Abstract
The transcription factor NF-E2-related factor (NRF2) is a key regulator of several enzymatic pathways, including cytoprotective enzymes in highly metabolic organs. In this review, we summarize the ongoing research related to NRF2 activity in cancer development, focusing on in vivo studies using NRF2 knockout (KO) mice, which have helped in defining the crucial role of NRF2 in chemoprevention. The lower cancer protection observed in NRF2 KO mice under calorie restriction (CR) suggests that most of the beneficial effects of CR on the carcinogenesis process are likely mediated by NRF2. We propose that future interventions in cancer treatment would be carried out through the activation of NRF2 in somatic cells, which will lead to a delay or prevention of the onset of some forms of human cancers, and subsequently an extension of health- and lifespan.
Keywords: calorie restriction, carcinogenesis, NRF2, phytochemicals
Introduction
Cancer is a major health problem in developed and industrialized countries that dramatically diminishes the quality of life and life expectancy. Cancer incidence has been increasing, particularly, during the last decades, becoming the second most common cause of death after heart disease and first in several subgroups of the population. For instance, in the United States of America, the lifetime probability of cancer diagnosis is about 44% for men and 37% for women. Moreover, cancer is the first cause of death among women aged 40–79 years and among men aged 60–79 years (Jemal et al., 2009). Despite the immense efforts that have been made in early diagnosis and in the improvement of treatment modalities, the mortality rates from most cancers have not significantly decreased in the past 30 years.
The tumour suppressor gene NRF2
The transcription factor NF-E2-related factor (NRF2) mediates the antioxidant response and decreases tumour susceptibility in most carcinogenesis models. It acts against spontaneous and induced carcinogenesis through the modulation of insulin/insulin-like growth factor-1 (IGF-1) signalling pathway, promoting survival (Henderson et al., 1998; Ramos-Gomez et al., 2001, 2003; Pearson et al., 2008). Moreover, during the last years NRF2 action has been suggested to be involved in many aged-related diseases, summarized in Table 1.
Table 1.
NRF2 and CR involvement in aged-related diseases
Abbreviations: CR, calorie restriction; NRF2, NF-E2-related factor.
NRF2 belongs to a basic region-leucine zipper (bZip)-type transcription factor family that shares a conserved structural ‘cap n collar’ domain (Moi et al., 1994; Itoh et al., 1995; Kensler et al., 2007). NRF2 was originally identified as an erythroid-restricted DNA-binding activity and is evolutionarily conserved (Kobayashi et al., 2002; Suzuki et al., 2005). One example is Caenorhabditis elegans, which has the NRF2 homologue SKN-1. This transcription factor controls the response of the worms to oxidative stress. Interestingly, there are some differences in the functioning of SKN-1 protein, working as a homodimer in this model. SKN-1 is expressed in the intestines and ASi neurons, where it acts in the upregulation of the calorie restriction (CR) metabolism and oxidative stress resistance metabolism, respectively (Onken and Driscoll, 2010). At least the role of NRF2 in glutathione synthesis, quinone reduction and protection from reactive oxygen species (ROS) is mediated by SKN-1 in C. elegans. Downstream targets of SKN-1 overlap with pathways regulated by CR and lifespan promoting an increased longevity and healthspan under SKN-1 overexpression in worms (Tullet et al., 2008). The homologue of NRF2 in Drosophila melanogaster has been proposed recently (Sykiotis and Bohmann, 2008). The gene CncC binds to the small musculoaponeurotic fibrosarcoma proteins and protects under oxidative treatments. The homologues in vertebrates have also been determined in several models such as Gallus gallus and Danio rerio (Maher and Yamamoto, 2010). Interestingly, to date there has not been any NRF2 homologues proposed in bacteria or yeast.
In mammals, this family is composed of four proteins, p45-NFE2, NRF1, NRF2 and NRF3, as well as two distantly related proteins termed Bach1 and Bach2 (Chan et al., 1993a, 1993b; Moi et al., 1994; Itoh et al., 1995; Oyake et al., 1996; Muto et al., 1998; Kobayashi et al., 1999). Interestingly, the ‘cap n collar’ genes knockout mice have provided an invaluable tool for studying the functions of these genes in vivo. Studies using NRF3 KO mice, the closest gene to NRF2, do not show phenotypical differences compared with control mice (Derjuga et al., 2004; Kobayashi et al., 2004b). NRF3 likely acts as a major regulator of phase 2 enzyme genes, as NRF3 is not expressed in most metabolic organs, such as the liver and intestine (Braun et al., 2002; Derjuga et al., 2004; Kobayashi et al., 2004b).
NRF2 is the most potent transcription factor of the ‘cap n collar’ family, activating downstream targets about 100-fold (Toki et al., 1997; Kobayashi et al., 1999). NRF2 signalling is central to efficient detoxification of reactive metabolites and ROS. NRF2 is induced by oxidative stress, which enhances protection against molecular damage that induces cancer development (Ingram et al., 1990).
NRF2 is an unstable protein that has an estimated half-life time of 30 min (McMahon et al., 2003). This protein is repressed in homeostatic conditions maintaining a low basal level of cytoprotective gene expression (Motohashi and Yamamoto, 2004; Motohashi et al., 2004). A lower proteasome activity leads to increasing levels of NRF2 and its downstream targets, promoting a pathological status. Nuclear accumulation of NRF2 is abolished when protein synthesis is blocked by cycloheximide treatment, establishing that NRF2 activity is mainly regulated by its own stability.
Regulation of NRF2 transcriptional activity
NRF2 signalling is regulated by compartmental segregation from the cytoplasm to the nucleus. Under homeostatic conditions, NRF2 is bound to a Kelch-like ECH-associated protein 1 (Keap1) dimer in the cytoplasm (Itoh et al., 1999; Tong et al., 2006a). The interaction between NRF2 and Keap1 has been shown by yeast two-hybrid screening (Itoh et al., 1999). Furthermore, immunohistochemical analyses have shown that Keap1 is associated with the actin cytoskeleton, which retains NRF2 in the cytoplasm (Kang et al., 2004).
The Kelch domain of Keap1 interacts with two distinct amino-acid sequences present in the N-terminal of NRF2: ETGE and DLG (Tong et al., 2006b; Hayes and McMahon, 2009). A sequential interaction process termed ‘hinge and latch’ mechanism has been hypothesized. The first interaction is through the ETGE, and subsequently the DLG docks onto the adjacent unoccupied Kelch-repeat domain. Keap1 sequesters NRF2 in the cytoplasm and acts as an adaptor enhancing the interaction of the Cullin 3-based E3-ubiquitin ligase complex 2 (Zhang and Hannink, 2003; Cullinan et al., 2004; Kobayashi et al., 2004a; McMahon et al., 2004; Furukawa and Xiong, 2005). This process leads to a continuous ubiquitination, proteasomal degradation and transcriptional repression of NRF2 by preventing its nuclear translocation (Itoh et al., 1999; Kobayashi et al., 2004a). Cul3-mediated NRF2 degradation has been shown in studies that show cytoplasmatic NRF2 accumulation in Cul3-silenced cells (Cullinan et al., 2004; Furukawa and Xiong, 2005).
There are two independent hypotheses that explain different mechanisms responsible for dissociation of Nrf2 from Keap1. The first mechanism Keap1 acts as a primary redox sensor that contains reactive cysteines. All cysteine residues in Keap1 are found to be highly conserved across species (Itoh et al., 1999; Eggler et al., 2005). Nitric oxide (NO) is a multifunctional messenger that has been shown to induce the release NRF2 from Keap1. For instance, heme oxygenase-1 (HO-1) expression is increased after NO exposure in smooth muscle cells in an NRF2/antioxidant response element (ARE)-dependent manner (Liu et al., 2007). NRF2 expression has shown an age-related decrease in rodent and human tissues (Suh et al., 2004; Shih and Yen, 2007; Collins et al., 2009; Duan et al., 2009), possibly leading to higher levels of ROS and increased risk of cancer. This decrease on NRF2 activity has been suggested to be related to a decrease in NO-dependent regulation of NRF2 levels during senescence due to a functional inactivation of NO by high levels of superoxide (Ungvari et al., 2008). NO levels are increased under CR conditions, whereas superoxide is decreased in CR in rodents (Yang et al., 2004). Moreover, high NO levels show cardioprotective effects in atherogenesis. Interestingly, NO mimetics have been proposed to ameliorate the age-related diseases such as Alzheimer’s disease and are under clinical trials (Thatcher et al., 2005). Oxidative modifications of these cysteines act as sensors for stresses, provoking the disruption of the Keap1-Nrf2 interaction and release of Nrf2, resulting in the stabilization and the accumulation of this protein into the nucleus (Dinkova-Kostova et al., 2002; Wakabayashi et al., 2004; Wilson et al., 2005).
The second mechanism that regulates NRF2 activity is mediated through post-transcriptional modification on Keap1-Nrf2 complex by several classes of kinases. The phosphorylation state of Nrf2 enhances the stability and/or release of Nrf2 from Keap1 (Huang et al., 2002). The specific kinases that are implicated in the regulation of Nrf2 activity include mitogen-activated protein kinase, phosphatidylinositol 3-kinase (PI3K), protein kinase R-like endoplasmic reticulum kinase (Cullinan et al., 2003) and protein kinase C (PKC). In vitro studies have shown that phosphorylation of Nrf2 by PKC promotes its dissociation from Keap1 (Huang et al., 2002; Bloom and Jaiswal, 2003; Numazawa et al., 2003). In this case, a mutation driving to a constitutively dephosphorylated NRF2 state (S40A) has been shown, which is the target site for PKC, and decreases Keap1 -NRF2 release. Then, inhibition of PI3K attenuates nuclear translocation of Nrf2 from the cytoplasm. Furthermore, Cullinan et al. (2003) showed that protein kinase R-like endoplasmic reticulum kinase phosphorylates Nrf2 and triggers dissociation from Keap1, resulting in increased nuclear translocation. Other studies showed that the phosphatase inhibitor okadaic acid increases Nrf2 accumulation and transcriptional activation, likely because phosphorylated proteins are less accessible to ubiquitin ligase (Nguyen et al., 2003; Ramos-Gomez et al., 2003).
Once NRF2 is released from Keap1 by any of these mechanisms, NRF2 can be imported into the nucleus (Dinkova-Kostova et al., 2002; Wakabayashi et al., 2004). On the basis of the repressive effect of Keap1 on NRF2 activity, loss of function of Keap1 KO mice was suggested to enhance a cellular cancer chemopreventive effect (Devling et al., 2005). However, some studies have indicated that Keap1 KO mice resulted in constitutively hyperactive NRF2 signalling owing to its nuclear localization (Wakabayashi et al., 2003, 2004; Okawa et al., 2006). This overexpression is lethal owing to obstructive lesions mediated by hyperkeratotic outgrowth of the oesophageal and forestomach epithelial cells. In young Keap1 KO mice, nuclear levels of NRF2 as well as its downstream targets were substantially higher than control mice. Interestingly, this phenotype is reversed in Keap1::NRF2 double KO mice (Wakabayashi et al., 2003). A specific conditional Keap1 KO in hepatocytes shows increased resistance against acute drug toxicity induced by acetaminophen and increased levels of NRF2-regulated antioxidative enzymes (Okawa et al., 2006).
