Abstract
Low-intensity pulsed ultrasound (LIPUS) has demonstrated its positive effects on osteogenic differentiation of mesenchymal stem cells and the proliferation and differentiation of osteoblasts, negative effects on osteoclast growth, and promotion of angiogenesis, leading to improvement of the tissue perfusion. Heat-shock proteins (HSPs) are initially identified as molecules encouraged and expressed by heat stress or chemical stress to cells and involved in the balance between differentiation and apoptosis of osteoblasts. However, it remains unclear if the effect of LIPUS on osteoblast differentiation could involve HSP expression and contribution. In this study, mouse calvarial osteoblasts were exposed to LIPUS at a frequency of 3.0 MHz by 30 mW/cm2 for 15 min or to 42°C heat shock for 20 min at day 3 of cell culture and examined for osteogenesis with pursuing induction of HSP27, HSP70, and HSP90. LIPUS as well as heat shock initially upregulated HSP90 and phosphorylation of Smad1 and Smad5, encouraging cell viability and proliferation at 24 h, enhancing mineralized nodule formation stronger by LIPUS after 10 days. However, HSP27, associated with BMP2-stimulated p38 mitogen-activated protein kinase during osteoblast differentiation, was downregulated by both stimulations at this early time point. Notably, these two stimuli maintained Smad1 phosphorylation with mineralized nodule formation even under BMP2 signal blockage. Therefore, LIPUS might be a novel inducer of osteoblastic differentiation through a noncanonical signal pathway. In conclusion, LIPUS stimulation enhanced cell viability and proliferation as early as 24 h after treatment, and HSP90 was upregulated, leading to dense mineralization in the osteoblast cell culture after 10 days.
Introduction
Low-intensity pulsed ultrasound (LIPUS) is a clinically established physiotherapeutic technique, approved by the Food and Drug Administration, used to accelerate the healing of bone fractures and delayed union or nonunion of bone. Its effectiveness has been demonstrated in numerous in vivo studies1–7 and supported by in vitro examinations using cell culture systems.8–12 LIPUS stimulation is a noninvasive, feasible, and economical method, and it has emerged as a safer alternative to biophysical approaches, especially for patients with bone plates or pacemakers.
Numerous studies have demonstrated its positive effects, such as osteogenic differentiation of mesenchymal stem cells, the proliferation and differentiation of osteoblasts, negative regulation of osteoclast growth, and the promotion of angiogenesis, which lead to improvement in bone tissue perfusion. The mechanism by which LIPUS induces these responses is unclear; however, what is known is that mechanical stress, such as ultrasound stimulation, is translated into biochemical signals.
Heat-shock proteins (HSPs) were initially identified as molecules expressed in cells in response to heat stress or chemical stress.13–18 They are classified into six families according to their estimated molecular weights: HSP20, HSP40, HSP60, HSP70, HSP90, and HSP100. These HSPs play fundamental roles in many physiologic and pathophysiologic processes, such as degradation of unstable proteins, control of regulatory proteins, and import and folding of proteins.19,20 Some HSPs are constitutively active, while others are induced only after exposure to stimuli, such as the inducible HSP72.21 HSP stimulation and increases in HSP expression have a cytoprotective role within the cell. HSPs are also being investigated for their contribution to cell status in basic and clinical studies.22–24 Furthermore, HSP induction might effectively reduce cellular injury, as it was recently demonstrated that activated HSPs accelerated the recovery of damaged cells and fatigue.25,26 HSPs are also associated with bone metabolism. HSP27, a low-molecular-weight HSP, was reported to regulate the balance between the differentiation and apoptosis of osteoblasts.27,28 Various physiological stresses are able to induce HSP27 expression in MC3T3 cells, an osteoblast-like cell line, although HSP27 expression levels differ by cell type.29–31 However, the exact mechanism of HSP27 induction in osteoblasts remains unclear. HSP70 and HSP90, members of high-molecular-weight HSP families, act as molecular chaperones, and they are implicated in protein folding, oligomerization, and translocation.32 They are also involved in osteogenic signal transduction. These HSPs, as well as HSP27, are stimulated by heat stress. HSP27 is induced through the activation of p38 mitogen-activated protein (MAP) kinase. This is followed by phosphorylation of intracellular Smads, which are important proteins for BMP-initiated osteogenesis; this phosphorylation stimulates HSP27 during osteoblastic differentiation in osteoblast-like MC3T3 cells. Unlike HSP27, HSP70 and HSP90 are reported to interfere with glucocorticoid signal transduction by binding directly to the glucocorticoid receptor.33 Therefore, HSP70 and HSP90 may be regulated by different signaling pathways, independent of BMP signal transduction, in MC3T3 cells during osteogenic events like bone fracture healing.
There are several reports indicating a synergistic interaction between ultrasound and heat that leads to induction of Hsp72, a HSP70 family member. This synergy was reported in human promyelocytic leukemia HL-60 cells when exposed to ultrasonic waves at 10 MHz for 10 min. However, there have been no definitive studies on HSPs to link this synergy to the regulation of osteogenesis. In this study, calvarial cells (osteoblasts) isolated and cultured from mouse neonates were exposed to LIPUS in vitro and examined for osteogenesis and induction of Hsp27, 70, and 90. The aim of this study is to understand the synergistic relationship between LIPUS and HSPs during osteogenesis. This work also suggests that LIPUS may be an effective therapy for bone healing in conditions where osteogenesis is compromised, such as perturbations in BMP signaling.
Materials and Methods
The Institutional Animal Care and Use Committee of Tokyo Medical and Dental University approved the protocol design and procedures (approval number 0150222A).
