Abstract
Reprograming of metabolism is one of the central hallmarks of cancer. The majority of cancer cells depend on high rates of glycolysis and glutaminolysis for their growth and survival. A number of oncogenes and tumor suppressors have been connected to the regulation of altered glucose and glutamine metabolism in cancer cells. For example, the oncogene c-Myc plays vital roles in cancer cell metabolic adaptation by directly regulating various genes that participate in aerobic glycolysis and glutaminolysis. Inhibitor of differentiation 1 (Id1) is a helix-loop-helix transcription factor that plays important roles in cell proliferation, differentiation, and cell fate determination. Overexpression of Id1 causes intestinal adenomas and thymic lymphomas in mice, suggesting that Id1 could function as an oncogene. Despite it being an oncogene, whether Id1 plays any prominent role in cancer cell metabolic reprograming is unknown. Here, we demonstrate that Id1 is strongly expressed in human and mouse liver tumors and in hepatocellular carcinoma (HCC) cell lines, whereas its expression is very low or undetectable in normal liver tissues. In HCC cells, Id1 expression is regulated by the MAPK/ERK pathway at the transcriptional level. Knockdown of Id1 suppressed aerobic glycolysis and glutaminolysis, suggesting that Id1 promotes a metabolic shift toward aerobic glycolysis. At the molecular level, Id1 mediates its metabolic effects by regulating the expression levels of c-Myc. Knockdown of Id1 resulted in down-regulation (∼75%) of c-Myc, whereas overexpression of Id1 strongly induced (3-fold) c-Myc levels. Interestingly, knockdown of c-Myc resulted in down-regulation (∼60%) of Id1, suggesting a positive feedback-loop regulatory mechanism between Id1 and c-Myc. Under anaerobic conditions, both Id1 and c-Myc are down-regulated (50–70%), and overexpression of oxygen-insensitive hypoxia-inducible factor 1α (Hif1α) or its downstream target Mxi1 resulted in a significant reduction of c-Myc and Id1 (∼70%), suggesting that Hif1α suppresses Id1 and c-Myc under anaerobic conditions via Mxi1. Together, our findings indicate a prominent novel role for Id1 in liver cancer cell metabolic adaptation.—Sharma, B. K., Kolhe, R., Black, S. M., Keller, J. R., Mivechi, N. F., Satyanarayana, A. Inhibitor of differentiation 1 transcription factor promotes metabolic reprogramming in hepatocellular carcinoma cells.
Keywords: glucose metabolism, glutamine metabolism, liver cancer, c-Myc, Hif1α
Cancer cells face a substantial bioenergetic challenge because they need to produce sufficient energy and at the same time synthesize various biomolecules in order to proliferate continuously. To meet these demands of proliferation, cancer cells undergo a metabolic reprogramming, one of the central hallmarks of cancer and a significant contributing factor to oncogenesis (1). The major difference between normal and cancer cells is how much glucose they consume and how they metabolize it. Many cancer cells depend on high rates of glucose uptake and convert most of the glucose to lactate instead of completely catabolizing glucose by oxidative phosphorylation (oxphos) even in the presence of sufficient oxygen, a phenomenon known as the Warburg effect (1, 2). Despite being energetically inefficient, this metabolic shift provides a steady supply of glycolytic intermediates as carbon-containing precursors for macromolecular biosynthesis while still meeting their energy requirements. The resulting shift to aerobic glycolysis in turn converts mitochondria into a biosynthetic machine that supports glucose-dependent lipid synthesis and nonessential amino acid production (3). In addition to glucose, glutamine can be used as a nutrient for cell growth and viability, and glutaminolysis, the catabolism of glutamine, is also an essential characteristic of cancer metabolic reprogramming. Glutaminolysis provides nitrogen for nucleotide and amino acid synthesis, carbon for tricarboxylic acid cycle intermediates, and NADPH for lipid and nucleotide synthesis, thereby playing a predominant role in biosynthetic pathways (3, 4). Together, cancer cells effectively remodel their glucose and glutamine metabolism for their proliferation and survival.
A number of studies have demonstrated that cancer cell nutrient uptake and metabolism are under the direct control of oncogenic signaling pathways. Moreover, various mutations that occur in cancer also control cancer cell metabolism, suggesting that oncogenes and tumor suppressors regulate metabolism as part of their mode of action (5). The oncogene c-Myc, for example, has emerged as one of the central regulators that actively engages in the metabolic reprogramming of cancer cells. c-Myc regulates an array of genes that participates in both glucose and glutamine transport and metabolism, such as HK2 (hexokinase 2), PFK (phosphofructokinase), ENO1 (enolase 1), glyceraldehyde 3-phosphate dehydrogenase, PGK1 (phosphoglycerate kinase 1), LDHA (lactase dehydrogenase A), PDK1 (pyruvate dehydrogenase kinase, isozyme 1), Glut1 (glucose transporter 1), glutaminase (GLS), and the glutamine transporters ASC amino acid transporter 2 and H. sapiens solute carrier family 38, member 5, thereby exerting tremendous influence on cancer cell metabolic reprogramming (6). Therefore, identifying factors that regulate c-Myc expression and/or its transcriptional activity is essential to developing therapeutic agents to target c-Myc and inhibit cancer cell metabolic reprogramming and suppress cancer cell growth.
Inhibitor of differentiation 1 (Id1, also known as Id1A or Id1-001) is a helix-loop-helix (HLH) transcription factor that plays an important role in a number of cellular processes such as cell proliferation, cellular differentiation, cell fate determination, neurogenesis, and hematopoiesis (7–10). The other Id1 isoform Id1B or Id1-002 is known to maintain cellular quiescence and promotes self-renewal and stem cell-like features (11). It has been shown that Id1 is strongly expressed in a number of human cancers such as breast, pancreas, cervical, ovarian, and prostate (12–14). Overexpression of Id1 causes intestinal adenomas and thymic lymphomas in mice, suggesting that Id1 functions as an oncogene (15, 16). Despite it being an oncogene, it is unknown whether Id1 plays any prominent role in cancer cell metabolic reprograming. Here, we report that Id1 is strongly expressed in liver tumors and in hepatocellular carcinoma (HCC) cell lines and promotes both aerobic glycolysis and glutaminolysis by regulating the expression levels of c-Myc in HCC cells.
MATERIALS AND METHODS
Human HCC samples
There were 20 formalin-fixed, paraffin-embedded cases of liver cancer (American Joint Committee on Cancer stages I–IV), 8 liver samples from patients who have cirrhosis, and 8 normal control liver samples retrieved from the pathology archives of Georgia Regents University under an approved institutional review board protocol. Archival blocks were retrieved, and slides were reviewed with clinical information on each entity. There were 7 μm sections with >50% lesion from each case used for staining and analysis.
Immunohistochemistry
For immunohistochemistry (IHC), slides were deparaffinized in xylol and microwave heated in 0.01 M citrate buffer for 16 min. After cooling for 20 min and washing in PBS, endogenous peroxidase was blocked with methanol containing 0.3% hydrogen peroxide for 30 min, followed by incubation with PBS containing 10% normal goat serum for 30 min. For detection of Id1 protein expression, specimens were incubated overnight at 4°C with Id1 rabbit mAb (#M087; CalBioreagents, San Mateo, CA, USA) at a dilution of 1:50. Based on the published literature (13), as a positive control for Id1 expression, IHC was performed on a sample of invasive squamous cell carcinoma from human cervix, which is known to have strong Id1 expression. Because Id1 is also strongly expressed in smooth muscle cells of vessels and endothelial cells, they served as an internal positive control (13). As a negative control, the primary antibody was replaced by normal, nonimmune rabbit serum. As a control for the staining procedure, IHC was carried out on a specimen of liver cancer with strong Id1 expression after blocking the antigen-binding site of the primary antibody by a corresponding blocking peptide (#sc-488p; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Mice
Mice were housed in a barrier facility under standard conditions with a 12 h light–dark cycle. Mice were handled in compliance with the National Institutes of Health guidelines for animal care and use. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Georgia Regents University. C57BL/6J background mice were used for this study.
Diethylnitrosamine-mediated induction of liver tumors in mice
Diethylnitrosamine (DEN), a chemical carcinogen, is used to induce liver tumorigenesis in C57BL/6J male mice. A single dose of 25 mg/kg body weight (0.2 ml) DEN (#N0756; Sigma-Aldrich, St. Louis, MO, USA) was injected intraperitoneally to 15-d-old newborn mice to initiate liver tumorigenesis. Mice were euthanized after 8 mo (n = 6), and liver tumors and nontumorous liver tissue were harvested and fixed in 10% neutral buffered formalin (#HT 501128-4L; Sigma-Aldrich) for further analysis.