Nuclear NRF2 dimerizes with a group of nuclear bZip proteins termed small musculoaponeurotic fibrosarcoma proteins (Itoh et al., 1995; Motohashi et al., 2004). This dimerization strongly activates the transcription of downstream targets by enhancing the specificity to bind to a cis-acting enhancer of the ARE contained in the promoters of these genes (Friling et al., 1990; Itoh et al., 1997; Shou et al., 2001; Yu and Kensler, 2005; Yamamoto et al., 2006). Interestingly, studies conducted in vitro indicate that the affinity of NRF2 heterodimeric complexes with small musculoaponeurotic fibrosarcomas to ARE sequences is similar, regardless of the phosphorylation state of NRF2 (Huang et al., 2002).
Enzymes regulated by NRF2
Microarray results suggest that more than 200 gene products are under the transcriptional control of NRF2. Downstream targets include antioxidative enzymes, enzymes responsible for the production of antioxidants, reducing equivalents, cofactors and also genes that are classified into different categories, like 26S proteasome subunits, PSMB5 subunit gene and some heat-shock proteins (Kwak et al., 2003a, b). The main classes of NRF2-regulated genes include antioxidative enzymes like NAD(P)H:quinone oxidoreductase (NQO1), epoxide hydrolase, aldehyde dehydrogenase, aldoketo reductase, catalase, HO-1 (Favreau and Pickett, 1991; Li and Jaiswal, 1992; Prestera et al., 1995; Thimmulappa et al., 2002; Kwak et al., 2003b; Leonard et al., 2006). Another family of enzymes is involved in glutathione homeostasis, including glutathione reductases, peroxir-edoxin, thioredoxin and thioredoxin reductases and glutathione peroxidase (Friling et al., 1990; Rushmore and Pickett, 1990; Reinhart and Pearson, 1993; Mulcahy and Gipp, 1995). It is well known that NRF2 also enhances toxin export through the multidrug response transporters, like the multiple drug resistance-associated protein, carboxyl esterase, esterase D, retinal oxidase/ aldehyde oxidase and carbonic anhydrase. Wassreman and others have shown the role of NRF2 in the upregulation of proteasome subunits and heat-shock proteins, such as heat-shock protein 40 and mitochondrial stress-70 protein, sequestosome 1 and ubiquitin C that recognizes and degrades damaged proteins (Wasserman and Fahl, 1997; Davies, 2001; Kwak et al., 2003a; Rangasamy et al., 2004). Even NRF2 appears to regulate the expression of other transcription factors, growth factors, receptors, molecular chaperons and its own expression, through two putative functional AREs identified in the NRF2 promoter (Kwak et al., 2002).
The protein products of these genes provide multiple layers of protection during cellular insults, collectively favouring cell survival. For instance, these enzymes are essential for neuronal survival because they block neurotoxicity derived from glutathione depletion, lipid peroxidation, intracellular calcium overload, excitotoxins and disruption of the mitochondrial electron transport chain (Shih et al., 2003; Lee et al., 2003a, 2005a). Moreover, NRF2 activation leads to an increased cellular energetics and redox potential (Lee et al., 2003b; Kraft et al., 2004; Nguyen et al., 2004). It is interesting to note that in the nervous system, NRF2-regulated genes are activated in astrocytes and also confer protection to neighbouring neurons (Calkins et al., 2005; Jakel et al., 2007). Also, induction of NRF2 expression in cultured endothelial cells results in a marked increase in NRF2-driven transcriptional activity leading to increased survival under oxidative stress treatments (Mostoslavsky et al., 2006). Multiple studies have shown that NRF2 KO mice show a reduced constitutive expression of downstream targets in the main tissues that reach the electrophilic response, such as the liver, intestine and forestomach (McMahon et al., 2001; Hayes et al., 2000; Thimmulappa et al., 2002; Wakabayashi et al., 2003).
Some toxic compounds, for example, diesel exhaust particles, induce NRF2 expression and its nuclear accumulation. In turn, several antioxidant and phase 2 enzymes, like HO-1 and some glutathione S-transferase (GST) subunits, are significantly upregulated. Less well documented, but perhaps equally important, the activation of the NRF2 pathway also evokes the downregulation of many genes. Of note, NRF2 inhibits inflammation through decreasing expression of the pro-inflammatory mediators cyclooxygenase-2, interleukin-lβ, interleukin-6 and tumour necrosis factor-α (Khor et al., 2006; Kensler et al., 2007; Hayes and McMahon, 2009).
Importance of NRF2 activity in cancer prevention
NRF2 signalling is involved in the upregulation of enzymes that mediate the detoxification of reactive metabolites and ROS (see Table 2). These enzymes enhance the protection against molecular damage and eventually lead to a lower cancer development (Ingram et al., 1990). Interestingly, there must be other different pathways that are not related to NRF2 activity that are able to regulate the longevity since CR increases longevity in NRF2 KO mice. To date, these specific pathways remain to be elucidated.
Table 2.
NRF2 downstream targets involved in cancer protection
| Gene | Role in cancer prevention/cancer growth |
References |
|---|---|---|
| GPX2 | Inflammatory response | Banning et al., 2008 |
| PRDX1 | Antioxidant | Cao et al., 2009 |
| PRDX61 | Antioxidant | Chang et al., 2007 |
| NQO1 | Antioxidant | Nolan et al., 2010 |
| CBR1 | Antioxidant | La1 et al., 2008 |
| CBR3 | Antioxidant | La1 et al., 2008 |
| CYP2B9 | Drug metabolism | Muguruma et al., 2006 |
| FMO2 | Drug metabolism | Fialka et al., 2008 |
| FMO3 | Drug metabolism | Bae et al., 2006 |
| GSTA1 | ROS protection | Nguyen et al., 2010 |
| GSTM1 | ROS protection | Nguyen et al., 2010 |
| GSTP1 | ROS protection | Nguyen et al., 2010 |
| GSTT1 | ROS protection | Nguyen et al., 2010 |
| MGST3 | ROS protection | Efferth and Volm, 2005 |
| ALDH3A1 | Metabolism | Patel et al., 2008 |
| GADD45G | DNA repair | Baguley, 2010 |
| HSP40 | Stress resistance | Mitra et al., 2009 |
| HSP70 | Stress resistance | Wang et al., 2010 |
Abbreviation: NRF2, NF-E2-related factor; ROS, reactive oxygen species.
Overexpression of NRF2 or its downstream detoxification enzymes by transfection protects cells against carcinogen-induced DNA damage and/or cytotoxicity (Fields et al., 1999). On the other hand, loss of expression of this gene or its targets induces sensitivity to DNA damage and carcinogenesis (Henderson et al., 1998; Ramos-Gomez et al., 2001, 2003). NRF2 KO mice are susceptible to a variety of oxidative insults, DNA adducts formation and cancer development, clearly indicating the critical contribution of NRF2 downstream targets to cellular protection. The potential of NRF2-regulated antioxidative response in protecting against two-stage induced cancer has been shown (Kwak et al., 2002). NRF2 KO mice showed increased skin oxidative damage during 12-O-tetradecanoylphorbol-13-acetate promotion, leading to an increased multiplicity and incidence of skin tumours (Xu et al., 2006). A decline in levels of NRF2 in aged organisms that promotes oxidative damage is well documented (Suh et al., 2004). In a rat model, a decline in transcriptional activity of NRF2 in aged rats is responsible for the significant decline in glutathione levels in the liver. Furthermore, age-related NRF2 inhibition is observed in Parkinson, Alzheimer, Huntington’s diseases and atherosclerosis models (Pratico and Delanty, 2000; Jenner, 2003). Genetic ablation of the NRF2 gene increases the size of the lesions, whereas transplantation of NRF2-overexpressing astrocytes reduces it (Calkins et al., 2005; Shih et al., 2005). Some NRF2 activators of the triterpenoid family have been shown to improve the phenotype of these neurodegenerative diseases, such as 2-cyano-3,12-dioxooleana-1,9-dien-olic acid (CDDO), CDDO-ethyl amide and CDDO-trifluoroethyl amide (Stack et al., 2010). Recently, the involvement of NRF2 in the pathogenesis of diabetes has also been shown. Hyper-glycaemic conditions in animals and human models are associated with an increased ROS production (Kiritoshi et al., 2003; Ye et al., 2004). NRF2 expression is decreased in athero-susceptible regions of the aorta (Zakkar et al., 2009). NRF2 activation leads to an increased antioxidant battery that ameliorates the diabetic complications and diabetes itself through ROS scavenging by NRF2 downstream targets. The known decreased NRF2 expression in the elderly could lead to diabetes (Suh et al., 2004). NRF2 activation by some phytochemicals, such as sulphoraphane and bardoxolone methyl, increase the expression on antioxidant proteins conferring an increased protection to hyperglycaemia (Xue et al., 2008). Furthermore, some studies have shown that NRF2 regulates inflammation process. NRF2-deficient mice show susceptibility to induced colitis, leading to a loss of colonic crypts, massive infiltration of inflammatory cells and anal bleeding (Khor et al., 2008).
The dark side of NRF2
It has been documented that Keap1 KO mice, which constitutively express NRF2, died within 3 weeks of birth. Lethality has been attributed to hyperkeratosis of the oesophagus and forestomach cells and overexpression of keratins K1, K6 and loricrin, resulting in oesophageal occlusion and subsequent malnutrition (Wakabayashi et al., 2003). However, hepatocyte-specific disruption of Keap1 does not seem to have any adverse effect in mice. These mice exhibit a normal phenotype and express high hepatic levels of NRF2 downstream targets conferring protection under acetaminophen and concanavalin A treatments (Okawa et al., 2006; Osburn et al., 2008).
Constitutive activation of NRF2 may have negative effects enhancing tumour cell protection against chemotherapy as shown in some non-synonymous polymorphisms that are determinant for susceptibility to cancer in humans (Palli et al., 2000). For instance, non-synonymous Keap1 alleles afflicting Keap1 binding to NRF2 have been characteristically observed in human lung tumour and Keap1 mutations have also been found in breast and gall bladder cancers (Nioi and Nguyen, 2007; Shibata et al., 2008). This fact promotes nuclear localization of NRF2 and constitutive expression of its downstream targets, which facilitates resistance of lung tumour cells to chemotherapeutic drugs (Padmanabhan et al., 2006; Singh et al., 2006; Tong et al., 2006b; Pearson et al., 2008).
CR induces NRF2 activity
It has been shown that NRF2 is responsible for most of the anticarcinogenic effects of CR (Pearson et al., 2008). CR, reduced calorie intake without malnutrition, prevents carcinogenesis in spontaneous, chemically induced and radiation-induced cancer in experimental models (Tannenbaum and Silverstone, 1953; Andreou and Morgan, 1981; Kritchevsky et al., 1984; Pollard et al., 1984; Boissonneault et al., 1986; Gross and Dreyfuss, 1986; Klurfeld et al., 1987; Lagopoulos and Stalder, 1987; Birt et al., 1991; Shimokawa et al., 1991; Kritchevsky, 2001; Hursting et al., 2003).
Early efforts to understand the interaction of reduced calorie intake and carcinogenesis have allowed researchers to begin making progress understanding the mechanisms behind these effects of CR. It is well known that CR decreases ROS production, enhances plasma membrane redox system, decreases inflammation process, induces modification in hormonal milieu and improves insulin signalling pathway, at least in part through the induction of NRF2 activity.
Given the importance of NRF2, our laboratory has focused on the study of this transcription factor in cancer and ageing research. We showed that NRF2 is responsible for most of the anticarcinogenic effects of CR in the two-stage carcinogenesis model. In our study, CR was not effective against chemically induced tumorigenesis in the NRF2 KO mice. Both ad libitum-fed NRF2 KO and CR NRF2 KO mice developed tumours more readily and reached total tumour incidence at the age of 30 weeks, whereas 40% CR wt mice did not show any papilloma up to week 42. Even tumour multiplicity was not significantly different between the CR-fed KO mice and the ad libitum-fed wild-type mice, suggesting that the anticarcinogenic effect of CR solely depends on the activity of NRF2. However, we showed that CR was able to extend lifespan and increased insulin sensitivity similarly in NRF2 KO and in wild-type mice. Interestingly, we were able to identify a molecular pathway that dissociates the prolongevity and anticarcinogenic effects of CR in mice.