Extraction of cells and cell culture
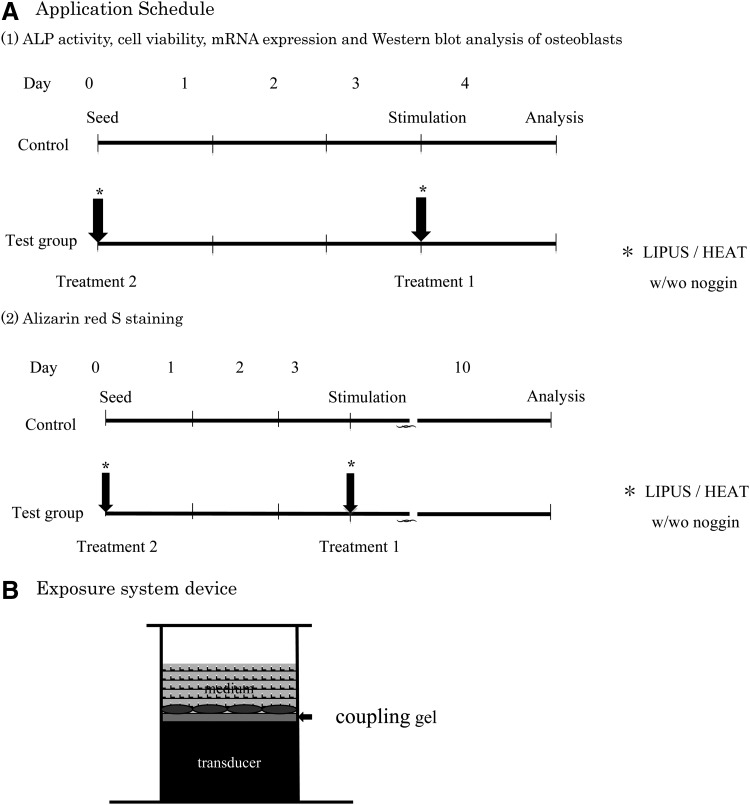
Osteoblasts were extracted from the calvaria by sequential collagenase digestion of 1-day-old neonatal mice. Briefly, the cells were cultured in an α-minimum essential medium (α-MEM; Invitrogen) containing 10% fetal bovine serum (FBS; Sigma-Aldrich) with 100 U/mL of penicillin and 100 U/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2:95% air. When semiconfluent, the cells were trypsinized and reseeded into 35-mm culture dishes at a concentration of 1 × 105 cells/cm2 in 1.2 mL of α-MEM containing 10% FBS. After cells were cultured for 72 h, they were exposed to two different stimuli: Group 1, LIPUS stimulation and Group 2, heat-shock treatment. The control group was cultured without LIPUS stimulation or heat-shock treatment. All groups, including controls, were cultured with supplemental Noggin (Sigma-Aldrich), a transient inhibitor of BMP signaling, at a concentration of 1.0 ng/mL, before stimulation. The experimental time schedule is shown in Figure 1.
FIG. 1.
Low-intensity pulsed ultrasound (LIPUS) application schedule (A) and LIPUS exposure system device (B).
LIPUS stimulation
LIPUS was applied to cultured osteoblasts for 15 min at room temperature, in a fresh medium without FBS, using an OSTEOTRON D2 sonicator (Ito Co., Ltd.) through gel on the bottom of the 35-mm culture dishes. Cells were exposed at a frequency of 3.0 MHz, and the temporal average intensity was 30 mW/cm2. The cells were placed back into the incubator for an additional 24 h before analysis.
Heat-shock stimulation
Osteoblasts were placed in a 42°C conditioning incubator for 20 min. The cells were then placed back into the original incubator for an additional 24 h before analysis.
Cell proliferation analysis
An osteoblast viability assay was performed using the PrestoBlue Cell Viability Reagent (Molecular Probes; Invitrogen) according to the manufacturer's protocol. The cells in suspension were seeded at 1 × 105 cells/well in a 96-well microtiter plate. After 24 h, the cells were washed and incubated with the PrestoBlue reagent for 10 min. The changes in cell viability were detected using absorbance spectroscopy at a wavelength of 570 nm. For further analysis of cell viability, another set of the cells was subjected for an apoptosis test by staining them at 24 h of the culture (LIVE and DEAD Cell Assay Kit ab115347; Abcam), by which live cells were detected with green fluorescence and dead with red through a Biozero BZ-8000 microscope (Keyence), and the numbers of live/dead cells were quantified.
Alkaline phosphatase (ALP) activity assay
Twenty-four hours after the LIPUS stimulation, the cells were assayed for the alkaline phosphatase (ALP) activity. The cells were washed twice with phosphate-buffered saline (PBS), sonicated with an OSTEOTRON D2 (Ito Physiotherapy and Rehabilitation Co., Ltd.), and stored at −80°C until the assay was performed. The ALP activity was measured as the release of p-nitrophenol from p-nitrophenylphosphate, pH 9.8, by optical density analysis at 405 nm using the SenoLyte pNPP Alkaline Phosphatase Assay Kit (AnaSpec).
ALP-positive cell staining
ALP-positive cells were stained using the ALP Stain kit (Wako). Cells were washed twice with PBS and fixed in 3.7% formalin for 10 min. Fixed cells were then rinsed with PBS twice and incubated in 1 mL staining solution at 37°C for 20 min to identify ALP-positive (blue) cells. Cells were then washed with PBS to stop the staining reaction. Digital images were captured using a Biozero BZ-8000 microscope (Keyence).
Alizarin red staining
To examine nodule formation, the cells were seeded in 35-mm dishes at a concentration of 1 × 105 cells/cm2 in an osteogenic medium supplemented with 10−8 M dexamethasone, 10 mM β-glycerophosphate (G-9891; Sigma-Aldrich), and 50 ng/mL ascorbic acid (013-12061; Wako Chemical) in 1.2 mL α-MEM containing 10% FBS. An early simulation protocol was also used where LIPUS and heat stress were administered simultaneously during cell seeding, when the cells were neither attached nor stable on the dishes. Mineralized nodules were stained after 10 days of culture. The staining solution was prepared by dissolving alizarin red S (1%) in 1:100 aluminum hydroxide in water, followed by filtration. Cells were washed twice with PBS and immersed in methanol for 10 min. After cells were rinsed in water, they were incubated with 500 μL of alizarin red S solution per well for 2 min until the mineralized nodules had stained red. The reaction was then terminated by washing the cells with water, to remove excessive staining precipitate and reagents. Digital images were captured using a light microscope (Biozero BZ-8000; Keyence). For quantification of the staining, nodules were destained using 10% cetylpyridinium chloride (CPC, C0732-100G; SIGMA-ALDRICH) in 10 mM sodium phosphate, pH 7.0, for 15 min at room temperature. Aliquots of extracts were diluted 10-fold in 10% CPC solution, and the concentration of alizarin red S was determined by measuring the absorbance at 562 nm on a multilabel counter (Wallac 1420 Arvo Sx).
