Abstract
Stability of endothelial cell (EC) adherens junctions (AJs) is central for prevention of tissue edema, the hallmark of chronic inflammatory diseases including acute respiratory distress syndrome. Here, we demonstrate a previously unsuspected role of sphingosine kinase 1 (SPHK1) in the mechanism by which transient receptor potential channel 1 (Trpc1)-mediated Ca2+ entry destabilizes AJs. Trpc1−/− monolayers showed a 2.2-fold increase in vascular endothelial (VE)-cadherin cell-surface expression above wild-type (WT) monolayers. Thrombin increased endothelial permeability (evident by a 5-fold increase in interendothelial gap area and 60% decrease in transendothelial electrical resistance) in WT but not Trpc1−/− ECs. Trpc1−/− mice resisted the hyperpermeability effects of the edemagenic agonists used and exhibited 60% less endotoxin-induced mortality. Because sphingosine-1-phosphate (S1P) strengthens AJs, we determined if TRPC1 functioned by inhibiting SPHK1 activity, which generates S1P. Intriguingly, Trpc1−/− ECs or ECs transducing a TRPC1-inactive mutant showed a 1.5-fold increase in basal SPHK1 expression compared with WT ECs, resulting in a 2-fold higher S1P level. SPHK1 inhibitor SK1-I decreased basal transendothelial electrical resistance more in WT ECs (48 and 72% reduction at 20 and 50 μM, respectively) than in Trpc1−/− ECs. However, SK1-I pretreatment rescued thrombin-induced EC permeability in Trpc1−/− ECs. Thus, TRPC1 suppression of basal SPHK1 activity enables EC-barrier destabilization by edemagenic agonists.—Tauseef, M., Farazuddin, M., Sukriti, S., Rajput, C., Meyer, J. O., Ramasamy, S. K., Mehta, D. Transient receptor potential channel 1 maintains adherens junction plasticity by suppressing sphingosine kinase 1 expression to induce endothelial hyperpermeability.
Keywords: acute lung injury, thrombin, TRPC, VE-cadherin
The vascular endothelium, forming the innermost layer of all blood vessels, has the vital task of supplying nutrients to the underlying tissue while maintaining normal tissue–fluid homeostasis and tissue surveillance by leukocytes (1–5). Adherens junctions (AJs) formed by tethering of vascular endothelial (VE)-cadherin between contiguous endothelial cells (ECs) play a key role in maintaining endothelial barrier integrity. AJs achieve this goal by selectively controlling the transendothelial flux of a wide range of molecules such as electrolytes, ions, and proteins (4–8). Loss of AJ function results in exudation of plasma proteins and inflammatory cells into the tissue, leading to chronic inflammatory diseases such as atherosclerosis and acute respiratory distress syndrome (3–5, 8). Cell-surface-localized VE-cadherin is a determinant of the AJs stability (7, 8). A balance between VE-cadherin endocytosis, recycling, and transcription dictates the amount of cell-surface VE-cadherin expressed (9). Edemagenic agonists, by reducing VE-cadherin cell-surface expression, destabilize AJs, resulting in endothelial hyperpermeability. However, the fundamental mechanisms regulating VE-cadherin cell-surface expression remain unclear.
An increase in intracellular Ca2+ induced by several inflammatory mediators such as thrombin, histamine, oxidants, and growth factors, is a well-known initial trigger for disruption of AJs (2, 5, 10–13). The TRPC (transient receptor potential canonical channel) family of ionic channels has been shown to play an important role in regulating Ca2+ entry in ECs (3, 5, 12, 14–17). TRPC1/4 induces Ca2+ entry in response to endoplasmic reticulum store depletion by inositol triphosphate (IP3) or thapsigargin (5, 14, 18, 19). TRPC3/6 mediates Ca2+ entry in a diacylglycerol (DAG)-dependent but store-independent fashion (3, 12, 14, 20). Evidence indicates that TRPC1/4 can disrupt AJs by inducing actin–myosin–driven endothelial contraction (5) or activating PKC-α (21). PKC-α can disrupt AJs via VE-cadherin endocytosis through phosphorylation of p120-catenin (22). Additionally, TRPC1 could interfere with the activation of Rac1-GTPase, which maintains VE-cadherin at the AJs in confluent endothelial monolayers (7, 23).
Sphingosine-1-phosphate (S1P) produced through phosphorylation of sphingosine by sphingosine kinase 1 (SPHK1) anneals AJs in a Rac1-dependent manner (1, 24–30). However, it remains unknown whether TRPC1-induced Ca2+ signaling mediates endothelial hyperpermeability by modulating VE-cadherin cell-surface expression and AJ stability through regulation of (SPHK1)-induced S1P generation. Here, we show that loss of TRPC1 augments cell-surface VE-cadherin expression and thereby prevents AJ disruption by thrombin or thapsigargin. Trpc1−/− mice resisted thrombin- and thapsigargin-induced hyperpermeability and exhibited 60% less mortality from endotoxin. Pharmacological inhibition of SPHK1 suffices to restore the hyperpermeability response in Trpc1−/− ECs, thus demonstrating a novel role of TRPC1 activity in down-regulating SPHK1 expression to optimize AJ stability and endothelial permeability increase.
MATERIALS AND METHODS
Animals
All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Illinois. A Trpc1−/− mice breeding pair in a C57Blk/6J background was initially obtained from National Institute of Environmental Health Sciences, National Institutes of Health (NIH; Bethesda, MD, USA). Trpc1−/− colony was maintained at the pathogen-free housing facility at University of Illinois at Chicago. C57Blk/6J mice acquired from The Jackson Laboratory (Bar Harbor, ME, USA) and bred at the University of Illinois at Chicago were used as wild-type (WT) controls. All experiments were done in 8- to 10-wk-old mice.
FuGene transfection reagent was procured from Promega (Madison, WI, USA). Anti–Alexa Fluor 488 antibodies and ProLong Gold antifade were obtained from Invitrogen (Carlsbad, CA, USA). Sphingosine kinase 1 inhibitor SK1-I was purchased from EnzoBiomol (Enzo Life Sciences, Farmingdale, NY, USA). Anti–VE-cadherin, anti-calcium release–activated calcium channel protein 1 (Orai1) anti-TRPC1 antibodies, and protein A/G agarose beads were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-STIM1 antibody was purchased from BD Biosciences (San Jose, CA, USA). Anti-TRPC4 and anti-TRPC6 antibodies were purchased from Everest Biotech (Upper Heyford, United Kingdom).
