Abstract
MicroRNAs (miRNAs) regulate multiple genes, often within the same pathway, fine-tuning expression of key factors and stabilizing gene networks against aberrant fluctuations. The demanding physiologic functions of photoreceptor cells and the retinal pigmented epithelium necessitate precise gene regulation to maintain their homeostasis and function, thus rendering these postmitotic cells vulnerable to premature death in retinal degenerative disorders. Recent studies of the physiologic impact of miRNAs in these cells clearly demonstrate that miRNAs are an essential component of that gene regulation. These important advances provide the foundation for future exploration of miRNA-regulated gene networks in the eye to facilitate the development of miRNA-targeted therapeutics to combat blinding diseases.—Sundermeier, T. R., Palczewski, K. The impact of microRNA gene regulation on the survival and function of mature cell types in the eye.
Keywords: retina, RPE, photoreceptor, retinal degeneration, gene therapy
Discovered over 2 decades ago in Caenorhabditis elegans, microRNAs (miRNAs) are now recognized as fundamental players in eukaryotic gene regulation (1, 2). miRNAs regulate gene expression at the posttranscriptional level through imperfect complementarity with target sites on cellular mRNAs. miRNA-target mRNA interactions recruit the miRNA-induced silencing complex (miRISC), which represses gene expression primarily by destabilizing the mRNA, but also through reducing the rate of protein translation. With more than 2000 miRNAs in humans regulating up to 60% of protein-encoding genes (3, 4), it is difficult to overstate the ubiquity of this mode of gene regulation in virtually every cellular process and disease pathology. Due to the simplicity of the early model systems involved in the discovery and characterization of miRNAs, much of the early work focused on their roles in tissue, organ, and organismal development and on their functions in transformed cell lines. In these model systems, the global impact of miRNAs as well as the importance of specific miRNAs was unmistakable.
In contrast, studies of the impact of miRNAs in postmitotic mammalian cell types have advanced more slowly, owing to both the increased complexity of higher eukaryotes and the generally less pronounced phenotypic defects associated with miRNA loss of function in mature tissues. Nevertheless, a clear trend is emerging from such studies. In mature organs and tissues, miRNAs tend not to affect the primary functions of cells, but rather have proven essential for coping with various forms of cellular and organismal stress (5–7). Consistent with the tendency of miRNA regulation to produce small effects on the expression of several different genes, often within the same pathway, miRNAs are thought to support the robustness of gene networks, buffering against fluctuations in gene expression due either to stochastic modulation or environmental stress (Fig. 1) (8–10). Such robustness is essential in tissues and cell types that encounter continuous environmental stress.
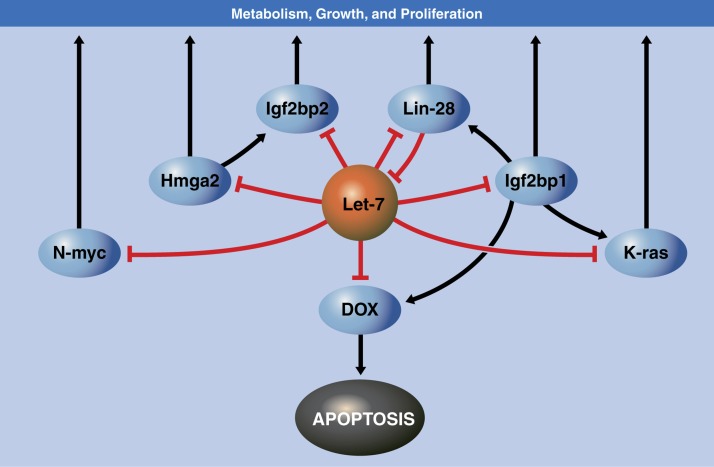
Figure 1.
miRNA-regulated gene networks and the systems pharmacology potential of therapeutic manipulation of miRNAs. Displayed is a simplified schematic of a gene network regulating survival, growth, and metabolism identified in cultured mammalian cells. The Let-7 miRNA, as a central node in this gene network, regulates several key genes shown in the pathway, modestly repressing their expression. Manipulating miRNAs therapeutically has the potential to deliver enhanced efficacy by simultaneously modulating multiple key players. In contrast to targeting a single protein, the relatively modest impact of miRNA binding on each individual target makes it unlikely that other interconnected pathways will be undesirably impacted. Researchers currently are analyzing visual system gene networks to identify attractive new targets for miRNA-based therapeutics.
Within the visual system, postmitotic photoreceptor and retinal pigmented epithelium (RPE) cells can be considered perpetually stressed as they must cope with exceptionally high rates of metabolism and protein synthesis, maintain viability under highly oxidizing conditions, deal with toxic byproducts of phototransduction, and shed or phagocytose 10% of each photoreceptor cell every day. These processes make these cells highly vulnerable to premature death, and their loss is characteristic of many retinal degenerative disorders. Thus, it is likely that miRNAs are required for the survival and function of photoreceptor and RPE cells. Consistent with this notion, specific roles for miRNAs in these labile, medically important visual system cell types have emerged in recent years (Fig. 2).
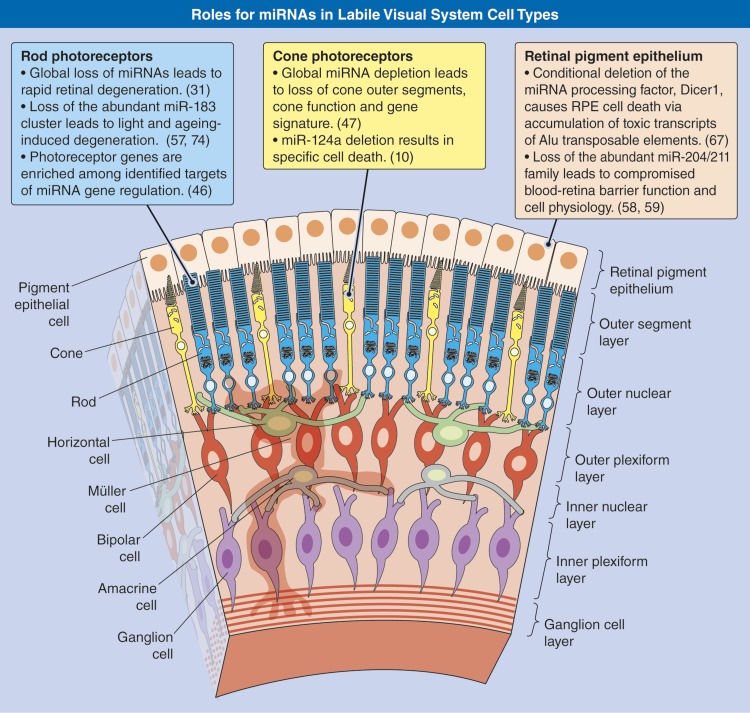
Figure 2.
Roles for miRNAs in labile visual system cell types. Artistic rendering of the laminar architecture of the mammalian retina/RPE highlighting recently revealed impacts of miRNAs in rods, cones, and the RPE, 3 cell types whose loss is a hallmark of most retinal degenerative disorders.
The demanding nature of photoreceptor and RPE cell functions in vision makes these irreplaceable postmitotic cells more susceptible to premature cell death, hence promoting their survival is a primary goal of the fight to combat a myriad of retinal degenerative disorders. Moreover, gene therapy approaches targeting visual system miRNAs represents an attractive strategy for developing novel interventions to counteract progressive vision loss due to the promised enhanced efficacy of miRNA-targeted therapeutics. This is due both to their relative ease of delivery and ability to influence multiple factors in the same pathway along with the relative accessibility and isolation of the visual system compartment (Fig. 3). The great promise of miRNAs as therapeutic targets for a broad class of retinal degenerative disorders will certainly continue to increase the pace of investigation of visual system miRNA gene networks and efforts to exploit them to therapeutic advantage.
Figure 3.
