Abstract
Background: Maternal hypothyroidism and/or hypothyroxinemia have been associated with child's poor neuropsychological development, but the results have been inconsistent.
Methods: The Northern Finland Birth Cohort 1986 included all expected births within a year (9362 women, 9479 children) from the two northernmost provinces of Finland. Maternal serum samples (n = 5791) were obtained in early pregnancy (M ± SD = 10.7 ± 2.8 weeks' gestation), and serum samples from their children were obtained at 16 years of age (n = 5829). All samples were analyzed for thyrotropin, free thyroxine (fT4), and thyroid peroxidase antibodies. The children's school performance was evaluated by their main teachers at eight years of age, as well as by the adolescents themselves at 16 years of age. Data on possible severe intellectual deficiency and mild cognitive limitation were collected from healthcare records and registries for all children. Logistic regression estimated the odds of poor school performance or severe intellectual deficiency/mild cognitive limitation associated with exposure to maternal thyroid dysfunction. The odds of poor school performance associated with the adolescents' own thyroid function at age 16 were also estimated. Results are presented as odds ratios (OR) with confidence intervals (CI), adjusted for maternal/family covariates and child's sex.
Results: Girls of mothers with subclinical hypothyroidism had more self-evaluated difficulties in mathematics than did girls of euthyroid mothers (OR 1.62 [CI 1.06–2.49]). Boys of hypothyroxinemic mothers repeated a school class more often than did boys of euthyroid mothers (OR 5.46 [CI 1.19–25.06]). Adolescents of hyperthyroid mothers had increased odds of poor self-evaluated performance in mathematics (OR 1.61 [CI 1.01–2.49]). Maternal thyroid dysfunction did not increase the odds of a child having severe intellectual deficiency/mild cognitive limitation. At 16 years of age, girls with hyperthyroidism by laboratory measurements had more difficulties in Finnish language (OR 2.82 [CI 1.42–5.61]) than did euthyroid girls. Boys with hypothyroxinemia by laboratory measurement had higher odds of having difficulties in Finnish and/or mathematics (OR 2.13 [CI 1.26–3.62]) than did euthyroid boys.
Conclusions: Maternal thyroid dysfunction during early pregnancy was associated with poorer scholastic performance of the adolescent. Additionally, adolescents' own thyroid dysfunction was associated with difficulties in school performance assessed by self-evaluation.
Introduction
During early pregnancy, a crucial time for neurodevelopment, the fetus is totally dependent on maternal thyroid hormone supply (1). Maternal thyroid hormones are needed for normal neuronal migration and maturation (2,3). Children of mothers with inadequately treated hypothyroidism or low free thyroxine (fT4) levels (hypothyroxinemia) during pregnancy have been shown to be at risk of lower full-scale IQ scores (4–7), reduced performance in motor skills (1,5,8,9), and increased reaction time (10). Maternal hypothyroxinemia has also been associated with child's poorer psychomotor development (5,8,11), a delay in expressive language and nonverbal cognition (12), and autism (13). Interestingly, maternal thyroid autoimmune disease has been associated with poorer IQ and overall mental scores in the offspring (5,14,15), but the mechanism underlying the association is unclear.
However, some studies have not revealed an association between maternal hypothyroidism or hypothyroxinemia and child's neurodevelopment or IQ (9,16–18). Treatment of maternal hypothyroidism with levothyroxine has improved children's IQ scores in some studies (4,17–19), but in a randomized trial of levothyroxine treatment for maternal hypothyroidism or hypothyroxinemia during pregnancy, IQ scores of children did not improve (16).
To the authors' knowledge, there are no previous studies evaluating the effect of early pregnancy maternal thyroid function on child's scholastic performance. To address this data gap, this study investigated the effects of maternal early pregnancy thyroid function and maternal thyroid antibody status on children's school performance. Additionally, the association between maternal thyroid function and prevalence of severe intellectual deficiency or mild cognitive limitation in the children was studied. Furthermore, since maternal thyroid function has been shown to associate with child's thyroid function (20), the association between child's thyroid function and school performance and attention deficit hyperactivity disorder (ADHD) in adolescence was studied.
Materials and Methods
Northern Finland Birth Cohort 1986
The prospective Northern Finland Birth Cohort 1986 (NFBC 1986) covers 99% of all births with calculated term between July 1, 1985, and June 30, 1986, drawn from the two northernmost provinces of Finland (9362 mothers, 9479 children). Maternal and family demographics, maternal health data, and data on pregnancy, delivery, and neonatal outcomes were collected during routine visits in communal free-of-charge maternity welfare clinics (participation rate 99.8%) and via questionnaires during the index pregnancy. All mothers were recruited to the study at 24 weeks' gestation, but their follow-up started at the first visit to a maternity welfare clinic at 8–12 weeks' gestation (21,22).
After birth, data on the health of cohort children and familial demographics were obtained via visits to communal child welfare clinics, questionnaires, and clinical examination, supplemented with data from national registers up to 16 years of age (22). The latest clinical examination with drawn blood samples was conducted at 16 years of age (20).
Informed consent was obtained from all subjects. The Ethics Committees of the Northern Ostrobothnia Hospital District and the National Institute for Health and Welfare approved this study.
The Finnish school system
All Finnish children attend a compulsory comprehensive school for nine years at 7–16 years of age. All education in Finland is free of charge, and the education system has high concordance in different regions according to Organization of Economic Cooperation and Development Program in Secondary Assessment surveys (Organization of Economic Cooperation and Development Program in Secondary Assessment; http://dx.doi.org/10.1787/9789264091450-e). Teachers in Finland are highly educated, with master's university degrees. The first six years of compulsory education are mainly taught by class teachers (only one main teacher per class), and years 7–9 are taught by specialized subject teachers. Most students with minor learning or adjustment problems attend regular education, but are entitled to remedial teaching. Students who cannot follow education because of a disability, illness, delayed development, or some other reason can be admitted to or transferred to special needs education. Whenever possible, special needs education is integrated into regular education or given in a special class. Each student with special learning needs has an individual teaching and learning plan.
Evaluation of child's scholastic performance and ADHD
When the cohort children were seven to eight years old, data concerning their growth, development, health, school and family type, and social situation were gathered from the parents using a postal questionnaire (response rate n = 8416; 90% of the NFBC 1986). Additionally, the main teachers of the children filled in questionnaires on learning difficulties (response rate n = 8525, 92% of the NFBC 1986), with questions on whether children had difficulties in reading, writing, and/or mathematics (impaired/unimpaired) (23).
At 16 years of age, the adolescents self-evaluated their school performance in Finnish language and mathematics (response rate n = 7344; 80% of the NFBC 1986) using three subscales of the Youth Self-Report (24), which has been shown to be a reliable data collection method in epidemiological research (25). As a part of the Youth Self-Report, adolescents compared their scholastic performance to their peers as “better than average,” “average,” “worse than average,” or “worse than most” (26). The self-evaluated school performance data have been shown to be comparable to the adolescents' actual school grades (Taanila et al., pers. commun.). The adolescents also reported whether they had repeated a school year at any time during their education.
A parental questionnaire administered when the adolescents were 16 years of age included a screening instrument for ADHD (the Strengths and Weaknesses of ADHD Symptoms and Normal Behavior [SWAN] scale; www.adhd.net). A total fo 530 NFBC 1986 adolescents were categorized as probable ADHD cases (27).