HCC cell lines
Hepa1-6 (CRL-1830) and HepG2 (HB-8065) cells were purchased from American Type Culture Collection (Manassas, VA, USA). The cells were cultured in DMEM (#SH30022.01; HyClone Laboratories, Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; #100106; Gemini Bio-Products, West Sacramento, CA, USA) in a humidified cell culture incubator (Heracell 150i; Thermo Fisher Scientific, Waltham, MA, USA) maintained at 37°C, 5% CO2, and 20% O2.
Transient transfection, short hairpin RNA, and overexpression vectors
Continuum Transfection Reagent (#400-700; Gemini Bio-Products) was used to transfect the cells with control, short hairpin RNA (shRNA), and overexpression vectors according to the manufacturer’s protocol. Control shRNA (shGL) and Id1-shRNA vectors were a kind gift from Jonathan Keller (National Cancer Institute, Frederick, MD, USA) and were described previously (17). GFP-mId1 and VL3 (Flag-Mad2) were a gift from Robert Benezra (#20963 and #16047) (9, 18), pcDNA3 mHIF-1α MYC (P402A/P577A/N813A) was a gift from Celeste Simon (#44028) (19), and pRetrosuper Myc shRNA was a gift from Martin Eilers (#15662; all from Addgene, Cambridge, MA, USA) (20).
Immunocytochemistry
Hepa1-6 cells cultured in 100 mm culture dishes were transferred onto coverslips in 6-well plates at a density of 1 × 105. After 12 h, cells were washed twice with cold PBS, fixed with ice-cold acetone:methanol (1:1) for 5 min, and washed 3 times with ice-cold PBS and incubated with an anti-Id1 (#BCH-1, 37-2; BioCheck, Foster City, CA, USA) antibody at 4°C overnight. The cells were then washed 3 times with PBS with 0.2% Tween 20 and incubated with Alexa Fluor 555 goat anti-rabbit (#A21428; Life Technologies, Carlsbad, CA, USA) secondary antibody for 1 h at room temperature. The coverslips were washed 3 times with PBS with 0.2% Tween 20, air-dried for 10 min, and mounted with fluorescence mounting medium (Vectashield H-1200; Vector Laboratories, Burlingame, CA, USA) containing DAPI. The optical single-slice images were captured with the Zeiss Axio fluorescence laser-scanning microscope (Carl Zeiss Gesellschaft mit beschränkter Haftung, Jena, Germany) and Olympus BX41 (Olympus America, Center Valley, PA, USA).
Cell cycle analysis by fluorescence-activated cell sorting
Hepa1-6 [passage 2 (P2)] cells that express either scramble shRNA or Id1-shRNA were cultured in 10 cm dishes. When the cells were 100% confluent, they were trypsinized and split into three 10 cm dishes. The cells were collected after 12, 24, and 36 h and analyzed for their cell cycle profile. Propidium iodide staining was performed as described elsewhere (21). Flow cytometry acquisition and analysis were carried out with a FACSCalibur flow cytometry system equipped with CellQuest Pro Software (both from BD Biosciences, San Jose, CA, USA).
Colony formation assays
For colony formation assays, 5 × 102 Hepa1-6 or 1 × 103 HepG2 cells were seeded in 6-well plates and maintained in complete medium. After 14 d, colonies in the plates were fixed with ice-cold methanol and stained with 0.5% crystal violet for 10 min.
AlamarBlue proliferation
AlamarBlue proliferation assays (Thermo Fisher Scientific) for Hepa1-6 and HepG2 cells were performed as described previously (22). The proliferation of P2 control and Id1-knockdown cells was analyzed in 96-well plates for 7 d. Triplicates of 1500 cells per well for each sample were seeded, and the proliferation rate was evaluated by measuring the fluorescence at 540/600 using a Synergy HTX Multi-Mode plate reader (BioTek Instruments, Winooski, VT, USA).
Xenograft tumor assay
Id1 knockdown and control Hepa1-6 cells (3 × 106) were injected subcutaneously into the athymic nude mice (strain code #490; Charles River Laboratories, Wilmington, MA, USA), and tumor growth was monitored daily. Mice were killed on d 15 as the tumor size reached >2.0 cm in diameter. Tumors were isolated from the nude mice, and weight and diameter were measured using a vernier caliper (Thermo Fisher Scientific). Tumor volume was calculated according to the following formula: volume (cm3) = 1/2(L × W2), where L and W are the length and width of the tumor, respectively.
Glucose uptake, lactate, and glutamate production
For glucose uptake assay, HepG2 cells growing in complete medium were replaced with glucose-free medium for 45 min. The cells were then incubated with the fluorescently labeled glucose analog 2-NBDG {2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose; #11046; Cayman Chemicals, Ann Arbor, MI, USA} for 30 min. Medium containing 2-NBDG was removed from the cells, which were washed twice with PBS, trypsinized, resuspended in PBS, and analyzed by the FACSCalibur flow cytometry system equipped with CellQuest Pro Software. Lactate production was measured by the Glycolysis Cell-Based Assay Kit (#600450; Cayman Chemicals) according to the manufacturer’s instructions. Glutamate levels were measured by the Glutamate Calorimetric Assay Kit (#K629-100; BioVision Incorporated, Milpitas, CA, USA) according to the manufacturer’s instructions.
Serum deprivation, cycloheximide, MG132, and inhibitor studies
For serum-deprivation experiments, HepG2 cells were cultured in 10 cm culture dishes (DMEM plus 10% FBS). When the cells were ∼70% confluent, they were washed twice with PBS, and complete medium was replaced with serum starvation medium (DMEM plus 0.1% FBS). The cells were harvested, and whole-cell protein lysates were prepared at the indicated time points. For cycloheximide and MG132 experiments, HepG2 cells were treated with a final concentration of 100 µM cycloheximide or 10 µM MG132, and the cells were harvested at the indicated time points for protein lysate preparation. For drug inhibitor experiments, Hepa1-6 and HepG2 cells were cultured in 10 cm culture dishes (DMEM plus 10% FBS) and treated with a 10 µM final concentration of either U0126 (MEK inhibitor), axitinib (VEGF inhibitor), or IWR-1-endo (β-catenin inhibitor) for 24 h. The cells were washed twice with PBS, and whole-cell lysates were prepared for further analysis.
Mitochondrial respiration
Mitochondrial respiration was measured in real time with the Seahorse Bioscience Extracellular Flux Analyzer (#XF24; North Billerica, MA, USA). The XF24 measures the oxygen consumption rate (OCR) as an indicator of respiration. Cells (3 × 104) were cultured overnight in custom XF24 microplates (#100777-604; Seahorse Bioscience). Just before measurements, cells were washed with DMEM (pH 7.4) supplemented with 5 mM glucose and 2 mM sodium pyruvate. The XF24 culture microplates were then incubated in a CO2-free XF prep station at 37°C for 45 min to allow temperature and pH calibration. After measuring basal OCR, oligomycin (1 µm final concentration, an ATP synthase inhibitor), carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (1 µm final concentration, an electron transport chain uncoupler), and rotenone plus antimycin A (1 µm final concentration of each, complex I and complex III inhibitors, respectively) were sequentially injected. Basal mitochondrial respiration, reserve respiratory capacity, and maximal respiratory capacity were then determined in picomoles of oxygen consumed per minute. All the drugs used were from the XF Cell Mito Stress Test Kit (#101706-100; Seahorse Bioscience).
RNA preparation, cDNA synthesis, and quantitative PCR
Total RNA from Hepa1-6 and HepG2 cells was prepared using Trizol Reagent (#15596-026; Life Technologies) according to the manufacturer’s instructions. Total RNA (2 µg) from each sample was reverse transcribed into cDNA using the RevertAid RT Kit (#K1691; Thermo Fisher Scientific) according to the manufacturer’s instructions. Quantitative PCR (qPCR) analysis was performed using Power SYBR Green PCR Master Mix (#4367659; Life Technologies) according to the manufacturer’s instructions in a 20 μl final reaction volume in 96-well plates (#4346907; Applied Biosciences, Life Technologies). The qPCR primers used are provided in Table 1.
TABLE 1.
