Abstract
Our understanding of the functions of ceramide signaling has advanced tremendously over the past decade. In this review, we focus on the roles and regulation of neutral sphingomyelinase 2 (nSMase2), an enzyme that generates the bioactive lipid ceramide through the hydrolysis of the membrane lipid sphingomyelin. A large body of work has now implicated nSMase2 in a diverse set of cellular functions, physiological processes, and disease pathologies. We discuss different aspects of this enzyme’s regulation from transcriptional, post-translational, and biochemical. Furthermore, we highlight nSMase2 involvement in cellular processes including inflammatory signaling, exosome generation, cell growth, and apoptosis, which in turn play important roles in pathologies such as cancer metastasis, Alzheimer’s disease, and other organ systems disorders. Lastly, we examine avenues where targeted nSMase2-inhibition may be clinically beneficial in disease scenarios.
Keywords: sphingomyelinase, ceramide, inflammation, apoptosis, exosomes
I. Introduction
Sphingomyelin hydrolysis is catalyzed by a class of enzymes referred to as sphingomyelinases (SMases) to generate ceramide, a bioactive lipid involved in diverse cellular processes (Hannun and Obeid, 2011, Ogretmen and Hannun, 2004). SMases are classified based on their pH optima of activity into acid, neutral, and alkaline subtypes. Of the four different mammalian neutral SMases that have been identified; neutral sphingomyelinase-2 (nSMase2) appears to be the predominant nSMase in cellular systems, physiologies, and pathologies (Airola and Hannun, 2013, Clarke et al. , 2011b, Goni and Alonso, 2002, Wu et al. , 2010). This review will focus on the roles and regulation of this enzyme emphasizing recent findings implicating nSMase2 in disease processes.
II. Characterization and regulation of nSMase2
A. Cloning
Cloning of nSMase2 revealed it as part of a protein superfamily that hydrolyzes phosphodiester linkages and requiring Mg2+ for activity. Both the human and mouse nSMase2 gene (SMPD3) encode for a 655 amino acid protein with a molecular mass of 71 kDa that contains an N-terminus with 2 hydrophobic segments and a C-terminus consisting of the catalytic site (Hofmann et al. , 2000). The mouse and human versions are very similar and share 90% sequence identity.
B. Basic biochemical properties
NSMase2 specifically hydrolyzes the phosphocholine-headgroup from sphingomyelin and does not exhibit Phospholipase c-type activity against phosphatidylcholine, lysophosphatidylcholine, platelet activating factor or lyso-platelet activating factor. Neutral pH and divalent cations (Mg2+ or Mn2+) are required for activity, while phosphatidylserine (PS) and unsaturated fatty acids stimulate enzymatic activity in vitro (Hofmann, Tomiuk, 2000). A later study found that sphingosylphosphocholine, the deacylated form of SM, can be hydrolyzed under detergent-free conditions by nSMase2 (Miura et al. , 2004).
C. Structural features
To date, there has not been a crystal structure reported for nSMase2. Therefore, our current understanding of the structure and mechanism of nSMase2 is based on investigations of related bacterial SMases. Three different structures of bacterial neutral SMases from the pathogenic organisms Bacillus cereus, Listeria ivanovii, and Staphylococcus aureus has confirmed neutral SMases belong to the DNase I type protein superfamily, which also includes inositol phosphatases (Ago et al. , 2006, Huseby et al. , 2007, Matsuo et al. , 1996, Openshaw et al. , 2005). NSMase2 shares relatively low sequence identity with the bacterial versions but most likely shares a similar protein fold and catalytic mechanism for SM hydrolysis (Ago, Oda, 2006, Openshaw, Race, 2005). Studies by Tani et al showed that the enzyme harbors two hydrophobic loops at the amino terminus rather than the sequence-predicted transmembrane segments (Tani and Hannun, 2007a).
D. Cellular localization
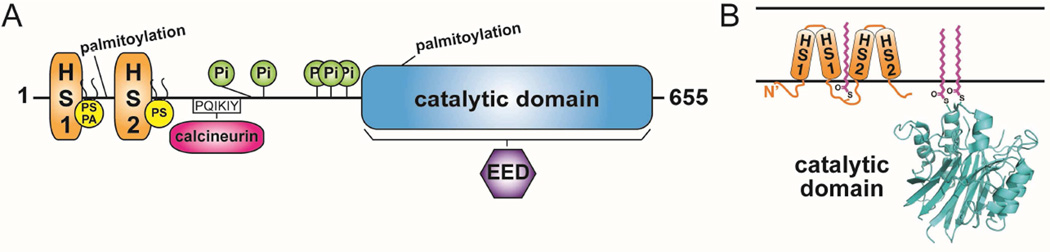
After initially cloning nSMase2, Hoffman et al. showed by antibody staining that nSMase2 localized to the Golgi apparatus in both PC12 and SH-SY5Y cells (Hofmann, Tomiuk, 2000). Subsequently, overexpressed nSMase2 was shown to localize to the plasma membrane (PM) in the confluence phase of MCF7 cells and in primary hepatocytes (Karakashian et al. , 2004, Marchesini et al. , 2004). Building on these findings, Tani et al. found that nSMase2 localized to the inner leaflet of the PM in confluent MCF7 cells and was palmitoylated at two Cysteine clusters (Tani and Hannun, 2007a, b). The first cluster encompasses Cys53, Cys54 and Cys59 that are present between the 2 hydrophobic segments of the N-terminus, and the second cluster encompasses Cys395 and Cys396, which are present at the beginning of the catalytic site (Tani and Hannun, 2007b). Studies with cycloheximide in subconfluent MCF7 cells demonstrated that the Golgi localization of overexpressed subconfluent nSMase2 is the result of two protein pools: newly synthesized protein and a second that recycles back from the PM through the endosomal system (Milhas et al. , 2010).
E. Regulation by anionic phospholipids
Prior to the cloning of the different isoforms of neutral SMases, anionic phospholipids (APLs) were found to increase neutral sphingomyelinase activity in rat hepatomas. Delipidated membranes treated with Triton X-100 recovered NSMase activity only upon addition of PS, phosphatidic acid, phosphatidylinositol but not the neutral lipids phosphatidylcholine or phosphatidylethanolamine (Tamiya-Koizumi and Kojima, 1986). These findings were later confirmed in a purified membrane bound neutral sphingomyelinase from rat brain (Liu et al. , 1998b). Following the cloning of nSMase2, biochemical characterization showed that the enzyme has a catalytic pH optimum of 7.5, with a strong stimulation by PS or cardiolipin and a requirement for either Mg2+ or Mn2+ for activity (Hofmann, Tomiuk, 2000, Marchesini et al. , 2003). The activity of the previously cloned neutral SMase1 was shown to be APL-independent (Tomiuk et al. , 2000), thus identifying nSMase2 as the previously characterized magnesium dependent neutral SMase that was activated by APLs. A detailed mechanistic study found that APLs bind to the N-terminus of nSMase2 at two distinct positively charged sites. The first site binds both PS and phosphatidic acid and required R33 and the amino acids 45–48 (KRQR). The second site selectively bound PS and required R92 and R93 (Wu et al. , 2011). Biochemically, APL binding was found to affect both the substrate affinity (Km) and rate of hydrolysis (Vmax) of nSMase2. Mutation of the APL binding sites altered the localization to the endoplasmic reticulum (ER). In yeast strains lacking the nSMase2 homolog Isc1, the wild type protein corrected sensitivity to hydroxyurea, while the mutant protein was unable to do so (Wu, Clarke, 2011) [Fig1].
Figure 1. Domain architecture and membrane topology of nSMase2.
(A) Domain architecture of nSMase2. Catalytic domain, blue; Hydrophobic segments 1 (HS1) and 2 (HS2), orange; Anionic phospholipid binding sites, yellow lipids; Phosphorylation sites (Pi), green; Calcineurin binding motif, magenta; EED binding region, purple; and palmitoylation sites are indicated. (B) Membrane topology of nSMase2 with palmitoylation sites, magenta. HS1 and HS2 associate with, but do not transverse, the membrane. A representative structure of the nSMase2 catalytic domain is shown from B. cereus sphingomyelinase (PDB ID 2DDT).
F. Regulation by phosphorylation
NSMase2 was originally identified as a phosphoprotein in human bronchial epithelial and A549 cells with phosphorylation occurring basally on serine residues (Filosto et al. , 2010). The serine/threonince phosphatase Calcineurin, (also known also as protein phosphatase 2B) was found to bind a PQIKIY motif between the N-terminus and the C-terminus to dephosphorylate nSMase2 (Filosto, Fry, 2010). The phosphorylation of nSMase2 under H2O2 stimulation in A549 cells occurs on 5 conserved serine residues: S173, S209, S291, S294 and S301, which are located near the calcineurin binding site. Alanine point mutants lacking the phosphorylation sites are not phosphorylated by H2O2 and are not activated in response to oxidative stress. In addition protein phosphorylation at these sites regulated the stability of nSMase2 in this cellular model (Filosto et al. , 2012). In addition, some of nSMase2 functional effects have been found to be dependent on protein kinases. For instance, p38 and PKCδ regulate the translocation of nSMase2 to the plasma membrane in response to TNF (Clarke et al. , 2007). Furthermore PKCδ role in this translocation is independent of effects on the activation of nSMase2 in response to TNF. (Clarke et al. , 2008). However, it is not known if these kinases phosphorylate nSMase2 directly or affect nSMase2 function through signaling pathways.
E. Transcriptional regulation of nSMase2
Recent insights into stimuli activating nSMase2 shed light on some aspects of its transcriptional regulation. The chemotherapeutics Daunorubicin and Camptothecin were found to induce nSMase2 transcription in MCF-7 breast cancer cells and K562 leukemia cells via activation of the putative promoter by the tansciption factors Sp1 and Sp3. The region of the promoter necessary for this activation was mapped to −147 bp upstream of exon 1 (Ito et al. , 2009). All-trans retinoic acid (ATRA) was also found to induce transcriptional activation of nSMase2 via the same mechanism (Ito et al. , 2012).
F. Tools for the study of nSMase2: inhibitors and mouse models
Initial studies of NSMase activity relied heavily on the natural occurring inhibitors Scyphostatin and Manumycin A that were originally identified from fungi and bacteria respectively (Arenz et al. , 2001, Nara et al. , 1999a, Zeeck et al. , 1987). These inhibitors had a broad NSMase inhibitory activity with no selectivity towards different NSMase isoforms. In addition, Scyphostatin had inhibited acid SMase activity but with a higher IC50 (Canals et al. , 2011, Nara et al. , 1999b). A high throughput screen for small molecule inhibitors of nSMase2 identified GW4869 as a selective nSMase2 inhibitor with an IC50 of 1 µM. GW4869 is a non-competitive nSMase2 inhibitor and its mechanism of action is thought to be through interference with APL activation of the enzyme (Canals, Perry, 2011, Luberto et al. , 2002). Since its identification, GW4869 has been the most commonly employed pharmacological tool to study nSMase2 function in cellular and animal models.
Mice models to study nSMase2 function were characterized at the beginning of the 21st century. Stoffel et al. generated the first SMPD3−/− mouse and showed that it has decreased neutral SMase activity in many liver and brain while the SMPD3−/− SMPD2−/−double knock out mouse, which deletes both nSMase1 and nSMase2, abolished all neutral SMase activity (Stoffel et al. , 2005). The fragilitas osseum (fro/fro) mouse model, which was originally identified in a chemical screen for bone defects, was shown by Aubin et al. to have a catalytically inactive nSMase2 due to deletion of the C-terminal 33 residues and has since been used as an additional model to study nSMase2 function. The original study reported the fro/fro mouse retained residual NSMase activity to almost 10% of the wild type (Aubin et al. , 2005). More recent reports have found overexpression of the fro/fro mutation in a cellular model did not increase nSMase2 activity. This is more consistent with the deletion of two catalytically conserved residues, D638 and H639, in the fro/fro mutatation (Clarke, Wu, 2011b, Wu, Clarke, 2010) and it was speculated that residual nSMase activity in the fro/fro mouse is due to the presence of other nSMase isoforms.