Assessment of child's intellectual problems
Children with intellectual problems were traced using maternal, peri-, and neonatal data collected via cohort questionnaires and during routine visits to free-of-charge maternity and child welfare clinics. The information collected was based on the routine clinical practice of referring a child for further examinations because of developmental or learning disorders. These data were linked with national register data (Hospital Discharge Register, Cause-of-Death Register, National Insurance, and Medication Reimbursement Register) and hospital, family counseling, public health center, and institutional health records. Data on psychometric test results were collected from hospitals, institutions for children with intellectual deficiencies, family counseling centers, and school psychologists. No separate evaluations or examinations were carried out for the purposes of the study. Psychometric test results (82% of the testing was conducted by using the Wechsler Intelligence Scale for Children-Revised) and other relevant records were requested from all social/healthcare units in the child's original and/or current living area (28).
A child was considered to have mild cognitive limitation if his/her IQ was ≥50 but ≤85 (29), and severe intellectual deficiency if his/her IQ was <50 (29), based on either the most recent standardized psychometric test result or on developmental assessment carried out on a clinical basis. In cases where no IQ estimate was available, hospital records were searched for assessment by a medical doctor or psychologist of the child's intellectual level. If no assessment was found, but it was evident that the child had intellectual deficiency on the basis of diagnosis of a disorder or a disease (e.g., chromosomal disorders, specific syndromes, brain anomalies), then the classification was made by clinical estimation. This procedure mainly concerned cases of neonatal and infant death (28).
Maternal thyroid function during pregnancy
The mothers in the NFBC 1986, as with all Finnish pregnant women, underwent infectious disease screening at their first visit to a maternity welfare clinic. The mean gestational age at sampling was 10.7 weeks (SD = 2.8). Leftover serum samples were stored in the Finnish Maternity Cohort at −25°C (30). The available samples (n = 5791, 61.1% of the NFBC 1986) were analyzed for thyrotropin (TSH), fT4, and thyroid peroxidase antibodies (TPO-Abs) using the Abbott Architect i2000 method (Abbott Diagnostics, Abbott Park, IL). Information on laboratory data collection (30) and the effect of long-term storage on these laboratory parameters has been reported previously (31). Maternal demographic characteristics and birth outcomes of mothers with and without laboratory analyses were similar (30). Ninety-eight mothers had a diagnosed and treated thyroid disease identified in medical chart review. Sixty-eight mothers were under some form of thyroid medication during the index pregnancy or had used thyroid medication previously.
The respective trimester-specific reference intervals for TSH and fT4 were 0.07–3.1 mIU/L and 11.4–22.4 pmol/L in the first trimester, and 0.10–3.5 mIU/L and 11.09–18.9 pmol/L in the second trimester (32). Mothers with a TPO-Ab concentration above the 95th percentile (>167.7 IU/mL) were deemed to be TPO-Ab-positive (32). Altogether, 5.1% of mothers were TPO-Ab positive (32).
Mothers were divided into four groups according to their serum fT4 and TSH concentrations (Table 1) (20): (i) euthyroidism: maternal TSH and fT4 within the reference intervals (n = 4746); (ii) hypothyroidism: TSH above the upper reference interval with low or normal fT4 concentrations (n = 358), of whom 40 had overt hypothyroidism and 318 had subclinical hypothyroidism; (iii) hypothyroxinemia: TSH within reference intervals with low fT4 concentrations (n = 67); (iv) hyperthyroidism: TSH below the lower reference interval with high or normal fT4 concentrations (n = 124), of whom 45 had overt hyperthyroidism and 79 had subclinical hyperthyroidism. The number of mothers with thyroid dysfunction may vary in different analyses due to missing questionnaire data.
Table 1.
Maternal and Family Characteristics by Maternal Thyroid Function
| Characteristics | Euthyroid (n = 4746) | Hypothyroid (n = 358) | Hypothyroxinemic (n = 67) | Hyperthyroid (n = 124) | NFBC 1986 with sufficient maternal serum samples (n = 5295) |
|---|---|---|---|---|---|
| Median maternal TSH concentration, mIU/L | 1.2 (0.7–1.7)* | 4.1 (3.6–5.8)* | 1.3 (0.9–2.0) | 0.03 (0.02–0.04)* | 1.2 (0.7–1.9) |
| Median maternal free T4, pmol/L | 15.1 (13.8–16.5) | 13.8 (12.5–15.2)* | 11.0 (10.7–11.2)* | 20.3 (17.8–22.7)* | 15.0 (13.7–16.5) |
| Median maternal TPO-Ab, IU/mL | 4.2 (3.0–6.2)* | 24.9 (4.8–295.5)* | 3.5 (2.3–6.0)* | 4.3 (3.3–6.1) | 4.3 (3.1–6.7) |
| M (SD) maternal age at birth, years | 28.1 (5.3) | 28.5 (5.4) | 29.8 (6.2)* | 30.0 (5.5)* | 28.2 (5.3) |
| >35 years, n (%) | 549 (11.6) | 45 (12.6) | 16 (23.9)* | 25 (20.2)* | 635 (12.0) |
| <20 years, n (%) | 177 (3.7) | 13 (3.6) | 4 (6.0) | 0 (0.0)* | 194 (3.7) |
| M (SD) BMI, kg/m2 | 22.2 (3.4) | 22.6 (3.6)* | 23.7 (4.8)* | 22.5 (3.4) | 22.2 (3.4) |
| Overweight/obese (BMI >25 kg/m2), n (%) | 737 (15.9) | 71 (20.1) | 16 (26.2)* | 23 (18.7) | 847 (16.4) |
| Smoking during pregnancy, n (%) | 1005 (21.3) | 48 (13.5)* | 18 (27.3) | 19 (15.4) | 1090 (20.7) |
| Maternal education | |||||
| ≥11 years, n (%) | 2562 (61.4) | 184 (59.2) | 33 (55.9) | 68 (59.6) | 2847 (61.1) |
| <11 years, n (%) | 1614 (38.6) | 127 (40.8) | 26 (44.1) | 46 (40.4) | 1813 (38.9) |
| Socioeconomic status of the family | |||||
| Professional, n (%) | 2842 (78.7) | 203 (78.7) | 41 (85.4) | 77 (80.2) | 3163 (78.8) |
| Skilled, n (%) | 599 (16.6) | 46 (17.8) | 4 (8.3) | 13 (13.5) | 662 (16.5) |
| Unskilled, n (%) | 26 (0.7) | 4 (1.6) | 1 (2.1) | 0 (0.0) | 31 (0.8) |
| Farmers, n (%) | 144 (4.0) | 5 (1.9) | 2 (4.2) | 6 (6.3) | 157 (3.9) |
| M (SD) gestational age at maternal serum sampling, weeks | 10.7 (2.8) | 10.7 (2.8) | 10.4 (2.7) | 10.9 (2.5) | 10.7 (2.8) |
| Preterm births (<37 weeks), n (%) | 164 (3.5) | 12 (3.4) | 4 (6.0) | 5 (4.0) | 185 (3.5) |
| Male children, n (%) | 2415 (50.9) | 187 (52.2) | 40 (59.7) | 45 (36.3)* | 2687 (50.7) |
| Children in the family when child was 8 years old (min–max), n | 3.2 (1–19) | 3.4 (1–17) | 4.1 (1–17) | 4.2 (1–15)* | 3.3 (1–19) |
The groups include mothers with child's eight-year and 16-year questionnaire data available.
Euthyroidism: maternal TSH and fT4 both between the reference intervals. Hypothyroidism: TSH above its upper limit with low or normal fT4 concentrations. Hyperthyroidism: TSH below its lower reference limit with high or normal fT4 concentrations. Hypothyroxinemia: TSH between its reference intervals with low fT4 concentrations.
p < 0.05, when the maternal thyroid function group was compared with all women with laboratory data: t test or the Mann–Whitney U test (continuous variables), χ2 test (categorical variables).
NFBC, the Northern Finland Birth Cohort; TSH, thyrotropin; fT4, free thyroxine; TPO-Ab, thyroid peroxidase antibodies; BMI, body mass index; IQR, interquartile range.