Therapeutic strategies for miRNA manipulation. (Top left) Schematic of miRNA gene regulation. miRNAs are transcribed in the nucleus as primary transcripts (pri-miRNAs) subject to 2 sequential cleavage steps, the first in the nucleus generating miRNA precursors (premiRNAs) and the second generating mature miRNAs in the cytoplasm. Mature miRNAs are bound to Argonaute family proteins, which assemble into miRISCs that recognize cellular mRNAs by base pairing to imperfectly complementary target sites, generally found in the 3′ UTR. miRISC recruitment results in repression of gene expression through mRNA destabilization and translational inhibition. (Top center) miRNA function may be inhibited in vivo through introduction of short oligonucleotides, chemically modified for enhanced stability and complementary to the mature miRNA sequence. (Top right) Similarly, miRNA mimics may be introduced that are processed by DICER into mature miRNAs that are identical to the endogenous miRNA. In this way, mature miRNA levels can be dramatically enhanced. (Bottom left) miRNA sponges are mRNAs bearing multiple target sites for an miRNA. They reduce miRNA activity by sequestering the mature miRNA. Sponge mRNAs may be employed as a gene therapy agent, introduced via cell-type-specific transfection approaches or viral gene transfer techniques. (Bottom right) In addition to synthetic miRNA mimics, increased miRNA expression can be accomplished via gene therapy approaches, with additional or replacement copies of miRNA genes introduced into the nucleus via viral gene transfer. This approach has the potential advantages of avoiding adverse host immune responses to oligonucleotides and permitting tunable, cell-type-specific expression through the use of appropriate gene promoters.
Here we review recent progress toward understanding the impact of miRNA gene regulation on the survival and function of postmitotic cell types in the mammalian retina, highlighting new insights derived from global and specific miRNA loss-of-function studies. We discuss how the functions of miRNAs can be best understood within a systems biology framework. Future directions for expansion of our knowledge regarding the physiologic impact of miRNAs in the visual system are discussed, along with the potential for translating this knowledge into a new class of treatment options to combat retinal degenerative diseases.
IDENTIFYING VISUAL SYSTEM miRNAs AND THEIR DIRECT TARGET miRNAs
miRNA expression analyses
As the deep evolutionary conservation of miRNAs became well established and functional roles for miRNA gene regulation were being clarified through studies in simpler model systems, interest emerged to determine whether miRNAs contribute to the regulation of visual system health and/or function. Initial efforts focused on identifying which miRNAs are abundantly expressed in intraocular compartments. A cloning and Sanger sequencing-based strategy was employed to identify small RNAs expressed in the newt eye and miRNA arrays were used to assess differential expression of miRNAs in the mouse cornea, lens, and retinal compartments (11, 12). The following year, more detailed microarray analysis identified 78 retinal miRNAs in mice including many that were significantly enriched in the retina as compared with brain and heart (13). Twelve of these miRNAs were also shown to be diurnally regulated, and RNA in situ hybridization studies revealed restricted expression patterns for many miRNAs within retinal layers (13–15). miRNA array approaches were later employed to survey changes in retinal miRNA profiles during retinal development (16). More recently, the retinal miRNA transcriptome was quantitatively analyzed by next-generation sequencing (NGS) approaches. Such studies have identified miRNAs exhibiting light-dependent expression patterns (17), miRNAs enriched in particular retinal cell types (18), and sequence length variants (isomiRs) among miRNAs in the retina and RPE/choroid (19). Subsequent studies have documented altered miRNA expression patterns in animal models of ocular pathologies including retinitis pigmentosa (20–22), congenital stationary night blindness (22), Leber congenital amaurosis (22), ischemia-induced retinal neovascularization (23), experimental autoimmune uveoretinitis (24), diabetic retinopathy induced either genetically or through streptozotocin treatment (25, 26), and retinoblastoma (26), all signifying potential roles for miRNAs in modulating blinding diseases.
Identifying direct miRNA target genes
Although identification of visual system-specific miRNAs has progressed for nearly a decade, far less information is available regarding the direct target genes that are repressed by these miRNAs. Direct miRNA targets were classically identified with in silico miRNA target prediction algorithms followed by their validation, usually in a simple cell culture-based model system. Though many bona fide visual system miRNA targets were identified in this manner, this approach has several limitations. First, target prediction algorithms suffer from high rates of both false-positives and false-negatives, though it is important to note that new insights into the mechanism of target site recognition by Argonaute/miRNA complexes promises significant improvement for the next generation of target prediction algorithms (27–29). Second, the large number of candidate targets identified in silico makes systematic validation impractical, thus leading to inherent bias in the genes selected for further analysis. Finally, and perhaps most importantly, miRNA target site selection depends on a host of cell-specific factors such as other transcripts with competing target sites, other RNA binding factors that could compete for binding sites on target mRNAs, differences in target mRNA folding or rates of translation, and so on. A systematic in situ approach is needed for reliable identification of miRNA targets in specific cell types and tissues to gain insights into the roles of miRNAs in regulating intertwined gene networks.
To overcome the above limitations, various techniques combining in vivo protein-RNA cross-linking with NGS technology have been developed to analyze the interaction of the Argonaute family of miRNA-binding proteins with cellular mRNAs to provide nonbiased snapshots of genes targeted by miRNA regulation in vivo (30–32). One recent report involved the use of this technology to improve understanding of the gene regulatory networks controlled by miRNAs in the mammalian retina. Argonaute high throughput sequencing of RNAs isolated by cross-linking immunoprecipitation of bovine retina identified 348 high-confidence miRNA target sites in 261 genes (33). The heterogeneity of the cow population coupled with the high stringency and strict requirement for conservation among biologic replicates probably limited this study to identifying only a fraction of the miRNA–target mRNA interactions that regulate retinal gene networks. Still, this work reliably identified a host of target genes warranting further research. One intriguing observation was that retinal miRNA targets showed significant enrichment of photoreceptor genes, highlighting the importance of miRNAs in these primary sensory neurons.
Unanswered questions
Like transcription factors and translational control genes, modulation in expression of a given miRNA impacts multiple target genes. miRNAs generally elicit modest effects on individual targets that are integrated to yield observable cellular consequences. Thus, miRNAs serve as gene regulatory nodes, directly impacting gene network output, buffering against aberrant modulations, or integrating multiple gene networks (Fig. 1). The inherent complexity of this mode of gene regulation presents challenges to understanding the physiologic roles of specific miRNAs, particularly in a complex in vivo setting. Although documenting miRNA expression profiles of ocular tissues and identifying expression changes that occur during development or in the context of various pathologies are essential for understanding the roles of miRNAs in vision, obtaining cell type-specific miRNA expression information would greatly simplify this process. Systematic application of miRNA in situ hybridization approaches to visual system miRNAs was particularly useful in this regard (15), though limited by probe quality and the inherent high background associated with small RNA hybridization techniques. More recently, 2 NGS-based approaches gained cell type-specific information on miRNA populations. Fluorescence-activated cell sorting was used to isolate cone photoreceptor populations for cone cell-specific small RNA sequencing analyses (33). Though limited by potential alterations in miRNA expression or stability due to mechanical disruption of retinal structure and the time required for sorting, this approach is a useful step toward cell type-specific miRNAome analysis. Small RNAseq analysis comparing the retinas of rod-specific Dicer conditional knockout (cKO) mice to control littermates was also used to identify miRNAs expressed in rods (18). This genetic approach yielded information about rod-specific miRNA populations in their in vivo settings, but is limited by the availability of cell type-specific cKO mouse models. With the continual advancement of NGS technologies, including a reduction in input RNA requirements and cost, a future challenge is to devise strategies for cell type-specific measurement of the dynamics of ocular miRNA expression to identify alterations associated with development and disease pathology.
Similarly, adapting high-throughput sequencing of RNAs isolated by cross-linking immunoprecipitation technology to the bovine retina is an exciting first step toward identifying which genes serve as physiologically important targets of miRNA gene regulation in the visual system. Applying this technology to laboratory model species under more controlled conditions should yield a more complete picture of ocular miRNA target genes. Moreover, technological advances enabling cell type-specific analysis of miRNA target genes would greatly facilitate unraveling the complexity of miRNA-regulated gene networks in vision. Analyzing gene networks in a cell type-specific manner within a mature in vivo tissue is challenging. But the well-characterized cell type functionalities and their genetic properties in the eye make applying this approach feasible in the near future.