The reduction of NRF2 downstream targets in NRF2 KO mice lead to increased DNA damage. Moreover, in these mice NQO1 expression as well as its enzymatic activity was markedly reduced under CR compared with wild-type CR animals. It is possible that not only NRF2, but also other CR-induced pathways increase NQO1 gene expression in NRF2 non-dependent manner. We observed that NQO1 mRNA levels were increased in NRF2 KO mice under CR compared with their ad libitum counterparts, whereas other downstream targets such as HO-1, glutamatecysteine ligase, catalytic subunit, GST A1 and glutathione peroxidase-1 were not increased in NRF2 KO under CR conditions.
On the other hand, we showed that CR does not require NRF2 for insulin sensitivity as well as lifespan prolongation in mice. Proposed hallmarks of tumour prevention were measured and we found that insulin sensitivity and corticosterone levels were improved and increased, respectively, in CR NRF2 KO mice compared with ad libitum-fed ones. This fact could explain the delay in tumour incidence in CR groups compared with ad libitum. Interestingly, in longevity studies we observed similar increase in median lifespan in CR NRF2 KO and ad libitum mice, allowing us to separate the NRF2-mediated anticarcinogenic beneficial effect of CR from CR-induced longevity extension (Pearson et al., 2008).
CR beneficial effects through the induction of NRF2 activity
During the early 1900s, some studies published the beneficial effects of underfeeding laboratory animals on transplanted and induced tumours (Moreschi, 1909; Rous, 1914). Since then, beneficial effects have been described on longevity, age-associated diseases, attenuation of functional declines, cognitive deterioration and carcinogenesis in many models (Hursting et al., 2003; Pollak, 2009a). SKN-1 (NRF2 orthologue) is upregulated in the ASIs neurons in C. elegans under CR. Its activity increases the metabolic activity and is required for longevity extension in this model (Bishop and Guarente, 2007). Even CR shows lifespan extension when initiated later in life in rodents (Weindruch and Walford, 1982).
During the last decade, long-term studies have been examining the health benefits of CR in non-human primates. Monkeys under CR showed a delayed onset of age-associated pathologies, significantly better glucose tolerance, less muscle loss, no type 2 diabetes, cardiovascular disease, and brain atrophy and 50% lower cancer incidence compared with their ad libitum counterparts (Hansen et al., 1995; Colman et al., 2009). Final results from several ongoing non-human primate studies will be achieved over the next decade. Their results will give researchers more clues about the beneficial effects of CR on cancer and ageing, and will allow them to perform future research (Ramsey et al., 2000; Mattison et al., 2007).
There are a few human studies suggesting beneficial effects of CR in humans. In the fifteenth century, Luigi Cornaro started a kind of CR when he was 40 years old. His diet was based on 400 g of food daily plus wine and daily exercise, he died at the age of 91, almost three times the average lifespan during this century in developed countries (Howell, 1987). Another documented case is the population of Okinawa during Second World War, who consumed fewer calories than their counterparts in the rest of Japan. During this time, they showed the lowest incidence of coronary heart disease, stroke, cancer and delayed ageing in the world (Suzuki, 2001; Willcox et al., 2007). Interestingly, the subsequent diet normalization have raised the incidence of the mentioned diseases and ageing up to regular rates (Miyagi et al., 2003). Furthermore, the beneficial effects in human studies are supported by studies of people on long-term CR, who show fewer signs of cardiovascular ageing (Holloszy and Fontana, 2007). Studies from Pennington Calerie team show that 6-month CR in humans decrease the levels of fasting insulin and core body temperature, two known biomarkers of longevity. Even more, it has been shown that energy expenditure is decreased and DNA fragmentation is lower owing to less damage to DNA. CR affects many pathways and leads to benefits for cancer development and other age-related diseases, summarized in Table 1. In the following sections, we will describe the present knowledge about molecular pathways improved by CR.
Decreased ROS production
CR decreases metabolic rate and oxidative damage. This effect is considered one of the major factors contributing to slowing the ageing process and preventing tumour formation. ROS production is achieved by products of metabolism mainly produced by mitochondrial oxidative phosphorylation as well as extracellular oxidant compounds. When their levels are exceeded, ROS modify cellular molecules, resulting in lipid peroxidation, DNA strand break, telomere shortening and protein carbonylation (Dexter et al., 1989; Halliwell, 1992, 1996b; Djuric and Kritschevsky, 1993; Shaw et al., 1995; Fitzmaurice et al., 1996; Halliwell, 1996a, 2001; Alam et al., 1997a,b; Stadtman and Berlett, 1998; Cakatay et al., 2001; Warita et al., 2001; Volchegorskii et al., 2004).
Oxidative damage eventually produces DNA mutations commonly identified in age-related diseases. These mutations may confer growth advantage and eventually cancer development. In order to defend against ROS, cells under CR induce a coordinated expression of transcription factors, NRF2 among them, that increase antioxidant enzymes, including phase II detoxification enzymes and phase III efflux transporters (Motohashi and Yamamoto, 2004; Klaassen and Slitt, 2005; Mandlekar et al., 2006).
Plasma membrane redox system improvement
One of the most important benefits of CR consists in the improvement of the plasma membrane redox system. CR enhances the activities and content of antioxidant compounds, which usually declines with age (Murakami and Johnson, 1996). Previous work carried out in our laboratory has shown that cytochrome b5 reductase and NQO1 expression and activities are increased in plasma membranes from rodent’s tissues under long-term CR (Manjgaladze et al., 1993; De Cabo et al., 2004; Hyun et al., 2006, 2007). Furthermore, the plasma membrane redox system also contributes to the regulation of the cellular redox homeostasis affecting NADH/NAD+ ratio and contributing to regulate survival (Jimenez-Hidalgo et al., 2009).
Improved apoptosis, inflammation and cell proliferation inhibition under CR
Cellular consequences of reduced energy intake in CR models are a decrease in inflammation, suppression of cell proliferation and encouraged apoptosis (Birt et al., 1998). CR modulates a gene expression shift associated with inflammation, cellular stress, fibrosis, apoptosis, type I enzymes, cell division and DNA replication processes. Reduced nutrient availability is sensed by AMP kinase and protein kinase B/AKT, which are activated when AMP/ATP ratio increases. Another enzymatic pathway downregulated by CR is the mammalian target of rapamycin, which only transduces signals from upstream pathways when intracellular nutrients concentration is adequate.
In the early 1900s it was shown that chronic inflammation increases carcinogenesis. Inflammation is required to maintain integrity when cells are damaged by an infection in animals (Philip et al., 2004). The inflammation process is initiated by a cascade of cytokines and chemokines that relay in the generation of oxidative stress that mutates DNA and promotes cancer (Lok et al., 1988; Hursting et al., 1994). Several studies have shown that CR promotes the reduction of inflammatory mediators, as tumour necrosis factor-α and interleukin-6 (Chandrasekar et al., 1995). Alternative day fasting protects from the age-induced inflammation, resulting in less DNA damage and protein carbonylation in rats through a decreased nuclear factor-κB DNA-binding activity (Castello et al., 2010). It has been shown that even short-term CR decrease pro-inflammatory gene expression in old rats (Jung et al., 2009).
The decrease of the DNA replication rate under CR makes cells less susceptible to DNA damage induced by carcinogens, and decreases oncogenic cells proliferation. The modulation in the regulation of these pathways by CR leads to a lower energy expenditure, protein translation and proliferation, increased apoptosis, growth of mitochondria and promotion of autophagy process, which prevent cancer by eliminating damaged proteins and whole organelles. These processes promote cell survival in CR models, decreasing the opportunity for a damaged cell to survive, which makes the organisms less susceptible to cancer development (Franke, 2008).
Shift of the hormonal milieu
The protective properties of CR include the modulation of the hormonal content. A 10-fold higher level of plasma corticosterone at 0700 hours in CR mice compared with ad libitum counterparts has been published. The adrenal gland, which produces corticosterone and dehydroepiandrosterone, is necessary for beneficial effects of CR in the lung- and skin-induced cancer (Pashko and Schwartz, 1992). Adrenal hormones inhibit stimulated epidermal DNA synthesis and tumour formation in two-stage cancer skin model. Studies in mice showed less papilloma accumulation in control mice under CR compared with CR adrenalectomyced ones (Stewart et al., 2005). Interestingly, corticosterone supplementation in water resulted in cancer prevention in both mice strains, suggesting that elevation of corticosterone in CR mice mediates the prevention of skin cancer. Furthermore, ad libitum adrenalectomyced mice showed elevated lymphosarcoma incidence compared with corticosterone supplemented ones, indicating an anticarcinogenic role of this hormone (Birt et al., 2004).
Another adrenal steroid, dehydroepiandrosterone, suppresses tumour formation and proliferation. Dehydroepiandrosterone administration in diet reproduces many of the beneficial effects of CR, including a repression of tumour development in several organs, and prolongs both mean and maximal lifespan of mice. It has been shown that dehydroepiandrosterone inhibits glucose-6-phosphate dehydrogenase, leading to a lower NADPH and ribose 5-phosphate levels with a consequent inhibition of deoxyribonucleotide synthesis (Gordon et al., 1987; Garcea et al., 1988; Shantz et al., 1989; Pashko et al., 1991).
The current hypothesis of glucocorticoid hormone function on cancer prevention is through the activation of the glucocorticoid receptor and increased glucocorticoid hormone, which drives to a decreased PKC activity (Birt et al., 1999). Then, low levels of 12-O-tetradeca-noylphorbol-13-acetate-dependent PKC inhibits MAP-1/Raf-1 pathway, leading to an attenuation in the induction of the activator protein-1 transcription factor, which is essential for CR prevention of mouse skin carcinogenesis.
Improvement of insulin signalling pathways
CR improves markers of diabetes such as insulin sensitivity. In rodents and primates, CR causes a decline in insulin relative to glucose concentrations, circulating insulin, IGF-1 receptor, IGF-1 (which fall at first but rebound to normal levels) and increased concentrations of IGFBP3 (Cohen and Hilf, 1974; Taub et al., 1987; Ruggeri et al., 1989; Breese et al., 1991; Masoro, 1995). The first evidence to support a role of insulin-like signals in the regulation of longevity and age-related diseases in mammals came from studies of mice with hereditary dwarfism (Ames dwarf) that showed low circulating IGF-1 (Brown-Borg et al., 1996).
Lower levels of these proteins lead to a lower activation of downstream kinases PI3K/protein kinase B, ultimately causing the dephosphorylation, nuclear translocation and activation of FOXO transcription factors. Moreover, high levels of IGFBP3 in CR animals induce pro-apoptotic and antiproliferative effects in cancer cells in an IGF-1 independent manner (Butt et al., 2002; Lee et al., 2005b; Kalaany and Sabatini, 2009). On the other hand, heightened activity of downstream insulin pathway in tumour cells drives a signal through receptors that reduces FOXO activity and promotes the growth and survival of cancer cells (Greer and Brunet, 2005; Pollak, 2009). There are some reported tumours that have developed escape mechanisms that allow them to evade to the beneficial effects of CR. Constitutively the deregulated activated insulin/IGF-1/IGF-1 receptor/PI3K signalling pathway is determinant of the sensitivity of tumours to CR (Tannenbaum and Silverstone, 1949; Weindruch and Walford, 1982; Cheney et al., 1983; Pugh et al., 1999; Kritchevsky, 2001; Sell, 2003; Thompson et al., 2003). Activation of PI3K or inactivation of its counterpart PTEN phosphatase results in the production of phosphorylated inositol lipids at the plasma membrane (Hennessy et al., 2005). These lipids act as secondary messengers, and provide docking sites for many intracellular proteins, resulting in the activation of a variety of downstream signalling molecules.