Adolescents' own thyroid function at 16 years of age
In 2001–2002, the cohort adolescents were invited to a clinical follow-up examination including blood sampling (participation rate 74%) (17). Altogether, 5829 (61.5%) samples from the adolescents were assayed for at least one thyroid function analyte (TSH, fT4, or TPO-Ab) in 2011, using the Abbott Architect i2000 method (17). Adolescents' TSH, fT4, and TPO-Ab concentrations were categorized on the basis of population-specific reference limits, being 0.64–3.74 mIU/L for TSH, 11.01–16.63 pmol/L for fT4, and ≤5.61 IU/mL for TPO-Ab (17). They were categorized as having euthyroid (n = 5256), hypothyroid (n = 216), hyperthyroid (n = 146), or hypothyroxinemic (n = 134) thyroid function test results based on their TSH and fT4 concentration, and as TPO-Ab-positive (n = 365) or -negative (n = 5369) based on their TPO-Ab concentrations.
Final study population
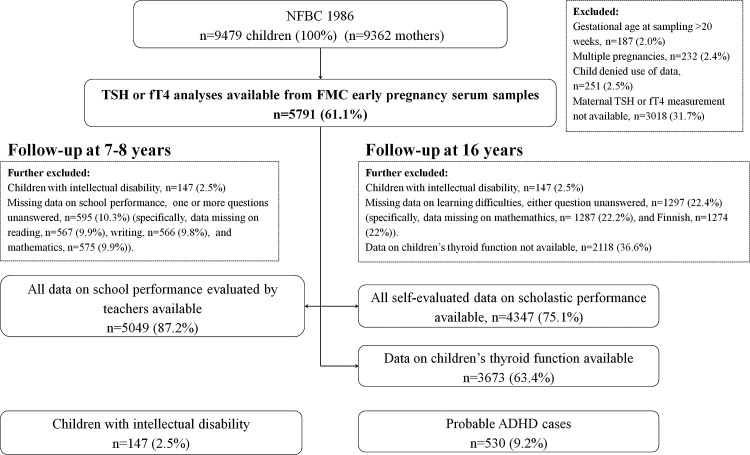
For analyses evaluating the association between maternal thyroid function and child's scholastic performance, multiple pregnancies (n = 232), those refusing use of data (n = 251), children with IQ ≤85 (n = 147), mothers with insufficient or missing sample for thyroid function analyses (n = 3004), and those with maternal gestational age at sampling >20 weeks (n = 187) were excluded. The final study population for these analyses consisted of 5069–5078 children with follow-up data on teacher-estimated school performance at eight years, or 4357–4370 adolescents with 16-year self-evaluations available (numbers vary due to missing data per outcome; Fig. 1). For the analyses of mild cognitive limitation and severe intellectual deficiency, the same exclusion criteria were used, but naturally the children with IQ ≤85 were retained in the analyses.
FIG. 1.
Flowchart of the study population in the Northern Finland Birth Cohort 1986. ADHD, attention deficit and hyperactivity disorder; FMC, Finnish Maternity Cohort; fT4, free thyroxine; NFBC 1986, The Northern Finland Birth Cohort 1986; TSH, thyroid stimulating hormone.
For the analyses of adolescents' thyroid function and self-evaluated scholastic performance, multiples (n = 232), those refusing use of data (n = 251), children missing data on thyroid function (n = 2118), and children with IQ ≤85 (n = 147) were excluded. The analyses were performed among 5246–5393 adolescents with thyroid function analyses and scholastic performance data available (numbers vary due to missing data per outcome).
Statistical analysis
Maternal and family characteristics of mothers in different thyroid function groups were compared to the whole NFBC 1986 cohort by using t-tests for continuous variables with normal distributions and Mann–Whitney U-test for those with non-Gaussian distributions. Categorical variables were compared by using chi-square tests.
Chi-square tests and logistic regression were used to estimate the prevalence and odds ratios (OR) with confidence intervals (CI) of poor scholastic performance, severe intellectual deficiency and mild cognitive limitation, and ADHD symptoms among children of mothers with thyroid dysfunction compared with children of euthyroid mothers. Analyses were adjusted for sex, number of children in the family at the time of scholastic performance evaluation (≥2 vs. 1), maternal smoking (yes vs. no), socioeconomic status of the family (professional, skilled, unskilled, and farmers), and maternal age (<20 or >35 years vs. 20–35 years). As the results of unadjusted and adjusted analyses were mostly similar, only the unadjusted analyses are presented in the tables. Any meaningful changes to the results after adjustments are reported in the text.
In sensitivity analyses, all data were analyzed by including and excluding TPO-Ab-positive mothers and mothers with diagnosed and treated thyroid disease during or prior to the index pregnancy. The data were stratified to term and preterm children to see if preterm birth modified the association.
Chi-square tests and logistic regression were used to evaluate the prevalence and ORs with CIs of poor scholastic performance and ADHD in adolescents with thyroid dysfunction in the laboratory analyses compared with euthyroid children. The results were adjusted for maternal TSH concentrations, and data were stratified by sex because of statistically significant interactions. As the results of unadjusted and adjusted analyses were similar, only the unadjusted analyses are present in the tables.
All statistical analyses were performed by using SPSS v18.0 software (IBM Corp., Armonk, NY).
Results
Maternal and family demographics
Demographic characteristics of the NFBC 1986 mothers during their index pregnancies are presented in Table 1. Hypothyroid mothers had a higher pre-pregnancy body mass index, and they smoked less often than did the total cohort. Mothers with overt hypothyroidism had slightly larger family size than did mothers with subclinical hypothyroidism. Hyperthyroid mothers were older than the total cohort was. Mothers with overt hyperthyroidism were older and had larger family size than mothers with subclinical hypothyroidism did. Hypothyroxinemic mothers had a higher pre-pregnancy body mass index and were older than the total cohort was.
Maternal thyroid dysfunction and child's scholastic performance
At eight years of age, there were 590 (11.6%) children with reading difficulties, 839 (16.5%) with writing difficulties, 416 (8.2%) with difficulties in mathematics, and 1062 (20.9%) with difficulties in at least one of these. No significant differences in scholastic problems at eight years of age were observed when children of mothers with thyroid dysfunction were compared to children of euthyroid mothers (Tables 2 and 3).
Table 2.
