Abstract
Ionizing radiation is a common therapeutic modality and following irradiation dermal changes, including fibrosis and atrophy, may lead to permanent changes. We have previously demonstrated that occupancy of A2A receptor (A2AR) stimulates collagen production, so we determined whether blockade or deletion of A2AR could prevent radiation-induced fibrosis. After targeted irradiation (40 Gy) of the skin of wild-type (WT) or A2AR knockout (A2ARKO) mice, the A2AR antagonist ZM241385 was applied daily for 28 d. In irradiated WT mice treated with the A2AR antagonist, there was a marked reduction in collagen content and skin thickness, and ZM241385 treatment reduced the number of myofibroblasts and angiogenesis. After irradiation, there is an increase in loosely packed collagen fibrils, which is significantly diminished by ZM241385. Irradiation also induced an increase in epidermal thickness, prevented by ZM241385, by increasing the number of proliferating keratinocytes. Similarly, in A2ARKO mice, the changes in collagen alignment, skin thickness, myofibroblast content, angiogenesis, and epidermal hyperplasia were markedly reduced following irradiation. Radiation-induced changes in the dermis and epidermis were accompanied by an infiltrate of T cells, which was prevented in both ZM241385-treated and A2ARKO mice. Radiation therapy is administered to a significant number of patients with cancer, and radiation reactions may limit this therapeutic modality. Our findings suggest that topical application of an A2AR antagonist prevents radiation dermatitis and may be useful in the prevention or amelioration of radiation changes in the skin.—Perez-Aso, M., Mediero, A., Low, Y. C., Levine, J., Cronstein, B. N. Adenosine A2A receptor plays an important role in radiation-induced dermal injury.
Keywords: ZM241385, skin, radiotherapy lesions, therapeutic
About half of patients with cancer are treated with radiation therapy, but radiation reactions are a limiting factor in radiation therapy (1). Although radiation toxicities have been described in many tissues, including skin (2) and lung (3), skin is particularly sensitive to radiation fibrosis (4), often referred to as radiation dermatitis (5), which compromises patients’ quality of life because of pain and premature interruption of radiation treatment, which in turn may impair control of disease (6–11). Because of unavoidable exposure during radiation therapy, the skin is commonly subject to fibrosis yielding stiff, discolored tissue with poor wound-healing characteristics (12). Indeed, as many as 95% of patients treated with radiation therapy for cancer will experience a skin reaction (13). Interestingly, patients with collagen vascular disease, particularly those with scleroderma, are believed to be at increased risk of developing late complications of fibrosis after radiation therapy (14–17). Thus, prevention of fibrosis may both enhance cosmesis and reduce symptoms following radiation therapy (18).
Radiation-induced fibrosis is a multicellular process that begins with the induction of and interaction between multiple growth factors and cytokines (11). The most radiosensitive cells in the body are those that are highly proliferative and sufficiently oxygenated. Therefore, skin is susceptible to radiation damage because it is a continuously renewing organ containing rapidly proliferating and maturing cells (5) such as basal keratinocytes, hair follicles, and melanocytes (9, 19). Radiation injury to the skin involves immediate damage to basal keratinocytes and hair follicle stem cells, and ionizing radiation destroys a percentage of basal keratinocytes, resulting in a disruption in the self-renewing property of the epidermis (9). However, acute radiation skin injury primarily involves cellular alterations and inflammation in the epidermis and the dermis leading to hyperproliferation of the epidermis and thickening of the stratum corneum (5). Although for many years radiation burns (i.e., radiation skin injury) have been treated using the same therapeutic measures applied to thermal burns, the pathophysiology of radiation burns differs so that there are unpredictable and successive inflammatory waves occurring weeks to years after radiation exposure (20, 21). Overall, the healing of radiation burns is unpredictable (5).
In addition to being a physical barrier, the skin provides a system of immune surveillance that maintains homeostasis due to key cells such as Langerhans cells (22), keratinocytes, and mast and T cells, which are also important players in the radiation-induced immune response (23–25). Ionizing radiation incites signaling between the epidermis and the dermis, so that the immediate damage to the basal keratinocytes results in increased formation of cytokines and chemokines that stimulate resident skin cells and recruit circulating immune cells (5).
Under normal physiologic conditions, the level of adenosine in the tissue environment is relatively low (26), but tissue destruction combined with damaged microcirculation and hypoxia leads to increases of extracellular adenosine (27). Increased extracellular generation of adenosine in the skin promotes dermal fibrosis (28) by increasing collagen synthesis via activation of the adenosine A2A receptor (A2AR) (29–31). Moreover, A2AR activation by adenosine facilitates the progression of fibrosing illnesses such as scleroderma and cirrhosis (32–35), and A2AR blockade or deletion prevents dermal fibrosis in mice treated with bleomycin, a model of diffuse dermal fibrosis (36). A2AR blockade also prevents scarring by reducing collagen content and misalignment (34). We, therefore, sought to analyze the impact of A2AR blockade and knockout (KO) in a murine model of radiation fibrosis.
MATERIALS AND METHODS
Animal model
Wild-type (WT) C57/BL6J male 6-wk-old mice or A2AR knockout (A2ARKO) on C57/BL6J background mice were anesthetized with inhaled isoflurane; the dorsal surface was shaved with an electric clipper followed by a depilatory agent. The dorsal skin was then irradiated with a single dose of 40 Gy, delivered by a standard linear accelerator (Clinac C2100; Varian Medical Systems, Palo Alto, CA, USA) by an isolated skin injury model that delivers clinically relevant radiation doses, causing reproducible skin fibrosis without systemic radiation exposure as previously described (12). After 24 h, 200 μl of the A2AR antagonist ZM241385 (Tocris Bioscience, Bristol, United Kingdom) (2.5 mg/ml in 3% carboxymethylcellulose; Sigma-Aldrich, St. Louis, MO, USA) was topically applied every day. To prevent the leakage, a pocket of 2 × 1 cm was patterned in a double-thickness DuoDerm dressing (ConvaTec, Bridgewater Township, NJ, USA). After 28 d, the mice were then euthanized, and the skin fold thickness was measured on the treated skin using skin calipers. The skin was excised, bisected, and fixed in 10% formalin to undergo routine histologic processing or homogenized for the hydroxyproline assay. All protocols were approved by the New York University School of Medicine Institutional Animal Care and Use Committee.
