Abstract
This scoping review was conducted to understand the extent, range, and nature of current research on adolescents and young adults (AYA) with cancer and distress, depression, and anxiety (DDA). This information is necessary to find and aggregate valuable data on the AYA population embedded in generalized studies of DDA. Keyword searches of six relevant electronic databases identified 2156 articles, with 316 selected for abstract review and 40 for full text review. Full-text reviews and data extraction resulted in 34 studies being included, which ranged widely in design, sample size, age-range categorization, analysis methods, DDA measurement tool, overall study rigor, and quality of evidence. Studies very seldom reported using theory to guide their age categorization, with only four studies giving any rationale for their age-group definitions. All 34 studies found a significant association between at least one DDA construct and the younger age group relative to the older age groups at some point along the cancer trajectory. However, age as an independent risk factor for DDA is still unclear, as the relationship could be confounded by other age-related factors. Despite the wide range of definitions and effect sizes in the studies included in this review, one thing is clear: adolescents and young adults, however defined, are a distinct group within the cancer population with an elevated risk of DDA. Widespread adoption of a standard AYA age-range definition will be essential to any future meta-analytical psycho-oncology research in this population.
Keywords: : younger age, distress, depression, anxiety, review
The high prevalence of distress, depression, and anxiety (DDA) within cancer patients and survivors has been a driving force in the development of the field of psychosocial oncology.1 As researchers have identified risk factors for DDA, high-risk populations have started to emerge. Adolescents and young adults (AYA) with cancer may be one such population. However, it can be difficult to interpret age-specific findings without consistent usage of clinically relevant and theory-derived conceptual age boundaries. In 2009, the National Cancer Institute defined the term “adolescent and young adult” as age 15–39 years, citing the relative lack of improvement in survival for this entire age range and the lack of a “home” in both research and healthcare for this demographic.2
Cancer incidence in AYAs has been increasing, but improvements in survival rates have been slow compared with children and older adults3,4 due to delayed diagnosis,5 age-specific psychological factors,6 low enrollment in clinical trials,7 unique tumor biology,8 and minimal communication between adult and pediatric oncologists, leading to suboptimal treatment protocols.9 It is important to understand if and how the unique biological, psychological, and social aspects of cancer in the AYA phase of life might put people with cancer in this age range at greater risk for DDA. Rich data about the AYA demographic are likely contained within the larger psycho-oncology literature traditionally divided along the lines of other patient characteristics, such as tumor group or time since diagnosis. To find and aggregate valuable data on the AYA population embedded in more generalized studies of DDA, researchers must develop a search strategy.
The primary rationale for summarizing risk-factor research in the DDA field from the AYA psycho-oncology perspective is that recreating this body of work with a relatively small subpopulation (AYAs account for approximately 10% of all cancer diagnoses10) would be difficult and redundant if enough data already exist within studies of a larger scope. Additionally, the comparison of DDA in the AYA group with other age groups, which occurs in these larger studies, may have implications for psychosocial resource allocation within cancer care and could influence practice and policy on a broader level. The purpose is to assess the “extent, range and nature of current research”11 examining the AYA age range as a risk factor for cancer-related DDA; clarify working definitions of age, conceptual boundaries, and other literature searching constructs12 for use in larger, systematic reviews on this topic; and to explore age-specific research gaps in DDA risk-factor literature.11 The primary research question that will guide this review process is:
RQ1: Are younger age or AYA risk factors for clinical DDA in cancer patients and survivors?
The secondary research questions, which further elucidate the scope of this inquiry, are:
RQ2: Do research databases and studies define younger age or AYAs with a concrete age range, and if so, what is the most common definition?
RQ3: In general, how often is younger age or AYAs cited as a risk factor for clinical DDA, and what is the magnitude of this increased risk?
RQ4. What are the potential confounders, mediators, or moderators of increased DDA as it relates to younger age or AYAs?
Methods
The analytic framework of this scoping review is based on the methodology proposed by Arksey and O'Malley11 and further refined by Levac et al.12 A scoping review summarizes the extent, range, and nature of a research field.12 It is different from a systematic review and meta-analysis in that it does not use a formal methodology to assess the quality of the studies included, is not exhaustive in its literature searching, and does not employ statistical methods to compare across studies.13 It is different from narrative and literature reviews in that analytical reinterpretation of the literature is required in an attempt to specify a future viable review.13
Search strategy
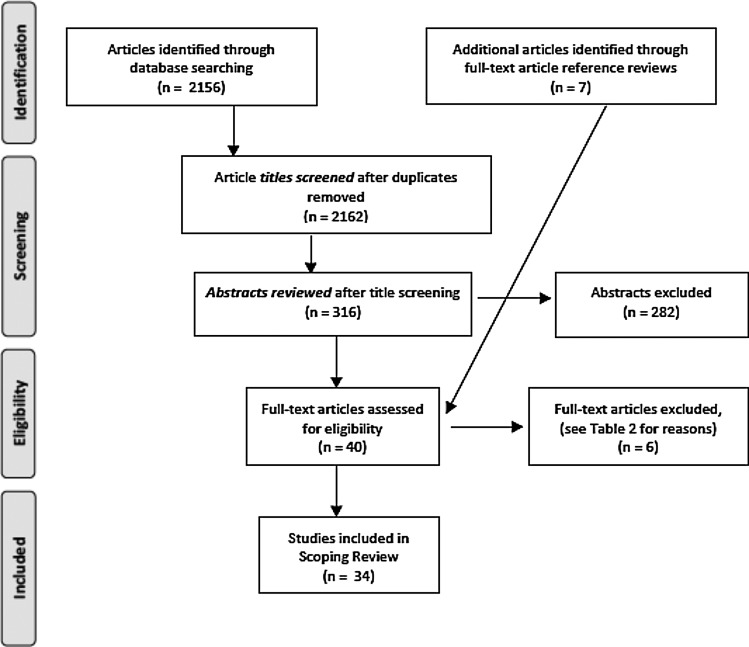
A preliminary search conducted during the initial conceptualization of this scoping review determined that “young* age” as a search term would greatly increase the sensitivity of the search and, despite the negative impact on search specificity, it was included with the search terms “adolescent*” and “young* adult*.” The primary research question was explored using the chosen search terms (presented in Table 1) to query the MEDLINE, PubMed, PsychINFO, CINHAL, Cochrane, and Web of Science databases. Only keyword searches were used, so that the search was as broad as possible and because the Medical Subject Headings (MeSH) are not categorized appropriately for a search of this nature. The PRISMA flow diagram (Fig. 1) illustrates the study selection, and highlights the iterative nature of the searching process.
Table 1.
Search Terms and Boolean Operators Used in Database Searches
| #1. Cancer | cancer* OR neoplasm* OR carcinoma* OR tumor* |
| AND | |
| #2. Risk factor | “risk factor*” OR predict* OR risk* or determinant* |
| AND | |
| #3. Distress | distress OR anxiety OR depression |
| AND | |
| #4. Age | “young* age*” OR “young* adult*” OR adolescent* OR teen* OR “young* people” |
FIG. 1.
PRISMA study selection flow diagram.
Inclusion criteria
The specific inclusion criterion that was applied during abstract and full-text review included: (1) the abstract directly mentioning either “young* age” or “adolescent* and young* adult*” as a risk factor in order to be selected for a full-text review; (2) the measurement of DDA by a widely used and systematically validated tool with standardized cutoffs; and (3) the analysis of DDA as a primary outcome variable and not as a risk factor for other health-related quality of life indices or comorbidities. The relative inclusion criteria were factors taken into account unsystematically during the abstract and full-text review that increased the relevance and quality of the studies selected. These relative inclusion criteria were: (1) a preference to include studies of tumor groups that are highly prevalent in the AYA demographic, and (2) study quality as determined by an informal evaluation of study characteristics, such as appropriate study design and analysis for research question, statistical power, and supporting evidence.
Exclusion criteria
A global filter was used to exclude: (1) studies that were not written in English, due to the monolingual study team; and (2) studies that were published before 1999. Using the 1999 cutoff date allowed us to leverage advancements in DDA measurement tools, larger population-based samples, and rigorous study methods in a mature field of research. Specific exclusion criteria were pediatric oncology studies that included children younger than 14 years of age, as this would not provide a sample with the appropriate age range for this scoping review. The only relative exclusion criteria were DDA studies of the prostate and lung tumor groups, which are very unlikely to include patients younger than 40 years old. In these cases, the search term “younger age” would not likely refer to the AYA age group that we wished to explore. However, studies that included multiple tumor groups along with lung or prostate were still eligible.