Human and mouse primers used for real-time qPCR
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| Human | ||
| 18S | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
| Id1 | GTAAACGTGCTGCTCTACGACATGA | AGCTCCAACTGAAGGTCCCTGA |
| c-Myc | AAACACAAACTTGAACAGCTAC | ATTTGAGGCAGTTTACATTATGG |
| Aldolase A | TCAACCACACTCCGTCCACG | GTAGCAAGTTCCGGTGCTTC |
| LDHA | AGCCCGATTCCGTTACCT | CACCAGCAACATTCATTCCA |
| HK2 | CAAAGTGACAGTGGGTGTGG | GCCAGGTCCTTCACTGTCTC |
| ENO1 | AAAGCTGGTGCCGTTGAGAA | GGTTGTGGTAAACCTCTGCTC |
| Enolase 2 | AGCCTCTACGGGCATCTATGA | TTCTCAGTCCCATCCAACTCC |
| PDK1 | CTGTGATACGGATCAGAAACCG | TCCACCAAACAATAAAGAGTGCT |
| PKM2 | ATGTCGAAGCCCATAGTGAA | TGGGTGGTGAATCAATGTCCA |
| GLUT1 | AACTCTTCAGCCAGGGTCCAC | CACAGTGAAGATGATGAAGAC |
| SLC1A5 | TCCTTCAATGAGGCCACCAT | TGCGGGTGAAGAGGAAGTAG |
| SLC38A3/SN1 | CTCACCTTCTACAACGGGGT | TGATACAAGTGAGCAGGCCA |
| SLC38A5/SN2 | CGTCCTACTCCATCCACCTC | TCCCTTCAAGAACCAGTCCC |
| Mouse | ||
| c-Myc | GGAAACCCCGACAGCCACGACG | GTGGGAAGCAGCTCGAATTT |
| Id1 | GGTCCGAGGCAGAGTATTACA | CCTGAAAAGTAAGGAAGGGGGA |
| 18S | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
PKM2, pyruvate kinase isozyme M2; SLC1A5, solute carrier family 1 (neutral amino acid transporter), member 5; SLC38A3/SN1, H. sapiens solute carrier family 38, member 3; SLC38A5/SN2, H. sapiens solute carrier family 38, member 5.
Subcellular protein fractionation
Nuclear and cytoplasmic protein fractions were prepared from Hepa1-6 and HepG2 cells by using the Subcellular Protein Fractionation Kit according to the manufacturer’s instructions (#78840; Thermo Fisher Scientific).
Hypoxia
For growth under hypoxic conditions, Hepa1-6 and HepG2 cells were incubated at 37°C in a modular chamber flushed with 1% O2, 5% CO2, and 94% N2. At the indicated time points, cells were harvested or transferred to normoxic conditions.
Immunoblotting and coimmunoprecipitations
Whole-cell protein lysates were prepared from cells using RIPA lysis buffer (#89901; Thermo Fisher Scientific) supplemented with a cOmplete Mini Protease Inhibitor Tablet (#11836153001; Roche Life Science, Indianapolis, IN, USA). For Western blot analysis, 50 µg protein was separated on NuPAGE precast gels (Invitrogen/Life Technologies), transferred using an XCell II Blot module (#090707-098; Invitrogen/Life Technologies) onto Immobilon-FL membranes (#IPFL00010; EMD Millipore, Billerica, MA, USA), and probed with specific primary antibodies: Mouse Id1 (#BCH-1, 37-2); Mouse/Human Id1 (#BCH-1/195-14; BioCheck); Mxi1/MAD2 (#A300-301A; Bethyl Laboratories, Montgomery, TX, USA); Myc-Tag (#2276; Cell Signaling Technology, Danvers, MA, USA); HK2 (#2867; Cell Signaling Technology); Aldolase A (#8060; Cell Signaling Technology); ENO1 (#3810; Cell Signaling Technology); Enolase 2 (#8171; Cell Signaling Technology); PDHK1 (#3820; Cell Signaling Technology); PFKB2 (#13045; Cell Signaling Technology); PFKB3 (#13123; Cell Signaling Technology); PFKL (#8175; Cell Signaling Technology); PGAM1 (phosphoglycerate mutase 1; #12098; Cell Signaling Technology); Phospho-c-Myc (Ser62) (#13748; Cell Signaling Technology); LDHA (#2012; Cell Signaling Technology); Glut1 (#32551; Abcam, Cambridge, MA, USA); Hif1α (hypoxia-inducible factor 1α; #MAB1536; R&D Systems, Minneapolis, MN, USA); c-Myc (#62928; Abcam); CoxIV (cytochrome c oxidase subunit IV; #16056; Abcam); ERK1/2 (#4695; Cell Signaling Technology); pERK1/2 (#4370; Cell Signaling Technology); HK2 (#2867S; Cell Signaling Technology); nuclear respiratory factor 2 (NRF2) (#sc-722; Santa Cruz Biotechnology); NRF1 (#sc-33771; Santa Cruz Biotechnology); Lamin A + C (#ab108922; Abcam); and β-actin (#A5441; Sigma-Aldrich). All the antibodies were used at a dilution of 1:1000 except Id1, which was used at 1:500 dilution, c-Myc at 1:4000 dilution, and β-actin at 1:10,000 dilution. The following IRDye-conjugated secondary antibodies were used: donkey anti-mouse IRDye 800CW (#926-32212), and donkey anti-rabbit IRDye 800CW (#926-32213; both from Li-Cor Biosciences, Lincoln, NE, USA). Li-Cor Biosciences Odyssey Classic Imager was used to detect Western blots. For coimmunoprecipitation assays (Id1/c-Myc), 750 μg protein from whole-cell lysates and 10 μl Protein A/G Plus-agarose immunoprecipitation reagent (sc-2003; Santa Cruz Biotechnology) were used.
Statistical analysis
The quantitative data for the experiments were performed in triplicates, repeated 3 times, and are presented as means ± sd. Statistical analyses were performed by the 2-tailed Student’s t test, and a value of P < 0.05 was considered statistically significant.
RESULTS
Id1 is strongly expressed in liver tumor tissues and in HCC cell lines
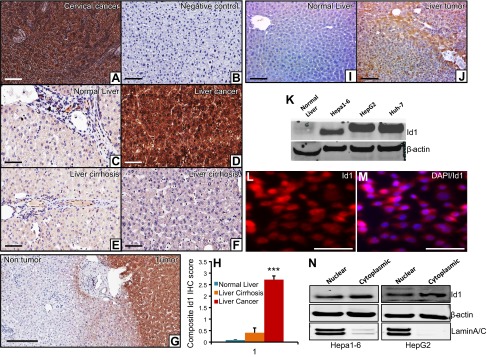
To determine whether Id1 has any role in liver tumorigenesis, we first measured the expression pattern of Id1 in normal liver and in human HCC samples by immunohistochemical (IHC) staining. We detected strong expression of Id1 in HCC samples compared with normal liver or liver cirrhosis tissues (Fig. 1A–G). To investigate whether Id1 is also expressed in liver tumors of mice, we induced liver tumors in C57BL/6J wild-type male mice by DEN and performed IHC staining of Id1 on nontumorous and liver tumor tissues. IHC staining revealed a strong expression of Id1 in the mouse liver tumor tissues, but no detectable staining in the normal liver of mice (Fig. 1H, I). Moreover, expression analysis of Id1 in different HCC cell lines, Hepa1-6 (mouse), HepG2 (human), and huh-7 (human), showed strong expression of Id1 in these cells compared with normal liver (Fig. 1J). In addition to Western blot analysis, immunocytochemical staining of Id1 in Hepa1-6 cells revealed strong expression of Id1 that appears to be mainly localized in the nucleus (Fig. 1K, L). To further confirm the localization pattern of Id1, we prepared cytoplasmic and nuclear protein fractions from Hepa1-6 and HepG2 cells, analyzed Id1 levels in each fraction, and detected the presence of Id1 in both compartments. Overall, our results suggest that Id1 might play a role in HCC.
Figure 1.