III. NSMase2 as a regulator of biological functions
NSMase2 has been implicated in many physiological processes as well as disease pathologies. In this section, we will review the most important biological functions relating to nSMase2 and highlight potential avenues for future research.
A. Inflammation
Response to Tumor necrosis factor-alpha (TNF-α)
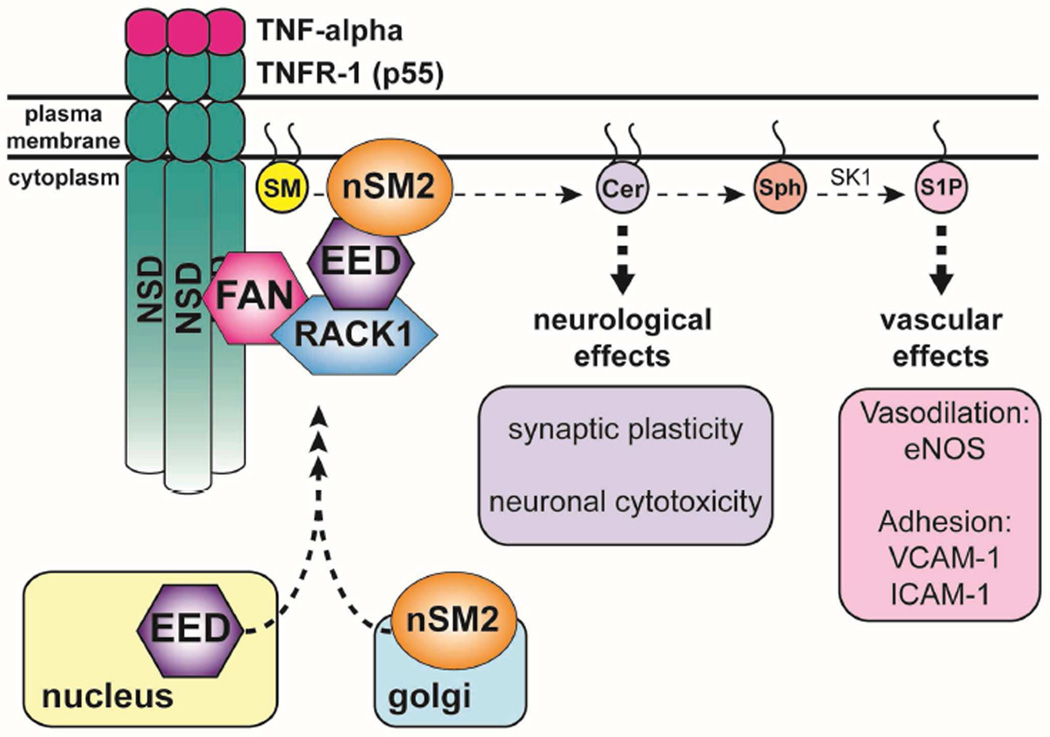
Activation of neutral sphingomyelinase in response to the cytokine TNF-α was initially observed in the HL-60 cell line. In these leukemia cells, TNF-α induces a rapid SM hydrolysis, peaking at 1 hour concomitant with an increase in ceramide and cellular differentiation into monocytes (Kim et al. , 1991). The initial discovery was replicated in a cell-free system (Dressler et al. , 1992) and it required the TNF-α receptor-1 (TNFR-1), commonly known as the p55 receptor (Adam et al. , 1996, Adam-Klages et al. , 1998, Belka et al. , 1995, Yang et al. , 1993). Mechanistically, a distinct region of the p55 receptor spanning amino acids 309–319 was found to mediate the increase in NSMase activity and was named the Neutral Sphingomyelinase Activation Domain (NSD) (Adam, Wiegmann, 1996). The 9 amino acid stretch of the NSD binds a protein termed Factor Associated with Neutral sphingomyelinase (FAN) that influences NSMase activity in response to TNF-α (Adam-Klages et al. , 1996, Adam-Klages, Schwandner, 1998). FAN is a protein of the WD-40 family and contains 5 WD repeats, which are common scaffolding motifs involved in coordinating multi-protein complex assemblies. The N-terminus of FAN is critical for NSMase activity as a truncation of FAN, which contained only the WD repeats, inhibits NSMase activation following TNF-α in a dominant negative fashion (Adam-Klages, Adam, 1996, Adam-Klages, Schwandner, 1998). Following that, Receptor for Activated C-Kinase 1 (RACK1) was identified as a FAN interaction partner using a yeast two hybrid system. The interaction between FAN and RACK1 was verified both in vitro and in cells and is dependent on the C-terminal WD repeats of both proteins. RACK1 was also shown to modulate N-SMase activation following TNF-α treatment (Tcherkasowa et al. , 2002). Subsequently, a yeast-two hybrid interaction mapping suggested the polycomb protein embryonic ectoderm development (EED) as a binding partner of nSMase2 via its C-terminus. Further investigation showed that EED also binds to RACK1 via the same region that interacts with nSMase2, and rapidly translocates from the nucleus to the PM after TNF-α treatment. The proposed model is one where EED interacts with RACK-1 and nSMase2 and brings them together in a multi-protein complex with TNFR1 through FAN, hence establishing EED as a critical modulator of nSMase2 activation following TNF-α (Philipp et al. , 2010).
In addition to the elucidation of the molecular partners, other investigations have focused on cellular factors that influence TNF-α activation of nSMase2. Liu et al. reported that the levels of glutathione (GSH), which can inhibit neutral SMase activity, are critical for TNF-a activation. Their model suggests that baseline inhibition of nSMase2 activity is due to physiologic levels of GSH and TNF-a treatment activates nSMase2 by decreasing levels of GSH (Liu et al. , 1998a). Further studies in A549 lung cells demonstrated that TNF-α induces a translocation of nSMase2 from the Golgi to the PM that was largely dependent on p38 MAPK (Clarke, Truong, 2007) and Protein Kinase C delta (PKC-δ) (Clarke, Guthrie, 2008). In vascular smooth muscle cells and fibroblasts, TNF-α activation of nSMase2 was shown to require the activation of the metallomatrix protease 2 (MMP2) from its inactive precursor form MT1-MMP2 by proteolysis mediated by Furin (Tellier et al. , 2007)
Functionally, the activation of nSMase2 has implications in the inflammatory response of TNF-α. Studies in HeLa cells showed that nSMase2 acts on the PI3K/Akt pathway to activate endothelial nitric oxide synthase (eNOS) (Barsacchi et al. , 2003). In HUVEC cells, this effect required the conversion of ceramide into sphingosine-1-phosphate (S1P), by the action of Sphingosine Kinase 1 (SK1) and eNOS activation regulated the expression of adhesion molecules like E-selection and VCAM-1 (De Palma et al. , 2006). Clarke et al. expanded on this theme and demonstrated that in A549 cells, nSMase2 inhibition attenuated TNF-α stimulation of VCAM-1 and ICAM-1 (Clarke, Truong, 2007). In addition to the vascular effects of TNF-α, nSMase2 mediates some of the effects of TNF-α on the neural system. TNF-α stimulates nSMase2-dependent ceramide production in primary hippocampal neurons that modulates synaptic plasticity through trafficking of NR1 subunits of N-methyl-D-aspartate receptors to lipid rafts (Wheeler et al. , 2009). Another report suggests that activation of nSMase2 in primary cortical neurons precedes reactive oxygen species (ROS) formation and damage to neurons (Barth et al. , 2012). It is interesting to note that the TNF-α derived vascular effects seem to require the sequential action of both nSMase2 and SK1, while in contrast the neuronal effects of TNF-α is dependent only on nSMase2 activation. This suggests that nSMase2-derived ceramide can have bioactive action by itself in response to TNF-α or can be metabolized into other lipids to induce differential responses [Fig2].
Figure 2. nSMase2 is a mediator of TNF-α signaling.
TNF-α induces nSMase2 activation and ceramide generation. Recruitment of nSMase2 to the plasma membrane occurs through formation of a complex between five proteins: nSMase2, EED, RACK1, FAN, and TNFR-1.
Response to Interleukin-1 beta (IL-1β)
The role of nSMase2 in inflammation has been studied extensively in response to another cytokine, IL-1β. IL-1β is a pro-inflammatory cytokine found to be secreted by inflammosomes, which is an early pathogenic feature of many liver and other organ systems diseases (Szabo and Csak, 2012). IL-1β was first reported to induce NSMase activity in rat hepatocytes (Nikolova-Karakashian et al. , 1997). Subsequent studies in murine cortices demonstrated that this activation requires the IL1-Receptor 1 (IL1-R1) based on the observation that IL1-R1 KO mice failed to increase NSMase activity after IL-1β stimulation (Nalivaeva et al. , 2000). The major isoform activated by IL-1β was demonstrated to be nSMase2 as overexpression of nSMase2 in IL-1β treated HepG2 cells enhanced phosphorylation of Janus Kinase (JNK) acutely (Karakashian, Giltiay, 2004). In the same system, nSMase2 overexpression suppressed phosphorylation and increased the stability of the Interleukin-1 receptor-associated kinase (IRAK-1) through activation of the protein phosphatase 2A (PP2A) family (Karakashian, Giltiay, 2004). Further studies identified the phosphatase isoform involved as PP2Ac and showed that nSMase-2-dependent dephosphorylation of IRAK-1 by PP2Ac delayed IRAK-1 ubiquitination, which led to its stabilization and amplification of the IL-1β response by nSMase2 (Dobierzewska et al. , 2011).
Functionally, the role of nSMase2 in the IL-1β response was elucidated through aging models. Aged hepatocytes display increased sensitivity to IL-1β stimulation that is translated via higher JNK phosphorylation as well as higher IRAK-1 stability (Rutkute et al. , 2007a). This is due to an increase in nSMase2 activity in aged hepatocytes that is dependent on decreased levels of GSH (Rutkute, Asmis, 2007a). Attenuation of nSMase2 levels by siRNA or by shRNA decreased IRAK-1 stability and JNK phosphorylation to restore a normal response to IL-1β in aged hepatocytes (Rutkute et al. , 2007b). Moreover in normal rat hepatocytes and in HEK 293 cells, nSMase2 was shown to stimulate post-transcriptional FOXO1, accumulation in the nucleus through its JNK/ERK activation in response to IL-1β (Dobierzewska et al. , 2012). In glial cells, nSMase2 was shown to be important in IL-1β dependent Src activation that mediates IL-6 secretion (Tsakiri et al. , 2008). Taken together, these results place nSMase2 as an attractive drug target in inflammatory diseases of the liver and other organ systems.
Response to Interferon gamma (IFN-γ)
IFN-γ is another cytokine that was studied in the induction of nSMase2 activity. IFN-γ was shown to initially increase NSMase activity with acute stimulations in HL-60 cells. (Kim, Linardic, 1991). Subsequently, nSMase2 was demonstrated to be the major NSMase activated in response to IFN-γ and induced translocation of PKC-δ to the Golgi in HeLa cells in a JAK1/2 dependent manner (Kajimoto et al. , 2001). In RAW264.7 macrophages IFN-γ activates an nSMase2 ➔ PP2A ➔ Akt/GSK ➔ Nitric oxide pathway (Tsai et al. , 2009); while in rat vascular smooth muscle cells, NSMase inhibition by 3-O-methyl sphingpmyelin prevented IFN-γ-mediated nitric oxide production. This is due to the inhibition of translocation of p65 subunit of NF-k κB to the nucleus and hence failure of the NF- κB signaling cascade to activate iNOS at the mRNA level (Hsieh et al. , 2011).
