Abstract
Purpose: Fertility preservation in a pediatric and teen female population is challenging because standard technologies of egg and embryo freezing may not be possible due to premenarcheal status. Ovarian tissue cryopreservation (OTC) with the intent of future ovarian tissue transplantation or in vitro follicle growth may be the only option to preserve fertility. The purpose of this study was to add to the general understanding of primordial follicle dynamics in young patients.
Methods: First, the unique infrastructure of the Oncofertility Consortium National Physicians Cooperative (OC-NPC) is described, which simultaneously drives clinical fertility preservation and basic research to explore and expand the reproductive options for those in need. Then, the OC-NPC research resource is used to perform a histological evaluation of ovarian tissue from 24 participants younger than 18 years of age.
Results: Primordial follicles, which comprise the ovarian reserve, were observed in all participant tissues, irrespective of variables, including age, diagnosis, previous treatment history, tissue size, and tissue processing methods. Primordial follicles were present in ovarian tissue, even in participants who had a previous history of exposure to chemotherapy and/or radiation treatment regimens, which placed them at risk for iatrogenic infertility or premature ovarian failure.
Conclusion: Primordial follicles were observed in ovarian tissue from all participants examined, despite population and tissue heterogeneity. These results increase the understanding of human follicle dynamics and support OTC as a promising fertility preservation modality in the young female population. Future studies to evaluate follicle quality within these tissues are warranted.
Keywords: : fertility preservation, oncofertility, ovarian tissue cryopreservation, ovary, follicle
Each day, 36 children are diagnosed with cancer in the United States and >40,000 children undergo treatment every year; the average age of diagnosis in pediatric patients is 6 years old.1 Because of advancements in therapy, survival rates for most childhood cancers have improved from 10% in the 1950s to currently >80% at 5 years and 75% at 10 years.2 Although more children and teens are surviving their cancer diagnoses and living into adulthood, recent data from the Childhood Cancer Survivor Study (CCSS) identifies gonadal failure as a long-term health concern.3,4 Indeed, the CCSS reported that 6.3% of childhood survivors experience acute ovarian failure immediately after treatment, and 8% experience premature ovarian failure at some point after the end of treatment.5,6 Preservation and restoration of fertility are key aspects for enhancing quality of life in this population, and studies have found that 60% of survivors desire to rear biological children and >65% of childhood or young adult cancer survivors have used or want information about infertility and options for having children.7–11 Thus, major emphasis has been placed on research in the area of childhood cancer survivors, directed not only at providing a cure for their cancer, but at a treatment approach that will allow for the best possible quality of life into adulthood. In addition, the American Society of Clinical Oncology (ASCO), the American Association of Pediatrics, the American Society for Reproductive Medicine (ASRM), and the Association of Pediatric Hematology/Oncology Nurses (APHON) have issued practice guidelines or recommendations that all patients and their parents, as part of education and informed consent before cancer therapy, should be made aware of potential effects on their reproductive function and referred to appropriate reproductive specialists.
Fertility interventions for children and teens facing life-preserving but fertility-threatening cancer treatments have evolved greatly over the past decade. Post-menarche patients can undergo ovarian stimulation with oocyte retrieval and cryopreservation similar to assisted reproductive technology (ART) treatments used for infertile couples.12,13 This process generally involves 8–14 days of ovarian stimulation followed by oocyte retrieval and cryopreservation, and chemotherapy can be started 1–2 days after oocyte retrieval. Although this is a potential fertility preservation option, providers are urged to evaluate the patient for their understanding of the procedure, and tools including the Adolescent Fertility Values Clarification Tool are useful for teens 12–18 years old.14 Ovarian tissue cryopreservation (OTC) is a potential alternative fertility preservation option that may be suitable for pre- and post-menarcheal girls. This technology, however, is still considered experimental and must be done with appropriate Institutional Review Board (IRB) approval. OTC involves the removal and cryopreservation of ovarian tissue, ideally prior to gonadotoxic chemotherapy or pelvic radiation, to allow options for future fertility.12,13 Once the patient has survived cancer, the ovarian tissue may be thawed and transplanted to restore endocrine function and/or fertility. This experimental technique has resulted in multiple live births worldwide to date, but it has the inherent risk of reseeding malignancies.15,16 To overcome the risk, methods are being developed to isolate immature follicles from fresh or cryopreserved ovarian tissue and grow them in vitro.12,17–19 Mature oocytes derived from this method could then be cryopreserved and used for ART to restore fertility.