The Prevalence of Scholastic Difficulties in Children of Mothers With and Without Thyroid Dysfunction at 8 and 16 Years of Age
| Euthyroid, n = 4831 | Hypothyroid, n = 365 (7.0%) | Hypothyroxinemic, n = 71 (1.4%) | Hyperthyroid, n = 124 (2.5%) | |
|---|---|---|---|---|
| na/nb(%) | na/nb(%) | na/nb(%) | na/nb(%) | |
| 8 years, teacher evaluation: | ||||
| Difficulties in reading | 498/4335 (11.5%) | 41/328 (12.5%) | 4/66 (6.1%) | 12/118 (10.2%) |
| Boys | 329/2189 (15.0%) | 29/173 (16.8%) | 3/39 (7.7%) | 8/42 (19.0%) |
| Girls | 169/2146 (7.9%) | 12/155 (7.7%) | 1/27 (3.7%) | 4/76 (5.3%) |
| Difficulties in writing | 711/4335 (16.4%) | 56/329 (17.0%) | 8/66 (12.1%) | 15/118 (12.7%) |
| Boys | 461/2186 (21.1%) | 39/173 (22.5%) | 5/39 (12.8%) | 10/42 (23.8%) |
| Girls | 250/2149 (11.6%) | 17/156 (10.9%) | 3/27 (11.1%) | 5/76 (6.6%) |
| Difficulties in mathematics | 353/4328 (8.2%) | 26/328 (7.9%) | 7/66 (10.6%) | 7/117 (6.0%) |
| Boys | 163/2180 (7.5%) | 15/172 (8.7%) | 4/39 (10.3%) | 2/42 (4.8%) |
| Girls | 190/2148 (8.8%) | 11/156 (7.1%) | 3/27 (11.1%) | 5/75 (6.7%) |
| Difficulties in at least one | 900/4337 (20.8%) | 73/329 (22.2%) | 12/66 (18.2%) | 23/117 (19.7%) |
| Boys | 540/2184 (24.7%) | 50/173 (28.9%) | 6/39 (15.4%) | 13/42 (31.0%) |
| Girls | 360/2153 (16.7%) | 23/156 (14.7%) | 6/27 (22.2%) | 10/75 (13.3%) |
| 16 years, self-evaluation: | ||||
| Difficulties in Finnish | 327/3752 (8.7%) | 21/262 (8.0%) | 3/54 (5.6%) | 6/103 (5.8%) |
| Boys | 241/1795 (13.4%) | 17/127 (13.4%) | 3/29 (10.3%) | 4/34 (11.8%) |
| Girls | 86/1957 (4.4%) | 4/135 (3.0%) | 0/25 | 2/69 (2.9%) |
| Difficulties in mathematics | 866/3739 (23.2%) | 62/261 (23.8%) | 14/55 (25.5%) | 33/103 (32.0%)* |
| Boys | 349/1790 (19.5%) | 20/127 (15.7%) | 7/30 (23.3%) | 9/34 (26.5%) |
| Girls | 517/1947 (26.6%) | 42/134 (31.3%) | 7/25 (28.0%) | 24/69 (34.8%) |
| Difficulties in Finnish or mathematics | 1026/3735 (27.5%) | 75/261 (28.7%) | 15/54 (27.8%) | 34/103 (33.0%) |
| Boys | 476/1790 (26.6%) | 33/127 (26.0%) | 8/29 (27.6%) | 10/34 (29.4%) |
| Girls | 550/1945 (28.3%) | 42/134 (31.3%) | 7/25 (28.0%) | 24/69 (34.8%) |
| Repeated a class | 61/3750 (1.6%) | 8/265 (3.0%) | 3/55 (5.5%) | 0/102 |
| Boys | 41/1790 (2.3%) | 5/129 (3.9%) | 3/30 (10.0%)* | 0/34 |
| Girls | 20/1960 (1.0%) | 3/136 (2.2%) | 0/25 | 0/68 |
The groups include mothers with 8- and/or 16-year questionnaire data available. Numbers may vary in different analyses due to missing questionnaire data.
Euthyroidism: maternal TSH and fT4 both between the reference intervals. Hypothyroidism: TSH above its upper limit with low or normal fT4 concentrations. Hyperthyroidism: TSH below its lower reference limit with high or normal fT4 concentrations. Hypothyroxinemia: TSH between its reference intervals with low fT4 concentrations.
Children with intelligence quotient ≤85 were excluded from analysis.
p < 0.05, when the prevalence of scholastic problems was compared using chi-square tests.
na/nb, number of children with a scholastic problem/total number of children with data on scholastic performance available in the maternal thyroid function group (and percentage).
Table 3.
The Odds Ratios of Child's Scholastic Difficulties at 8 and 16 Years of Age by Maternal Thyroid Status
| Hypothyroid, n = 365 (7.0%) | Hypothyroxinemic, n = 71 (1.4%) | Hyperthyroid, n = 124 (2.5%) | |
|---|---|---|---|
| 8-year questionnaire: | |||
| Difficulties in reading | 1.10 [0.78–1.55] | 0.50 [0.18–1.37] | 0.87 [0.48–1.60] |
| Boys | 1.14 [0.75–1.73] | 0.47 [0.14–1.54] | 1.33 [0.61–2.90] |
| Girls | 0.98 [0.53–1.81] | 0.45 [0.06–3.34] | 0.65 [0.24–1.80] |
| Difficulties in writing | 1.05 [0.78–1.41] | 0.70 [0.33–1.48] | 0.74 [0.43–1.28] |
| Boys | 1.09 [0.75–1.58] | 0.55 [0.21–1.42] | 1.17 [0.57–2.40] |
| Girls | 0.93 [0.55–1.56] | 0.95 [0.28–3.18] | 0.54 [0.21–1.34] |
| Difficulties in mathematics | 0.97 [0.64–1.47] | 1.34 [0.61–2.95] | 0.72 [0.33–1.55] |
| Boys | 1.18 [0.68–2.06] | 1.41 [0.50–4.03] | 0.62 [0.15–2.58] |
| Girls | 0.78 [0.42–1.47] | 1.29 [0.38–4.32] | 0.74 [0.29–1.85] |
| Difficulties in at least one | 1.09 [0.83–1.43] | 0.85 [0.45–1.59] | 0.93 [0.59–1.48] |
| Boys | 1.24 [0.88–1.74] | 0.55 [0.23–1.33] | 1.37 [0.70–2.64] |
| Girls | 0.86 [0.55–1.36] | 1.42 [0.57–3.55] | 0.77 [0.39–1.51] |
| 16-year questionnaire: | |||
| Repeated a class | 1.88 [0.89–3.98] | 3.49 [1.06–11.48]* | NA |
| Boys | 1.72 [0.67–4.43] | 4.74 [1.38–16.25]* | NA |
| Girls | 2.19 [0.64–7.46] | NA | NA |
| Difficulties in Finnish | 0.91 [0.58–1.45] | 0.62 [0.19–1.99] | 0.65 [0.28–1.49] |
| Boys | 1.00 [0.59–1.69] | 0.74 [0.22–2.48] | 0.86 [0.30–2.46) |
| Girls | 0.66 [0.24–1.84] | NA | 0.65 [0.16–2.70] |
| Difficulties in mathematics | 1.03 [0.77–1.39] | 1.13 [0.62–2.09] | 1.56 [1.03–2.38]* |
| Boys | 0.77 [0.47–1.26] | 1.26 [0.54–2.96] | 1.49 [0.69–3.22] |
| Girls | 1.26 [0.87–1.84] | 1.08 [0.45–2.59] | 1.48 [0.89–2.45] |
| Difficulties in Finnish or mathematics | 1.07 [0.81–1.41] | 1.02 [0.56–1.85] | 1.30 [0.86–1.97] |
| Boys | 0.97 [0.64–1.46] | 1.05 [0.46–2.39] | 1.15 [0.55–2.42] |
| Girls | 1.16 [0.79–1.69] | 0.99 [0.41–2.38] | 1.35 [0.82–2.24] |
Data shown are odds ratios (OR) and confidence intervals (CI). The groups include mothers with 8- and/or 16-year questionnaire data available. Children with an IQ ≤85 were excluded from analysis.
Hypothyroidism: TSH above its upper limit with low or normal fT4 concentrations. Hyperthyroidism: TSH below its lower reference limit with high or normal fT4 concentrations. Hypothyroxinemia: TSH between its reference intervals with low fT4 concentrations.
ORs with 95% CIs were calculated by way of logistic regression to estimate the risk of poorer scholastic performance associated with maternal thyroid dysfunction during pregnancy.
p < 0.05.
NA, not applicable due to lack of subjects; IQ, intelligence quotient.
