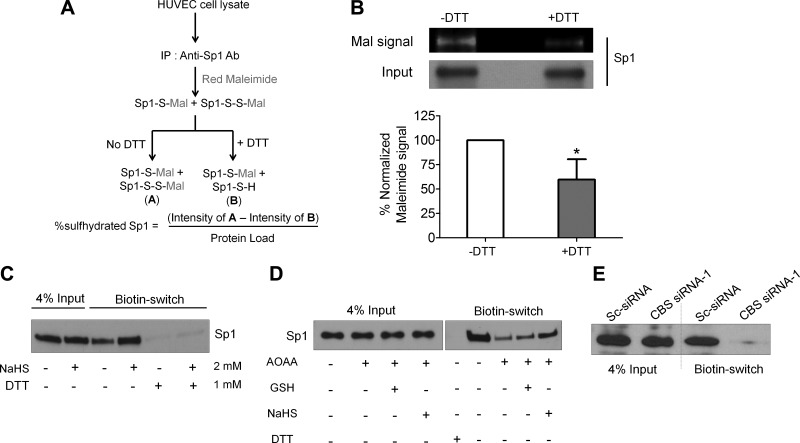
Sulfhydration stabilizes and enhances binding of SP1 to the VEGFR-2 promoter
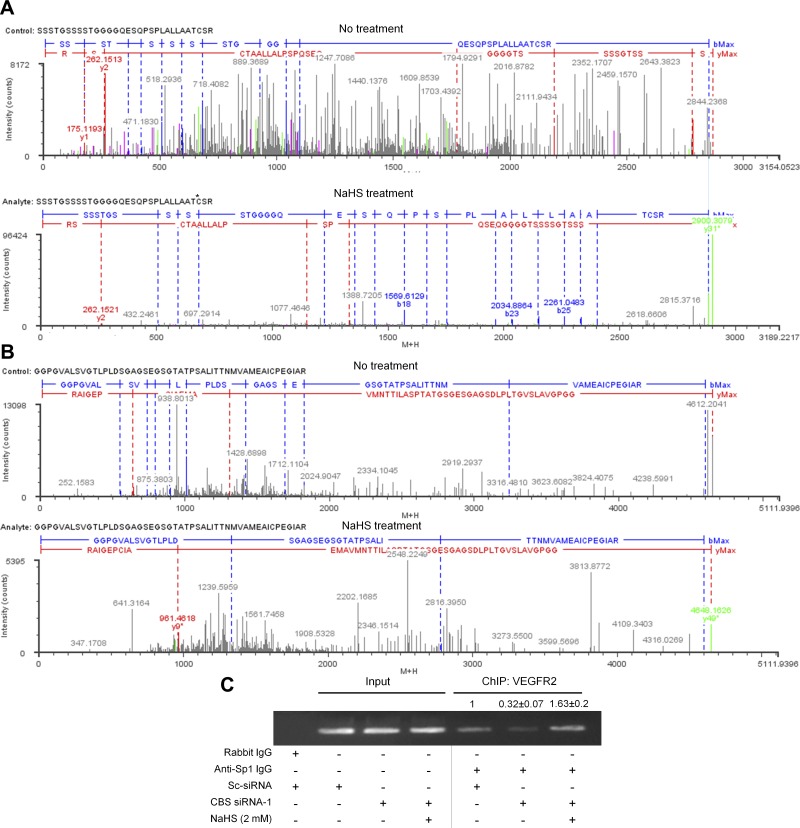
Given that H2S can modify proteins through S-sulfhydration (24), we performed the maleimide assay in which the -SH and -SSH groups but not S-S in a protein react with fluorescent maleimide (27) (Fig. 7A). Upon treatment of the maleimide-protein conjugate with DTT, the disulfide bonds in -SSH are cleaved leaving the free sulfhydryl groups modified with maleimide. Upon addition of DTT, we noted a 40% loss in the immunoprecipitated Sp1-maleimide signal suggesting that this was the proportion of sulfhydrated endogenous Sp1 in HUVECs (Fig. 7B). Using the modified biotin-switch assay (23), we then determined if Sp1 could be sulfhydrated by NaHS. This assay is based on the principle that free thiol groups (-SH) in a protein can be blocked using MMTS yielding -SSMe, without affecting -SSH groups. These free -SSH groups are then modified with N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio)propionamide (reversible with DTT), and upon immunoprecipitation with streptavidin, pull down the sulfhydrated proteins. We observed that endogenous Sp1 sulfhydration increased by 2-fold upon NaHS treatment and DTT almost completely reduced the biotin-Sp1 complex resulting in loss of the immunoprecipitated protein (Fig. 7C). We next demonstrated that inhibiting CBS function by AOAA, dramatically decreased (4.5-fold) sulfhydrated Sp1 levels that could be significantly rescued by NaHS but not GSH (Fig. 7D). Such a dramatic decrease in endogenous sulfhydrated Sp1 was also observed in siRNA-mediated CBS silenced cells compared with the control (Fig. 7E). Finally, to identify the putative sulfhydration sites on Sp1, we analyzed untreated or 100 µM NaHS-treated purified full-length human Sp1 protein by mass spectrometry (Fig. 7F, and Fig. 8A, B). Mass spectrometry analysis revealed that of total 11 cysteines on Sp1, 2 of them are potential site of sulfhydration, Cys68 (within the repressor domain) and Cys755 (within domain D). Also, 56.3% of the detected peptides were sulfhydrated at Cys68 by NaHS, but they remained completely unmodified in