Abstract
Background
N-terminal-pro-B-type natriuretic peptide (NT-proBNP) and cardiac troponin T (TnT) predict cardiovascular disease (CVD) risk in a variety of populations. Whether their predictive value varies by ethnicity is unknown. We sought to determine: whether NT-proBNP and TnT improve prediction of incident coronary heart disease (CHD) and CVD, independent of CVD risk factors, in a multi-ethnic population; whether NT-proBNP improves prediction compared to the Framingham Risk Score (FRS) or the Pooled Cohort Risk Equation (PCRE); and whether a second NT-proBNP further improves prediction.
Methods
NT-proBNP and TnT were measured in 5592 Multi-Ethnic Study of Atherosclerosis white, black, Hispanic and Chinese participants (60% nonwhite, mean age 62.3±10.3) in 2000–2002 and 2004–2005. We evaluated adjusted risk of incident CHD and CVD based on baseline and change in biomarker concentration.
Results
Participants were followed through 2011 and incurred 370 CVD events (232 CHD). NT-proBNP and TnT concentrations varied by ethnicity. NT-proBNP and TnT were associated with an increased risk of events (adjusted HR for CHD [95% CI] for 5th versus other 4 quintiles of NT-proBNP, 2.03[1.50–2.76]; HR for CHD for detectable versus undetectable TnT, 3.95[2.29–6.81]). NT-proBNP improved risk prediction and classification compared to the FRS and the PCRE. Change in NT-proBNP was independently associated with events (HR for CHD per unit increase in ΔlogNT-proBNP, 1.95[1.16–3.26]). None of the observed associations varied by ethnicity.
Conclusions
NT-proBNP and TnT are predictors of incident CHD, independent of established risk factors and ethnicity, in a multi-ethnic population without known CVD. Change in NT-proBNP may add additional prognostic information.
Keywords: cardiovascular disease, coronary heart disease, ethnicity, race, natriuretic peptides, risk classification, biomarkers
Introduction
A significant number of individuals who are at risk for developing cardiovascular disease (CVD) are not currently identified by traditional screening methods. Blood-based biomarkers provide an attractive adjunctive methodology for identifying individuals at higher risk for adverse cardiovascular events. Natriuretic peptide (including B-type natriuretic peptide [BNP] and the N-terminal fragment of proBNP [NT-proBNP]) and cardiac troponin are associated with long-term risk of cardiovascular outcomes in the general population.1–13 In addition, measuring serial concentrations of natriuretic peptides 2 to 3 years may further improve prediction of CVD.9 However, the incidence and prevalence of CVD varies by ethnicity within the United States;14 ethnicity may also affect natriuretic peptide and troponin concentrations.15 For instance, prior studies have suggested that African Americans may have relatively lower natriuretic peptide concentrations compared to non-Hispanic whites,15, 16 though other studies have not found any difference.17–19 Regardless of whether natriuretic peptide concentrations vary by race/ethnicity, little is known about whether their association with incident CVD varies by race/ethnicity. Though some studies of natriuretic peptides for CVD risk prediction have focused on non-white populations,20 and others have included moderate numbers of a single minority group, there are sparse multiethnic data within a single study, and none in Hispanic or Chinese populations. In addition, little is known about the prognostic value of troponin and serial natriuretic peptide concentrations in various minority groups. The purpose of the present analysis was to determine whether NT-proBNP (single and serial measures) and TnT are predictive of incident CVD in a diverse cohort from the Multi-Ethnic Study of Atherosclerosis (MESA), a prospective, population-based study of white, black, Hispanic, and Chinese individuals without clinical CVD at baseline. We also wanted to determine whether the addition of these biomarkers to established CVD risk prediction scores, including the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) Pooled Cohort Equation, could improve performance of the risk score.
Methods
Study Population
MESA is a multicenter, prospective, population-based study that was designed to investigate the prevalence, correlates, and progression of sub clinical CVD in asymptomatic individuals of four ethnicities in the United States. A detailed description of the study methods has been published previously.21 Briefly, between July 2000 and August 2002, 6814 men and women who identified themselves as white, African-American, Hispanic, or Chinese, were 45 to 84 years old, and were free of clinically apparent CVD were recruited from portions of 6 communities in the United States.
Individuals with a history of physician-diagnosed myocardial infarction (MI), angina, heart failure, stroke, or transient ischemic attack, or having undergone an invasive procedure for CVD were excluded from participation. The institutional review boards at all participating centers approved the study, and all participants gave informed consent. The present analysis was approved by the institutional review board of the University of California, San Diego. The trial was registered at www.clinicaltrials.gov (Registration Number: NCT00005487).
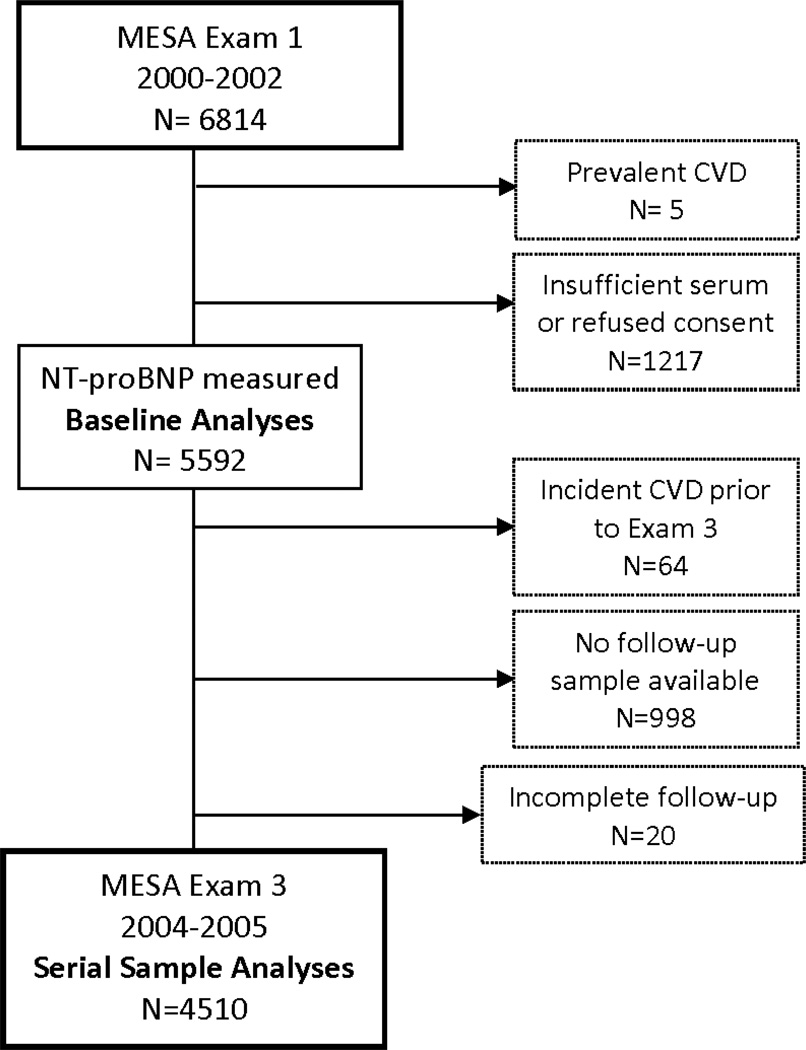
Of the 6814 MESA participants, 5 were excluded for prevalent CVD; of the remaining 6809 study participants, consent to participate in industry-funded research and sufficient serum from the baseline Exam 1 in 2000–2002 for measurement of NT-proBNP was available in 5592 (82.1%) (Figure 1). Of these, 4510 were included in serial sample analyses based upon complete follow-up without incident CVD through Exam 3 (2004–2005) and sufficient serum for a subsequent NT-proBNP.
Figure 1.
Flow diagram of MESA participants
Primary Outcomes
Details regarding the MESA processes and criteria for identifying, investigating, and classifying cardiovascular events have been previously reported.22 Participants were followed for clinical events via telephone interview every 9 to 12 months through 2011.
Outcomes were incident coronary heart disease (CHD), and incident CVD. CHD was defined as fatal or non-fatal MI, or resuscitated cardiac arrest. CVD was defined as CHD, stroke, or stroke death.
Assay Methods
All samples were stored at −70° C and were thawed prior to testing (maximum of 3 freeze-thaw cycles). NT-proBNP and cardiac troponin T (TnT) were measured in serum collected at the baseline Exam 1 and at Exam 3 using the Elecsys 2010 system (Roche Diagnostics, Indianapolis, IN). All analyses were performed at a core lab (Veteran’s Affairs San Diego Healthcare System, La Jolla, CA). The TnT assay is a contemporary-sensitive, but not a “highly sensitive” troponin assay. Prior studies have shown that measurements of NT-proBNP using this assay do not change after 5 freeze-thaw cycles.23 Intra-assay and inter-assay coefficients of variation at various concentrations of NT-proBNP and TnT have been previously reported.15, 24, 25 The analytical measurement range for NT-proBNP was 5–35,000 pg/mL, and for TnT was 0.01–25.0 ng/ml.