Analysis
One researcher (M.L.) completed the database searching (Table 1) and completed the title review of 2156 articles. Of these, 316 articles were selected for abstract review and transferred into a reference management program (Endnote v6). Two researchers (M.L. and V.D.) jointly screened all the abstracts and, through consensus, selected 33 full-text articles for eligibility assessment. Following the independent review of these full-text articles, M.L. and V.D. met to discuss the inclusion of each article based on the inclusion/exclusion criteria and the relevance of the study to the research questions. In addition, any potential studies identified post hoc through a reference review of the full-text articles were assessed for eligibility at this time (n = 7). Articles that produced disagreements were sent to another member of the study team to review (J.G.-D.) with that decision being final. As a result, six studies were excluded, with a total of 34 articles included (exclusion rationale provided in Table 2). As suggested by Levac et al.,12 after full-text article selection, M.L. and V.D. independently extracted data from the same five articles and compared their results to determine whether the data extraction approach was consistent with the research question and purpose. Once the necessary modifications were completed, M.L. finished the data extraction, consulting V.D. and J.G.-D. as needed.
Table 2.
Rationale for Study Exclusion After Full-Text Review
| Author | Date | Country | Design | Rationale |
|---|---|---|---|---|
| Stava CJ, Lopez A, Vassilopoulou-Sellin R | 2006 | United States | Cross-sectional | This study explored prevalence of specific health and psychosocial problems but did not actually measure DDA using a standardized tool |
| Wilson KG, Chochinov HM, Skirko MG, et al. | 2007 | Canada | Cross-sectional | Demographics showed that an insignificant number of participants included in the study fit the AYA demographic |
| Neilson K, Pollard A, Boonzaier A, et al. | 2013 | Australia | Longitudinal | Demographic table showed that there were fewer than five people included in the study that fit the AYA demographic |
| Kim J-H, Yoon S, Won W-Y, et al. | 2013 | South Korea | Cross-sectional | Performance status was primary outcome and DDA were used as independent variables predicting performance status |
| Giese-Davis J, Waller A, Carlson LE, et al. | 2012 | Canada | Longitudinal | Depression and anxiety were not primary outcomes and only used for correlations with number of practical and psychosocial problems |
| Fossa SD, Dahl AA, Loge JH | 2003 | Norway | Cross-sectional | Depression and anxiety were not primary outcomes and only used as independent variables predicting chronic fatigue |
Results
Full data extraction of the 34 selected studies, including age-specific findings related to DDA, is provided in Table 3 with a summary table of study characteristics provided in Table 4. In general, the included studies ranged widely in design, sample size, age range, analysis methods, DDA measurement tool, overall study rigor, and quality of evidence. There was cross-cultural representation with studies from 11 countries in addition to representation of every major tumor group. However, the most prominent tumor group by far was breast cancer (n = 15, 41.1%). All the longitudinal studies followed patients from treatment into survivorship (post-treatment) with a majority of cross-sectional studies (n = 19) assessing DDA in patients (n = 14, 76.3%) and a smaller number assessing DDA in post-treatment survivors (n = 5, 26.3%). Overall, 25 studies (73.5%) included patients and 13 studies (37.1%) included cancer survivors. The majority of studies were <5 years old (55.9%). However, publication dates of the included studies ranged from 1999 to 2014.
Table 3.
Data Extraction for Selected Studies
| Authors | Year | Origin | Study design | Sample | Age range definition and analysis (description) | Relevant primary outcome measure (construct or subscales used) | Age-specific findings (DDA) |
|---|---|---|---|---|---|---|---|
| Arden-Close E, Gidron Y, Moss-Morris R | 2008 | United Kingdom | Systematic review | 18 studies of ovarian cancer patients and survivors | N/A | Strength of the evidence was based on the quality and consistency of findings | Strong evidence for a relationship between younger age, being diagnosed with more advanced disease, more physical symptoms. and shorter time since diagnosis with increased levels of anxiety and depression |
| Avis NE, Levine B, Naughton MJ, Case LD, Naftalis E, Van Zee KJ | 2013 | United States | Longitudinal | 554 patients/survivors, stage I–III breast cancer | Age groups defined a priori: 25–44, 45–54, 55–64, 65–74. Included in model as categorical variable (not given) | BDI-1A (depression) | At baseline, younger women have increased levels of depression compared with older women (24–44 BDI (SD) = 11.2 (6.9), 65–74 BDI (SD) = 6.3 (6.2), p < 0.0001). Age was not independently associated with average level of or change in depression over time (p = 0.23). However, because younger age is closely associated with other highly significant independent predictors of depression (illness intrusiveness, chemotherapy with doxorubicin, pain), young women are more likely to present with depression than older women were. |
| Bardwell WA, Natarajan L, Dimsdale JE, et al. | 2006 | United States | Retrospective cohort | 2595 survivors, stage I–III breast cancer | Age groups defined a priori: <50, 50–59.9, ≥60. Included in model as categorical variable (M = 53, range 28–74) | CES-Dsf (short form, depression), RAND-36 (distress) | Before the entry of psychosocial variables, younger age was a significant risk factor for elevated depressive symptoms (<50 OR = 1 vs. ≥60 OR = 0.401, p < 0.001), while other objective cancer-related variables were not. However, after inclusion of psychosocial variables in the model, younger age was no longer a significant predictor. |
| Bodurka-Bevers D, Basen-Engquist K, Carmack CL, et al. | 2000 | United States | Cross-sectional | 246 patients, stage I–IV epithelial ovarian cancer | Age groups defined a priori: <50, ≥50. Age modeled as categorical variable (M = 56.7, range 22–76) | CES-D (depression), Spielberger State–Trait Anxiety Inventory (anxiety) | The younger age group was significantly more likely to be depressed than the older age group was (regression adjusted percentages, <50 = 25% and ≥50 = 13%), but no significant relationship with anxiety and younger/older age was observed |
| Boehmer U, Glickman M, Winter M | 2012 | United States | Cross-sectional | 257 heterosexual and 69 lesbian survivors, stage I–III breast cancer | Age modeled as continuous variable (lesbian M [SD] = 55.9 [8.3], heterosexual M [SD] = 62 [11]) | HADS (anxiety, depression) | Significant negative associations with older age predicting both lower anxiety (β = −0.14, SE = 0.02) and depression levels (β = −0.07, SE = 0.02), while no association with sexual orientation was found |
| Bumbasirevic U, Bojanic N, Pekmezovic T, et al. | 2013 | Serbia | Cross-sectional | 202 survivors, testicular cancer | Age modeled as continuous variable (M [SD] = 35.5 [9.5], range 19–66) | BDI-II (depression) | Age was the only statistically significant risk factor in the development of depression in this sample (OR = 3.2 [95% CI 1.3–8.1], p = 0.012). Other examined factors (level of education, employment status, marital status, duration of follow-up, tumor type, and treatment type) did not show statistical significance. |
| Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A | 2005 | United Kingdom | Prospective cohort | 170 patients/survivors, stage I–III breast cancer | Age modeled as continuous variable (M [SD] = 48.4 [7.8]) | SCID with DSM III (depression, anxiety) | Younger age was slightly associated with higher levels of depression (hazard ratio = 0.96 [0.93–0.99], p < 0.01) in the long term (2–5 years post-diagnosis) but not in immediate (1–4 months post-diagnosis) and medium term (4 months–2 years post-diagnosis). |
| Cohen M, Baziliansky S, Beny A | 2014 | Israel | Cross-sectional | 92 survivors, stage II–III mixed sex colorectal cancer | Age modeled as continuous variable (M [SD] = 58 [11.96], range 27–87) | BSI-18 (anxiety, depression, GSI = distress) | Depression and anxiety were negatively and strongly associated with age (r = −0.35 and −0.39, respectively, p < 0.001). When global distress (GSI) was regressed on age (β = 0.42, p < 0.001), this original strong association became non-significant when resilience was entered into the regression (β = 0.12. p > 0.05). Thus, resilience mediated the relation of age to the GSI distress score. |