Involvement in phagocytosis
NSMase2 has a described role in phagocytes, which are typical cells of acute inflammation that engulf bacterial cells or other small cells. Polymorphonuclear cells, of which the phagocytic neutrophils constitute the major portion, display a plasma membrane NSMase activity that increases during phagocytosis (Hinkovska-Galcheva et al. , 1998). Inhibition of nSMase2 by GW4869 causes loss of directional motility and migration in these cells (Sitrin et al. , 2011). Finally, peptidoglycan, a major component of bacterial cell wall, was shown to induce nSMase2 activity in human macrophages that leads to the activation of p38 MAPK and NF-κB to increase cyclooxygenase-2 expression (Chen et al. , 2009).
B. Involvement in pulmonary pathophysiology
Recent findings have demonstrated a functional regulation of nSMase2 in response to lung insults and in certain lung diseases. Initially, Castillo et al. reported that H2O2 stimulation and ROS formation activates nSMase2 to mediate apoptosis in human airway epithelium, while glutathione (GSH) pre-treatment prevents apoptosis (Castillo et al. , 2007). Building on this in a pathophysiological context, it was found that cigarette smoking, a potent ROS-generating lung insult, increased NSMase activity acutely in immortalized human airway epithelium leading to apoptosis (Levy et al. , 2009). GSH pre-treatment or siRNA to nSMase2 were found to inhibit apoptosis, concordant with the previous study. Consistent with the cellular studies, mouse models of cigarette smoke exposure showed an accumulation of ceramide in lungs of mice 1–3 weeks after exposure, causing apoptosis (Filosto et al. , 2011). There was a simultaneous increase in nSMase2 protein expression in both bronchial epithelium and alveolar septae of mice that was prevented by pretreatment with the ROS scavenger N-acetylcysteine (NAC) (Filosto, Castillo, 2011). One major consequence of chronic smoking is the development of chronic obstructive pulmonary disease (COPD), a clinical entity comprising two distinct pathologies, namely emphysema and chronic bronchitis. Conflicting results have been reported on the involvement of nSMase2 in the pathophysiology of emphysema. Poirier et al. showed increased morphological changes that resemble emphysematous changes in lungs of fro/fro mice in the absence of any insult (Poirier et al. , 2012). These changes included alveolar distention and increased lung compliance by pulmonary functional testing, which suggests that lack of nSMase2 activity in the fro/fro mice mediates emphysematous changes (Poirier, Berdyshev, 2012). In contrast, Filosto et al. reported increased nSMase2 activity in the alveolar space of emphysematous patients (Filosto, Castillo, 2011). Tibboel et al. observed in an elastase-treatment model of emphysema that there was an nSMase2-independent increase in ceramide levels in broncheo-alveolar lavage fluid at day 2 to 5 post-treatment (Goldkorn et al. , 2014). NSMase2 has also been implicated in hypoxic pulmonary vasoconstriction, which is a physiologic pulmonary smooth muscle vasoconstriction in response to poor perfusion in order to shunt the blood supply to better-oxygenated alveoli (Cogolludo et al. , 2009). Hypoxia induces a GW4869-inhibited acute ceramide increase and pulmonary arterial smooth muscle contraction (Cogolludo, Moreno, 2009). Further elucidation of the mechanism revealed that nSMase2 activation in rat pulmonary arteries in response to hypoxia generated ROS and was prevented by blockage of the mitochondrial NADPH oxidase (Frazziano et al. , 2011). Finally, Lin et al. suggested a role for nSMase2 and sphingosine kinase in antagonizing anti-apoptotic properties of lipopolysaccharide during acute lung injury, which improved lung function and survival in these patients (Lin et al. , 2011). Taken together, these studies suggest a profound role of nSMase2 in modulation of lung responses to pathologies.
C. Involvement in circulatory and cardiac pathophysiology
nSMase2 in circulatory conditions
NSMase2 has been implicated in the development of hypertension. Initial findings pointed to activation of a membrane NSMase in response to acute changes in vascular pressure associated with NSMase-dependent ERK1/2 and MEK1/2 phosphorylation (Czarny et al. , 2003, Czarny and Schnitzer, 2004). Applying this finding to animal models, young and old rat aortas and mesenteric vessels were studied. Old aortas lost acetylcholine mediated vascular relaxation, which was associated with inhibitory phosphorylation on eNOS (Smith et al. , 2006). NSMase activity was also increased, and GW4869 treatment improved the vasomotor action of aged vessels (Ohanian et al. , 2014, Smith, Visioli, 2006). Furthermore, the inhibitory effect on eNOS was recapitulated in a mouse model of ER stress that showed increased nSMase2 activity associated with inactivation of eNOS. NSMase2 overexpression mimicked ER stress and resulted in decreased NO production, a potent vasodilator. (Chaube et al. , 2012, Mogami et al. , 2005). These effects suggest that activation of nSMase2 is associated with a vasoconstrictor phenotype. In that context, the effect of the physiologic vasoconstrictors endothelin-1 (ET-1) on nSMase2 was studied. ET-1 activates nSMase2 to induce VCAM-1 expression in rat small mesenteric artery (Ohanian et al. , 2012). Probing the system further, the effect of dietary Mg2+ deficiency was studied. Mg2+ deficiency has been demonstrated experimentally and is clinically associated with hypertension. In rats, a Mg2+ deficient diet for 21 days increased NSMase activity in both aortic smooth muscle and the left ventricle (Altura et al. , 2013). This was reversed by supplementing the diet with magnesium and resulted in alterations of telomerase activity that correlated with NSMase inhibition (Altura, Shah, 2013, Shah et al. , 2014). Taken together, this presents nSMase2 as a potential target for pharmacological inhibition in clinical hypertension.
Studies have implicated nSMase2 in various other circulatory pathologies. Transplant vasculopathy is a major cause of transplant rejection and is mediated by anti-HLA antibodies of the host. Anti-HLA activates nSMase2 via MMP2 in smooth muscle cells and produces a mitogenic signaling that leads to thickening of the intima of the vessels (Galvani et al. , 2011). The MMP2 ➔ nSMase2 ➔ mitogenic activation cascade is also found in endothelial ECV304 cells in response to urokinase. Here, integrinα5β3 activation of MMP2 is a critical step for nSMase2 activation(Maupas-Schwalm et al. , 2009). Finally, nSMase2 activity has implications in circulatory changes in dyslipidemia. Reports suggest that ApoC-1 enriched HDL particles, which are HDL particles with limited peripheral absorption, leads to an nSMase2-dependent apoptosis, when exogenously added to aortic smooth muscle cells (Kolmakova et al. , 2004)
nSMase2 in cardiac conditions
The literature on nSMase2 in cardiac pathologies is not thoroughly developed, but seems to suggest that activation of nSMase2 occurs in cardiac conditions and is, at least partly, responsible for cytotoxic outcomes. Early studies reported that following ischemia, reperfusion caused acute activation of a Mg2+-dependent NSMase in rat cardiac myocytes (Hernandez et al. , 2000), which was prevented by pre-treatment with NAC, and NSMase-derived ceramide activated the JNK pathway (Hernandez, Discher, 2000). Furthermore, post-myocardial infarction rat hearts displayed reduced GSH and its repletion using NAC resulted in inhibition of a nSMase2/Bcl-2/caspase3 axis and decreased oxidative stress resulting improved left ventricular function (Adamy et al. , 2007). Another report suggested that obese type II diabetic patients have increased serum NSMase activity but that serum ceramide levels were unaffected (Baranowski et al. , 2010).
C. Involvement in neurobiology and neuropathology
Response to neurotrophins
Neurotrophins are a family of four mammalian proteins that promote neuronal survival and function through 2 classes of receptor mediated signaling; the p75 neurotrophin receptor, which is a common receptor for all neurotrophins and the Tropomyosin-receptor kinase (Trk) receptors that selectively bind specific neurotrophins. Initial studies demonstrated activation of the sphingomyelin cycle following neurotrophin treatment in T9 gliomas and PC12 cells (Dobrowsky et al. , 1995, Dobrowsky et al. , 1994). Application of the neurotrophin Nerve Growth Factor (NGF) to cultured hippocampal neurons produces ceramide via nSMase2 activation to engage apoptosis (Brann et al. , 2002). NSMase2 activation requires the p75 receptor and increases phosphorylation of JNK (Brann, Tcherpakov, 2002). This effect is recapitulated in adult motor neurons as NGF-induced apoptosis is blocked by the nSMase inhibitors manumycin A and GW4869 (Pehar et al. , 2007). p75 activation of nSMase2 also increases action potentials in capsaicin sensitive sensory neurons through increased current in tetrodoxin resistant sodium channels and suppression of a delayed rectifier outward potassium current (Zhang et al. , 2002). Other data suggest that nSMase2-derived ceramide may also act upstream of p75 receptors. Peng et al. showed that penta-acetyl geniposide induces NSMase activity in C6 gliomas with ceramide generation that is inhibited by GW4869 (Peng et al. , 2006). NGF and p75 are upregulated in that setting in an nSMase2-dependent manner (Peng, Huang, 2006). Moreover, the role of nSMase2-derived ceramide in apoptosis/survival in response to neurotrophins has recently been clarified. Candalija et al. reported a protective, cell survival-promoting role of nSMase2 in response to the neurotrophin brain-derived neutrophic factor in granule neurons and NGF in PC12 cells (Candalija et al. , 2014). However, unlike previous studies, the increase in cell survival stemmed from activation of the Trk receptors (Candalija, Cubi, 2014). This leads to a possible model where activation of the p75 low affinity receptor in response to NGF promotes an apoptotic nSMase2-dependent signal, while neurotrophin activation of the Trk receptors promotes a pro-survival nSMase2-dependent signal.
Activation of neuronal death
A body of literature suggests the involvement of nSMase2-derived ceramide in neuronal death. Ethanol activates nSMase2 to produce glial cell death and signals through the ERK, JNK and p38 MAPK pathways in astrocytes (Pascual et al. , 2003). On the other hand H2O2 causes apoptotic cell death via activation of nSMase2 in primary oligodendrocyte, a finding that sheds light on one of the contributing factors to the exacerbation of multiple sclerosis, a demyelinating disease involving oligodendrocytes (Jana and Pahan, 2007). Kainic acid-induced status epilepticus increases pro-apototic Bax and decreases anti-apoptotic Bcl-2 levels simultaneous with nSMase2 activation (Mikati et al. , 2008). Finally, oxidized phosphatidylcholine increases nSMase2 at the mRNA level in rat oligodendrocytes and leads to the activation of caspase-3 and caspase-8 (Qin et al. , 2009).
Alzheimer’s disease
There is growing interest in the involvement of nSMase2 in the pathogenesis of Alzheimer’s disease. Initial reports showed that Aβ25–35 peptide upregulated NSMase activity in rat primary astrocytes and neurons causing cell death (Ayasolla et al. , 2004, Ju et al. , 2005). Further studies suggested that Aβ1–42 and Aβ1–40 peptides activated nSMase2 to negatively control gamma secretase activity (Grimm et al. , 2005). Recently, inhibition of phospholipase D2 was shown to abrogate Aβ-nSMase2 activation in SH-SY5Y cells (Tanabe et al. , 2014). The importance of exosomal secretion of Aβ peptide mediated by nSMase2 is beginning to gain appreciation (Dinkins et al. , 2014, Wang et al. , 2012, Yuyama et al. , 2012). It is thought that exosomes released from microglia prevent Aβ oligomerization, through unknown mechanisms and that the absence of exosomes leads to a soluble Aβ peptide that produces neuronal death (Yuyama, Sun, 2012).