Other Covariates
Medical history, anthropometric measurements, and laboratory data for the present study were taken from Exam 1 of the MESA cohort (July 2000 to August 2002), as previously described.21 Hypertension was defined as systolic blood pressure (SBP) >140 mmHg, diastolic blood pressure (DBP) >90 mmHg, or use of antihypertensive medication. Diabetes was defined as a fasting glucose ≥126 mg/dL or use of hypoglycemic medications. Current smoking was defined as having smoked a cigarette in the last 30 days. Coronary artery calcification (CAC) was measured by coronary computed tomography as previously described.22 Estimated glomerular filtration rate (GFR) was calculated using the simplified Modification of Diet in Renal Disease study formula.26
Statistical methods
Measured variables are presented as means ± standard deviation (SD) or medians (interquartile range), as appropriate. NT-proBNP concentrations were not normally distributed and were log10-transformed for continuous analyses. Chi-square tests and t-tests tests were used to test for differences between quintiles of NT-proBNP in categorical and continuous variables, respectively. The Jonckheere-Terpstra test was used to compare medians across quintiles of NT-proBNP.
Single-predictor associations between the clinical variables listed in Table 1 and logNT-proBNP were determined by linear regression analyses; predictors of detectable TnT were determined by Spearman’s correlation analyses. Backward multivariable regression analysis including variables with significant individual associations was used to determine which covariates were independently associated with logNT-proBNP, while backward logistic regression analysis was used to determine multivariable associations with detectable TnT; race was then entered in stepwise fashion.
Table I.
Characteristics of participants by baseline NT-proBNP concentration
| Overall n=5592 (4.90–11699 pg/mL) |
1st n=1120 (4.90–19.24 pg/mL) |
2nd n=1119 (19.25–40.86 pg/mL) |
3rd n=1119 (40.87–71.00 pg/mL) |
4th n=1119 (71.01–135.40 pg/mL |
5th n=1115 (135.41–11699 pg/mL) |
p-value | |
|---|---|---|---|---|---|---|---|
| Age (years) | 62.3 ± 10.3 | 55.7 ± 8.1 | 59.1 ± 9.1 | 62.0 ± 9.7 | 64.8 ± 9.7 | 69.6 ± 9.2 | <0.001 |
| Male | 48.5% | 71.6% | 55.9% | 45.0% | 36.8% | 33.2% | <0.001 |
| Race/Ethnicity | <0.001 | ||||||
| Non-Hispanic White | 39.6% | 25.2% | 36.9% | 40.4% | 45.6% | 50.0% | |
| Chinese | 13.3% | 18.0% | 13.0% | 14.1% | 12.4% | 8.8% | |
| African-American | 24.2% | 32.9% | 25.9% | 22.5% | 19.4% | 20.2% | |
| Hispanic | 23.0% | 23.9% | 24.2% | 23.0% | 22.6% | 21.0% | |
| Risk factors | |||||||
| Body mass index (kg/ m2) | 23.2 ±5.5 | 28.8 ±5.0 | 28.6±5.4 | 28.3±5.7 | 27.8±5.5 | 27.6±5.6 | <0.001 |
| Current smoking | 12.5% | 16.6% | 13.7% | 11.7% | 10.5% | 9.9% | <0.001 |
| Hypertension | 47.7% | 31.3% | 39.6% | 46.6% | 52.0% | 69.3% | <0.001 |
| Diabetes | 12.5% | 14.6% | 13.0% | 10.4% | 11.1% | 13.2% | 0.02 |
| Family history of Ml | 42.1% | 36.9% | 40.8% | 42.5% | 42.9% | 47.4% | <0.001 |
| Hemodynamic parameters | |||||||
| SBP(mmHg) | 126±21 | 119±15 | 122±18 | 126±21 | 120±21 | 138±26 | <0.001 |
| DBP(mmHg) | 72±10 | 74±9 | 73±10 | 72±10 | 70±10 | 70±11 | <0.001 |
| Heart rate (bpm) | 63±10 | 65±10 | 64±10 | 63±10 | 62±10 | 62±10 | <0.001 |
| Current use of cardiovascular medication | |||||||
| ACB or ARB | 17.5% | 14.2% | 15.9% | 16.8% | 17.7% | 23.1% | <0.001 |
| Aspirin (3+times/week) | 20.1% | 13.3% | 19.4% | 18.4% | 22.0% | 27.2% | <0.001 |
| Beta-blocker | 9.8% | 3.8% | 4.2% | 7.4% | 10.4% | 23.1% | <0.001 |
| Diuretic | 13.0% | 8.3% | 10.8% | 11.7% | 13.4% | 20.6% | <0.001 |
| Anti-hypertensive | 32.6% | 23.9% | 26.5% | 32.1% | 32.9% | 47.6% | <0.001 |
| Statin | 14.7% | 13.1% | 14.1% | 14.1% | 14.7% | 17.6% | 0.04 |
| Laboratory values | |||||||
| LDL cholesterol (mg/dL) | 117±31 | 121 ±32 | 120±31 | 116±31 | 116±30 | 112±32 | <0.001 |
| HDL cholesterol (mg/dL)* | 48(19) | 44(14) | 46(16) | 49(19) | 51(20) | 53(21) | <0.001 |
| Total cholesterol (mg/dL) | 194±35 | 197±37 | 194±35 | 193±37 | 194±34 | 192±36 | 0.08 |
| Triglycerides (mg/ dL)* | 113(84) | 123(102) | 116(88) | 111(85) | 108(76) | 109(80) | <0.001 |
| eGFR(mL/min/1.73m2) | 81 ±19 | 87±16 | 83±22 | 82±17 | 79±17 | 74±19 | <0.001 |
| Detectable troponin T | 1.5% | 0.2% | 0.6% | 0.8% | 0.8% | 4.9% | <0.001 |
| NT-proBNP(pg/mL)* | 54(88) | 9(9) | 30(11) | 55(15) | 96(31) | 214(147) | <0.001 |
| CAC Agatston Score =0 | 50.9% | 40.4% | 46.4% | 48.5% | 53.7% | 65.6% | <0.001 |
| CACAgatston score* | 92 | 49 | 77 | 84 | 93 | 156 | <0.001 |
| Incident CHD† | 232(4.4) | 27(2.5) | 34(3.2) | 38(3.6) | 42(4.0) | 91 (9.4) | <0.001 |
| Incident CVD† | 370(7.1) | 44(4.1) | 48(4.5) | 68(6.4) | 67(6.4) | 143(15.1) | <0.001 |
ACS, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAC coronary artery calcification; CHD, coronary heart disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; Ml, myocardial infarction; SBP, systolic blood pressure.
Cell values represent mean ± SD or percent, except where indicated.
Median (interquartile range)
n( n per 1000 years)
Differences in event rates over time by quintile of NT-proBNP, and stratified by ethnicity, were plotted with Kaplan-Meier curves, and tested for significance with long-rank tests. For time-to-event analyses, NT-proBNP was modeled both by quintiles and as a log-transformed linear variable. Cox proportional hazards models were used to estimate the association between initial NT-proBNP and time to the main outcomes of interest (CHD or CVD) after adjusting for covariates in a series of models. Model 1 adjusted for demographic factors (age, sex, and ethnicity); Model 2 included the covariates of Model 1 plus clinical factors (current smoking, family history of heart attack, diabetes, use of statins, use of anti-hypertensive medication, body mass index, systolic blood pressure, high density lipoprotein (HDL)-cholesterol, low density lipoprotein (LDL)-cholesterol, and glomerular filtration rate); Model 3 included the covariates of Model 2 plus troponin category (detectable vs. undetectable); Model 4 included the covariates of Model 3 plus CAC category (Agatston score non-zero vs. zero). In addition, we evaluated 2 other models to determine whether logNT-proBNP improves upon 2 established cardiovascular risk scores, and limited the analysis to participants not taking cholesterol-lowering medications at baseline. Model 5 adjusted for the Framingham Risk Score. For CHD outcomes we used the JAMA Framingham risk survival model for 10-year risk of hard CHD,27 and for CVD outcomes we used the Framingham Global CVD Risk formula for 10-year risk of CVD.28 Model 6 (calculated only for CVD outcome analyses) adjusted for the Pooled Cohort Equation for estimation of 10-year risk of hard atherosclerotic CVD.29 For each of these analyses, NT-proBNP was modelled both as a continuous variable with the hazard ratio (HR) reported per 1 unit logNT-proBNP, and also as a categorical variable (HR for the 5th quintile versus the other 4 quintiles). Formal and graphical methods were used to test the assumption of proportional hazards. Effect modification by race was estimated by testing of appropriate multiplicative interaction terms, and by evaluating each ethnic group separately.