| Costanzo ES, Lutgendorf SK, Mattes ML, et al. | 2007 | United States | Longitudinal | 89 survivors, stage 0–III breast cancer | Age modeled as continuous variable but graphically displayed in quartiles, with 32–47 being the youngest quartile (M = 55.0, range 32–89) | CES-D (depression), IES (distress) | Overall participants in this study were not highly distressed. However, in both univariate and multivariate models, younger age predicted greater depression (F = 9.62, p = 0.003 and F = 8.68, p = 0.004, respectively) and distress (F = 12.12, p = 0.001 and F = 6.42, p = 0.02, respectively). Age was a robust predictor, with younger women significantly more likely to experience DDA. |
| Dunn J, Ng SK, Holland J, et al. | 2013 | Australia | Longitudinal | 1884 patients/survivors, colorectal cancer followed for 5 years | Age groups defined a priori: 20–49, 50–59, 60–69, 70–80. Age modeled as continuous variable but frequencies of distress reported using age definitions (20–49 age group = 144, 8.5% of total sample) | BSI-18 (anxiety, depression), GSI (distress) | The 20–49 age group was much more likely to have a trajectory consistently above the GSI cutoff for clinical distress vs. a below cutoff trajectory (adjusted OR = 2.82 [95% CI 1.9–4.1]). On both the depression and anxiety subscales, the 20–49 age group was also more likely to have trajectory consistently above clinical cutoffs vs. below cutoff trajectory (adjusted OR = 1.36 [95% CI 1.1–1.7]), p < 0.001 and OR = 1.41 [95% CI 1.2–1.7], p < 0.001, respectively). |
| Enns A, Waller A, Groff SL, Bultz BD, Fung T, Carlson LE | 2013 | Canada | Longitudinal | 480 patients/survivors, mixed sex and diagnosis | Age groups defined post hoc using median (61.7) to define “younger” and “older” groups. Modeled as a categorical variable (M [SD] = 60.4 [12.30]) | DT (distress), PSSCAN (anxiety, depression) | Compared with the “never” distressed subgroup, younger patients were more likely to report occasional distress (χ2 = 9.27, p = 0.003), and in multivariate analysis, this association remained significant (β = 0.578, OR = 0.56 [95% CI 0.34–0.93], p < 0.05). Younger patients reported more continuous and occasional anxiety (χ2 = 19.28, p < 0.001 and χ2 = 18.92, p < 0.001, respectively) than the “never anxious” subgroup, and this was confirmed in multivariate analysis (β = 0.822, OR = 0.44 [95% CI 0.26–0.76], p < 0.05). Younger patients were more likely to report occasional depression than they were to report “never” being depressed (χ2 = 7.15, p = 0.008). However, in the multivariate analysis, age did not remain a significant predictor of depression subgroup membership (β = 0.319, OR = 0.73 [95% CI 0.43–1.22], p > 0.05). |
| Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE | 2003 | United States | Cross-sectional | 577 survivors, stage 0–II breast cancer | Age at diagnosis defined post hoc, no justification given: 25–34, 35–39, 40–44, 45–51. χ2 test used to compare across age groups at diagnosis. Current age at survey modeled as continuous variable (current age M [SD] = 49.5, range 30–61.6) | CES-D (depression), PANAS (depression) | Scores on the CES-D and the PANAS were consistent with more depressive symptomatology (25–34 = 28.6% clinically depressed, 35–39 = 31.2% clinically depressed, p = 0.06) and more negative affect (25–34 M [SD] = 19.6 [8.1], 35–39 M [SD] = 18.8 [7.6], p = 0.03) in the youngest women compared with the older age groups ≥40, persisting many years after diagnosis. |
| Hart SL, Charles ST | 2013 | United States | Longitudinal | 139 patients, stage I–IV mixed sex colorectal cancer | Age modeled as a continuous variable for CES-D but defined post hoc, using median split to define “younger” (28–59) and “older” (60–89) age groups, to represent change in negative affect graphically (M [SD] = 58.7 [13.6], range 28–89) | CES-D (depression), PANAS (negative affect/depression) | Older age was related to lower levels of negative affect (r = −0.24, p < 0.01) and depressive symptoms, γ = −0.15 (0.05), t(134) = −3.36, p < 0.01. Younger age was related to a steeper decrease in negative affect over time. However, this more rapid decline with age is moderated by level of threat appraisal, γ = −0.15 (0.06), t(249) = −3.07, p < 0.01. |
| Hipkins J, Whitworth M, Tarrier N, Jayson G | 2004 | United Kingdom | Longitudinal | 63 patients, stage I–IIII ovarian cancer, 63 partners | Age modeled as continuous variable (M [SD] = 58.2 [11.5], range 29–79) | HADS (anxiety, depression) | Anxiety at time 1 and younger age (β [SE] =−0.109 [0.48], t = −2.285, p = 0.027) were significantly and independently associated with anxiety at time 2 (F[2, 47] = 34.9, p < 0.001), with these two variables explaining 58% of the variance. No association was found between age and depression. |
| Hopwood P, Sumo G, Mills J, Haviland J, Bliss JM, Group STM | 2010 | United Kingdom | Longitudinal | 2208 patients, stage I–IV breast cancer | Age groups defined a priori: 20–39, 40–49, 50–59, 60–69, 70–89. Age modeled as continuous variable frequencies of depression and anxiety reported using age definitions (M [SD] = 56.9 [10.4], range 26–86) | HADS (anxiety, depression) | Women aged <50 at baseline had higher rates of borderline and case levels of anxiety compared with women ≥50 (41.5% vs. 29.6) while depression rates were similar between the age groups (14.9% vs. 11.0%, respectively). Anxiety status improved with follow-up time (p = 0.041), while younger age (p < 0.001) and worse baseline depression (borderline or case vs. normal category, p < 0.001) were significant in predicting worse anxiety over time. |
| Jadoon NA, Munir W, Shahzad MA, Choudhry ZS | 2010 | Pakistan | Cross-sectional | 150 patients, mixed sex and diagnosis 268 general population | Age groups defined a priori: ≤40, >40. Age modeled as categorical variable (patient M [SD] = 40.85 [16.46], control M [SD] = 39.58 [11.74]) | Aga Khan University Anxiety and Depression Scale (anxiety, depression) | Age ≤40 years was found to increase the odds of having depression and anxiety significantly (>40 OR = 0.46 [95% CI 0.23–0.91], p = 0.027). High prevalence of depression and anxiety in cancer patients vs. health controls was reported (66.0% vs. 40.7%, p < 0.001). Cancer patients were 2.83 times more likely to have psychological distress [95% CI 1.89–4.25]. |
| Jones JM, Cheng T, Jackman M, Rodin G, Walton T, Catton P | 2010 | Canada | Cross-sectional | 440 patients, stage 0–III breast cancer | Age groups defined a priori: 18–49, 50–59, 60+. Age modeled as categorical variable (18–49, n = 151; 50–59, n = 144; 60+, n = 145) | POMS-SF (depression), MOS-HDS (distress) | 18–49 age group had significantly higher depression (p < 0.0001) and distress (p < 0.001) scores compared with women >50. In multivariate analysis, age group (β = −0.204, t = −4.21, p < 0.0001), perceived preparedness (β = 0.09, t = 2.23, p = 0.03), and self-efficacy (β = −0.57, t = −14.21, p < 0.0001) accounted for 36% (F = 59.71, p < 0.0001) of the variance in depression scores. Self-efficacy (β = −0.45, t =−10.24, p < 0.0001) and age group (β = −0.30, t = −6.13, p < 0.0001) accounted for 26% (F = 51.54, p < 0.0001) of the variance in global distress. |
| Kornblith AB, Powell M, Regan MM, et al. | 2007 | United States | Longitudinal | 252 survivors, stage I–III breast and endometrial cancer | Age groups defined a priori: ≤55, 65+. Age modeled as categorical variable (M [SD] for age at diagnosis and age at interview reported for each age group and tumor group) | HADS (anxiety, depression, global distress) | At baseline, the ≤55 group had significantly higher levels of global distress (χ2 = 9.86, p = 0.002) and anxiety (χ2 = 11.84, p = 0.0008) than the 65+ group. No significant age group differences were found in depression scores. There were no significant changes for those scoring above clinical cutoffs for anxiety, depression, or global distress in cancer survivors (time since diagnosis M [SD] = 3.7 [1.9]) over a 1-year period. |
| Krok JL, Baker TA, McMillan SC | 2013 | United States | Cross-sectional | 232 patients, mixed sex and diagnosis | Age groups defined a priori: <60, ≥60 (based on previous study). Age modeled as categorical variable (<60, n = 133; ≥60, n = 99) | MSAS-PSYCH (subscale—distress) | Younger adults reported more pain (81% vs. 66%, p < 0.01), greater pain severity (M [SD] = 2.62 [1.05] vs. 2.23 [1.08], p < 0.05), and higher pain-related distress (M [SD] = 2.79 [1.17] vs. 2.30 [1.26], p < 0.05) compared with older patients. |
| Kwak M, Zebrack BJ, Meeske KA, et al. | 2013 | United States | Longitudinal | 215 patients, mixed sex and diagnosis | Age groups defined a priori based on developmental life stage theory: 14–17, 18–25, 26–39. Age modeled as categorical variable but was insignificant (M [SD] = 23.6 [8.9], range 14–39) | BSI-18 (anxiety, depression), GSI (distress) | No significant differences in baseline DDA and change over time were observed as a function of age at diagnosis. A statistically significant decline in distress over 1 year (β [SE] = −0.89 [0.44], p = 0.42) was attenuated by the increase in distress from 6 to 12 months post-diagnosis (6-month M [SD] = 53.66 [0.75] vs. 12-month M [SD] = 55.11 [0.83]). No statistically significant decline in depression and anxiety over was observed due to similar increases from 6 to 12 months post-diagnosis, suggesting two distinct peaks of DDA corresponding to diagnosis and transition to off-treatment survivorship. |