Although OTC followed by transplantation or in vitro follicle growth both represent promising fertility preservation methods for pediatric and teen patients, the general knowledge of ovarian tissue biology in this young population remains limited because such tissue is not readily available for investigation. This study documents how the unique infrastructure of the National Physicians Cooperative of the Oncofertility Consortium (OC-NPC) has enabled collection of pediatric and teen ovarian tissue samples for research as part of a multicenter protocol for fertility preservation. Using this unique repository, a histological evaluation was performed to assess whether variables—including age, cancer diagnosis, history of previous treatment, whether an entire ovary was removed or if an ovarian biopsy was done, or if the tissue was transported prior to cryopreservation—impacted whether follicles were detected in the tissue. The research samples obtained through the OC-NPC, therefore, provide an important resource to understand follicle dynamics in the specific context of fertility preservation. Such knowledge is necessary so that young patients can be counseled more effectively in terms of treatment options and new investigational technologies can be tailored to this specific population. This is of increasing importance as the majority of females who undergo OTC elect to store their ovarian tissue long term beyond the initial period of cryostorage.20
Materials and Methods
Human ovarian research tissue acquisition
The OC-NPC facilitates both clinical OTC and basic fertility preservation research. Human ovarian tissue for research purposes was obtained from females undergoing ovarian tissue removal for OTC through clinical sites that are part of the National Physician's Cooperative of the Oncofertility Consortium (oncofertility.northwestern.edu; Supplementary Table S1 and Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/jayao). OC-NPC sites serve the purpose of facilitating basic research using human ovarian tissue. The ultimate goal of this basic research is to expand fertility preservation options for individuals in need through increased understanding of the human ovary and development of new technologies. These twin goals are accomplished via a team science approach involving basic scientists, oncologists, REI specialists, allied health professionals, patients, families, and others.21,22 Through these coordinated partnerships, specific IRB-approved protocols are implemented in which 80% of the removed ovarian tissue is cryopreserved for the participant's future use to restore reproductive function and 20% is donated to basic research after de-identification (Supplementary Fig. S1B). The amount of ovarian tissue removed from an individual can be a small biopsy or a whole ovary, depending on site-specific clinical practices as well as case-specific variables. Research tissue from these investigational protocols has led to a series of breakthroughs in in vitro follicle growth and in vitro maturation technologies.17,19,23
For this particular study, following informed consent under IRB-approved protocols, all tissue designated for basic research was processed at Northwestern University (Chicago, IL). Ovarian tissue that was removed at local OC-NPC sites (Chicago, IL) was brought to the research laboratory within 2–4 hours of removal and processed immediately upon arrival. For all other cases, ovarian tissue was transported to Northwestern University in SAGE OTC Holding Media (Copper Surgical, Trumball, CT) at 4°C for up to 24 hours. It has been shown that these transport conditions do not compromise the function of the primordial follicles.17,24–27 For the subset of research samples described in this study (N = 24), small pieces of fresh tissue (up to 10%) were fixed and processed for histological analysis as described below (Supplementary Fig. S1B).
Participant data acquisition
This study includes information from participants who enrolled in OTC protocols through the OC-NPC between May 2007 and April 2014. Basic demographic and health information for each participant was obtained at the time of enrollment. This information included type of cancer and/or diagnosis, and previous treatment (specifically all treatments prior to the OTC procedure). For participants who had received prior chemotherapy, the regimen name, number of cycles of chemotherapy, and the total cumulative doses were recorded. For participants who had received radiation, the dose and field were recorded. For participants who underwent surgery, the type and extent of surgery was recorded. More detailed data for the specific participant subset included in the histological analysis are documented in Table 1 and Supplementary Table S2 (N = 24).
Table 1.
Participant Characteristics
| ID | Age (years) | Diagnosis | Previous therapy | Specimen type | Specimen source |
|---|---|---|---|---|---|
| No prior treatment to OTC | |||||
| A | 11 | Fanconi anemia | None | Ovary | Transport |
| B | 11 | SCA | None | Ovary | Transport |
| C | 14 | MDS | None | Biopsy | Transport |
| D | 15 | MDL | None | Biopsy | Transport |
| E | 16 | NHL | None | Ovary | Local |
| F | 16 | Breast EWS | None | Biopsy | Local |
| G | 17 | HL | None | Ovary | Local |
| H | 17 | Lymphoma | None | Ovary | Transport |
| I | 17 | HL | None | Ovary | Transport |
| Prior treatment to OTC—low risk for infertility or POF | |||||
| J | 3 | CML | Chemo | Biopsy | Transport |
| K | 8 | AML | Chemo | Biopsy | Transport |
| L | 8 | Thalassemia | Chemo | Ovary | Transport |
| M | 12 | ALL | Chemo | Biopsy | Transport |
| N | 14 | ALL | Chemo | Biopsy | Transport |
| O | 15 | Idiopathic AA | Chemo | Ovary | Transport |
| P | 16 | ALL | Chemo + radiation | Ovary | Local |
| Q | 17 | Nasopharyngeal RMS | Radiation | Ovary | Transport |
| R | 17 | HL | Chemo + radiation | Ovary | Local |
| S | 17 | ALL | Chemo | Biopsy | Transport |
| Prior treatment to OTC—high risk for infertility or POF | |||||
| T | 2 | NBL | Chemo + surgery | Ovary | Transport |
| U | 6 | ALL | Chemo + radiation | Biopsy | Transport |
| V | 8 | ALL | Chemo | Biopsy | Transport |
| W | 9 | Vaginal RMS | Chemo + radiation + surgery | Ovary | Local |
| X | 16 | Recurrent EWS | Chemo | Ovary | Local |
AA, aplastic anemia; ALL, acute lymphoblastic lymphoma; CML, chronic myeloid leukemia; EWS, Ewing's sarcoma; HL, Hodgkin lymphoma; MDL, medulloblastoma; MDS, myelodysplastic syndrome; NBL, neuroblastoma; NHL, non-Hodgkin lymphoma; OTC, ovarian tissue cryopreservation; POF, premature ovarian failure; RMS, rhabdomyosarcoma; SCA, sickle cell anemia.