Other neurological functions
Effects of nSMase2 on normal synaptic regulation was studied in models of dopamine uptake where it was shown that nSMase2 inhibition or downregulation by siRNA decreased dopamine uptake in PC12 cells (Kim et al. , 2010) with Hsp60 acting as an interaction partner that negatively regulates nSMase2 in that system (Ahn et al. , 2013). This paved the way to more in depth studies of involvement of nSMase2 in dopaminergic pathologies such as Parkinson’s disease. Physiologically, nSMase2 inhibition by GW4869 delayed formation of spatial reference memory in mice without affecting episodic-like memory; the likely mechanism is through the modification of the subunit composition of N-methyl-D-aspartate receptors (Tabatadze et al. , 2010). Also, a role for nSMase2 was found in inflammation following ischemia/reperfusion injuries in the brain. Astrocytes, but not neurons, displayed increased nSMase2 activity following ischemia/reperfusion with nSMase2-dependent generation of the pro-inflammatory cytokines TNF-α, Interleukin-1 and Interleukin-6 (Gu et al. , 2013). Finally, a potential role for nSMase2-derived ceramide was recently described in embryonic stem cells and embryonic stem cell-derived neural progenitors. In these cells, ceramide induce ciliogenesis, a critical step in differentiation that was inhibited by GW4869 and fumonisin B1, an inhibitor of ceramide synthase. NSMase2-derived ceramide prevented the activation of histone deacetylase-6, Aurora A, and sequestered protein kinase C into apicolateral domains preventing its activation (He et al. , 2014). Taken together, these findings suggest a profound role of nSMase2 in neuronal pathologies. This will pave way for research understanding more in depth mechanisms of neuronal regulation by nSMase2, but also for the development of specific inhibitors of nSMase2 as novel strategies of treatment of neurological diseases.
D. NSMase2-mediated release of exosomes
Recently, a body of literature emerged involving nSMase2 in exosome release through a non-canonical pathway independent of ESCRT protein complexes. Exosomes are 40–100 nm vesicles that are released from the cell by the fusion of multivesicular endosomes to the plasma membrane. The resulting secreted exosome carries cellular contents such as protein, RNA, and lipids and serves to influence biological functions of neighboring cells through the transfer of these contents (Thery et al. , 2002). In tumors, nSMase2 was initially shown to mediate exosomal release from HEK293 containing mir16 and mir146 (Kosaka et al. , 2010). The functional significance of this was demonstrated by showing that blocking nSMase-2 mediated exosomal release from 4T1 breast xenografts reduced lung metastasis through perturbation of endothelial function ( Kosaka et al. , 2013). Isolated exosomes from these tumors increased HUVEC tube formation and migration through exosomal transfer of mir-210 (Kosaka, Iguchi, 2013). However, this function seems to be tumor specific as another reports suggested that in androgen-resistant prostatic cell line PC-3, nSMase2 does not play a role in exosome release and instead suggested a role of glycolipids in that release(Phuyal et al. , 2014).
The role of nSMase2 in Alzheimer’s disease is mediated at least partly through exosomes. Primary astrocytes from murine cortices treated with Aβ25–35 or Aβ1–42 died along with induction of PAR-4, production of ceramide and caspase3 activation (Wang, Dinkins, 2012). The PAR4 along with the ceramide was found to localize to exosomes, and that finding was not present in the fro/fro mice (Wang, Dinkins, 2012). Treating wild-type mice and 5XFAD Alzheimer’s disease mouse model with GW4869 resulted in lower exosomes in the brain, with the 5XFAD mice displaying lower whole brain concentration of Aβ1–42 (Dinkins, Dasgupta, 2014). Finally, nSMase2 mediated exosomal transfer was found to be important in antigen presentation. Antigen presenting cells secrete nSMase2-dependent exosomes, enriched in small RNA species, which regulate antigen presentation (Mittelbrunn et al. , 2011).
E. Role of nSMase2 in cancer
The role of sphingolipids in cancer is a subject that has gained attention recently. While the involvement of nSMase2 in chemotherapeutic-mediated cytotoxicity has been the subject of intense examination, the contribution of nSMase2 in the pathogenesis and perpetuation of human tumors is less well understood (Henry et al. , 2013). In leukemia, mutations of the nSMase2 gene, SMPD3, were identified in 5% of acute myeloid leukemia and 6% of acute lymphoid leukemia. Some of these mutations perturbed the stability or localization of the enzyme (Kim et al. , 2008). In breast cancer, SMPD3 was one of 55 genes with more than 4 fold upregulation in tumor vasculature of luminal A type breast cancer versus control matched breast tissue (Bhati et al. , 2008). Moreover, the metastasis of 4T1 murine breast tumors was found to be dependent on nSMase2-mediated exosome transfer of miR-210 (Kosaka, Iguchi, 2013). Moreover, a recent study identified nSMase2 as a possible tumor suppressor in hepatocellular carcinoma as genome-wide analysis revealed hypermethylation of the gene (SMPD3) in human samples. Further experiments demonstrated that overexpression of nSMase2 in these cells slowed growth while its downregulation promoted cell invasion and migration (Revill et al. , 2013).
From a therapeutic standpoint, the early hints at a role for nSMase2 mediating effects from tumor therapy came from studies that found stimulation of NSMase activity following 1-beta-D-Arabinofuranosylcytosine (Ara-C) peaking at 30 minutes with increase in ceramide in HL-60 cells (Strum et al. , 1994). A similar effect was seen with ionizing radiation (IR) (Haimovitz-Friedman et al. , 1994). Erythromyeloblastic cell lines that are resistant to IR fail to activate NSMase and do not undergo apoptosis ( Bruno et al. , 1998) while c-kit activation, which confers resistant to IR, prevents nSMase2 activation (Bhati, Patterson, 2008, Maddens et al. , 2002). Following that, daunorubicin, etoposide, paclitaxel and ara-C were found to activate nSMase2 in different cell lines (Henry, Moller, 2013, Plo et al. , 2001, Sumitomo et al. , 2002) via a PKC-dependent mechanism, to mediate the cytotoxic effects of these chemotherapeutics (Plo, Lautier, 2001, Sumitomo, Ohba, 2002). Compounds with anti-tumor properties that are not used in clinics have also been studied. Polyphenols (t-PER and QUER) activate nSMase2 (Ferrer et al. , 2007), while nSMase2-dependent apoptosis was described in K562 and MOLT-4 in response to Withanolide D (Mondal et al. , 2010), and in K562 and HT29 in response to Protopanaxadiol (Park et al. , 2013). Finally, compounds derivative of p53 reactivation and induction of massive apoptosis (PRIMA-1), possess nSMase2-mediated cytotoxic properties against lung cells (Soans et al. , 2014). The cytotoxic effect of nSMase2 activation can also mediate some side effects of chemotherapeutics. Gentamycin-induced ototoxicity is prevented by GW4869, as pre-treatment with this inhibitor decreased outer hair loss in organs of Corti explants (Chi et al. , 2014).
F. Role of nSMase2 in cell death
One of the earliest described biological consequences of ceramide formation is cell death. NSMase2 activation to engage cell death machinery has been described both in acute (minutes to a few hours) and in prolonged (>24 hours) stimulations. Early on NSMase-derived ceramide action on cell death was defined in TNF-α mediated apoptosis. Activation of NSMase was shown initially in response to TNF-α (Kim, Linardic, 1991), before Obeid et al. demonstrated that TNF-α induced apoptosis and exogenous addition of C2 ceramide mimicked that effect (Obeid et al. , 1993), and similar results were shown using exogenous sphingomyelinases (Jarvis et al. , 1994) . Further characterization was done using bacterial sphingomyelinase as a tool mimicking NSMase activation in cells. Zhang et al. showed that exogenous application of Bacillus Cereus cloned sphingomyelinase resulted in cell death with activation of PARP (Zhang et al. , 1997). Other members of the TNF receptor family such as FAS have been also shown to mediate activation of NSMase to cause cellular apoptosis. Agonist of Fas induced accumulation of ceramide with an increase in membrane bound SMase activity and DNA fragmentation in Fas sensitive cells, a finding that was mimicked with exogenous addition of ceramide in Fas resistant cells (Skowronski et al. , 1996, Tepper et al. , 1995). Many stumuli were subsequently shown to activate NSMase. In WEHI231 cells, cross-linking of IgM increases membrane sphingomyelinase activity and ceramide production causing apoptosis, preventable by overexpression of Bcl-xL (Wiesner et al. , 1997). PC12 cells activate sphingomyelinase in response to hypoxia to produce apoptosis peaking at 24 hours. Similar to WEHI231s, this is blocked by overexpression of the anti-apoptotic Bcl2 (Yoshimura et al. , 1998), or by pre-treatment with NAC or GSH (Yoshimura et al. , 1999). This observation was consolidated in C6 glioma cells where Bcl-2 overexpression prevented etoposide-induced nSMase2 activation (Sawada et al. , 2000). Work by Chipuk et al. studied how NSMase-derived ceramide cooperate with Bax and Bcl-2 to promote apoptosis. Isolated mitochondria where found to be resistant to release of cytochrome c following cleaved caspase 8 (C8-BID) treatment. A restoration of the sensitivity occurred only when the microsomal fraction (containing NSMase activity) was added to the mitochondrial fraction, and the resensitization was blocked by GW4869.
While the use of GW4869 in that study suggests the involvement of nSMase2 as the NSMase isoform responsible for the ceramide generation, the lack of data on microsomal nSMase2 interacting with mitochondria coupled with the unavailability of data relating to GW4869 inhibition of the recently cloned mitochondrial-associated NSMase lead to the conclusion that further studies are required to determine the NSMase isoform responsible for the biology described.
The effector pathway of apoptotic cell death is through activation of caspases. The role of caspase activation in nSMase2-mediated cell death has been reported with conflicting results. While most studies suggest caspase activation follows the activation of nSMase2 (Meyers-Needham et al. , 2012), some define caspase activation as a pre-requisite for nSMase2 activation. For example, inhibition of caspase-3 prevented nSMase2 activation in HL-60 cells following sodium nitroprusside treatment and addition of recombinant caspase-3 activates NSMase activity in a cell free system (Takeda et al. , 1999).
One disease process in which nSMase2-mediated apoptosis seems to be relevant is diabetes mellitus (DM). One of the proposed mechanisms of pathogenesis of DM is ER stress and the activation of the unfolded protein response. Initial findings showed that basal nSMase2 was activated in Akita mice, a mouse model of ER stress (Lei et al. , 2010). Subsequently pancreatic β islets treated with thapsigargin were shown to undergo apoptosis with activation of nSMase2 at the message level (Lei et al. , 2012) and that nSMase2 upregulation is mediated by iPLA2 (Lei et al. , 2013, Lei, Zhang, 2012). Finally, phosphatidylinositol ether lipid analogs (PIAs) induce secretion of pro-apoptotic factors in non-exosomes nanovesicles in an nSMase2-dependent manner (Gills et al. , 2012).
G. Role of nSMase2 in cell differentiation and growth arrest
The role of NSMase activity in cell differentiation was examined prior to the cloning of the different isoforms of NSMase. Vitamin D-induced cell differentiation in HL60 leukemia cells was accompanied by activation of a neutral sphingomyelinase and ceramide generation, while the addition of an exogenous SMase potentiated the differentiation effect of subthreshold Vitamin D (Okazaki et al. , 1989) with ceramide being identified as the sphingolipid responsible for that effect (Okazaki et al. , 1990). The effect of NSMase activation on HL60 monocytic differentiation was also shown in response to TNF (Kim, Linardic, 1991) and the later characterization of nSMase2 as the major NSMase activated by TNF suggest this effect is nSMase2 specific. Later studies expanded on the role of NSMase in cell differentiation, with the effect of neurotrophins on the activation of nSMase2 (refer to section C) and the potential role of that in neuronal differentiation being the most characterized of these functions.
The study of nSMase2 in modulation of the cell cycle has gained momentum recently with the discovery of stimuli that activate nSMase2 to produce growth arrest. While ceramide has been described to produce a growth arrest phenotype, among its many biological functions, studies of nSMase2-derived ceramide has lagged behind (Hannun and Obeid, 2011). MCF7 breast cancer cells overexpressing nSMase2 have a similar growth phenotype to control cells in the exponential phase. However, upon serum starvation they get retained in G0/G1 to a greater extent than cells overexpressing control plasmid. Moreover, cell confluence upregulates nSMase2 to arrest cells in G0/G1 with hypophosphorylation of the retinoblastoma protein and induction of p21. Downregulation of nSMase2 by siRNA bypasses this phenotype (Marchesini, Osta, 2004). Later studies demonstrated nSMase2-dependent dephosphorylation of beta catenin on T41/S45 via activation of PPC1γ in confluent cells (Marchesini et al. , 2007). Another stimulus that induces nSMase2-dependent growth arrest is All-Trans Retinoic Acid (ATRA). ATRA induces activation of nSMase2 at the transcriptional level in cells with functional retinoic acid receptor-α to arrest them in G1 phase of cell cycle (Somenzi et al. , 2007). The mechanism is thought to be through the dephosphorylation of S6K, independent of PP2A (Clarke et al. , 2011a).