EC culture
Mouse lung ECs were isolated as described elsewhere (3, 17). Briefly, blood-free mouse lungs were minced, digested with collagenase at 37°C for 45 min, triturated, and centrifuged at 3000 rpm. Cell suspension was incubated with platelet-endothelial cell adhesion molecule 1–coated DynaBeads (Thermo Fisher Scientific, Grand Island, NY, USA) for 1 h, after which ECs were magnetically sorted. Isolated ECs were plated on a fibronectin-coated T-25 flask and cultured with DMEM containing endothelial growth supplement. Isolated mouse lung ECs were >90% pure (3).
Confocal staining
Cells stimulated with thrombin for the indicated times were fixed and incubated with the indicated antibody and counterstained with donkey anti-goat Alexa Fluor 488. Cells were viewed with a 63× 1.2 NA objective and a Zeiss LSM 510 confocal microscope (Carl Zeiss GmbH, Jena, Germany) (31).
Transfection of cells
cDNA was transduced into human pulmonary artery endothelial cells (HPAECs) by electroporation using an Amaxa nucleofector kit, (Lonza, Basel, Switzerland) according to the manufacturer’s protocol (3, 31).
Immunoprecipitation
Lungs or ECs were lysed in modified RIPA buffer, and equal amounts of protein were immunoprecipitated with appropriate antibodies overnight at 4°C, followed by the addition of protein A/G agarose beads for 4 h at 4°C, as described previously (3, 31).
RT-PCR
Total RNA was isolated from mouse lungs ECs using Trizol agent (Invitrogen) according to the manufacturer’s instructions. RNA was quantified spectrophotometrically and reverse transcribed using specific primers to determine the expression of various TRPC channels (3, 17, 20, 28).
Cytosolic Ca2+ measurements
An increase in intracellular Ca2+ was measured using the Ca2+-sensitive fluorescent dye Fura 2-AM as described elsewhere (3, 12).
Assessment of lung vascular permeability to albumin
Evans blue–conjugated albumin (EBA) (20 mg/kg) was injected retro-orbitally 30 min before euthanizing the mice to determine vascular permeability, as described previously (3, 28, 31, 32). Briefly, blood was obtained from the right ventricle of the heart into heparinized syringes, and plasma was separated. Lungs were homogenized. Lung lysates and plasma were incubated with 2 volumes of formamide for 18 h at 55 to 60°C, then centrifuged at 5000 g for 30 min. The optical density of the supernatant was determined spectrophotometrically at 620 nm (Evans blue) and 740 nm (hemoglobin correction). EBA extravasation was calculated as EBA influx in lung vs. that in plasma.
Measurement of transendothelial electrical resistance
WT or TRPC1-null ECs were seeded on 8-well gold-plated electrodes (Applied Biosciences, Carlsbad, CA, USA). After formation of monolayer, cells were serum deprived for 1 h, then treated with indicated inhibitors for a duration of 30 min to 2 h (for SK1-I) or 2 h (for pertussis toxin); basal resistances were recorded as described elsewhere (28, 31). Cells were then stimulated with 50 nM thrombin, and alterations in transendothelial electrical resistance (TER) were determined. Data were calculated by electric cell-substrate impedance sensing software (ECIS; Applied Biophysics, Inc., Troy, NY, USA) and normalized to baseline resistance.
Measurement of vessel filtration coefficient
Mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg body weight) and xylazin (15 mg/kg body weight). Lungs were collected, and microvessel permeability was determined in isogravimetric lungs by determining the microvascular filtration coefficient (Kf,c) (3, 28, 31, 33). Briefly, outflow pressure was elevated by 10 cm H2O for 20 min in isogravimetric perfused lungs. The lung wet weight gain during this time, which is the net fluid accumulation, was recorded. At the end of each experiment, lung dry weight was determined. Kf,c (ml ⋅ min−1 ⋅ cm H2O ⋅ g dry wt−1) was calculated from the slope of the recorded weight change normalized to the pressure change and lung dry weight.
Lung edema determination
Left lungs from the same mice used for Evans blue albumin extravasation were excised and completely dried in a 60°C oven overnight for calculation of lung wet–dry ratio (28, 34).
Measurement of sphingosine kinase activity
HPAECs or mouse lung ECs were homogenized in buffer [containing, in millimolar, 20 Tris (pH 7.4), 20% glycerol, 1 mercaptoethanol, 1 EDTA, 1 sodium orthovanadate, 40 β-glycerophosphate, 15 NaF, 1 phenylmethylsulfonyl fluoride, 0.5 4-deoxypyridoxine, and 10 µg/ml each of leupeptin, aprotinin, and soybean trypsin inhibitor]. Lysates containing 40 µg of protein were then mixed in a total volume of 190 µl homogenizing buffer plus 10 µl of 1 mM sphingosine and 10 µl of [32P] ATP (10 µCi, 20 mM), and SPHK activity was determined as described (28). Sphingosine kinase–specific activity was calculated and expressed as picomoles of S1P formed per minute (unit) per milligram of protein.
Analysis of S1P levels
HPAECs or mouse lung ECs were homogenized in buffer [containing, in millimolar, 20 PIPES, 150 NaCl, 1 EGTA, 1% (v/v) Triton X-100, 1.5 MgCl2, 0.1% SDS, 1 sodium orthovanadate, 1× protease inhibitor cocktail (without EDTA), pH 7], were applied to the ELISA plate at 30 µg protein per well. S1P levels were analyzed using a S1P competitive ELISA kit (Echelon Biosciences, Salt Lake City, UT, USA) according to the manufacturer’s instructions.
SPHK1 promoter activity
HPAECs were cotransfected with control vector or TRPC1-inactive mutant along with GeneCopoeia GLuc-On human SPHK1 promoter luciferase reporter vector (GeneCopoeia, Rockville, MD, USA). The Gaussia luciferase (GLuc) and secreted alkaline phosphatase activity were determined using secrete-pair dual luminescence assay kit (GeneCopoeia) as described in manufacturer’s protocol. GLuc activity was normalized taking secreted alkaline phosphatase activity as an internal control.
Statistical analysis
Comparisons between experimental groups were made by 1-way ANOVA and post hoc testing. Differences in mean values were considered significant at P < 0.05.