the untreated Sp1 protein (Fig.7F, Fig. 8A, B). On the other hand, 17.2% of the detected peptides had Cys755 sulfhydrated in the untreated Sp1, which further increased to 32.8% upon NaHS treatment (Fig.7F, Fig. 8A, B), suggesting that H2S-producing enzymes, CBS, CSE, and 3-MST that are all present in the bacteria are capable of modifying the human Sp1 (45). To further substantiate whether the H2S-stabilized form of Sp1 could actually bind the VEGFR-2 promoter, we conducted ChIP assays using PCR primers encompassing the four Sp1 binding sites. Compared with the control, silencing CBS resulted in significant loss of Sp1 binding to the VEGFR-2 promoter, which reverted beyond control in the CBS-silenced, NaHS-supplemented ECs (Fig. 8C) (densitometry; control: 1 vs. CBS siRNA: 0.32 vs. CBS si + NaHS: 1.63). Together these results substantiate that endogenous Sp1 is sulfhydrated by H2S produced by the enzymatic action of CBS. Sulfhydrated Sp1 is stable and functionally active in that it can bind promoter regions and drive transcription of responsive genes.
Figure 7.
Sulfhydration of Sp1 augments DNA binding to the VEGFR-2 promoter. A) Schematic representation for detection of sulfhydration of endogenous Sp1 by maleimide assay. B) Maleimide assay to determine the extent of endogenous Sp1 sulhydration in HUVECs. Decrease in red maleimide signal upon DTT treatment establishes sulfhydration of endogenous Sp1. Data represent means ± sd (n = 3), 2-tailed Student’s t test. *P ≤ 0.05. C) Modified biotin switch assay to ascertain sulfhydration of Sp1 in untreated and 2 mM NaHS-treated HUVECs for 6 h. D) Modified biotin-switch assay to determine effect of silencing CBS function with 2 mM AOAA (24 h) on sulfhydration of Sp1and the rescue of Sp1 sulfhydration status with reduced GSH (2 mM) and NaHS (2 mM) treated for 21 h. E) Modified biotin switch assay to ascertain sulfhydration of Sp1 in scrambled control siRNA or CBS siRNA-1-treated HUVECs after 48 h transfection. F) LC-MS/MS analysis of purified full-length Sp1 protein with or without treatment of 100 µM NaHS for 30 min reveals sulfhydration of Cys68 and Cys755. The modified cysteine are labeled red and oxidized methionine are labeled blue.