| Linden W, Vodermaier A, MacKenzie R, Greig D | 2012 | Canada | Cross-sectional | 10,153 patients, mixed sex and diagnosis | Age groups defined a priori: 19–49, 50–69, 70+. Age was used a categorical variable in prevalence estimates and odds ratios. No regression modeling (M [SD] = 58.9 [14.6]) | PSSCAN (anxiety, depression) | Across tumor groups, older age (50–69, 70+) was associated with less anxiety (r = −0.15) and depression (r = −0.12), but only weak age associations with distress were found, never explaining >2.5% of the variance. Prevalence of anxiety and depression was 25.7% and 17.7% for the youngest, 18.9% and 12.6% for the middle, and 11.8% and 8.2% for the oldest age group (p < 0.001 with 70+ as reference group). Various age-related differences emerged within tumor groups. |
| Loquai C, Scheurich V, Syring N, et al. | 2013 | Germany | Cross-sectional | 520 patients, stage I–IV mixed sex melanoma | Age modeled as continuous variable (M [SD] = 58.5 [14.0], range 18–89) | DT (distress) | Distress scores decreased with increasing age (OR = 0.97 [95% CI 0.96–0.98] per year). There was no significant association of distress with demographic data apart from younger age and employment state. |
| Luutonen S, Vahlberg T, Eloranta S, Hyvari H, Salminen E | 2011 | Finland | Cross-sectional | 297 patients, stage I–II breast cancer | Age groups defined a priori, approximating menopausal status: <53, ≥53. Age modeled as categorical variable (M [SD] = 57.8 [9.9], range 18–89) | BDI (depression), DT (distress) | Younger patients (<53) had significantly higher DT and BDI scores than older patients did (p = 0.003, p = 0.047, respectively). Younger age group was associated with perceived inadequacy of psychosocial support (44.9% of patients <53 years vs. 17.7% of patients ≥53 years, p < 0.001) |
| Mehnert A, Koch U | 2008 | Germany | Cross-sectional | 1083 survivors, stage I–IV breast cancer | Age groups defined a priori: ≤50, 51–65, 66+. Age modeled as a continuous variable and used as a categorical variable in comparative gen. pop. significance testing (M [SD] = 68.1 [9.8], range 31–81) | HADS (Anxiety, Depression) | Older patients (66+) were found to have less anxiety (p = 0.004, η2 = 0.01) but higher levels of depression (p = 0.004, η2 = 0.01) than younger patients (≤50). In multivariate regression, younger age was a significant predictor of psychological comorbidity (either anxiety or depression; β [SE] = −0.02 [0.01], OR = 0.98 [95% CI 0.96–1.00], p ≤ 0.005) |
| Osborne RH, Elsworth GR, Hopper JL | 2003 | Australia | Case control | 731 patients, stage I–IV breast cancer | Age groups defined a priori: 23–34, 35–39, 40–44, 45–49, 50–54, 55–60. Age used as a categorical variable in prevalence estimates, crude odds ratios, and continuous variable in modeling (M [SD] = 43.5 [8.2], range 23–60) | HADS (anxiety, depression) | Risk of a depression score of ≥8 was not associated with age. No overall trend of decreasing anxiety with age was found when age was treated as a categorical variable or as a continuous variable. However, in the multivariate analysis, younger age was independently and significantly associated with increased risk of an anxiety score of ≥8 (slope, effect size, and p-value not reported). |
| Politi M, Enright T, Weihs K | 2007 | United States | Cross-sectional | 91 patients, stage I–III breast cancer | No age grouped defined. Age modeled as a continuous variable (M [SD] = 51 [10.3], range 34–80) | POMS-TMDS (distress) | Regression analysis found age to be significantly and independently associated with distress (β = −0.30, p < 0.01), with age and emotional acceptance explaining 29% of the variance (F = 16.43, p < 0.0001). Younger women reported higher levels of distress than older women did. |
| Prieto JM, Blanch J, Atala J, et al. | 2006 | Spain | Longitudinal | 220 patients, mixed sex hematological cancer | No age grouped defined. Age modeled as a continuous variable (M = 38, range 16–65) | SCID with DSM-IV (pooled depression/anxiety disorder variable) | In the multivariate baseline model predicting post-admission psychiatric disorder (depression/anxiety), younger age (OR = 0.97 [95% CI 0.94–1.00], p = 0.049) emerged as a weak independent risk factor. However, in the full model, younger age only demonstrated a close to significant association (p = 0.056). |
| Salvo N, Zeng L, Zhang L, et al. | 2012 | Canada | Retrospective cohort | 1439 stage IV patients, mixed sex and diagnosis | No age grouped defined. Age modeled as a continuous variable (median = 69, range 21–95) | ESAS (anxiety, depression) | Univariate regression of anxiety scores included younger age (OR = 0.99 [95% CI 0.98–0.99], p = 0.0002) as a small but independent and statistically significant demographic predictor, which retained its significance in the multivariate analysis (OR = 0.987 [95% CI 0.98–0.99], p = 0.003) No association between age and depression was found. |
| Senf B, Brandt H, Dignass A, Kleinschmidt R, Kaiser J | 2010 | Germany | Cross-sectional | 478 patients, mixed sex and diagnosis | No age grouped defined. Age modeled as a continuous variable (median = 63, range 18–95) | PO-Bado SF/BC (interviewer-rated distress), QSC-R23 (self-rated distress) | Age was the only sociodemographic variable that differentiated by distress in this sample (distressed age M [SD] = 61.3 [12.3], non-distressed age M [SD] = 64.2 [12.3], t[458] = 2.52, p = 0.012). However, in multivariate regression analysis, age was no longer a significant predictor of distress level (β [SE] = −0.02 [0.02], p = 0.23). |
| Sheppard VB, Harper FWK, Davis K, Hirpa F, Makambi K | 2014 | United States | Cross-sectional | 82 patients, stage I–III breast cancer | Age group defined post hoc: ≤50, >50. Age modeled as a continuous variable (M [SD] = 53.7 [11.1]) | HADS (anxiety, depression) | ≤50 age group compared with >50 group had higher depression levels that suggest borderline or caseness of psychological morbidity (32.3% vs. 13.7%; p = 0.045). However, difference in anxiety levels for the two groups was not significant (35.5% vs. 19.6%; p = 0.110). In the final multivariate model, age was not significantly associated with anxiety (β = −0.174, p = 0.097) but was significantly associated with depression (β = −0.217, p = 0.033). |
| Strong V, Waters R, Hibberd C, et al. | 2007 | United Kingdom | Cross-sectional | 3071 patients, mixed sex and diagnosis | Age groups defined a priori: <65, ≥65. Age modeled as a continuous variable (median = 62, range 18.2–93.1) | HADS (anxiety, depression, global distress) | In multivariate analysis, ≥65 was a significant independent predictor of global distress (OR = 0.71 [95% CI 0.59–0.85], p = 0.0002), with younger age being associated with higher odds of clinical distress. The associations with clinically significant anxiety and depression were similar to those with clinically significant emotional distress (depression or anxiety). |
| Vodermaier A, Linden W, MacKenzie R, Greig D, Marshall C | 2011 | Canada | Cross-sectional | 3850 patients, mixed sex and diagnosis | No age grouped defined. Age modeled as a continuous variable (M = 60.7) | PSSCAN (anxiety, depression) | In the multivariate model for the breast and GI tumor group, age was a significant independent predictor of both anxiety (breast, β = −0.063, p < 0.001 and GI β = −0.042, p < 0.001) and depression (breast β = −0.043, p < 0.001 and GI β = −0.030, p < 0.001). (Prostate and lung excluded from scoping review.) |
| Wenzel LB, Fairclough DL, Brady MJ, et al. | 2000 | United States | Cross-sectional | 304 patients, stage I–III breast cancer | Age groups defined a priori, approximating menopausal status: ≤ 50,> 50. Age analyzed as both a categorical and continuous variable, but only categorical reported (≤50, n = 161,> 50, n = 143) | CES-D (depression) | After controlling for treatment differences, 32% (SE = 0.037) of ≤50 patients experience depressive symptoms in the clinical range compared with 20% (SE = 0.040) of patients aged >50 (p = 0.041) |
| Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S | 2001 | United States | Cross-sectional | 4496 patients, mixed sex and diagnosis | Age groups defined a priori: <20, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, ≥80. Age modeled as a categorical and continuous variable (median = 57, range 19–95) | BSI-18 GSI (distress) | As a categorical variable, no significant relationship between age and distress was found. When analyzed as continuous variables, significant inverse relationships between age and distress were detected (r = −0.06, p < 0.05), but the low correlation indicated weak relationships. It was observed that the younger age groups (<30) possessed a higher level of global distress, which leveled off from age 30 to 60, and then began to decline again for 60+ groups. |
Table 4.