Reverse transcription polymerase chain reaction analysis
Total RNA was isolated with the Trizol® reagent (Life Technologies, Inc.) and then reverse transcribed using the SuperScriptTM first-strand synthesis system for reverse transcription polymerase chain reaction (RT-PCR) (Life Technologies) according to the manufacturer's instructions. The osteoblast-related gene expression was determined using real-time quantitative RT-PCR. Polymerase chain reaction (PCR) amplification primers are listed in Table 1. SYBR Green-based real-time PCR analysis was carried out with the ABI Prism 7300 Sequence Detection System (Applied Biosystems). Gene expression levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) within the same sample using formula: target amount = 2−ΔΔCT and then this value was further divided with the values of the control group.
Table 1.
Primer Pairs Used for Real-Time PCR
| Gene | Forward and reverse primers | GenBank™ accession number | Fragment length (bp) |
|---|---|---|---|
| GAPDH | ACCCAGAAGACTGTGGATGG | NM_001289726 | 186 |
| CACATTGGGGGTAGGAACAC | |||
| HSP27 | CCCAGTGAATCCCCTGTCTA | NM_001164708.1 | 179 |
| CCCCCAGGTTTTGGTTTATT | |||
| HSP70 | TGCTGATCCAGGTGTACGAG | NM_010478.2 | 204 |
| CGTTGGTGATGGTGATCTTG | |||
| HSP90 | GGCATCGATGAAGATGAGGT | NM_008302.3 | 196 |
| ACATGAGCAGAGAGCCAGGT | |||
| Smad1 | TCTTTCTGAAGTGGGCTTTC | NM_008539.3 | 185 |
| CAGCCAGCATACAGTTTCAG | |||
| Smad5 | TGTGCTCCTTGTGCAGATAA | NM_008541.3 | 221 |
| TGGTGCTCTACGAGACCTTC | |||
| OSX | CCCACCTAACAGGAGGATTT | NM_130458.3 | 200 |
| CACTGGAATGGAGTGAAACC | |||
| Colla1 | TGCTGTTCTTGGGGGACTAC | NM_001001849.1 | 192 |
| GCCATAGAGGGGTGTTCTCA |
HSP, heat-shock protein; PCR, polymerase chain reaction.
Western blotting
Western blot analysis was used to investigate the correlation between expression of HSP27, HSP70, HSP90, Smad1, or Smad5 and the LIPUS stimulation (30 mW/cm2 for 15 min) or thermal stress (42°C for 20 min). For the Smads, cells were lysed by centrifugation and the total lysate was used for the analysis. The culture medium (1.2 mL) was collected for HSP analysis. Each sample was dialyzed against distilled water and lyophilized. Two aliquots per group, each of which was mixture from three culture wells, were dissolved in 20 μL running buffer, were denatured at 70°C for 10 min, fractionated on 4–12% NuPAGE Bis-Tris gels (Invitrogen), and transferred to PVDF membranes. Following incubation with polyclonal antibodies and then anti-rabbit IgG secondary antibodies, the protein complexes were detected with the Chromogenic Western Blot Immunodetection Kit (WesternBreeze; Invitrogen) according to the manufacturer's instructions. Intensities of the developed bands were calibrated by image analyzing software (ImageJ 1.42q; NIH) and expressed in ratios.
Statistical analysis
Statistical analysis was performed using the statistics software package 14.0 SPSS for Windows (SPSS, Inc.). Results are expressed as mean ± standard deviation. The differences in the means were analyzed by Student's t-test and a p-value <0.05 was considered statistically significant.
Results
Osteoblast viability and ALP activity
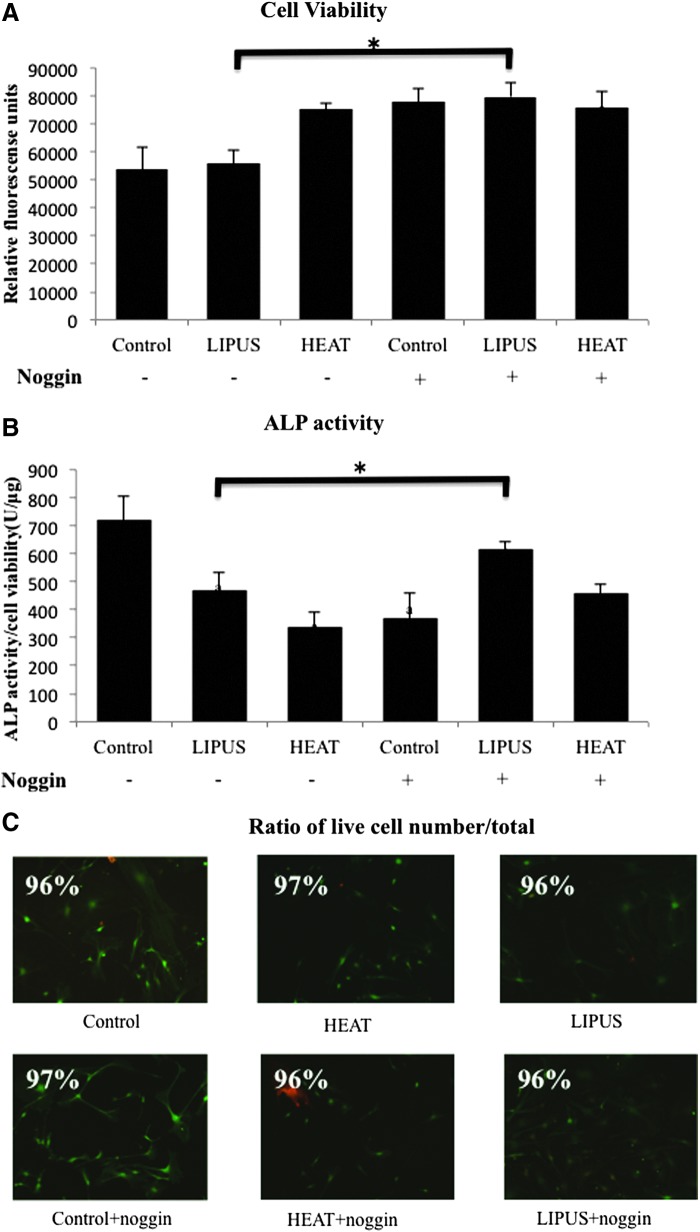
As shown in Figure 2A, osteoblasts quickly responded to the heat shock, significantly increasing cell viability; however, LIPUS stimulation did not increase cell viability at this early time point. As predicted, BMP2 signal blockage by Noggin showed an elevated cell viability, which was observed in all groups. On the other hand, when the ALP activity was normalized to cell viability, it was dramatically reduced by both LIPUS and heat shock stimulations (Fig. 2B). These results suggested that cellular metabolism had shifted to proliferation rather than differentiation after LIPUS stimulation or heat shock treatment. Most notably, the ALP activity detected in the absence of Noggin treatment demonstrated recovery of ALP activity with LIPUS stimulation and a slight recovery with heat shock treatment. ALP-positive cells were visualized with nitro blue tetrazolium chloride. Intense staining was obvious in control osteoblasts, although this group contained the fewest cells (Fig. 2C).