ROLES OF miRNAs IN VISUAL SYSTEM DEVELOPMENT
Initial studies of the roles of miRNAs in the eye focused mainly on ocular development in insect, amphibian, and fish model systems. In fact, eye development has proven to be a powerful model system for studying the roles of miRNAs in regulating the complex gene networks involved in cell type specification. The insect model system was used to identify the role of miR-7 in regulating photoreceptor specification through targeting the Yan transcription factor and also to discern roles for bantam miRNA in regulating apoptosis and proliferation during development (34, 35). Dissection of the complex gene regulatory networks governed by miR-7 during Drosophila eye development provided early evidence for miRNAs conferring robustness on gene regulatory networks, demonstrating that miRNA can buffer gene network output against environmental challenges or stochastic modulation (36). Similarly, a study of Xenopus eye development yielded insights into specific functions of miRNAs in regulating the timing of differentiation of distinct cell types from a progenitor population. Inactivation of the miRNA processing enzyme, DICER, in frogs led to defects in cell cycle progression, survival of retinal progenitors, and timing of differentiation of retinal cell types (37). In addition, miRNA-mediated repression of translation of the homeobox genes otx2 and vsx1 was suggested to be involved in coupling the rate of progenitor proliferation with the differentiation of bipolar cells in the developing frog retina (38). miR-24 was also shown to regulate eye morphogenesis in Xenopus by repressing the expression of proapoptotic factors (39). Finally, the physiologic impact of miR-204 was established in the medaka fish model system, where morpholino-induced inactivation of this miRNA led to microphthalmia, lens defects, and aberrant dorsal-ventral patterning producing coloboma, mediated by affecting a diverse set of genes involved in various pathways (40, 41). miR-204 expression and activity are reportedly intertwined with Pax6, the master regulator of eye development, as miR-204 expression is regulated by Pax6 in both fish and mice, and miR-204 also feeds back in Pax6 indirectly through targeting of the transcription factor Meis2 (40, 42). Interestingly, a heterozygous single nucleotide seed region substitution mutation in the miR-204 gene has been linked to bilateral ocular coloboma in human patients, and introduction of this miR-204 variant in medaka fish had similar developmental consequences likely mediated through a mutant miRNA gain-of-function mechanism (43).
miRNA biogenesis involves 2 sequential cleavage steps. Primary miRNA transcripts fold into hairpin structures that are recognized by the nuclear microprocessor complex, where the RNA binding protein DGCR8 recruits the RNA endonuclease DROSHA to release the hairpin structure known as a pre-miRNA. pre-miRNAs then are exported to the cytoplasm where they undergo a second cleavage step catalyzed by the nuclease DICER. The classic approach to delineating the functional impact of miRNA gene regulation is to inactivate one of these miRNA processing factors specifically in a cell type or tissue of interest. Dicer knockout leads to embryonic lethality in mice (44), but visual system-specific cKO mouse models have yielded insights about this type of gene regulation in vision. Characterization of several retina-specific DICER cKO mouse models has provided important information about the impact of miRNAs on mammalian eye development. A mosaic pattern of Cre recombinase expression and Dicer gene excision in chx10 Cre recombinase-driven Dicer cKO mice resulted in a comparatively mild phenotype as compared with subsequently developed retina-specific Dicer cKOs, but provided the first evidence that miRNAs play a role in maintaining retinal function and homeostasis (45). These mice evidenced consistent abnormalities in electroretinogram (ERG) responses accompanied by progressive disorganization of retinal morphology and retinal degeneration. αPax6 Cre recombinase-driven retina-specific Dicer cKO mice showed Cre recombinase expression and Dicer disruption in large continuous regions within the developing retina that resulted in earlier onset developmental defects (46). This more robust DICER loss revealed defects in cell-type specification with increased production of premature ganglion and horizontal cell types and failure to differentiate the later-born rod photoreceptor and Mueller cell fates, a phenotype partially attributable to the loss of miRNA-dependent maintenance of Notch signaling (47). A similar pattern was later observed in an α-Cre recombinase-dependent cKO mouse line with DICER deficiency in the neuroretinal compartment of the optic cup (48). Rx-Cre recombinase-driven Dicer cKO resulted in microphthalmia and defects in ganglion cell axon pathfinding at the optic chiasm (16). Roles for individual miRNAs in mammalian eye development have also been characterized more recently. In mice deletion of the locus encoding miR-124a-1, resulting in loss of the dominant source of miR-124a, elicited death of cone photoreceptors during retinal development, due at least in part to dysregulation of the homeobox gene Lhx2 (49). miR-132 was shown to be involved in branching of retinal ganglion cell axons through targeting p250GAP in mouse retina cultures (50). In an elegant study of broad scope, researchers in the Reh laboratory demonstrated that the let-7, miR-9, and miR-125 families are involved in cell-type specification in the mouse retina, regulating the switch in competence of retinal progenitors to differentiate into later-born retinal cell types. Finally, another groundbreaking recent study demonstrated that proper timing of miR-183 cluster expression during retinal development in mice, regulated by a retina-specific long noncoding RNA, is essential for establishing proper retinal laminar architecture (51).
ROLES OF miRNAs IN POSTMITOTIC ROD AND CONE PHOTORECEPTORS AND THE RPE
Studies of miRNAs in eye development in model systems ranging from insects to mammals have clearly revealed general principles regarding the functions of miRNAs within gene regulatory networks, and the basic principles underlying developmental cell type specification. Moreover, due to the well-characterized miRNA expression changes in models of visual system pathology coupled with the emergence of miRNA manipulation as a viable therapeutic option, a firm grasp of the physiologic roles of miRNAs in mature retinal cell types could result in novel therapeutic options for a host of devastating blinding diseases. Recent reports are beginning to unravel the roles of miRNAs in mature photoreceptors and the RPE (Fig. 2).
The physiology of photoreceptors and the RPE presents a unique set of challenges necessitating precisely tuned gene regulatory networks to maintain cell viability and support specific functional requirements. Photoreceptor cells are the primary sensory neurons of vision, capturing photons of light and converting this information into electrical signals to be processed by the central nervous system. The high metabolic demand associated with maintenance of the rod photoreceptor dark current, the continuous need to synthesize large amounts of opsin proteins to maintain their high concentration in continuously renewed outer segments, along with demands associated with regenerating visual chromophore and coping with toxic byproducts of this process, combine to render these postmitotic cells highly vulnerable to premature death from mutational or environmental challenges. A monolayer of cells lying adjacent to photoreceptor outer segments, the RPE ensures the health and function of rods and cones. RPE cells must overcome similar challenges associated with supporting the visual (retinoid) cycle, while simultaneously maintaining ionic and osmotic balance in the outer retina, providing nutrients from the choroidal blood supply, maintaining the blood retina barrier, and phagocytizing 10% of the photoreceptor outer segment layer each day (making these the most active phagosomes in the body). RPE cells are especially vulnerable to premature death caused by aging and environmental or mutational challenges, and their loss accounts for a large fraction of retinal degenerative diseases. Mounting evidence suggests that miRNAs are needed to cope with cellular stress, hence they are likely critical for the survival and function of photoreceptor cells and the RPE, making them attractive therapeutic targets for blinding diseases. Several recent reports have supported this hypothesis, identifying roles for miRNAs in the survival and function of vulnerable postmitotic visual system cell types.