Id1 is strongly expressed in liver tumors. A) As a known positive control, a cervical cancer specimen that shows strong Id1 expression was used. B) As an experimental negative control, the antigen-binding site of the Id1 antibody was blocked by a specific blocking peptide. C) Normal liver section displays weak expression of Id1. D) Strong intense expression of Id1 in liver cancer specimen. E and F) Weak-to-moderate Id1 expression was detected in liver cirrhotic samples. Images were captured using the Olympus BX43X (Tokyo, Japan) at 200× magnification. G) Representative Id1 IHC staining photograph displays strong intense staining of Id1 in the human liver tumor tissue and very weak-to-no staining in the surrounding nontumorous tissue. H) Id1 expression was determined semiquantitatively by assessing the percentage of tumor cells and the staining intensity. Two pathologists analyzed all slides, and the percentage of tumor cells stained for Id1 was documented. Intensity of staining was graded as absent (0), weak (1), moderate (2), and strong (3). The percentage of positive cells was counted in a range of 0–100%. The product of the 2 scores was calculated as a composite Id1 IHC score. All the liver cancer cases showed strong (3+) expression of Id1 (n = 20) (D). In the cirrhotic livers, 10–85% of cells showed weak (1) staining for Id1 (n = 8 (E and F). In the normal liver sections, 5–10% of cells displayed weak (1) staining for Id1 (n = 8) (C). A comparison of Id1 expression in these 3 groups was shown as a composite Id1 IHC score. I and J) Representative photographs show no detectable Id1 expression in normal mouse liver (I) and strong Id1 expression in a liver tumor (n = 6) (J). K) Western blot shows the expression level of Id1 and β-actin in normal mouse liver and in 3 HCC cell lines. L and M) Immunocytochemical staining of Id1 in Hepa1-6 cells shows strong nuclear as well as some cytoplasmic staining. N) Western blot shows the expression level of Id1 in the nuclear and cytoplasmic compartments of Hepa1-6 and HepG2 cell lines. β-Actin is used as a loading control that is present in both compartments, whereas Lamin A/C was used as a protein fractionation quality control because it is present only in the nucleus. Scale bars, 200 µm (A–G, I, and J) and 100 µm (L and M).
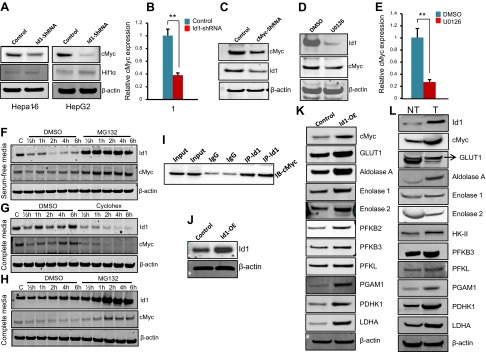
Id1 expression is regulated by the MAPK/ERK mitogenic pathway in HCC cells
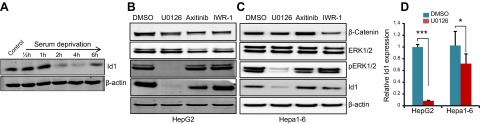
Id (inhibitor of DNA-binding/differentiation) proteins are very unstable, with a half-life of ∼40 min, and undergo rapid turnover facilitated by the ubiquitin–proteasome pathway (23). Because the Id1 protein undergoes rapid turnover, we asked whether Id1 expression is regulated by mitogen signaling in HCC cells. To explore this possibility, we serum deprived HepG2 cells and observed no changes in Id1 protein levels for up to 1 h of serum deprivation, but thereafter, Id1 rapidly disappeared (Fig. 2A), indicating that Id1 expression is dependent on the presence of mitogens. To investigate which specific mitogenic pathway is responsible for inducing the expression of Id1 in HCC cells, we treated HepG2 and Hepa1-6 cells with MEK (U0126), VEGF (axitinib), and Wnt/β-catenin (IWR-1-endo) inhibitors. We detected impaired ERK phosphorylation and down-regulation of Id1 in U0126-treated cells, whereas Id1 expression is not significantly affected in response to axitinib or IWR-1-endo–treated cells (Fig. 2B, C). These results suggest that the presence of pERK is necessary and that the MAPK/ERK pathway regulates Id1 expression in HCC cells. To further investigate whether the MAPK/ERK pathway regulates Id1 expression at the transcriptional or posttranscriptional level, we treated HepG2 and Hepa1-6 cells with U0126 and analyzed Id1 mRNA levels by qPCR and detected a significant reduction in Id1 mRNA levels in MEK inhibitor-treated cells compared with DMSO-treated cells (Fig. 2D). These results together suggest that enhanced expression of Id1 in HCC cells is due to constitutive transcriptional activation of Id1 regulated by the MAPK/ERK signaling pathway.
Figure 2.
MAPK/ERK signaling regulates Id1 expression. A) Western blot shows the expression level of Id1 and β-actin at the indicated time points after serum deprivation in HepG2 cells. B and C) Western blots show the expression levels of β-catenin, ERK1/2, pERK1/2, Id1, and β-actin in HepG2 (B) or Hepa1-6 (C) cells treated with MEK (U0126), VEGF (axitinib), and β-catenin (IWR-1-endo) inhibitors or DMSO for 24 h. D) Id1 mRNA transcript levels in HepG2 and Hepa1-6 cells after treatment with DMSO or U0126 for 24 h.
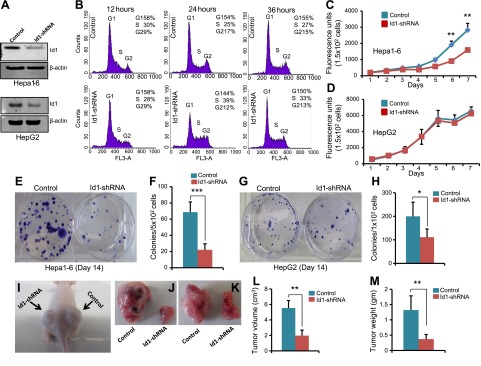
Id1 knockdown gradually suppresses cell proliferation and impairs colony formation
Strong expression of Id1 in liver tumors and in HCC cells prompted us to ask whether Id1 is required for the proliferation of cells. To investigate this, we knocked down Id1 in Hepa1-6 and HepG2 cell lines by shRNA. We achieved ∼80% of Id1 knockdown in Id1-shRNA cells compared with control cells (Fig. 3A). Analysis of cell cycle kinetics in response to Id1 knockdown revealed no clear differences in the percentage of cells in G1, S, and G2/M phases between control and Id1-shRNA cells (Fig. 3B), suggesting that Id1 knockdown does not cause abrupt cell cycle arrest. Next, we asked whether lack of Id1 impacts the short- or long-term proliferative capacity of HCC cells. We measured the overall proliferation rate of control and Id1-knockdown Hepa1-6 and HepG2 cells for up to 7 d by AlamarBlue proliferation assays and up to 14 d by colony formation assays. In Hepa1-6 cells, we did not detect any significant difference in proliferation until d 5, but the proliferation of Id1-knockdown cells started to decline between d 5 and 7 (Fig. 3C). In contrast, we did not detect any significant difference in the overall proliferation between control and Id1-knockdown HepG2 cells (Fig. 3D). However, we observed a significant reduction in the number of colonies formed by Id1-knockdown Hepa1-6 and HepG2 cells compared with control cells (Fig. 3E–H). Moreover, Id1 knockdown in HCC cells followed by subcutaneous xenograft in nude mice revealed a significant reduction in xenograft tumor growth in Id1-knockdown cells compared with control cells (Fig. 3I–M). Together, these results suggest that Id1 knockdown does not have an immediate effect on cell cycle initiation and progression, but in the long term, the proliferative capacity of HCC cells is gradually impaired in the absence of Id1.
Figure 3.
Id1 knockdown suppresses in vitro colony formation and in vivo tumor growth. A) Western blot shows the expression level of Id1 and β-actin in control and Id1-knockdown (Id1-shRNA) Hepa1-6 and HepG2 cells. B) Cell cycle profiles of Hepa1-6 cells measured by fluorescence-activated cell sorting (FACS) analysis. Id1 was knocked down by shRNA in Hepa1-6 cells. Fully confluent P2 cells were split into 1–3 plates, and the cell cycle profiles were measured by propidium iodide staining followed by FACS analysis at the indicated time points in control and Id1-shRNA cells. C and D) Line graphs show the overall proliferation rate of Hepa1-6 and HepG2 control and Id1-shRNA cells measured by AlamarBlue proliferation assay. E) Representative pictures of crystal violet-stained colonies formed by control and Id1-shRNA Hepa1-6 cells. F) Average number of colonies formed by control and Id1-shRNA Hepa1-6 cells. G) Representative pictures of crystal violet-stained colonies formed by control and Id1-shRNA HepG2 cells. H) Average number of colonies formed by control and Id1-shRNA HepG2 cells. I–K) Representative photographs show subcutaneous xenograft growth in nude mice (I) and the isolated tumors on d 15 (J and K). Control or Id1-shRNA Hepa1-6 cells (3 × 106) were injected subcutaneously into 7-wk-old nude mice. L and M) Bar graphs show tumor volume (L) and weight (M) on d 15 (n = 5). Experiments were done in triplicates and repeated 3 times. *P < 0.05; **P < 0.005; ***P < 0.0005.