Summary Table of Included Studies
| Paper characteristics | n (%) | Paper characteristics | n (%) |
|---|---|---|---|
| Study design | Country | ||
| Cross-sectional | 19 (55.9%) | United States | 13 (39.3%) |
| Longitudinal | 10 (28.6) | Canada | 5 (14.7%) |
| Prospective/retrospective cohort | 3 (8.8%) | United Kingdom | 5 (14.7%) |
| Systematic review | 1 (2.9%) | Australia | 2 (5.9%) |
| Case control | 1 | Serbia | 1 (2.9%) |
| Spain | 1 | ||
| Journal | Israel | 1 | |
| Psycho-oncology | 5 (14.7%) | Norway | 1 |
| Journal of Clinical Oncology | 4 (11.8%) | Pakistan | 1 |
| Supportive Care in Cancer | 3 (8.8%) | Germany | 3 |
| British Journal of Cancer | 3 | Finland | 1 |
| Journal of Psychosocial Oncology | 2 (5.9%) | ||
| European Journal of Cancer | 2 | Tumor groups sampled | |
| British Medical Journal | 1 (2.9%) | Breast | 15 (41.1%) |
| Journal of Geriatric Oncology | 1 | Mixed diagnoses | 10 (28.6%) |
| Journal of Consulting and Clinical Psychology | 1 | Ovarian | 3 (8.8%) |
| Gynecologic Oncology | 1 | Colorectal | 3 |
| Health Psychology | 1 | Testicular | 1 (2.9%) |
| British Journal of Health Psychology | 1 | Endometrial | 1 |
| The Breast | 1 | Melanoma | 1 |
| BMC Cancer | 1 | Hematological | 1 |
| Journal of Hospice & Palliative Nursing | 1 | ||
| Journal of Affective Disorders | 1 | Year published | |
| PLOS One | 1 | 1999 | 1 (2.9%) |
| Journal of Psychosomatic Research | 1 | 2000–2004 | 5 (17.6%) |
| Clinical Oncology | 1 | 2005–2009 | 9 (26.5%) |
| Cancer | 1 | 2010–2014 | 19 (55.9%) |
| Breast Cancer Research and Treatment | 1 | ||
| Radiotherapy and Oncology | 1 | Time since diagnosis* | |
| On-treatment (patient) | 25 (73.5%) | ||
| Treatment of age in analysis | Post-treatment (survivor) | 13 (37.1%) | |
| Categorical | 15 (44.1%) | ||
| Continuous | 21 (61.8%) | ||
Does not sum to 34, as some longitudinal studies followed people from the time of treatment up to 5 years.
Only one paper focused specifically on the AYA (15–39 years) demographic.14 However, all of the studies included in this review had AYA patients in their samples, as shown by the descriptive statistics (age ranges, see Table 3). Table 5 presents an overview of the age range and number of the AYA or younger age participants in the studies that reported this information. In these 15 studies alone, there were approximately 7000 AYAs included in the samples. The exact number of AYAs, using the “15–39” definition, is impossible to know without direct access to individual participant data.
Table 5.
Size of AYA or Younger Age Sample in Studies Where Reported
| Author | Age definition | n |
|---|---|---|
| Avis NE, Levine B, Naughton MJ, Case LD, Naftalis E, Van Zee KJ | 25–44 | 132 |
| Bardwell WA, Natarajan L, Dimsdale JE, et al. | <50 | 958 |
| Dunn J, Ng SK, Holland J, et al. | 20–49 | 144 |
| Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE | 25–39 | 135 |
| Hopwood P, Sumo G, Mills J, Haviland J, Bliss JM, Group STM | 20–39 | 127 |
| Jadoon NA, Munir W, Shahzad MA, Choudhry ZS | ≤40 | 78 |
| Jones JM, Cheng T, Jackman M, Rodin G, Walton T, Catton P | 18–49 | 151 |
| Kornblith AB, Powell M, Regan MM, al. | ≤55 | 111 |
| Kwak M, Zebrack BJ, Meeske KA, et al. | 15–39 | 215 |
| Linden W, Vodermaier A, MacKenzie R, Greig D | 19–49 | 2523 |
| Luutonen S, Vahlberg T, Eloranta S, Hyvari H, Salminen E | <53 | 77 |
| Osborne RH, Elsworth GR, Hopper JL | 23–39 | 305 |
| Strong V, Waters R, Hibberd C, et. al. | <65 | 1793 |
| Wenzel LB, Fairclough DL, Brady MJ, et al. | ≤50 | 161 |
| Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S | <20 | 14 |
| 20–29 | 167 | |
| 30–39 | 525 | |
| Total | 7616 | |
Most studies reported prevalence and correlations using age as a categorical variable, but in predictive modeling, most included age as a continuous variable (n = 21, 61.8%), with a smaller number including it as a categorical variable (n = 13, 38.2%) and two studies doing both.15,16 In all papers selected, age was just one of many potential predictors or correlations explored, with just over one third of the studies specifically looking for an a priori age effect (n = 12, 35.3%). Every paper reviewed included a wide range of covariates as potential effect measure mediators or moderators of the cancer/DDA relationship. Most studies assessed more than one DDA construct (n = 27, 79.4%), but only five studies used a single tool with subscales (HADS, BSI) to assess both clinical depression and anxiety, as well as a combined score assessing global distress.14,17–20 Two papers analyzed DDA scores as both continuous and categorical variables using clinical cutoffs to define caseness and overall scores to indicate DDA symptomatology.14,18,19
An overview of the age-specific keywords and Medical Subject Headings (MeSH) used by articles identified during database searches is provided in Table 6. The most common age-specific keywords and MeSH used were adult(s) (n = 13), middle aged (n = 13), and aged (n = 10). The most common AYA-specific keywords and MeSH used were young adult (n = 6), adolescent (n = 5), young adulthood (n = 3), and thirties (20–39 years, n = 3). As suggested by Levac et al.,12 the discussion of these results is organized thematically, using the research questions to provide a framework for applying meaning to the results of this scoping review.
Table 6.