Ovarian tissue fixation and histological analysis
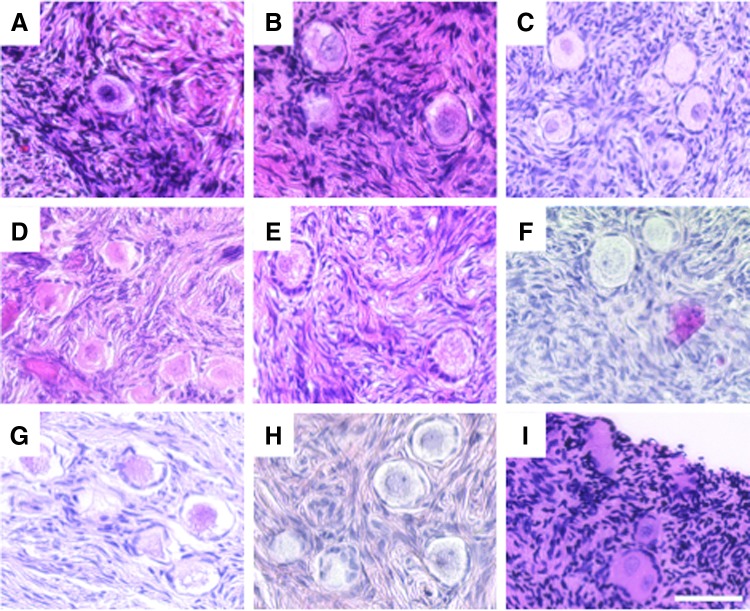
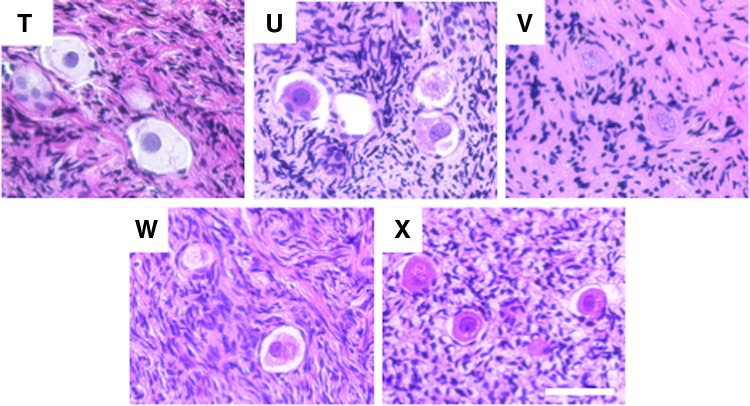
Ovarian tissue from each participant was fixed in 10% neutral buffered formalin overnight at 4°C (Table 1 and Supplementary Table S2). All tissue processing and hematoxylin and eosin (H&E) staining was performed by the Northwestern University Center for Reproductive Sciences Histology Core as described previously.17 Briefly, fixed tissue was processed using an automated tissue processor (Leica Biosystems, Buffalo Grove, IL) and embedded in paraffin. Serial sections were cut (5 μm) and stained with H&E using an Autostainer XL (Leica Biosystems). Stained histological sections were imaged and analyzed using an EVOS FL Auto Cell Imaging system (Life Technologies, Grand Island, NY). The participant samples were divided into three treatment categories based on the reported medical history of each participant.28–31 These categories included: (1) no treatment prior to OTC, (2) exposure to treatments prior to OTC that are considered low risk for infertility or premature ovarian failure (POF), and (3) exposure to treatments prior to OTC that are considered high risk for infertility or premature ovarian failure (POF; Table 1, Supplementary Table S2, and Fig. 2–4). A minimum of five slides were analyzed for each participant, and the presence or absence of follicles was annotated for each sample. Primordial follicles were defined as an oocyte surrounded by an incomplete layer of squamous granulosa cells, and primary follicles were identified as enlarged oocytes surrounded by a single complete layer of cuboidal granulosa cells.
FIG. 2.
Histological evaluation reveals primordial follicles in ovarian tissue from all participants with no prior exposure to gonadotoxic therapies. Representative standard hematoxylin and eosin (H&E) staining of ovarian tissue histological sections from pediatric and teen participants who had no previous treatment prior to undergoing OTC. The image labels correspond to the participant ID. More detailed information about each participant can be found in Table 1. Scale bar is 50 μm.
FIG. 3.
Histological evaluation reveals primordial follicles in ovarian tissue from participants who were exposed to low-risk gonadotoxic treatments prior to OTC. Representative standard H&E staining of ovarian tissue histological sections from pediatric and teen participants who had previous treatment prior to undergoing OTC that is considered to be low risk for infertility or premature ovarian failure. The image labels correspond to the participant ID. More detailed information about each participant can be found in Table 1 and Supplementary Table S2. Scale bar is 50 μm.
FIG. 4.
Histological evaluation reveals primordial follicles in ovarian tissue from participants who were exposed to high-risk gonadotoxic treatments prior to OTC. Representative standard H&E staining of ovarian tissue histological sections from pediatric and teen participants who had previous treatment prior to undergoing OTC that is considered to be high risk for infertility or premature ovarian failure. The image labels correspond to the participant ID. More detailed information about each participant can be found in Table 1 and Supplementary Table S2. Scale bar is 50 μm.