Adenosine release from skin
Skin biopsy specimens, taken at the indicated time after irradiation, were washed in PBS containing antibiotics (penicillin, 200 U/L; streptomycin, 200 μg/L; and amphotericin B, 50 μg/L), cut into small pieces, and incubated in DMEM (containing the same antibiotic concentration as before) at 37°C, 5% CO2. After 2 h of incubation, supernatants were collected, and adenosine was extracted and quantified by HPLC. Results were expressed as picomoles of adenosine per milligram of tissue (35).
Hydroxyproline assay
A total of 10 mg skin specimens was hydrolyzed in 1 ml of 6 N HCl (Thermo Fisher Scientific, Pittsburgh, PA, USA) at 120°C. After evaporation, 1 ml water was added, and pH was adjusted with 0.1 N NaOH (Sigma-Aldrich). A total of 100 µl of a 1:50 dilution of the supernatant was mixed with 250 µl chloramine solution (1.3% chloramine-T (Sigma-Aldrich), 10% propanol (Thermo Fisher Scientific), and 80% citrate-acetate buffer) during 20 min at room temperature, followed by addition of 250 µl Ehrlich solution (Sigma-Aldrich) and incubation at 60°C for 20 min. Absorbance was measured at 550 nm. Standard curves (0–100 µg/ml) were generated using reagent hydroxyproline (Sigma-Aldrich) as a standard.
Morphometric dermal measurements
Skin fold thickness was measured using skin calipers. The epidermal thickness and the fat layer thickness were measured from hematoxylin and eosin (H&E) skin cross sections with the SlidePath Digital Image Hub (DIH; version 3.0 software; Leica Biosystems, Buffalo Grove, IL, USA).
Histology, immunocytochemistry, and image analysis
Paraffin sections were stained with H&E, Picrosirius red [method of Puchtler (37)], or immunohistochemistry with antibodies α-SMA (smooth muscle actin; Abcam, Cambridge, MA, USA), CD68 (AbD Serotech, Raleigh, NC, USA), CD31 (BD Pharmingen, San Diego, CA, USA), Ki67 (Thermo Fisher Scientific) clone SP6, caspase-3 (Cell Signaling Technology, Danvers, MA, USA), CD45R (BD Biosciences, San Diego, CA, USA) clone RA3-6B2, CD3 (Ventana Medical Systems, Tucson, AZ, USA) clone 2GV6, and T-cell receptor, γ/δ (Abcam) clone GL-3. Endogenous peroxidase activity was blocked with hydrogen peroxide. Tissue sections were digested with alkaline endopeptidase for 12 min at 42°C, and primary antibodies were incubated overnight at room temperature. Primary antibody was detected with secondary anti-rabbit (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-rat (BD Pharmingen) followed by SigmaFast 3′3′-diaminobenzidine (Sigma-Aldrich) development, with hematoxylin nuclear counterstaining. Appropriate negative controls were included with the study sections. Slides were acquired using a Leica SCN400 Digital Slide Scanner (Leica Microsystems). Cells were counted using the SlidePath DIH. For Picrosirius red, polarized images were collected with a Nikon DS-Fi1 camera (Tokyo, Japan), and images were quantified with SigmaScan Pro v5 software (Systat Software, Inc., San Jose, CA, USA). The green color threshold was applied to sections of the same size for all the images, and the area for each color was normalized to nonirradiated vehicle controls.
Statistical analysis
Statistical differences were determined using a t test or repeated measures ANOVA carried out using GraphPad Software (La Jolla, CA, USA) on a personal computer. The α nominal level was set at 0.05 in all cases. A value of P < 0.05 was considered significant.
RESULTS
A2AR pharmacologic blockade and KO prevent the radiation-induced skin fibrosis
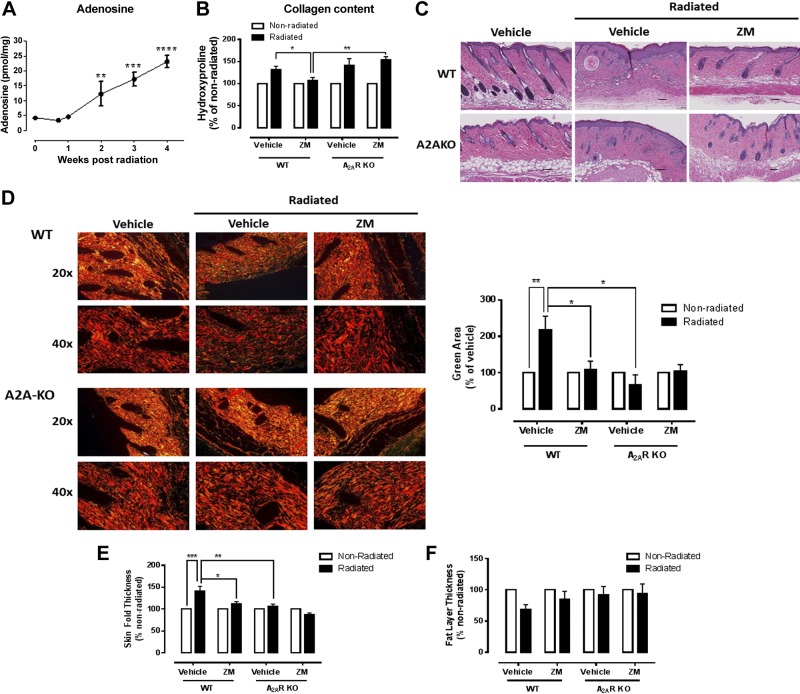
Mice were radiated in an isolated skin injury model that delivers clinically relevant radiation doses, causing reproducible skin fibrosis without systemic radiation exposure (12). We found that radiation promoted extracellular adenosine release from the skin that increased over time (Fig. 1A). In addition, there was a profound increase of collagen accumulation in the irradiated skin, measured as hydroxyproline content, that was prevented by daily topical application of the A2AR antagonist ZM241385 (2.5 mg/ml) in the WT mice, but not in the A2ARKO mice (Fig. 1B). Similarly, there was excessive accumulation of collagen and other extracellular matrix components with destruction of the normal tissue architecture and loss of function (10), as seen in the H&E-stained sections (Fig. 1C), which was prevented by both ZM241385 application and A2ARKO. In agreement, the alignment of collagen fibers, measured as the green birefringent area in the Picrosirius red–stained tissue viewed under polarized light, was severely disrupted in irradiated mice, and this change was significantly improved by A2AR blockade with ZM241385 or A2AR knockdown (Fig. 1D). Another measurement of skin fibrosis, skin thickness, was dramatically increased after radiation injury, and this change was also significantly reduced by ZM241385 treatment in the WT mice, whereas no increase in skin fold thickness was observed following radiation of the A2ARKO mice (Fig. 1E).