The c-statistic, modified for survival-time analyses, was used to assess the incremental gain in risk discrimination from the addition of logNT-proBNP to baseline models. Cox model increment tests were used to assess whether prediction improved with the addition of logNT-proBNP. Integrated discrimination improvement (IDI) and the continuous net reclassification index (NRI>0) for the addition of logNT-proBNP to each model were calculated based on the methods of Pencina et al.30 For discrimination and reclassification analyses, we estimated risk at 10 years.
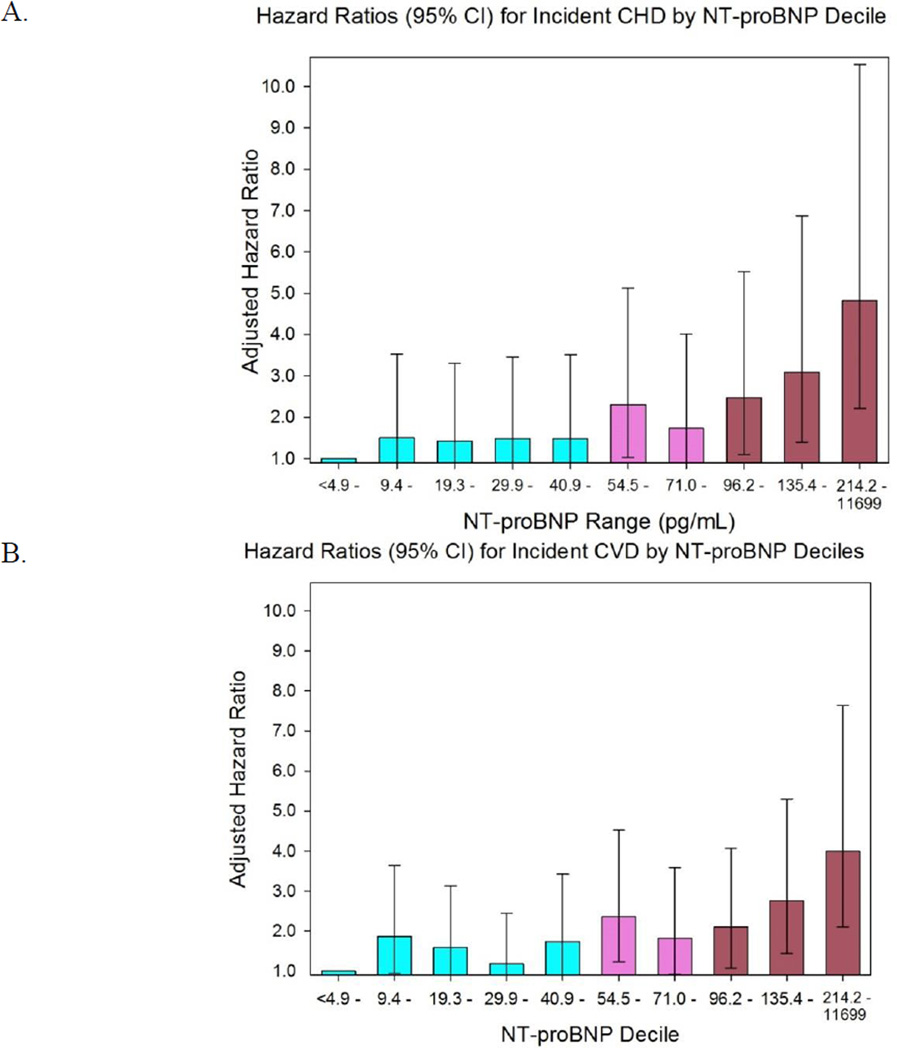
For analyzing serial NT-proBNP concentrations, we modelled the change in NT-proBNP as both a continuous and a categorical variable in separate approaches. For the former, we determined the difference in logNT-proBNP between the 3rd and baseline visits, and adjusted all analyses for baseline logNT-proBNP. For the latter, we adapted the model of deFilippi et al and first determined the optimal NT-proBNP cut-point for increased CVD risk.9 This was done in 2 ways: first, via receiver operator characteristic (ROC) curve analysis, and second, by plotting the adjusted hazard ratio (HR) for each decile of NT-proBNP (Figure 2). From these analyses, a cut-point of 80 pg/mL was identified visually, corresponding to a value between the 7th and 8th decile. For individuals with an initial NT-proBNP <80 pg/mL, the risk of incident CHD and CVD was determined for: (1) those whose subsequent NT-proBNP decreased by at least 25%; and (2) those whose NT-proBNP increased by at least 25%, to a concentration ≥80 pg/mL, compared to those with neither change. For individuals with an initial NT-proBNP ≥80 pg/mL, the risk of incident CHD and CVD was similarly determined for: (1) those whose NT-proBNP decreased by at least 25%, to a concentration <80 pg/mL; and (2) those whose NT-proBNP increased by at least 25%, compared to those with neither change. The 25% threshold for change was based upon the reported intra-individual variability of NT-proBNP in stable chronic heart failure patients, and the threshold used in previous studies.9, 31
Figure 2.
Adjusted hazard ratios for incident CHD (A) and CVD (B) by decile of NT-proBNP. Hazard ratios are adjusted for age, sex, race, current smoking (Y/N), family history of heart attack, diabetes, use of anti-hypertensive therapy, use of statin therapy, body mass index, systolic blood pressure, LDL-cholesterol, HDL-cholesterol, and glomerular filtration rate.
Finally, we developed a simple multimarker score which incorporated serial measurements of both NT-proBNP and TnT, and was equal to the number of markers that were persistently elevated or rising (0, 1, or 2) at visits 1 and 3. Kaplan Meier curves, Cox proportional hazard models, and discrimination and reclassification analyses (based on risk estimated at 7 years) are presented, as described above.
Statistical analysis was performed with SPSS version 19.0 (SPSS Inc., Chicago, Illinois). All p-values were 2-tailed, and p<0.05 was considered statistically significant. This study was supported by the National Heart, Lung, and Blood Institute grant (R01-HL66075-01), the Multi-Ethnic Study of Atherosclerosis study contracts (N01-HC-95162, N01-HC-95168, and N01-HC-95169), and Roche Diagnostics. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Subject Characteristics
The baseline characteristics of participants overall and by quintile of NT-proBNP are shown in Table I. The mean age was 62 ± 10 years, and 49% were male. Approximately one quarter of participants were African-American and one quarter were Hispanic, while a higher percentage were non-Hispanic white and a lower percentage were Chinese-American. Nearly half (48%) had a history of hypertension, and 13% had diabetes. The median NT-proBNP concentration was 54 pg/mL (24–112 pg/mL). Overall, 51% of participants had a non-zero CAC score, and 1.5% had detectable TnT (>0.01 ng/mL). African-Americans were the most likely to have detectable TnT (2.7%), followed by Hispanics (1.9%) and non-Hispanic whites (0.9%); Chinese-Americans had the lowest prevalence of detectable TnT (0.3%) (p<0.001). Prevalence of detectable TnT still varied significantly by race after adjusting for age, sex, and GFR (p<0.001). Supplementary Table I shows the characteristics of the 5592 participants included in baseline analyses versus the 1217 who were excluded, as well as the 4510 included in the serial sample analyses compared to the 1082 with only a single NT-proBNP measurement. Individuals included in baseline analyses were on average one year younger, more likely to be male, and varied slightly in other baseline variables compared to individuals who were excluded. Individuals included in serial sample analyses were somewhat younger and healthier overall, compared to those with only baseline measurements.
Univariable and Multivariable Correlates of NT-proBNP and TnT
As expected, individuals with higher NT-proBNP concentrations were older, had lower BMI, and were more likely to be female (Tables I and II). They also tended to have a lower GFR, and a higher prevalence of detectable TnT and non-zero CAC score. Non-Hispanic white individuals were more likely to have higher NT-proBNP concentrations.
Table II.