H. Role of nSMase2 in post-natal growth and bone development
Stoffel et al. initially described the severe growth retardation in the SMPD3−/− mice starting at embryonic day 14 and accompanied with hypoplasia of all organs except the brain. They also described delayed bone ossification present in the mice (Stoffel, Jenke, 2005). Later, studies on the fro/fro mice showed a reduction in mineralized tissue in long and flat bones. The hypertrophic chondrocytes in long bones persist and do not undergo either apoptosis or mineralization in long bones. The localized expression of SMPD3 corrected this phenotype (Khavandgar et al. , 2011). The mechanism by which nSMase2 acts to control chondrocyte maturation and mineralization was further studied to describe two key pathways. The first pertained to hyaluronan synthesis, an essential process in normal bone maturation. Fro/fro fibroblasts were found to have increased hyaluronan with upregulation of hyaluronan synthase-2 (HAS-2) (Qin et al. , 2012). In ATDC5 cells, a common cellular model of chondrocyte maturation, HAS-2 was found to be downregulated during maturation and siRNA to nSMase2 prevented HAS-2 downregulation ( Kakoi et al. , 2014). Another mechanism thought to partially contribute to the phenotype is the effect on Pi3K/Akt pathway. Fro/fro mice were found to have higher levels of phosphor-Akt and phosphp-S6K and knockdown of nSMase2 in ATDC5 cells during differentiation further potentiated this increase (Kakoi, Maeda, 2014, Qin, Berdyshev, 2012).
IV. Conclusion
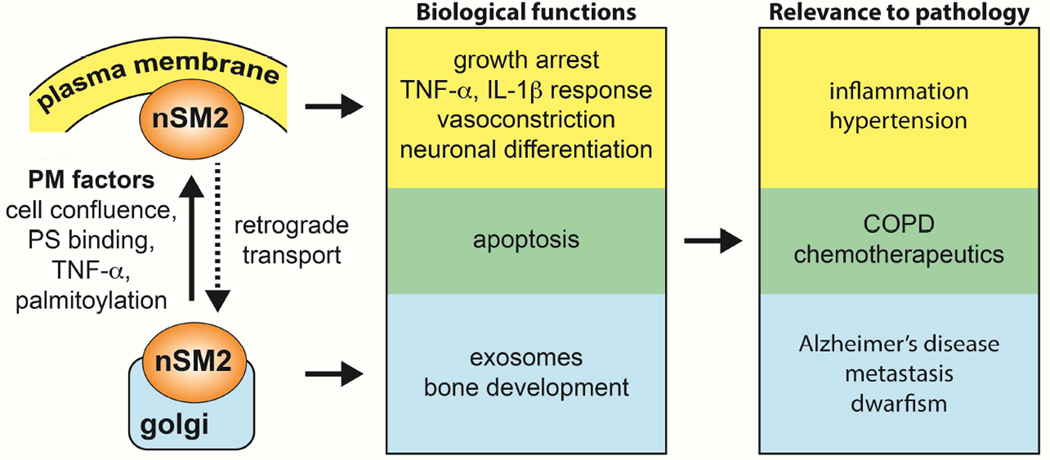
A number of tools developed over the past two decades have enlarged our understanding of the regulation, enzymology and biologic functions of nSMase2. However, many questions still remain [Fig.3]. The determination of a crystal structure of this enzyme may answer basic questions regarding the enzymology and the mechanism of activation. There is a need to understand cellular mechanisms of regulation of nSMase2 and the specific mechanisms by which ceramide and other down stream lipid metabolites mediate the various actions of this critical enzyme. More in depth studies are also needed to transition biological findings into clinical studies with the anticipation that this enzyme could be a drug target for the treatment of several disease processes.
Figure 3. Summary of nSMase2 functions and relevance to pathology.
Association of nSMase2 function in different cellular compartments. Plasma membrane, yellow; Golgi, blue; Unknown, green.
Acknowledgments
This work is partly supported by NIH grant R37 GM43825 to YAH and F32GM100679 to MVA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam D, Wiegmann K, Adam-Klages S, Ruff A, Kronke M. A novel cytoplasmic domain of the p55 tumor necrosis factor receptor initiates the neutral sphingomyelinase pathway. The Journal of biological chemistry. 1996;271:14617–14622. doi: 10.1074/jbc.271.24.14617. [DOI] [PubMed] [Google Scholar]
- Adam-Klages S, Adam D, Wiegmann K, Struve S, Kolanus W, Schneider-Mergener J, et al. FAN, a novel WD-repeat protein, couples the p55 TNF-receptor to neutral sphingomyelinase. Cell. 1996;86:937–947. doi: 10.1016/s0092-8674(00)80169-5. [DOI] [PubMed] [Google Scholar]
- Adam-Klages S, Schwandner R, Adam D, Kreder D, Bernardo K, Kronke M. Distinct adapter proteins mediate acid versus neutral sphingomyelinase activation through the p55 receptor for tumor necrosis factor. Journal of leukocyte biology. 1998;63:678–682. doi: 10.1002/jlb.63.6.678. [DOI] [PubMed] [Google Scholar]
- Adamy C, Mulder P, Khouzami L, Andrieu-abadie N, Defer N, Candiani G, et al. Neutral sphingomyelinase inhibition participates to the benefits of N-acetylcysteine treatment in post-myocardial infarction failing heart rats. Journal of molecular and cellular cardiology. 2007;43:344–353. doi: 10.1016/j.yjmcc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Ago H, Oda M, Takahashi M, Tsuge H, Ochi S, Katunuma N, et al. Structural basis of the sphingomyelin phosphodiesterase activity in neutral sphingomyelinase from Bacillus cereus. The Journal of biological chemistry. 2006;281:16157–16167. doi: 10.1074/jbc.M601089200. [DOI] [PubMed] [Google Scholar]
- Ahn KH, Kim SK, Choi JM, Jung SY, Won JH, Back MJ, et al. Identification of Heat Shock Protein 60 as a Regulator of Neutral Sphingomyelinase 2 and Its Role in Dopamine Uptake. PloS one. 2013;8:e67216. doi: 10.1371/journal.pone.0067216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airola MV, Hannun YA. Sphingolipid metabolism and neutral sphingomyelinases. Handbook of experimental pharmacology. 2013:57–76. doi: 10.1007/978-3-7091-1368-4_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura BM, Shah NC, Shah GJ, Li W, Zhang A, Zheng T, et al. Magnesium deficiency upregulates sphingomyelinases in cardiovascular tissues and cells: cross-talk among proto-oncogenes, Mg(2+), NF-kappaB and ceramide and their potential relationships to resistant hypertension, atherogenesis and cardiac failure. International journal of clinical and experimental medicine. 2013;6:861–879. [PMC free article] [PubMed] [Google Scholar]
- Arenz C, Thutewohl M, Block O, Waldmann H, Altenbach HJ, Giannis A. Manumycin A and its analogues are irreversible inhibitors of neutral sphingomyelinase. Chembiochem : a European journal of chemical biology. 2001;2:141–143. doi: 10.1002/1439-7633(20010202)2:2<141::AID-CBIC141>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Aubin I, Adams CP, Opsahl S, Septier D, Bishop CE, Auge N, et al. A deletion in the gene encoding sphingomyelin phosphodiesterase 3 (Smpd3) results in osteogenesis and dentinogenesis imperfecta in the mouse. Nature genetics. 2005;37:803–805. doi: 10.1038/ng1603. [DOI] [PubMed] [Google Scholar]
- Ayasolla K, Khan M, Singh AK, Singh I. Inflammatory mediator and beta-amyloid (25-35)-induced ceramide generation and iNOS expression are inhibited by vitamin E. Free radical biology & medicine. 2004;37:325–338. doi: 10.1016/j.freeradbiomed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Baranowski M, Blachnio-Zabielska A, Hirnle T, Harasiuk D, Matlak K, Knapp M, et al. Myocardium of type 2 diabetic and obese patients is characterized by alterations in sphingolipid metabolic enzymes but not by accumulation of ceramide. Journal of lipid research. 2010;51:74–80. doi: 10.1194/jlr.M900002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsacchi R, Perrotta C, Bulotta S, Moncada S, Borgese N, Clementi E. Activation of endothelial nitric-oxide synthase by tumor necrosis factor-alpha: a novel pathway involving sequential activation of neutral sphingomyelinase, phosphatidylinositol-3’ kinase, and Akt. Molecular pharmacology. 2003;63:886–895. doi: 10.1124/mol.63.4.886. [DOI] [PubMed] [Google Scholar]
- Barth BM, Gustafson SJ, Kuhn TB. Neutral sphingomyelinase activation precedes NADPH oxidase-dependent damage in neurons exposed to the proinflammatory cytokine tumor necrosis factor-alpha. Journal of neuroscience research. 2012;90:229–242. doi: 10.1002/jnr.22748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belka C, Wiegmann K, Adam D, Holland R, Neuloh M, Herrmann F, et al. Tumor necrosis factor (TNF)-alpha activates c-raf-1 kinase via the p55 TNF receptor engaging neutral sphingomyelinase. The EMBO journal. 1995;14:1156–1165. doi: 10.1002/j.1460-2075.1995.tb07099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhati R, Patterson C, Livasy CA, Fan C, Ketelsen D, Hu Z, et al. Molecular characterization of human breast tumor vascular cells. The American journal of pathology. 2008;172:1381–1390. doi: 10.2353/ajpath.2008.070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann AB, Tcherpakov M, Williams IM, Futerman AH, Fainzilber M. Nerve growth factor-induced p75-mediated death of cultured hippocampal neurons is age-dependent and transduced through ceramide generated by neutral sphingomyelinase. The Journal of biological chemistry. 2002;277:9812–9818. doi: 10.1074/jbc.M109862200. [DOI] [PubMed] [Google Scholar]
- Bruno AP, Laurent G, Averbeck D, Demur C, Bonnet J, Bettaieb A, et al. Lack of ceramide generation in TF-1 human myeloid leukemic cells resistant to ionizing radiation. Cell death and differentiation. 1998;5:172–182. doi: 10.1038/sj.cdd.4400330. [DOI] [PubMed] [Google Scholar]
- Canals D, Perry DM, Jenkins RW, Hannun YA. Drug targeting of sphingolipid metabolism: sphingomyelinases and ceramidases. British journal of pharmacology. 2011;163:694–712. doi: 10.1111/j.1476-5381.2011.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candalija A, Cubi R, Ortega A, Aguilera J, Gil C. Trk receptors need neutral sphingomyelinase activity to promote cell viability. FEBS letters. 2014;588:167–174. doi: 10.1016/j.febslet.2013.11.032. [DOI] [PubMed] [Google Scholar]
- Castillo SS, Levy M, Thaikoottathil JV, Goldkorn T. Reactive nitrogen and oxygen species activate different sphingomyelinases to induce apoptosis in airway epithelial cells. Experimental cell research. 2007;313:2680–2686. doi: 10.1016/j.yexcr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Chaube R, Kallakunta VM, Espey MG, McLarty R, Faccenda A, Ananvoranich S, et al. Endoplasmic reticulum stress-mediated inhibition of NSMase2 elevates plasma membrane cholesterol and attenuates NO production in endothelial cells. Biochimica et biophysica acta. 2012;1821:313–323. doi: 10.1016/j.bbalip.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Chen BC, Chang HM, Hsu MJ, Shih CM, Chiu YH, Chiu WT, et al. Peptidoglycan induces cyclooxygenase-2 expression in macrophages by activating the neutral sphingomyelinase-ceramide pathway. The Journal of biological chemistry. 2009;284:20562–20573. doi: 10.1074/jbc.M109.028084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi LN, Tabuchi K, Nakamagoe M, Nakayama M, Nishimura B, Hara A. Ceramide/sphingomyelin cycle involvement in gentamicin-induced cochlear hair cell death. Archives of toxicology. 2014 doi: 10.1007/s00204-014-1259-x. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, et al. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CJ, Guthrie JM, Hannun YA. Regulation of neutral sphingomyelinase-2 (nSMase2) by tumor necrosis factor-alpha involves protein kinase C-delta in lung epithelial cells. Molecular pharmacology. 2008;74:1022–1032. doi: 10.1124/mol.108.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CJ, Mediwala K, Jenkins RW, Sutton CA, Tholanikunnel BG, Hannun YA. Neutral sphingomyelinase-2 mediates growth arrest by retinoic acid through modulation of ribosomal S6 kinase. The Journal of biological chemistry. 2011a;286:21565–21576. doi: 10.1074/jbc.M110.193375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CJ, Truong TG, Hannun YA. Role for neutral sphingomyelinase-2 in tumor necrosis factor alpha-stimulated expression of vascular cell adhesion molecule-1 (VCAM) and intercellular adhesion molecule-1 (ICAM) in lung epithelial cells: p38 MAPK is an upstream regulator of nSMase2. The Journal of biological chemistry. 2007;282:1384–1396. doi: 10.1074/jbc.M609216200. [DOI] [PubMed] [Google Scholar]