FIG. 2.
Alkaline phosphatase (ALP) activity and viability of osteoblasts. Osteoblasts show changes in their viabilities in different stimuli. (A) The viabilities were determined numerically by colorimetric absorbance at a wavelength of 570 nm. (B) Quantitative ALP activity was normalized to cell viability in each sample. Data are expressed as mean ± SD (n = 3). a, versus control without Noggin (−), and b, versus control with Noggin (+). a and *: p < 0.05 was recognized to be significant. (C) A live/dead assay was performed at 24 h after each stimulation. Live/dead cells were detected in green/red fluorescence, respectively. There were no significant differences in the live/dead cell numbers among all conditions.
Mineralized nodule formation
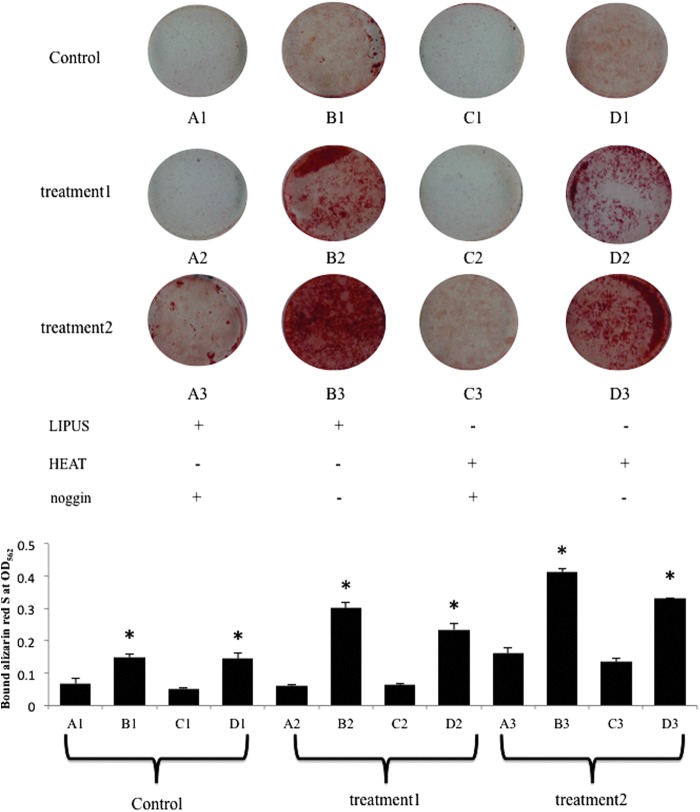
LIPUS stimulation and heat shock treatment showed stronger alizarin red S staining than the unstimulated controls, suggesting that osteoblasts accumulated more mineralized nodules in the test groups (Fig. 3). Furthermore, nodule formation was enhanced using the early stimulation protocol, in which LIPUS and heat shock were applied to the osteoblasts simultaneously with cell seeding at day 0 (Fig. 3). Notably, it was apparent that LIPUS stimulation drove mineral accumulation even under the Noggin administration by the early stimulation protocol (Fig. 3).
FIG. 3.
Alizarin red S staining. Mineralized nodule formation. Calcium nodules (red) were observed using Alizarin red S staining. LIPUS-treated cell culture. Heat-shock-treated cell culture. Treatment 1 followed the experimental time schedule indicated in Figure 1. In treatment 2, the cells received the stimuli at day 0 when the cells were seeded on the dishes. Control cells were cultured in the osteogenic medium without the stimulations. Quantitative analysis of Alizarin red S staining was performed by measuring the absorbance of destained Alizarin red S at 562 nm. The results are expressed as mean ± standard deviation (SD) (n = 3). *, versus A1 and C1. p < 0.05 was recognized to be significant.