Figure 8.
A, B) Charge-reduced, isotope-deconvoluted MSE spectra (fragment ion matched profiles) of untreated and 100 µM NaHS-treated full-length Sp1 protein. *The site of sulfhydration. C) ChIP assay to determine relative Sp1 binding to the VEGFR-2 promoter in scrambled or CBS siRNA- or CBS siRNA + NaHS (2 mM)-treated HUVECs.
DISCUSSION
Loss of CBS function is associated with a wide variety of pathologic conditions in humans, including cardiovascular disease, ischemic heart disease, atherothrombosis, coronary artery disease, and peripheral vascular complications (9). Mechanistic studies to decipher impaired endothelial function, however, have focused on supraphysiologic concentrations of Hcy in vitro (15, 17, 18, 50, 51). Clinically, hyperhomocysteinemia is classified into 3 groups; moderate (0.015–0.03 mM), intermediate (0.03–0.1 mM), and severe (>0.1 mM). In addition, of the total plasma Hcy concentration; approximately 75 to 85% is protein-bound and only 15 to 25% is in the acid-soluble free form (52). Prior studies that have reported an unfolded protein response and programmed cell death in HUVECs, typically used 3–5 mM Hcy, a concentration several-fold higher than the total Hcy concentration expected in the severe HHcy group (51). Another report demonstrating activation of stress induced activating transcription factor 3 and JNK in HUVECs again utilized 3 mM Hcy with no functional phenotype shown (18). Yet other studies have utilized adenine, erythro-9-(2-hydroxy-3-nonyl) adenine, and Hcy together with the supposition that formation of S-adenosyl Hcy might be responsible for EC dysfunction (53, 54). More recently, Chiku and coworkers have suggested that CSE in hyperhomocysteinemia patients generates excessive H2S through α,γ-elimination and γ-elimination of Hcy, which could be responsible for associated cardiovascular pathology (55). Although the observations reported are suggestive of a causal role for Hcy in endothelial dysfunction, clinical trials using vitamin supplementation though lowered Hcy levels did not alter disease outcome (13, 14). We find that Hcy even at relatively higher concentrations of 0.002–2 mM do not significantly (P > 0.05) affect proliferation of ECs or phospho-eNOS levels. Transient knockdown of CBS using siRNA did not significantly alter total homocysteinylated proteins likely due to remethylation of Hcy to methionine, a competitive pathway that exists in normal physiology. However, a significant reduction in levels of total GSH and H2S upon inhibition of CBS, with concomitant increase in cellular ROS levels, were observed, indicating that their production are highly dependent on the transsulfuration pathway mediated by the enzymatic action CBS in ECs. In this context, recent reports are suggestive of a proangiogenic role of H2S (44). Typically, treatment with the H2S donor, NaHS, has been shown to significantly increase collateral vessel growth, capillary density, and regional tissue blood flow in a rat model of hind limb ischemia (56). Also using both in vitro and in vivo approaches, Papapetropoulos and coworkers established that endogenous and exogenous H2S stimulates EC-related angiogenic properties through a KATP channel/MAPK pathway (22).