Figure 1.
Impact of A2AR blockade and KO on radiation-induced fibrosis. A) Adenosine levels were measured after irradiation at the indicated time points. B) Total collagen content. ZM, ZM241385. C) H&E histologies. D) Collagen alignment and packaging by Picrosirius red stain (magnifications of 20 and 40×). E, F) Skin thickness (E) and (F) fat layer thickness were determined as described under Materials and Methods. Scale bars, 100 μm. Data represent means ± sem of 4–10 animals per condition. *P < 0.05, **P < 0.01, ***P < 0.001 by ANOVA with Bonferroni’s posttest.
Previous studies have shown that in many fibrosing conditions in the skin, such as bleomycin-induced dermal fibrosis, marked atrophy of the subcutaneous adipose tissue is seen (38), and radiation treatment induced a significant decrease in the subcutaneous adipose layer in this model. Treatment with the A2AR antagonist ZM241385 application prevented loss of the subcutaneous adipose layer, and no decrease was found upon radiation of the A2ARKO mice (Fig. 1C, F), although the changes in the A2ARKO mice did not significantly differ from WT mice.
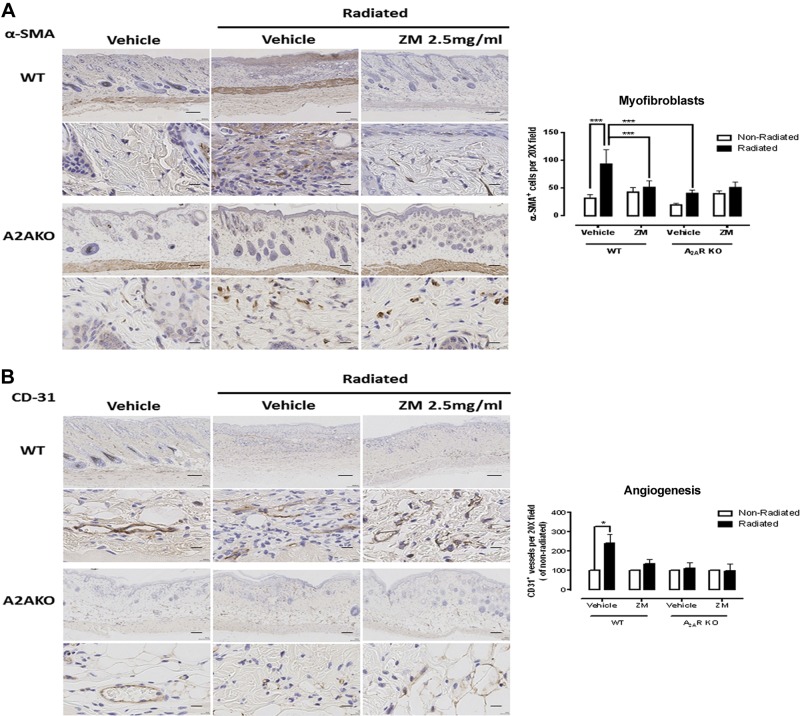
Consistent with the changes in skin matrix, radiation treatment stimulated a marked increase of the myofibroblast population (α-SMA+ cells, Fig. 2A) as well as increased angiogenesis (CD31+ vessels, Fig. 2B), which are both increased in fibrotic skin (39–41). Similar to collagen content and organization and skin thickening, both myofibroblast accumulation and excessive angiogenesis were prevented by ZM241385, and deletion of A2AR partially prevented the radiation-induced increase in myofibroblasts and angiogenesis. The modest increase in α-SMA+ cells in the dermis of ZM241385-treated mice that had not been irradiated was not significant.
Figure 2.
Impact of A2AR blockade and KO on myofibroblast population and angiogenesis upon irradiation. Immunohistochemistry was performed to determine the content of myofibroblasts (α-SMA+ cells) (A) and angiogenesis (CD31+ vessels) (B). ZM, ZM241385. Scale bars, 100 μm (top panels) and 20 μm (bottom panels). Cells and vessels per ×20 field were counted, and data represent means ± se of 4–10 animals per condition. *P < 0.05, ***P < 0.001 by ANOVA with Bonferroni’s posttest.
Thus, blockade or deletion of adenosine A2AR prevents skin fibrosis associated with radiation injury.
A2AR pharmacologic blockade and deletion prevent epidermal hyperplasia following skin irradiation
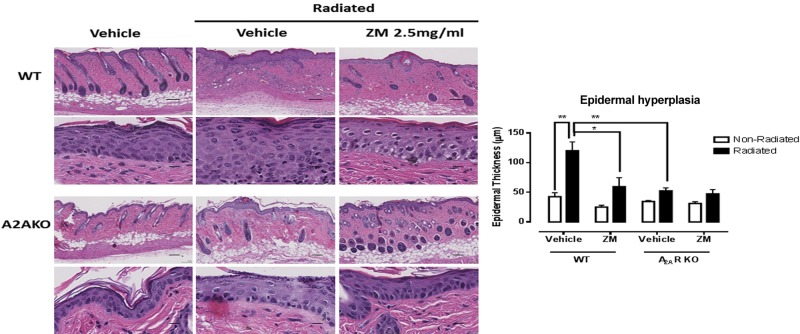
It has previously been reported that radiation injury promotes epithelial hyperplasia in the skin (42), and we observed similar epithelial hyperplasia following irradiation (Fig. 3). We were surprised to find that treatment with ZM241385 completely blocked epithelial hyperplasia. Similarly, there was no epithelial hyperplasia in the irradiated A2ARKO mice either.