Age-Specific Keywords, Limiters, and MeSH Used by Selected Articles
| Adult(s) | 13 | Thirties (30–39 years) | 3 |
| Middle aged | 13 | Middle age (40–64 years) | 3 |
| Aged | 10 | Age | 3 |
| Age factors | 6 | Aged (65 years and older) | 2 |
| Young adult | 6 | Aging | 1 |
| Aged, 80 and over | 6 | Older | 1 |
| Adolescent | 5 | Age differences | 1 |
| Adulthood (18 years and older) | 3 | Age-related differences | 1 |
| Young adulthood (18–29 years) | 3 |
Are younger age or AYA risk factors for clinical DDA in cancer patients?
Age is consistently cited as a potential risk factor for both clinical and subclinical levels of DDA, and this association has been thoroughly explored over the past 15 years. However, although researchers found younger age to be a more important risk factor of DDA than many other independent variables, the overall findings are difficult to interpret. A systematic review of DDA in ovarian cancer patients21 found strong evidence for younger age as a predictor of elevated anxiety and depression (five good-quality studies and one average study vs. one average study and one good-quality study finding no association). In this review, all 34 studies found a significant association between at least one DDA construct and the younger age group relative to the older age groups at some point along the cancer trajectory. Of the cross-sectional studies, 14 found younger age to be significantly associated with one or all of the DDA constructs in patients,15,16,20,22–32 while five studies found similar associations in cancer survivors.17,33–36
For younger age associations with change over time in DDA, there are mixed results. Of the longitudinal studies (n = 10), one found a significant age–time interaction, with clinically anxious younger people improving faster than older groups,37 while one study did not find a significant association between younger age and change in clinical depression over time.38 Other longitudinal studies with wide age ranges did not explicitly explore or report the DDA–time–younger age interaction. However, one high-quality study, using growth modeling to characterize change over time in DDA, found that while younger age was consistently associated with trajectories above clinical levels for all three constructs of DDA, young adults were also significantly associated with DDA trajectories that started very high, decreased between 1 and 3 years, and then elevated significantly again between 3 and 5 years.18 Similarly, one paper looking specifically at young adults found that global distress was very high at diagnosis, decreased at 6 months, and then increased significantly again near the end of treatment.14 The potential for a curvilinear trajectory of DDA in the AYA demographic could pose some methodological challenges when measuring change over time using traditional regression models, and this will need to be explored further in future research.
In the 34 studies reviewed, age is only occasionally associated with increased levels of clinical depression, while it is consistently associated with higher levels of clinical anxiety and global distress. This apparent difference could be explained by Trait–State Theory, with an AYA's mood state (global distress, anxiety) being more reactive to a diagnosis of cancer than that in older groups, while people with underlying depressive tendencies are similarly impacted by a cancer diagnosis, regardless of age.39 This has implications for future psychosocial intervention development for AYAs; an intervention targeted to reduce global distress and anxiety may be more clinically effective and produce greater effect sizes.
A limitation of this review is that 15/34 studies were of breast cancer, so the generalizability of younger age associations with DDA could be limited. However, the studies of other tumor groups all found similar results, increasing the likelihood that younger age is a risk factor, regardless of type of diagnosis and treatment. Additionally, the younger age association seems to be generalizable across cultures (studies from 11 countries were included), race/ethnicity,31 and minority groups.33 Specifically, one study of African American women with breast cancer found strong correlations between younger age and both anxiety and depression, similar to that observed in Caucasian breast cancer patients.31 Another study that compared lesbian and heterosexual women found younger age was associated with increased levels of DDA in both groups, while membership in the lesbian minority group was not.33 Overall, the results of this scoping review provide consistent evidence that younger age is a potential risk factor for DDA, regardless of tumor group, race/ethnicity, culture, or minority group. This has important implications for psycho-oncology departments that are structured using traditional biomedical organization by tumor group and not by patient characteristics. If younger age is a specific and well-recognized risk factor for DDA, an AYA psychosocial specialist should be considered at all major tertiary cancer centers.
Do research databases and studies define younger age or AYAs with a concrete age range, and if so, what is the most common definition?
Database searches to identify studies that are either AYA-specific or relevant to this population present many methodological challenges. First, the embedded age limiters and MeSH that could be used to narrow searches are entirely different in each database, with none of them fitting the 15–39 AYA age range put forward by the NCI. As illustrated in Table 6, databases often categorize age by 10–12-year groups, with “young adulthood” representing 18–29 and “thirties” representing 30–39. There is also a clear distinction between adult and adolescent at 18 years of age, with the adolescent group including those aged 13–17. Consequently, it is challenging to include the lowest end of the AYA age range (16–18) in searches while simultaneously excluding the ages outside of this range (14–15). Second, the keywords used to encompass this age group are extremely varied (17 different age-related MeSH and keywords used to describe studies relevant to this demographic), non-specific in nature, and arbitrarily applied, as discovered by comparing the keywords of similar studies. Of the 34 studies in this review, only six used “young adult” and five used “adolescent” as keywords, despite their specific relevance to this population. While it is recognized that the AYA demographic or younger age was not a specific focus of many of these studies, more consistent keyword, age limiter, and MeSH conventions based on internationally recognized and theory-driven definitions of age groups would be beneficial.
The studies reviewed very seldom reported using theory to guide their age categorization. Only four studies gave any rationale for their age-group definitions, with an approximation of pre/post-menopausal status cited twice15,28 and developmental life stage cited twice.14,38 Other studies defined their age categories post hoc as “younger” and “older” using a median split.37,40 Two papers defined their “younger” group as <60 years25 and <65 years.20 These crude, non-theoretical methods of age-group categorization are problematic for a couple of reasons. First, they could be masking the actual prevalence estimates and effect sizes (when modeling age as a categorical variable) in the AYA group by diluting the high DDA prevalence in the youngest of the “younger” group (<39) with a moderate to low DDA prevalence in the oldest of the “younger” group (≥40). Second, the lack of specificity in these widely defined age groups limits the clinical implications of any age-related finding. For example, to an adult oncologist, whose average patient is 60 years old,26 “younger” would mean a patient in their 40s and 50s, as they are the most prevalent younger patients younger than the mean age of 60. These semantic issues are important when conveying age-specific DDA findings to clinicians.
Additionally, widely ranging age-group definitions make it very difficult to pool aggregate data, and therefore stymie AYA-specific meta-analyses. In a field of research consistently limited by small sample sizes,41 difficult recruitment,42 and little funding,43 this is a significant loss in research potential. This potential is demonstrated by the finding that only 15 studies in this review contained data for approximately 7000 AYAs—a sample size that would be extremely difficult to recruit in any single study of this population. While it is possible to do meta-analyses of individual-level patient data, this method requires access to raw data, which can be difficult and time-consuming to obtain.44 For this reason, widespread adoption of the NCI AYA definition or some consideration of developmental theories such as the Socioemotional-Selectivity Theory,45 Life-Course Theory,46 or other young adulthood developmental theories looking at chronological and subjective age47,48 would be useful in defining standard age groups to be used in meta-analytical psycho-oncology research.
In light of these findings, defining the most common definition, as stated in the research question, is very difficult. Most of the heterogeneity was expressed in the upper-age limits, with six studies defining their upper AYA age limit at 39 years old, seven studies defining their upper age limit at 49 years old, and seven papers setting their upper age limit between 50 and 60 years old (including studies using a median split to define age groups). Very few studies defined a lower limit for their AYA age group, with six studies setting the limit between 20 and 25 years old and four studies setting the limit between 15 and 19 years old. Using these very basic prevalence groups, the most common AYA age range definition was 20–39 years, or using descriptive language, patients in their “20s or 30s,” which is somewhat congruent with what was expressed in the age limiters, keywords, and MeSH used as descriptors. In general, researchers and databases define AYAs as patients in their 20s and 30s, excluding the younger range of the 15–39 definition. Pragmatically, this means that future reviews or meta-analyses of this demographic could systematically exclude the lower end of the AYA age range, and this likelihood will limit information relevant for researchers to understand AYAs' needs fully.
If it exists, what is the magnitude of the increased risk of clinical DDA in the younger age or AYA age group?