The current hypothesis points towards a hallmark of CR-insensitive tumours that consists of increased PI3K/ protein kinase B activity and decreased FOXO-mediated transcription through phosphorylation, allowing tumour cells to proliferate in the absence of IGF-1 or insulin. Thus, PI3K-activating mutations are sufficient to induce resistance to CR benefits. Then, it is possible that insulin resistance in type 2 diabetes treatments might be beneficial in preventing tumours with activated PI3K/inactive PTEN pathway.
NRF2 activators
Owing to the difficult adaptation of humans to a CR diet similar to that performed under laboratory conditions, the upregulation of NRF2 has been proposed as a potential target to evaluate for CR mimetics. Then, activation of NRF2 and downstream targets by administration of some phytochemicals is a crucial target for tumour prevention. Epidemiological studies have clearly documented that some phytochemical’s action is linked to a lower risk of many types of cancers (Chen et al., 2000; Chan and Giovannucci, 2001). NRF2 signaling pathway has been a target for chemoprevention even before its molecular characterization by Wattenberg (1972), and several studies showed a markedly attenuated efficacy of various chemopreventive agents in NRF2 KO mice. Detoxification enzymes are expressed constitutively at low levels, but can be greatly enhanced in response to exposure to some phytochemical compounds that activate NRF2 transcription factor, summarized in Tables 2 and 3 (Hong and Sporn, 1997). Some NRF2 activators are currently under clinical trials in the study of aged-related diseases, such as CDDO-methyl ether and oltipraz (Kensler and Helzlsouer, 1995; Nagaraj et al., 2010). Interesting data support these clinical trials in cancer studies; CDDO-methyl ether, also known as bardoxolone methyl, induces apoptosis in lung cancer cells (Lapillonne et al., 2003; Iida et al., 2004; Hyer et al., 2005).
Table 3.
NRF2 activators
| Compound | References |
|---|---|
| Dithiolethiones (oltipraz) |
Ansher et al., 1986; Kwak et al., 2001; Pietsch et al., 2003; Ramos-Gomez et al., 2001; Iida et al., 2004 |
| Isothiocyanates (sulphoraphan) |
Heiss et al., 2001; Brigelius-Flohe and Banning, 2006; Dinkova-Kostova et al., 2004; Fahey et al., 1997; Gerhauser et al., 1997; Hu et al., 2006; Juge et al., 2007; Zhang et al., 1994; Campbell et al., 2004; Clinton et al., 1996; Chiao et al., 2004; Giovannucci et al., 1995; Hecht et al., 1995; Lii et al., 2010; Miller et al., 2002 |
| Triterpenoids (bardoxolone methyl) |
Dinkova-Kostova et al., 2001; Yates et al., 2007 |
| Curcumin | Shen et al., 2006 |
| Resveratrol | Leifert and Abeywardena, 2008; Udenigwe et al., 2008 |
| Ethoxyquindiet | Cabral and Neal, 1983; Nair et al., 2006 |
| Methyl ester and ethyl amide | Yates et al., 2007 |
| Piceatannol | Lee et al., 2010a |
| Simvastatin | Chartoumpekis et al., 2010 |
| Metformin | Onken and Driscoll, 2010 |
Abbreviation: NRF2, NF-E2-related factor.
For example, dithiolethiones increase NRF2 activity and lead to the detoxification activity of GST, NQO1, ferritin H and L, as well as glutathione reductase downstream targets (Ansher et al., 1986; Kwak et al., 2001; Ramos-Gomez et al., 2001; Pietsch et al., 2003). The most studied compound of this family, oltipraz, completely failed to protect the NRF2 KO mice, indicating the importance of NRF2 activity in chemo-protection. (Ramos-Gomez et al., 2001; Iida et al., 2004). An increase in glutathione levels in the liver, kidney and forestomach of mice was observed after oltipraz supplementation. Finally, the induced enzymes protect against cancer and reduce 10 times the volume of the liver occupied by preneoplastic foci at the same time that hepatic aflatoxin–DNA adduct formation is markedly reduced (Kensler et al., 1987). Interestingly, there was no effect on tumour burden in NRF2-deficient mice. Clinical trials have shown that oltipraz modulates the activities of both conjugating/detoxication enzymes as well as cytochrome P450s.
Sulphoraphan is a potent isothiocyanate formed following myrosinase-catalyzed metabolism of glucosinolates and is present in high concentrations in broccoli sprouts and other crucifers. Isothiocyanates show a strong anti-inflammatory activity, probably achieved through inhibition of the nuclear factor-κB signalling pathway (Heiss et al., 2001). It was found to be a potent activator of the NRF2-regulated response preventing tumorigenesis through improved activity of GSTs and NQO1 (Zhang et al., 1994; Fahey et al., 1997; Gerhauser et al., 1997; Dinkova-Kostova et al., 2004; Brigelius-Flohe and Banning, 2006; Hu et al., 2006; Juge et al., 2007). Dietary supplementation with sulphoraphan may be associated with a lower risk of prostate and colon cancer in mammals (Giovannucci et al., 1995; Hecht et al., 1995; Clinton et al., 1996; Miller et al., 2002; Campbell et al., 2004; Chiao et al., 2004).
Triterpenoids are also very potent activators of NRF2 and are able to activate NQO1 enzyme activity in vitro (Dinkova-Kostova et al., 2001). Studies in transgenic reporter mice with the NQO1 ARE linked to a luciferase gene localized ARE activation in metabolic organs such as the kidney, salivary gland, liver and intestine (Yates et al., 2007).
Curcumin, from turmeric, induces the expression of NRF2 downstream targets, like heme oxygenase-1 enzyme in human cells, and its anti-inflammatory activity has been shown to inhibit carcinogenesis in preclinical animal models (Shen et al., 2006).
Resveratrol has been shown to reduce inflammation, possess cardioprotective and vasoprotective properties in a NRF2-dependent induction, which confers cancer prevention as shown in several preclinical animal models (Leifert and Abeywardena, 2008; Udenigwe et al., 2008).
Ethoxyquindiet supplementation inhibited liver carcinogenesis in rats exposed to anatoxin Bl (Cabral and Neal, 1983). It has been subsequently shown that the induction of hepatic cytoprotective enzymes by these antioxidants is mediated by NRF2 signalling (Nair et al., 2006).
The methyl ester and ethyl amide derivatives are less documented, but it has been shown that they are able to induce NRF2-regulated cytoprotective genes in the lung and have been studied in a post-initiation lung cancer mouse model (Yates et al., 2007).
Metformin is a biguanide drug commonly used to treat type 2 diabetes that has been noted to extend healthspan of non-diabetic mice. In C. elegans, metformin dietary supplementation extends median lifespan through SKN-1 pathway in a conserved biochemical mechanism that acts like a CR mimetic (Onken and Driscoll, 2010).
Concluding remarks
During the last years, researchers have been trying to determine the mechanisms whereby dietary intake modulates cancer and ageing. The transcription factor NRF2 is activated under CR and induces cancer protection. Increased understanding of these physiological mechanisms would offer the potential to develop mechanism-based interventions to promote longevity, healthy ageing and lower cancer incidence (Figure 1). It has been shown that longevity and cancer share some enzymatic pathways. Existing therapies designed to produce antiageing effects are likely also to have a cancer preventive effect and vice versa. In order to develop a preventive strategy for cancer treatment in humans a periodic fasting or intermittent CR has been proposed to be attainable and beneficial (Williams et al., 1998; Johnson et al., 2007)). In any case, the adaptation of humans to a CR pattern similar to that we performed with mice in our laboratory (ranging from 10 to 40% restriction in food daily intake) is difficult to obtain. We propose NRF2 as a potential target to evaluate and develop CR mimetics.
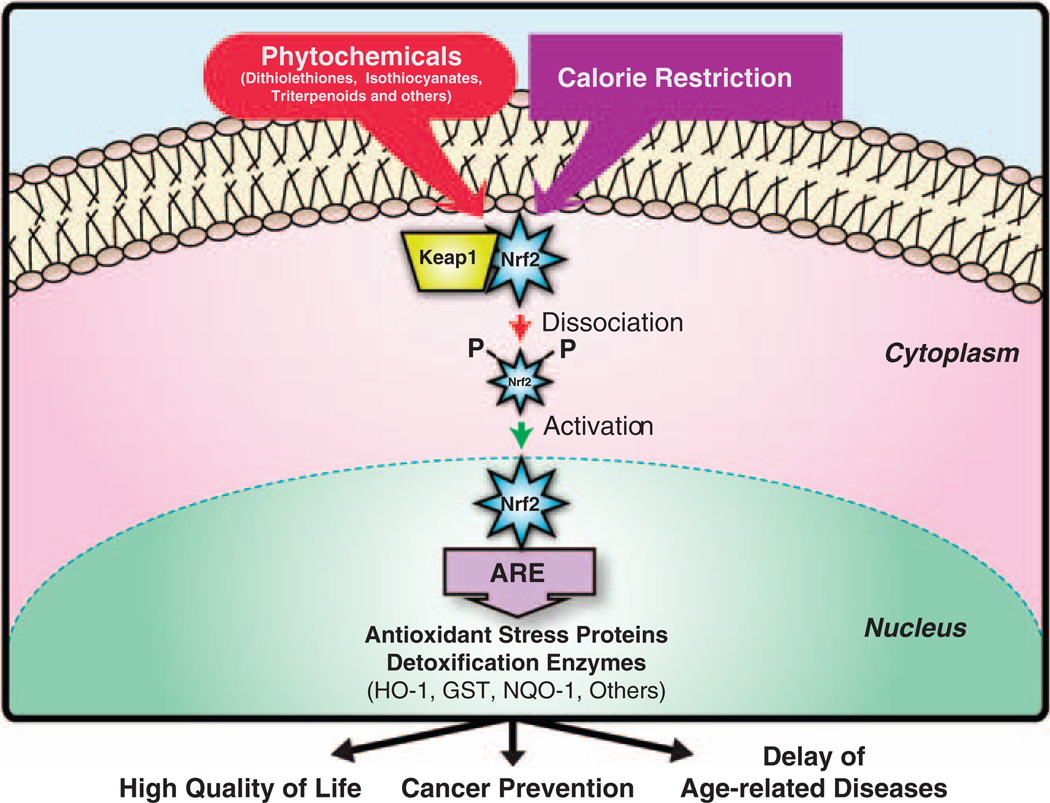
Figure 1.
Diagram of the activation of NRF2. Calorie restriction and a number of phytochemicals are able to induce the release of NRF2 from Keap1, allowing it to enter into the nucleus. Once in the nucleus, NRF2 binds to ARE sequences in the promoter of antioxidant and detoxifying enzymes, inducing their expression. Increased levels of these NRF2 downstream targets prevent age-related increases in cancer and ameliorates other age-related diseases, leading to an improved quality of life.