Results
Increasing numbers of pediatric and teen females have undergone OTC at OC-NPC sites
The OC-NPC is a nationwide network of sites committed to providing fertility preservation options to men, women, and children who are at risk of future infertility (Supplementary Fig. S1A and Supplementary Table S1; oncofertility.northwestern.edu).21 One mission of the OC-NPC is to facilitate the use of investigational fertility preservation procedures, such as OTC, by assisting sites with relevant technical training and regulatory and compliance support. To date, 33 OC-NPC sites offer their patients OTC under IRB-approved protocols, and 19 of these sites are approved to perform OTC in participants younger than 18 years of age, which includes pediatric and teen populations (Supplementary Fig. S1A and Supplementary Table S1). Between its inception in 2007 and early 2014, the number of participants who have enrolled in OTC protocols has steadily increased, with >150 participants having undergone the procedure (Fig. 1A). In recent years, there has been a shift in the participant population electing to undergo this experimental procedure. There has been a decrease in females older than 18 years of age potentially due in part to the designation of egg freezing as a standard fertility preservation option in 2013 (Fig. 1A) and due to the increasing information available to reproductive endocrinology and infertility (REI) practices on the reproductive needs of this patient group.32,33 During this same time interval, there has been a corresponding increase in the number of females younger than 18 years old who have participated in this investigational technology potentially due to growing awareness of and access to fertility preservation options (Fig. 1A).
FIG. 1.
There is a shift toward pediatric and teen usage of ovarian tissue cryopreservation (OTC). (A) Since its inception in 2007, the number of participants enrolled to undergo OTC has steadily increased (red line). In recent years, there has been a shift in the participant population electing to undergo this experimental procedure. There has been a decrease in females older than 18 years old (gray bars), and a corresponding increase in the number of females younger than 18 years old (black bars). (B) A histogram illustrating the age distribution of participants younger than 18 years old who have undergone investigational OTC protocols through the NPC. (C) The distribution of clinical diagnoses (malignant and non-malignant) of pediatric and teen populations that have elected to undergo OTC through the National Physicians Cooperative (NPC). (D) The treatment status of pediatric and teen populations prior to ovarian tissue removal and OTC (chemotherapy, radiation, disease modifying agent, or a combination of these).
The OC-NPC infrastructure has facilitated recruitment of pediatric and teen participants nationwide. Between May 2007 and April 2014, a total of 63 pediatric and teen participants underwent OTC. These participants ranged in age from 1 year to 17 years old, with a mean age of 11.7 ± 0.6 years old (Fig. 1B). These participants had diverse diagnoses that necessitated OTC as a fertility preservation option due to conditions or treatments thereof that were potentially fertility threatening. The majority of participants had hematological malignancies (40%), but other reported conditions included solid tumors (35%), bone marrow failure (14%), hemoglobinopathy (8%), and metabolic disease (1.5%; Fig. 1C). Of note, there has been a recent increase in the use of pediatric and teen use of OTC within the NPC for non-malignant conditions where treatment is gonadotoxic, including Hurler's syndrome, thalassemia, sickle cell anemia, idiopathic aplastic anemia, and Diamond–Blackfan anemia.
Although it is ideal to remove and cryopreserve ovarian tissue prior to any exposure to chemotherapy, radiation, and/or disease modifying agent, this is not always possible. As evidenced by the OC-NPC pediatric and teen experience, 46% of participants had not received any medical treatment prior to OTC (Fig. 1D). However, the remainder of participants had been exposed to some form of chemotherapy, radiation therapy, disease modifying agent, or combination before preserving their fertility via OTC (Fig. 1D, Table 1, and Supplementary Table S2).
Ovarian tissue from pediatric and teen OTC participants contains pre-antral follicles
The interest in this study was in establishing whether pre-antral follicles were present routinely in ovarian tissue from pediatric and teen participants undergoing OTC. To determine this, a simple morphological assessment of human ovarian research tissue was performed from a subset of pediatric and teen OC-NPC participants for whom fixed histological samples were available (N = 24; Supplementary Fig. S1B). This subset of participants was a representative cross-section of the total pediatric population who had undergone OTC with respect to participant-specific variables, including age, diagnosis, and previous treatment history (Table 1 and Supplementary Table S2). In addition, 10/24 participants had undergone a biopsy to obtain ovarian tissue for cryopreservation, whereas the remainder had at least one whole ovary removed (Table 1). Moreover, the ovarian tissue designated for research from 17/24 participants was maintained at 4°C for up to 24 hours post-removal to allow for transport between NPC sites and the research laboratory (Table 2).
Of the participant-specific variables that existed in this subset, analysis focused on previous treatment history because much work has been done demonstrating that different treatment regimens have distinct degrees of gonadotoxicity.34–38 This occurs through targeted impacts of radiation therapy (type, dose, frequency, field) and chemotherapy agents (type, dose, frequency) on key ovarian components including the germ cells, somatic cells, stroma, or vasculature. Surprisingly, evidence was found of primordial and/or early-activated primary follicles in all participant samples examined in the different treatment categories (Figs. 2–4). Larger follicles were also observed—including pre-antral and early antral follicles—in three of the samples examined, but these were limited to participants who had no prior treatment or exposure to only low-risk treatments (Fig. 5).
FIG. 5.