Age-specific findings as they relate to DDA are presented in Table 3. There is a wide range of values associated with AYA or younger age, depending on what is being reported (e.g., prevalence, correlations, odds/hazard ratios, or slopes and p-values). Prevalence estimates based on caseness in the AYA or younger age group depended heavily on the DDA construct being measured, but ranged from 25%22 to 32%15 for clinical depression and from 35.5%31 to 41.6%49 for clinical anxiety. In almost all age-group comparisons of DDA prevalence, AYA was statistically greater (see Table 3). Still, despite these large differences in prevalence estimates, some studies found only slightly higher odds of having DDA in the AYA group. Salvo et al.,54 Prieto et al.,55 and Mehnert and Koch36 found younger age to be a weak independent predictor of depression or anxiety (OR = 0.987 [95% CI 0.98–0.99], OR = 0.97 [95% CI 0.94–1.00], and OR = 0.98 [95% CI 0.96–1.00], respectively). The strongest longitudinal study found a weak hazard ratio of just 0.96 [95% CI 0.93–0.99].50 However, the dilution of effect sizes, which were mentioned earlier, must be taken into account when interpreting these results. These studies often used very wide, non-theoretically defined age groups, and this may be contributing the smaller than expected effect sizes. One study corroborating this idea is Jadoon et al.23 that used >40 to define its younger and older age groups and found much greater odds of the AYA group having clinical anxiety or depression (OR = 0.46 [95% CI 0.23–0.91]). Another study34 that did not analyze age as a categorical variable but studied a much younger sample overall (Mage [SD] = 35.5 [9.5] years, range 19–66 years) found a large effect size (OR = 3.2 [95% CI 1.3–8.1]) and indicated that younger age was the only statistically significant risk factor in the development of depression in their sample. Despite the heterogeneity in the magnitude of the association, it is clear that AYAs have elevated DDA levels above both the general population and older cancer patients/survivors, and therefore age-appropriate psychosocial care should be considered a priority.
What are the potential confounders, mediators, or moderators of increased DDA as it relates to younger age or AYAs?
Of the eight studies that did multivariate modeling, four found younger age to be a significant independent predictor of at least one DDA construct.24,40,51,52 Alternatively, five studies found that while age was a significant risk factor for at least one DDA construct in univariate or preliminary models, in the full multivariate analysis, these age effects were no longer significant.17,30,40,53,54 These results suggest that while younger age is definitely an important predictor of increased DDA in both patients and survivors, this relationship could be confounded by other age-specific factors. However, these results could also be compromised by researchers inserting many collinear covariates into an equation not powered to be robust to those analyses. Some of the potential confounding variables that were identified in this review include resilience,17 illness intrusiveness,38 and other psychosocial variables such as social support, strain, and optimism.53 As noted by Avis et al.,38 despite the fact that age was not independently associated with depression in their study, in the real world, younger women are still more likely to present with depression because they are more likely to present with factors that are independently associated with depression. While the exact nature of the relationship between younger age and DDA does need to be clarified so that high-risk individuals can be identified and targeted for intervention, the consistently observed, overall association between younger age and elevated DDA is relevant for the organization and delivery of psychosocial care and the general targeting of younger age groups for psychosocial intervention.
Conclusions
This scoping review only touched the surface of a very complex and multifaceted field. It is extremely difficult to categorize and summarize adequately such a wide-ranging body of literature, even from the narrow perspective of younger age, DDA, and cancer. The wide scope of this review and the lack of in-depth analysis do not allow specific findings to be clarified for the separate constructs of DDA, which are recognized as distinct, despite occurring along the same continuum. Additionally, the methodology of this study does not allow the potential of detection bias in the DDA measurement tools used to be assessed. Finally, the lack of a formal study quality assessment and the time-limited literature search introduces a risk of bias; specifically, there is danger that the mere existence of certain studies, rather than their intrinsic quality, could have biased the conclusions.2 Nevertheless, the purpose of a scoping review is to assess the extent, range, and nature of a field, with the end goal of specifying a future viable review,12 and this potential bias and incomplete analysis is an expected limitation.
Younger age or AYA associations with DDA in cancer have been extensively explored in the literature. Many studies included in this review did not specifically explore the AYA demographic, but were relevant to this population because they included participants in this age range. Bringing together this wide-ranging body of research and distilling it into clinically meaningful policies and recommendations will be extremely difficult. Perhaps, due to the heterogeneity of age-range conventions, analysis techniques, covariates, and other indicators, the only way to do this effectively will be through the meta-analysis of individual patient data and not through traditional meta-analysis techniques using aggregate data. AYA psychosocial oncology research is very difficult for a number of reasons, and leveraging the AYA data collected in wider samples will be very important to the future of this field.
Despite the wide range of findings in the studies included in this review, one thing is clear: AYAs, however defined, are a distinct group within the cancer population with an elevated risk of DDA. These findings have not yet translated into clinical practice with AYA-specific psychosocial screening and support implemented routinely in tertiary cancer centers in North America. Luutonen et al.28 found in their sample that 44.9% of the younger group (≤53) reported inadequate psychosocial support compared with only 17.7% of the older group. This could mean that AYAs are more distressed, depressed, or anxious then older patients, that current programs and models of care are not sufficiently meeting the needs of this demographic, or both. The results of this review suggest that the time has come for targeted psychosocial interventions for AYAs to become the standard of care in oncology settings.
Acknowledgments
We acknowledge scholarship support from the Psychosocial Oncology Research Training Fellowship to Michael Lang and salary support for Janine Giese-Davis from Alberta Cancer Foundation and The Enbridge Chair in Psychosocial Oncology held by Linda E. Carlson. We also acknowledge the manuscript editing support of Kate Collie, PhD.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Watson M, Dunn J, Holland JC. Review of the history and development in the field of psychosocial oncology. Int Rev Psychiatry. 2014;26(1):128–35 [DOI] [PubMed] [Google Scholar]
- 2.Adolescent and Young Adult Oncology Progress Review Group. Closing the gap: research and cancer care imperatives for adolescents and young adults with cancer (NIH Publication No. 06-6067). Bethesda, MD: Department of Health and Human Services, National Institutes of Health, National Cancer Institute, and the LIVESTRONG Young Adult Alliance; August 2006. Accessed April30, 2014 from: http://planning.cancer.gov/library/AYAO_PRG_Report_2006_FINAL.pdf [Google Scholar]
- 3.Lawrence L. Survival lags for adolescents, young adults with cancer. Hem/Onc Today. 2011;12(21) [Google Scholar]
- 4.Bleyer A. Latest estimates of survival rates of the 24 most common cancers in adolescent and young adult Americans. J Adolesc Young Adult Oncol. 2011;1(1):37–42 [DOI] [PubMed] [Google Scholar]
- 5.Dang-Tan T, Trottier H, Mery LS, et al. Delays in diagnosis and treatment among children and adolescents with cancer in Canada. Pediatr Blood Cancer. 2008;51(4):468–74 [DOI] [PubMed] [Google Scholar]
- 6.Zebrack B, Isaacson S. Psychosocial care of adolescent and young adult patients with cancer and survivors. J Clin Oncol. 2012;30(11):1221–6 [DOI] [PubMed] [Google Scholar]
- 7.Bleyer A. The adolescent and young adult gap in cancer care and outcome. Curr Probl Pediatr Adolesc Health Care. 2005;35(5):182–217 [DOI] [PubMed] [Google Scholar]
- 8.Bleyer A, Barr R, Hayes-Lattin B, et al. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8(4):288–98 [DOI] [PubMed] [Google Scholar]