At 16 years of age, 374 (8.6%) adolescents evaluated themselves as being worse than average in Finnish language, whereas 1020 (23.4%) considered themselves worse than average in mathematics, and 1204 (27.7%) worse than average in either Finnish or mathematics; 78 (1.8%) adolescents had repeated a class. Adolescents of hypothyroid mothers had similar outcomes as those of euthyroid mothers (Tables 2 and 3). However, when mothers with hypothyroidism were stratified to overt and subclinical hypothyroidism, adolescents of mothers with subclinical hypothyroidism had higher prevalence and unadjusted odds of having difficulties in mathematics (33.6% vs. 26.6%, OR 1.40 [CI 0.95–2.08], adjusted OR 1.62 [CI 1.06–2.49]) and having repeated a school class than those of euthyroid mothers (3.4% vs. 1.6%, OR 2.14 [CI 1.01–4.53], adjusted OR 2.02 [CI 0.78–5.21]).
Adolescents of hyperthyroid mothers more often had self-reported difficulties in mathematics than those of euthyroid mothers (32.0% vs. 23.2%, OR 1.56 [CI 1.03–2.38], adjusted OR 1.61 [CI 1.01–2.49]; Tables 2 and 3). The numbers were insufficient to study overt and subclinical hyperthyroidism separately (data not shown).
Adolescents of hypothyroxinemic mothers had a higher prevalence and unadjusted odds of ever having repeated a school class than did those of euthyroid mothers (5.5% vs. 1.6%, OR 3.49 [CI 1.06–11.48]; Tables 2 and 3). After adjustments, the findings were no longer statistically significant (OR 3.50 [CI 0.81–15.21]). Interestingly, all adolescents of mothers with hypothyroxinemia who repeated a class were boys, who had a significantly higher prevalence and odds of repeating a class than did boys of euthyroid mothers (10.0% vs. 2.3%, OR 4.74 [CI 1.38–16.25], adjusted OR 5.46 [CI 1.19–25.06]; Tables 2 and 3).
Maternal thyroid function and severe intellectual deficiency/mild cognitive limitation
A total of 147 children were identified with an IQ ≤85, and 142 children had an IQ ≤85 based on IQ testing and five based on a diagnosis. Of these, 25 (0.4%) had severe intellectual deficiency (IQ <50; 14 boys and 11 girls) and 117 (2.0%) had mild cognitive limitation (50 ≤ IQ ≤85; 82 boys and 35 girls). Maternal thyroid dysfunction was not associated with the prevalence and odds of the child's severe intellectual deficiency or mild cognitive limitation (Tables 4 and 5). Adjusted results were similar but were attenuated due to small sample sizes (data not shown).
Table 4.
Prevalence of Children's Intellectual Problems in Maternal Thyroid Function Groups in the NFBC 1986
| Maternal thyroid function groups | ||||
|---|---|---|---|---|
| Euthyroidism, n = 4957 | Hypothyroid, n = 375 (7.0%) | Hypothyroxinemic, n = 73 (1.5%) | Hyperthyroid, n = 127 (2.5%) | |
| na/nb | ||||
| Mild cognitive limitation | 100/4931 (2.0%) | 8/373 (2.1%) | 2/71 (2.7%) | 2/126 (1.6%) |
| Boys | 73/2527 (2.9%) | 4/198 (2.0%) | 2/44 (4.5%) | 1/45 (2.2%) |
| Girls | 27/2403 (1.1%) | 4/175 (2.3%) | 0/29 | 1/81 (1.2%) |
| Severe intellectual deficiency | 22/4853 (0.5%) | 1/366 (0.3%) | 0/71 | 1/125 (0.8%) |
| Boys | 11/2465 (0.4%) | 1/195 (0.5%) | 0/42 | 1/45 (2.2%) |
| Girls | 11/2387 (0.5%) | 0/171 | 0/29 | 0/80 |
| All children with IQ ≤85 | 126/4831 (2.5%) | 10/375 (2.7%) | 2/73 (2.7%) | 3/127 (2.4%) |
| Boys | 87/2541 (3.4%) | 6/200 (3.0%) | 2/44 (4.5%) | 2/46 (4.3%) |
| Girls | 49/2415 (1.6%) | 4/175 (2.3%) | 0/29 | 1/81 (1.2%) |
The groups include mothers with information on IQ available. Mild cognitive limitation: IQ ≥50 but ≤85. Severe ID: IQ <50. Euthyroidism: maternal TSH and fT4 both between the reference intervals. Hypothyroidism: TSH above its upper limit with low or normal fT4 concentrations. Hyperthyroidism: TSH below its lower reference limit with high or normal fT4 concentrations. Hypothyroxinemia: TSH between its reference intervals with low fT4 concentrations.
na/nb, number of children with a scholastic problem/total number of children with data on scholastic performance available in the maternal thyroid function group (and percentage); ID: intellectual deficiency.
Table 5.
Maternal Thyroid Function Parameters in Early Pregnancy and Child's Risk of Being Intellectually Disabled
| Maternal thyroid function groups | |||
|---|---|---|---|
| Hypothyroxinemic, n = 73 | Hyperthyroid, n = 127 | ||
| Hypothyroid, n = 375 | Odds ratio (95% confidence intervals) | ||
| Mild cognitive limitation | 1.08 [0.52–2.24] | 1.40 [0.34–5.78] | 0.79 [0.19–3.25] |
| Boys | 0.70 [0.25–1.94] | 1.63 [0.39–6.88] | 0.76 [0.10–5.61] |
| Girls | 2.15 [0.74–6.23] | NA | 1.15 [0.15–8.57] |
| Severe ID | 0.55 [0.07–4.07] | NA | 1.63 [0.22–12.15] |
| Boys | 1.06 [0.14–8.19] | NA | 4.69 [0.50–36.79] |
| Girls | NA | NA | NA |
| IQ ≤85 | 1.05 [0.55–2.02] | 1.08 [0.26–4.45] | 0.93 [0.29–2.96] |
| Boys | 0.87 [0.38–2.02] | 1.34 [0.32–5.64] | 1.28 [0.31–5.37] |
| Girls | 1.43 [0.50–4.04] | NA | 0.76 [0.10–5.61] |
Data shown are OR [CI].
Mild cognitive limitation: IQ ≥50 but ≤85. Severe ID: IQ <50. Hypothyroidism: TSH above its upper limit with low or normal fT4 concentrations. Hyperthyroidism: TSH below its lower reference limit with high or normal fT4 concentrations. Hypothyroxinemia: TSH between its reference intervals with low fT4 concentrations.
ORs with 95% CIs were calculated by way of logistic regression to estimate the risk of mild cognitive limitation and severe ID associated with maternal thyroid dysfunction during pregnancy.
Sensitivity analyses
All results remained similar after excluding preterm births, TPO-Ab-positive mothers, and mothers with diagnosed and/or treated thyroid disease during the index pregnancy. Maternal thyroid dysfunction was not associated with adolescents' ADHD symptoms at 16 years of age (data not shown).
Thyroid function of adolescents at 16 years of age and self-evaluated scholastic performance
Girls with hyperthyroid thyroid function test results more often self-reported having difficulties in Finnish (11.1% vs. 4.2%, OR 2.82 [CI 1.42–5.61]) than did girls with euthyroidism (Table 6). Boys with hypothyroxinemic test results more often self-reported having difficulties in Finnish and/or mathematics (43.1% vs. 26.2%, OR 2.13 [CI 1.26–3.62]) than did boys with euthyroidism (Table 6). No statistically significant increase was observed in the prevalence of ADHD among adolescents with abnormal thyroid status compared to adolescents with euthyroidism (data not shown). However, after stratifying data by sex, there were more probable hyperactive ADHD cases among girls with hyperthyroid test results than there were among euthyroid girls (7.1% vs. 3.0%, p = 0.04), before excluding those with an IQ ≤85. After that, the difference was not statistically significant. Hypothyroxinemic boys had statistically significantly more ADHD symptoms (10.2% vs. 5.2%) than euthyroid boys. The results were similar after adjustments.
Table 6.