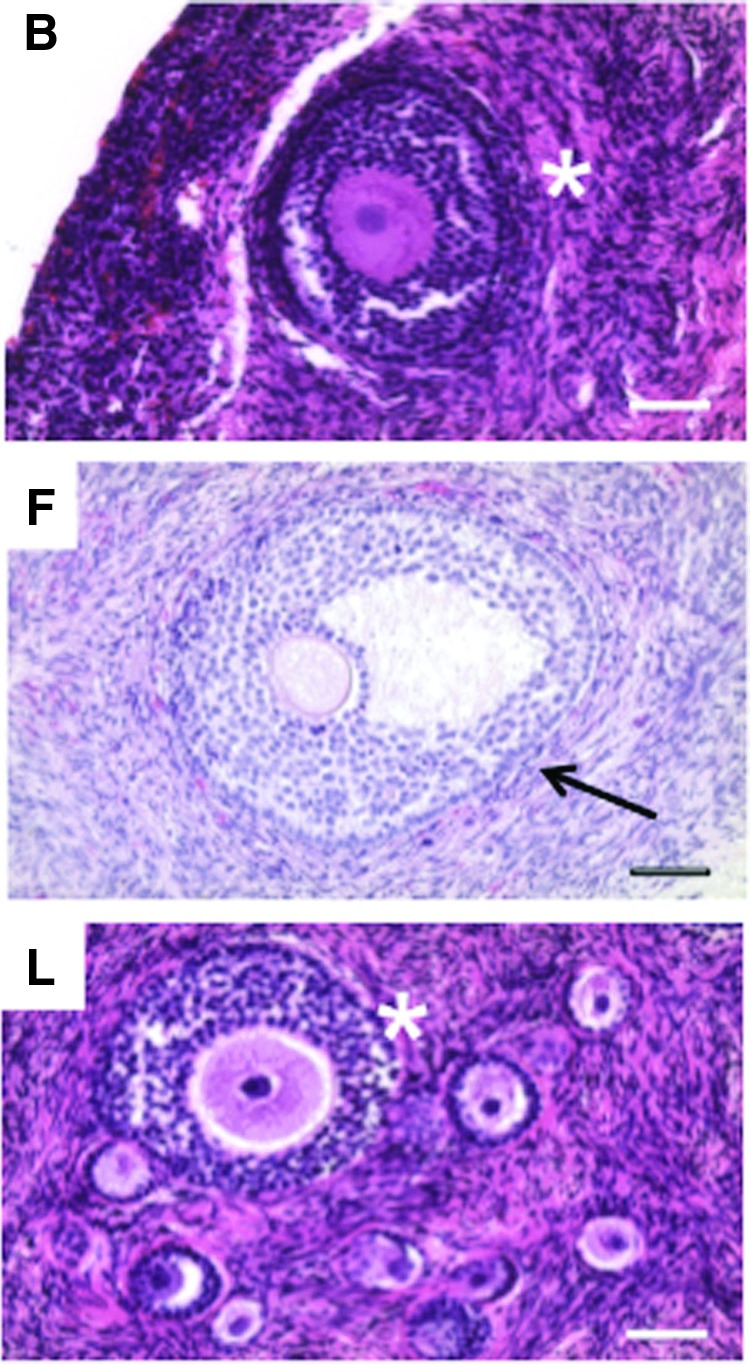
Histological evaluation reveals the presence of activated and growing follicles in a subset of participant ovarian tissue. In addition to primordial follicles, growing pre-antral follicles were observed in a total of three participants who had either not received prior treatment to gonadotoxic therapy (participants B and F) or had been exposed to treatment that would be considered low risk for infertility or premature ovarian failure (participant L). Multilayer secondary follicles are highlighted with asterisks, and the antral follicle is highlighted with an arrow. The image labels correspond to the participant ID. More detailed information about each participant can be found in Table 1 and Supplementary Table S2. Scale bar is 50 μm.
Although some of the participant samples had oocytes that appeared morphologically abnormal, with the most common feature being shrinkage of the oocyte cytoplasm away from the somatic cells, it was not possible to distinguish whether this was due to a fixation artifact or whether it was due to a true cellular defect (Figs. 2G–I; 3N and S; and 4U and W). Importantly, the altered morphology observed was not associated with a particular treatment history group or any other variable. In addition, some samples had evidence of atypical stromal cell organization characterized by a dispersed instead of compact morphology (Figs. 2D, E, and G; 3M, N, and Q; and 4V). Such changes in the local ovarian microenvironment may ultimately influence follicle development and quality. Nevertheless, these results taken together indicate that ovarian tissue from pediatric and teen populations that is removed for OTC is highly likely to contain follicles that may have the potential to restore or contribute to reproductive function in later years. Larger more comprehensive studies, however, are needed to determine just how resilient the pediatric ovarian reserve is to factors such as age, diagnosis, previous treatment history, and tissue processing methods.
Discussion
The most prominent finding in the present analysis was that pre-antral follicles were present in histological sections of ovarian tissue from all 24 participants examined. This is striking for several reasons. First, the ovarian tissue that is designated for research is not obtained from the same region of the ovary for each participant, and, in some cases, the research tissue was a portion of a small biopsy and not an entire ovary. Therefore, the fragment that was selected for histology was random in nature. Second, the human ovarian cortex, which is enriched in primordial follicles, does not have a uniform follicle density.39 There can be significant variability—up to orders of magnitude differences—in follicle number per cortical fragment even in the ovarian cortex from a given individual.39 Typically, primordial follicles are found in clusters in human ovaries rather than being evenly distributed throughout the tissue.17 Finally, the participants were largely heterogeneous in terms of factors known to affect follicle number, including age and prior exposure to potentially gonadotoxic therapies.13 Thus, it is intriguing that it was possible to document the presence of follicles in all samples examined. Follicles within these tissue fragments were not quantified, however, due to the inherent limitations of interpreting the results because of the factors listed above. Nevertheless, these data increase the knowledge about follicle dynamics in a reproductively young female population undergoing fertility preservation. The findings underscore the potential resilience of the primordial follicle population to extraordinary conditions and may reflect the relatively large ovarian reserve in this age group.