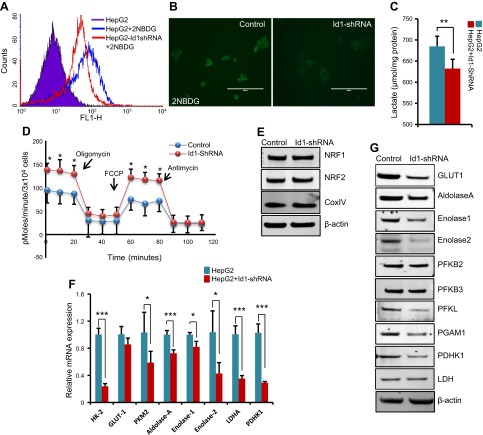
Id1 knockdown suppresses aerobic glycolysis
Knockdown of Id1 did not result in abrupt cell cycle arrest but rather caused a gradual impairment of HCC cell proliferation (Fig. 3B–H). Therefore, we reasoned that lack of Id1 causes certain changes in cells that ultimately result in impaired cell proliferation. In order to continuously proliferate, cancer cells reprogram their metabolism and depend on high rates of glycolysis and glutaminolysis to meet the demands of energy production and biomass accumulation (1, 2). We asked whether Id1 is required for metabolic reprograming and whether knockdown of Id1 could interfere with metabolic adaptation, leading to impaired HCC cell proliferation. To investigate this possibility, we first measured glucose uptake and lactate production rates between control and Id1-shRNA cells. By using the fluorescently labeled glucose analog 2-NBDG, we detected reduced glucose uptake in Id1-shRNA cells compared with control cells (Fig. 4A, B). Consequently, we detected significantly reduced lactate levels in Id1-shRNA cell cultures compared with control cells (Fig. 4C). These observations prompted us to ask whether Id1 knockdown causes the cells to switch from aerobic glycolysis to oxphos. Thus, we measured OCRs between the control and Id1-knockdown cells and detected significantly increased basal OCRs as well as maximum respiratory capacity in Id1-knockdown cells compared with control cells (Fig. 4D). To explore whether increased oxygen consumption in Id1-knockdown cells is due to an increase in mitochondrial content, we measured the expression levels of the mitochondrial marker protein CoxIV, and NRF1 and NRF2 that play important roles in mitochondrial biogenesis, and found no detectable differences between control and Id1-shRNA cells (Fig. 4E). These results together suggest that Id1 knockdown could suppress aerobic glycolysis and increase oxphos without affecting total mitochondrial content. To explore whether glycolytic activity is indeed reduced in response to Id1 knockdown, we analyzed the relative expression levels of mRNA transcripts encoding proteins involved in glycolysis by qPCR. We detected significant down-regulation of HK2, pyruvate kinase isozyme M2, Aldolase-A, ENO1, Enolase 2, LDHA, and PDHK1 in Id1-knockdown cells compared with control cells (Fig. 4F). Further expression analysis at the protein level revealed significant down-regulation of Glut1, Aldolase A, ENO1, Enolase 2, PFKL, and PGAM1 glycolytic enzymes in Id1-shRNA cells compared with control cells. We also detected significantly reduced levels of PDHK1, which promotes conversion of pyruvate to lactate, in Id1-shRNA cells compared with control cells (Fig. 4G). These results suggest that Id1 promotes aerobic glycolysis by regulating the expression levels of glycolytic enzymes, and knockdown of Id1 leads to reduced aerobic glycolysis due to diminished expression of glycolytic genes.
Figure 4.
Id1 knockdown increases oxygen consumption and reduces glycolytic gene expression. A) Glucose uptake in control HepG2 and Id1-shRNA HepG2 cells, measured by fluorescence-activated cell sorting following 30 min exposure to 2-NBDG. B) A small quantity of cells was also spread on glass slides and analyzed by fluorescence microscopy showing reduced 2-NBDG fluorescence in Id1-shRNA cells. Scale bar, 100 μm. C) Bar graph shows the amount of lactate secreted from HepG2 and Id1-shRNA HepG2 cells. D) OCR of control and Id1-shRNA HepG2 cells measured by the Seahorse Bioscience XF24 analyzer under basal conditions followed by sequential addition of oligomycin (1 µM), carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP; 0.3 µM), and antimycin. OCR is indicative of mitochondrial respiration (mean ± sd; n = 3). After measuring the basal OCR, oligomycin and FCCP were introduced to the cells. Oligomycin is an inhibitor of F1/F0 ATP synthase and blocks oxphos-linked respiration. Oligomycin-treated cells produce ATP only via glycolysis and, thus, provide a measure of maximum glycolytic potential. FCCP is a chemical uncoupler, and FCCP-stimulated oxygen consumption reflects the number and electron transport activity of mitochondria, providing a measure of maximum respiratory capacity. E) Western blot analysis of NRF1, NRF2, and CoxIV in control and Id1-shRNA HepG2 cells. β-Actin was used as loading control. F) mRNA levels of indicated genes in the control and Id1-shRNA HepG2 cells. G) Western blot analysis shows the expression levels of the indicated proteins in control and Id1-shRNA HepG2 cells. Expression data are means ± sd (n = 3). *P < 0.05; **P < 0.005; ***P < 0.0005.
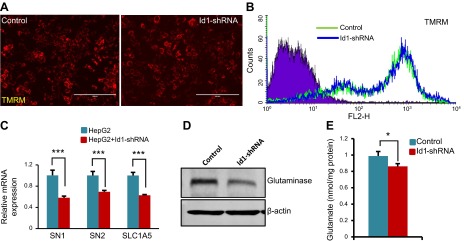
Id1 knockdown suppresses glutaminolysis
Cancer cells, in addition to glucose, also utilize large amounts of glutamine. Glutamine is metabolized by glutaminolysis, and the first step of this process is catalyzed by the enzyme GLS, which converts glutamine to glutamate (4). Cancer cells convert their mitochondria into biosynthetic machines and synthesize various macromolecules such as lipids, amino acids, and nucleotides by utilizing the metabolic intermediates of glutaminolysis (3). Because glucose uptake and glycolytic activity are reduced and oxphos is increased in response to Id1 knockdown, we asked whether glutaminolysis is also affected by lack of Id1. We first measured mitochondrial activity in control and Id1-knockdown cells by tetramethylrhodamine (TMRM), a cationic dye that is readily sequestered by active mitochondria. By both fluorescence microscopy and fluorescence-activated cell sorting analysis, we detected similar levels of TMRM staining in control and Id1-knockdown cells (Fig. 5A, B). To study whether Id1 knockdown affects glutamine uptake and metabolism, we measured expression levels of the glutamine transporters H. sapiens solute carrier family 38, member 3, H. sapiens solute carrier family 38, member 5, and solute carrier family 1 (neutral amino acid transporter), member 5 by qPCR and detected a significant reduction in the mRNA levels of all 3 glutamine transporters in Id1-knockdown cells compared with control cells (Fig. 5C). In addition, we also detected reduced GLS enzyme levels in Id1-shRNA cells compared with control cells (Fig. 5D). Consequently, we observed a reduction in cellular glutamate levels in Id1-shRNA cells compared with control cells (Fig. 5E). These results together suggest that lack of Id1 leads to reduced expression of glutamine transporters and GLS enzyme, leading to decreased glutamine uptake and metabolism in HCC cells.
Figure 5.
Reduced expression of glutamine receptors and glutamate production in Id1-knockdown cells. A) Representative TMRM-stained fluorescent mitochondrial images of control HepG2 and Id1-shRNA knockdown HepG2 cells. Scale bar, 100 μm. B) Fluorescence-activated cell sorting analysis of unstained HepG2 cells (violet) and TMRM-stained control and Id1-shRNA knockdown HepG2 cells. C) mRNA levels of indicated genes in control and Id1-shRNA HepG2 cells. D) Western blot shows the expression level of GLS and β-actin in control and Id1-knockdown HepG2 cells. E) Bar graph displays the levels of glutamate in control and Id1-shRNA HepG2 cells. Expression data are means ± sd (n = 3). *P < 0.05; ***P < 0.0005.