RESULTS
Loss of TRPC1 augments VE-cadherin cell surface expression and prevents disruption of AJs by thrombin
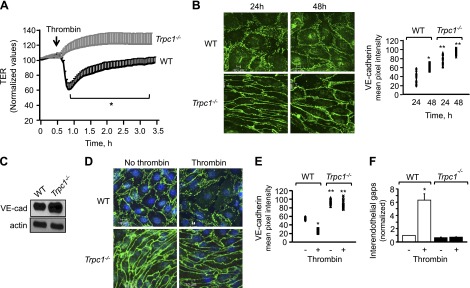
Given that Ca2+ entry plays an important role in disrupting AJs and thereby increasing endothelial permeability (5, 13, 16, 17, 35), we determined changes in TER, a dynamic assessment of AJ opening and closing (28, 31, 36), in EC monolayers isolated from WT or TRPC1-null mouse lungs. As expected, thrombin decreased TER by 60% in control ECs, indicating an increase in endothelial permeability (Fig. 1A). However, thrombin failed to decrease TER in TRPC1-deficient ECs; rather, we observed ∼20% increase in TER, which suggested that loss of TRPC1 strengthened AJs.
Figure 1.
Deletion of TRPC1 augments cell-surface VE-cadherin expression, which stabilizes AJs and prevents increase in endothelial permeability by thrombin. A) Changes in TER after stimulation of indicated ECs with thrombin. *Values lower than Trpc1−/− ECs; P < 0.05. B) In parallel, ECs were stained with anti–VE-cadherin antibody to determine AJs integrity. Top, Representative image of AJs at indicated times. Bottom, Mean ± sd of VE-cadherin cell surface pixel intensity; n = 3 per group. *Values higher than WT ECs at 24 h; **values higher than WT ECs at each of indicated times; P < 0.05. C) VE-cadherin protein expression in indicated ECs using anti-VE-cadherin antibody. Actin was used as loading control. Immunoblot represent data from experiments repeated at least 3 times. D–F) VE-cadherin cell-surface intensity and interendothelial gap formation after 5 min thrombin challenge of WT or TRPC1-null ECs. D) Representative image. E, F) Means ± sd of VE-cadherin pixel intensity and interendothelial gap formation from at least 10 individual cells per experiment. Each experiment was repeated at least 3 to 4 times. *Values different than WT ECs without thrombin or Trpc1−/− ECs; **values different than WT EC after thrombin stimulation or no thrombin stimulation; P < 0.05.
Next, we immunostained WT or TRPC1-null cells with an anti–VE-cadherin antibody to determine if loss of TRPC1 enhanced AJ stability, which in turn occluded the permeability-increasing effects of thrombin. Intriguingly, we found a 2.2-fold increase in cell-surface VE-cadherin expression in TRPC1-null cells compared with WT cells (Fig. 1B). We also confirmed a 2-fold increase in VE-cadherin expression by performing immunoblot analysis of TRPC1-null cells (Fig. 1C). Thrombin disrupted AJs, leading to a 5-fold increase in gap area in WT ECs (Figs. 1D–F). However, thrombin failed to induce gap formation in Trpc1−/− ECs (Figs. 1D–F). These findings demonstrate that TRPC1 down-regulates basal VE-cadherin cell-surface expression and thereby mediates the permeability increase induced by thrombin.
Loss of TRPC1 prevents increase in vascular permeability
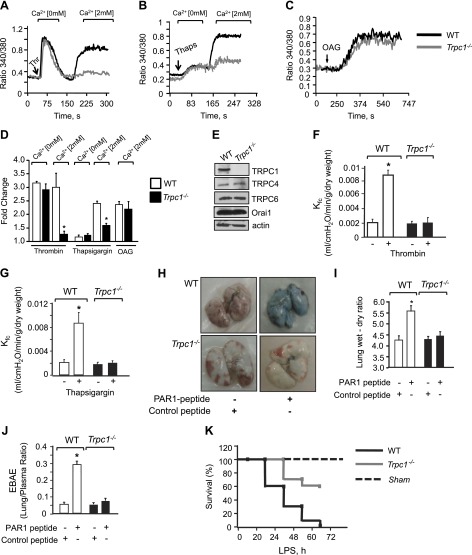
We next explored the in vivo relevance of TRPC1-mediated suppression of VE-cadherin expression and thereby AJ stability. We compared lung vascular permeability increase in WT and Trpc1−/− mice in response to thrombin or thapsigargin (3, 33). First, we confirmed in these studies that TRPC1 is required for regulation of Ca2+ entry induced upon endoplasmic reticulum store depletion because loss of TRPC1 did not alter thrombin- or thapsigargin-induced Ca2+ release from endoplasmic reticulum (Fig. 2A, B, D) but inhibited Ca2+ entry by ∼85% (Fig. 2A, B, D). Furthermore, loss of TRPC1 had no effect on Ca2+ entry induced by 1-oleoyl-2-acetyl-sn-glycerol, a cell-permeable analog of DAG (3) (Fig. 2C, D). The expression profile of TRPC4, TRPC6, and ORAI1 remained unchanged (Fig. 2E).
Figure 2.
Loss of TRPC1 impairs vascular permeability in vivo. Intracellular Ca2+ in response to thrombin (A) or thapsigargin (B) in Ca2+-free medium followed by re-addition of 2 mM extracellular Ca2+ to activate Ca2+ entry. C) Cells were activated using OAG in presence of 2 mM Ca2. Each representative tracing is average response of 20 to 25 cells. D) Plot showing means ± sd of fold change in steady-state intracellular Ca2+ after addition or without addition of extracellular Ca2+ in presence of indicated agonists; n = 4/group. *Values different from WT ECs; P < 0.05. E) Immunoblot showing expression of channels in WT or TRPC1-null cells using appropriate antibodies. Immunoblot with anti-actin antibody was used as loading control. Immunoblot represent data from multiple experiments. F, G) Kf,c in WT or Trpc1−/− mice lungs after perfusion with 10 µM PAR-1 agonist peptide (F) or 2 µM thapsigargin (G). Plot shows means ± sem from 3 to 4 individual experiments. *Significant increase in Kf,c; P < 0.05. H–J) Lung vascular permeability in response to PAR-1 agonist (1 mg/kg) or control peptide as determined by EBAE from lungs vs. plasma (H, I) and lung wet–dry ratio (J). H) Representative image of PAR-1-induced Evans blue accumulation in lungs. I and J) Means ± sem of changes in EBAE and lung edema from 3 to 4 individual experiments. *Significant increase from control peptide group. J) Mice were challenged intraperitoneally (40 mg/kg body weight) with LPS, and their survival was assessed by log-rank test, n = 10.