Figure 3.
A2AR blockade or KO prevents the epidermal hyperplasia following irradiation. H&E stains were performed in skin cross sections, and the epidermal thickness was measured with the SlidePath DIH software. ZM, ZM241385. Scale bars, 100 μm (top panels) and 20 μm (bottom panels). Data represent means ± se of 3–5 animals per condition. *P < 0.05, **P < 0.01 by ANOVA with Bonferroni’s posttest.
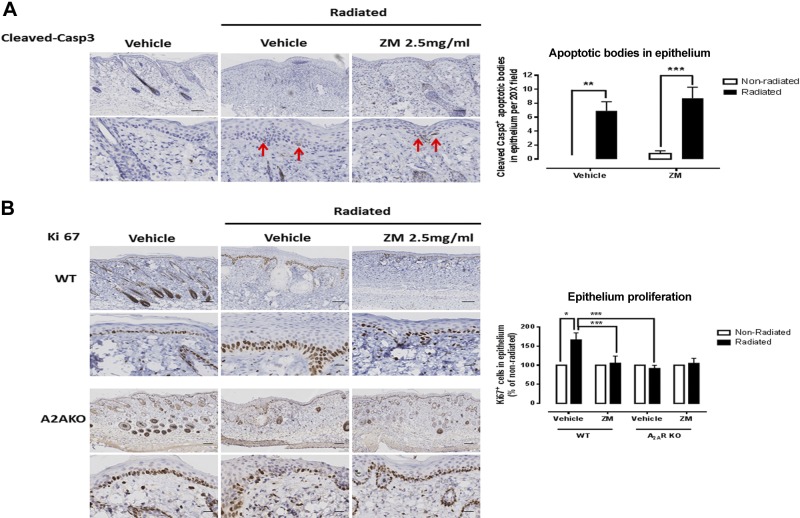
We therefore determined whether epithelial hyperplasia following radiation was mediated by an alteration of either keratinocyte proliferation or apoptosis. There was an increase in both apoptotic bodies (cleaved caspase-3+) in the epithelium (Fig. 4) and in proliferating basal keratinocytes (Ki67+ cells). However, A2AR blockade with ZM241385 prevented only the excessive proliferation of basal keratinocytes, but not the increased apoptosis, which suggests that A2AR contributes to epithelial hyperplasia by increasing the basal keratinocyte proliferation rate rather than by altering apoptosis. Consistent with this finding, we observed no increase in keratinocyte proliferation in A2ARKO mice following radiation.
Figure 4.
A2AR blockade or KO prevents basal keratinocyte proliferation but not increased apoptosis after irradiation. Immunohistochemistry was performed to determine the content of (A) apoptosis [cleaved caspase-3+ (Casp3)] and (B) proliferating keratinocytes (Ki67+ basal keratinocytes). ZM, ZM241385. Red arrows (A) indicate apoptotic bodies. Scale bars, 100 μm (top panels) and 20 μm (bottom panels). Cells and apoptotic bodies per ×20 field were counted, and data represent means ± se of 3–5 animals per condition. *P < 0.05, **P < 0.01, ***P < 0.001 by ANOVA with Bonferroni’s posttest.
A2AR pharmacologic blockade and KO prevent the increase in T-cell infiltrate associated with radiation injury in the skin
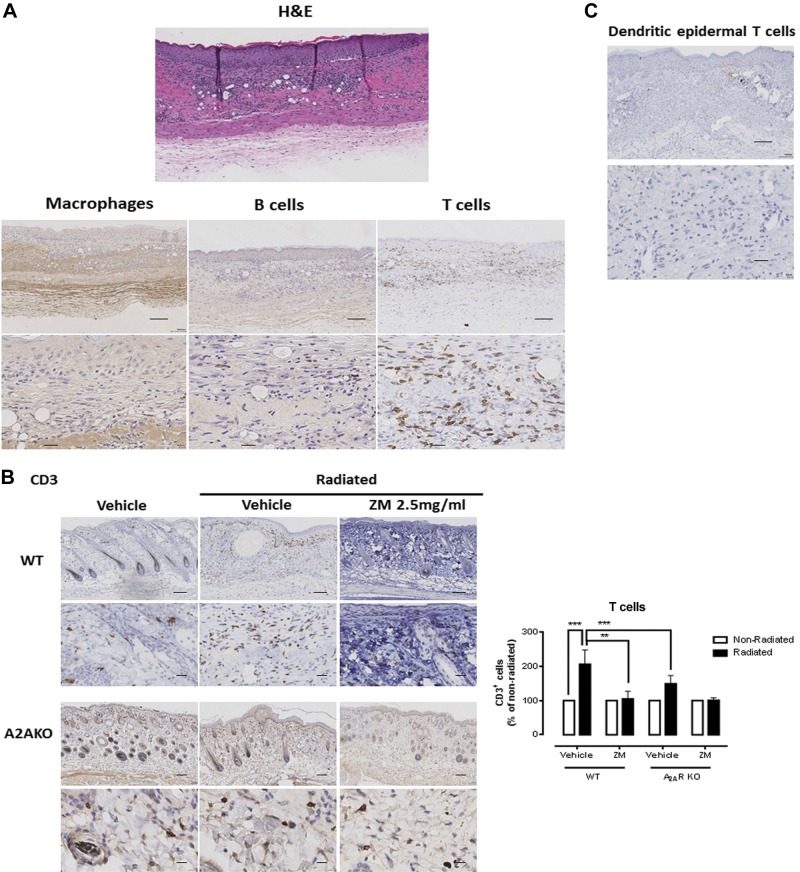
In the irradiated skin, there was an abundant cellular infiltrate in the dermis (Fig. 5A). We therefore determined the identity of these cells with specific markers for macrophages (CD68), B cells (CD45), and T cells (CD3); essentially none of the cells was positive for CD68 or for CD45, whereas the majority was CD3+. Surprisingly, daily application of ZM241385 to irradiated skin significantly prevented the accumulation of T cells, and no such infiltrate was seen in the irradiated A2ARKO mice (Fig. 5B). The modest increase in CD3+ T cells seen in the nonirradiated A2ARKO mice did not achieve statistical significance. These results indicate that the fibrosis and epithelial hyperplasia, which are mediated by A2AR, are accompanied by an infiltrate of T cells in irradiated skin, and all of these changes depend, either directly or indirectly, on A2AR stimulation.