Id1 promotes metabolic adaptation by regulating c-Myc expression
We have identified that Id1 knockdown results in down-regulation of both glycolysis and glutaminolysis genes (Figs. 4 and 5). Id proteins are HLH proteins and directly bind to basic-helix-loop-helix (bHLH) transcription factors and regulate their transcriptional activity. Therefore, we reasoned that Id1 could regulate the transcription of glycolysis and glutaminolysis genes by regulating the expression and/or transcriptional activity of a bHLH transcription factor involved in the regulation of glycolysis and glutaminolysis genes and cancer cell metabolic reprogramming. A number of studies have demonstrated that c-Myc and Hif1α, both of which are bHLH proteins, regulate the expression of a large number of glycolysis genes under aerobic and anaerobic conditions, respectively (24). Therefore, we asked whether Id1 controls the expression of glycolysis and glutaminolysis genes by regulating c-Myc and/or Hif1α. To this end, we first analyzed the expression levels of c-Myc and Hif1α proteins in control and Id1-knockdown HCC cells and observed a relatively low expression of Hif1α and no detectable difference between control and Id1-knockdown cells (Fig. 6A). In contrast, we detected strong expression of c-Myc in control cells and a significant down-regulation of c-Myc in Id1-knockdown cells (Fig. 6A), suggesting that Id1 regulates c-Myc levels. Next, we asked whether Id1 regulates c-Myc at the transcriptional or posttranscriptional level. We analyzed c-Myc mRNA transcript levels by qPCR and detected a significant reduction in c-Myc mRNA levels in Id1-knockdown cells compared with control cells (Fig. 6B), suggesting that Id1 regulates c-Myc at the transcriptional level. Because c-Myc regulates the transcription of a myriad of genes regulating diverse cellular processes (25), we asked whether c-Myc also regulates Id1. Knockdown of c-Myc by shRNA in HepG2 cells led to down-regulation of Id1 (Fig. 6C), suggesting that c-Myc, in fact, can regulate Id1 expression. Because the MAPK/ERK pathway regulates Id1 expression (Fig. 2B–D), we asked whether the MAPK/ERK pathway functions upstream of both Id1 and c-Myc. We treated HepG2 cells with DMSO or the MEK inhibitor, U0126, and detected down-regulation of both Id1 and c-Myc proteins in U0126-treated cells compared with DMSO-treated cells (Fig. 6D). Similar to down-regulation of Id1 mRNA in response to U0126 (Fig. 2D), c-Myc mRNA levels were also significantly reduced in U0126-treated cells compared with DMSO treatment (Fig. 6E). Together, these results suggest that Id1 and c-Myc regulate each other in a positive feedback-loop mechanism and that the MAPK/ERK pathway functions upstream of both Id1 and c-Myc.
Figure 6.
Id1 knockdown leads to down-regulation of c-Myc. A) Western blot analysis of c-Myc and HIF1α in control and Id1-shRNA Hepa1-6 and HepG2 cells. β-Actin was used as loading control. B) mRNA levels of c-Myc in control and Id1-shRNA HepG2 cells. C) Western blot shows the protein levels of c-Myc and Id1 in control and c-Myc-shRNA HepG2 cells. D) Western blot shows the protein levels of Id1 and c-Myc in DMSO- or U0126-treated HepG2 cells. E) mRNA levels of c-Myc in DMSO- or U0126-treated cells. F) Western blot shows the protein levels of Id1 and c-Myc in DMSO- or MG132-treated HepG2 cells in serum-free medium. G) Western blot shows the protein levels of Id1 and c-Myc in DMSO- or cycloheximide (cyclohex)-treated HepG2 cells in complete medium. H) Western blot shows the protein levels of Id1 and c-Myc in DMSO- or MG132-treated HepG2 cells in complete medium. I) Coimmunoprecipitation followed by Western blot shows a direct interaction between Id1 and c-Myc. Ten percent immunoprecipitation reaction was used as input. J) Western blot analysis of Id1 and β-actin in control and Id1-overexpressed Hepa1-6 cells. K) Western blot analysis of indicated proteins in control and Id1-overexpressed Hepa1-6 cells. L) Representative Western blots show the expression pattern of indicated proteins in mouse nontumorous (NT) and liver tumor (T) tissues (n = 3). Expression data are means ± sd (n = 3). **P < 0.005.
Id1 and c-Myc are highly labile proteins with a half-life of ∼30 and ∼20 min, respectively, and undergo ubiquitin proteasome-mediated degradation (23, 26). Therefore, we asked whether Id1 and c-Myc are mostly regulated at the transcriptional level in HCC cells, or whether protein stability is also a method to control their regulation. To investigate this possibility, we performed a cycloheximide chase and MG132 proteasome inhibitor studies. When HepG2 cells were serum deprived and treated with MG132, we detected down-regulation of Id1 and c-Myc in the serum-deprived condition and accumulation of both Id1 and c-Myc as early as 30 min after treatment with MG132 (Fig. 6F). In addition, when we treated cells in complete medium with cycloheximide or MG132, we detected immediate down-regulation of Id1 and c-Myc in the cycloheximide-treated cells and accumulation of both Id1 and c-Myc in MG132-treated cells as early as 30 min after treatment (Fig. 6G, H), suggesting that the protein stability of Id1 and c-Myc is not significantly increased in HCC cells.
Id proteins are known to interact with bHLH transcription factors and negatively regulate their transcriptional activity (27). Because c-Myc is a bHLH protein, we asked whether Id1 directly interacts with c-Myc and affects its transcriptional activity. By coimmunoprecipitation, we have detected a direct interaction between Id1 and c-Myc (Fig. 6I). To investigate whether this Id1 interaction affects the transcriptional activity of c-Myc, we overexpressed mouse Id1 in Hepa1-6 cells and observed a significant increase in c-Myc expression levels and its downstream targets such as Glut1, Aldolase A, ENO1, Enolase 2, PGAM1, PDHK1, and LDHA (Fig. 6K). These results suggest that overexpression of Id1 induces c-Myc expression, and although Id1 directly binds to c-Myc, it does not suppress c-Myc transcriptional activity because the majority of c-Myc downstream target gene expression is strongly induced in Id1-overexpressed cells. Moreover, not only in HCC cell lines but also in human and mouse liver tumors, Id1 is strongly expressed (Fig. 1). Therefore, we asked how c-Myc and its downstream target genes are expressed in liver tumors where Id1 expression is very high and in nontumorous tissues where Id1 expression is very low. Our analysis revealed increased levels of c-Myc and several c-Myc downstream target genes in mouse liver tumors compared with surrounding nontumorous tissues (Fig. 6L), suggesting that c-Myc and its downstream target gene expression coincide with Id1 expression.
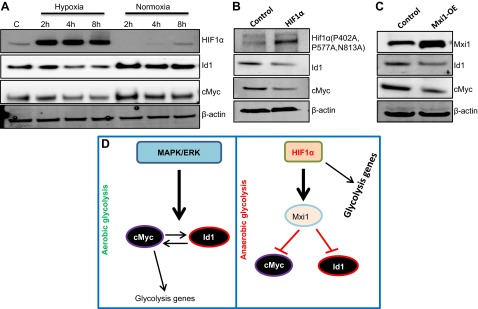
Hif1α down-regulates Id1 and c-Myc through Mxi1 under anaerobic conditions
In HCC cells, under aerobic conditions, the expression levels of Id1 and c-Myc are very high, whereas the Hif1α level is very low (Figs. 3A and 6A). Therefore, we were unable to investigate the existence of any possible regulatory mechanisms between Id1 and Hif1α. To evaluate whether Id1 regulates Hif1α or vice versa, we exposed HCC cells to hypoxia because it is well established that Hif1α is strongly induced in hypoxic conditions (28). As expected, we observed strong induction of Hif1α as early as 2 h after exposing the cells to hypoxia and an immediate down-regulation when cells were transferred back to normoxia (Fig. 7A). Most interestingly, in contrast to Hif1α, both Id1 and c-Myc were down-regulated in hypoxia and strongly induced when cells were transferred from hypoxia to normoxia. These observations prompted us to ask whether Id1 and c-Myc are sensitive to oxygen or Hif1α directly suppresses both Id1 and c-Myc. To this end, we overexpressed an oxygen-insensitive form of Hif1α under normoxic conditions in HepG2 cells and detected down-regulation of both Id1 and c-Myc, suggesting that under anaerobic conditions, Hif1α down-regulates Id1 and c-Myc. Previously, it was shown that Hif1α down-regulates c-Myc by recruiting Mxi1, which suppresses expression of c-Myc (29, 30). Therefore, we asked whether Mxi1 also suppresses the expression of Id1. We observed a down-regulation of both Id1 and c-Myc in response to Mxi1 overexpression in HepG2 cells. Together, these results suggest that unlike in aerobic glycolysis where Id1 induces c-Myc expression and promotes glycolytic activity, Id1 may not have a significant role in anaerobic glycolysis because Id1 is down-regulated by Hif1α via Mxi1.