We next determined the Kf,c, an ex vivo measure of lung vascular permeability (28, 33), in TRPC1-null and WT murine lungs. Activation of thrombin receptor protease activating receptor 1 (PAR-1) by a selective PAR-1 agonist peptide produced a 4-fold increase in Kf,c in WT mouse lungs but failed to elicit any increase in Trpc1−/− lungs (Fig. 2F). Similarly, thapsigargin significantly increased Kf,c in WT lungs, but such a response was absent in Trpc1−/− lungs (Fig. 2G). In confirmatory in vivo studies, we quantified EBA extravasation (EBAE) into the lung parenchyma and lung wet–dry weight ratio to determine the role of TRPC1 in increasing lung vascular permeability after PAR-1 activation. Lung vascular albumin permeability was the same in WT and Trpc1−/− mice receiving a scrambled PAR-1 peptide (Fig. 2H–J). Intravenously delivered PAR-1 peptide produced edema in WT lungs (Fig. 2H, I) and increased EBAE (Fig. 2H, J). In the absence of TRPC1, the PAR-1 agonist failed to increase lung vascular permeability and edema formation (Fig. 2H–J).
Because sepsis can lead to lung injury, we next investigated whether the stabilized endothelial barrier in TRPC1-null mice prevents mortality caused by the endotoxin LPS. LPS (40 mg/kg i.p.) resulted in 90% mortality in WT mice within 50 h, while 80% of Trpc1−/− mice remained viable at this time and 60% of the TRPC1-null mice survived even after 72 h (Fig. 2K). Thus, TRPC1 loss increased mouse survival from sepsis.
TRPC1-mediated Ca2+ entry suppresses SPHK1 expression and S1P generation to modulate AJs stability
S1P generated through SPHK1-induced phosphorylation of sphingosine anneals AJs (24, 28, 37–39). We next addressed the possibility that loss of TRPC1 augments VE-cadherin cell-surface expression and AJ stability by increasing SPHK activity and S1P generation. Intriguingly, compared with WT cells, basal SPHK activity and S1P levels were increased by 8-fold and 2.5-fold, respectively, in Trpc1−/− ECs (Fig. 3A, B). Quantitative PCR analysis showed that TRPC1 deficiency indeed markedly increased SPHK1 expression at both the mRNA and protein levels (Fig. 3C, D). As a control, we also determined SPHK2 expression, which is a minor source of S1P generation in ECs (28, 37–39). As expected, TRPC1 deletion had no significant effect on SPHK2 expression (Fig. 3C, D).
Figure 3.
TRPC1 deletion induces SPHK1 expression and S1P generation. SPHK activity (A) or S1P generation (B) after stimulation with or without thrombin of WT and TRPC1-null ECs. C) SPHK mRNA expression in Trpc1−/− or WT EC. *Values different from WT ECs (P < 0.05). D) SPHK protein expression using specific antibodies. E) Changes in TER in ECs upon pertussis toxin addition and after stimulation with thrombin. *Values different from vehicle; **values different from vehicle-treated cells after thrombin challenge; P < 0.05.
SPHK1-induced S1P enhances barrier function by activating S1P receptor 1, which in turn acts through the heterotrimeric G protein Gi (28, 37–39). We reasoned that if S1P is the functional important mediator in the case of TRPC1-null ECs, then inhibition of Gi should abolish its barrier-enhancing effects in TRPC1-null cells. Thus, we inhibited Gi function using pertussis toxin in TRPC1-null cells and assessed changes in TER without or with thrombin exposure. We found that treatment of TRPC1-null ECs with pertussis toxin restored endothelial permeability increase to the levels seen in WT cells both under basal conditions and after thrombin challenge (Fig. 3E).
To address the possibility that TRPC1-mediated Ca2+ entry regulates SPHK1 expression and signaling, we transduced HPAECs with a TRPC1 pore-defective mutant, which cannot mediate Ca2+ entry (40). Transduction of the TRPC1 pore mutant attenuated store-operated calcium entry by 65% (Fig. 4A). As with TRPC1-null cells, expression of the TRPC1 pore mutant enhanced basal SPHK1 expression at the mRNA and protein levels and increased S1P levels (Fig. 4B–D). We also observed a 2-fold increase in VE-cadherin protein levels by immunoblot analysis (Fig. 4C). Impairment of TRPC1 activity additionally caused a 3-fold increase in SPHK1 promoter activity, indicating that loss of TRPC1 activity enhances SPHK1 transcription, resulting in S1P generation basally, which in turn strengthens AJs so as to prevent permeability increase by edemagenic agonists.
Figure 4.
TRPC1-mediated Ca2+ entry suppresses SPHK1 activity and S1P generation of S1P. Intracellular Ca2+ in response to thapsigargin in HPAECs transduced with dominant negative TRPC1 or control vector following incubation in Ca2+-free medium or 2 mM extracellular Ca2+. A) Plot shows means ± sd of fold increase in steady-state Ca2+ after addition or without addition of extracellular Ca2+ in presence of thapsigargin. *Significant increase in intracellular Ca2+, P < 0.05. B) Plot showing means ± sd of fold increase in SPHK1 and SPHK2 mRNA expression relative to GAPDH in HPAECs transducing indicated mutants; n = 3. C) SPHK1, SPHK2, and VE-cadherin levels in HPAECs transducing dominant negative TRPC1 or control vector. Actin was used as loading control. D) S1P levels in HPAECs expressing indicated mutants. *Values different from WT ECs; P < 0.05. E) SPHK1 promoter activity in HPAECs cotransfected along with dominant negative TRPC1 or control vector. *Values different from WT ECs; P < 0.05.