Figure 5.
A2AR blockade or KO prevents the increased infiltrate of T cells after irradiation. A) Immunohistochemistry was performed in irradiated cross sections. The increased cellular infiltrate (H&E stain) was identified as CD3+ (T cells), but CD68− (macrophages) and CD45− (B cells). B) A2AR blockade or KO prevents the increased T-cell infiltrate after irradiation. ZM, ZM241385. Cells per ×20 field were counted, and data represent means ± se of 5–8 animals per condition. ***P < 0.001 and **P < 0.01 by ANOVA with Bonferroni’s posttest. C) The inflammatory infiltrate was TCRγδ− (dendritic epidermal T cells). Scale bars, 100 μm (top panels) and 20 μm (bottom panels).
It has been previously reported that in skin pathologies such as parapsoriasis or mycosis fungoides, the epithelial hyperplasia is accompanied by a dramatic increase of a dendritic epidermal T-cell (TCRγδ+ cells) infiltrate located between the epidermis and the dermis (43), and the dendritic epidermal T cells are necessary for epidermal thickening via release of keratinocyte growth factors that promote basal keratinocyte proliferation (44). We therefore determined whether the increased infiltrate of T cells found in the radiation-injured skin was composed of dendritic epidermal T cells. Surprisingly, in our model, there were no TCRγδ+ cells in the cellular infiltrate (Fig. 5C).
DISCUSSION
Fibrosis is a common late side effect of radiotherapy (radiation therapy) treatment for cancer and is considered to be a dose-limiting factor (1). Because of unavoidable exposure during radiation therapy, the skin is commonly subject to fibrosis resulting in thickened, stiff, and discolored tissue with poor wound-healing characteristics (12). Because we have previously reported that A2AR promotes wound healing (45), skin fibrosis progression (46), and excessive scarring (34) by, in part, stimulating collagen production by dermal fibroblasts (29–31, 36), we hypothesized that A2AR plays a role in radiation-induced skin injury, which is characterized by skin fibrosis (4, 5). We tested the effect of an A2AR antagonist and A2AR deletion in a radiation-induced skin injury model leading to reproducible skin fibrosis without systemic radiation exposure (12) and found that, following irradiation, skin released increasing adenosine over time and that adenosine induced skin fibrosis and epithelial hyperplasia via stimulation of the A2AR.
As expected, in the model studied, there was a significant increase in the total collagen content, skin thickness, and collagen misalignment (Fig. 1). Consistent with these changes in the skin, we observed an increase in the myofibroblast population and angiogenesis (Fig. 2). Upon A2AR blockade by daily topical application of the specific antagonist ZM241385, these 5 indicators of skin fibrosis were significantly prevented. Interestingly, in addition to acting as an A2AR antagonist, ZM241385 can also act as an inverse agonist at A2AR. In the experiments reported here, we have no evidence as to whether inverse agonism or direct antagonism of A2AR was responsible for prevention of radiation dermatitis, and further experiments with antagonists that do not act as inverse agonists would be required to determine which mechanism was important. Nonetheless, radiation of the A2ARKO mice did not elicit increases of collagen misalignment, skin thickness, myofibroblast content, and angiogenesis, and adenosine release from skin increases dramatically following radiation, suggesting that A2AR antagonism might be sufficient to prevent/treat radiation changes. Although A2ARKO did not prevent increases in total collagen content, taken together, our findings strongly suggest that A2AR plays a significant role in radiation-induced skin fibrosis.
ZM241385 is not totally selective for A2ARs and may also antagonize A2B receptors. In some tissues [e.g., the lung and the penis (47, 48)], stimulation of A2B receptors promotes fibrosis as well as A2AR ligation. In contrast, in the heart, ligation of A2B receptors inhibits fibrosis and fibroblast production of matrix (49, 50). Although our studies do not rule out a role for A2B receptor blockade in preventing fibrosis, the lack of change in the skin of the irradiated A2ARKO mice suggests that A2AR blockade is sufficient to diminish radiation-induced changes.
Adenosine release from skin increased over time after irradiation and was maximal at 4 wk. Although our experiments did not elucidate the mechanism for continuing adenosine release, it is unlikely that increasing cell necrosis underlies the increasing adenosine release because little evidence of increasing necrosis was observed in the skin harvested 4 wk after irradiation. Extracellular adenosine is derived, for the most part, from adenine nucleotides released from cells that are converted by the action of ectonucleotidases to adenosine (51–54). A variety of noxious stimuli, such as hypoxia or oxidants, induce adenine nucleotide release, which leads to an increase in extracellular adenosine levels (55, 56). Extracellular adenosine is both a substrate for adenosine deaminase, present in many biologic fluids, and cellular uptake via ent1, where it is converted to adenine nucleotides. Moreover, ligation of adenosine receptors down-regulates adenosine uptake, which leads to a further enhancement in extracellular adenosine levels (57). Based on the forgoing, we speculate that the increase in adenosine release from irradiated skin results from increased generation of extracellular adenosine combined with diminished adenosine utilization.
The skin injury was accompanied by a dramatic increase of epithelial thickness (Fig. 3). This finding is consistent with previously reported hyperproliferation of the epithelium, thickening of the stratum corneum, and transepidermal water loss, a measure of skin barrier integrity (58, 59). Interestingly, A2AR blockade or deletion dramatically reduced epithelial hyperplasia and basal keratinocyte proliferation following irradiation. Because neither blockade nor deletion of A2AR affected keratinocyte apoptosis, our findings indicate that, either directly or indirectly, A2AR stimulation provokes epithelial hyperplasia by increasing proliferation.