Here we showed that silencing CBS inhibited VEGF-induced proliferation and migration in ECs, though Hcy levels were not significantly affected. Extending our observations to the molecular level, we established that the reduced response to VEGF was primarily due to loss of VEGFR-2 and NRP-1 at the transcriptional level, which remained unaltered with Hcy. Furthermore, reduced activation of VEGF-induced eNOS in CBS-deficient cells was a function of impaired VEGFR-2/NRP-1 signaling, though an additional role for H2S cannot be ruled out because promotion of eNOS phosphorylation by H2S-mediated sulfhydration has been demonstrated recently (57). Mechanistically, the transcriptional loss of VEGFR-2 and NRP-1 in CBS-silenced ECs is caused by decreased binding of Sp1 to the respective promoters. The decrease in Sp1 protein levels could be due to the altered redox state or lack of H2S in CBS-silenced ECs. Although the effects of decreased GSH on the VEGF/VEGFR-2 signaling axis have not been studied, enhanced ROS signaling in ECs has been reported to either up-regulate expression of VEGF/VEGFR-2 or promote phosphorylation of VEGFR-2 in a ligand-independent manner (58, 59), neither of which was observed in CBS-deficient ECs. Interestingly, though low concentrations of GSH restored Sp1 in CBS-silenced ECs, VEGFR-2 or NRP-1 levels remained unaffected, whereas NaHS supplementation restored Sp1 along with VEGFR-2 and NRP-1, indicating that H2S-mediated sulfhydration stabilizes and prevents proteasome-mediated degradation of Sp1. In addition, decreased Sp1 transcription in CBS-silenced ECs corroborates previous studies suggesting autoregulation (47). Of particular interest is Cys755 located within domain D that plays an important role in synergistic activation of transcription by Sp1 on promoters with multiple GC boxes (60). Cys68 is located within the repressor domain (aa 1–82) of Sp1 that is regulated by sumoylation resulting in cleavage of the N-terminal repressor domain thereby activating transcription (61). Besides regulating the transcriptional activity of Sp1, our results also suggest an essential role of these 2 cysteine residues in governing the stability of the Sp1 protein as a whole, which remains to be further elucidated through site-directed mutational analysis. Finally, the sulfhydrated, stabilized Sp1 is functionally competent in that it can rescue VEGFR-2 and NRP-1 levels in CBS-silenced ECs concomitant with VEGF-induced proliferation and migration phenotypes.
In conclusion, our work emphasizes the importance of CBS-mediated protein S-sulfhydration in maintaining vascular health and function and purports possible H2S-donor based therapies for treatment of endothelial dysfunction in CBS deficiency.
Acknowledgments
This work was supported by U.S. National Institutes of Health, National Heart, Lung, and Blood Institute Grant RO1HL120585. The authors thank Dr. Virginie Sjoelund (Mass Spectrometry Facility, University of Oklahoma Health Sciences Center Core Laboratories) for Sp1 sulfhydration analysis.
Glossary
- 3-MST
3-mercaptopyruvate sulfotransferase
- AOAA
amino-oxyacetic acid
- BSA
bovine serum albumin
- CBS
cystathionine β-synthase
- ChIP
chromatin immunoprecipitation
- CSE
cystathionine γ lyase
- EBM
endothelial basal medium
- EC
endothelial cells
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSH
glutathione
- Hcy
homocysteine
- HEN
Hepes-EDTA-NaOH
- HHcy
hyperhomocysteinemia
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MMTS
methyl methanethiosulfonate
- NaHS
sodium hydrosulfide
- NRP
neuropilin
- OSE
ovarian surface epithelial cell
- PAG
N-propargylglycine