The activation of NRF2 and downstream targets by dietary factors is a crucial mechanism for tumour prevention owing to the potential to shift the metabolic balance, increasing NRF2 response and leading to the prevention of cancer development. Furthermore, it would be reasonable that the development of new nontoxic more potent NRF2-activating compounds will attenuate the carcinogenesis process. Although the NRF2-activating agents are present in short-time exposure, downstream induction is maintained for some days after exposure and cells respond to NRF2-activating agents repeatedly. This fact allows us to support a non-chronic NRF2 activator supplementation, which would improve cancer treatment and decrease possible phytochemical toxicity in organisms. A plausible cancer prevention strategy could be based on a similar approach that includes moderate CR, physical activity and cytoprotective supplementation. The next step in future investigations would be developing a mechanism to express NRF2 in non-cancer cells, while maintaining a lower NRF2 activation in preneoplastic or neoplastic cells, thus leading to selective toxicity in malignant cells.
Acknowledgements
AM-M and RdC are supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. The work was partially supported by Junta de Andalucía International Projects, CVI 4887 and CVI-276, NIH Grant 1R01AG028125-01A1 and FIS Grant PI080500 of the Ministry of Health, Spain. We thank Alex Sossong and Andrew Levette for critiquing the manuscript.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J Neurochem. 1997a;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, et al. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997b;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Reisman SA, Yeager RL, Goedken MJ, Klaassen CD. Nuclear factor erythroid 2-related factor 2 deletion impairs glucose tolerance and exacerbates hyperglycemia in type 1 diabetic mice. J Pharmacol Exp Ther. 2010;333:140–151. doi: 10.1124/jpet.109.162271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou KK, Morgan PR. Effect of dietary restriction on induced hamster cheek pouch carcinogenesis. Arch Oral Biol. 1981;26:525–531. doi: 10.1016/0003-9969(81)90011-x. [DOI] [PubMed] [Google Scholar]
- Ansher SS, Dolan P, Bueding E. Biochemical effects of dithiolthiones. Food Chem Toxicol. 1986;24:405–415. doi: 10.1016/0278-6915(86)90205-x. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- auf dem Keller U, Huber M, Beyer TA, Kumin A, Siemes C, Braun S, et al. Nrf transcription factors in keratinocytes are essential for skin tumor prevention but not for wound healing. Mol Cell Biol. 2006;26:3773–3784. doi: 10.1128/MCB.26.10.3773-3784.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SY, Choi SK, Kim KR, Park CS, Lee SK, Roh HK, et al. Effects of genetic polymorphisms of MDR1, FMO3 and CYP1A2 on susceptibility to colorectal cancer in Koreans. Cancer Sci. 2006;97:774–779. doi: 10.1111/j.1349-7006.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley BC. Multiple drug resistance mechanisms in cancer. Mol Biotechnol. 2010;46:308–316. doi: 10.1007/s12033-010-9321-2. [DOI] [PubMed] [Google Scholar]
- Banning A, Kipp A, Schmitmeier S, Lowinger M, Florian S, Krehl S, et al. Glutathione peroxidase 2 inhibits cyclooxygenase-2-mediated migration and invasion of HT-29 adenocarcinoma cells but supports their growth as tumors in nude mice. Cancer Res. 2008;68:9746–9753. doi: 10.1158/0008-5472.CAN-08-1321. [DOI] [PubMed] [Google Scholar]
- Bartke A, Masternak MM, Al-Regaiey KA, Bonkowski MS. Effects of dietary restriction on the expression of insulin-signaling-related genes in long-lived mutant mice. Interdiscip Top Gerontol. 2007;35:69–82. doi: 10.1159/000096556. [DOI] [PubMed] [Google Scholar]
- Birt DF, Merrill AH, Jr, Barnett T, Enkvetchakul B, Pour PM, Liotta DC, et al. Inhibition of skin carcinomas but not papillomas by sphingosine, N-methylsphingosine, and N-acetylsphingosine. Nutr Cancer. 1998;31:119–126. doi: 10.1080/01635589809514690. [DOI] [PubMed] [Google Scholar]
- Birt DF, Pelling JC, White LT, Dimitroff K, Barnett T. Influence of diet and calorie restriction on the initiation and promotion of skin carcinogenesis in the SENCAR mouse model. Cancer Res. 1991;51:1851–1854. [PubMed] [Google Scholar]
- Birt DF, Pinch HJ, Barnett T, Phan A, Dimitroff K. Inhibition of skin tumor promotion by restriction of fat and carbohydrate calories in SENCAR mice. Cancer Res. 1993;53:27–31. [PubMed] [Google Scholar]
- Birt DF, Przybyszewski J, Wang W, Stewart J, Liu Y. Identification of molecular targets for dietary energy restriction prevention of skin carcinogenesis: an idea cultivated by Edward Bresnick. J Cell Biochem. 2004;91:258–264. doi: 10.1002/jcb.10741. [DOI] [PubMed] [Google Scholar]
- Birt DF, Yaktine A, Duysen E. Glucocorticoid mediation of dietary energy restriction inhibition of mouse skin carcinogenesis. J Nutr. 1999;129:571S–574S. doi: 10.1093/jn/129.2.571S. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/ accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxido-reductase-1 gene expression. J Biol Chem. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- Boissonneault GA, Elson CE, Pariza MW. Net energy effects of dietary fat on chemically induced mammary carcinogenesis in F344 rats. J Natl Cancer Inst. 1986;76:335–338. [PubMed] [Google Scholar]
- Braun S, Hanselmann C, Gassmann MG, auf dem Keller U, Born-Berclaz C, Chan K, et al. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol. 2002;22:5492–5505. doi: 10.1128/MCB.22.15.5492-5505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Ingram RL, Sonntag WE. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J Gerontol. 1991;46:B180–B187. doi: 10.1093/geronj/46.5.b180. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohe R, Banning A. Part of the series: from dietary antioxidants to regulators in cellular signaling and gene regulation. Sulforaphane and selenium, partners in adaptive response and prevention of cancer. Free Radic Res. 2006;40:775–787. doi: 10.1080/10715760600722643. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Burton NC, Kensler TW, Guilarte TR. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology. 2006;27:1094–1100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Butt AJ, Fraley KA, Firth SM, Baxter RC. IGF-binding protein-3-induced growth inhibition and apoptosis do not require cell surface binding and nuclear translocation in human breast cancer cells. Endocrinology. 2002;143:2693–2699. doi: 10.1210/endo.143.7.8876. [DOI] [PubMed] [Google Scholar]
- Cabral JR, Neal GE. The inhibitory effects of ethoxyquin on the carcinogenic action of anatoxin Bl in rats. Cancer Lett. 1983;19:125–132. doi: 10.1016/0304-3835(83)90146-5. [DOI] [PubMed] [Google Scholar]
- Cakatay U, Telci A, Kayali R, Tekeli F, Akcay T, Sivas A. Relation of oxidative protein damage and nitrotyrosine levels in the aging rat brain. Exp Gerontol. 2001;36:221–229. doi: 10.1016/s0531-5565(00)00197-2. [DOI] [PubMed] [Google Scholar]
- Calkins MJ, Jakel RJ, Johnson DA, Chan K, Kan YW, Johnson JA. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci USA. 2005;102:244–249. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JK, Canene-Adams K, Lindshield BL, Boileau TW, Clinton SK, Erdman JW., Jr Tomato phytochemicals and prostate cancer risk. J Nutr. 2004;134:3486S–3492S. doi: 10.1093/jn/134.12.3486S. [DOI] [PubMed] [Google Scholar]
- Cao J, Schulte J, Knight A, Leslie NR, Zagozdzon A, Bronson R, et al. Prdxl inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello L, Froio T, Maina M, Cavallini G, Biasi F, Leonarduzzi G, et al. Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-kB activation. Free Radic Biol Med. 2010;48:47–54. doi: 10.1016/j.freeradbiomed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, et al. Cis–trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomarkers Prev. 1996;5:823–833. [PubMed] [Google Scholar]
- Cohen ND, Hilf R. Influence of insulin on growth and metabolism of 7,12-dimethylbenz(alpha)anthracene-induced mammary tumors. Cancer Res. 1974;34:3245–3252. [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, et al. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JM, Giovannucci EL. Vegetables, fruits, associated micronutrients, and risk of prostate cancer. Epidemiol Rev. 2001;23:82–86. doi: 10.1093/oxfordjournals.epirev.a000799. [DOI] [PubMed] [Google Scholar]
- Chan JY, Han XL, Kan YW. Cloning of Nrf1, an NF-E2-related transcription factor, by genetic selection in yeast. Proc Natl Acad Sci USA. 1993a;90:11371–11375. doi: 10.1073/pnas.90.23.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JY, Han XL, Kan YW. Isolation of cDNA encoding the human NF-E2 protein. Proc Natl Acad Sci USA. 1993b;90:11366–11370. doi: 10.1073/pnas.90.23.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar B, McGuff HS, Aufdermorte TB, Troyer DA, Talal N, Fernandes G. Effects of calorie restriction on transforming growth factor beta 1 and proinflammatory cytokines in murine Sjogren’s syndrome. Clin Immunol Immunopatholl. 1995;6:291–296. doi: 10.1006/clin.1995.1128. [DOI] [PubMed] [Google Scholar]
- Chang XZ, Li DQ, Hou YF, Wu J, Lu JS, Di GH, et al. Identification of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast Cancer Res. 2007;9:R76. doi: 10.1186/bcr1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoumpekis D, Ziros PG, Psyrogiannis A, Kyriazopoulou V, Papavassiliou AG, Habeos IG. Simvastatin lowers reactive oxygen species level by Nrf2 activation via PI3K/Akt pathway. Biochem Biophys Res Commun. 2010;396:463–466. doi: 10.1016/j.bbrc.2010.04.117. [DOI] [PubMed] [Google Scholar]
- Chen C, Yu R, Owuor ED, Kong AN. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res. 2000;23:605–612. doi: 10.1007/BF02975249. [DOI] [PubMed] [Google Scholar]
- Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, et al. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem. 2003;278:703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- Cheney KE, Liu RK, Smith GS, Meredith PJ, Mickey MR, Walford RL. The effect of dietary restriction of varying duration on survival, tumor patterns, immune function, and body temperature in B10C3F1 female mice. J Gerontol. 1983;38:420–430. doi: 10.1093/geronj/38.4.420. [DOI] [PubMed] [Google Scholar]
- Chiao JW, Wu H, Ramaswamy G, Conaway CC, Chung FL, Wang L, et al. Ingestion of an isothiocyanate metabolite from cruciferous vegetables inhibits growth of human prostate cancer cell xenografts by apoptosis and cell cycle arrest. Carcinogenesis. 2004;25:1403–1408. doi: 10.1093/carcin/bgh136. [DOI] [PubMed] [Google Scholar]
- Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA., Jr Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res. 2007;101:723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- De Cabo R, Cabello R, Rios M, Lopez-Lluch G, Ingram DK, Lane MA, et al. Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver. Exp Gerontol. 2004;39:297–304. doi: 10.1016/j.exger.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Derjuga A, Gourley TS, Holm TM, Heng HH, Shivdasani RA, Ahmed R, et al. Complexity of CNC transcription factors as revealed by gene targeting of the Nrf3 locus. Mol Cell Biol. 2004;24:3286–3294. doi: 10.1128/MCB.24.8.3286-3294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devling TW, Lindsay CD, McLellan LI, McMahon M, Hayes JD. Utility of siRNA against Keap1 as a strategy to stimulate a cancer chemopreventive phenotype. Proc Natl Acad Sci USA. 2005;102:7280–7285A. doi: 10.1073/pnas.0501475102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, et al. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neuro chem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- Ding Y, Choi KJ, Kim JH, Han X, Piao Y, Jeong JH, et al. Endogenous hydrogen peroxide regulates glutathione redox via nuclear factor erythroid 2-related factor 2 downstream of phospha-tidylinositol 3-kinase during muscle differentiation. Am J Pathol. 2008;172:1529–1541. doi: 10.2353/ajpath.2008.070429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Fahey JW, Talalay P. Chemical structures of inducers of nicotinamide quinone oxidoreductase 1 (NQO1) Methods Enzymol. 2004;382:423–448. doi: 10.1016/S0076-6879(04)82023-8. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci USA. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuric Z, Kritschevsky D. Modulation of oxidative DNA damage levels by dietary fat and calories. Mutat Res. 1993;295:181–190. doi: 10.1016/0921-8734(93)90019-y. [DOI] [PubMed] [Google Scholar]