Child's Thyroid Function at 16 Years of Age and Prevalence and Odds of Self-Evaluated Poor Scholastic Performance
| Thyroid function group | |||||||
|---|---|---|---|---|---|---|---|
| Euthyroid | Hypothyroid | Hypothyroxinemic | Hyperthyroid | ||||
| n = 5256 (89.3%) | n = 216 (3.7%) | n = 134 (2.3%) | n = 146 (2.5%) | ||||
| Child has difficulties in | na/nb | na/nb | OR [CI] | na/nb | OR [CI] | na/nb | OR [CI] |
| Finnish | 422/4801 (8.8%) | 14/204 (6.9%) | 0.77 [0.44–1.33] | 12/113 (10.6%) | 1.23 [0.67–2.26] | 17/133 (12.8%) | 1.52 [0.91–2.56] |
| Boys | 318/2347 (13.5%) | 9/102 (8.8%) | 0.62 [0.31–1.24] | 11/58 (19.0%) | 1.49 [0.77–2.91] | 7/43 (16.3%) | 1.24 [0.55–2.81] |
| Girls | 104/2453 (4.2%) | 5/102 (4.9%) | 1.16 [0.46–2.92] | 1/55 (1.8%) | 0.42 [0.06–3.05] | 10/90 (11.1%) | 2.82 [1.42–5.61]* |
| Mathematics | 1068/4779 (22.3%) | 41/204 (20.1%) | 0.87 [0.62–1.24] | 25/113 (22.1%) | 0.99 [0.63–1.55] | 30/131 (22.9%) | 1.03 [0.68–1.56] |
| Boys | 451/2336 (19.3%) | 15/102 (14.7%) | 0.72 [0.41–1.26] | 13/43 (30.2%) | 1.73 [0.98–3.08] | 7/43 (16.3%) | 0.81 [0.36–1.84] |
| Girls | 616/2442 (25.2%) | 26/102 (25.5%) | 1.01 [0.64–1.60] | 8/55 (14.5%) | 0.51 [0.24–1.07] | 23/88 (26.1%) | 1.05 [0.65–1.70] |
| Finnish and/or mathematics | 1270/4812 (26.4%) | 48/204 (23.5%) | 0.86 [0.62–1.19] | 34/113 (30.1%) | 1.20 [0.80–1.80] | 40/133 (30.1%) | 1.20 [0.82–1.75] |
| Boys | 617/2354 (26.2%) | 20/102 (19.6%) | 0.69 [0.42–1.13] | 25/58 (43.1%) | 2.13 [1.26–3.62]* | 13/43 (30.2%) | 1.22 [0.63–2.35] |
| Girls | 652/2457 (26.5%) | 28/102 (27.5%) | 1.05 [0.67–1.63] | 9/55 (16.4%) | 0.54 [0.26–1.11] | 27/90 (30.0%) | 1.19 [0.75–1.88] |
| Has repeated a class | 86/4912 (1.8%) | 2/205 (1.0%) | 0.55 [0.14–2.26] | 2/123 (1.6%) | 0.93 [0.23–3.81] | 1/138 (0.7%) | 0.41 [0.06–2.96] |
| Boys | 58/2345 (2.5%) | 2/101 (2.0%) | 0.80 [0.19–3.31] | 0/58 (0.0%) | NA | 1/43 (2.3%) | 0.94 [0.13–6.94] |
| Girls | 26/2452 (1.1%) | 0/102 (0.0%) | NA | 1/56 (1.8%) | 1.69 [0.23–12.73] | 0/91 (0.0%) | NA |
Children with intelligence quotient ≤85 excluded.
Euthyroidism: TSH and fT4 both between the reference intervals. Hypothyroidism: TSH above its upper limit with low or normal fT4 concentrations. Hyperthyroidism: TSH below its lower reference limit with high or normal fT4 concentrations. Hypothyroxinemia: TSH between its reference intervals with low fT4 concentrations.
Total sum of boys and girls may vary due to missing data.
Statistically significant OR value.
na/nb, number of children with a scholastic problem/total number of children with data on scholastic performance available.
ORs with 95% CIs were calculated by way of logistic regression to estimate the risk of poor scholastic performance when having abnormal thyroid hormone concentrations.
Discussion
In this large, prospective, population-based cohort study, an association was found between maternal hypothyroxinemia, maternal subclinical hypothyroidism, and the odds of a child repeating a class in school. When considering a single school subject, maternal hyperthyroidism had a small adverse effect on the adolescents' mathematical skills. In addition, adolescent's own thyroid function at 16 years of age as evaluated by laboratory measurements seemed to have some effect on scholastic success, as girls with hyperthyroidism more often had difficulties in Finnish language and boys with hypothyroxinemia had more problems in mathematics and/or Finnish language than those with normal thyroid function did. Maternal thyroid dysfunction during pregnancy did not have an effect on a child's odds of severe intellectual deficiency or mild cognitive limitation.
This study is the first in which the effect of abnormal maternal thyroid function parameters during early pregnancy on a child's performance in everyday school life has been evaluated with a long follow-up. In this study, maternal hypothyroxinemia and maternal subclinical hypothyroidism were associated with a small increase in the odds of repeating a class in school. Previously, Haddow et al. proposed an association between untreated maternal hypothyroidism during pregnancy and an adverse intellectual outcome in children aged seven to nine years of age (4). In some studies, treatment of maternal hypothyroidism with levothyroxine has improved children's IQ scores (4,17–19), but in a randomized trial of levothyroxine treatment for maternal hypothyroidism or hypothyroxinemia during pregnancy, IQ scores of children did not improve (16). Maternal hypothyroidism and/or child's congenital hypothyroidism has been associated with smaller hippocampal size in magnetic resonance imaging and with slightly decreased performance in child's total memory testing scores (33). A strong association was seen between maternal hypothyroidism and child's decreased everyday memory function by parental reporting (33).
Previous studies have also shown a possible association between maternal hypothyroxinemia and a child's poorer cognitive performance or lower IQ compared with children of euthyroid mothers (8,34,35). Maternal hypothyroxinemia has been associated with increased odds of a child's expressive language delay at 2.5 years of age (12), decreased nonverbal IQ scores (4.3 points lower) in offspring (36), and predict reduced performance in reaction time tests independently of maternal TSH concentrations (10). In the current study, abnormal maternal thyroid function parameters did not affect a child's risk of having mild cognitive limitation or severe intellectual deficiency, but it was not possible to study less subtle effects of maternal thyroid dysfunction on child's IQ. Similar to the present findings, one previous study found no association between maternal thyroid hormones during pregnancy and child's neurodevelopment at 5.5 years of age (15). There is no explanation of why children of mothers with subclinical hypothyroidism or hypothyroxinemia have higher odds of repeating a school class, but it could be due to subtle changes in the child's cognitive functions or due to increased prevalence of behavioral difficulties in these children (37). The association, however, requires further studies.
No previous studies on the effect of a child's own thyroid function on scholastic performance exist. In the present study, adolescents were categorized based on laboratory findings, and it was found that hypothyroxinemic boys had a higher prevalence of problems in both Finnish language and mathematics, and hyperthyroid girls more often had difficulties in Finnish language than euthyroid boys and girls did. This finding cannot be explained with any certain etiology. Probable hyperactive subtype of ADHD was more prevalent in trend among girls with hyperthyroid laboratory measurements than it was among euthyroid girls. Also, hypothyroxinemic boys had more ADHD symptoms. It could be speculated that an adolescent's own thyroid dysfunction might expose them to learning or attention difficulties. In the present study, maternal hyperthyroidism was not associated with the adolescents' ADHD symptoms at 16 years of age, but the number of adolescents in the maternal thyroid function groups was small. Maternal hyperthyroidism had, however, a small adverse effect on the adolescents' mathematical skills. In a Danish cohort study, maternal hyperthyroidism has also been associated with child's increased risk of having a diagnosis of ADHD (38). These findings merit further research.