Functions of miRNAs in mammalian rod and cone photoreceptors
cKO of miRNA biogenesis factors in mice has firmly established that miRNA gene regulation is essential for the function of both rods and cones. Mature rod photoreceptor-specific Dicer cKO mice were recently characterized that exhibited progressive retinal degeneration (18). Morphologic changes began with disorganization of the outer segment layer in 8-wk-old animals and progressed to nearly complete loss of photoreceptor nuclei in 14-wk-old mice. Analysis of phototransduction kinetics and visual cycle competence in 4-wk-old mice (after loss of mature rod miRNAs, but prior to morphologic changes) revealed no defects in either of these processes, and dark rearing did not impact retinal degeneration. This suggested that loss of rod photoreceptor miRNAs caused a primary defect in supporting the survival of these sensory neurons.
The most abundant miRNA family in the mature mouse retina is the miR-183 cluster, a polycistronic miRNA cluster comprised of miR-96, -182, and -183. These miRNAs are expressed primarily in sensory organs including the retina, hair cells of the inner ear, olfactory and lingual epithelium, and mechanosensory neurons in dorsal root ganglia (13, 34, 52, 53). Within the eye, they are enriched in rod and cone photoreceptors and their expression varies with exposure to light (13, 14, 17). Expression of these miRNAs is decreased in multiple mouse models of retinitis pigmentosa (21). In a miRNA sponge transgenic mouse model, inactivation of these miRNAs specifically in rods, resulted in increased sensitivity to bright light-induced retinal degeneration, suggesting that these miRNAs promoted the survival of rods after light stress (54). This concept was further supported by identifying the gene encoding the apoptosis factor Caspase 2 as a direct miR-183 cluster target. Later, the importance of the miR-183 cluster in photoreceptors was confirmed by disrupting these miRNAs with a gene trap approach (14). This strategy confirmed the increased retinal susceptibility to light damage and also revealed age-induced retinal degeneration, further strengthening the notion that this miRNA family promoted the survival of stressed photoreceptors. This mouse line also showed defects in ERG responses and alterations in the expression of genes encoding synaptic proteins and phototransduction factors.
Global loss of miRNAs in cone photoreceptors also led to striking consequences, as progressive loss of cone outer segments and photopic ERG responses was documented in a mature cone photoreceptor-specific Dgcr8 cKO mouse model (33). DGCR8 is apparently stabilized in cones, delaying significant loss of mature miRNAs until after postnatal day (P)30. However, these animals still showed loss of cone outer segments, increased inner segment diameters, enlarged mitochondria, and decreased photopic ERG responses by P60. Consistent with loss of cone outer segments, cone opsin staining was dramatically reduced, but surprisingly, so were cone opsin mRNA levels in isolated cones. miRNA expression analysis identified miR-182 as by far the most abundantly expressed cone miRNA, accounting for more than 60% of small RNA reads, with cocistronic miR-183 representing an additional 4%. Not surprising based on their abundance in cones, reintroduction of these miRNAs by subretinal injection of conditional adeno-associated viruses (AAVs) prevented outer segment disruption and restored cone opsin levels. Intriguingly, addition of miR-183 cluster miRNAs, both in vivo into P60 cKO mouse retinas (where cone outer segments were already lost) and in vitro into ES cell-derived retinal cultures, induced localization of opsin protein at the distal tips of photoreceptor cells, in the latter case forming rudimentary outer segment disk structures. These results highlight the potential of using miRNA manipulation to stimulate outer segment restoration in retinal degenerative diseases. Gene expression analyses revealed a progressive decline in the expression of phototransduction factors, and a slow general decline in the expression of cone-specific transcripts. These results are consistent with the reported differential expression of phototransduction factors and defects in photopic ERG responses reported for miR-183 cluster gene trap mice. Interestingly, genes involved in synaptic function were largely unchanged in cone-specific DGCR8 cKO mice, suggesting that differential expression of these factors in the miR-183 cluster gene trap animals was likely secondary to developmental defects.
Impact of miRNAs in the RPE
Findings about miRNAs in general, and the miR-183 cluster in particular, in rod and cone photoreceptor cells have been largely consistent. In contrast, studies of the impact of miRNAs and miRNA processing factors in the retinal pigment epithelium (RPE) have produced some seemingly contradictory results. Numerous studies performed on RPE cells isolated from human patients or RPE-derived cell culture lines indicate functions for miRNAs in fundamental RPE cellular activities. miRNA expression analyses comparing human fetal RPE to various other tissues identified miR-204 and -211 as the miRNAs most abundantly expressed in the RPE, and both these miRNAs were significantly down-regulated in dedifferentiated human fetal RPE cells (55, 56). Inhibition of either miRNA led to a decrease in transepithelial electrical resistance in human fetal RPE monolayers, increased human fetal RPE proliferation, and dysregulation of genes associated with tight junction integrity, indicating that these miRNAs could affect the physiology and barrier function of RPE cells (55, 56). Inhibition of both miRNAs resulted in decreased expression of RPE-specific genes and a concomitant increase in expression of genes associated with epithelial to mesenchymal transition coupled with changes in morphology, suggesting that loss of these miRNAs results in de-differentiation of mature RPE cells (55). Evidence from primary isolated human RPE cells highlighted miRNA expression alterations in response to inflammatory stimuli and reactive oxygen species (57–59). miR-23a was significantly down-regulated in the RPE derived from patients with age-related macular degeneration and manipulation of this miRNA modulated the sensitivity to apoptosis of RPE-derived cell lines (60). miR-34a modulated the proliferation and migration of cultured RPE cell lines (61). Moreover, miR-184 was down-regulated in cultured RPE cells derived from age-related macular degeneration patients and inhibition of this miRNA in an RPE-derived cell line produced defects in phagocytic activity (62).
RPE-specific loss of miRNAs was studied in vivo with 3 miRNA biogenesis factor cKO mouse models that consistently revealed a requirement for these genes to support RPE health and function. Dct-Cre recombinase-driven Dicer cKO mice exhibited Cre recombinase expression in the ocular pigmented cell lineage starting around embryonic day 9.5 and resulted in embryonic developmental anomalies including flattening of the ciliary body at embryonic day 18.5 and failure to develop an iris by P8 (48). Subsequent detailed investigation of RPE patterning and gene expression revealed that although the RPE monolayer hexagonal morphology and polarization were preserved through P11 (the latest age analyzed), both a reduction in cell size and a complete lack of pigmentation had occurred (63). Immunostaining revealed a severe reduction in the expression of visual cycle genes along with failure to form photoreceptor outer segments, aberrant rhodopsin localization, and subsequent photoreceptor apoptosis. Many of these phenotypes, including the reduced expression of visual cycle genes and rhodopsin mislocalization in rod photoreceptor cells, were also observed in Dct-Cre recombinase-driven Dgcr8 cKO mice, indicating that the phenotype is dependent on loss of miRNAs rather than any noncanonical DICER activity. Array-based gene expression analysis confirmed a reduction in visual cycle gene expression and also revealed differential expression of factors involved in melanosome biogenesis and cell adhesion. These in vivo results corroborated many previous findings from primary RPE culture and cell line studies, including the loss of RPE barrier function upon inhibition of the abundant miR-204 and -211 and miRNA-dependent control of RPE cell proliferation (55, 56, 61). But previous studies indicating dedifferentiation of RPE cells upon inhibition of miR-204 and -211 would have predicted a more severe loss of RPE cells in the absence of miRNAs in vivo. This discrepancy could arise from inherent differences in primary cultured RPE cells as compared RPE cells developing in a canonical in vivo setting, counteracting effects of loss of other RPE miRNAs, or differences between human and mouse RPE cells. One high profile recent study reported down-regulation of DICER in the RPE of geographic atrophy patients (a late stage of dry age-related macular degeneration) and examined the impact of loss of factors involved in miRNA biogenesis and function specifically in the mature RPE (64). With Cre recombinase expression beginning at P10 and peaking at P28, Best1-Cre recombinase-driven Dicer cKO mice exhibited aberrant RPE morphology and RPE cell death (65). This phenotype was recapitulated by AAV-mediated delivery of a Best1-Cre recombinase expression cassette into mice carrying conditional Dicer alleles. However, this phenotype is reportedly independent of DICER’s canonical function in miRNA maturation, as subretinal injection of the identical Best1-Cre recombinase AAV into mice carrying conditional alleles for either the microprocessor components Drosha or Dgcr8, or the miRNA-binding protein Ago2 did not develop changes in RPE morphology. Similarly, Ago1, Ago3, and Ago4 knockout mice had normal RPEs, and RPE morphologic changes were not observed in mice lacking the miRNA processing enhancer, Tarbp2. DICER loss reportedly causes RPE degeneration from failure to degrade toxic transcripts of Alu transposable elements. Subsequent studies investigated the downstream events associated with Alu RNA toxicity and evaluated various therapeutic strategies to combat this pathology (66–70).