Up-regulated basal SPHK1 activity prevents endothelial permeability increase by thrombin in TRPC1-null ECs
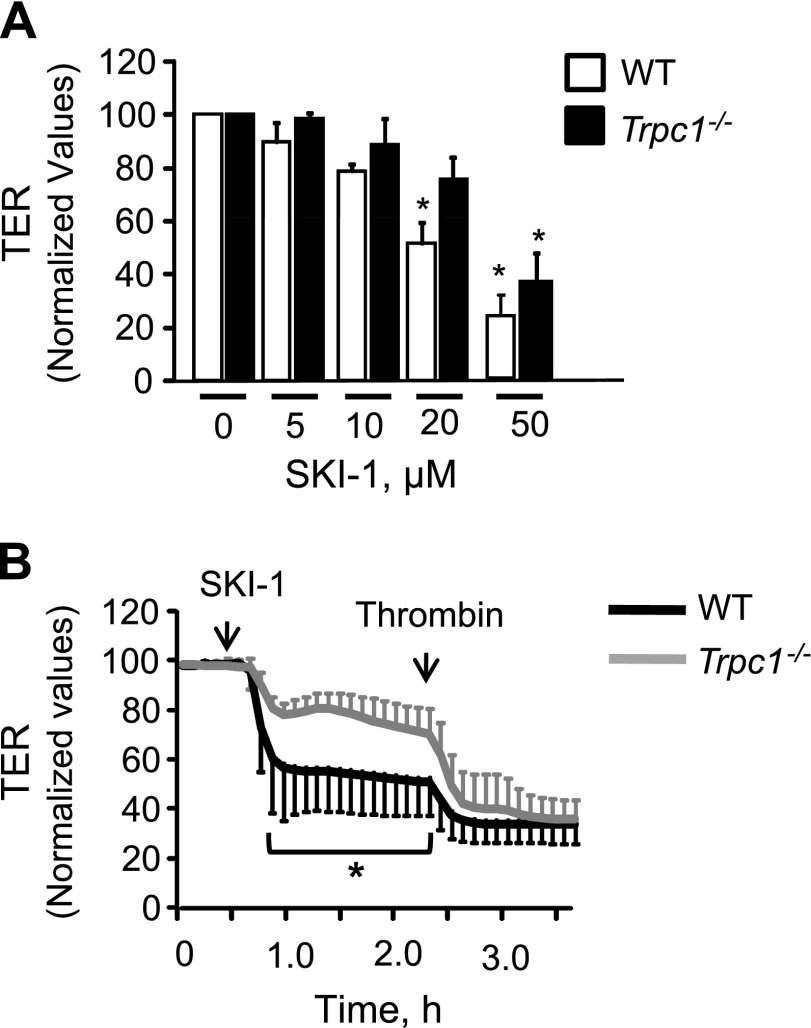
To address if basal SPHK1-induced S1P synthesis was sufficient to maintain EC permeability in the absence of agonists and to prevent agonist-induced permeability increase in TRPC1-null cells, we inhibited SPHK1 activity using SK1-I, a specific small molecule inhibitor of SPHK1 (41, 42). SK1-I has a Ki value of 10 μM. Thus, we pretreated Trpc1−/− or WT ECs with SK1-I at concentrations ranging from 5 to 50 μM, and changes in TER were determined. We found that treatment of WT ECs with 5 to 10 μM SK1-I produced no significant decrease in TER values (Fig. 5A). SK1-I produced a 48% drop in TER at 20 μM and a 72% reduction at 50 μM in WT ECs. In contrast, SK1-I only produced a 24% decrease in TER in TRPC1-null cells at 20 μM and a 63% decrease at 50 μM SK1-I, dovetailing with the findings above that TRPC1 suppresses basal SPHK1 activity (Fig. 5A). When we pretreated TRPC1-null ECs with 20 μM SK1-I, we found that resultant partial inhibition of SPHK1 was sufficient to restore thrombin’s effect on permeability to the level seen in WT cells (Fig. 5B).
Figure 5.
Inhibition of SPHK1 activity rescues endothelial barrier disruption in TRPC1-null ECs and lungs. A) Dose–response curve of SPHK1 inhibitor, SK1-I in altering TER. SK1-I was added onto WT or TRPC1-null ECs after stable baseline was achieved. Plot shows means ± sd of changes in TER values normalized to TER values at time zero. *Values different from WT ECs; P < 0.05. B) SK1-I treatment rescues thrombin-induced barrier dysfunction in TRPC1-null ECs. Thrombin was added after 2 h treatment with 20 μM SK1-I. *Values different from WT ECs; P < 0.05.
DISCUSSION
A new concept emerging from the present study is that TRPC1 activity holds SPHK1 constitutively in a suppressed state to enable edemagenic agonists to induce vascular leak. We showed that loss of TRPC1 in ECs enhanced SPHK1 expression and S1P generation in conjunction with increased VE-cadherin cell-surface expression, thereby preventing the permeability increase induced by thrombin or thapsigargin. Because these findings were recapitulated in HPAECs transducing the inactive TRPC1 pore mutant, we conclude that Ca2+ entry through TRPC1 was required to suppress SPHK1 expression. A causal relationship between TRPC1 activity and SPHK1 expression was indicated because inhibition of SPHK1 resulted in an increase in basal endothelial permeability in WT ECs and also rescued the permeability increase induced by edemagenic agonists in TRPC1-null ECs.
AJs formed by VE-cadherin driven homotypic adhesion between ECs maintain endothelial barrier function. AJ stability depends on the amount of cell-surface VE-cadherin expressed, which in turn is dictated by endocytosis, degradation, recycling, and de novo synthesis of cadherins (9, 22, 43–46). We showed that loss of TRPC1 enhanced VE-cadherin cell surface expression and overall VE-cadherin protein expression. On the basis of imaging studies, the induced protein was shown to localize at the cell surface, and importantly, VE-cadherin did not internalize after thrombin challenge. These findings suggest that in naive endothelium, TRPC1 is constitutively active to modulate VE-cadherin cell-surface levels in such a way that AJs remain susceptible to edemagenic agents.
The normal suppression of SPHK1 appears to be specific to the TRPC1 channel. Edemagenic agonists can activate TRPC via store-dependent or store-independent pathways through the second messenger’s inositol IP3 and DAG (12, 47). We showed that thrombin and thapsigargin failed to increase lung vascular permeability in TRPC1-null mice. We also showed that loss of TRPC1 spared DAG-induced Ca2+ entry. DAG specifically activates store-independent channels such as TRPC6 (3, 12, 48). In the present study, transduction of a TRPC1 pore mutant was able to induce SPHK1 expression, and inhibition of SPHK1 by SK1-I rescued the permeability defect in TRPC1-null ECs. Moreover, we showed that expression of TRPC4, TRPC6, and ORAI1 remained unaltered. Thus, these findings suggest that TRPC1 functioned by suppressing SPHK1 expression.
We also showed that loss of TRPC1 reduced mice mortality from LPS-induced sepsis. LPS disrupts barrier function and induces inflammatory signaling and sepsis by activating multiple cascades (3, 43, 49, 50). LPS is known to induce TRPC1 expression (51–54). On the basis of the inverse relationship between TRPC1 and SPHK1 activity emerging from current studies, we could argue that LPS, by increasing TRPC1 expression and thereby TRPC1 activity, will further dampen SPHK1 activity and signaling exaggerating barrier disruption and hence sepsis mortality. Thus, up-regulated SPHK1 expression and downstream stabilization of AJs in TRPC1-null mice was responsible for decreased mortality after sepsis.
Several studies showed that S1P enhances endothelial barrier function by strengthening AJs in a Gi- and Rac1-dependent manner (23, 24, 27, 28, 37). The blockade of Gi signaling in the present study with pertussis toxin is consistent with the concept that S1P enhanced AJ stability and thereby counteracted thrombin-induced permeability increase in TRPC1-deficient ECs in a Gi-dependent manner.