The presence of primordial follicles in all participant tissue samples examined in this study, although promising, is not a guarantee that this tissue will have sufficient ovarian potential to support puberty, endocrine health, or fertility. In fact, a recent study demonstrated that human prepubertal ovaries contain a high proportion of abnormal non-growing follicles that have a reduced ability to grow in vitro.40 These findings are consistent with the observations of altered primordial follicle morphology in some of the participant tissues. A shortcoming of this study is that it is limited to morphologic assessment of follicles within histological sections. Thus, while follicles are present in all tissues, it is not known whether differences exist in quality measures. Moreover, the possibility cannot be discounted that the surrounding vasculature and/or stroma are impacted in these samples. Assessing functional differences in human ovarian tissue is difficult because of the lack of comprehensive and predictive assays. There are several methods that have been used in attempts to quantify ovarian tissue quality, including staining for markers of cell death, proliferation, survival, and viability.17,41 In vitro follicle growth is another useful tool in which ovarian follicles are cultured, and parameters such as follicle growth and survival, cell differentiation, hormone production, ovulation, and production of a fertilizable-gamete can be tracked.42,43 Finally, an in vivo system that is used to evaluate tissue quality is xenotransplantation, in which small pieces of human ovarian tissue are transplanted into a host species (typically immunocompromised mice) and follicle development, endocrine function, and mature gamete production are monitored over a period of months.44,45 Although these assays can provide important information about how specific conditions affect ovarian tissue function, they can be time-consuming, costly, and inefficient or impractical for application with human material. Moreover, the results from these assays may not be representative or predictive of the quality of the tissue as a whole.
The unequivocal test of ovarian tissue quality is whether endocrine function and fertility can be restored post-transplantation and how long the tissue remains functional. To date, there have been upwards of 30 females worldwide who have experienced restoration of fertility following transplantation.16 This success has been reported following orthotopic and heterotopic transplantation, as well as following use of slow frozen or vitrified ovarian tissue.16,46,47 Based on combined data from three centers in Europe, ovarian tissue transplant resulted in pregnancies in 11/60 cases.16,48 However, in general, it is difficult to gauge the overall success rate of ovarian tissue transplantation because failures are rarely reported in the literature, and thus the denominator of attempted cases is largely unknown. Several in vivo transplants have occurred in pediatric patients, allowing entry into puberty.49–51 However, the ongoing fertility status of these individuals remains to be determined. In the pediatric and teen population, it may take decades before the quality of the removed tissue is understood, given the potential time delay between the OTC procedure and when these young individuals would be ready to use the tissue to restore reproductive potential. Of note, it was recently reported that a live birth was obtained following transplantation of ovarian tissue that had been harvested from an individual and cryopreserved prior to menarche.48
Based on the data gathered through the OC-NPC, it is clear that the use of OTC has increased substantially over the past several years in pediatric and teen populations for both oncologic and non-oncologic indications. This is likely due to several reasons, including a heightened awareness of fertility preservation options among patients and families, an increase in clinical teams and referral networks that offer fertility preservation, strong advocacy groups that support fertility preservation, as well as more explicit and updated fertility preservation guidelines. Given these trends in OTC usage and the clinical, ethical, and legal ramifications of removing ovarian tissue from a minor who may be prepubertal, it is imperative that the fertility preservation field continues to make strides in establishing evidence-based guidelines for who would most benefit from transplantation or in developing investigational technologies that protect follicles without necessitating the extreme measure of ovarian tissue removal.52 Potential medical mitigation strategies include the use of gonadotropin releasing hormone (GnRH) agonists to shut down the hypothalamic–pituituary–gonadropon axis. Keeping ovarian function suppressed may have a protective effect, as has been recently suggested in a randomized control trial of breast cancer patients receiving chemotherapy with or without goserelin.53 However, the broad efficacy of this method of fertility preservation remains controversial.54 In addition to GnRH agonists, fertoprotective adjuvant therapies that inhibit apoptosis and prevent germ cell loss in response to gonadotoxic exposures (sphingosine-1-phosphate, FTY720, and imatinib) have shown promise in several preclinical rodent and primate models of radiation and chemotherapy-induced damage.55–58 An important limitation of emerging technologies, which rely on anti-apoptotic agents, is that the surviving germ cells may have significant DNA damage. This would restrict the usefulness of these protected germ cells to providing endocrine support rather than also restoring fertility. A recent study has demonstrated the fertoprotective nature of bortezomib, a drug that prevents doxyrubicin accumulation in the ovary, thereby mitigating doxyrubicin-induced DNA damage and preserving fertility in a rodent model.59 In addition to fertoprotective adjuvant therapies, another active area of research is the development of fertoprotective “smart drugs” that target the cancer or disease while minimizing toxicity to the ovary and germ cells. An example of such a drug is nanobin encapsulated arsenic trioxide for the treatment of lymphoma, which has been tested in a preclinical rodent study.60