Unanswered questions
In contrast to rod-specific Dicer cKO mice, no cone cell loss was reported in Dgcr8 cKO mice. Instead, miRNAs were shown to be required for cone outer segment preservation as well as maintenance of the cone photoreceptor cell fate (18, 33). However, in both cases, outer segment disruption was the earliest observed morphologic change, suggesting a conserved role for miR-183 cluster miRNAs in maintaining photoreceptor outer segments. Rescue experiments demonstrated that miR-183 cluster miRNAs could restore cone outer segment maintenance, but global miR-183 cluster loss-of-function studies suggest that they are not strictly required for outer segment homeostasis in either rods or cones (33, 54, 71). Delineating the specific roles of these miRNAs in this process will surely be a focus of future studies. The observation that postmitotic rod photoreceptor-specific Dicer cKO causes a far more severe phenotype than loss of the miR-183 cluster in mature rods indicates that additional miRNAs are important for rod survival (18, 54, 71). Identifying these miRNAs and discerning their specific functions on rod gene networks promoting homeostasis are compelling avenues for future investigation.
Global miRNA loss-of-function in both rods and cones resulted in dramatic changes in the morphology and function of these sensory neurons. Interestingly, more than 60% of reads from small RNAseq analyses of isolated cones mapped to miR-182 (33). Similarly, 37% of reads from retina-wide small RNAseq analyses of the rod-dominated mouse retina in control animals mapped to miR-182 (18). In both analyses, read counts for miR-182 were orders of magnitude larger than counts for the cocistronic miR-96 and -183. It is unclear whether a second promoter element drives expression of miR-182 independently of the other miRNAs (miR-182 is the third miRNA in this cluster, relatively distant from the other 2), or if these miRNAs undergo differential rates of processing or decay. Rapid amplification of cDNA ends for 3′ and 5′ identified 2 transcript variants, with all 3 miR-183 cluster miRNAs intronic in one and their positions undefined in the other (72). More intriguingly, 2 separate miR-183 cluster loss-of-function models showed dramatic morphologic changes after loss of these 3 miRNAs (54, 71) whose exogenous delivery ameliorated morphologic and functional changes in cone-specific DGCR8 cKO mice (33). However, a previously reported miR-182 knockout mouse model revealed no observable morphologic changes in the retina, and microarray analyses revealed no significant expression alterations (72). Possibly the expression/stability of miR-96 and -183 are enhanced in the absence of miR-182 and other cluster miRNAs perform redundant functions (the seed region sequences of these 3 miRNAs are strikingly similar). This is an interesting avenue for future investigation. More intriguing is the potential for combining cell type-specific information about direct miRNA target genes in rods and cones with analyses of global transcriptome changes that occur in the absence of miRNAs in these cells. Such an approach could provide significant insight into the gene networks through which miR-183 cluster miRNAs promote rod survival and preserve cone outer segment homeostasis and cell fate.
Mature RPE-specific Dicer1 cKO mice developed retinal degeneration that was reportedly driven by loss of Dicer1 activity in removing toxic transcripts of Alu-like transposable elements (64). Although this activity precluded use of this model to investigate the roles of miRNAs in the mature RPE, the lack of consequences reported for subsequent AAV-mediated disruption of other factors involved in miRNA biogenesis and function suggests that miRNAs do not play fundamental roles in the mature RPE (64). This is quite a surprising observation given the defined roles of individual miRNAs in maintaining RPE barrier function and cell fate in primary cultured RPE cells (55, 56). The apparent inconsistency possibly results from species differences or differences in cell physiology associated with isolation and culturing of RPE cells. Even more likely is that either the virus-assisted recombination strategy employed to disrupt Drosha and Dgcr8 did not adequately inactivate the function of these factors in the RPE, or the loss of microprocessor activity in the RPE was slower to develop than the loss of DICER. Neither the efficacy of Drosha and Dgcr8 excision and subsequent loss of mature miRNAs nor the time course of the analysis was outlined for the report in question (64). But a subsequent report demonstrated that high stability of DGCR8 protein in cone photoreceptors led to a delayed clearance of this protein after Cre recombinase-dependent exon excision (33). This property could extend to other components of the nuclear microprocessor complex, including DROSHA. The functional redundancy of mammalian Argonaute family proteins is well documented (73), such that loss of these factors would not be expected to yield nearly as severe consequences as DICER loss. It is clear that loss of DICER in the RPE is accompanied by accumulation of Alu element transcripts and that exogenous delivery of these RNAs has a severe impact on RPE cell health in vivo. It is unclear whether theses toxic Alu transcripts serve a physiologically relevant purpose in the RPE or if these transposable elements are simply intracellular pathogens that must be controlled through DICER activity. One possibility is that DICER cleavage is involved in the biogenesis of shorter mature Alu RNA species that are functionally important. For example, in stem cells DICER-dependent maturation of retinoic acid-induced, Alu element-derived small RNAs reportedly resulted in reduced stability of a subset of mRNAs that participate in regulating cell proliferation (74). It is also likely that miRNAs are essential for RPE cell function and homeostasis. Studying the impact of losing individual miRNAs in the mature RPE in vivo will surely clarify this point, and the continuous systematic development of miRNA loss-of-function mouse models will soon greatly facilitate these types of analyses (75, 76).
miRNAs AND SYSTEMS PHARMACOLOGY: HARNESS miRNA GENE REGULATION TO COMBAT RETINAL DEGENERATION
Extensive emerging evidence highlights essential roles for miRNAs in supporting the homeostasis and function of ocular cell types that are both crucial for our vision and highly vulnerable to death resulting from ageing, gene mutations, or environmental insults. miRNA-based therapeutics have inherent potential advantages over other therapeutic strategies (Fig. 3). First, as miRNAs often target multiple genes in the same pathway, a single miRNA-directed therapeutic agent could impact multiple relevant target genes. In addition, miRNA mimics and antago-miRs act in the cytoplasm, making their delivery less challenging than traditional gene therapies. Finally, modulating miRNA activity with modified nucleic acid-based therapeutic agents requires relatively short sequences, thereby facilitating their delivery. For these reasons, development of miRNA replacement therapies to restore expression levels of selected pathologically dysregulated miRNAs is emerging as an attractive novel option to treat retinal diseases.
Taking into account both preclinical and clinical development stages, the cost of bringing a single novel drug to market is massive. Moreover, the risk of failure due to unanticipated side effects is also high. For these reasons, many researchers are turning to alternative drug development strategies, including a systems pharmacological approach. Delivery of smaller doses of multiple therapeutic agents has the potential to yield powerful synergistic effects on a targeted pathway, while simultaneously minimizing the impact on any given target and the potential for unwanted side effects on other linked pathways. Such a strategy, using U.S. Food and Drug Administration-approved drugs, has recently shown great promise in preclinical development to impact various retinal degenerative disorders (77, 78). However, one important limitation of this polypharmacological approach is that not all disease-modulating pathways are “druggable” with traditional small molecule therapeutics.