- qRT-PCR
quantitative RT-PCR
- ROS
reactive oxygen species
- siRNA
small interfering RNA
- Sp1
specificity protein 1
- VEGFR
VEGF receptor
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Banerjee R., Zou C. G. (2005) Redox regulation and reaction mechanism of human cystathionine-beta-synthase: a PLP-dependent hemesensor protein. Arch. Biochem. Biophys. 433, 144–156 [DOI] [PubMed] [Google Scholar]
- 2.Jhee K. H., Kruger W. D. (2005) The role of cystathionine beta-synthase in homocysteine metabolism. Antioxid. Redox Signal. 7, 813–822 [DOI] [PubMed] [Google Scholar]
- 3.Janosík M., Kery V., Gaustadnes M., Maclean K. N., Kraus J. P. (2001) Regulation of human cystathionine beta-synthase by S-adenosyl-L-methionine: evidence for two catalytically active conformations involving an autoinhibitory domain in the C-terminal region. Biochemistry 40, 10625–10633 [DOI] [PubMed] [Google Scholar]
- 4.Wallace J. L., Wang R. (2015) Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 14, 329–345 [DOI] [PubMed] [Google Scholar]
- 5.Vitvitsky V., Mosharov E., Tritt M., Ataullakhanov F., Banerjee R. (2003) Redox regulation of homocysteine-dependent glutathione synthesis. Redox Rep. 8, 57–63 [DOI] [PubMed] [Google Scholar]
- 6.Miles E. W., Kraus J. P. (2004) Cystathionine beta-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J. Biol. Chem. 279, 29871–29874 [DOI] [PubMed] [Google Scholar]
- 7.Kraus J. P., Janosík M., Kozich V., Mandell R., Shih V., Sperandeo M. P., Sebastio G., de Franchis R., Andria G., Kluijtmans L. A., Blom H., Boers G. H., Gordon R. B., Kamoun P., Tsai M. Y., Kruger W. D., Koch H. G., Ohura T., Gaustadnes M. (1999) Cystathionine beta-synthase mutations in homocystinuria. Hum. Mutat. 13, 362–375 [DOI] [PubMed] [Google Scholar]
- 8.McCully K. S. (1969) Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am. J. Pathol. 56, 111–128 [PMC free article] [PubMed] [Google Scholar]
- 9.Beard R. S. Jr., Bearden S. E. (2011) Vascular complications of cystathionine β-synthase deficiency: future directions for homocysteine-to-hydrogen sulfide research. Am. J. Physiol. Heart Circ. Physiol. 300, H13–H26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yap S., Naughten E. R., Wilcken B., Wilcken D. E., Boers G. H. (2000) Vascular complications of severe hyperhomocysteinemia in patients with homocystinuria due to cystathionine beta-synthase deficiency: effects of homocysteine-lowering therapy. Semin. Thromb. Hemost. 26, 335–340 [DOI] [PubMed] [Google Scholar]
- 11.Brattström L., Wilcken D. E. (2000) Homocysteine and cardiovascular disease: cause or effect? Am. J. Clin. Nutr. 72, 315–323 [DOI] [PubMed] [Google Scholar]
- 12.Weiss N., Heydrick S., Zhang Y. Y., Bierl C., Cap A., Loscalzo J. (2002) Cellular redox state and endothelial dysfunction in mildly hyperhomocysteinemic cystathionine beta-synthase-deficient mice. Arterioscler. Thromb. Vasc. Biol. 22, 34–41 [DOI] [PubMed] [Google Scholar]
- 13.Bønaa K. H., Njølstad I., Ueland P. M., Schirmer H., Tverdal A., Steigen T., Wang H., Nordrehaug J. E., Arnesen E., Rasmussen K., Investigators N. T.; NORVIT Trial Investigators (2006) Homocysteine lowering and cardiovascular events after acute myocardial infarction. N. Engl. J. Med. 354, 1578–1588 [DOI] [PubMed] [Google Scholar]
- 14.Ebbing M., Bleie Ø., Ueland P. M., Nordrehaug J. E., Nilsen D. W., Vollset S. E., Refsum H., Pedersen E. K., Nygård O. (2008) Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA 300, 795–804 [DOI] [PubMed] [Google Scholar]