Both SPHK1 and SPHK2 induce S1P generation (26, 55). However, we previously showed that SPHK1 is primarily responsible for S1P generation in ECs and facilitates barrier recovery after injury by edemagenic agonists (28). Thus, we surmised that loss of TRPC1 may have stabilized AJ function by modulating SPHK1 activity. In support of this concept, we showed that ECs lacking TRPC1 exhibited increased basal SPHK1 activity and higher S1P levels. We also showed that thrombin increased barrier function in TRPC1-null ECs, consistent with our earlier observations that thrombin can activate SPHK1 and thereby S1P generation in ECs to promote barrier reannealing (28). We further showed that TRPC1-mediated Ca2+ entry was required to induce basal SPHK1 activity and S1P generation because expression of a pore-deleted TRPC1 mutant increased SPHK1 promoter activity, SPHK1 expression, and S1P levels. We also showed that SK1-I, a specific inhibitor of SPHK1 (41, 56–58), was comparatively less effective in disrupting basal barrier function in TRPC1-null ECs, especially at 20 µM, supporting the central concept that loss of TRPC1 augments basal SPHK1 expression and thereby increases AJs integrity. The question arose that preexisting S1P should be able to ligate S1PR1 and protect against barrier dysfunction even though no new S1P was being made as a result of inhibition of SPHK1 by SK1-I. Consistently, we showed that SK1-I could only impair barrier function in WT ECs at a dose much higher than its known Ki (41, 58), indicating that preexisting S1P was sufficient to prevent barrier dysfunction at lower doses of SK1-I. The advantage of using a pharmacologic approach was the ability to observe the immediate effects of altering SPHK1 activity on barrier function. Overall, these studies established the causality between TRPC1 expression and SPHK1 function because pharmacologic inhibition of SPHK1 activity was sufficient to rescue the permeability defect in TRPC1-null cells after PAR-1 activation.
The mechanism by which TRPC1 normally suppresses SPHK1 expression remains to be parsed out. Because TRPC1 deletion induced SPHK1 expression without affecting SPHK2 expression, we infer that a Ca2+-sensitive transcription factor must be involved in the control of SPHK1 expression. The SPHK1 promoter contains STAT-6, SP1, and EGR-2 binding sites (59). Future studies will be required to delineate whether TRPC1 activity suppresses SPHK1 expression by modulating these transcription factors.
In summary, our findings identify a key role of TRPC1-mediated Ca2+ entry in controlling basal SPHK1 expression and downstream S1P generation and VE-cadherin stability, thereby enabling edemagenic agonists to induce an increase in vascular permeability. Thus, targeting TRPC1 activity could provide a novel strategy for up-regulating S1P levels in pathologies complicated by leaky blood vessels.
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grants HL-71794, HL-84153, and HL-060678. The authors thank I. S Ambudkar (NIH) for providing TRPC1 mutant constructs and V. Natarajan (University of Illinois, Chicago, IL, USA) for his gift of SPHK1 luciferase promoter. The authors also thank L. Birnbaumer (NIH) and A. Dietrich (Walther-Straub-Institute for Pharmacology and Toxicology, Munich, Germany) for providing TRPC1−/− mice.
Glossary
- AJ
adherens junction
- DAG
diacylglycerol
- EBA
Evans blue–conjugated albumin
- EBAE
Evans blue–conjugated albumin extravasation
- EC
endothelial cell
- GLuc
Gaussia luciferase
- HPAEC
human pulmonary arterial endothelial cells
- Kf,c
microvascular filtration coefficient
- NIH
National Institutes of Health
- Orai1
calcium release-activated calcium channel protein
- PAR-1
protease activating receptor-1
- S1P
sphingosine-1-phosphate
- SPHK1
sphnigosine kinase 1
- TER
transendothelial electrical resistance
- TRPC1
transient receptor potential channel 1
- VE
vascular endothelial
- WT
wild-type
REFERENCES
- 1.Chavez A., Smith M., Mehta D. (2011) New insights into the regulation of vascular permeability. Int. Rev. Cell Mol. Biol. 290, 205–248 [DOI] [PubMed] [Google Scholar]
- 2.Sukriti S., Tauseef M., Yazbeck P., Mehta D. (2014) Mechanisms regulating endothelial permeability. Pulm. Circ. 4, 535–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tauseef M., Knezevic N., Chava K. R., Smith M., Sukriti S., Gianaris N., Obukhov A. G., Vogel S. M., Schraufnagel D. E., Dietrich A., Birnbaumer L., Malik A. B., Mehta D. (2012) TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J. Exp. Med. 209, 1953–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta D., Ravindran K., Kuebler W. M. (2014) Novel regulators of endothelial barrier function. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L924–L935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta D., Malik A. B. (2006) Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 86, 279–367 [DOI] [PubMed] [Google Scholar]
- 6.Komarova Y. A., Huang F., Geyer M., Daneshjou N., Garcia A., Idalino L., Kreutz B., Mehta D., Malik A. B. (2012) VE-cadherin signaling induces EB3 phosphorylation to suppress microtubule growth and assemble adherens junctions. Mol. Cell 48, 914–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daneshjou N., Sieracki N., van Nieuw Amerongen G. P., Conway D. E., Schwartz M. A., Komarova Y. A., Malik A. B. (2015) Rac1 functions as a reversible tension modulator to stabilize VE-cadherin trans-interaction. J. Cell Biol. 208, 23–32. Erratum in: J. Cell Biol. 2015;209:181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong H., Gao X., Feng S., Siddiqui M. R., Garcia A., Bonini M. G., Komarova Y., Vogel S. M., Mehta D., Malik A. B. (2014) Evidence of a common mechanism of disassembly of adherens junctions through Gα13 targeting of VE-cadherin. J. Exp. Med. 211, 579–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowalczyk A. P., Nanes B. A. (2012) Adherens junction turnover: regulating adhesion through cadherin endocytosis, degradation, and recycling. Subcell. Biochem. 60, 197–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta D., Ahmmed G. U., Paria B. C., Holinstat M., Voyno-Yasenetskaya T., Tiruppathi C., Minshall R. D., Malik A. B. (2003) RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. J. Biol. Chem. 278, 33492–33500 [DOI] [PubMed] [Google Scholar]