In agreement with previous work, epithelial hyperplasia, increased vascularization, and myofibroblast accumulation were accompanied by an infiltrate of inflammatory cells (42), which we identified as CD3+ T cells, but not macrophages or B cells (Fig. 5A), and surprisingly, cellular infiltration into the dermis was prevented by both A2AR blockade with the antagonist ZM241385, and A2ARKO (Fig. 5B). The modest increase in CD3+ T cells seen in the nonirradiated A2ARKO mice did not achieve statistical significance. Because A2AR stimulation suppresses most T-cell immunologic responses [reviewed in (60, 61)], the reduction in the T-cell infiltrate by A2AR blockade or KO is likely an indirect result of the reduction in the radiation-induced changes in fibroblasts and angiogenesis.
Dermal pathologies such as mycosis fungoides and parapsoriasis exhibit a dramatic epithelial hyperplasia along with a marked increase in an infiltrate of CD3+ cells bearing γδTCR (43). These cells are dendritic epidermal T cells, a unique subset of unconventional lymphocytes that secrete keratinocyte growth factors inducing keratinocyte proliferation and epidermal thickening (44). Surprisingly, the infiltrate of CD3+ T cells found in this study stained negative for γδTCR. Further studies will therefore be required to elucidate the identity of this inflammatory infiltrate, which may help to understand the differences between skin pathologies that share the epithelial hyperplasia but differ in the inflammatory signature.
The results reported here indicate that pharmacologic blockade or deletion of the A2AR prevents radiation-induced dermal fibrosis in a murine model of irradiation-induced fibrosis by preventing increases in skin thickness, collagen content and misalignment, myofibroblast population, and angiogenesis. Surprisingly, the epidermal hyperplasia driven by a dramatic increase in proliferating basal keratinocytes and the associated increase in the dermal T-cell infiltrate following irradiation injury is also prevented by A2AR blockade or deletion. Therefore, targeting the A2AR may be useful in the prevention or amelioration of radiation changes in the skin, a serious clinical problem for many patients.
Acknowledgments
This work was supported by grants from the U.S. National Institutes of Health (NIH) National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR56672 and AR68593), New York University–Health and Hospitals Corporation Clinical and Translational Science Institute (UL1TR000038), the Laura and Isaac Perlmutter Cancer Center, and NIH National Cancer Institute Cancer Center Support grant (P30CA016087).
Glossary
- A2AR
A2A receptor
- A2ARKO
A2A receptor knockout
- DIH
Digital Image Hub
- H&E
hematoxylin and eosin
- KO
knockout
- SMA
smooth muscle actin
- WT
wild-type
REFERENCES
- 1.Holler V., Buard V., Gaugler M. H., Guipaud O., Baudelin C., Sache A., Perez Mdel. R., Squiban C., Tamarat R., Milliat F., Benderitter M. (2009) Pravastatin limits radiation-induced vascular dysfunction in the skin. J. Invest. Dermatol. 129, 1280–1291 [DOI] [PubMed] [Google Scholar]
- 2.Bentzen S. M., Thames H. D., Overgaard M. (1989) Latent-time estimation for late cutaneous and subcutaneous radiation reactions in a single-follow-up clinical study. Radiother. Oncol. 15, 267–274 [DOI] [PubMed] [Google Scholar]
- 3.McDonald S., Rubin P., Phillips T. L., Marks L. B. (1995) Injury to the lung from cancer therapy: clinical syndromes, measurable endpoints, and potential scoring systems. Int. J. Radiat. Oncol. Biol. Phys. 31, 1187–1203 [DOI] [PubMed] [Google Scholar]
- 4.Hopewell J. W. (1990) The skin: its structure and response to ionizing radiation. Int. J. Radiat. Biol. 57, 751–773 [DOI] [PubMed] [Google Scholar]
- 5.Ryan J. L. (2012) Ionizing radiation: the good, the bad, and the ugly. J. Invest. Dermatol. 132, 985–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan W., MacDougall R. H., Kerr G. R., Downing D. (1996) Adverse effect of treatment gaps in the outcome of radiotherapy for laryngeal cancer. Radiother. Oncol. 41, 203–207 [DOI] [PubMed] [Google Scholar]
- 7.Robertson C., Robertson A. G., Hendry J. H., Roberts S. A., Slevin N. J., Duncan W. B., MacDougall R. H., Kerr G. R., O’Sullivan B., Keane T. J. (1998) Similar decreases in local tumor control are calculated for treatment protraction and for interruptions in the radiotherapy of carcinoma of the larynx in four centers. Int. J. Radiat. Oncol. Biol. Phys. 40, 319–329 [DOI] [PubMed] [Google Scholar]
- 8.Salvo N., Barnes E., van Draanen J., Stacey E., Mitera G., Breen D., Giotis A., Czarnota G., Pang J., De Angelis C. (2010) Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr. Oncol. 17, 94–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McQuestion M. (2011) Evidence-based skin care management in radiation therapy: clinical update. Semin. Oncol. Nurs. 27, e1–e17 [DOI] [PubMed] [Google Scholar]
- 10.Dormand E. L., Banwell P. E., Goodacre T. E. (2005) Radiotherapy and wound healing. Int. Wound J. 2, 112–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodemann H. P., Bamberg M. (1995) Cellular basis of radiation-induced fibrosis. Radiother. Oncol. 35, 83–90 [DOI] [PubMed] [Google Scholar]
- 12.Lee J. W., Tutela J. P., Zoumalan R. A., Thanik V. D., Nguyen P. D., Varjabedian L., Warren S. M., Saadeh P. B. (2010) Inhibition of Smad3 expression in radiation-induced fibrosis using a novel method for topical transcutaneous gene therapy. Arch. Otolaryngol. Head Neck Surg. 136, 714–719 [DOI] [PubMed] [Google Scholar]
- 13.De Conno F., Ventafridda V., Saita L. (1991) Skin problems in advanced and terminal cancer patients. J. Pain Symptom Manage. 6, 247–256 [DOI] [PubMed] [Google Scholar]