- 15.Jiang X., Yang F., Tan H., Liao D., Bryan R. M. Jr., Randhawa J. K., Rumbaut R. E., Durante W., Schafer A. I., Yang X., Wang H. (2005) Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler. Thromb. Vasc. Biol. 25, 2515–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang S. S., Wong P. W., Malinow M. R. (1992) Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Annu. Rev. Nutr. 12, 279–298 [DOI] [PubMed] [Google Scholar]
- 17.Hossain G. S., van Thienen J. V., Werstuck G. H., Zhou J., Sood S. K., Dickhout J. G., de Koning A. B. L., Tang D., Wu D., Falk E., Poddar R., Jacobsen D. W., Zhang K., Kaufman R. J., Austin R. C. (2003) TDAG51 is induced by homocysteine, promotes detachment-mediated programmed cell death, and contributes to the cevelopment of atherosclerosis in hyperhomocysteinemia. J. Biol. Chem. 278, 30317–30327 [DOI] [PubMed] [Google Scholar]
- 18.Cai Y., Zhang C., Nawa T., Aso T., Tanaka M., Oshiro S., Ichijo H., Kitajima S. (2000) Homocysteine-responsive ATF3 gene expression in human vascular endothelial cells: activation of c-Jun NH(2)-terminal kinase and promoter response element. Blood 96, 2140–2148 [PubMed] [Google Scholar]
- 19.Werstuck G. H., Lentz S. R., Dayal S., Hossain G. S., Sood S. K., Shi Y. Y., Zhou J., Maeda N., Krisans S. K., Malinow M. R., Austin R. C. (2001) Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J. Clin. Invest. 107, 1263–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang R. (2011) Signaling pathways for the vascular effects of hydrogen sulfide. Curr. Opin. Nephrol. Hypertens. 20, 107–112 [DOI] [PubMed] [Google Scholar]
- 21.Hoefer I. E. (2007) Something is rotten in the state of angiogenesis -- H2S as gaseous stimulator of angiogenesis. Cardiovasc. Res. 76, 1–2 [DOI] [PubMed] [Google Scholar]
- 22.Papapetropoulos A., Pyriochou A., Altaany Z., Yang G., Marazioti A., Zhou Z., Jeschke M. G., Branski L. K., Herndon D. N., Wang R., Szabó C. (2009) Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 106, 21972–21977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mustafa A. K., Gadalla M. M., Sen N., Kim S., Mu W., Gazi S. K., Barrow R. K., Yang G., Wang R., Snyder S. H. (2009) H2S signals through protein S-sulfhydration. Sci. Signal. 2, ra72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul B. D., Snyder S. H. (2012) H₂S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 13, 499–507 [DOI] [PubMed] [Google Scholar]
- 25.Vandiver M. S., Paul B. D., Xu R., Karuppagounder S., Rao F., Snowman A. M., Ko H. S., Lee Y. I., Dawson V. L., Dawson T. M., Sen N., Snyder S. H. (2013) Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 4, 1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y., Yang R., Liu X., Zhou Y., Qu C., Kikuiri T., Wang S., Zandi E., Du J., Ambudkar I. S., Shi S. (2014) Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration. Cell Stem Cell 15, 66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sen N., Paul B. D., Gadalla M. M., Mustafa A. K., Sen T., Xu R., Kim S., Snyder S. H. (2012) Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell 45, 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsson A. K., Dimberg A., Kreuger J., Claesson-Welsh L. (2006) VEGF receptor signalling - in control of vascular function. Nat. Rev. Mol. Cell Biol. 7, 359–371 [DOI] [PubMed] [Google Scholar]
- 29.Shalaby F., Rossant J., Yamaguchi T. P., Gertsenstein M., Wu X. F., Breitman M. L., Schuh A. C. (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376, 62–66 [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki T., Kitsukawa T., Bekku Y., Matsuda Y., Sanbo M., Yagi T., Fujisawa H. (1999) A requirement for neuropilin-1 in embryonic vessel formation. Development 126, 4895–4902 [DOI] [PubMed] [Google Scholar]