- Duan W, Zhang R, Guo Y, Jiang Y, Huang Y, Jiang H, et al. Nrf2 activity is lost in the spinal cord and its astrocytes of aged mice. In vitro Cell Dev Biol Anim. 2009;45:388–397. doi: 10.1007/s11626-009-9194-5. [DOI] [PubMed] [Google Scholar]
- Efferth T, Volm M. Glutathione-related enzymes contribute to resistance of tumor cells and low toxicity in normal organs to artesunate. In vivo. 2005;19:225–232. [PubMed] [Google Scholar]
- Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci USA. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favreau LV, Pickett CB. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- Fialka F, Gruber RM, Hitt R, Opitz L, Brunner E, Schliephake H, et al. CPA6, FMO2, LGI1, SIAT1 and TNC are differentially expressed in early- and late-stage oral squamous cell carcinoma—a pilot study. Oral Oncol. 2008;44:941–948. doi: 10.1016/j.oraloncology.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Fields WR, Morrow CS, Doehmer J, Townsend AJ. Expression of stably transfected murine glutathione S-transferase A3-3 protects against nucleic acid alkylation and cytotoxicity by aflatoxin B1 in hamster V79 cells expressing rat cytochrome P450-2B1. Carcinogenesis. 1999;20:1121–1125. doi: 10.1093/carcin/20.6.1121. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice PS, Shaw IC, Kleiner HE, Miller RT, Monks TJ, Lau SS, et al. Evidence for DNA damage in amyotrophic lateral sclerosis. Muscle Nerve. 1996;19:797–798. [PubMed] [Google Scholar]
- Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- Friling RS, Bensimon A, Tichauer Y, Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci USA. 1990;87:6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Rocl ligase. Mol Cell Biol. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea R, Daino L, Frassetto S, Cozzolino P, Ruggiu ME, Vannini MG, et al. Reversal by ribo- and deoxyribonucleosides of dehydroepiandrosterone-induced inhibition of enzyme altered foci in the liver of rats subjected to the initiation-selection process of experimental carcinogenesis. Carcinogenesis. 1988;9:931–938. doi: 10.1093/carcin/9.6.931. [DOI] [PubMed] [Google Scholar]
- Gerhauser C, You M, Liu J, Moriarty RM, Hawthorne M, Mehta RG, et al. Cancer chemopreventive potential of sulforamate, a novel analogue of sulforaphane that induces phase 2 drug-metabolizing enzymes. Cancer Res. 1997;57:272–278. [PubMed] [Google Scholar]
- Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87:1767–1776. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- Gordon GB, Shantz LM, Talalay P. Modulation of growth, differentiation and carcinogenesis by dehydroepiandrosterone. Adv Enzyme Regul. 1987;26:355–382. doi: 10.1016/0065-2571(87)90023-9. [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Gross L, Dreyfuss Y. Inhibition of the development of radiation-induced leukemia in mice by reduction of food intake. Proc Natl Acad Sci USA. 1986;83:7928–7931. doi: 10.1073/pnas.83.20.7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Mitchell-Raymundo F, Yang H, Ikeno Y, Nelson J, Diaz V, et al. Dietary restriction reduces atherosclerosis and oxidative stress in the aorta of apolipoprotein E-deficient mice. Mech Ageing Dev. 2002;123:1121–1131. doi: 10.1016/s0047-6374(02)00008-8. [DOI] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Free radicals, proteins and DNA: oxidative damage versus redox regulation. Biochem Soc Trans. 1996a;24:1023–1027. doi: 10.1042/bst0241023. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radio Res. 1996b;25:57–74. doi: 10.3109/10715769609145656. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- Hansen BC, Ortmeyer HK, Bodkin NL. Prevention of obesity in middle-aged monkeys: food intake during body weight clamp. Obes Res. 1995;3(Suppl 2):199s–204s. doi: 10.1002/j.1550-8528.1995.tb00464.x. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Chanas SA, Henderson CJ, McMahon M, Sun C, Moffat GJ, et al. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem Soc Trans. 2000;28:33–41. doi: 10.1042/bst0280033. [DOI] [PubMed] [Google Scholar]
- Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Chung FL, Richie JP, Jr, Akerkar SA, Borukhova A, Skowronski L, et al. Effects of watercress consumption on metabolism of a tobacco-specific lung carcinogen in smokers. Cancer Epidemiol Biomarkers Prev. 1995;4:877–884. [PubMed] [Google Scholar]
- Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR. Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc Natl Acad Sci USA. 1998;95:5275–5280. doi: 10.1073/pnas.95.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997;278:1073–1077. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- Howell TH. The art of living long by Luigi Cornaro. Age Ageing. 1987;16:194–195. doi: 10.1093/ageing/16.3.194. [DOI] [PubMed] [Google Scholar]
- Hu R, Xu C, Shen G, Jain MR, Khor TO, Gopalkrishnan A, et al. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett. 2006;243:170–192. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;111:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- Hursting SD, Perkins SN, Phang JM. Calorie restriction delays spontaneous tumorigenesis in p53-knockout transgenic mice. Proc Natl Acad Sci USA. 1994;91:7036–7040. doi: 10.1073/pnas.91.15.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyer ML, Croxton R, Krajewska M, Krajewski S, Kress CL, Lu M, et al. Synthetic triterpenoids cooperate with tumor necrosis factor-related apoptosis-inducing ligand to induce apoptosis of breast cancer cells. Cancer Res. 2005;65:4799–4808. doi: 10.1158/0008-5472.CAN-04-3319. [DOI] [PubMed] [Google Scholar]
- Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci USA. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun DH, Hunt ND, Emerson SS, Hernandez JO, Mattson MP, de Cabo R. Up-regulation of plasma membrane-associated redox activities in neuronal cells lacking functional mitochondria. J Neurochem. 2007;100:1364–1374. doi: 10.1111/j.1471-4159.2006.04411.x. [DOI] [PubMed] [Google Scholar]
- Iida K, Itoh K, Kumagai Y, Oyasu R, Hattori K, Kawai K, et al. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M, et al. Dietary restriction and aging: the initiation of a primate study. J Gerontol. 1990;45:B148–B163. doi: 10.1093/geronj/45.5.b148. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Itoh K, Igarashi K, Hayashi N, Nishizawa M, Yamamoto M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol Cell Biol. 1995;15:4184–4193. doi: 10.1128/mcb.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakel RJ, Townsend JA, Kraft AD, Johnson JA. Nrf2-mediated protection against 6-hydroxydopamine. Brain Res. 2007;1144:192–201. doi: 10.1016/j.brainres.2007.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S26–S36. doi: 10.1002/ana.10483. discussion S36–38. [DOI] [PubMed] [Google Scholar]
- Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, Zhang DD. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59:850–860. doi: 10.2337/db09-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Hidalgo M, Santos-Ocana C, Padilla S, Villalba JM, Lopez-Lluch G, Martin-Montalvo A, et al. NQR1 controls lifespan by regulating the promotion of respiratory metabolism in yeast. Aging Cell. 2009;8:140–151. doi: 10.1111/j.1474-9726.2009.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KJ, Lee EK, Kim JY, Zou Y, Sung B, Heo HS, et al. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflamm Res. 2009;58:143–150. doi: 10.1007/s00011-008-7227-2. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MI, Kobayashi A, Wakabayashi N, Kim SG, Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc Natl Acad Sci USA. 2004;101:2046–2051. doi: 10.1073/pnas.0308347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanninen K, Heikkinen R, Malm T, Rolova T, Kuhmonen S, Leinonen H, et al. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106:16505–16510. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Egner PA, Dolan PM, Groopman JD, Roebuck BD. Mechanism of protection against anatoxin tumorigenicity in rats fed 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione (oltipraz) and related 1,2-dithiol-3-thiones and 1,2-dithiol-3-ones. Cancer Res. 1987;47:4271–4277. [PubMed] [Google Scholar]
- Kensler TW, Helzlsouer KJ. Oltipraz: clinical opportunities for cancer chemoprevention. J Cell Biochem Suppl. 1995;22:101–107. doi: 10.1002/jcb.240590813. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66:11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- Khor TO, Yu S, Kong AN. Dietary cancer chemopreventive agents—targeting inflammation and Nrf2 signaling pathway. Planta Med. 2008;74:1540–1547. doi: 10.1055/s-0028-1088303. [DOI] [PubMed] [Google Scholar]
- Kiritoshi S, Nishikawa T, Sonoda K, Kukidome D, Senokuchi T, Matsuo T, et al. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes. 2003;52:2570–2577. doi: 10.2337/diabetes.52.10.2570. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Umemura T, Kanki K, Kodama Y, Kitamoto S, Saito K, et al. Increased susceptibility to hepatocarcinogenicity of Nrf2-deficient mice exposed to 2-amino-3-methylimidazo[4,5-f]quinoline. Cancer Sci. 2007;98:19–24. doi: 10.1111/j.1349-7006.2006.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, Slitt AL. Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab. 2005;6:309–328. doi: 10.2174/1389200054633826. [DOI] [PubMed] [Google Scholar]
- Klurfeld DM, Weber MM, Kritchevsky D. Inhibition of chemically induced mammary and colon tumor promotion by caloric restriction in rats fed increased dietary fat. Cancer Res. 1987;47:2759–2762. [PubMed] [Google Scholar]
- Kobayashi A, Ito E, Toki T, Kogame K, Takahashi S, Igarashi K, et al. Molecular cloning and functional characterization of a new Cap’n’ collar family transcription factor Nrf3. J Biol Chem. 1999;214:6443–6452. doi: 10.1074/jbc.274.10.6443. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004a;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Ohta T, Yamamoto M. Unique function of the Nrf2-Keap1 pathway in the inducible expression of antioxidant and detoxifying enzymes. Methods Enzymol. 2004b;378:273–286. doi: 10.1016/S0076-6879(04)78021-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Itoh K, Suzuki T, Osanai H, Nishikawa K, Katoh Y, et al. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;1:807–820. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritchevsky D. Caloric restriction and cancer. J Nutr Sci Vitaminol (Tokyo) 2001;47:13–19. doi: 10.3177/jnsv.47.13. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D, Weber MM, Klurfeld DM. Dietary fat versus caloric content in initiation and promotion of 7,12-dimethylben-z(a)anthracene-induced mammary tumorigenesis in rats. Cancer Res. 1984;44:3174–3177. [PubMed] [Google Scholar]
- Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol. 2002;22:2883–2892. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Itoh K, Yamamoto M, Sutter TR, Kensler TW. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1, 2-dimethiole-3-thione. Mol Med. 2001;7:135–145. [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol. 2003a;23:8786–8794. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003b;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- Lagopoulos L, Stalder R. The influence of food intake on the development of diethylnitrosamine-induced liver tumours in mice. Carcinogenesis. 1987;8:33–37. doi: 10.1093/carcin/8.1.33. [DOI] [PubMed] [Google Scholar]
- Lai S, Sandanaraj E, Wong ZW, Ang PC, Wong NS, Lee EJ, et al. CBR1 and CBR3 pharmacogenetics and their influence on doxorubicin disposition in Asian breast cancer patients. Cancer Sci. 2008;99:2045–2054. doi: 10.1111/j.1349-7006.2008.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapillonne H, Konopleva M, Tsao T, Gold D, McQueen T, Sutherland RL, et al. Activation of peroxisome proliferator-activated receptor gamma by a novel synthetic triterpenoid 2-cyano-3,12-dioxooleana-l,9-dien-28-oic acid induces growth arrest and apoptosis in breast cancer cells. Cancer Res. 2003;63:5926–5939. [PubMed] [Google Scholar]
- Lee HH, Park SA, Almazari I, Kim EH, Na HK, Surh YJ. Piceatannol induces heme oxygenase-1 expression in human mammary epithelial cells through activation of ARE-driven Nrf2 signaling. Arch Biochem Biophys. 2010a;501:142–150. doi: 10.1016/j.abb.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003a;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, et al. Nrf2, a multi-organ protector? FASEB J. 2005a;19:1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- Lee JM, Shih AY, Murphy TH, Johnson JA. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003b;278:37948–37956. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- Lee KW, Ma L, Yan X, Liu B, Zhang XK, Cohen P. Rapid apoptosis induction by IGFBP-3 involves an insulin-like growth factor-independent nucleomitochondrial translocation of RXRal-pha/Nur77. J Biol Chem. 2005b;280:16942–16948. doi: 10.1074/jbc.M412757200. [DOI] [PubMed] [Google Scholar]
- Lee SK, Lee CY, Kook YA, Lee SK, Kim EC. Mechanical stress promotes odontoblastic differentiation via the heme oxygenase-1 pathway in human dental pulp cell line. Life Sci. 2010b;86:107–114. doi: 10.1016/j.lfs.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Leifert WR, Abeywardena MY. Cardioprotective actions of grape polyphenols. Nutr Res. 2008;28:729–737. doi: 10.1016/j.nutres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, et al. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 2006;20:2624–2626. doi: 10.1096/fj.06-5097fje. [DOI] [PubMed] [Google Scholar]
- Li Y, Jaiswal AK. Regulation of human NAD(P)H:quinone oxidoreductase gene. Role of API binding site contained within human antioxidant response element. J Biol Chem. 1992;267:15097–15104. [PubMed] [Google Scholar]
- Lii CK, Liu KL, Cheng YP, Lin AH, Chen HW, Tsai CW. Sulforaphane and alpha-lipoic acid upregulate the expression of the pi class of glutathione S-transferase through c-jun and Nrf2 activation. J Nutr. 2010;140:885–892. doi: 10.3945/jn.110.121418. [DOI] [PubMed] [Google Scholar]
- Liu XM, Peyton KJ, Ensenat D, Wang H, Hannink M, Alam J, et al. Nitric oxide stimulates heme oxygenase-1 gene transcription via the Nrf2/ARE complex to promote vascular smooth muscle cell survival. Cardiovasc Res. 2007;75:381–389. doi: 10.1016/j.cardiores.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok E, Nera EA, Iverson F, Scott F, So Y, Clayson DB. Dietary restriction, cell proliferation and carcinogenesis: a preliminary study. Cancer Lett. 1988;38:249–255. doi: 10.1016/0304-3835(88)90016-x. [DOI] [PubMed] [Google Scholar]
- Love R. Calorie restriction may be neuroprotective in AD and PD. Lancet Neurol. 2005;4:84. doi: 10.1016/s1474-4422(05)00985-3. [DOI] [PubMed] [Google Scholar]
- Maher J, Yamamoto M. The rise of antioxidant signaling—the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol. 2010;244:4–15. doi: 10.1016/j.taap.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Mandlekar S, Hong JL, Kong AN. Modulation of metabolic enzymes by dietary phytochemicals: a review of mechanisms underlying beneficial versus unfavorable effects. Curr Drug Metab. 2006;7:661–675. doi: 10.2174/138920006778017795. [DOI] [PubMed] [Google Scholar]
- Manjgaladze M, Chen S, Frame LT, Seng JE, Duffy PH, Feuers RJ, et al. Effects of caloric restriction on rodent drug and carcinogen metabolizing enzymes: implications for mutagenesis and cancer. Mutat Res. 1993;295:201–222. doi: 10.1016/0921-8734(93)90021-t. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Lees HA, Wohlgemuth SE, Leeuwenburgh C. Sarcopenia of aging: underlying cellular mechanisms and protection by calorie restriction. Biofactors. 2009;35:28–35. doi: 10.1002/biof.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Antiaging action of caloric restriction: endocrine and metabolic aspects. Obes Res. 1995;3(Suppl 2):241s–247s. doi: 10.1002/j.1550-8528.1995.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Lane MA, Ingram DK. Dietary restriction in aging nonhuman primates. Interdiscip Top Gerontol. 2007;35:137–158. doi: 10.1159/000096560. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Will caloric restriction and folate protect against AD and PD? Neurology. 2003;60:690–695. doi: 10.1212/01.wnl.0000042785.02850.11. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Lee J, Guo Z. Suppression of brain aging and neurodegenerative disorders by dietary restriction and environmental enrichment: molecular mechanisms. Mech Ageing Dev. 2001;122:757–778. doi: 10.1016/s0047-6374(01)00226-3. [DOI] [PubMed] [Google Scholar]
- McKiernan SH, Bua E, McGorray J, Aiken J. Early-onset calorie restriction conserves fiber number in aging rat skeletal muscle. FASEB J. 2004;18:580–581. doi: 10.1096/fj.03-0667fje. [DOI] [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, et al. The Cap‘n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J Biol Chem. 2004;279:31556–31567. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- Miller EC, Giovannucci E, Erdman JW, Jr, Bahnson R, Schwartz SJ, Clinton SK. Tomato products, lycopene, and prostate cancer risk. Urol Clin N Am. 2002;29:83–93. doi: 10.1016/s0094-0143(02)00020-4. [DOI] [PubMed] [Google Scholar]
- Mitra A, Shevde LA, Samant RS. Multi-faceted role of HSP40 in cancer. Clin Exp Metastasis. 2009;26:559–567. doi: 10.1007/s10585-009-9255-x. [DOI] [PubMed] [Google Scholar]
- Miyagi S, Iwama N, Kawabata T, Hasegawa K. Longevity and diet in Okinawa, Japan: the past, present and future. Asia Pac J Public Health. 2003;15(Suppl):S3–S9. doi: 10.1177/101053950301500S03. [DOI] [PubMed] [Google Scholar]
- Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreschi C. Beziehungen zwischen Ernahrung und Tumor-wachstum. Z fur Immunitatsforsch. 1909;2:661–675. [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Katsuoka F, Engel JD, Yamamoto M. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc Natl Acad Sci USA. 2004;101:6379–6384. doi: 10.1073/pnas.0305902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Muguruma M, Nishimura J, Jin M, Kashida Y, Moto M, Takahashi M, et al. Molecular pathological analysis for determining the possible mechanism of piperonyl butoxide-induced hepatocarcino-genesis in mice. Toxicology. 2006;228:178–187. doi: 10.1016/j.tox.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Mulcahy RT, Gipp JJ. Identification of a putative antioxidant response element in the 5’-flanking region of the human gamma-glutamylcysteine synthetase heavy subunit gene. Biochem Biophys Res Commun. 1995;209:227–233. doi: 10.1006/bbrc.1995.1493. [DOI] [PubMed] [Google Scholar]
- Murakami S, Johnson TE. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genetics. 1996;143:1207–1218. doi: 10.1093/genetics/143.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Hoshino H, Madisen L, Yanai N, Obinata M, Karasuyama H, et al. Identification of Bach2 as a B-cell-specific partner for small maf proteins that negatively regulate the immunoglobulin heavy chain gene 3′ enhancer. EMBO J. 1998;17:5734–5743. doi: 10.1093/emboj/17.19.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–1823. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Xu C, Shen G, Hebbar V, Gopalakrishnan A, Hu R, et al. Pharmacogenomics of phenolic antioxidant butylated hydroxyanisole (BHA) in the small intestine and liver of Nrf2 knockout and C57BL/6J mice. Pharm Res. 2006;23:2621–2637. doi: 10.1007/s11095-006-9099-x. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Huang HC, Yang CS, Pickett CB. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem. 2003;278:4536–4541. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic Biol Med. 2004;37:433–441. doi: 10.1016/j.freeradbiomed.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Janssen MJ, van Oijen MG, Bergevoet SM, te Morsche RH, van Asten H, et al. Genetic polymorphisms in GSTA1, GSTP1, GSTT1, and GSTM1 and gastric cancer risk in a Vietnamese population. Oncol Res. 2010;18:349–355. doi: 10.3727/096504010x12626118080064. [DOI] [PubMed] [Google Scholar]
- Nioi P, Nguyen T. A mutation of Keap1 found in breast cancer impairs its ability to repress Nrf2 activity. Biochem Biophys Res Commun. 2007;362:816–821. doi: 10.1016/j.bbrc.2007.08.051. [DOI] [PubMed] [Google Scholar]
- Nolan KA, Scott KA, Barnes J, Doncaster J, Whitehead RC. Stratford IJ Pharmacological inhibitors of NAD(P)H quinone oxidoreductase, NQO1: structure/activity relationships and functional activity in tumour cells. Biochem Pharmacol. 2010;80:977–981. doi: 10.1016/j.bcp.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Numazawa S, Ishikawa M, Yoshida A, Tanaka S, Yoshida T. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. Am J Physiol Cell Physiol. 2003;285:C334–C342. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the Keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- Onken B, Driscoll M. Metformin induces a dietary restrictionlike state and the oxidative stress response to extend C. elegans healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osburn WO, Yates MS, Dolan PD, Chen S, Liby KT, Sporn MB, et al. Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol Sci. 2008;104:218–227. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyake T, Itoh K, Motohashi H, Hayashi N, Hoshino H, Nishizawa M, et al. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol Cell Biol. 1996;16:6083–6095. doi: 10.1128/mcb.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, et al. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Palli D, Vineis P, Russo A, Berrino F, Krogh V, Masala G, et al. Diet, metabolic polymorphisms and DNA adducts: the EPIC-Italy cross-sectional study. Int J Cancer. 2000;87:444–451. doi: 10.1002/1097-0215(20000801)87:3<444::aid-ijc21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Pashko LL, Lewbart ML, Schwartz AG. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-promoted skin tumor formation in mice by 16 alpha-fluoro-5-androsten-17-one and its reversal by deoxyribonucleosides. Carcinogenesis. 1991;12:2189–2192. doi: 10.1093/carcin/12.11.2189. [DOI] [PubMed] [Google Scholar]
- Pashko LL, Schwartz AG. Reversal of food restriction-induced inhibition of mouse skin tumor promotion by adrenalectomy. Carcinogenesis. 1992;13:1925–1928. doi: 10.1093/carcin/13.10.1925. [DOI] [PubMed] [Google Scholar]
- Patel M, Lu L, Zander DS, Sreerama L, Coco D, Moreb JS. ALDH1A1 and ALDH3A1 expression in lung cancers: correlation with histologic type and potential precursors. Lung Cancer. 2008;59:340–349. doi: 10.1016/j.lungcan.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci USA. 2008;105:2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol. 2004;14:433–439. doi: 10.1016/j.semcancer.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Pietsch EC, Chan JY, Torti FM, Torti SV. Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. J Biol Chem. 2003;278:2361–2369. doi: 10.1074/jbc.M210664200. [DOI] [PubMed] [Google Scholar]