Individual and Multivariable Covariates of logNT-proBNP and Detectable TnT
| NT-proBNP Univariable |
NT-proBNP Multivariable |
Detectable TnT Univariable |
Detectable TnT Multivariable | |||||
|---|---|---|---|---|---|---|---|---|
| r | P | P | P | r | P | Odds Ratio (95% CI) |
P | |
| Age (years) | 0.46 | <0.001 | 0.31 | <0.001 | 0.09 | <0.001 | ||
| Male sex | −0.27 | <0.001 | −0.20 | <0.001 | 0.07 | <0.001 | 5.36(3.10–9.25) | <0.001 |
| Race/Ethnicity | <0.001 | <0.001 | ||||||
| Non-Hispanic White | 0.17 | <0.001 | Reference | - | −0.04 | 0.005 | Reference | - |
| African-American | −0.11 | <0.001 | −0.14 | <0.001 | 0.06 | <0.001 | 3.25(2.00–5.27) | <0.001 |
| Hispanic | −0.02 | 0.20 | 0.05 | 0.001 | 0.02 | 0.17 | 1.35(0.80–230) | 0.26 |
| Chinese | −0.09 | <0.001 | −0.09 | <0.001 | −0.04 | 0.004 | 0.30(0.10–0.87) | 0.03 |
| Risk factors | ||||||||
| Body mass index (kg/ m2) | −0.08 | <0.001 | 0.03 | 0.03 | ||||
| Current smoking | −0.07 | <0.001 | −0.02 | 0.08 | ||||
| Diabetes | −0.006 | 0.65 | 0.10 | <0.001 | 3.00(1.75–5.16) | <0.001 | ||
| Hemodynamic parameters | ||||||||
| SBP(mmHg) | 0.29 | <0.001 | 0.24 | <0.001 | 0.06 | <0.001 | ||
| DBP(mmHg) | −0.11 | <0.001 | −0.13 | <0.001 | 0.03 | 0.02 | ||
| Heart rate(bpm) | −0.12 | <0.001 | −0.10 | <0.001 | 0.001 | 0.94 | ||
| Current use of cardiovascular medication | ||||||||
| ACB or ARB | 0.09 | <0.001 | 0.08 | <0.001 | ||||
| Aspirin (3+ times/week) | 0.11 | <0.001 | 0.04 | 0.002 | ||||
| Beta-blocker | 0.23 | <0.001 | 0.13 | <0.001 | 0.04 | 0.008 | ||
| Duretic | 0.12 | <0.001 | 0.05 | <0.001 | ||||
| Laboratory values | ||||||||
| LDL cholesterol (mg/dL) | −0.10 | <0.001 | −0.08 | <0.001 | −0.02 | 0.25 | ||
| HDL cholesterol (mg/dg* | 0.21 | <0.001 | 0.09 | <0.001 | −0.03 | 0.03 | ||
| Total cholesterol (mg/dL) | −0.04 | 0.003 | −0.02 | 0.07 | ||||
| Triglyoerides (mg/dL)* | −0.09 | <0.001 | −0.004 | 0.76 | ||||
| eGFR(mL/min/1.73m2) | −0.26 | <0.001 | −0.04 | <0.001 | −0.08 | <0.001 | 0.97(0.95–0.98) | <0.001 |
| NT-proBNP (pg/mg)* | N/A | N/A | 0.13 | <0.001 | 10.18(6.20–16.72) | <0.001 | ||
| Detectable troponin T | 0.16 | <0.001 | 0.13 | <0.001 | N/A | N/A | N/A | - |
| CACAgatst on Score >0 | 0.17 | <0.001 | 0.06 | <0.001 | ||||
| CAC Agatston score* | 0.15 | <0.001 | N/A | - | 0.07 | <0.001 | N/A | - |
Abbreviations as in Table 1.
r = correlation coeffident
β = standardized regression coefficient
Adjusted R2 =0.42 for IgNT-proBNP and 0.34 for TnT
Log transformed
On multivariable analysis, variables that remained associated with higher NT-proBNP were older age; female sex; race/ethnicity; higher SBP, HDL-cholesterol; lower DBP, heart rate, LDL-cholesterol, and GFR; use of a beta blocker; and detectable TnT (Table II). All together, these variables accounted for approximately 40% of the variability in NT-proBNP (R2=0.42). African-Americans and Chinese-Americans had lower NT-proBNP concentrations compared to non-Hispanic whites and Hispanics, on multivariable analysis.
Multivariable predictors of detectable TnT levels included male sex, race/ethnicity, diabetes, lower GFR, and higher NT-proBNP (R2=0.34) (Table II). On multivariable analysis, African-Americans were more likely to have detectable TnT while Chinese-Americans were less likely.
Baseline NT-proBNP, TnT, and Outcomes
Participants were followed for a median of 10.2 (1.1) years and a maximum of 11.5 years. During this time there were 232 (4.1%) incident CHD events (71 fatal) including 221 participants with MI and 19 with resuscitated cardiac arrest; and 370 (6.6%) incident CVD events (102 fatal), including 156 strokes (some participants had more than one event). Event rates were highest for Hispanics (5.0 and 8.2 events per 1000 person-years for CHD and CVD, respectively), and lowest for Chinese-Americans (2.3 and 3.9 events per 1000 person-years).
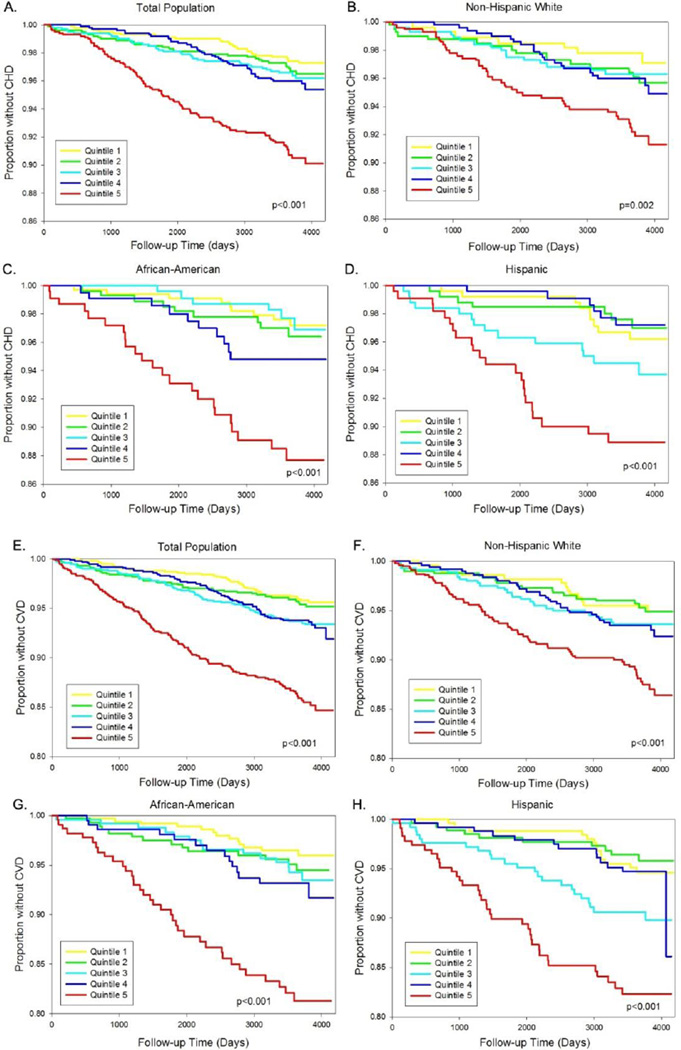
On Kaplan Meyer analysis, higher concentration of NT-proBNP was associated with an increased risk of incident CHD and CVD (p<0.01 for both), with an especially high risk seen for those in the highest quintile (Figure 3, A & E). When stratified by ethnic group results were similar (Figure 3, B–D & F–H).
Figure 3.
Kaplan-Meier plots based on quintile of NT-proBNP, by ethnicity. The plots show risk of incident CHD and CVD among (A, E) all participants, (B, F) non-Hispanic whites, (C, G) African Americans, and (D, H) Hispanics.
Multivariable Cox proportional hazard models were used to evaluate the adjusted risk of CHD or CVD for the highest quintile versus the other 4 quintiles (Table III), as well as for each unit increase in logNT-proBNP (Supplementary Table II). After adjusting for age, sex, and ethnicity, NT-proBNP was strongly and significantly associated with both CHD and CVD (Model 1). This association remained strong after further adjusting for clinical variables (Model 2) and TnT (Model 3). In these models, TnT was also an independent predictor of both outcomes. Individuals with detectable TnT had a 4.0 times increased risk of CHD (95% confidence interval [CI] 2.3–6.8, p<0.001), and a 3.0 times increased risk of CVD (95% CI 1.8–4.8, p<0.001) compared to individuals with undetectable TnT. With the further addition of CAC score (Model 4), all three markers (NT-proBNP, TnT, and CAC) remained independently and significantly associated with both outcomes.
Table III.