Effect of LIPUS on early expression of HSPs and osteogenesis-related genes
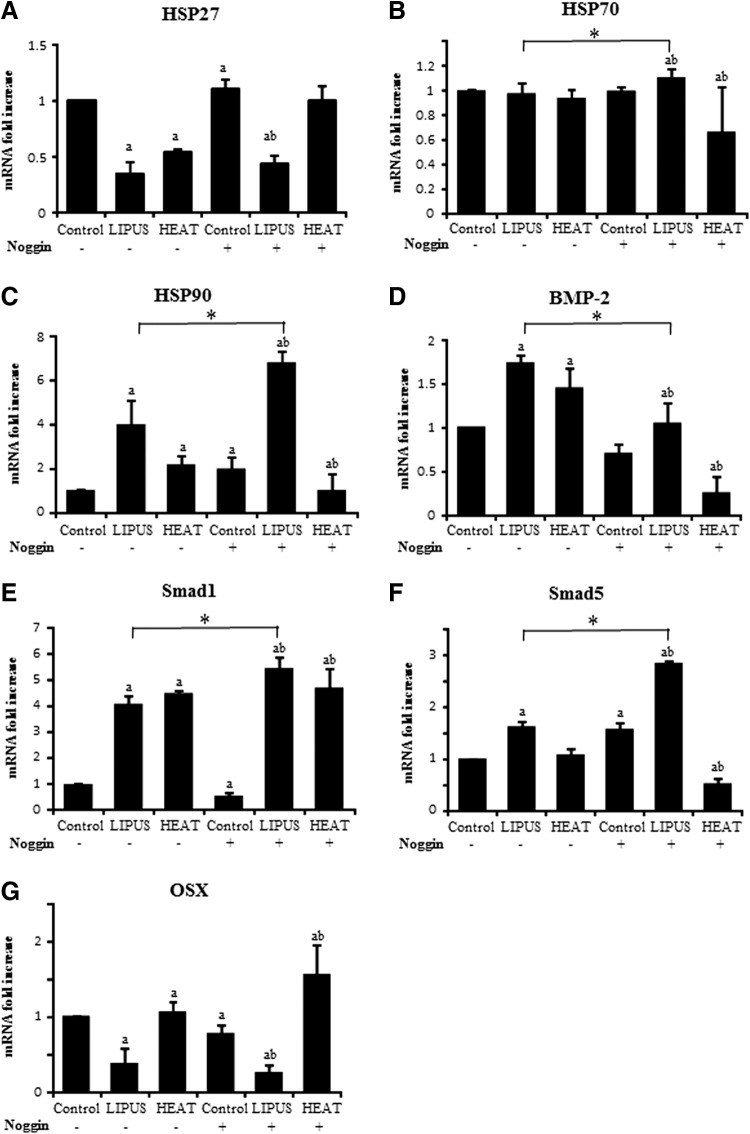
The RT-PCR results showed that HSP27 gene expression was downregulated in the LIPUS- and heat-stimulated osteoblasts (Fig. 4); however, when the culture medium was supplemented with Noggin to antagonize BMP signaling, HSP27 mRNA transcription was decreased only by LIPUS stimulation. In contrast, HSP90 gene expression was upregulated upon LIPUS or heat stimulation, and only LIPUS stimulation dramatically upregulated HSP90 gene expression in the presence of Noggin. Conversely, HSP90 gene expression dropped down by the supply of Noggin on heat stimulation. On the other hand, HSP70 mRNA levels remained stable throughout the experiment.
FIG. 4.
mRNA expression. The mRNA levels of (A) heat-shock proteins (HSP)27, (B) HSP70, (C) HSP90, (D) BMP2, (E) Smad1, (F) Smad5, and (G) OSX were measured from total RNA extracted from the osteoblasts of 24-h culture. mRNA expression levels are expressed as fold difference of value in the groups to value in control without Noggin (−). Data are expressed as mean ± SD (n = 3). a, versus control without Noggin (−) and b, versus control with Noggin (+). a, b, and *: p < 0.05 was recognized to be significant.
Osteogenic gene expression was also examined. Bmp2 was upregulated after LIPUS stimulation or heat shock treatment. In the presence of Noggin, Bmp2 was decreased with LIPUS and heat stimulation as expected, but there was an even further decrease with heat treatment in the presence of Noggin. Smad1 was also upregulated by both treatments; however, Smad5 expression was increased only after LIPUS stimulation, especially in the presence of Noggin. Again, heat in the presence of Noggin significantly decreased Smad5 expression. Interestingly, OSX, a marker of osteoblast differentiation, showed contrasting gene expression levels between LIPUS stimulation and heat shock; it was downregulated by LIPUS stimulation and upregulated by heat, with or without exposure to Noggin.
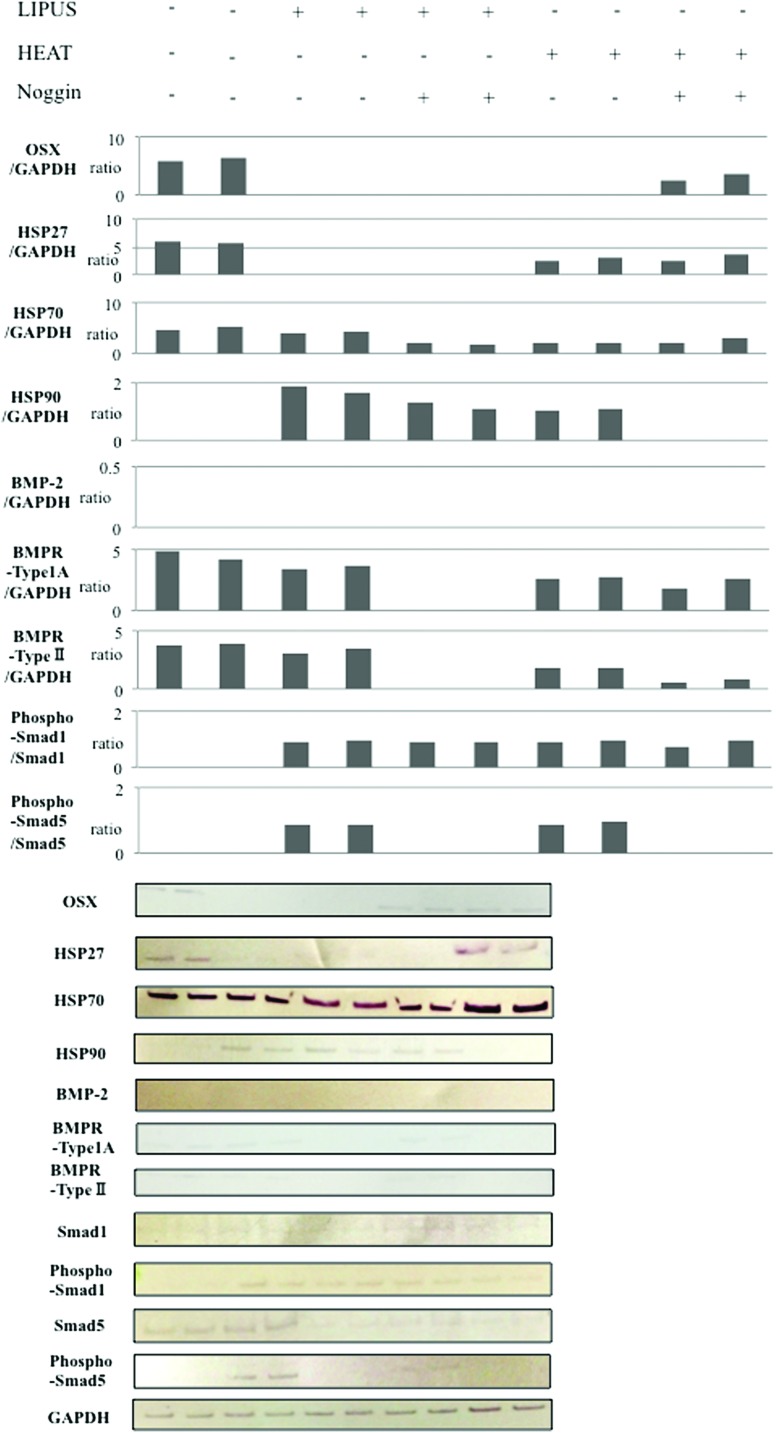
HSP27, HSP70, HSP90, Smad1, Smad5, and BMP2 immunoblot analysis
Western blot analysis was performed for HSP27, HSP70, HSP90, BMP2, BMPR Type IA, BMPR Type II, Smad1, phosphorylated Smad1, Smad5, phosphorylated Smad5, OSX, and GAPDH (Fig. 5). The expression tendencies were consistent with the mRNA expression results, where the HSP27 level decreased after LIPUS stimulation and heat shock treatment; however, only LIPUS stimulation downregulated HSP27 protein expression when Noggin was present. HSP70 protein was detected under all experimental conditions, and their expression levels remained stable. HSP90, undetectable without the stimuli, was stimulated by LIPUS or heat; however, Noggin not only canceled but also blocked HSP90 strongly against the heat shock stimulation. Furthermore, both Smad1 and Smad5 were only weakly detected. It was noteworthy that the phosphorylation of Smad5 was inhibited by Noggin. BMP2 synthesis showed little or undetectable levels in all treatments. On the other hand, both BMPRs expressions were maintained in each stimulation, but inhibited by Noggin. OSX was undetectable by both LIPUS and heat stimulation, but slightly observed only when the cultured cells were exposed to the heat with Noggin.
FIG. 5.