- Pollak M. Macronutrient intake and cancer: how does dietary restriction influence tumor growth and why should we care? Cancer Prev Res (Phila Pa) 2009;2:698–701. doi: 10.1158/1940-6207.CAPR-09-0134. [DOI] [PubMed] [Google Scholar]
- Pollard M, Luckert PH, Pan GY. Inhibition of intestinal tumorigenesis in methylazoxymethanol-treated rats by dietary restriction. Cancer Treat Rep. 1984;68:405–408. [PubMed] [Google Scholar]
- Pratico D, Delanty N. Oxidative injury in diseases of the central nervous system: focus on Alzheimer’s disease. Am J Med. 2000;109:577–585. doi: 10.1016/s0002-9343(00)00547-7. [DOI] [PubMed] [Google Scholar]
- Prestera T, Talalay P, Alam J, Ahn YI, Lee PJ, Choi AM. Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: regulation by upstream antioxidant-responsive elements (ARE) Mol Med. 1995;1:827–837. [PMC free article] [PubMed] [Google Scholar]
- Pugh TD, Oberley TD, Weindruch R. Dietary intervention at middle age: caloric restriction but not dehydroepiandrosterone sulfate increases lifespan and lifetime cancer incidence in mice. Cancer Res. 1999;59:1642–1648. [PubMed] [Google Scholar]
- Qin W, Zhao W, Ho L, Wang J, Walsh K, Gandy S, et al. Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer’s disease-type amyloid neuropathology and spatial memory deterioration. Ann N Y Acad Sci. 2008;1147:335–347. doi: 10.1196/annals.1427.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Gomez M, Dolan PM, Itoh K, Yamamoto M, Kensler TW. Interactive effects of nrf2 genotype and oltipraz on benzo[a]pyrene-DNA adducts and tumor yield in mice. Carcinogenesis. 2003;24:461–467. doi: 10.1093/carcin/24.3.461. [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, et al. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart J, Pearson WR. The structure of two murine class-mu glutathione transferase genes coordinately induced by butylated hydroxyanisole. Arch Biochem Biophys. 1993;303:383–393. doi: 10.1006/abbi.1993.1299. [DOI] [PubMed] [Google Scholar]
- Rous P. The influence of diet on transplanted and spontaneous tumors. J Exp Med. 1914;20:433–451. doi: 10.1084/jem.20.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri BA, Klurfeld DM, Kritchevsky D, Furlanetto RW. Caloric restriction and 7,12-dimethylbenz(a)anthracene-induced mammary tumor growth in rats: alterations in circulating insulin, insulin-like growth factors I and II, and epidermal growth factor. Cancer Res. 1989;49:4130–4134. [PubMed] [Google Scholar]
- Rushmore TH, Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- Sell C. Caloric restriction and insulin-like growth factors in aging and cancer. Horm Metab Res. 2003;35:705–711. doi: 10.1055/s-2004-814156. [DOI] [PubMed] [Google Scholar]
- Shantz LM, Talalay P, Gordon GB. Mechanism of inhibition of growth of 3T3-L1 fibroblasts and their differentiation to adipocytes by dehydroepiandrosterone and related steroids: role of glucose-6-phosphate dehydrogenase. Proc Natl Acad Sci USA. 1989;86:3852–3856. doi: 10.1073/pnas.86.10.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Ince PG, Falkous G, Mantle D. Oxidative damage to protein in sporadic motor neuron disease spinal cord. Ann Neurol. 1995;38:691–695. doi: 10.1002/ana.410380424. [DOI] [PubMed] [Google Scholar]
- Shen G, Xu C, Hu R, Jain MR, Gopalkrishnan A, Nair S, et al. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5:39–51. doi: 10.1158/1535-7163.MCT-05-0293. [DOI] [PubMed] [Google Scholar]
- Shibata T, Kokubu A, Gotoh M, Ojima H, Ohta T, Yamamoto M, et al. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008;135:1358–1368. doi: 10.1053/j.gastro.2008.06.082. e1–4. [DOI] [PubMed] [Google Scholar]
- Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, et al. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem. 2005;280:22925–22936. doi: 10.1074/jbc.M414635200. [DOI] [PubMed] [Google Scholar]
- Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, et al. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih PH, Yen GC. Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology. 2007;8:71–80. doi: 10.1007/s10522-006-9033-y. [DOI] [PubMed] [Google Scholar]
- Shimokawa I, Yu BP, Masoro EJ. Influence of diet on fatal neoplastic disease in male Fischer 344 rats. J Gerontol. 1991;46:B228–B232. doi: 10.1093/geronj/46.6.b228. [DOI] [PubMed] [Google Scholar]
- Shou M, Dai R, Cui D, Korzekwa KR, Baillie TA, Rushmore TH. A kinetic model for the metabolic interaction of two substrates at the active site of cytochrome P450 3A4. J Biol Chem. 2001;276:2256–2262. doi: 10.1074/jbc.M008799200. [DOI] [PubMed] [Google Scholar]
- Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, et al. Dysfunctional KEAP1–NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack C, Ho D, Wille E, Calingasan NY, Williams C, Liby K, et al. Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington′s disease. Free Radic Biol Med. 2010;49:147–158. doi: 10.1016/j.freeradbiomed.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab Rev. 1998;30:225–243. doi: 10.3109/03602539808996310. [DOI] [PubMed] [Google Scholar]
- Stewart JW, Koehler K, Jackson W, Hawley J, Wang W, Au A, et al. Prevention of mouse skin tumor promotion by dietary energy restriction requires an intact adrenal gland and glucocorticoid supplementation restores inhibition. Carcinogenesis. 2005;26:1077–1084. doi: 10.1093/carcin/bgi051. [DOI] [PubMed] [Google Scholar]
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci USA. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M. Cultural climate and social custom for longevity region, Okinawa] Nippon Ronen Igakkai Zasshi. 2001;38:163–165. [PubMed] [Google Scholar]
- Suzuki T, Takagi Y, Osanai H, Li L, Takeuchi M, Katoh Y, et al. Pi class glutathione S-transferase genes are regulated by Nrf 2 through an evolutionarily conserved regulatory element in zebrafish. Biochem J. 2005;388:65–73. doi: 10.1042/BJ20041860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum A, Silverstone H. The genesis and growth of tumors; effects of varying the proportion of protein (casein) in the diet. Cancer Res. 1949;9:162–173. [PubMed] [Google Scholar]
- Tannenbaum A, Silverstone H. Nutrition in relation to cancer. Adv Cancer Res. 1953;1:451–501. doi: 10.1016/s0065-230x(08)60009-3. [DOI] [PubMed] [Google Scholar]
- Taub R, Roy A, Dieter R, Koontz J. Insulin as a growth factor in rat hepatoma cells. Stimulation of proto-oncogene expression. J Biol Chem. 1987;262:10893–10897. [PubMed] [Google Scholar]
- Thatcher GR, Bennett BM, Reynolds JN. Nitric oxide mimetic molecules as therapeutic agents in Alzheimer’s disease. Curr Alzheimer Res. 2005;2:171–182. doi: 10.2174/1567205053585945. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- Thompson HJ, Zhu Z, Jiang W. Dietary energy restriction in breast cancer prevention. J Mammary Gland Biol Neoplasia. 2003;8:133–142. doi: 10.1023/a:1025743607445. [DOI] [PubMed] [Google Scholar]
- Toki T, Itoh J, Kitazawa J, Arai K, Hatakeyama K, Akasaka J, et al. Human small Maf proteins form heterodimers with CNC family transcription factors and recognize the NF-E2 motif. Oncogene. 1997;14:1901–1910. doi: 10.1038/sj.onc.1201024. [DOI] [PubMed] [Google Scholar]
- Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006a;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong KI, Kobayashi A, Katsuoka F, Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem. 2006b;387:1311–1320. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, et al. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenigwe CC, Ramprasath VR, Aluko RE, Jones PJ. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev. 2008;66:445–454. doi: 10.1111/j.1753-4887.2008.00076.x. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volchegorskii IA, Shemyakov SE, Turygin VV, Malinovskaya NV. The age dynamics of monoamine oxidase activity and levels of lipid peroxidation products in the human brain. Neurosci Behav Physiol. 2004;34:303–305. doi: 10.1023/b:neab.0000018736.84877.4f. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, et al. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Wang H, Xing J, Wang F, Han W, Ren H, Wu T, et al. Expression of Hsp27 and Hsp70 in lymphocytes and plasma in healthy workers and coal miners with lung cancer. J Huazhong Univ Sci Technol Med Sci. 2010;30:415–420. doi: 10.1007/s11596-010-0441-5. [DOI] [PubMed] [Google Scholar]
- Warabi E, Takabe W, Minami T, Inoue K, Itoh K, Yamamoto M, et al. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radic Biol Med. 2007;42:260–269. doi: 10.1016/j.freeradbiomed.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Warita H, Hayashi T, Murakami T, Manabe Y, Abe K. Oxidative damage to mitochondrial DNA in spinal motoneurons of transgenic ALS mice. Brain Res Mol Brain Res. 2001;89:147–152. doi: 10.1016/s0169-328x(01)00029-8. [DOI] [PubMed] [Google Scholar]
- Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci USA. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattenberg LW. Inhibition of carcinogenic and toxic effects of polycyclic hydrocarbons by phenolic antioxidants and ethoxyquin. J Natl Cancer Inst. 1972;48:1425–1430. [PubMed] [Google Scholar]
- Weed JL, Lane MA, Roth GS, Speer DL, Ingram DK. Activity measures in rhesus monkeys on long-term calorie restriction. Physiol Behav. 1997;62:97–103. doi: 10.1016/s0031-9384(97)00147-9. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- Westerbeek ZW, Hepple RT, Zernicke RF. Effects of aging and caloric restriction on bone structure and mechanical properties. J Gerontol A. 2008;63:1131–1136. doi: 10.1093/gerona/63.11.1131. [DOI] [PubMed] [Google Scholar]
- Wilson LA, Gemin A, Espiritu R, Singh G. ets-1 is transcriptionally up-regulated by H2O2 via an antioxidant response element. FASEB J. 2005;19:2085–2087. doi: 10.1096/fj.05-4401fje. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Willcox DC, Todoriki H, Fujiyoshi A, Yano K, He Q, et al. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci. 2007;1114:434–455. doi: 10.1196/annals.1396.037. [DOI] [PubMed] [Google Scholar]
- Williams KV, Mullen ML, Kelley DE, Wing RR. The effect of short periods of caloric restriction on weight loss and glycemic control in type 2 diabetes. Diabetes Care. 1998;21:2–8. doi: 10.2337/diacare.21.1.2. [DOI] [PubMed] [Google Scholar]
- Xu C, Huang MT, Shen G, Yuan X, Lin W, Khor TO, et al. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumor-igenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008;57:2809–2817. doi: 10.2337/db06-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Kyo M, Kamiya T, Tanaka T, Engel JD, Motohashi H, et al. Predictive base substitution rules that determine the binding and transcriptional specificity of Maf recognition elements. Genes Cells. 2006;11:575–591. doi: 10.1111/j.1365-2443.2006.00965.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Shi M, Story J, Richardson A, Guo Z. Food restriction attenuates age-related increase in the sensitivity of endothelial cells to oxidized lipids. J Gerontol A. 2004;59:316–323. doi: 10.1093/gerona/59.4.b316. [DOI] [PubMed] [Google Scholar]
- Yates MS, Tauchi M, Katsuoka F, Flanders KC, Liby KT, Honda T, et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007;6:154–162. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, et al. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–1343. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- Yu X, Kensler T. Nrf2 as a target for cancer chemoprevention. Mutat Res. 2005;591:93–102. doi: 10.1016/j.mrfmmm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Zakkar M, Van der Heiden K, Luong le A, Chaudhury H, Cuhlmann S, Hamdulay SS, et al. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vase Biol. 2009;29:1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]