Remarkably similar principles govern the mode of action of miRNAs within the gene networks they regulate. miRNAs serve as central nodes in gene regulatory networks, and their modest regulation of multiple factors has important consequences for gene network outputs, such as buffering against aberrant stochastic or environmental fluctuations (8–10). Therefore, by modulating the expression of individual miRNAs, cells use an approach analogous to systems pharmacology, selectively modulating certain pathways without negatively impacting the crosstalk of individual network nodes with other pathways. One could think of miRNAs as nature’s systems pharmacology agents, tailored to the demands of individual cell types and circumstances and prophylactically administered to gene networks to combat potential environmental, genetic, and stochastic insults. From this perspective, it is easy to see why miRNA loss-of-function studies often reveal changes only under conditions of stress (5–7). This property of miRNAs makes their therapeutic manipulation an even more attractive idea. However, rational manipulation of miRNAs to produce a selective impact on the output of pathologically dysregulated gene networks will require a precise in vivo cell type-specific understanding of the canonical functions of miRNA pathways. This includes genome-wide knowledge of direct target genes and the pathways they regulate, along with a systematic understanding of the molecular mechanisms underlying disease pathology. The ongoing revolution in genomics initiated by the advent of NGS, makes collection of this type of information with high precision on a genome-wide scale feasible in the near future. With its well-studied genetics and cell type functionality, along with its relative isolation and accessibility, the eye is an ideal organ for the development of such an approach. Many innovative studies cited here provide a strong foundation for future development of miRNA-based therapies to prevent or ameliorate blinding diseases.
Acknowledgments
The authors thank Susie Suh (Case Western Reserve University) for comments on this manuscript. This work was supported by funding from the U.S. National Institutes of Health, National Eye Institute (EY022326), and the Arnold and Mabel Beckman Foundation. K.P. is John H. Hord Professor of Pharmacology. The authors declare no conflicts of interest.
Glossary
- AAV
adeno-associated virus
- cKO
conditional knockout
- ERG
electroretinogram
- miRNA
microRNA
- miRISC
miRNA-induced silencing complex
- NGS
next-generation sequencing
- P
postnatal day
- RPE
retinal pigmented epithelium
REFERENCES
- 1.Lee R. C., Feinbaum R. L., Ambros V. (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854 [DOI] [PubMed] [Google Scholar]
- 2.Wightman B., Ha I., Ruvkun G. (1993) Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–862 [DOI] [PubMed] [Google Scholar]
- 3.Friedman R. C., Farh K. K., Burge C. B., Bartel D. P. (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ENCODE Project Consortium (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schober A., Nazari-Jahantigh M., Weber C. (2015) MicroRNA-mediated mechanisms of the cellular stress response in atherosclerosis. Nat. Rev. Cardiol. 12, 361–374 [DOI] [PubMed] [Google Scholar]
- 6.Emde A., Hornstein E. (2014) miRNAs at the interface of cellular stress and disease. EMBO J. 33, 1428–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung A. K., Sharp P. A. (2010) MicroRNA functions in stress responses. Mol. Cell 40, 205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidigal J. A., Ventura A. (2015) The biological functions of miRNAs: lessons from in vivo studies. Trends Cell Biol. 25, 137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebert M. S., Sharp P. A. (2012) Roles for microRNAs in conferring robustness to biological processes. Cell 149, 515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Posadas D. M., Carthew R. W. (2014) MicroRNAs and their roles in developmental canalization. Curr. Opin. Genet. Dev. 27, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan D. G., Oliveira-Fernandes M., Lavker R. M. (2006) MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol. Vis. 12, 1175–1184 [PubMed] [Google Scholar]
- 12.Makarev E., Spence J. R., Del Rio-Tsonis K., Tsonis P. A. (2006) Identification of microRNAs and other small RNAs from the adult newt eye. Mol. Vis. 12, 1386–1391 [PubMed] [Google Scholar]
- 13.Xu S., Witmer P. D., Lumayag S., Kovacs B., Valle D. (2007) MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J. Biol. Chem. 282, 25053–25066 [DOI] [PubMed] [Google Scholar]
- 14.Karali M., Peluso I., Marigo V., Banfi S. (2007) Identification and characterization of microRNAs expressed in the mouse eye. Invest. Ophthalmol. Vis. Sci. 48, 509–515 [DOI] [PubMed] [Google Scholar]
- 15.Karali M., Peluso I., Gennarino V. A., Bilio M., Verde R., Lago G., Dollé P., Banfi S. (2010) miRNeye: a microRNA expression atlas of the mouse eye. BMC Genomics 11, 715.1–715.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hackler L. Jr., Wan J., Swaroop A., Qian J., Zack D. J. (2010) MicroRNA profile of the developing mouse retina. Invest. Ophthalmol. Vis. Sci. 51, 1823–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krol J., Busskamp V., Markiewicz I., Stadler M. B., Ribi S., Richter J., Duebel J., Bicker S., Fehling H. J., Schübeler D., Oertner T. G., Schratt G., Bibel M., Roska B., Filipowicz W. (2010) Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell 141, 618–631 [DOI] [PubMed] [Google Scholar]
- 18.Sundermeier T. R., Zhang N., Vinberg F., Mustafi D., Kohno H., Golczak M., Bai X., Maeda A., Kefalov V. J., Palczewski K. (2014) DICER1 is essential for survival of postmitotic rod photoreceptor cells in mice. FASEB J. 28, 3780–3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soundara Pandi S. P., Chen M., Guduric-Fuchs J., Xu H., Simpson D. A. (2013) Extremely complex populations of small RNAs in the mouse retina and RPE/choroid. Invest. Ophthalmol. Vis. Sci. 54, 8140–8151 [DOI] [PubMed] [Google Scholar]
- 20.Loscher C. J., Hokamp K., Kenna P. F., Ivens A. C., Humphries P., Palfi A., Farrar G. J. (2007) Altered retinal microRNA expression profile in a mouse model of retinitis pigmentosa. Genome Biol. 8, R248.1–R248.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loscher C. J., Hokamp K., Wilson J. H., Li T., Humphries P., Farrar G. J., Palfi A. (2008) A common microRNA signature in mouse models of retinal degeneration. Exp. Eye Res. 87, 529–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genini S., Guziewicz K. E., Beltran W. A., Aguirre G. D. (2014) Altered miRNA expression in canine retinas during normal development and in models of retinal degeneration. BMC Genomics 15, 172.1–172.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen J., Yang X., Xie B., Chen Y., Swaim M., Hackett S. F., Campochiaro P. A. (2008) MicroRNAs regulate ocular neovascularization. Mol. Ther. 16, 1208–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishida W., Fukuda K., Higuchi T., Kajisako M., Sakamoto S., Fukushima A. (2011) Dynamic changes of microRNAs in the eye during the development of experimental autoimmune uveoretinitis. Invest. Ophthalmol. Vis. Sci. 52, 611–617 [DOI] [PubMed] [Google Scholar]
- 25.Kovacs B., Lumayag S., Cowan C., Xu S. (2011) MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Invest. Ophthalmol. Vis. Sci. 52, 4402–4409 [DOI] [PubMed] [Google Scholar]
- 26.Kandalam M. M., Beta M., Maheswari U. K., Swaminathan S., Krishnakumar S. (2012) Oncogenic microRNA 17-92 cluster is regulated by epithelial cell adhesion molecule and could be a potential therapeutic target in retinoblastoma. Mol. Vis. 18, 2279–2287 [PMC free article] [PubMed] [Google Scholar]