- Clarke CJ, Wu BX, Hannun YA. The neutral sphingomyelinase family: identifying biochemical connections. Advances in enzyme regulation. 2011b;51:51–58. doi: 10.1016/j.advenzreg.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogolludo A, Moreno L, Frazziano G, Moral-Sanz J, Menendez C, Castaneda J, et al. Activation of neutral sphingomyelinase is involved in acute hypoxic pulmonary vasoconstriction. Cardiovascular research. 2009;82:296–302. doi: 10.1093/cvr/cvn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarny M, Liu J, Oh P, Schnitzer JE. Transient mechanoactivation of neutral sphingomyelinase in caveolae to generate ceramide. The Journal of biological chemistry. 2003;278:4424–4430. doi: 10.1074/jbc.M210375200. [DOI] [PubMed] [Google Scholar]
- Czarny M, Schnitzer JE. Neutral sphingomyelinase inhibitor scyphostatin prevents and ceramide mimics mechanotransduction in vascular endothelium. American journal of physiology Heart and circulatory physiology. 2004;287:H1344–H1352. doi: 10.1152/ajpheart.00222.2004. [DOI] [PubMed] [Google Scholar]
- De Palma C, Meacci E, Perrotta C, Bruni P, Clementi E. Endothelial nitric oxide synthase activation by tumor necrosis factor alpha through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: a novel pathway relevant to the pathophysiology of endothelium. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:99–105. doi: 10.1161/01.ATV.0000194074.59584.42. [DOI] [PubMed] [Google Scholar]
- Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiology of aging. 2014;35:1792–1800. doi: 10.1016/j.neurobiolaging.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobierzewska A, Giltiay NV, Sabapathi S, Karakashian AA, Nikolova-Karakashian MN. Protein phosphatase 2A and neutral sphingomyelinase 2 regulate IRAK-1 protein ubiquitination and degradation in response to interleukin-1beta. The Journal of biological chemistry. 2011;286:32064–32073. doi: 10.1074/jbc.M111.238030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobierzewska A, Shi L, Karakashian AA, Nikolova-Karakashian MN. Interleukin 1beta regulation of FoxO1 protein content and localization: evidence for a novel ceramide-dependent mechanism. The Journal of biological chemistry. 2012;287:44749–44760. doi: 10.1074/jbc.M112.378836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowsky RT, Jenkins GM, Hannun YA. Neurotrophins induce sphingomyelin hydrolysis. Modulation by co-expression of p75NTR with Trk receptors. The Journal of biological chemistry. 1995;270:22135–22142. doi: 10.1074/jbc.270.38.22135. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- Dressler KA, Mathias S, Kolesnick RN. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992;255:1715–1718. doi: 10.1126/science.1313189. [DOI] [PubMed] [Google Scholar]
- Ferrer P, Asensi M, Priego S, Benlloch M, Mena S, Ortega A, et al. Nitric oxide mediates natural polyphenol-induced Bcl-2 down-regulation and activation of cell death in metastatic B16 melanoma. The Journal of biological chemistry. 2007;282:2880–2890. doi: 10.1074/jbc.M605934200. [DOI] [PubMed] [Google Scholar]
- Filosto S, Ashfaq M, Chung S, Fry W, Goldkorn T. Neutral sphingomyelinase 2 activity and protein stability are modulated by phosphorylation of five conserved serines. The Journal of biological chemistry. 2012;287:514–522. doi: 10.1074/jbc.M111.315481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosto S, Castillo S, Danielson A, Franzi L, Khan E, Kenyon N, et al. Neutral sphingomyelinase 2: a novel target in cigarette smoke-induced apoptosis and lung injury. American journal of respiratory cell and molecular biology. 2011;44:350–360. doi: 10.1165/rcmb.2009-0422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosto S, Fry W, Knowlton AA, Goldkorn T. Neutral sphingomyelinase 2 (nSMase2) is a phosphoprotein regulated by calcineurin (PP2B) The Journal of biological chemistry. 2010;285:10213–10222. doi: 10.1074/jbc.M109.069963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazziano G, Moreno L, Moral-Sanz J, Menendez C, Escolano L, Gonzalez C, et al. Neutral sphingomyelinase NADPH oxidase reactive oxygen species. Role in acute hypoxic pulmonary vasoconstriction. Journal of cellular physiology. 2011;226:2633–2640. doi: 10.1002/jcp.22611. [DOI] [PubMed] [Google Scholar]
- Galvani S, Trayssac M, Auge N, Thiers JC, Calise D, Krell HW, et al. A key role for matrix metalloproteinases and neutral sphingomyelinase-2 in transplant vasculopathy triggered by anti-HLA antibody. Circulation. 2011;124:2725–2734. doi: 10.1161/CIRCULATIONAHA.111.021790. [DOI] [PubMed] [Google Scholar]
- Gills JJ, Zhang C, Abu-Asab MS, Castillo SS, Marceau C, LoPiccolo J, et al. Ceramide mediates nanovesicle shedding and cell death in response to phosphatidylinositol ether lipid analogs and perifosine. Cell death & disease. 2012;3:e340. doi: 10.1038/cddis.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldkorn T, Filosto S, Chung S. Lung Injury and Lung Cancer Caused by Cigarette Smoke-Induced Oxidative Stress: Molecular Mechanisms and Therapeutic Opportunities Involving the Ceramide-Generating Machinery and Epidermal Growth Factor Receptor. Antioxidants & redox signaling. 2014 doi: 10.1089/ars.2013.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni FM, Alonso A. Sphingomyelinases: enzymology and membrane activity. FEBS letters. 2002;531:38–46. doi: 10.1016/s0014-5793(02)03482-8. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M, et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nature cell biology. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- Gu L, Huang B, Shen W, Gao L, Ding Z, Wu H, et al. Early activation of nSMase2/ceramide pathway in astrocytes is involved in ischemia-associated neuronal damage via inflammation in rat hippocampi. Journal of neuroinflammation. 2013;10:109. doi: 10.1186/1742-2094-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovitz-Friedman A, Kan CC, Ehleiter D, Persaud RS, McLoughlin M, Fuks Z, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. The Journal of experimental medicine. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Many ceramides. The Journal of biological chemistry. 2011;286:27855–27862. doi: 10.1074/jbc.R111.254359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Wang G, Wakade S, Dasgupta S, Dinkins M, Kong JN, et al. Primary cilia in stem cells and neural progenitors are regulated by neutral sphingomyelinase 2 and ceramide. Molecular biology of the cell. 2014;25:1715–1729. doi: 10.1091/mbc.E13-12-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B, Moller C, Dimanche-Boitrel MT, Gulbins E, Becker KA. Targeting the ceramide system in cancer. Cancer letters. 2013;332:286–294. doi: 10.1016/j.canlet.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Hernandez OM, Discher DJ, Bishopric NH, Webster KA. Rapid activation of neutral sphingomyelinase by hypoxia-reoxygenation of cardiac myocytes. Circulation research. 2000;86:198–204. doi: 10.1161/01.res.86.2.198. [DOI] [PubMed] [Google Scholar]
- Hinkovska-Galcheva V, Kjeldsen L, Mansfield PJ, Boxer LA, Shayman JA, Suchard SJ. Activation of a plasma membrane-associated neutral sphingomyelinase and concomitant ceramide accumulation during IgG-dependent phagocytosis in human polymorphonuclear leukocytes. Blood. 1998;91:4761–4769. [PubMed] [Google Scholar]
- Hofmann K, Tomiuk S, Wolff G, Stoffel W. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5895–5900. doi: 10.1073/pnas.97.11.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CY, Hsu MJ, Hsiao G, Wang YH, Huang CW, Chen SW, et al. Andrographolide enhances nuclear factor-kappaB subunit p65 Ser536 dephosphorylation through activation of protein phosphatase 2A in vascular smooth muscle cells. The Journal of biological chemistry. 2011;286:5942–5955. doi: 10.1074/jbc.M110.123968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huseby M, Shi K, Brown CK, Digre J, Mengistu F, Seo KS, et al. Structure and biological activities of beta toxin from Staphylococcus aureus. Journal of bacteriology. 2007;189:8719–8726. doi: 10.1128/JB.00741-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Murakami M, Furuhata A, Gao S, Yoshida K, Sobue S, et al. Transcriptional regulation of neutral sphingomyelinase 2 gene expression of a human breast cancer cell line, MCF-7, induced by the anti-cancer drug, daunorubicin. Biochimica et biophysica acta. 2009;1789:681–690. doi: 10.1016/j.bbagrm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Ito H, Tanaka K, Hagiwara K, Kobayashi M, Hoshikawa A, Mizutani N, et al. Transcriptional regulation of neutral sphingomyelinase 2 in all-trans retinoic acid-treated human breast cancer cell line, MCF-7. Journal of biochemistry. 2012;151:599–610. doi: 10.1093/jb/mvs037. [DOI] [PubMed] [Google Scholar]
- Jana A, Pahan K. Oxidative stress kills human primary oligodendrocytes via neutral sphingomyelinase: implications for multiple sclerosis. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2007;2:184–193. doi: 10.1007/s11481-007-9066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis WD, Kolesnick RN, Fornari FA, Traylor RS, Gewirtz DA, Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju TC, Chen SD, Liu CC, Yang DI. Protective effects of S-nitrosoglutathione against amyloid beta-peptide neurotoxicity. Free radical biology & medicine. 2005;38:938–949. doi: 10.1016/j.freeradbiomed.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Kajimoto T, Ohmori S, Shirai Y, Sakai N, Saito N. Subtype-specific translocation of the delta subtype of protein kinase C and its activation by tyrosine phosphorylation induced by ceramide in HeLa cells. Molecular and cellular biology. 2001;21:1769–1783. doi: 10.1128/MCB.21.5.1769-1783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakoi H, Maeda S, Shinohara N, Matsuyama K, Imamura K, Kawamura I, et al. Bone morphogenic protein (BMP) signaling up-regulates neutral sphingomyelinase 2 to suppress chondrocyte maturation via the Akt protein signaling pathway as a negative feedback mechanism. The Journal of biological chemistry. 2014;289:8135–8150. doi: 10.1074/jbc.M113.509331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakashian AA, Giltiay NV, Smith GM, Nikolova-Karakashian MN. Expression of neutral sphingomyelinase-2 (NSMase-2) in primary rat hepatocytes modulates IL-beta-induced JNK activation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:968–970. doi: 10.1096/fj.03-0875fje. [DOI] [PubMed] [Google Scholar]
- Khavandgar Z, Poirier C, Clarke CJ, Li J, Wang N, McKee MD, et al. A cell-autonomous requirement for neutral sphingomyelinase 2 in bone mineralization. The Journal of cell biology. 2011;194:277–289. doi: 10.1083/jcb.201102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Linardic C, Obeid L, Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha gamma-interferon Specific role in cell differentiation. The Journal of biological chemistry. 1991;266:484–489. [PubMed] [Google Scholar]