- 9.Ram R, Wolach O, Vidal L, et al. Adolescents and young adults with acute lymphoblastic leukemia have a better outcome when treated with pediatric-inspired regimens: systematic review and meta-analysis. Am J Hematol. 2012;87(5):472–8 [DOI] [PubMed] [Google Scholar]
- 10.Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer. Cancer. 2006;107(7 Suppl):1645–55 [DOI] [PubMed] [Google Scholar]
- 11.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32 [Google Scholar]
- 12.Levac D, Colquhoun H, O'Brien K. Scoping studies: advancing the methodology. Implementation Sci. 2010;5(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26(2):91–108 [DOI] [PubMed] [Google Scholar]
- 14.Kwak M, Zebrack BJ, Meeske KA, et al. Trajectories of psychological distress in adolescent and young adult patients with cancer: a 1-year longitudinal study. J Clin Oncol. 2013;31(17):2160–6 [DOI] [PubMed] [Google Scholar]
- 15.Wenzel LB, Fairclough DL, Brady MJ, et al. Age-related differences in the quality of life of breast carcinoma patients after treatment. Cancer. 1999;86(9):1768–74 [PubMed] [Google Scholar]
- 16.Zabora J, Brintzenhof-Szoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28 [DOI] [PubMed] [Google Scholar]
- 17.Cohen M, Baziliansky S, Beny A. The association of resilience and age in individuals with colorectal cancer: an exploratory cross-sectional study. J Geriatr Oncol. 2014;5(1):33–9 [DOI] [PubMed] [Google Scholar]
- 18.Dunn J, Ng SK, Holland J, et al. Trajectories of psychological distress after colorectal cancer. Psychooncology. 2013;22(8):1759–65 [DOI] [PubMed] [Google Scholar]
- 19.Kornblith AB, Powell M, Regan MM, et al. Long-term psychosocial adjustment of older vs younger survivors of breast and endometrial cancer. Psychooncology. 2007;16(10):895–903 [DOI] [PubMed] [Google Scholar]
- 20.Strong V, Waters R, Hibberd C, et al. Emotional distress in cancer patients: the Edinburgh Cancer Centre symptom study. Br J Cancer. 2007;96(6):868–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arden-Close E, Gidron Y, Moss-Morris R. Psychological distress and its correlates in ovarian cancer: a systematic review. Psychooncology. 2008;17(11):1061–72 [DOI] [PubMed] [Google Scholar]
- 22.Bodurka-Bevers D, Basen-Engquist K, Carmack CL, et al. Depression, anxiety, and quality of life in patients with epithelial ovarian cancer. Gynecol Oncol. 2000;78(3):302–8 [DOI] [PubMed] [Google Scholar]
- 23.Jadoon NA, Munir W, Shahzad MA, Choudhry ZS. Assessment of depression and anxiety in adult cancer outpatients: a cross-sectional study. BMC Cancer. 2010;10:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones JM, Cheng T, Jackman M, et al. Self-efficacy, perceived preparedness, and psychological distress in women completing primary treatment for breast cancer. J Psychosoc Oncol. 2010;28(3):269–90 [DOI] [PubMed] [Google Scholar]
- 25.Krok JL, Baker TA, McMillan SC. Age differences in the presence of pain and psychological distress in younger and older cancer patients. Hosp Palliat Nurs. 2013;15(2):107–13 [Google Scholar]
- 26.Linden W, Vodermaier A, MacKenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141(2–3):343–51 [DOI] [PubMed] [Google Scholar]
- 27.Loquai C, Scheurich V, Syring N, et al. Screening for distress in routine oncological care-a survey in 520 melanoma patients. PLoS One. 2013;8(7):e66800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luutonen S, Vahlberg T, Eloranta S, et al. Breast cancer patients receiving postoperative radiotherapy: distress, depressive symptoms and unmet needs of psychosocial support. Radiother Oncol. 2011;100(2):299–303 [DOI] [PubMed] [Google Scholar]
- 29.Politi M, Enright T, Weihs K. The effects of age and emotional acceptance on distress among breast cancer patients. Support Care Cancer. 2007;15(1):73–9 [DOI] [PubMed] [Google Scholar]
- 30.Senf B, Brandt H, Dignass A, et al. Psychosocial distress in acute cancer patients assessed with an expert rating scale. Support Care Cancer. 2010;18(8):957–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheppard VB, Harper FWK, Davis K, et al. The importance of contextual factors and age in association with anxiety and depression in black breast cancer patients. Psychooncology. 2014;23(2):143–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vodermaier A, Linden W, MacKenzie R, et al. Disease stage predicts post-diagnosis anxiety and depression only in some types of cancer. Br J Cancer. 2011;105(12):1814–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boehmer U, Glickman M, Winter M. Anxiety and depression in breast cancer survivors of different sexual orientations. J Consult Clin Psychol. 2012;80(3):382–95 [DOI] [PubMed] [Google Scholar]
- 34.Bumbasirevic U, Bojanic N, Pekmezovic T, et al. Health-related quality of life, depression, and sexual function in testicular cancer survivors in a developing country: a Serbian experience. Support Care Cancer. 2013;21(3):757–63 [DOI] [PubMed] [Google Scholar]
- 35.Ganz PA, Greendale GA, Petersen L, et al. Breast cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol. 2003;21(22):4184–93 [DOI] [PubMed] [Google Scholar]
- 36.Mehnert A, Koch U. Psychological comorbidity and health-related quality of life and its association with awareness, utilization, and need for psychosocial support in a cancer register-based sample of long-term breast cancer survivors. J Psychosom Res. 2008;64(4):383–91 [DOI] [PubMed] [Google Scholar]
- 37.Hart SL, Charles ST. Age-related patterns in negative affect and appraisals about colorectal cancer over time. Health Psychol. 2013;32(3):302–10 [DOI] [PubMed] [Google Scholar]
- 38.Avis NE, Levine B, Naughton MJ, et al. Age-related longitudinal changes in depressive symptoms following breast cancer diagnosis and treatment. Breast Cancer Res Treat. 2013;139(1):199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts BW, Walton KE, Viechtbauer W. Patterns of mean-level change in personality traits across the life course: a meta-analysis of longitudinal studies. Psychol Bull. 2006;132(1):1–25 [DOI] [PubMed] [Google Scholar]
- 40.Enns A, Waller A, Groff SL, et al. Risk factors for continuous distress over a 12-month period in newly diagnosed cancer outpatients. J Psychosoc Oncol. 2013;31(5):489–506 [DOI] [PubMed] [Google Scholar]
- 41.Barr RD. Adolescents, young adults, and cancer—the international challenge. Cancer. 2011;117(S10):2245–9 [DOI] [PubMed] [Google Scholar]
- 42.Hendricks-Ferguson VL, Cherven BO, Burns DS, et al. Recruitment strategies and rates of a multi-site behavioral intervention for adolescents and young adults with cancer. J Pediatr Health Care. 2013;27(6):434–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eaton G, Greenberg M, Joblin K. The forgotten generation. Report card on cancer in Canada, Cancer Advocacy Coalition of Canada; Februrary 2007 Accessed April30, 2014 from: www.canceradvocacy.ca/reportcard/2007/Report Card on Cancer in Canada 2007.pdf
- 44.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:cc221. [DOI] [PubMed] [Google Scholar]
- 45.Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: a theory of socioemotional selectivity. Am Psychol. 1999;54(3):165–81 [DOI] [PubMed] [Google Scholar]
- 46.Elder GH. The life course as developmental theory. Child Dev. 1998;69(1):1–12 [PubMed] [Google Scholar]
- 47.Arnett JJ. Emerging adulthood: a theory of development from the late teens through the twenties. Am Psychol. 2000;55(5):469–80 [PubMed] [Google Scholar]
- 48.Galambos NL, Turner PK, Tilton-Weaver LC. Chronological and subjective age in emerging adulthood: the crossover effect. J Adolesc Res. 2005;20(5):538–56 [Google Scholar]
- 49.Hopwood P, Sumo G, Mills J, et al. The course of anxiety and depression over 5 years of follow-up and risk factors in women with early breast cancer: results from the UK Standardisation of Radiotherapy Trials (START). Breast. 2010;19(2):84–91 [DOI] [PubMed] [Google Scholar]
- 50.Burgess C, Cornelius V, Love S, et al. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330(7493):702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costanzo ES, Lutgendorf SK, Mattes ML, et al. Adjusting to life after treatment: distress and quality of life following treatment for breast cancer. Br J Cancer. 2007;97(12):1625–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hipkins J, Whitworth M, Tarrier N, Jayson G. Social support, anxiety and depression after chemotherapy for ovarian cancer: a prospective study. Br J Health Psychol. 2004;9(4):569–81 [DOI] [PubMed] [Google Scholar]
- 53.Bardwell WA, Natarajan L, Dimsdale JE, et al. Objective cancer-related variables are not associated with depressive symptoms in women treated for early-stage breast cancer. J Clin Oncol. 2006;24(16):2420–7 [DOI] [PubMed] [Google Scholar]
- 54.Salvo N, Zeng L, Zeng M, et al. Frequency of reporting and predictive factors for anxiety and depression in patients with advanced cancer. J Clin Oncol. 2012;24(2):139–148 [DOI] [PubMed] [Google Scholar]
- 55.Prieto JM, Blanch J, Atala J, et al. Stem cell transplantation: risk factors for psychiatric morbidity. Eur J Cancer Care. 2006;42(4):514–20 [DOI] [PubMed] [Google Scholar]