Western blot analysis. Western blotting for the HSP: HSP27, HSP70, and HSP90; osteogenic transcription factors: Smad1, Smad5, phospho-Smad1, phospho-Smad5, and OSX; and BMP2 and BMPR Type IA/II were performed using their respective polyclonal antibodies. The cultured cells were collected for Smad1, Smad5, phospho-Smad1, phospho-Smad5, OSX, and BMPR Type IA/II detection, and the culture medium was assayed for the HSPs and BMP2 secreted from the osteoblasts 24 h after the stimulation. Intensities of the developed bands were semiquantified by image analyzing software. Color images available online at www.liebertpub.com/tea
Discussion
LIPUS is used clinically to accelerate fracture healing.3,5,34–37 Azuma et al. demonstrated a LIPUS-mediated enhancement in fracture repair using an animal model.38,39 Many studies examining the effects of LIPUS on cultured cells used similar experimental parameters, such as stimulus power, duration, and ultrasound frequency.40–44 The focus of this study was to determine whether LIPUS stimulates the expression of HSP leading to osteoblast activation and whether osteoblast stimulation could activate osteogenic signal transduction, independent of the canonical BMP signaling pathway. The experimental protocol used in the present study was based on the precedent manuals, although in this study, LIPUS exposure was concentrated into a one-time treatment at 30 mW/cm2 for 15 min. Nevertheless, the experimental results suggest a mechanism by which LIPUS may initialize osteogenesis and enhance mineralization after 10 days through heat shock protein syntheses. Although different exposures such as 45 and 60 mW/cm2 were examined rather than 30 mW/cm2, those stronger exposures did not accelerate the differentiation or proliferation of the cultured cells, and the design used in the current study just followed similar in vitro studies.40,42,45
Heat alters the induction of HSP27, HSP70, and HSP90 in osteoblasts.13 HSP70 expression in cultured human bone marrow cells was increased by the application of mild heat (39°C). This was also observed in MG-63 cells, not only at a temperature of 39°C but also at temperatures as low as 33°C. On the other hand, it was reported that simvastatin, a member of the statin family, rapidly activated p38 MAP kinase in MC3T3 cells, affecting neither HSP70 nor HSP90, but increasing HSP27 levels to markedly promote osteoblast differentiation.46 Furthermore, simvastatin promotes osteoblast viability and differentiation through Smad1 phosphorylation through the BMP2 signaling pathway,47 which is required for osteogenesis. Another report showed the enhancement of mineralization in osteoblast cells after HSP27 gene transfer when the culture media were supplemented with recombinant BMP4.48 These data help formulate a hypothesis that directing osteogenesis in osteoblastic cells may be intimately associated with BMP2 synthesis through HSP27 expression. Many studies have demonstrated that LIPUS promotes osteoblast differentiation in vitro. In this study, LIPUS stimulation was used to induce osteogenic differentiation of mouse calvaria-derived cells. Therefore, LIPUS and heat shock, using relatively high heat (42°C), elevated HSP90 and lowered HSP27 at 24 h, in contrast to simvastatin stimulation. This suggests that mechanical stress, such as heat or LIPUS, does not induce the low-molecular-weight HSP27, but it instead activates the high-molecular weight HSP90 that acts as a molecular chaperone and protects cells subjected to hazardous conditions.49 Hence, these mechanical stressors may activate not only the BMP canonical signaling pathway but possibly also another signal pathway, which might be different from the p38 MAP kinase-related noncanonical signal pathway, to promote osteoblastic differentiation.