Hazard Ratio for incident CHD and CVD in 5th quintile of NT-proBNP versus other 4 quintiles.
| P | HR for 5th Quintile NT-proBNP vs other 4 Quintiles(95%CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | P | White | P | AA | P | Hispanic | P | Chinese | P | ||
| CHD | (232/5592) | (101/2214) | (57/1352) | (58/1284) | (16/742) | ||||||
| Model 1 | 2.25(1.68–3.02) | <0.001 | 1.77(1.13–277) | 0.01 | 3.61 (2.04–6.38) | <0.001 | 2.54(1.42–4.53) | 0.002 | 1.35(0.44–4.15) | 0.61 | |
| Model 2 | 2.22(1.64–3.00) | <0.001 | 1.88(1.18–3.00) | 0.008 | 3.13(1.71–572) | <0.001 | 2.12(1.15–3.92) | 0.02 | 1.41(0.41–4.81) | 0.58 | |
| Model 3 | |||||||||||
| HR for 5th Quintle NT-proBNP | 2.03(1.50–276) | <0.001 | 1.84(1.15–294) | 0.01 | 2.78(1.50–516) | 0.001 | 1.66(0.87–3.20) | 0.13 | 1.46(0.44–4.91) | 0.54 | |
| HR for detectable TnT | 3.95(2.29–6.81) | <0.001 | 2.49(0.75–8.30) | 0.14 | 5.79(226–14.81) | <0.001 | 4.60(1.58–13.40) | 0.005 | N/A* | N/A | |
| Model 4 | |||||||||||
| HR for 5th quintile NT-proBNP | 1.99(1.45–270) | <0.001 | 1.83(1.14–295) | 0.01 | 2.67(1.44–4.95) | 0.002 | 1.56(0.82–297) | 0.18 | 1.20(0.34–4.20) | 0.77 | |
| HR for detectable TnT | 3.94(2.30–6.77) | <0.001 | 2.30(0.69–762) | 0.17 | 6.25(244–16.05) | <0.001 | 5.00(1.75–14.28) | 0.003 | N/A* | N/A | |
| HR for detectable CAC | 2.62(1.81–3.80) | <0.001 | 3.00(1.60–562) | 0.001 | 2.02(1.09–3.76) | 0.03 | 2.81(1.29–6.12) | 0.009 | 8.14(1.04–63.81) | 0.046 | |
| Model 5 | 2.78(2.06–3.77) | <0.001 | 2.19(1.40–3.44) | 0.001 | 4.27(2.36–773) | <0.001 | 2.09(1.08–4.07) | 0.03 | 3.51(1.05–11.72) | 0.04 | |
| CVD | (370/5592) | (157/2214) | (91/1352) | (95/1284) | (27/742) | ||||||
| Model 1 | 2.06(1.63–260) | <0.001 | 1.50(1.05–215) | 0.03 | 3.41 (2.17–537) | <0.001 | 2.37(1.50–3.73) | <0.001 | 1.34(0.53–3.36) | 0.53 | |
| Model 2 | 1.88(1.48–240) | <0.001 | 1.45(0.99–211) | 0.05 | 2.93(1.81–4.72) | <0.001 | 1.92(1.19–3.12) | 0.008 | 1.69(0.61–4.71) | 0.31 | |
| Model 3 | |||||||||||
| HR for 5th Quintile NT-proBNP | 1.78(1.39–227) | <0.001 | 1.43(0.98–208) | 0.06 | 2.73(1.68–4.45) | <0.001 | 1.59(0.96–262) | 0.07 | 1.75(0.64–4.79) | 0.28 | |
| HR for detectable TnT | 2.96(1.84–4.77) | <0.001 | 2.27(0.81–6.35) | 0.12 | 2.74(1.20–6.28) | 0.02 | 5.24(223–1232) | <0.001 | N/A* | N/A | |
| Model 4 | |||||||||||
| HR for 5th quintile NT-proBNP | 1.75(1.37–223) | <0.001 | 1.42(0.98–208) | 0.07 | 2.65(1.63–4.30) | <0.001 | 1.55(0.94–253) | 0.08 | 1.56(0.56–4.36) | 0.40 | |
| HR for detectable TnT | 2.96 (1.84–4.76) | <0.001 | 2.13(0.76–593) | 0.15 | 2.87(1.25–6.58) | 0.01 | 5.75(249–13.30) | <0.001 | N/A* | N/A | |
| HR for detectable CAC | 1.88(1.44–246) | <0.001 | 2.02(1.28–3.19) | 0.002 | 1.65(1.02–266) | 0.04 | 2.11(1.23–3.63) | 0.007 | 2.49(0.90–6.88) | 0.08 | |
| Model 5 | 2.28(1.79–289) | <0.001 | 1.88(1.31–269) | 0.001 | 2.78(1.74–4.46) | <0.001 | 2.41(1.47–3.95) | <0.001 | 2.11(0.81–552) | 0.13 | |
| Model 6 | 1.75(1.37–224) | <0.001 | 1.40(0.96–203) | 0.08 | 2.56(1.58–4.15) | <0.001 | 1.72(1.03–288) | 0.04 | 1.46(0.54–3.95) | 0.46 | |
AA, African American; CAC, coronary artery calcium; CHD, coronary heart disease; CVD, cardiovascular disease; TnT, troponin T.
Model 1 - age, sex, ethnicity (except in ethnicity-specific analyses)
Model 2 - Model 1 + clinical variables
Model 3 - Model 2 + troponin T (detectable vs undetectable)
Model 4 - Model 3 + CAC (yes/ no)
Model 5 - Framingham risk score (log-transformed; limited to n=4630 participants not taking cholesterol-lowering medication)
Model 6 (for CV D only) - Pooled Cohort Equation (log-transformed; limited to n=4630 participants not taking cholesterol - lowering medication )
Only 2 Chinese individuals had detectable troponin T.
No differences in the associations of NT-proBNP or TnT were observed among ethnicities for either outcome (tests of interaction: p>0.10 for all), though there was insufficient power to detect significant differences in some ethnicities, and the relative strengths of NT-proBNP versus TnT appeared to vary by ethnic group.
Baseline NT-proBNP and Improvement in Model Performance
To assess model accuracy, the c-statistic was calculated for each model without and then with the addition of NT-proBNP. As shown in Table IV, the addition of NT-proBNP to the model with clinical risk factors modestly improved the c-statistic from 0.758 to 0.770 for CHD (p<0.001), and from 0.760 to 0.769 (p<0.001) for CVD. The IDI was also calculated, and showed that NT-proBNP improved risk discrimination compared to clinical risk factors alone, for both outcomes. Finally, we assessed the ability of NT-proBNP to improve risk classification using the continuous NRI>0. The use of NT-proBNP on top of clinical risk factors resulted in a net 27% improvement in classification of CHD risk, and a net 20% improvement in classification of CVD risk (p<0.001 for both). The reclassification improvement in both cases resulted from a combination of accurate upward reclassification of risk among individuals who went on to have events (the “event NRI”), as well as from correct downgrading of risk among individuals who remained event-free (the “non-event NRI”).
Table IV.
AUC NR>0 and ID of Models with and without NT-pro BNP and Change in NT-pro BNP for Predicting CHD and CVD
| C-statistic | NR>0) | IDI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHD | CVD | CHD | CVD | |||||||||||
| Model | CHD | CVD | Overall | Event | Non-Event | P | Overall | Event | Non-Event | P | IDI | P | IDI | P |
| Clinical Risk Factors | 0.758(0.731–0.785) | 0.760(0.739–0.781) | N/A | N/A | ||||||||||
| + NT-proBNP | 0.770(0.741–0.798) | 0.769(0.748–0.791) | 26.7% | 15.9% | 10.8% | <0.001 | 19.9% | 12.6% | 7.3% | <0.001 | 0.018 | <0.001 | 0.017 | <0.001 |
| +change in NT-proBNP | 0.789(0.756–0.821) | 0.777(0.752–0.802) | 26.8% | −14.6% | 41.4% | 0.002 | 20.4% | −18.0% | 38.4% | 0.004 | 0.016 | 0.02 | 0.006 | 0.15 |
| Framingham Risk Score | 0.739(0.706–0.773) | 0.744(0.719–0.769) | N/A | N/A | ||||||||||
| + NT-proBNP | 0.767(0.735–0.800) | 0.762(0.736–0.787) | 28.8% | 16.3% | 12.5% | <0.001 | 21.0% | 13.8% | 7.2% | <0.001 | 0.025 | <0.001 | 0.022 | <0.001 |
| Fooled Cohort Equation | N/A | 0.753(0.728–0.778) | N/A | N/A | ||||||||||
| + NT-proBNP | N/A | 0.757(0.731–0.782) | N/A | 9.3% | 12.7% | −3.3% | 0.06 | N/A | 0.012 | <0.001 | ||||
IDI, integrated discrimination index; NR>0, net reclassification improvement (continuous); other abbreviations as in Table 1.
Addition of NT-proBNP to Established Risk Scores
We also evaluated whether the addition of a baseline NT-proBNP could improve the prognostic ability of 2 established cardiovascular risk scores, the Framingham Risk Score (Model 5) and the Pooled Cohort Risk Equation (Model 6) for estimation of 10-year cardiovascular risk. For these 2 models, analyses were limited to the 4630 participants not taking cholesterol-lowering medications at baseline. Among all participants taken together, as well as among each ethnicity separately, NT-proBNP significantly and independently predicted incident CHD and CVD, above and beyond the Framingham Risk Score (Table III). To a lesser degree, NT-proBNP was also a significant independent predictor of CVD events after adjusting for the Pooled Cohort Risk Equation (Table III).