- Kim SK, Ahn KH, Ji JE, Choi JM, Jeon HJ, Jung SY, et al. Neutral sphingomyelinase 2 induces dopamine uptake through regulation of intracellular calcium. Cellular signalling. 2010;22:865–870. doi: 10.1016/j.cellsig.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Kim WJ, Okimoto RA, Purton LE, Goodwin M, Haserlat SM, Dayyani F, et al. Mutations in the neutral sphingomyelinase gene SMPD3 implicate the ceramide pathway in human leukemias. Blood. 2008;111:4716–4722. doi: 10.1182/blood-2007-10-113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmakova A, Kwiterovich P, Virgil D, Alaupovic P, Knight-Gibson C, Martin SF, et al. Apolipoprotein C-I induces apoptosis in human aortic smooth muscle cells via recruiting neutral sphingomyelinase. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:264–269. doi: 10.1161/01.ATV.0000112036.72200.ac. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. The Journal of biological chemistry. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. The Journal of biological chemistry. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Bone RN, Ali T, Wohltmann M, Gai Y, Goodwin KJ, et al. Genetic modulation of islet beta-cell iPLA(2)beta expression provides evidence for its impact on beta-cell apoptosis and autophagy. Islets. 2013;5:29–44. doi: 10.4161/isl.23758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Zhang S, Barbour SE, Bohrer A, Ford EL, Koizumi A, et al. Spontaneous development of endoplasmic reticulum stress that can lead to diabetes mellitus is associated with higher calcium-independent phospholipase A2 expression: a role for regulation by SREBP-1. The Journal of biological chemistry. 2010;285:6693–6705. doi: 10.1074/jbc.M109.084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Zhang S, Bohrer A, Barbour SE, Ramanadham S. Role of calcium-independent phospholipase A(2)beta in human pancreatic islet beta-cell apoptosis. American journal of physiology Endocrinology and metabolism. 2012;303:E1386–E1395. doi: 10.1152/ajpendo.00234.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Khan E, Careaga M, Goldkorn T. Neutral sphingomyelinase 2 is activated by cigarette smoke to augment ceramide-induced apoptosis in lung cell death. American journal of physiology Lung cellular and molecular physiology. 2009;297:L125–L133. doi: 10.1152/ajplung.00031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WC, Lin CF, Chen CL, Chen CW, Lin YS. Inhibition of neutrophil apoptosis via sphingolipid signaling in acute lung injury. The Journal of pharmacology and experimental therapeutics. 2011;339:45–53. doi: 10.1124/jpet.111.181560. [DOI] [PubMed] [Google Scholar]
- Liu B, Andrieu-Abadie N, Levade T, Zhang P, Obeid LM, Hannun YA. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-alpha-induced cell death. The Journal of biological chemistry. 1998a;273:11313–11320. doi: 10.1074/jbc.273.18.11313. [DOI] [PubMed] [Google Scholar]
- Liu B, Hassler DF, Smith GK, Weaver K, Hannun YA. Purification and characterization of a membrane bound neutral pH optimum magnesium-dependent and phosphatidylserine-stimulated sphingomyelinase from rat brain. The Journal of biological chemistry. 1998b;273:34472–34479. doi: 10.1074/jbc.273.51.34472. [DOI] [PubMed] [Google Scholar]
- Luberto C, Hassler DF, Signorelli P, Okamoto Y, Sawai H, Boros E, et al. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. The Journal of biological chemistry. 2002;277:41128–41139. doi: 10.1074/jbc.M206747200. [DOI] [PubMed] [Google Scholar]
- Maddens S, Charruyer A, Plo I, Dubreuil P, Berger S, Salles B, et al. Kit signaling inhibits the sphingomyelin-ceramide pathway through PLC gamma 1: implication in stem cell factor radioprotective effect. Blood. 2002;100:1294–1301. [PubMed] [Google Scholar]
- Marchesini N, Jones JA, Hannun YA. Confluence induced threonine41/serine45 phospho-beta-catenin dephosphorylation via ceramide-mediated activation of PP1cgamma. Biochimica et biophysica acta. 2007;1771:1418–1428. doi: 10.1016/j.bbalip.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini N, Luberto C, Hannun YA. Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism. The Journal of biological chemistry. 2003;278:13775–13783. doi: 10.1074/jbc.M212262200. [DOI] [PubMed] [Google Scholar]
- Marchesini N, Osta W, Bielawski J, Luberto C, Obeid LM, Hannun YA. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. The Journal of biological chemistry. 2004;279:25101–25111. doi: 10.1074/jbc.M313662200. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Yamada A, Tsukamoto K, Tamura H, Ikezawa H, Nakamura H, et al. A distant evolutionary relationship between bacterial sphingomyelinase and mammalian DNase I. Protein science : a publication of the Protein Society. 1996;5:2459–2467. doi: 10.1002/pro.5560051208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupas-Schwalm F, Bedel A, Auge N, Grazide MH, Mucher E, Thiers JC, et al. Integrin alpha(v)beta(3), metalloproteinases, and sphingomyelinase-2 mediate urokinase mitogenic effect. Cellular signalling. 2009;21:1925–1934. doi: 10.1016/j.cellsig.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Meyers-Needham M, Lewis JA, Gencer S, Sentelle RD, Saddoughi SA, Clarke CJ, et al. Off-target function of the Sonic hedgehog inhibitor cyclopamine in mediating apoptosis via nitric oxide-dependent neutral sphingomyelinase 2/ceramide induction. Molecular cancer therapeutics. 2012;11:1092–1102. doi: 10.1158/1535-7163.MCT-11-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikati MA, Zeinieh M, Habib RA, El Hokayem J, Rahmeh A, El Sabban M, et al. Changes in sphingomyelinases, ceramide, Bax, Bcl(2), and caspase-3 during and after experimental status epilepticus. Epilepsy research. 2008;81:161–166. doi: 10.1016/j.eplepsyres.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Milhas D, Clarke CJ, Idkowiak-Baldys J, Canals D, Hannun YA. Anterograde and retrograde transport of neutral sphingomyelinase-2 between the Golgi and the plasma membrane. Biochimica et biophysica acta. 2010;1801:1361–1374. doi: 10.1016/j.bbalip.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature communications. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Gotoh E, Nara F, Nishijima M, Hanada K. Hydrolysis of sphingosylphosphocholine by neutral sphingomyelinases. FEBS letters. 2004;557:288–292. doi: 10.1016/s0014-5793(03)01523-0. [DOI] [PubMed] [Google Scholar]
- Mogami K, Kishi H, Kobayashi S. Sphingomyelinase causes endothelium-dependent vasorelaxation through endothelial nitric oxide production without cytosolic Ca(2+) elevation. FEBS letters. 2005;579:393–397. doi: 10.1016/j.febslet.2004.11.100. [DOI] [PubMed] [Google Scholar]
- Mondal S, Mandal C, Sangwan R, Chandra S, Mandal C. Withanolide D induces apoptosis in leukemia by targeting the activation of neutral sphingomyelinase-ceramide cascade mediated by synergistic activation of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase. Molecular cancer. 2010;9:239. doi: 10.1186/1476-4598-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalivaeva NN, Rybakina EG, Pivanovich I, Kozinets IA, Shanin SN, Bartfai T. Activation of neutral sphingomyelinase by IL-1beta requires the type 1 interleukin 1 receptor. Cytokine. 2000;12:229–232. doi: 10.1006/cyto.1999.0547. [DOI] [PubMed] [Google Scholar]
- Nara F, Tanaka M, Hosoya T, Suzuki-Konagai K, Ogita T. Scyphostatin, a neutral sphingomyelinase inhibitor from a discomycete, Trichopeziza mollissima: taxonomy of the producing organism, fermentation, isolation, and physico-chemical properties. The Journal of antibiotics. 1999a;52:525–530. doi: 10.7164/antibiotics.52.525. [DOI] [PubMed] [Google Scholar]
- Nara F, Tanaka M, Masuda-Inoue S, Yamasato Y, Doi-Yoshioka H, Suzuki-Konagai K, et al. Biological activities of scyphostatin, a neutral sphingomyelinase inhibitor from a discomycete, Trichopeziza mollissima. The Journal of antibiotics. 1999b;52:531–535. doi: 10.7164/antibiotics.52.531. [DOI] [PubMed] [Google Scholar]
- Nikolova-Karakashian M, Morgan ET, Alexander C, Liotta DC, Merrill AH., Jr Bimodal regulation of ceramidase by interleukin-1beta. Implications for the regulation of cytochrome p450 2C11. The Journal of biological chemistry. 1997;272:18718–18724. doi: 10.1074/jbc.272.30.18718. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nature reviews Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Ohanian J, Forman SP, Katzenberg G, Ohanian V. Endothelin-1 stimulates small artery VCAM-1 expression through p38MAPK-dependent neutral sphingomyelinase. Journal of vascular research. 2012;49:353–362. doi: 10.1159/000336649. [DOI] [PubMed] [Google Scholar]
- Ohanian J, Liao A, Forman SP, Ohanian V. Age-related remodeling of small arteries is accompanied by increased sphingomyelinase activity and accumulation of long-chain ceramides. Physiological reports. 2014;2 doi: 10.14814/phy2.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw AE, Race PR, Monzo HJ, Vazquez-Boland JA, Banfield MJ. Crystal structure of SmcL, a bacterial neutral sphingomyelinase C from Listeria. The Journal of biological chemistry. 2005;280:35011–35017. doi: 10.1074/jbc.M506800200. [DOI] [PubMed] [Google Scholar]
- Park B, Lee YM, Kim JS, Her Y, Kang JH, Oh SH, et al. Neutral sphingomyelinase 2 modulates cytotoxic effects of protopanaxadiol on different human cancer cells. BMC complementary and alternative medicine. 2013;13:194. doi: 10.1186/1472-6882-13-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Valles SL, Renau-Piqueras J, Guerri C. Ceramide pathways modulate ethanol-induced cell death in astrocytes. Journal of neurochemistry. 2003;87:1535–1545. doi: 10.1046/j.1471-4159.2003.02130.x. [DOI] [PubMed] [Google Scholar]
- Pehar M, Vargas MR, Robinson KM, Cassina P, Diaz-Amarilla PJ, Hagen TM, et al. Mitochondrial superoxide production and nuclear factor erythroid 2-related factor 2 activation in p75 neurotrophin receptor-induced motor neuron apoptosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:7777–7785. doi: 10.1523/JNEUROSCI.0823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CH, Huang CN, Hsu SP, Wang CJ. Penta-acetyl geniposide induce apoptosis in C6 glioma cells by modulating the activation of neutral sphingomyelinase-induced p75 nerve growth factor receptor and protein kinase Cdelta pathway. Molecular pharmacology. 2006;70:997–1004. doi: 10.1124/mol.106.022178. [DOI] [PubMed] [Google Scholar]
- Philipp S, Puchert M, Adam-Klages S, Tchikov V, Winoto-Morbach S, Mathieu S, et al. The Polycomb group protein EED couples TNF receptor 1 to neutral sphingomyelinase. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1112–1117. doi: 10.1073/pnas.0908486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuyal S, Hessvik NP, Skotland T, Sandvig K, Llorente A. Regulation of exosome release by glycosphingolipids and flotillins. The FEBS journal. 2014;281:2214–2227. doi: 10.1111/febs.12775. [DOI] [PubMed] [Google Scholar]
- Plo I, Lautier D, Casteran N, Dubreuil P, Arock M, Laurent G. Kit signaling and negative regulation of daunorubicin-induced apoptosis: role of phospholipase Cgamma. Oncogene. 2001;20:6752–6763. doi: 10.1038/sj.onc.1204877. [DOI] [PubMed] [Google Scholar]