- 27.Chandradoss S. D., Schirle N. T., Szczepaniak M., MacRae I. J., Joo C. (2015) A dynamic search process underlies MicroRNA targeting. Cell 162, 96–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salomon W. E., Jolly S. M., Moore M. J., Zamore P. D., Serebrov V. (2015) Single-molecule imaging reveals that Argonaute reshapes the binding properties of its nucleic acid guides. Cell 162, 84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jo M. H., Shin S., Jung S. R., Kim E., Song J. J., Hohng S. (2015) Human Argonaute 2 has diverse reaction pathways on target RNAs. Mol. Cell 59, 117–124 [DOI] [PubMed] [Google Scholar]
- 30.Chi S. W., Zang J. B., Mele A., Darnell R. B. (2009) Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature 460, 479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaskiewicz L., Bilen B., Hausser J., Zavolan M. (2012) Argonaute CLIP--a method to identify in vivo targets of miRNAs. Methods 58, 106–112 [DOI] [PubMed] [Google Scholar]
- 32.Hafner M., Lianoglou S., Tuschl T., Betel D. (2012) Genome-wide identification of miRNA targets by PAR-CLIP. Methods 58, 94–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busskamp V., Krol J., Nelidova D., Daum J., Szikra T., Tsuda B., Jüttner J., Farrow K., Scherf B. G., Alvarez C. P., Genoud C., Sothilingam V., Tanimoto N., Stadler M., Seeliger M., Stoffel M., Filipowicz W., Roska B. (2014) miRNAs 182 and 183 are necessary to maintain adult cone photoreceptor outer segments and visual function. Neuron 83, 586–600 [DOI] [PubMed] [Google Scholar]
- 34.Li X., Carthew R. W. (2005) A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye. Cell 123, 1267–1277 [DOI] [PubMed] [Google Scholar]
- 35.Thompson B. J., Cohen S. M. (2006) The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell 126, 767–774 [DOI] [PubMed] [Google Scholar]
- 36.Li X., Cassidy J. J., Reinke C. A., Fischboeck S., Carthew R. W. (2009) A microRNA imparts robustness against environmental fluctuation during development. Cell 137, 273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Decembrini S., Andreazzoli M., Barsacchi G., Cremisi F. (2008) Dicer inactivation causes heterochronic retinogenesis in Xenopus laevis. Int. J. Dev. Biol. 52, 1099–1103 [DOI] [PubMed] [Google Scholar]
- 38.Decembrini S., Bressan D., Vignali R., Pitto L., Mariotti S., Rainaldi G., Wang X., Evangelista M., Barsacchi G., Cremisi F. (2009) MicroRNAs couple cell fate and developmental timing in retina. Proc. Natl. Acad. Sci. USA 106, 21179–21184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker J. C., Harland R. M. (2009) microRNA-24a is required to repress apoptosis in the developing neural retina. Genes Dev. 23, 1046–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conte I., Carrella S., Avellino R., Karali M., Marco-Ferreres R., Bovolenta P., Banfi S. (2010) miR-204 is required for lens and retinal development via Meis2 targeting. Proc. Natl. Acad. Sci. USA 107, 15491–15496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conte I., Merella S., Garcia-Manteiga J. M., Migliore C., Lazarevic D., Carrella S., Marco-Ferreres R., Avellino R., Davidson N. P., Emmett W., Sanges R., Bockett N., Van Heel D., Meroni G., Bovolenta P., Stupka E., Banfi S. (2014) The combination of transcriptomics and informatics identifies pathways targeted by miR-204 during neurogenesis and axon guidance. Nucleic Acids Res. 42, 7793–7806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaham O., Gueta K., Mor E., Oren-Giladi P., Grinberg D., Xie Q., Cvekl A., Shomron N., Davis N., Keydar-Prizant M., Raviv S., Pasmanik-Chor M., Bell R. E., Levy C., Avellino R., Banfi S., Conte I., Ashery-Padan R. (2013) Pax6 regulates gene expression in the vertebrate lens through miR-204. PLoS Genet. 9, e1003357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conte I., Hadfield K. D., Barbato S., Carrella S., Pizzo M., Bhat R. S., Carissimo A., Karali M., Porter L. F., Urquhart J., Hateley S., O’Sullivan J., Manson F. D., Neuhauss S. C., Banfi S., Black G. C. (2015) MiR-204 is responsible for inherited retinal dystrophy associated with ocular coloboma. Proc. Natl. Acad. Sci. USA 112, E3236–E3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernstein E., Kim S. Y., Carmell M. A., Murchison E. P., Alcorn H., Li M. Z., Mills A. A., Elledge S. J., Anderson K. V., Hannon G. J. (2003) Dicer is essential for mouse development. Nat. Genet. 35, 215–217 [DOI] [PubMed] [Google Scholar]
- 45.Damiani D., Alexander J. J., O’Rourke J. R., McManus M., Jadhav A. P., Cepko C. L., Hauswirth W. W., Harfe B. D., Strettoi E. (2008) Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina. J. Neurosci. 28, 4878–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Georgi S. A., Reh T. A. (2010) Dicer is required for the transition from early to late progenitor state in the developing mouse retina. J. Neurosci. 30, 4048–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Georgi S. A., Reh T. A. (2011) Dicer is required for the maintenance of notch signaling and gliogenic competence during mouse retinal development. Dev. Neurobiol. 71, 1153–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis N., Mor E., Ashery-Padan R. (2011) Roles for Dicer1 in the patterning and differentiation of the optic cup neuroepithelium. Development 138, 127–138 [DOI] [PubMed] [Google Scholar]
- 49.Sanuki R., Onishi A., Koike C., Muramatsu R., Watanabe S., Muranishi Y., Irie S., Uneo S., Koyasu T., Matsui R., Chérasse Y., Urade Y., Watanabe D., Kondo M., Yamashita T., Furukawa T. (2011) miR-124a is required for hippocampal axogenesis and retinal cone survival through Lhx2 suppression. Nat. Neurosci. 14, 1125–1134 [DOI] [PubMed] [Google Scholar]
- 50.Marler K. J., Suetterlin P., Dopplapudi A., Rubikaite A., Adnan J., Maiorano N. A., Lowe A. S., Thompson I. D., Pathania M., Bordey A., Fulga T., Van Vactor D. L., Hindges R., Drescher U. (2014) BDNF promotes axon branching of retinal ganglion cells via miRNA-132 and p250GAP. J. Neurosci. 34, 969–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krol J., Krol I., Alvarez C. P., Fiscella M., Hierlemann A., Roska B., Filipowicz W. (2015) A network comprising short and long noncoding RNAs and RNA helicase controls mouse retina architecture. Nat. Commun. 6, 7305.1–7305.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuhn S., Johnson S. L., Furness D. N., Chen J., Ingham N., Hilton J. M., Steffes G., Lewis M. A., Zampini V., Hackney C. M., Masetto S., Holley M. C., Steel K. P., Marcotti W. (2011) miR-96 regulates the progression of differentiation in mammalian cochlear inner and outer hair cells. Proc. Natl. Acad. Sci. USA 108, 2355–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aldrich B. T., Frakes E. P., Kasuya J., Hammond D. L., Kitamoto T. (2009) Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience 164, 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu Q., Sun W., Okano K., Chen Y., Zhang N., Maeda T., Palczewski K. (2011) Sponge transgenic mouse model reveals important roles for the microRNA-183 (miR-183)/96/182 cluster in postmitotic photoreceptors of the retina. J. Biol. Chem. 286, 31749–31760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adijanto J., Castorino J. J., Wang Z. X., Maminishkis A., Grunwald G. B., Philp N. J. (2012) Microphthalmia-associated transcription factor (MITF) promotes differentiation of human retinal pigment epithelium (RPE) by regulating microRNAs-204/211 expression. J. Biol. Chem. 287, 20491–20503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang F. E., Zhang C., Maminishkis A., Dong L., Zhi C., Li R., Zhao J., Majerciak V., Gaur A. B., Chen S., Miller S. S. (2010) MicroRNA-204/211 alters epithelial physiology. FASEB J. 24, 1552–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kutty R. K., Samuel W., Jaworski C., Duncan T., Nagineni C. N., Raghavachari N., Wiggert B., Redmond T. M. (2010) MicroRNA expression in human retinal pigment epithelial (ARPE-19) cells: increased expression of microRNA-9 by N-(4-hydroxyphenyl)retinamide. Mol. Vis. 16, 1475–1486 [PMC free article] [PubMed] [Google Scholar]