- 11.Cioffi D. L., Stevens T. (2006) Regulation of endothelial cell barrier function by store-operated calcium entry. Microcirculation 13, 709–723 [DOI] [PubMed] [Google Scholar]
- 12.Singh I., Knezevic N., Ahmmed G. U., Kini V., Malik A. B., Mehta D. (2007) Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J. Biol. Chem. 282, 7833–7843 [DOI] [PubMed] [Google Scholar]
- 13.Jho D., Mehta D., Ahmmed G., Gao X. P., Tiruppathi C., Broman M., Malik A. B. (2005) Angiopoietin-1 opposes VEGF-induced increase in endothelial permeability by inhibiting TRPC1-dependent Ca2 influx. Circ. Res. 96, 1282–1290 [DOI] [PubMed] [Google Scholar]
- 14.Beech D. J. (2013) Characteristics of transient receptor potential canonical calcium-permeable channels and their relevance to vascular physiology and disease. Circ. J. 77, 570–579 [DOI] [PubMed] [Google Scholar]
- 15.Dietrich A., Kalwa H., Gudermann T. (2010) TRPC channels in vascular cell function. Thromb. Haemost. 103, 262–270 [DOI] [PubMed] [Google Scholar]
- 16.Cioffi D. L., Lowe K., Alvarez D. F., Barry C., Stevens T. (2009) TRPing on the lung endothelium: calcium channels that regulate barrier function. Antioxid. Redox Signal. 11, 765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiruppathi C., Freichel M., Vogel S. M., Paria B. C., Mehta D., Flockerzi V., Malik A. B. (2002) Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circ. Res. 91, 70–76 [DOI] [PubMed] [Google Scholar]
- 18.Earley S., Brayden J. E. (2015) Transient receptor potential channels in the vasculature. Physiol. Rev. 95, 645–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cioffi D. L. (2011) Redox regulation of endothelial canonical transient receptor potential channels. Antioxid. Redox Signal. 15, 1567–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietrich A., Kalwa H., Rost B. R., Gudermann T. (2005) The diacylgylcerol-sensitive TRPC3/6/7 subfamily of cation channels: functional characterization and physiological relevance. Pflugers Arch. 451, 72–80 [DOI] [PubMed] [Google Scholar]
- 21.Ahmmed G. U., Mehta D., Vogel S., Holinstat M., Paria B. C., Tiruppathi C., Malik A. B. (2004) Protein kinase Calpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J. Biol. Chem. 279, 20941–20949 [DOI] [PubMed] [Google Scholar]
- 22.Vandenbroucke St Amant E., Tauseef M., Vogel S. M., Gao X. P., Mehta D., Komarova Y. A., Malik A. B. (2012) PKCα activation of p120-catenin serine 879 phospho-switch disassembles VE-cadherin junctions and disrupts vascular integrity. Circ. Res. 111, 739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta D., Konstantoulaki M., Ahmmed G. U., Malik A. B. (2005) Sphingosine 1-phosphate-induced mobilization of intracellular Ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J. Biol. Chem. 280, 17320–17328 [DOI] [PubMed] [Google Scholar]
- 24.Sammani S., Moreno-Vinasco L., Mirzapoiazova T., Singleton P. A., Chiang E. T., Evenoski C. L., Wang T., Mathew B., Husain A., Moitra J., Sun X., Nunez L., Jacobson J. R., Dudek S. M., Natarajan V., Garcia J. G. (2010) Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. Am. J. Respir. Cell Mol. Biol. 43, 394–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maceyka M., Harikumar K. B., Milstien S., Spiegel S. (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 22, 50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiegel S., Milstien S. (2011) The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 11, 403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chavez A., Schmidt T. T., Yazbeck P., Rajput C., Desai B., Sukriti S., Giantsos-Adams K., Knezevic N., Malik A. B., Mehta D. (2015) S1PR1 Tyr143 phosphorylation downregulates endothelial cell surface S1PR1 expression and responsiveness. J. Cell Sci. 128, 878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tauseef M., Kini V., Knezevic N., Brannan M., Ramchandaran R., Fyrst H., Saba J., Vogel S. M., Malik A. B., Mehta D. (2008) Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ. Res. 103, 1164–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X., Ma S. F., Wade M. S., Acosta-Herrera M., Villar J., Pino-Yanes M., Zhou T., Liu B., Belvitch P., Moitra J., Han Y. J., Machado R., Noth I., Natarajan V., Dudek S. M., Jacobson J. R., Flores C., Garcia J. G. (2013) Functional promoter variants in sphingosine 1-phosphate receptor 3 associate with susceptibility to sepsis-associated acute respiratory distress syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L467–L477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathew B., Jacobson J. R., Berdyshev E., Huang Y., Sun X., Zhao Y., Gerhold L. M., Siegler J., Evenoski C., Wang T., Zhou T., Zaidi R., Moreno-Vinasco L., Bittman R., Chen C. T., LaRiviere P. J., Sammani S., Lussier Y. A., Dudek S. M., Natarajan V., Weichselbaum R. R., Garcia J. G. (2011) Role of sphingolipids in murine radiation-induced lung injury: protection by sphingosine 1-phosphate analogs. FASEB J. 25, 3388–3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knezevic N., Tauseef M., Thennes T., Mehta D. (2009) The G protein betagamma subunit mediates reannealing of adherens junctions to reverse endothelial permeability increase by thrombin. J. Exp. Med. 206, 2761–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McVerry B. J., Peng X., Hassoun P. M., Sammani S., Simon B. A., Garcia J. G. (2004) Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am. J. Respir. Crit. Care Med. 170, 987–993 [DOI] [PubMed] [Google Scholar]
- 33.Vogel S. M., Gao X., Mehta D., Ye R. D., John T. A., Andrade-Gordon P., Tiruppathi C., Malik A. B. (2000) Abrogation of thrombin-induced increase in pulmonary microvascular permeability in PAR-1 knockout mice. Physiol. Genomics 4, 137–145 [DOI] [PubMed] [Google Scholar]
- 34.Barnard J. W., Biro M. G., Lo S. K., Ohno S., Carozza M. A., Moyle M., Soule H. R., Malik A. B. (1995) Neutrophil inhibitory factor prevents neutrophil-dependent lung injury. J. Immunol. 155, 4876–4881 [PubMed] [Google Scholar]