- Poirier C, Berdyshev EV, Dimitropoulou C, Bogatcheva NV, Biddinger PW, Verin AD. Neutral sphingomyelinase 2 deficiency is associated with lung anomalies similar to emphysema. Mammalian genome : official journal of the International Mammalian Genome Society. 2012;23:758–763. doi: 10.1007/s00335-012-9419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Berdyshev E, Poirer C, Schwartz NB, Dawson G. Neutral sphingomyelinase 2 deficiency increases hyaluronan synthesis by up-regulation of Hyaluronan synthase 2 through decreased ceramide production and activation of Akt. The Journal of biological chemistry. 2012;287:13620–13632. doi: 10.1074/jbc.M111.304857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Testai FD, Dawson S, Kilkus J, Dawson G. Oxidized phosphatidylcholine formation and action in oligodendrocytes. Journal of neurochemistry. 2009;110:1388–1399. doi: 10.1111/j.1471-4159.2009.06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revill K, Wang T, Lachenmayer A, Kojima K, Harrington A, Li J, et al. Genome-wide methylation analysis and epigenetic unmasking identify tumor suppressor genes in hepatocellular carcinoma. Gastroenterology. 2013;145:1424–1435. doi: 10.1053/j.gastro.2013.08.055. e1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkute K, Asmis RH, Nikolova-Karakashian MN. Regulation of neutral sphingomyelinase-2 by GSH: a new insight to the role of oxidative stress in aging-associated inflammation. Journal of lipid research. 2007a;48:2443–2452. doi: 10.1194/jlr.M700227-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkute K, Karakashian AA, Giltiay NV, Dobierzewska A, Nikolova-Karakashian MN. Aging in rat causes hepatic hyperresposiveness to interleukin-1beta which is mediated by neutral sphingomyelinase-2. Hepatology. 2007b;46:1166–1176. doi: 10.1002/hep.21777. [DOI] [PubMed] [Google Scholar]
- Sawada M, Nakashima S, Banno Y, Yamakawa H, Takenaka K, Shinoda J, et al. Influence of Bax or Bcl-2 overexpression on the ceramide-dependent apoptotic pathway in glioma cells. Oncogene. 2000;19:3508–3520. doi: 10.1038/sj.onc.1203699. [DOI] [PubMed] [Google Scholar]
- Shah NC, Shah GJ, Li Z, Jiang XC, Altura BT, Altura BM. Short-term magnesium deficiency downregulates telomerase, upregulates neutral sphingomyelinase and induces oxidative DNA damage in cardiovascular tissues: relevance to atherogenesis, cardiovascular diseases and aging. International journal of clinical and experimental medicine. 2014;7:497–514. [PMC free article] [PubMed] [Google Scholar]
- Sitrin RG, Sassanella TM, Petty HR. An obligate role for membrane-associated neutral sphingomyelinase activity in orienting chemotactic migration of human neutrophils. American journal of respiratory cell and molecular biology. 2011;44:205–212. doi: 10.1165/rcmb.2010-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronski EW, Kolesnick RN, Green DR. Fas-mediated apoptosis and sphingomyelinase signal transduction: the role of ceramide as a second messenger for apoptosis. Cell death and differentiation. 1996;3:171–176. [PubMed] [Google Scholar]
- Smith AR, Visioli F, Frei B, Hagen TM. Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging cell. 2006;5:391–400. doi: 10.1111/j.1474-9726.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- Soans E, Evans SC, Cipolla C, Fernandes E. Characterizing the Sphingomyelinase Pathway Triggered by PRIMA-1 Derivatives in Lung Cancer Cells with Differing p53 Status. Anticancer research. 2014;34:3271–3283. [PubMed] [Google Scholar]
- Somenzi G, Sala G, Rossetti S, Ren M, Ghidoni R, Sacchi N. Disruption of retinoic acid receptor alpha reveals the growth promoter face of retinoic acid. PloS one. 2007;2:e836. doi: 10.1371/journal.pone.0000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W, Jenke B, Block B, Zumbansen M, Koebke J. Neutral sphingomyelinase 2 (smpd3) in the control of postnatal growth and development. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4554–4559. doi: 10.1073/pnas.0406380102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strum JC, Small GW, Pauig SB, Daniel LW. 1-beta-D-Arabinofuranosylcytosine stimulates ceramide and diglyceride formation in HL-60 cells. The Journal of biological chemistry. 1994;269:15493–15497. [PubMed] [Google Scholar]
- Sumitomo M, Ohba M, Asakuma J, Asano T, Kuroki T, Asano T, et al. Protein kinase Cdelta amplifies ceramide formation via mitochondrial signaling in prostate cancer cells. The Journal of clinical investigation. 2002;109:827–836. doi: 10.1172/JCI14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Csak T. Inflammasomes in liver diseases. Journal of hepatology. 2012;57:642–654. doi: 10.1016/j.jhep.2012.03.035. [DOI] [PubMed] [Google Scholar]
- Tabatadze N, Savonenko A, Song H, Bandaru VV, Chu M, Haughey NJ. Inhibition of neutral sphingomyelinase-2 perturbs brain sphingolipid balance and spatial memory in mice. Journal of neuroscience research. 2010;88:2940–2951. doi: 10.1002/jnr.22438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Tashima M, Takahashi A, Uchiyama T, Okazaki T. Ceramide generation in nitric oxide-induced apoptosis. Activation of magnesium-dependent neutral sphingomyelinase via caspase-3. The Journal of biological chemistry. 1999;274:10654–10660. doi: 10.1074/jbc.274.15.10654. [DOI] [PubMed] [Google Scholar]
- Tamiya-Koizumi K, Kojima K. Activation of magnesium-dependent, neutral sphingomyelinase by phosphatidylserine. Journal of biochemistry. 1986;99:1803–1806. doi: 10.1093/oxfordjournals.jbchem.a135659. [DOI] [PubMed] [Google Scholar]
- Tanabe F, Nakajima T, Ito M. Involvement of diacylglycerol produced by phospholipase D activation in Abeta-induced reduction of sAPPalpha secretion in SH-SY5Y neuroblastoma cells. Biochemical and biophysical research communications. 2014;446:933–939. doi: 10.1016/j.bbrc.2014.03.038. [DOI] [PubMed] [Google Scholar]
- Tani M, Hannun YA. Analysis of membrane topology of neutral sphingomyelinase 2. FEBS letters. 2007a;581:1323–1328. doi: 10.1016/j.febslet.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani M, Hannun YA. Neutral sphingomyelinase 2 is palmitoylated on multiple cysteine residues. Role of palmitoylation in subcellular localization. The Journal of biological chemistry. 2007b;282:10047–10056. doi: 10.1074/jbc.M611249200. [DOI] [PubMed] [Google Scholar]
- Tcherkasowa AE, Adam-Klages S, Kruse ML, Wiegmann K, Mathieu S, Kolanus W, et al. Interaction with factor associated with neutral sphingomyelinase activation, a WD motif-containing protein, identifies receptor for activated C-kinase 1 as a novel component of the signaling pathways of the p55 TNF receptor. Journal of immunology. 2002;169:5161–5170. doi: 10.4049/jimmunol.169.9.5161. [DOI] [PubMed] [Google Scholar]
- Tellier E, Negre-Salvayre A, Bocquet B, Itohara S, Hannun YA, Salvayre R, et al. Role for furin in tumor necrosis factor alpha-induced activation of the matrix metalloproteinase/sphingolipid mitogenic pathway. Molecular and cellular biology. 2007;27:2997–3007. doi: 10.1128/MCB.01485-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nature reviews Immunology. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Tomiuk S, Zumbansen M, Stoffel W. Characterization and subcellular localization of murine and human magnesium-dependent neutral sphingomyelinase. The Journal of biological chemistry. 2000;275:5710–5717. doi: 10.1074/jbc.275.8.5710. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Kai JI, Huang WC, Wang CY, Wang Y, Chen CL, et al. Glycogen synthase kinase-3beta facilitates IFN-gamma-induced STAT1 activation by regulating Src homology-2 domain-containing phosphatase 2. Journal of immunology. 2009;183:856–864. doi: 10.4049/jimmunol.0804033. [DOI] [PubMed] [Google Scholar]
- Tsakiri N, Kimber I, Rothwell NJ, Pinteaux E. Interleukin-1-induced interleukin-6 synthesis is mediated by the neutral sphingomyelinase/Src kinase pathway in neurones. British journal of pharmacology. 2008;153:775–783. doi: 10.1038/sj.bjp.0707610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Dinkins M, He Q, Zhu G, Poirier C, Campbell A, et al. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD) The Journal of biological chemistry. 2012;287:21384–21395. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D, Knapp E, Bandaru VV, Wang Y, Knorr D, Poirier C, et al. Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. Journal of neurochemistry. 2009;109:1237–1249. doi: 10.1111/j.1471-4159.2009.06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner DA, Kilkus JP, Gottschalk AR, Quintans J, Dawson G. Anti-immunoglobulin-induced apoptosis in WEHI 231 cells involves the slow formation of ceramide from sphingomyelin and is blocked by bcl-XL. The Journal of biological chemistry. 1997;272:9868–9876. doi: 10.1074/jbc.272.15.9868. [DOI] [PubMed] [Google Scholar]
- Wu BX, Clarke CJ, Hannun YA. Mammalian neutral sphingomyelinases: regulation and roles in cell signaling responses. Neuromolecular medicine. 2010;12:320–330. doi: 10.1007/s12017-010-8120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BX, Clarke CJ, Matmati N, Montefusco D, Bartke N, Hannun YA. Identification of novel anionic phospholipid binding domains in neutral sphingomyelinase 2 with selective binding preference. The Journal of biological chemistry. 2011;286:22362–22371. doi: 10.1074/jbc.M110.156471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Costanzo M, Golde DW, Kolesnick RN. Tumor necrosis factor activation of the sphingomyelin pathway signals nuclear factor kappa B translocation in intact HL-60 cells. The Journal of biological chemistry. 1993;268:20520–20523. [PubMed] [Google Scholar]
- Yoshimura S, Banno Y, Nakashima S, Hayashi K, Yamakawa H, Sawada M, et al. Inhibition of neutral sphingomyelinase activation and ceramide formation by glutathione in hypoxic PC12 cell death. Journal of neurochemistry. 1999;73:675–683. doi: 10.1046/j.1471-4159.1999.0730675.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Banno Y, Nakashima S, Takenaka K, Sakai H, Nishimura Y, et al. Ceramide formation leads to caspase-3 activation during hypoxic PC12 cell death. Inhibitory effects of Bcl-2 on ceramide formation and caspase-3 activation. The Journal of biological chemistry. 1998;273:6921–6927. doi: 10.1074/jbc.273.12.6921. [DOI] [PubMed] [Google Scholar]
- Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. The Journal of biological chemistry. 2012;287:10977–10989. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeck A, Schroder K, Frobel K, Grote R, Thiericke R. The structure of manumycin. ICharacterization, structure elucidation and biological activity. The Journal of antibiotics. 1987;40:1530–1540. doi: 10.7164/antibiotics.40.1530. [DOI] [PubMed] [Google Scholar]
- Zhang P, Liu B, Jenkins GM, Hannun YA, Obeid LM. Expression of neutral sphingomyelinase identifies a distinct pool of sphingomyelin involved in apoptosis. The Journal of biological chemistry. 1997;272:9609–9612. doi: 10.1074/jbc.272.15.9609. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na(+) current and delayed rectifier K(+) current in rat sensory neurons. The Journal of physiology. 2002;544:385–402. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]