Figure 7.
Hif1α down-regulates Id1 and c-Myc. A) Western blot analysis of HIF1α, Id1, and c-Myc in hypoxic and normoxic conditions. B) Western blots display the expression of oxygen-insensitive HIF1α detected by Myc-tag antibody, Id1, and c-Myc in control and Hif1α-overexpressed HepG2 cells. C) Western blots show Mxi1, Id1, and c-Myc proteins in control and Mxi1-overexpressed HepG2 cells. β-Actin was used as loading control. D) According to our proposed model, the MAPK/ERK pathway functions upstream to Id1 and c-Myc. Once induced, Id1 and c-Myc positively regulate each other’s expression in a positive feedback-loop mechanism to promote aerobic glycolysis in HCC cells. Under hypoxic conditions, Hif1α suppresses both Id1 and c-Myc by recruiting Mxi1, thereby controlling anaerobic glycolysis.
DISCUSSION
Id1 has been known to play essential roles in cell proliferation, cellular differentiation, cell fate determination, hematopoiesis, and neurogenesis (31–33). In the current work, we have demonstrated that Id1 controls aerobic glycolysis and glutaminolysis in HCC cells. We detected strong expression of Id1 in human and mouse liver tumor tissues and in HCC cell lines, and its expression is mainly regulated by the MAPK/ERK pathway at the transcriptional level. Previously, in osteosarcoma, it was shown that the stability of Id1 protein is increased by a ubiquitin-specific peptidase 1–mediated deubiquitination mechanism (34). However, in HCC cells, we observed immediate down-regulation of Id1 by cycloheximide chase experiments, suggesting that the stability of Id1 is not significantly extended in HCC cells. These observations highlight the existence of alternative Id1 regulatory mechanisms in different cancer cells.
Cancer cells exhibit increased metabolic activity compared with normal cells by predominantly utilizing glucose and glutamine to support their unrestrained growth and survival. This dependency on high rates of glucose uptake, lactate production, and glutamine utilization provides bioenergetic and biosynthetic advantages (1, 2, 4). We demonstrated that knockdown of Id1 by shRNA in HCC cells resulted in reduced glucose uptake and lactate production and decreased expression of various glycolytic enzymes both at the mRNA and protein level. These observations suggest that Id1 promotes aerobic glycolysis in HCC cells, and knockdown of Id1 results in suppression of glycolytic activity. Consequently, the OCR, which is an indicator of mitochondrial respiration, is increased in Id1-knockdown cells, suggesting that the cells switched from aerobic glycolysis to oxphos in response to Id1 knockdown. Although cancer cells typically switch to aerobic glycolysis, they still depend on sustained mitochondrial activity, and glutamine catabolism (glutaminolysis) maintains mitochondrial integrity and the NADPH levels necessary for redox homeostasis and macromolecular synthesis (2, 4). We demonstrated that, in addition to glycolysis, Id1 knockdown resulted in reduced expression of glutamine transporters and GLS enzyme leading to decreased glutamate production, suggesting that glutaminolysis is also suppressed in response to loss of Id1. As a result, the growth of HCC cells is significantly impaired in vitro as well as in vivo. Interestingly, how quickly the metabolic changes effect cell proliferation appears to differ from one cell type to the other because the growth of Hepa1-6 cells started to decline earlier than HepG2 cells in vitro.
Another important question we have answered is how Id1 regulates various enzymes and transporters that participate in glycolysis and glutaminolysis. One of the central regulators of aerobic glycolysis and glutaminolysis is c-Myc, which activates the transcription of various glycolytic and glutaminolysis genes both directly and indirectly via posttranscriptional mechanisms (6, 35–37). Conversely, Hif1α predominantly regulates glycolysis under anaerobic conditions (24, 38). Both c-Myc and Hif1α are bHLH transcription factors. Id proteins are known to bind to and negatively regulate bHLH transcription factors (7, 31, 39). Because c-Myc and Hif1α are bHLH proteins, it is entirely possible that Id1 directly interacts with either c-Myc or Hif1α or both, thereby directly controlling their transcriptional activity. Thus, Id1 could indirectly influence glycolysis and glutaminolysis in cancer cells. Under aerobic conditions, Hif1α expression is extremely low; nevertheless, we observed strong expression of c-Myc in both HCC cell lines. By coimmunoprecipitation, we detected a direct interaction between Id1 and c-Myc. Surprisingly, however, Id1 binding to c-Myc does not appear to have any negative effect on the transcriptional activity of c-Myc. This is because the overexpression of Id1 in HCC cells resulted in induced expression of not only c-Myc but also c-Myc’s downstream target genes Glut1, PFK, ENO1, PGK1, LDHA, and PDHK1, indicating that the Id1 interaction with c-Myc does not suppress c-Myc transcriptional activity. Furthermore, c-Myc protein levels were positively correlated with Id1 protein levels. Knockdown of Id1 led to down-regulation of c-Myc, whereas overexpression of Id1 resulted in increased expression of c-Myc, suggesting that Id1 regulates c-Myc expression. Most interestingly, knockdown of c-Myc resulted in down-regulation of Id1, indicating the existence of a positive feedback-loop regulatory mechanism between Id1 and c-Myc. Overall, our data suggest that the MAPK/ERK pathway functions upstream to both Id1 and c-Myc; however, whether MAPK/ERK signaling first induces Id1 or c-Myc is unclear because Id1 and c-Myc positively induce each other’s expression, and knockdown of one leads to down-regulation of the other. Together, our results reveal a novel mutually cooperative positive regulatory mechanism between Id1 and c-Myc in HCC cells. The feedback loop between Id1 and c-Myc appears to be essential to promote aerobic glycolysis because knockdown of Id1 breaks down this feedback loop, leading to down-regulation of c-Myc and suppression of aerobic glycolysis (Fig. 7D). Under anaerobic conditions, Hif1α is known to promote c-Myc degradation and suppresses c-Myc expression by recruiting Mxi1 (30, 40). Interestingly, we found that, similar to c-Myc, Id1 is down-regulated under hypoxic conditions, and Hif1α promotes Id1 down-regulation by essentially utilizing the mechanism, via Mxi1. Thus, it appears that Id1 and c-Myc, by positively regulating each other’s expression, regulate aerobic glycolysis and glutaminolysis, whereas under anaerobic conditions, Hif1α suppresses both Id1 and c-Myc in order to take over the regulation of glycolysis (Fig. 7D). In conclusion, our study indicates that Id1 could potentially serve as a prognostic marker for human HCC. Moreover, our results demonstrate a novel function of Id1 in cancer cell metabolic adaptation and highlight the existence of a novel regulatory mechanism between Id1 and c-Myc to promote glycolysis.
Acknowledgments
The authors thank Jingling Yuan, Ashis Mondal, and Kimya Jones for technical help and Dr. Rhea-Beth Markowitz for reviewing and editing the manuscript. This research is supported by a grant from the U.S. National Institutes of Health (NIH) National Cancer Institute Grant K22CA168828 to A.S. and NIH National Heart, Lung, and Blood Institute Grant HL060190 to S.M.B. The authors declare no conflicts of interest.