The strengths of the present study are the carefully designed cohort setting and the Finnish maternity care and schooling systems. The maternal serum samples were mainly drawn in the first trimester and were studied in a single laboratory. In addition, the authors have previously published their own thyroid hormone reference intervals for their pregnant population (32). The excellent participation rate as regards the questionnaires at eight (92%) and 16 years of age (80%) led to >5000 mother–child pairs with teachers' evaluations of the children's scholastic performance, the adolescents' self-evaluation at 16 years of age, and maternal thyroid function test results available. Most of the children with intellectual problems have had their IQ tested, and the etiologies of their problems are known (28). In most of the cohort children with an IQ of ≤85, there is a prenatal cause, and in only 33.6% of cases is the cause unknown. Nearly all (98.1%) of the cohort children attended public schools, and because all Finnish teachers are university educated, the teachers' evaluations can be considered to be very reliable. The self-evaluation questionnaire administered at 16 years of age, the Youth Self-Report (24), has been validated in epidemiological research. Because there is comprehensive data collection as regards the NFBC 1986, it was possible to adjust for the most important confounders. The effect of the country's iodine status must also be acknowledged, since even in some countries previously considered as iodine sufficient, iodine deficiency has reoccurred (39). Iodine insufficiency during pregnancy should not confound the results because in Finland iodine supplementation has been in use since the 1940s (40,41). In 1986, Finland had the highest iodine intake of all European countries (approximately 300 μg/day) (42), and at that time, Finland was considered as iodine sufficient.
As for limitations, some outcomes were rare in the cohort. The effect of prematurity on scholastic performance and the risk of having intellectual problems could not be properly analyzed because of the low prematurity rate. However, the results did not change after excluding prematurely born children. Some maternal serum samples were lacking, but it was found that mothers without laboratory data did not differ from the rest with regard to background factors (30). It is acknowledged that serum fT4 measurements by way of immunoassays may not be totally reliable during pregnancy, although they are clinically used in addition to TSH measurements (43). Fortunately, it was possible to use population- and trimester-specific reference intervals to define maternal thyroid function groups, as currently recommended when gold-standard methods are not possible (44).
In conclusion, abnormal maternal thyroid function status during early pregnancy increased adolescents' odds of repeating a class at school and also had some effect on the adolescents' performance in the Finnish language and mathematics at 16 years of age. It did not, however, increase a child's odds of having an intellectual problem. Additionally, adolescents' own abnormal thyroid function status had some effect on their self-evaluated school performance.
Acknowledgments
We gratefully thank all cohort participants for enabling NFBC 1986 research at the University of Oulu. We also thank Sarianna Vaara, Aljona Amelina, Jenna Aavavirta, and all other personnel from the National Institute for Health and Welfare, and Tuula Ylitalo from the Institute of Health Sciences, Oulu University, for their valuable work regarding the NFBC 1986 and the Finnish Maternity Cohort serum bank. We also appreciated the chance to learn from Dr. Ulla Heikura's previous excellent work on the NFBC 1986 children's intellectual problems, and Dr. Tanja Nordström's and Anja Taanila's work on children's scholastic and ADHD-related problems in the NFBC 1986. This work was supported in part by grants from the Academy of Finland, the Alma and K.A. Snellman Foundation (Oulu, Finland), the European Commission (Framework 5 award QLG1-CT-2000-001643), Euro-BLS, the Jalmari and Rauha Ahokas Foundation (Finland), the Northern Ostrobothnia Hospital District (Finland), the Finnish Medical Foundation, and the Finnish Medical Association of Clinical Chemistry.
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.de Escobar GM, Obregon MJ, del Rey FE. 2004. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab 18:225–248 [DOI] [PubMed] [Google Scholar]
- 2.Cao XY, Jiang XM, Dou ZH, Rakeman MA, Zhang ML, O'Donnell K, Ma T, Amette K, DeLong N, DeLong GR. 1994. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N Engl J Med 331:1739–1744 [DOI] [PubMed] [Google Scholar]
- 3.Auso E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P. 2004. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology 145:4037–4047 [DOI] [PubMed] [Google Scholar]
- 4.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O'Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. 1999. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341:549–555 [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C, Teng X, Guo R, Wang H, Li J, Chen Y, Wang W, Chawinga M, Zhang L, Yang L, Zhao Y, Hua T. 2010. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol (Oxf) 72:825–829 [DOI] [PubMed] [Google Scholar]
- 6.Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, Xu YH, Tao FB. 2011. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab 96:3234–3241 [DOI] [PubMed] [Google Scholar]
- 7.Smit BJ, Kok JH, Vulsma T, Briet JM, Boer K, Wiersinga WM. 2000. Neurologic development of the newborn and young child in relation to maternal thyroid function. Acta Paediatr 89:291–295 [PubMed] [Google Scholar]
- 8.Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. 2003. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 59:282–288 [DOI] [PubMed] [Google Scholar]
- 9.Craig WY, Allan WC, Kloza EM, Pulkkinen AJ, Waisbren S, Spratt DI, Palomaki GE, Neveux LM, Haddow JE. 2012. Mid-gestational maternal free thyroxine concentration and offspring neurocognitive development at age two years. J Clin Endocrinol Metab 97:E22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finken M, van Eijsden M, Loomans E, Vrijkotte T, Rotteveel J. 2013. Maternal hypothyroxinemia in early pregnancy predicts reduced performance in reaction time tests in 5- to 6-year-old offspring. J Clin Endocrinol Metab 98:1417–1426 [DOI] [PubMed] [Google Scholar]
- 11.Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL. 1999. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 50:149–155 [DOI] [PubMed] [Google Scholar]
- 12.Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, Hooijkaas H, de Muinck Keizer-Schrama SM, Hofman A, Jaddoe VV, Visser W, Steegers EA, Verhulst FC, de Rijke YB, Tiemeier H. 2010. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 95:4227–4234 [DOI] [PubMed] [Google Scholar]
- 13.Román GC, Ghassabian A, Bongers-Schokking JJ, Jaddoe VW, Hofman A, de Rijke YB, Verhulst FC, Tiemeier H. 2013. Association of gestational maternal hypothyroxinemia and increased autism risk. Ann Neurol 74:733–742 [DOI] [PubMed] [Google Scholar]
- 14.Pop VJ, de Vries E, van Baar AL, Waelkens JJ, de Rooy HA, Horsten M, Donkers MM, Komproe IH, van Son MM, Vader HL. 1995. Maternal thyroid peroxidase antibodies during pregnancy: a marker of impaired child development? J Clin Endocrinol Metab 80:3561–3566 [DOI] [PubMed] [Google Scholar]