- 35.Kusaba T., Okigaki M., Matui A., Murakami M., Ishikawa K., Kimura T., Sonomura K., Adachi Y., Shibuya M., Shirayama T., Tanda S., Hatta T., Sasaki S., Mori Y., Matsubara H. (2010) Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc. Natl. Acad. Sci. USA 107, 19308–19313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajput C., Kini V., Smith M., Yazbeck P., Chavez A., Schmidt T., Zhang W., Knezevic N., Komarova Y., Mehta D. (2013) Neural Wiskott-Aldrich syndrome protein (N-WASP)-mediated p120-catenin interaction with Arp2-Actin complex stabilizes endothelial adherens junctions. J. Biol. Chem. 288, 4241–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y. D., Ohkawara H., Rehman J., Wary K. K., Vogel S. M., Minshall R. D., Zhao Y. Y., Malik A. B. (2009) Bone marrow progenitor cells induce endothelial adherens junction integrity by sphingosine-1-phosphate-mediated Rac1 and Cdc42 signaling. Circ. Res. 105, 696–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natarajan V., Dudek S. M., Jacobson J. R., Moreno-Vinasco L., Huang L. S., Abassi T., Mathew B., Zhao Y., Wang L., Bittman R., Weichselbaum R., Berdyshev E., Garcia J. G. (2013) Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. Am. J. Respir. Cell Mol. Biol. 49, 6–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong Y., Hla T. (2014) S1P control of endothelial integrity. Curr. Top. Microbiol. Immunol. 378, 85–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ong H. L., Cheng K. T., Liu X., Bandyopadhyay B. C., Paria B. C., Soboloff J., Pani B., Gwack Y., Srikanth S., Singh B. B., Gill D. L., Ambudkar I. S. (2007) Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J. Biol. Chem. 282, 9105–9116 Erratum in: J. Biol Chem. 2007;282:27556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paugh S. W., Paugh B. S., Rahmani M., Kapitonov D., Almenara J. A., Kordula T., Milstien S., Adams J. K., Zipkin R. E., Grant S., Spiegel S. (2008) A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood 112, 1382–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song L., Xiong H., Li J., Liao W., Wang L., Wu J., Li M. (2011) Sphingosine kinase-1 enhances resistance to apoptosis through activation of PI3K/Akt/NF-kappaB pathway in human non–small cell lung cancer. Clin. Cancer Res. 17, 1839–1849 [DOI] [PubMed] [Google Scholar]
- 43.Gong H., Rehman J., Tang H., Wary K., Mittal M., Chaturvedi P., Zhao Y. Y., Komarova Y. A., Vogel S. M., Malik A. B. (2015) HIF2α signaling inhibits adherens junctional disruption in acute lung injury. J. Clin. Invest. 125, 652–664, erratum 1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gavard J. (2014) Endothelial permeability and VE-cadherin: a wacky comradeship. Cell Adhes. Migr. 8, 158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dejana E., Orsenigo F., Lampugnani M. G. (2008) The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 121, 2115–2122 [DOI] [PubMed] [Google Scholar]
- 46.Goel M., Sinkins W. G., Schilling W. P. (2002) Selective association of TRPC channel subunits in rat brain synaptosomes. J. Biol. Chem. 277, 48303–48310 [DOI] [PubMed] [Google Scholar]
- 47.Kini V., Chavez A., Mehta D. (2010) A new role for PTEN in regulating transient receptor potential canonical channel 6–mediated Ca2+ entry, endothelial permeability, and angiogenesis. J. Biol. Chem. 285, 33082–33091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hofmann T., Obukhov A. G., Schaefer M., Harteneck C., Gudermann T., Schultz G. (1999) Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263 [DOI] [PubMed] [Google Scholar]
- 49.Kaneider N. C., Leger A. J., Agarwal A., Nguyen N., Perides G., Derian C., Covic L., Kuliopulos A. (2007) “Role reversal” for the receptor PAR1 in sepsis-induced vascular damage. Nat. Immunol. 8, 1303–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angus D. C., van der Poll T. (2013) Severe sepsis and septic shock. N. Engl. J. Med. 369, 840–851 [DOI] [PubMed] [Google Scholar]
- 51.DebRoy A., Vogel S. M., Soni D., Sundivakkam P. C., Malik A. B., Tiruppathi C. (2014) Cooperative signaling via transcription factors NF-κB and AP1/c-Fos mediates endothelial cell STIM1 expression and hyperpermeability in response to endotoxin. J. Biol. Chem. 289, 24188–24201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sundivakkam P. C., Freichel M., Singh V., Yuan J. P., Vogel S. M., Flockerzi V., Malik A. B., Tiruppathi C. (2012) The Ca(2+) sensor stromal interaction molecule 1 (STIM1) is necessary and sufficient for the store-operated Ca(2+) entry function of transient receptor potential canonical (TRPC) 1 and 4 channels in endothelial cells. Mol. Pharmacol. 81, 510–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paria B. C., Vogel S. M., Ahmmed G. U., Alamgir S., Shroff J., Malik A. B., Tiruppathi C. (2004) Tumor necrosis factor-alpha-induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L1303–L1313 [DOI] [PubMed] [Google Scholar]
- 54.Paria B. C., Bair A. M., Xue J., Yu Y., Malik A. B., Tiruppathi C. (2006) Ca2+ influx induced by protease-activated receptor-1 activates a feed-forward mechanism of TRPC1 expression via nuclear factor-kappaB activation in endothelial cells. J. Biol. Chem. 281, 20715–20727 [DOI] [PubMed] [Google Scholar]
- 55.Wadgaonkar R., Patel V., Grinkina N., Romano C., Liu J., Zhao Y., Sammani S., Garcia J. G., Natarajan V. (2009) Differential regulation of sphingosine kinases 1 and 2 in lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L603–L613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harikumar K. B., Yester J. W., Surace M. J., Oyeniran C., Price M. M., Huang W. C., Hait N. C., Allegood J. C., Yamada A., Kong X., Lazear H. M., Bhardwaj R., Takabe K., Diamond M. S., Luo C., Milstien S., Spiegel S., Kordula T. (2014) K63-linked polyubiquitination of transcription factor IRF1 is essential for IL-1-induced production of chemokines CXCL10 and CCL5. Nat. Immunol. 15, 231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim E. Y., Sturgill J. L., Hait N. C., Avni D., Valencia E. C., Maceyka M., Lima S., Allegood J., Huang W. C., Zhang S., Milstien S., Conrad D., Spiegel S. (2014) Role of sphingosine kinase 1 and sphingosine-1-phosphate in CD40 signaling and IgE class switching. FASEB J. 28, 4347–4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marfe G., Mirone G., Shukla A., Di Stefano C. (2015) Sphingosine kinases signalling in carcinogenesis. Mini Rev. Med. Chem. 15, 300–314 [DOI] [PubMed] [Google Scholar]
- 59.Maceyka M., Sankala H., Hait N. C., Le Stunff H., Liu H., Toman R., Collier C., Zhang M., Satin L. S., Merrill A. H. Jr., Milstien S., Spiegel S. (2005) SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 280, 37118–37129 [DOI] [PubMed] [Google Scholar]