Even with the inherent limitations of this study, it is promising to find that pre-antral follicles were observed in ovarian tissue from all participants examined, despite population heterogeneity. Primordial follicles were even observed in the ovarian tissue of participants who had previous treatments that placed them in a high-risk category for iatrogenic infertility or premature ovarian insufficiency. At this time, OTC may be a promising modality to preserve fertility and endocrine function, especially in a pediatric and teen population. However, given the rapid pace with which the oncofertility field is growing, ongoing scrutiny of emerging technologies is essential to ensure that patients are being providing with the safest and most efficacious treatment options.22
Supplementary Material
Acknowledgments
We thank K. Smith and B. Smith for coordinating NPC research tissue and data acquisition, and J. Pahnke for coordinating research tissue processing. We are grateful to M. Romero and K. Barreto for histological expertise, and E. Bulun, and R. Woodruff for technical assistance. We also wish to acknowledge K. Ebbert and B. Kong for valuable discussions. This work ultimately to improve fertility preservation options for those with fertility threatening conditions could not have been done without the participants and NPC sites who generously provided human ovarian tissue for basic research. This work was supported by the Center for Reproductive Health After Disease (P50HD076188) from the National Institutes of Health National Center for Translational Research in Reproduction and Infertility (NCTRI).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ries LAG, Melbert D, Krapcho M, et al. SEER cancer statistics review, 1975–2004. Accessed April1, 2015 from: http://seer.cancer.gov/csr/1975_2004
- 2.Pui CH, Gajjar AJ, Kane JR, Qaddoumi IA, Pappo AS. Challenging issues in pediatric oncology. Nat Rev Clin Oncol. 2011;8(9):540–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton SE, Najita JS, Ginsburg ES, et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2013;14(9):873–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford JS, Kawashima T, Whitton J, et al. Psychosexual functioning among adult female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32(28):3126–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armuand GM, Wettergren L, Rodriguez-Wallberg KA, Lampic C. Desire for children, difficulties achieving a pregnancy, and infertility distress 3 to 7 years after cancer diagnosis. Support Care Cancer. 2014;22(10):2805–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metzger ML, Meacham LR, Patterson B, et al. Female reproductive health after childhood, adolescent, and young adult cancers: guidelines for the assessment and management of female reproductive complications. J Clin Oncol. 2013;31(9):1239–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns KC, Boudreau C, Panepinto JA. Attitudes regarding fertility preservation in female adolescent cancer patients. J Pediatr Hematol Oncol. 2006;28(6):350–4 [DOI] [PubMed] [Google Scholar]
- 8.Gorman JR, Bailey S, Pierce JP, Su HI. How do you feel about fertility and parenthood? The voices of young female cancer survivors. J Cancer Surviv. 2012;6(2):200–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mersereau JE, Goodman LR, Deal AM, Gorman JR, Whitcomb BW, Su HI. To preserve or not to preserve: how difficult is the decision about fertility preservation? Cancer. 2013;119(22):4044–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schover LR. Patient attitudes toward fertility preservation. Pediatr Blood Cancer. 2009;53(2):281–4 [DOI] [PubMed] [Google Scholar]
- 11.Zebrack BJ, Casillas J, Nohr L, Adams H, Zeltzer LK. Fertility issues for young adult survivors of childhood cancer. Psycho-oncology. 2004;13(10):689–99 [DOI] [PubMed] [Google Scholar]
- 12.De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384(9950):1302–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan FE, Jozefik JK, Kim AM, Hirshfeld-Cytron J, Woodruff TK. The gynecologist has a unique role in providing oncofertility care to young cancer patients. US Obstet Gynecol. 2011;6(1):24–34 [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn GP, Murphy D, Knapp CA, Christie J, Phares V, Wells KJ. Coping styles of female adolescent cancer patients with potential fertility loss. J Adolesc Young Adult Oncol. 2013;2(2):66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastings L, Beerendonk CC, Westphal JR, et al. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Hum Reprod Update. 2013;19(5):483–506 [DOI] [PubMed] [Google Scholar]
- 16.Donnez J, Dolmans MM, Pellicer A, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99(6):1503–13 [DOI] [PubMed] [Google Scholar]
- 17.Laronda MM, Duncan FE, Hornick JE, et al. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet. 2014;31(8):1013–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril. 2013;99(6):1523–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu M, Barrett SL, West-Farrell E, et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Human Reprod. 2009;24(10):2531–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macklon KT, Ernst E, Andersen AN, Andersen CY. Cryobanking of human ovarian tissue: do women still want their tissue stored beyond 5 years? Reprod Biomed Online. 2014;29(4):452–6 [DOI] [PubMed] [Google Scholar]
- 21.Woodruff TK. The Oncofertility Consortium—addressing fertility in young people with cancer. Nat Rev Clin Oncol. 2010;7(8):466–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodruff TK. From the bench to bedside to babies: translational medicine made possible by funding multidisciplinary team science. J Assist Reprod Genet. 2013;30(10):1249–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan FE, Hornick JE, Lampson MA, Schultz RM, Shea LD, Woodruff TK. Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell. 2012;11(6):1121–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dittrich R, Lotz L, Keck G, et al. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97(2):387–90 [DOI] [PubMed] [Google Scholar]
- 25.Ernst E, Bergholdt S, Jorgensen JS, Andersen CY. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25(5):1280–1 [DOI] [PubMed] [Google Scholar]