Risk discrimination was improved with the addition of NT-proBNP to the Framingham Risk Score (c-statistic for CHD went from 0.739 to 0.767, p<0.001; CVD, 0.744 to 0.762, p<0.001), and was slightly improved when added to the Pooled Cohort Equation (0.753 to 0.757, p<0.001) (Table IV). A similar pattern was seen when discrimination was assessed using the IDI. NT-proBNP also improved reclassification compared to the Framingham Risk Score, and resulted from improvement in both the event and the non-event NRI. In contrast, the improvement in reclassification seen with the addition of NT-proBNP to the Pooled Cohort Equation resulted entirely from an increase in the event NRI.
Changes in NT-proBNP Over Time and Outcomes
Characteristics of the participants based on change in NT-proBNP over a mean of 3.2 ± 0.3 years are shown in Supplementary Table III. Change in NT-proBNP varied by ethnicity. Non-Hispanic whites were more likely than other ethnic groups to have a significant decrease, while African-Americans were more likely to have a significant increase in NT-proBNP.
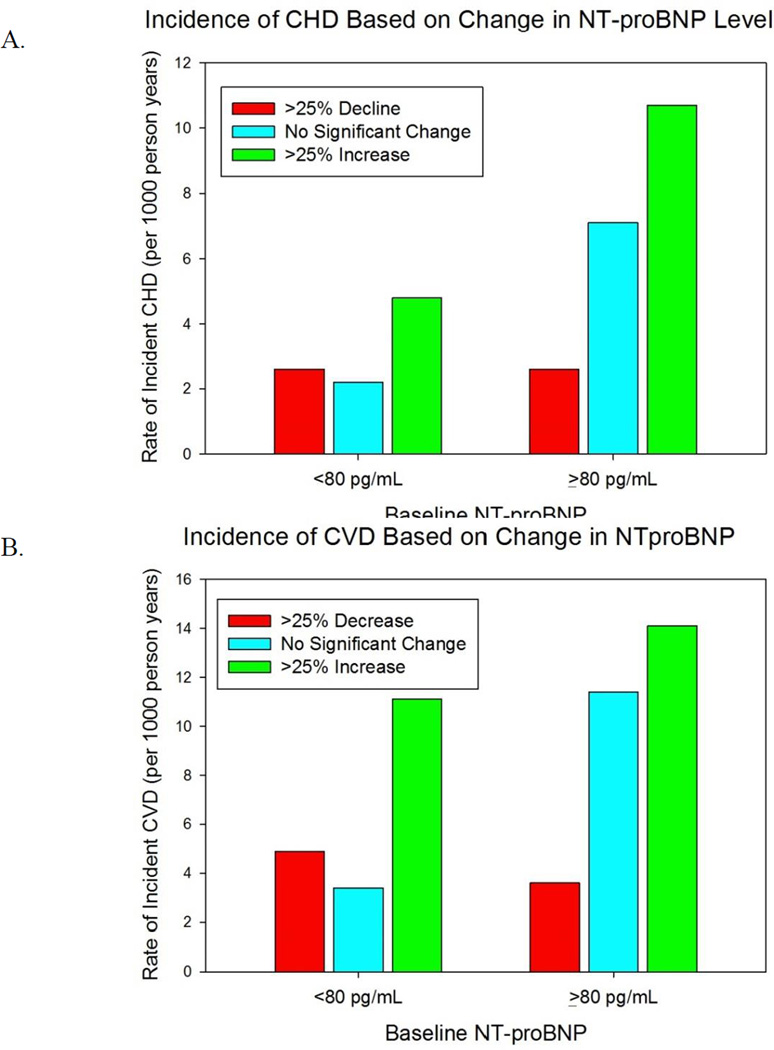
The rate of incident CHD and CVD based upon change in NT-proBNP is shown in Figure 4. Regardless of initial NT-proBNP, an increase of at least 25% on serial measurement was associated with an increased incidence of CHD and CVD. Individuals with an initially elevated NT-proBNP ≥80 pg/mL whose concentration dropped by at least 25% to a value <80 pg/mL had a lower incidence of events than those whose concentration failed to significantly drop. Among individuals with an initially low concentration, a 25% or higher drop in NT-proBNP did not further reduce the incidence of future events. On multivariable Cox proportional hazard analyses, a similar pattern was seen (Table V).
Figure 4.
Incidence rate of CHD (A) and CVD (B) based on change in NT-proBNP. Change in NT-proBNP is defined among those with a baseline NT-proBNP ≥80 pg/ml as a decrease in NT-proBNP of at least 25% or an increase of at least 25% to a concentration ≥80 pg/ml. Change in NT-proBNP is defined among those with a baseline NT-proBNP <80 pg/ml as either a decline of at least 25% to a concentration <80 pg/mL or an increase of >25%.
Table V.
Hazard Ratio for incident CHD and CVD by change in NT- proBNP
| ΔNT-proBNP as a continuous variable |
Baseline NT-proBMP<80 pg/mL |
Baseline NT-proBNP≥80pg/mL |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=4510 HR(95% CI)* |
P | >25% decrease n=497(11%) HR(95%CI) |
P | No significant change n=1863(41%) HR(95%CI) |
>25% increase to>80pg/mL n=623(14%) HR(95%a) |
P | >25% deaease to<80pg/mL n=278(6%) HR(95%CI) |
P | No significant change n=678 (15%) HR(95%CI) |
>25% increase n=571 (13%) HR(95%CI) |
P | ||
| CHD | 134 events | 9 | 29 | 20 | 5 | 32 | 39 | ||||||
| Model 1 | 2.40(1.43–3.92) | 0.001 | 1.26(0.58–2.77) | 0.56 | Reference | 2.03(1.07–387) | 0.03 | 0.58(022–1.54) | 0.27 | Reference | 1.50(093–2.43) | 0.10 | |
| Model 2 | 1.95(1.16–3.26) | 0.01 | 1.21(0.55–2.66) | 0.64 | Reference | 1.69(089–323) | 0.11 | 0.63(0.24–1.68) | 0.36 | Reference | 1.37(084–2.23) | 0.21 | |
| Model 3 | |||||||||||||
| HR for ΔNT-proBNP | 1.88(1.13–3.12) | 0.02 | 1.04(0.46–2.35) | 0.93 | Reference | 1.80(094–345) | 0.08 | 0.63(0.24–1.70) | 0.36 | Reference | 1.36(083–2.22) | 0.22 | |
| HR for detedable TnT | 3.40(1.54–7.51) | 0.002 | 9.66(2.72–34.34), p<0.001 | 2.20 (0.80–6.04), p=0.13 | |||||||||
| Model 4 | |||||||||||||
| HR for ΔNT-proBNP | 1.84(1.11–3.07) | 0.02 | 1.00(0.44–2.27) | 0.99 | Reference | 1.77(092–341) | 0.09 | 0.66(0.25–1.76) | 0.41 | Reference | 1.38(085–2.25) | 0.19 | |
| HR for detedable TnT | 3.14(1.42–6.94) | 0.005 | 8.80(2.46–31.47), p=0.001 | 2.20 (0.76–5.76), p=0.15 | |||||||||
| HR for detedable CAC | 2.77(1.72–4.46) | <0.001 | 2.82(1.43–5.58), p=0.003 | 2.44(1.25–4.77), 0=0.009 | |||||||||
| Model 5 | 2.63(1.48–4.68) | 0.001 | 1.51(0.64–3.59) | 0.35 | Reference | 1.84(085–399) | 0.12 | 0.26(0.06–1.11) | 0.07 | Reference | 1.58(092–2.74) | 0.10 | |
| CVD | 216 events | 17 | 44 | 46 | 7 | 51 | 51 | ||||||
| Model 1 | 2.27(1.52–3.39) | <0.001 | 1.52(0.85–2.74) | 0.16 | Reference | 2.87(1.80–4.58) | <0.001 | 0.51(0.22–1.14)) | 0.10 | Reference | 1.21(081–1.91) | 0.34 | |
| Model 2 | 1.73(1.15–2.61) | 0.009 | 1.50(0.83–2.70) | 0.18 | Reference | 2.33(1.46–372) | <0.001 | 0.55(024–1.25) | 0.15 | Reference | 1.09(072–1.62) | 0.69 | |
| Model 3 | |||||||||||||
| HR for ΔNT-proBNP | 1.70(1.13–2.56) | 0.01 | 1.39(0.76–2.52) | 0.28 | Reference | 2.39(1.49–383) | <0.001 | 0.56(024–1,26) | 0.16 | Reference | 1.09(072–1.63) | 0.69 | |
| HR for detedable TnT | 2.66(1.29–5.28) | 0.008 | 5.03(1.49–16.99), p=0.009 | 1.77(0.71–4.38), p=0.22 | |||||||||
| Model 4 | |||||||||||||
| HR for ΔNT-proBNP | 1.66(1.11–2.50) | 0.01 | 1.38(0.76–2.52) | 0.29 | Reference | 2.37(1.48–380) | <0.001 | 0.57(025–1.30) | 0.18 | Reference | 1.10(074–1.65) | 0.64 | |
| HR for detedable TnT | 2.52(1.23–5.19) | 0.01 | 4.69(1.39–15.88), p=0.01 | 1.75(0.71–4.32), p=0.22 | |||||||||
| HR for detedable CAC | 2.01(1.42–2.84) | <0.001 | 1.62(1.02–2.56), p=0.04 | 2.47(1.43–4.29), p=0.001 | |||||||||
| Model 5 | 2.66(1.71–4.14) | <0.001 | 1.85(0.99–3.47) | 0.05 | Reference | 2.79(1.64–4.73) | <0.001 | 0.35(0.12–1.00) | 0.05 | Reference | 1.25(0.81–1.93) | 0.32 | |
| Model 6 | 2.55(1.62–4.02) | <0.001 | 1.69(0.89–3.20) | 0.11 | Reference | 2.78(1.61–4.80) | <0.001 | 0.39(013–1.11) | 0.08 | Reference | 1.37(0.81–2.13) | 0.17 | |
Model 1 - age, sex, race (except in race-specific analyses), and baseline NTproBNP
Model 2 - Model 1 + clinical variables + time between visits
Model 3 - Model 2 + troponin T
Model 4 - Model 3 + CAC
Model 5 - Framingham risk score + baseline NT-proBNP (limited to the n=3736 participants not taking cholesterol- lowering medication )
Model 6 (for CV D only) - Pooled Cohort Equation + baseline NT-proNP (limited to the n=3736 participants not taking cholesterol - lowering medication)
Hazard ratio is for 1 unit increase in the change in logNT-proBNP from baseline to follow-up
When change in NT-proBNP was modelled as a continuous variable it was a significant independent predictor of both outcomes above and beyond clinical risk factors, TnT, and CAC score (Table V). The predictive utility of change in NT-proBNP did not vary by ethnicity (p-value for interaction: 0.47 for CHD, and 0.66 for CVD). The incremental gain in model performance with the addition of change in NT-proBNP to the clinical risk score, however, was modest. There was significant improvement in the c-statistic (from 0.770 to 0.789 for CHD, and from 0.769 to 0.777 for CVD, p<0.001 for both). While the NRI>0 showed significant improvement in overall reclassification (net gain of 27% for CHD and 20% for CVD), there was only improvement in reclassification for non-events.