- 14.Abu-Shakra M., Lee P. (1993) Exaggerated fibrosis in patients with systemic sclerosis (scleroderma) following radiation therapy. J. Rheumatol. 20, 1601–1603 [PubMed] [Google Scholar]
- 15.Phan C., Mindrum M., Silverman C., Paris K., Spanos W. (2003) Matched-control retrospective study of the acute and late complications in patients with collagen vascular diseases treated with radiation therapy. Cancer J. 9, 461–466 [DOI] [PubMed] [Google Scholar]
- 16.Chen A. M., Obedian E., Haffty B. G. (2001) Breast-conserving therapy in the setting of collagen vascular disease. Cancer J. 7, 480–491 [PubMed] [Google Scholar]
- 17.Morris M. M., Powell S. N. (1997) Irradiation in the setting of collagen vascular disease: acute and late complications. J. Clin. Oncol. 15, 2728–2735 [DOI] [PubMed] [Google Scholar]
- 18.Kumar S., Kolozsvary A., Kohl R., Lu M., Brown S., Kim J. H. (2008) Radiation-induced skin injury in the animal model of scleroderma: implications for post-radiotherapy fibrosis. Radiat. Oncol. 3, 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendelsohn F. A., Divino C. M., Reis E. D., Kerstein M. D. (2002) Wound care after radiation therapy. Adv. Skin Wound Care 15, 216–224 [DOI] [PubMed] [Google Scholar]
- 20.Bey E., Prat M., Duhamel P., Benderitter M., Brachet M., Trompier F., Battaglini P., Ernou I., Boutin L., Gourven M., Tissedre F., Créa S., Mansour C. A., de Revel T., Carsin H., Gourmelon P., Lataillade J. J. (2010) Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen. 18, 50–58 [DOI] [PubMed] [Google Scholar]
- 21.Lataillade J. J., Doucet C., Bey E., Carsin H., Huet C., Clairand I., Bottollier-Depois J. F., Chapel A., Ernou I., Gourven M., Boutin L., Hayden A., Carcamo C., Buglova E., Joussemet M., de Revel T., Gourmelon P. (2007) New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen. Med. 2, 785–794 [DOI] [PubMed] [Google Scholar]
- 22.Valladeau J., Saeland S. (2005) Cutaneous dendritic cells. Semin. Immunol. 17, 273–283 [DOI] [PubMed] [Google Scholar]
- 23.Kalesnikoff J., Galli S. J. (2008) New developments in mast cell biology. Nat. Immunol. 9, 1215–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller K., Meineke V. (2011) Radiation-induced mast cell mediators differentially modulate chemokine release from dermal fibroblasts. J. Dermatol. Sci. 61, 199–205 [DOI] [PubMed] [Google Scholar]
- 25.Stelekati E., Bahri R., D’Orlando O., Orinska Z., Mittrücker H. W., Langenhaun R., Glatzel M., Bollinger A., Paus R., Bulfone-Paus S. (2009) Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity 31, 665–676 [DOI] [PubMed] [Google Scholar]
- 26.Sitkovsky M. V., Lukashev D., Apasov S., Kojima H., Koshiba M., Caldwell C., Ohta A., Thiel M. (2004) Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 22, 657–682 [DOI] [PubMed] [Google Scholar]
- 27.Zarek P. E., Huang C. T., Lutz E. R., Kowalski J., Horton M. R., Linden J., Drake C. G., Powell J. D. (2008) A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 111, 251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández P., Perez-Aso M., Smith G., Wilder T., Trzaska S., Chiriboga L., Franks A. Jr, Robson S. C., Cronstein B. N., Chan E. S. (2013) Extracellular generation of adenosine by the ectonucleotidases CD39 and CD73 promotes dermal fibrosis. Am. J. Pathol. 183, 1740–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan E. S., Liu H., Fernandez P., Luna A., Perez-Aso M., Bujor A. M., Trojanowska M., Cronstein B. N. (2013) Adenosine A(2A) receptors promote collagen production by a Fli1- and CTGF-mediated mechanism. Arthritis Res. Ther. 15, R58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Aso M., Fernandez P., Mediero A., Chan E. S., Cronstein B. N. (2014) Adenosine 2A receptor promotes collagen production by human fibroblasts via pathways involving cyclic AMP and AKT but independent of Smad2/3. FASEB J. 28, 802–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Aso M., Mediero A., Cronstein B. N. (2013) Adenosine A2A receptor (A2AR) is a fine-tune regulator of the collagen1:collagen3 balance. Purinergic Signal. 9, 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katebi M., Fernandez P., Chan E. S., Cronstein B. N. (2008) Adenosine A2A receptor blockade or deletion diminishes fibrocyte accumulation in the skin in a murine model of scleroderma, bleomycin-induced fibrosis. Inflammation 31, 299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazzerini P. E., Natale M., Gianchecchi E., Capecchi P. L., Montilli C., Zimbone S., Castrichini M., Balistreri E., Ricci G., Selvi E., Garcia-Gonzalez E., Galeazzi M., Laghi-Pasini F. (2012) Adenosine A2A receptor activation stimulates collagen production in sclerodermic dermal fibroblasts either directly and through a cross-talk with the cannabinoid system. J. Mol. Med. 90, 331–342 [DOI] [PubMed] [Google Scholar]
- 34.Perez-Aso M., Chiriboga L., Cronstein B. N. (2012) Pharmacological blockade of adenosine A2A receptors diminishes scarring. FASEB J. 26, 4254–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan E. S., Montesinos M. C., Fernandez P., Desai A., Delano D. L., Yee H., Reiss A. B., Pillinger M. H., Chen J. F., Schwarzschild M. A., Friedman S. L., Cronstein B. N. (2006) Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br. J. Pharmacol. 148, 1144–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan E. S., Fernandez P., Merchant A. A., Montesinos M. C., Trzaska S., Desai A., Tung C. F., Khoa D. N., Pillinger M. H., Reiss A. B., Tomic-Canic M., Chen J. F., Schwarzschild M. A., Cronstein B. N. (2006) Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum. 54, 2632–2642 [DOI] [PubMed] [Google Scholar]