Glossary
- 2-NBDG
2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose
- bHLH
basic-helix-loop-helix
- CoxIV
cytochrome c oxidase subunit IV
- DEN
diethylnitrosamine
- ENO1
enolase 1
- FBS
fetal bovine serum
- GLS
glutaminase
- Glut1
glucose transporter 1
- HCC
hepatocellular carcinoma
- Hif1α
hypoxia-inducible factor 1α
- HK2
hexokinase 2
- HLH
helix-loop-helix
- Id
inhibitor of DNA-binding/differentiation
- Id1
inhibitor of differentiation 1
- IHC
immunohistochemistry
- LDHA
lactase dehydrogenase A
- NRF1
nuclear respiratory factor 1
- NRF2
nuclear respiratory factor 2
- OCR
oxygen consumption rate
- oxphos
oxidative phosphorylation
- P2
passage 2
- PDK1
pyruvate dehydrogenase kinase, isozyme 1
- PFK
phosphofructokinase
- PGAM1
phosphoglycerate mutase 1
- PGK1
phosphoglycerate kinase 1
- qPCR
quantitative PCR
- shRNA
short hairpin RNA
- TMRM
tetramethylrhodamine
REFERENCES
- 1.DeBerardinis R. J., Lum J. J., Hatzivassiliou G., Thompson C. B. (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7, 11–20 [DOI] [PubMed] [Google Scholar]
- 2.Dang C. V. (2012) Links between metabolism and cancer. Genes Dev. 26, 877–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn C. S., Metallo C. M. (2015) Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab. 3, 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hensley C. T., Wasti A. T., DeBerardinis R. J. (2013) Glutamine and cancer: cell biology, physiology, and clinical opportunities. J. Clin. Invest. 123, 3678–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine A. J., Puzio-Kuter A. M. (2010) The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330, 1340–1344 [DOI] [PubMed] [Google Scholar]
- 6.Miller D. M., Thomas S. D., Islam A., Muench D., Sedoris K. (2012) c-Myc and cancer metabolism. Clin. Cancer Res. 18, 5546–5553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton J. D. (2000) ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J. Cell Sci. 113, 3897–3905 [DOI] [PubMed] [Google Scholar]
- 8.Perk J., Iavarone A., Benezra R. (2005) Id family of helix-loop-helix proteins in cancer. Nat. Rev. Cancer 5, 603–614 [DOI] [PubMed] [Google Scholar]
- 9.Nam H. S., Benezra R. (2009) High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell 5, 515–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry S. S., Zhao Y., Nie L., Cochrane S. W., Huang Z., Sun X. H. (2007) Id1, but not Id3, directs long-term repopulating hematopoietic stem-cell maintenance. Blood 110, 2351–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manrique I., Nguewa P., Bleau A. M., Nistal-Villan E., Lopez I., Villalba M., Gil-Bazo I., Calvo A. (2015) The inhibitor of differentiation isoform Id1b, generated by alternative splicing, maintains cell quiescence and confers self-renewal and cancer stem cell-like properties. Cancer Lett. 356(2 Pt B), 899–909 [DOI] [PubMed] [Google Scholar]
- 12.Maruyama H., Kleeff J., Wildi S., Friess H., Büchler M. W., Israel M. A., Korc M. (1999) Id-1 and Id-2 are overexpressed in pancreatic cancer and in dysplastic lesions in chronic pancreatitis. Am. J. Pathol. 155, 815–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schindl M., Oberhuber G., Obermair A., Schoppmann S. F., Karner B., Birner P. (2001) Overexpression of Id-1 protein is a marker for unfavorable prognosis in early-stage cervical cancer. Cancer Res. 61, 5703–5706 [PubMed] [Google Scholar]
- 14.Ouyang X. S., Wang X., Lee D. T., Tsao S. W., Wong Y. C. (2002) Over expression of ID-1 in prostate cancer. J. Urol. 167, 2598–2602 [PubMed] [Google Scholar]
- 15.Wice B. M., Gordon J. I. (1998) Forced expression of Id-1 in the adult mouse small intestinal epithelium is associated with development of adenomas. J. Biol. Chem. 273, 25310–25319 [DOI] [PubMed] [Google Scholar]
- 16.Kim D., Peng X. C., Sun X. H. (1999) Massive apoptosis of thymocytes in T-cell-deficient Id1 transgenic mice. Mol. Cell. Biol. 19, 8240–8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner K., Zhang P., Rosenbauer F., Drescher B., Kobayashi S., Radomska H. S., Kutok J. L., Gilliland D. G., Krauter J., Tenen D. G. (2006) Absence of the transcription factor CCAAT enhancer binding protein alpha results in loss of myeloid identity in bcr/abl-induced malignancy. Proc. Natl. Acad. Sci. USA 103, 6338–6343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wassmann K., Liberal V., Benezra R. (2003) Mad2 phosphorylation regulates its association with Mad1 and the APC/C. EMBO J. 22, 797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu C. J., Sataur A., Wang L., Chen H., Simon M. C. (2007) The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol. Biol. Cell 18, 4528–4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popov N., Wanzel M., Madiredjo M., Zhang D., Beijersbergen R., Bernards R., Moll R., Elledge S. J., Eilers M. (2007) The ubiquitin-specific protease USP28 is required for MYC stability. Nat. Cell Biol. 9, 765–774 [DOI] [PubMed] [Google Scholar]
- 21.Satyanarayana A., Hilton M. B., Kaldis P. (2008) p21 Inhibits Cdk1 in the absence of Cdk2 to maintain the G1/S phase DNA damage checkpoint. Mol. Biol. Cell 19, 65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed S. A., Gogal R. M. Jr, Walsh J. E. (1994) A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170, 211–224 [DOI] [PubMed] [Google Scholar]
- 23.Bounpheng M. A., Dimas J. J., Dodds S. G., Christy B. A. (1999) Degradation of Id proteins by the ubiquitin-proteasome pathway. FASEB J. 13, 2257–2264 [PubMed] [Google Scholar]
- 24.Gordan J. D., Thompson C. B., Simon M. C. (2007) HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell 12, 108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eilers M., Eisenman R. N. (2008) Myc’s broad reach. Genes Dev. 22, 2755–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sears R. C. (2004) The life cycle of C-myc: from synthesis to degradation. Cell Cycle 3, 1133–1137 [PubMed] [Google Scholar]
- 27.O’Toole P. J., Inoue T., Emerson L., Morrison I. E., Mackie A. R., Cherry R. J., Norton J. D. (2003) Id proteins negatively regulate basic helix-loop-helix transcription factor function by disrupting subnuclear compartmentalization. J. Biol. Chem. 278, 45770–45776 [DOI] [PubMed] [Google Scholar]
- 28.Wang G. L., Jiang B. H., Rue E. A., Semenza G. L. (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92, 5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corn P. G., Ricci M. S., Scata K. A., Arsham A. M., Simon M. C., Dicker D. T., El-Deiry W. S. (2005) Mxi1 is induced by hypoxia in a HIF-1-dependent manner and protects cells from c-Myc-induced apoptosis. Cancer Biol. Ther. 4, 1285–1294 [DOI] [PubMed] [Google Scholar]
- 30.Zhang H., Gao P., Fukuda R., Kumar G., Krishnamachary B., Zeller K. I., Dang C. V., Semenza G. L. (2007) HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11, 407–420 [DOI] [PubMed] [Google Scholar]
- 31.Lasorella A., Uo T., Iavarone A. (2001) Id proteins at the cross-road of development and cancer. Oncogene 20, 8326–8333 [DOI] [PubMed] [Google Scholar]
- 32.Zebedee Z., Hara E. (2001) Id proteins in cell cycle control and cellular senescence. Oncogene 20, 8317–8325 [DOI] [PubMed] [Google Scholar]
- 33.Sikder H. A., Devlin M. K., Dunlap S., Ryu B., Alani R. M. (2003) Id proteins in cell growth and tumorigenesis. Cancer Cell 3, 525–530 [DOI] [PubMed] [Google Scholar]
- 34.Williams S. A., Maecker H. L., French D. M., Liu J., Gregg A., Silverstein L. B., Cao T. C., Carano R. A., Dixit V. M. (2011) USP1 deubiquitinates ID proteins to preserve a mesenchymal stem cell program in osteosarcoma. Cell 146, 918–930 [DOI] [PubMed] [Google Scholar]
- 35.Gao P., Tchernyshyov I., Chang T. C., Lee Y. S., Kita K., Ochi T., Zeller K. I., De Marzo A. M., Van Eyk J. E., Mendell J. T., Dang C. V. (2009) c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458, 762–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shim H., Dolde C., Lewis B. C., Wu C. S., Dang G., Jungmann R. A., Dalla-Favera R., Dang C. V. (1997) c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc. Natl. Acad. Sci. USA 94, 6658–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osthus R. C., Shim H., Kim S., Li Q., Reddy R., Mukherjee M., Xu Y., Wonsey D., Lee L. A., Dang C. V. (2000) Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem. 275, 21797–21800 [DOI] [PubMed] [Google Scholar]
- 38.Dang C. V., Kim J. W., Gao P., Yustein J. (2008) The interplay between MYC and HIF in cancer. Nat. Rev. Cancer 8, 51–56 [DOI] [PubMed] [Google Scholar]
- 39.Benezra R., Davis R. L., Lassar A., Tapscott S., Thayer M., Lockshon D., Weintraub H. (1990) Id: a negative regulator of helix-loop-helix DNA binding proteins. Control of terminal myogenic differentiation. Ann. N. Y. Acad. Sci. 599, 1–11 [DOI] [PubMed] [Google Scholar]
- 40.Huang L. E. (2008) Carrot and stick: HIF-alpha engages c-Myc in hypoxic adaptation. Cell Death Differ. 15, 672–677 [DOI] [PubMed] [Google Scholar]