Multimarker Score
Using serial sampling of NT-proBNP and TnT from visit 1 and visit 3, there was an incremental risk associated with the number of markers (0, 1, or 2) that were persistently elevated or rising (Supplementary Figure 1, and Supplementary Table IV). Addition of the multimarker score to a model that included baseline NT-proBNP and change in NT-proBNP resulted in modest improvement in risk discrimination as assessed by the c-statistic (for incidence CVD more so than for CHD) and in both the event and non-event NRI (Supplementary Table V).
Discussion
In this large, multi-ethnic cohort of asymptomatic individuals, NT-proBNP is a significant independent predictor of incident CHD and CVD above and beyond clinical risk factors, and provides complementary information to TnT and CAC score, irrespective of ethnicity. Furthermore, a second NT-proBNP measurement approximately 3 years later further improves prediction among all ethnic groups studied.
A few previous studies have evaluated the prognostic utility of serial NT-proBNP measurements. A study of 817 community-dwelling 70 year-olds from the PIVUS study found that an increase in NT-proBNP over 5 years was associated with increased mortality, while none of the 45 subjects with decreasing NT-proBNP died in the ensuing 5 years.32 Among older, predominantly white and African-American community-dwelling individuals (mean age 73 years) from the Cardiovascular Health Study (CHS), NT-proBNP concentrations were found to fluctuate, and these fluctuations indicate a change in risk in the same direction as the change in biomarker.9 In that study, the optimal cut-point was 190 pg/mL. In the present study of younger individuals, the optimal cut-point was expectedly lower (80 pg/mL). We found a similar pattern as was seen in the CHS. Individuals with an initially low NT-proBNP whose subsequent concentration increased, as well as individuals with initially high concentrations whose concentrations persisted or increased, each showed a graded increase in risk. On the other hand, individuals with an initially low NT-proBNP (<80 pg/mL) whose subsequent concentration was even lower did not have a concordant drop in their already low risk. Our study adds to the earlier literature by extending the predictive utility of serial NT-proBNP measurements in asymptomatic individuals to multiple ethnic groups, and demonstrates that the risk of CHD and CVD associated with this biomarker can be interpreted without specifically accounting for race or ethnicity.
We have previously shown that NT-proBNP concentrations in asymptomatic individuals vary by ethnicity, and are predictive of incident congestive heart failure across ethnicities, independent of heart failure risk factors, left ventricular hypertrophy, and interim MI.15 This study demonstrates that in addition to heart failure, individuals with elevated NT-proBNP (or TnT) are also at risk for atherosclerotic cardiovascular disease, with a similar association strength. The relative contribution of stroke to the overall CVD outcome was not specifically evaluated in this study, but is a subject of interest for future investigations.
In the present study, detectable TnT was predictive of incident CHD and CVD overall as well as among individual ethnic groups, although conclusions could not be drawn for Chinese-Americans in whom there was a very low prevalence of detectable troponin. These findings are notable given that we used a contemporary, clinically relevant TnT. Prior studies have suggested that the difference in troponin concentrations between healthy US and Asian populations is very small after adjusting for other clinical factors.33, 34 We found that African-Americans had a higher prevalence and Chinese-Americans had a lower prevalence of detectable TnT than non-Hispanic whites, which was not explained by other measured covariates.
Despite its predictive ability, the overall low prevalence of detectable TnT in this study limited its clinical value. The addition of TnT to a prediction model that included clinical risk factors and NT-proBNP resulted in a reduction in the accuracy of risk classification (i.e. a negative NRI). This misclassification was the result of undetectable troponin among individuals who went on to develop events. With only 1.5% of individuals having detectable TnT, the assay was likely not sensitive enough to detect subclinical risk among most of the at-risk individuals in this population. On the other hand, our multimarker score, which incorporated serial TnT measurements, resulted in significant improvement in reclassification compared with clinical risk factors and serial NT-proBNP measurements. It is possible that troponin would have even better predictive performance if we had used a highly sensitive troponin (with detectable concentrations in 50% or more of the population),35 instead of a “contemporary-sensitive” troponin, which would allow for finer distinctions in troponin concentrations. Future studies evaluating highly sensitive troponin in MESA participants will be able to better inform us about the correlates and predictive value of cardiac troponin among various ethnic groups. In addition, prior studies of community-dwelling individuals have suggested that troponin is more predictive of incident congestive heart failure than of future atherosclerotic CVD.10 Heart failure was not included as an outcome in the present analyses.
A novel aspect of this study is our evaluation of whether NT-proBNP could improve upon the predictive ability and performance of the Framingham Risk Score or the Pooled Cohort Risk Equation. The Pooled Cohort Risk Equation was recently adopted as the preferred method for evaluating 10-year CVD risk and for determining an individual’s eligibility for statin therapy, by the 2013 guidelines of the ACC and the AHA for the management of cholesterol.29 The new recommendations have come with some degree of controversy.36 A major point of contention is the notion that, based upon the new prevention guidelines, a very large number of people will be eligible for statin therapy (up to 49% of all adults, and 87% of men 60 to 75 years) despite the fact that a large number of these will never actually experience a cardiovascular event.37 Thus, there is a potential role for a biomarker that could accurately result in downgrading of risk. We found that the addition of NT-proBNP significantly improved predictive ability and model performance of the Framingham Risk Score. The effect when NT-proBNP was added to the Pooled Cohort Risk Equation, however, was modest, and resulted in significant improvement of only the event, but not the non-event NRI. According to these results, in a population like MESA, NT-proBNP would not be useful for accurately downgrading risk or refining statin recommendations as determined by current guidelines.
This study has several limitations and strengths. Limitations include the limited number of events when participants were stratified by race and by category of change in NT-proBNP. Because of this, we were unable to reliably estimate risk for Chinese-Americans. Also, consistent with prior MESA publications, we focused on hard CHD and CVD endpoints; the absence of intermittent claudication and heart failure from our composite CVD endpoint, both of which were included in the Framingham Global CVD composite endpoint, may limit the utility of comparisons between risk models.28 In addition, we used a contemporary-sensitive – but not highly sensitive – cardiac troponin assay. Despite the lower prevalence of detectable troponin with this assay compared with highly sensitive assays, we nonetheless found strong and significant associations between TnT and outcomes. We would expect the association to be even stronger if a more sensitive assay were used. Use of this assay could also be regarded as a strength, in that it is a clinically relevant marker used regularly in daily practice. Other strengths of the present study include its prospective design and the well-characterized cohort with rigorous adjudication of outcomes.