- 37.Heap B. J., Kiernan J. A. (1973) Histological, histochemical and pharmacological observations on mast cells in the stomach of the rat. J. Anat. 115, 315–325 [PMC free article] [PubMed] [Google Scholar]
- 38.Wu M., Melichian D. S., Chang E., Warner-Blankenship M., Ghosh A. K., Varga J. (2009) Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-gamma. Am. J. Pathol. 174, 519–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinz B., Gabbiani G. (2010) Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol. Rep. 2, 78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh M. H., Oh S. Y., Yu J., Myers A. C., Leonard W. J., Liu Y. J., Zhu Z., Zheng T. (2011) IL-13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. J. Immunol. 186, 7232–7242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wynn T. A. (2008) Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flanders K. C., Sullivan C. D., Fujii M., Sowers A., Anzano M. A., Arabshahi A., Major C., Deng C., Russo A., Mitchell J. B., Roberts A. B. (2002) Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am. J. Pathol. 160, 1057–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bordignon M., Belloni Fortina A., Pigozzi B., Alaibac M. (2008) γδ T cells as potential contributors to the progression of parapsoriasis to mycosis fungoides. Mol. Med. Rep. 1, 485–488 [PubMed] [Google Scholar]
- 44.Jameson J., Ugarte K., Chen N., Yachi P., Fuchs E., Boismenu R., Havran W. L. (2002) A role for skin gammadelta T cells in wound repair. Science 296, 747–749 [DOI] [PubMed] [Google Scholar]
- 45.Montesinos M. C., Gadangi P., Longaker M., Sung J., Levine J., Nilsen D., Reibman J., Li M., Jiang C. K., Hirschhorn R., Recht P. A., Ostad E., Levin R. I., Cronstein B. N. (1997) Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J. Exp. Med. 186, 1615–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernández P., Trzaska S., Wilder T., Chiriboga L., Blackburn M. R., Cronstein B. N., Chan E. S. (2008) Pharmacological blockade of A2A receptors prevents dermal fibrosis in a model of elevated tissue adenosine. Am. J. Pathol. 172, 1675–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong H., Belardinelli L., Maa T., Zeng D. (2005) Synergy between A2B adenosine receptors and hypoxia in activating human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 32, 2–8 [DOI] [PubMed] [Google Scholar]
- 48.Wen J., Jiang X., Dai Y., Zhang Y., Tang Y., Sun H., Mi T., Phatarpekar P. V., Kellems R. E., Blackburn M. R., Xia Y. (2010) Increased adenosine contributes to penile fibrosis, a dangerous feature of priapism, via A2B adenosine receptor signaling. FASEB J. 24, 740–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubey R. K., Gillespie D. G., Jackson E. K. (1998) Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts: role of A2B receptors. Hypertension 31, 943–948 [DOI] [PubMed] [Google Scholar]
- 50.Chen Y., Epperson S., Makhsudova L., Ito B., Suarez J., Dillmann W., Villarreal F. (2004) Functional effects of enhancing or silencing adenosine A2b receptors in cardiac fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 287, H2478–H2486 [DOI] [PubMed] [Google Scholar]
- 51.Möser G. H., Schrader J., Deussen A. (1989) Turnover of adenosine in plasma of human and dog blood. Am. J. Physiol. 256, C799–C806 [DOI] [PubMed] [Google Scholar]
- 52.Deussen A., Bading B., Kelm M., Schrader J. (1993) Formation and salvage of adenosine by macrovascular endothelial cells. Am. J. Physiol. 264, H692–H700 [DOI] [PubMed] [Google Scholar]
- 53.Huang D. Y., Vallon V., Zimmermann H., Koszalka P., Schrader J., Osswald H. (2006) Ecto-5′-nucleotidase (cd73)-dependent and -independent generation of adenosine participates in the mediation of tubuloglomerular feedback in vivo. Am. J. Physiol. Renal Physiol. 291, F282–F288 [DOI] [PubMed] [Google Scholar]
- 54.Romio M., Reinbeck B., Bongardt S., Hüls S., Burghoff S., Schrader J. (2011) Extracellular purine metabolism and signalling of CD73-derived adenosine in murine Treg and Teff cells. Am. J. Physiol. Cell Physiol. 301, C530–C539 [DOI] [PubMed] [Google Scholar]
- 55.Gödecke S., Roderigo C., Rose C. R., Rauch B. H., Gödecke A., Schrader J. (2012) Thrombin-induced ATP release from human umbilical vein endothelial cells. Am. J. Physiol. Cell Physiol. 302, C915–C923 [DOI] [PubMed] [Google Scholar]
- 56.Islam M. R., Uramoto H., Okada T., Sabirov R. Z., Okada Y. (2012) Maxi-anion channel and pannexin 1 hemichannel constitute separate pathways for swelling-induced ATP release in murine L929 fibrosarcoma cells. Am. J. Physiol. Cell Physiol. 303, C924–C935 [DOI] [PubMed] [Google Scholar]
- 57.Nagy L. E., Diamond I., Casso D. J., Franklin C., Gordon A. S. (1990) Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J. Biol. Chem. 265, 1946–1951 [PubMed] [Google Scholar]
- 58.Jensen J. M., Gau T., Schultze J., Lemmnitz G., Fölster-Holst R., May T., Abels C., Proksch E. (2011) Treatment of acute radiodermatitis with an oil-in-water emulsion following radiation therapy for breast cancer: a controlled, randomized trial. Strahlenther. Onkol. 187, 378–384 [DOI] [PubMed] [Google Scholar]
- 59.Schmuth M., Sztankay A., Weinlich G., Linder D. M., Wimmer M. A., Fritsch P. O., Fritsch E. (2001) Permeability barrier function of skin exposed to ionizing radiation. Arch. Dermatol. 137, 1019–1023 [PubMed] [Google Scholar]
- 60.Cronstein B. N. (2005) Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol. Rev. 57, 163–172 [DOI] [PubMed] [Google Scholar]
- 61.Sitkovsky M., Lukashev D., Deaglio S., Dwyer K., Robson S. C., Ohta A. (2008) Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. Br. J. Pharmacol. 153(Suppl 1), S457–S464 [DOI] [PMC free article] [PubMed] [Google Scholar]