Mechanical stresses placed on bone tissue cause osteoblasts to respond in various ways,50 like promoting BMP production. Suzuki et al. showed a significant increase in BMP2 mRNA levels in rat osteosarcoma cell line, an osteoblastic cell line.45 Notably, LIPUS stimulation in this study enhanced nodule formation in cell culture, as demonstrated in other reports, which presented stronger Alizarin red S staining than the heat shock (Fig. 3). Furthermore, the mineral accumulation was weak, but detected more in the LIPUS treatment, under the Noggin supplemented condition (Fig. 3). This stimulated BMP2 mRNA expression as early as 24 h of culture. LIPUS stimulation led to the highest levels of mineralization, although heat shock treatment also enhanced nodule formation over control levels after 10 days of culture. However, OSX gene expression was downregulated by LIPUS, while heat shock slightly increased its expression after 24 h. Conversely, cell viability quickly responded to the heat shock stimulus with a concomitant increase as the ALP activity decreased. This result was also induced by LIPUS stimulation, suggesting that LIPUS stimulation and heat shock accelerated cell proliferation. This may have masked cell differentiation events during the early stages of osteogenesis, leading to cellular mineral accumulation afterward. One report found that phosphorylation of Smad1 was evident in cultured cells after 30 min of statin treatment, and the expression of BMP2 might stimulate BMP2 receptors through autocrine or paracrine signaling.47 In our study, both Smad1 and Smad5 mRNAs were drastically increased by LIPUS stimulation; on the other hand, the heat shock elevated only Smad1 gene expression (Fig. 4). Other studies have demonstrated that p38 MAP-kinase-dependent osteoblast differentiation is important,46,51,52 even though OSX mRNA expression was only suppressed by LIPUS stimulation at 24 h in our study. However, the strongest mineral accumulation was apparent in LIPUS-treated osteoblasts. Therefore, the subsequent upregulation of BMP2 gene accompanied by both Smad1 and Smad5 genes, following LIPUS stimulation, may have activated BMP2-dependent osteoblast differentiation afterward together by 10 days. The critical time when OSX expression increases should be examined in a future study since we have no detailed information about the later gene expression profiles.
Studies showed that variants of the BMP2 gene and altered BMP2 signal transaction are closely associated with osteoporosis and osteoporotic fractures.53,54 Therefore, the blockage of BMP2 signaling by interfering with BMP2 receptor binding may cause downstream gene activation that promotes osteoblast differentiation to be dysfunctional. In the present study, Noggin, a BMP antagonist, inhibited osteogenic gene expression. Notably, when the BMP2 signal is blocked, LIPUS stimulation elevated both Smad1 and Smad5 gene expression considerably, while heat shock treatment enhanced only Smad1 with unaltered Smad5 under the signal blockage. OSX mRNA expression showed the same pattern or downregulation by LIPUS and upregulation by heat shock during the early 24-h period. However, cell viability was immediately enhanced in the short term, resulting in a decrease of ALP activity in all test groups, including controls that were exposed to Noggin. Therefore, although both types of stimulation promoted osteoblast viability, cells may become even more proliferative after LIPUS stimulation by suppressing OSX mRNA expression. Theoretically, the levels of BMP2 ligand and its receptors should not be influenced by Noggin. Surprisingly, our results show that BMP2 transcription rapidly decreased after heat shock treatment in the presence of Noggin, whereas LIPUS accelerated BMP2 mRNA expression under similar conditions; however, BMP2 protein was not well visualized by Western blot analysis, assuming one possible reason that the protein amount might not reach an immunodetectable level substantially. Remarkably, when the BMP signal pathway had been inhibited by Noggin, LIPUS stimulation as well as heat shock maintained the phosphorylation of Smad1 and inhibited HSP27, but stimulated HSP90 instead, suggesting that the phosphorylation might be activated in a different signal pathway from the p38 MAP-kinase-related noncanonical pathway to induce Smad1 phosphorylation. On the other hand, these two stimulations demonstrated Smad5 phosphorylation inhibition with Noggin treatment (Fig. 5). This hypothesis of another noncanonical transduction is also supported by a result demonstrating abandon of HSP27 and, instead, encouragement of HSP90, which was specific to both LIPUS and heat shock (Fig. 5). Interestingly, recovery of HSP27 and significant decrease of HSP90 were observed only by heat shock under Noggin treatment, in which heat shock might play a role to compensate for the signal blockage.
The current experiment demonstrated feasibility of LIPUS upon avoidance of the Noggin-mediated BMP2 signal blockage by observing mineralized nodule formation, mRNA expression, and protein syntheses of interest in the cell culture. On the other hand, how OSX could become activated by BMP2 stimulated by LIPUS in an autocrine or paracrine manner remains unexplored. As Smad1 phosphorylation was maintained even under BMP signal blockage by Noggin in the present study, LIPUS might be a novel inducer of osteoblastic differentiation under critical conditions in which BMP signals are suppressed congenitally or posteriorly due to accidental blockage of BMP binding to its receptors. Our results strongly suggested that LIPUS initially stimulated high-molecular-weight HSP90 and BMP2 gene expressions without the elevation of OSX expression at 24 h. This might not be enough to understand the mechanism by which LIPUS accelerated osteogenesis through a noncanonical pathway accompanied by downregulation of low-molecular-weight HSP27. However, we speculated that LIPUS might contribute to quick propagation of osteoblasts by HSP90 elevation rather than differentiation at the early time point. Furthermore, increased Smad1, Smad5, and BMP2 gene expressions may signal preparation for mineralized nodule formation observed after 10 days. We additionally administered early stimulation of both the LIPUS and heat shock to the osteoblasts during cell seeding at day 0, which showed an increased mineralization. This suggests that the timing of stimulus application is important. In conclusion, LIPUS stimulation enhanced cell viability and proliferation as early as 24 h after treatment, and HSP90 was upregulated, leading to dense mineralization in the osteoblast cell culture after 10 days.
Acknowledgment
The authors acknowledge Dr. Michiko Suzuki, a Research Assistant in Oral Implantology and Regenerative Dental Medicine, Tokyo Medical and Dental University, Tokyo, Japan, for technical advice.
Disclosure Statement
No competing financial interests exist.