- 26.Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod. 2012;27(6):1801–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosendahl M, Schmidt KT, Ernst E, et al. Cryopreservation of ovarian tissue for a decade in Denmark: a view of the technique. Reprod Biomed Online. 2011;22(2):162–71 [DOI] [PubMed] [Google Scholar]
- 28.Chemaitilly W, Mertens AC, Mitby P, et al. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab. 2006;91(5):1723–8 [DOI] [PubMed] [Google Scholar]
- 29.Chiarelli AM, Marrett LD, Darlington G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964–1988 in Ontario, Canada. Am J Epidemiol. 1999;150(3):245–54 [DOI] [PubMed] [Google Scholar]
- 30.Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab. 2003;88(11):5307–14 [DOI] [PubMed] [Google Scholar]
- 31.Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98(13):890–6 [DOI] [PubMed] [Google Scholar]
- 32.Noyes N, Labella PA, Grifo J, Knopman JM. Oocyte cryopreservation: a feasible fertility preservation option for reproductive age cancer survivors. J Assist Reprod Genetics. 2010;27(8):495–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Practice Committees of American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43 [DOI] [PubMed] [Google Scholar]
- 34.Wallace WH. Oncofertility and preservation of reproductive capacity in children and young adults. Cancer. 2011;117(10 Suppl):2301–10 [DOI] [PubMed] [Google Scholar]
- 35.Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys. 2009;73(5):1304–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adriaens I, Smitz J, Jacquet P. The current knowledge on radiosensitivity of ovarian follicle development stages. Hum Reprod Update. 2009;15(3):359–77 [DOI] [PubMed] [Google Scholar]
- 37.Turan V, Oktay K. Sexual and fertility adverse effects associated with chemotherapy treatment in women. Expert Opin Drug Saf. 2014;13(6):775–83 [DOI] [PubMed] [Google Scholar]
- 38.Ginsberg JP. Educational paper: the effect of cancer therapy on fertility, the assessment of fertility and fertility preservation options for pediatric patients. Eur J Pediatr. 2011;170(6):703–8 [DOI] [PubMed] [Google Scholar]
- 39.Schmidt KL, Byskov AG, Nyboe Andersen A, Muller J, Yding Andersen C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18(6):1158–64 [DOI] [PubMed] [Google Scholar]
- 40.Anderson RA, McLaughlin M, Wallace WH, Albertini DF, Telfer EE. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod. 2014;29(1):97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ting AY, Yeoman RR, Campos JR, et al. Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system. Hum Reprod. 2013;28(5):1267–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12(10):2739–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75(6):916–23 [DOI] [PubMed] [Google Scholar]
- 44.Lotz L, Liebenthron J, Nichols-Burns SM, et al. Spontaneous antral follicle formation and metaphase II oocyte from a non-stimulated prepubertal ovarian tissue xenotransplant. Reprod Biol Endocrinol. 2014;12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luyckx V, Scalercio S, Jadoul P, et al. Evaluation of cryopreserved ovarian tissue from prepubertal patients after long-term xenografting and exogenous stimulation. Fertil Steril. 2013;100(5):1350–7 [DOI] [PubMed] [Google Scholar]
- 46.Kawamura K, Cheng Y, Suzuki N, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110(43):17474–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donnez J, Dolmans MM. Transplantation of ovarian tissue. Best practice and research. Clin Obstet Gynecol. 2014;28(8):1188–97 [DOI] [PubMed] [Google Scholar]
- 48.Demeestere I, Simon P, Dedeken L, Moffa F, Tsépélidis S, Brachet C, Delbaere A, Devreker F, Ferster A. Live birth after autograph of ovarian tissue cryopreserved during childhood. Hum Reprod. 2015; Epub ahead of print. DOI: 10.1093/humrep/dev128 [DOI] [PubMed] [Google Scholar]
- 49.Anderson RA, Hindmarsh PC, Wallace WH. Induction of puberty by autograft of cryopreserved ovarian tissue in a patient previously treated for Ewing sarcoma. Eur J Cancer. 2013;49(13):2960–1 [DOI] [PubMed] [Google Scholar]
- 50.Ernst E, Kjaersgaard M, Birkebaek NH, Clausen N, Andersen CY. Case report: stimulation of puberty in a girl with chemo- and radiation therapy induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. Eur J Cancer. 2013;49(4):911–14 [DOI] [PubMed] [Google Scholar]
- 51.Poirot C, Abirached F, Prades M, Coussieu C, Bernaudin F, Piver P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet. 2012;379(9815):588. [DOI] [PubMed] [Google Scholar]
- 52.Wallace WH, Smith AG, Kelsey TW, Edgar AE, Anderson RA. Fertility preservation for girls and young women with cancer: population-based validation of criteria for ovarian tissue cryopreservation. Lancet Oncol. 2014;15(10):1129–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore HC, Unger JM, Phillips KA, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. New Engl J Med. 2015;372(10):923–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blumenfeld Z, von Wolff M. GnRH-analogues and oral contraceptives for fertility preservation in women during chemotherapy. Hum Reprod Update. 2008;14(6):543–52 [DOI] [PubMed] [Google Scholar]
- 55.Kim SY, Cordeiro MH, Serna VA, et al. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013;20(8):987–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li F, Turan V, Lierman S, Cuvelier C, De Sutter P, Oktay K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum Reprod. 2014;29(1):107–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morita Y, Perez GI, Paris F, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6(10):1109–14 [DOI] [PubMed] [Google Scholar]
- 58.Zelinski MB, Murphy MK, Lawson MS, et al. In vivo delivery of FTY720 prevents radiation-induced ovarian failure and infertility in adult female nonhuman primates. Fertil Steril. 2011;95(4):1440–5, e1441–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roti Roti EC, Ringelstetter AK, Kropp J, Abbott DH, Salih SM. Bortezomib prevents acute doxorubicin ovarian insult and follicle demise, improving the fertility window and pup birth weight in mice. PloS One. 2014;9(9):e108174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahn RW, Barrett SL, Raja MR, et al. Nano-encapsulation of arsenic trioxide enhances efficacy against murine lymphoma model while minimizing its impact on ovarian reserve in vitro and in vivo. PloS One. 2013;8(3):e58491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.