- 58.Kutty R. K., Nagineni C. N., Samuel W., Vijayasarathy C., Hooks J. J., Redmond T. M. (2010) Inflammatory cytokines regulate microRNA-155 expression in human retinal pigment epithelial cells by activating JAK/STAT pathway. Biochem. Biophys. Res. Commun. 402, 390–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kutty R. K., Nagineni C. N., Samuel W., Vijayasarathy C., Jaworski C., Duncan T., Cameron J. E., Flemington E. K., Hooks J. J., Redmond T. M. (2013) Differential regulation of microRNA-146a and microRNA-146b-5p in human retinal pigment epithelial cells by interleukin-1β, tumor necrosis factor-α, and interferon-γ. Mol. Vis. 19, 737–750 [PMC free article] [PubMed] [Google Scholar]
- 60.Lin H., Qian J., Castillo A. C., Long B., Keyes K. T., Chen G., Ye Y. (2011) Effect of miR-23 on oxidant-induced injury in human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 52, 6308–6314 [DOI] [PubMed] [Google Scholar]
- 61.Hou Q., Tang J., Wang Z., Wang C., Chen X., Hou L., Dong X. D., Tu L. (2013) Inhibitory effect of microRNA-34a on retinal pigment epithelial cell proliferation and migration. Invest. Ophthalmol. Vis. Sci. 54, 6481–6488 [DOI] [PubMed] [Google Scholar]
- 62.Murad N., Kokkinaki M., Gunawardena N., Gunawan M. S., Hathout Y., Janczura K. J., Theos A. C., Golestaneh N. (2014) miR-184 regulates ezrin, LAMP-1 expression, affects phagocytosis in human retinal pigment epithelium and is downregulated in age-related macular degeneration. FEBS J. 281, 5251–5264 [DOI] [PubMed] [Google Scholar]
- 63.Ohana R., Weiman-Kelman B., Raviv S., Tamm E., Pasmanik-Chor M., Rinon A., Netanely D., Shamir R., Salomon A. S., Ashery-Padan R. (2015) MicroRNAs of the RPE are essential for RPE differentiation and photoreceptor maturation. Development 142, 2487–2498 [DOI] [PubMed] [Google Scholar]
- 64.Kaneko H., Dridi S., Tarallo V., Gelfand B. D., Fowler B. J., Cho W. G., Kleinman M. E., Ponicsan S. L., Hauswirth W. W., Chiodo V. A., Karikó K., Yoo J. W., Lee D. K., Hadziahmetovic M., Song Y., Misra S., Chaudhuri G., Buaas F. W., Braun R. E., Hinton D. R., Zhang Q., Grossniklaus H. E., Provis J. M., Madigan M. C., Milam A. H., Justice N. L., Albuquerque R. J., Blandford A. D., Bogdanovich S., Hirano Y., Witta J., Fuchs E., Littman D. R., Ambati B. K., Rudin C. M., Chong M. M., Provost P., Kugel J. F., Goodrich J. A., Dunaief J. L., Baffi J. Z., Ambati J. (2011) DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature 471, 325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iacovelli J., Zhao C., Wolkow N., Veldman P., Gollomp K., Ojha P., Lukinova N., King A., Feiner L., Esumi N., Zack D. J., Pierce E. A., Vollrath D., Dunaief J. L. (2011) Generation of Cre transgenic mice with postnatal RPE-specific ocular expression. Invest. Ophthalmol. Vis. Sci. 52, 1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tarallo V., Hirano Y., Gelfand B. D., Dridi S., Kerur N., Kim Y., Cho W. G., Kaneko H., Fowler B. J., Bogdanovich S., Albuquerque R. J., Hauswirth W. W., Chiodo V. A., Kugel J. F., Goodrich J. A., Ponicsan S. L., Chaudhuri G., Murphy M. P., Dunaief J. L., Ambati B. K., Ogura Y., Yoo J. W., Lee D. K., Provost P., Hinton D. R., Núñez G., Baffi J. Z., Kleinman M. E., Ambati J. (2012) DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell 149, 847–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dridi S., Hirano Y., Tarallo V., Kim Y., Fowler B. J., Ambati B. K., Bogdanovich S., Chiodo V. A., Hauswirth W. W., Kugel J. F., Goodrich J. A., Ponicsan S. L., Hinton D. R., Kleinman M. E., Baffi J. Z., Gelfand B. D., Ambati J. (2012) ERK1/2 activation is a therapeutic target in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 109, 13781–13786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kerur N., Hirano Y., Tarallo V., Fowler B. J., Bastos-Carvalho A., Yasuma T., Yasuma R., Kim Y., Hinton D. R., Kirschning C. J., Gelfand B. D., Ambati J. (2013) TLR-independent and P2X7-dependent signaling mediate Alu RNA-induced NLRP3 inflammasome activation in geographic atrophy. Invest. Ophthalmol. Vis. Sci. 54, 7395–7401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y., Tarallo V., Kerur N., Yasuma T., Gelfand B. D., Bastos-Carvalho A., Hirano Y., Yasuma R., Mizutani T., Fowler B. J., Li S., Kaneko H., Bogdanovich S., Ambati B. K., Hinton D. R., Hauswirth W. W., Hakem R., Wright C., Ambati J. (2014) DICER1/Alu RNA dysmetabolism induces caspase-8-mediated cell death in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 111, 16082–16087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gelfand B. D., Wright C. B., Kim Y., Yasuma T., Yasuma R., Li S., Fowler B. J., Bastos-Carvalho A., Kerur N., Uittenbogaard A., Han Y. S., Lou D., Kleinman M. E., McDonald W. H., Núñez G., Georgel P., Dunaief J. L., Ambati J. (2015) Iron toxicity in the retina requires Alu RNA and the NLRP3 inflammasome. Cell Rep. 11, 1686–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lumayag S., Haldin C. E., Corbett N. J., Wahlin K. J., Cowan C., Turturro S., Larsen P. E., Kovacs B., Witmer P. D., Valle D., Zack D. J., Nicholson D. A., Xu S. (2013) Inactivation of the microRNA-183/96/182 cluster results in syndromic retinal degeneration. Proc. Natl. Acad. Sci. USA 110, E507–E516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin Z. B., Hirokawa G., Gui L., Takahashi R., Osakada F., Hiura Y., Takahashi M., Yasuhara O., Iwai N. (2009) Targeted deletion of miR-182, an abundant retinal microRNA. Mol. Vis. 15, 523–533 [PMC free article] [PubMed] [Google Scholar]
- 73.Landthaler M., Gaidatzis D., Rothballer A., Chen P. Y., Soll S. J., Dinic L., Ojo T., Hafner M., Zavolan M., Tuschl T. (2008) Molecular characterization of human Argonaute-containing ribonucleoprotein complexes and their bound target mRNAs. RNA 14, 2580–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu Q., Tanasa B., Trabucchi M., Li W., Zhang J., Ohgi K. A., Rose D. W., Glass C. K., Rosenfeld M. G. (2012) DICER- and AGO3-dependent generation of retinoic acid-induced DR2 Alu RNAs regulates human stem cell proliferation. Nat. Struct. Mol. Biol. 19, 1168–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park C. Y., Jeker L. T., Carver-Moore K., Oh A., Liu H. J., Cameron R., Richards H., Li Z., Adler D., Yoshinaga Y., Martinez M., Nefadov M., Abbas A. K., Weiss A., Lanier L. L., de Jong P. J., Bluestone J. A., Srivastava D., McManus M. T. (2012) A resource for the conditional ablation of microRNAs in the mouse. Cell Rep. 1, 385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prosser H. M., Koike-Yusa H., Cooper J. D., Law F. C., Bradley A. (2011) A resource of vectors and ES cells for targeted deletion of microRNAs in mice. Nat. Biotechnol. 29, 840–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Y., Palczewski K. (2015) Systems pharmacology links GPCRs with retinal degenerative disorders. [E-pub ahead of print] Annu. Rev. Pharmacol. Toxicol. 10.1146/annurev-pharmtox-010715-103033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y., Palczewska G., Mustafi D., Golczak M., Dong Z., Sawada O., Maeda T., Maeda A., Palczewski K. (2013) Systems pharmacology identifies drug targets for Stargardt disease-associated retinal degeneration. J. Clin. Invest. 123, 5119–5134 [DOI] [PMC free article] [PubMed] [Google Scholar]