Conclusions
Among asymptomatic individuals of multiple ethnicities, NT-proBNP and TnT are significant, independent predictors of incident CHD and CVD above and beyond clinical risk factors. Change in NT-proBNP may provide additional prognostic information. Whether these biomarkers can meaningfully improve upon current methods of risk stratification for primary CVD prevention is not clear.
Supplementary Material
Acknowledgments
Sources of Funding:
This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, by grants UL1-TR-000040 and UL1-TR-001079 from NCRR, and by Roche Diagnostics. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Abbreviations List
- AUC
area under the curve
- BMI
body mass index
- CAC
coronary artery calcification
- CHD
coronary heart disease
- CHS
Cardiovascular Health Study
- CI
confidence interval
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- GFR
glomerular filtration rate
- HDL
high density lipoprotein
- HR
hazard ratio
- IDI
integrated discrimination improvement
- LDL
low density lipoprotein
- MESA
Multi-Ethnic Study of Atherosclerosis
- MI
myocardial infarction
- NRI
net reclassification improvement
- NT-proBNP
N-terminal pro B-type natriuretic peptide
- PIVUS
Prospective Investigation of the Vasculature in Uppsala Seniors study
- ROC
receiver operator characteristic
- SBP
systolic blood pressure
- SD
standard deviation
- TnT
troponin T
Footnotes
Clinical Trial Registration - URL: http://www.clinicaltrials.gov. Unique identifier: NCT00005487.
Disclosures:
Roche Diagnostics provided the reagents for the biomarker analyses in this study, but had no input into the study design, analyses, or manuscript. AB: No disclosures. ASM: Has received research support from Abbott, Alere, BG Medicine, Critical Diagnostic, Roche Diagnostics; and consulting fees from BG Medicine and Sphingotec. CRD: Receives investigator initiated grant support from Roche Diagnostics and Critical Diagnostics. Receives consulting/honorarium from Roche Diagnostics, Siemens, Radiometer and HDL. DS: No disclosures. HB: No disclosures. JACL: No disclosures. LBD has received research supplies from Critical Diagnostics, speaking fees from Roche Diagnostics and Critical Diagnostics and has served as a consultant for diaDexus and Alere. MC: Grant funding diaDexus. Consultant Daiichi Sankyo, Merck. MHC: No disclosures. OS: No disclosures. PC: No disclosures. PG: No disclosures. RPT: No disclosures.
References
- 1.McKie PM, Rodeheffer RJ, Cataliotti A, Martin FL, Urban LH, Mahoney DW, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide: biomarkers for mortality in a large community-based cohort free of heart failure. Hypertension. 2006;47(5):874–880. doi: 10.1161/01.HYP.0000216794.24161.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293(13):1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 3.Olsen MH, Hansen TW, Christensen MK, Gustafsson F, Rasmussen S, Wachtell K, et al. N-terminal pro-brain natriuretic peptide, but not high sensitivity C-reactive protein, improves cardiovascular risk prediction in the general population. Eur Heart J. 2007;28(11):1374–1381. doi: 10.1093/eurheartj/ehl448. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 5.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358(20):2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 6.Daniels LB, Laughlin GA, Clopton P, Maisel AS, Barrett-Connor E. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol. 2008;52(6):450–459. doi: 10.1016/j.jacc.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zethelius B, Johnston N, Venge P. Troponin I as a predictor of coronary heart disease and mortality in 70-year-old men: a community-based cohort study. Circulation. 2006;113(8):1071–1078. doi: 10.1161/CIRCULATIONAHA.105.570762. [DOI] [PubMed] [Google Scholar]
- 8.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304(22):2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55(5):441–450. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123(13):1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blankenberg S, Zeller T, Saarela O, Havulinna AS, Kee F, Tunstall-Pedoe H, et al. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: the MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation. 2010;121(22):2388–2397. doi: 10.1161/CIRCULATIONAHA.109.901413. [DOI] [PubMed] [Google Scholar]
- 12.Di Angelantonio E, Chowdhury R, Sarwar N, Ray KK, Gobin R, Saleheen D, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120(22):2177–2187. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 13.Rutten JH, Mattace-Raso FU, Steyerberg EW, Lindemans J, Hofman A, Wieberdink RG, et al. Amino-terminal pro-B-type natriuretic peptide improves cardiovascular and cerebrovascular risk prediction in the population: the Rotterdam study. Hypertension. 2010;55(3):785–791. doi: 10.1161/HYPERTENSIONAHA.109.143313. [DOI] [PubMed] [Google Scholar]
- 14.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi EY, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, et al. N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure: Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2012;5(6):727–734. doi: 10.1161/CIRCHEARTFAILURE.112.968701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniels LB, Clopton P, Potocki M, Mueller C, McCord J, Richards M, et al. Influence of age, race, sex, and body mass index on interpretation of midregional pro atrial natriuretic peptide for the diagnosis of acute heart failure: results from the BACH multinational study. Eur J Heart Fail. 2012;14(1):22–31. doi: 10.1093/eurjhf/hfr157. [DOI] [PubMed] [Google Scholar]
- 17.Montagnana M, Lippi G, Salvagno GL, Guidi GC. Reference ranges and diagnostic thresholds of laboratory markers of cardiac damage and dysfunction in a population of apparently healthy black Africans. Clin Chem Lab Med. 2008;46(5):714–716. doi: 10.1515/cclm.2008.130. [DOI] [PubMed] [Google Scholar]
- 18.Krauser DG, Chen AA, Tung R, Anwaruddin S, Baggish AL, Januzzi JL., Jr Neither race nor gender influences the usefulness of amino-terminal pro-brain natriuretic peptide testing in dyspneic subjects: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. J Card Fail. 2006;12(6):452–457. doi: 10.1016/j.cardfail.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Kapuku GK, Davis HC, Thomas P, Januzzi J, Harshfield GA. Does the relationship between natriuretic hormones and diastolic function differ by race? Am J Med Sci. 2012;344(2):96–99. doi: 10.1097/MAJ.0b013e31823673fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doi Y, Ninomiya T, Hata J, Hirakawa Y, Mukai N, Ikeda F, et al. N-terminal pro-brain natriuretic peptide and risk of cardiovascular events in a Japanese community: the Hisayama study. Arterioscler Thromb Vasc Biol. 2011;31(12):2997–3003. doi: 10.1161/ATVBAHA.111.223669. [DOI] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 22.Lakoski SG, Greenland P, Wong ND, Schreiner PJ, Herrington DM, Kronmal RA, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA) Arch Intern Med. 2007;167(22):2437–2442. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
- 23.Ordonez-Llanos J, Collinson PO, Christenson RH. Amino-terminal pro-B-type natriuretic peptide: analytic considerations. Am J Cardiol. 2008;101(3A):9–15. doi: 10.1016/j.amjcard.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Roche Diagnostics. Elecsys proBNP package insert. Indianapolis, IN. 2003 [Google Scholar]
- 25.Roche Diagnostics. Elecsys Troponin T package insert. Indianapolis, IN. 2004 [Google Scholar]
- 26.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 27.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 28.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 29.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr, Gibbons R, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 31.Schou M, Gustafsson F, Nielsen PH, Madsen LH, Kjaer A, Hildebrandt PR. Unexplained week-to-week variation in BNP and NT-proBNP is low in chronic heart failure patients during steady state. Eur J Heart Fail. 2007;9(1):68–74. doi: 10.1016/j.ejheart.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Eggers KM, Venge P, Lind L. Prognostic usefulness of the change in N-terminal pro B-type natriuretic peptide levels to predict mortality in a single community cohort aged >/= 70 years. Am J Cardiol. 2013;111(1):131–136. doi: 10.1016/j.amjcard.2012.08.058. [DOI] [PubMed] [Google Scholar]
- 33.Gaggin HK, Dang PV, Do LD, deFilippi CR, Christenson RH, Lewandrowski EL, et al. Reference interval evaluation of high-sensitivity troponin T and N-terminal B-type natriuretic peptide in Vietnam and the US: The North South East West Trial. Clin Chem. 2014;60(5):758–764. doi: 10.1373/clinchem.2013.216275. [DOI] [PubMed] [Google Scholar]
- 34.Aw TC, Phua SK, Tan SP. Measurement of cardiac troponin I in serum with a new high-sensitivity assay in a large multi-ethnic Asian cohort and the impact of gender. Clin Chim Acta. 2013;422:26–28. doi: 10.1016/j.cca.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 35.Daniels LB. Making sense of high sensitivity troponin assays and their role in clinical care. Curr Cardiol Rep. 2014;16(4):471. doi: 10.1007/s11886-014-0471-x. [DOI] [PubMed] [Google Scholar]
- 36.Statins: new US guideline sparks controversy. Lancet. 9906;382:1680. doi: 10.1016/S0140-6736(13)62405-8. [DOI] [PubMed] [Google Scholar]
- 37.Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, Williams K, Neely B, Sniderman AD, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370(15):1422–1431. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.