- 15.Williams FL, Watson J, Ogston SA, Visser TJ, Hume R, Willatts P. 2013. Maternal and umbilical cord levels of T4, FT4, TSH, TPOAb, and TgAb in term infants and neurodevelopmental outcome at 5.5 years. J Clin Endocrinol Metab 98:829–838 [DOI] [PubMed] [Google Scholar]
- 16.Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, Chiusano E, John R, Guaraldo V, George LM, Perona M, Dall'Amico D, Parkes AB, Joomun M, Wald NJ. 2012. Antenatal thyroid screening and childhood cognitive function. N Engl J Med 366:493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Momotani N, Iwama S, Momotani K. 2012. Neurodevelopment in children born to hypothyroid mothers restored to normal thyroxine (T4) concentration by late pregnancy in Japan: no apparent influence of maternal T4 deficiency. J Clin Endocrinol Metab 97:1104–1108 [DOI] [PubMed] [Google Scholar]
- 18.Downing S, Halpern L, Carswell J, Brown RS. 2012. Severe maternal hypothyroidism corrected prior to the third trimester is associated with normal cognitive outcome in the offspring. Thyroid 22:625–630 [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Momotani N, Noh JY, Ishikawa N, Takebe K, Ito K. 1994. Maternal hypothyroidism during early pregnancy and intellectual development of the progeny. Arch Intern Med 154:785–787 [PubMed] [Google Scholar]
- 20.Päkkilä F, Männistö T, Surcel H, Ruokonen A, Bloigu A, Pouta A, Hartikainen A, Vääräsmäki M, Järvelin M, Suvanto E. 2013. Maternal thyroid dysfunction during pregnancy and thyroid function of her child in adolescence. J Clin Endocrinol Metab 98:965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Järvelin MR, Hartikainen-Sorri AL, Rantakallio P. 1993. Labour induction policy in hospitals of different levels of specialisation. Br J Obstet Gynaecol 100:310–315 [DOI] [PubMed] [Google Scholar]
- 22.Järvelin M, Elliott P, Kleinschmidt I, Martuzzi M, Grundy C, Hartikainen AL, Rantakallio P. 1997. Ecological and individual predictors of birthweight in a northern Finland birth cohort 1986. Paediatr Perinat Epidemiol 11:298–312 [DOI] [PubMed] [Google Scholar]
- 23.Taanila A, Ebeling H, Kotimaa A, Moilanen I, Järvelin MR. 2004. Is a large family a protective factor against behavioural and emotional problems at the age of 8 years? Acta Paediatr 93:508–517 [DOI] [PubMed] [Google Scholar]
- 24.Achenbach T. 2001. Manual for the ASEBA School-Age Forms and Profiles, University of Vermont, Research Center for Children, Youth and Families, Burlington, VT [Google Scholar]
- 25.Broberg AG, Ekeroth K, Gustafsson PA, Hansson K, Hagglof B, Ivarsson T, Larsson B. 2001. Self-reported competencies and problems among Swedish adolescents: a normative study of the YSR. Youth Self Report. Eur Child Adolesc Psychiatry 10:186–193 [DOI] [PubMed] [Google Scholar]
- 26.Hurtig T, Taanila A, Ebeling H, Miettunen J, Moilanen I. 2005. Attention and behavioural problems of Finnish adolescents may be related to the family environment. Eur Child Adolesc Psychiatry 14:471–478 [DOI] [PubMed] [Google Scholar]
- 27.Smalley SL, McGough JJ, Moilanen IK, Loo SK, Taanila A, Ebeling H, Hurtig T, Kaakinen M, Humphrey LA, McCracken JT, Varilo T, Yang MH, Nelson SF, Peltonen L, Järvelin MR. 2007. Prevalence and psychiatric comorbidity of attention-deficit/hyperactivity disorder in an adolescent Finnish population. J Am Acad Child Adolesc Psychiatry 46:1575–1583 [DOI] [PubMed] [Google Scholar]
- 28.Heikura U, Linna SL, Olsen P, Hartikainen AL, Taanila A, Järvelin MR. 2005. Etiological survey on intellectual disability in the northern Finland birth cohort 1986. Am J Ment Retard 110:171–180 [DOI] [PubMed] [Google Scholar]
- 29.Heikura U, Hartikainen A, Nordström T, Pouta A, Taanila A, Järvelin M. 2013. Maternal hypertensive disorders during pregnancy and mild cognitive limitations in the offspring. Paediatr Perinat Epidemiol 27:188–198 [DOI] [PubMed] [Google Scholar]
- 30.Männistö T, Vääräsmäki M, Pouta A, Hartikainen A, Ruokonen A, Surcel H, Bloigu A, Järvelin M, Suvanto E. 2009. Perinatal outcome of children born to mothers with thyroid dysfunction or antibodies: a prospective population-based cohort study. J Clin Endocrinol Metab 94:772–779 [DOI] [PubMed] [Google Scholar]
- 31.Männistö T, Surcel H, Bloigu A, Ruokonen A, Hartikainen A, Järvelin M, Pouta A, Vääräsmäki M, Suvanto E. 2007. The effect of freezing, thawing, and short- and long-term storage on serum thyrotropin, thyroid hormones, and thyroid autoantibodies: implications for analyzing samples stored in serum banks. Clin Chem 53:1986–1987 [DOI] [PubMed] [Google Scholar]
- 32.Männistö T, Surcel H, Ruokonen A, Vääräsmäki M, Pouta A, Bloigu A, Järvelin M, Hartikainen A, Suvanto E. 2011. Early pregnancy reference intervals of thyroid hormone concentrations in a thyroid antibody-negative pregnant population. Thyroid 21:291–298 [DOI] [PubMed] [Google Scholar]
- 33.Willoughby KA, McAndrews MP, Rovet JF. 2014. Effects of maternal hypothyroidism on offspring hippocampus and memory. Thyroid 24:576–584 [DOI] [PubMed] [Google Scholar]
- 34.Oken E, Braverman LE, Platek D, Mitchell ML, Lee SL, Pearce EN. 2009. Neonatal thyroxine, maternal thyroid function, and child cognition. J Clin Endocrinol Metab 94:497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Julvez J, Alvarez-Pedrerol M, Rebagliato M, Murcia M, Forns J, Garcia-Esteban R, Lertxundi N, Espada M, Tardon A, Riano Galan I, Sunyer J. 2013. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology 24:150–157 [DOI] [PubMed] [Google Scholar]
- 36.Ghassabian A, El Marroun H, Peeters RP, Jaddoe VW, Hofman A, Verhulst FC, Tiemeier H, White T. 2014. Downstream effects of maternal hypothyroxinemia in early pregnancy: nonverbal IQ and brain morphology in school-age children. J Clin Endocrinol Metab 99:2383–2390 [DOI] [PubMed] [Google Scholar]
- 37.Päkkilä F, Männistö T, Pouta A, Hartikainen A, Ruokonen A, Surcel H, Bloigu A, Vääräsmäki M, Järvelin M, Moilanen I, Suvanto E. 2014. The impact of gestational thyroid hormone concentrations on ADHD symptoms of the child. J Clin Endocrinol Metab 99:E1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen SL, Laurberg P, Wu CS, Olsen J. 2014. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG 121:1365–1374 [DOI] [PubMed] [Google Scholar]
- 39.Andersson M, Karumbunathan V, Zimmermann MB. 2012. Global iodine status in 2011 and trends over the past decade. J Nutr 142:744–750 [DOI] [PubMed] [Google Scholar]
- 40.Lamberg BA, Haikonen M, Mäkelä M, Jukkara A, Axelson E, Welin MG. 1981. Further decrease in thyroidal uptake and disappearance of endemic goitre in children after 30 years of iodine prophylaxis in the east of Finland. Acta Endocrinol 98:205–209 [DOI] [PubMed] [Google Scholar]
- 41.Erkkola M, Karppinen M, Järvinen A, Knip M, Virtanen SM. 1998. Folate, vitamin D, and iron intakes are low among pregnant Finnish women. Eur J Clin Nutr 52:742–748 [DOI] [PubMed] [Google Scholar]
- 42.Lamberg BA. 1986. Endemic goitre in Finland and changes during 30 years of iodine prophylaxis. Endocrinol Exp 20:35–47 [PubMed] [Google Scholar]
- 43.Feldt-Rasmussen U, Bliddal Mortensen AS, Rasmussen A, Boas M, Hilsted L, Main K. 2011. Challenges in interpretation of thyroid function tests in pregnant women with autoimmune thyroid disease. J Thyroid Res 2011:598712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W; American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum 2011. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 21:1081–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]