- 31.Wu N., Siow Y. L., O K. (2010) Ischemia/reperfusion reduces transcription factor Sp1-mediated cystathionine beta-synthase expression in the kidney. J. Biol. Chem. 285, 18225–18233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharya R., Kwon J., Li X., Wang E., Patra S., Bida J. P., Bajzer Z., Claesson-Welsh L., Mukhopadhyay D. (2009) Distinct role of PLCbeta3 in VEGF-mediated directional migration and vascular sprouting. J. Cell Sci. 122, 1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhattacharyya S., Saha S., Giri K., Lanza I. R., Nair K. S., Jennings N. B., Rodriguez-Aguayo C., Lopez-Berestein G., Basal E., Weaver A. L., Visscher D. W., Cliby W., Sood A. K., Bhattacharya R., Mukherjee P. (2013) Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS One 8, e79167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qipshidze N., Metreveli N., Mishra P. K., Lominadze D., Tyagi S. C. (2012) Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. Int. J. Biol. Sci. 8, 430–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Campenhout A., Moran C. S., Parr A., Clancy P., Rush C., Jakubowski H., Golledge J. (2009) Role of homocysteine in aortic calcification and osteogenic cell differentiation. Atherosclerosis 202, 557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganapathy P. S., Moister B., Roon P., Mysona B. A., Duplantier J., Dun Y., Moister T. K., Farley M. J., Prasad P. D., Liu K., Smith S. B. (2009) Endogenous elevation of homocysteine induces retinal neuron death in the cystathionine-beta-synthase mutant mouse. Invest. Ophthalmol. Vis. Sci. 50, 4460–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chompoosor A., Saha K., Ghosh P. S., Macarthy D. J., Miranda O. R., Zhu Z. J., Arcaro K. F., Rotello V. M. (2010) The role of surface functionality on acute cytotoxicity, ROS generation and DNA damage by cationic gold nanoparticles. Small 6, 2246–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kondo S., Tanaka N., Kubota S., Mukudai Y., Yosimichi G., Sugahara T., Takigawa M. (2006) Novel angiogenic inhibitor DN-9693 that inhibits post-transcriptional induction of connective tissue growth factor (CTGF/CCN2) by vascular endothelial growth factor in human endothelial cells. Mol. Cancer Ther. 5, 129–137 [DOI] [PubMed] [Google Scholar]
- 39.Paul, B. D., Snyder, S. H. (2015) Protein sulfhydration. In Methods in Enzymology, Vol. 555 (Enrique, C., and Lester, P., eds.), pp. 79–90, Academic Press Waltham, MA, USA [DOI] [PubMed] [Google Scholar]
- 40.Bao L., Vlcek C., Paces V., Kraus J. P. (1998) Identification and tissue distribution of human cystathionine beta-synthase mRNA isoforms. Arch. Biochem. Biophys. 350, 95–103 [DOI] [PubMed] [Google Scholar]
- 41.Bełtowski J. (2005) Protein homocysteinylation: a new mechanism of atherogenesis? Postepy Hig Med Dosw (Online) 59, 392–404 [PubMed] [Google Scholar]
- 42.Jakubowski H., Zhang L., Bardeguez A., Aviv A. (2000) Homocysteine thiolactone and protein homocysteinylation in human endothelial cells: implications for atherosclerosis. Circ. Res. 87, 45–51 [DOI] [PubMed] [Google Scholar]
- 43.Chen X., Jhee K. H., Kruger W. D. (2004) Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J. Biol. Chem. 279, 52082–52086 [DOI] [PubMed] [Google Scholar]
- 44.Szabó C., Papapetropoulos A. (2011) Hydrogen sulphide and angiogenesis: mechanisms and applications. Br. J. Pharmacol. 164, 853–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shatalin K., Shatalina E., Mironov A., Nudler E. (2011) H2S: a universal defense against antibiotics in bacteria. Science 334, 986–990 [DOI] [PubMed] [Google Scholar]
- 46.Soker S., Miao H. Q., Nomi M., Takashima S., Klagsbrun M. (2002) VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J. Cell. Biochem. 85, 357–368 [DOI] [PubMed] [Google Scholar]
- 47.Nicolás M., Noé V., Jensen K. B., Ciudad C. J. (2001) Cloning and characterization of the 5′-flanking region of the human transcription factor Sp1 gene. J. Biol. Chem. 276, 22126–22132 [DOI] [PubMed] [Google Scholar]
- 48.Patterson C., Perrella M. A., Hsieh C. M., Yoshizumi M., Lee M. E., Haber E. (1995) Cloning and functional analysis of the promoter for KDR/flk-1, a receptor for vascular endothelial growth factor. J. Biol. Chem. 270, 23111–23118 [DOI] [PubMed] [Google Scholar]
- 49.Wu X., Bishopric N. H., Discher D. J., Murphy B. J., Webster K. A. (1996) Physical and functional sensitivity of zinc finger transcription factors to redox change. Mol. Cell. Biol. 16, 1035–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roybal C. N., Yang S., Sun C. W., Hurtado D., Vander Jagt D. L., Townes T. M., Abcouwer S. F. (2004) Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J. Biol. Chem. 279, 14844–14852 [DOI] [PubMed] [Google Scholar]
- 51.Zhang C., Cai Y., Adachi M. T., Oshiro S., Aso T., Kaufman R. J., Kitajima S. (2001) Homocysteine induces programmed cell death in human vascular endothelial cells through activation of the unfolded protein response. J. Biol. Chem. 276, 35867–35874 [DOI] [PubMed] [Google Scholar]
- 52.Durand P., Prost M., Loreau N., Lussier-Cacan S., Blache D. (2001) Impaired homocysteine metabolism and atherothrombotic disease. Laboratory Invest 81, 645–672 [DOI] [PubMed] [Google Scholar]
- 53.Castro R., Rivera I., Martins C., Struys E. A., Jansen E. E., Clode N., Graça L. M., Blom H. J., Jakobs C., de Almeida I. T. (2005) Intracellular S-adenosylhomocysteine increased levels are associated with DNA hypomethylation in HUVEC. J. Mol. Med. 83, 831–836 [DOI] [PubMed] [Google Scholar]
- 54.Wang H., Yoshizumi M., Lai K., Tsai J. C., Perrella M. A., Haber E., Lee M. E. (1997) Inhibition of growth and p21ras methylation in vascular endothelial cells by homocysteine but not cysteine. J. Biol. Chem. 272, 25380–25385 [DOI] [PubMed] [Google Scholar]
- 55.Chiku T., Padovani D., Zhu W., Singh S., Vitvitsky V., Banerjee R. (2009) H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 284, 11601–11612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang M. J., Cai W. J., Li N., Ding Y. J., Chen Y., Zhu Y. C. (2010) The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid. Redox Signal. 12, 1065–1077 [DOI] [PubMed] [Google Scholar]
- 57.Altaany Z., Ju Y., Yang G., Wang R. (2014) The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci. Signal. 7, ra87 [DOI] [PubMed] [Google Scholar]
- 58.Warren C. M., Ziyad S., Briot A., Der A., Iruela-Arispe M. L. (2014) A ligand-independent VEGFR2 signaling pathway limits angiogenic responses in diabetes. Sci. Signal. 7, ra1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arbiser J. L., Petros J., Klafter R., Govindajaran B., McLaughlin E. R., Brown L. F., Cohen C., Moses M., Kilroy S., Arnold R. S., Lambeth J. D. (2002) Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc. Natl. Acad. Sci. USA 99, 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pascal E., Tjian R. (1991) Different activation domains of Sp1 govern formation of multimers and mediate transcriptional synergism. Genes Dev. 5, 1646–1656 [DOI] [PubMed] [Google Scholar]
- 61.Spengler M. L., Brattain M. G. (2006) Sumoylation inhibits cleavage of Sp1 N-terminal negative regulatory domain and inhibits Sp1-dependent transcription. J. Biol. Chem. 281, 5567–5574 [DOI] [PubMed] [Google Scholar]