References
- 1.Cook S.D., Ryaby J.P., McCabe J., Frey J.J., Heckman J.D., and Kristiansen T.K. Acceleration of tibia and distal radius fracture healing in patients who smoke. Clin Orthop Relat Res 198, 337, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Duarte L.R. The stimulation of bone growth by ultrasound. Arch Orthop Trauma Surg 101, 153, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Heckman J.D., Ryaby J.P., McCabe J., Frey J.J., and Kilcoyne R.F. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am 76, 26, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Klug W., Franke W.G., and Knoch H.G. Scintigraphic control of bone-fracture healing under ultrasonic stimulation: an animal experimental study. Eur J Nucl Med 11, 494, 1986 [DOI] [PubMed] [Google Scholar]
- 5.Kristiansen T.K., Ryaby J.P., McCabe J., Frey J.J., and Roe L.R. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am 79, 961, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Takikawa S., Matsui N., Kokubu T., Tsunoda M., Fujioka H., Mizuno K., et al. . Low-intensity pulsed ultrasound initiates bone healing in rat nonunion fracture model. J Ultrasound Med 20, 197, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Tanzer M., Harvey E., Kay A., Morton P., and Bobyn J.D. Effect of noninvasive low intensity ultrasound on bone growth into porous-coated implants. J Orthop Resch 14, 901, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Maddi A., Hai H., Ong S.T., Sharp L., Harris M., and Meghji S. Long wave ultrasound may enhance bone regeneration by altering OPG/RANKL ratio in human osteoblast-like cells. Bone 39, 283, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Naruse K., Mikuni-Takagaki Y., Azuma Y., Ito M., Oota T., Kameyama K., et al. . Anabolic response of mouse bone-marrow-derived stromal cell clone ST2 cells to low-intensity pulsed ultrasound. Biochem Biophys Res Commun 268, 216, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Sena K., Leven R.M., Mazhar K., Sumner D.R., and Virdi A.S. Early gene response to low-intensity pulsed ultrasound in rat osteoblastic cells. Ultrasound Med Biol 31, 703, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Warden S.J., Favaloro J.M., Bennell K.L., McMeeken J.M., Ng K.W., Zajac J.D., et al. . Low-intensity pulsed ultrasound stimulates a bone-forming response in UMR-106 cells. Biochem Biophys Res Commun 286, 443, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Takayama T., Suzuki N., Ikeda K., Shimada T., Suzuki A., Maeno M., et al. . Low-intensity pulsed ultrasound stimulates osteogenic differentiation in ROS 17/2.8 cells. Life Sci 80, 965, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Nover L. HSFs and HSPs—a stressful program on transcription factors and chaperones. Stress proteins and the heat shock response, sponsored by Cold Spring Harbor Laboratory, Cold Spring Harbor, NY USA, April 29-May 2, 1991. New Biol 3, 855, 1991 [PubMed] [Google Scholar]
- 14.Nover L., and Hightower L. Heat shock and development. Introduction. Results Probl Cell Differ 17, 1, 1991 [PubMed] [Google Scholar]
- 15.Mathew A., and Morimoto R.I. Role of the heat-shock response in the life and death of proteins. Ann N Y Acad Sci 851, 99, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Welch W.J. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev 72, 1063, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Pockley A.G. Heat shock proteins, inflammation, and cardiovascular disease. Circulation 105, 1012, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Menoret A., Chaillot D., Callahan M., and Jacquin C. Hsp70, an immunological actor playing with the intracellular self under oxidative stress. Int J Hyperthermia 18, 490, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Garrido C., Gurbuxani S., Ravagnan L., and Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun 286, 433, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Zylicz M., and Wawrzynow A. Insights into the function of Hsp70 chaperones. IUBMB Life 51, 283, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Kregel K.C. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92, 2177, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Ciocca D.R., and Calderwood S.K. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 10, 86, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Issels R.D. Hyperthermia adds to chemotherapy. Eur J Cancer 44, 2546, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Zhang H.G., Mehta K., Cohen P., and Guha C. Hyperthermia on immune regulation: a temperature's story. Cancer Lett 271, 191, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Naito H., Powers S.K., Demirel H.A., and Aoki J. Exercise training increases heat shock protein in skeletal muscles of old rats. Med Sci Sports Exerc 33, 729, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Yamada P., Amorim F., Moseley P., and Schneider S. Heat shock protein 72 response to exercise in humans. Sports Med 38, 715, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Tiffee J.C., Griffin J.P., and Cooper L.F. Immunolocalization of stress proteins and extracellular matrix proteins in the rat tibia. Tissue Cell 32, 141, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Leonardi R., Barbato E., Paganelli C., and Lo Muzio L. Immunolocalization of heat shock protein 27 in developing jaw bones and tooth germs of human fetuses. Calcif Tissue Int 75, 509, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Lavoie J.N., Gingras-Breton G., Tanguay R.M., and Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem 268, 3420, 1993 [PubMed] [Google Scholar]
- 30.Blackburn R.V., Galoforo S.S., Berns C.M., Armour E.P., McEachern D., Corry P.M., et al. . Comparison of tumor growth between hsp25- and hsp27-transfected murine L929 cells in nude mice. Int J Cancer 72, 871, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Hedges J.C., Dechert M.A., Yamboliev I.A., Martin J.L., Hickey E., Weber L.A., et al. . A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem 274, 24211, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Gething M.J., and Sambrook J. Protein folding in the cell. Nature 355, 33, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Pratt W.B., Scherrer L.C., Hutchison K.A., and Dalman F.C. A model of glucocorticoid receptor unfolding and stabilization by a heat shock protein complex. J Steroid Biochem Mol Biol 41, 223, 1992 [DOI] [PubMed] [Google Scholar]
- 34.Nolte P.A., van der Krans A., Patka P., Janssen I.M., Ryaby J.P., and Albers G.H. Low-intensity pulsed ultrasound in the treatment of nonunions. J Trauma 51, 693; discussion -3, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Mayr E., Mockl C., Lenich A., Ecker M., and Ruter A. [Is low intensity ultrasound effective in treatment of disorders of fracture healing?]. Unfallchirurg 105, 108, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Leung K.S., Lee W.S., Tsui H.F., Liu P.P., and Cheung W.H. Complex tibial fracture outcomes following treatment with low-intensity pulsed ultrasound. Ultrasound Med Biol 30, 389, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Gebauer D., Mayr E., Orthner E., and Ryaby J.P. Low-intensity pulsed ultrasound: effects on nonunions. Ultrasound Med Biol 31, 1391, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Azuma Y., Ito M., Harada Y., Takagi H., Ohta T., and Jingushi S. Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus. J Bone Miner Res 16, 671, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Pilla A.A., Mont M.A., Nasser P.R., Khan S.A., Figueiredo M., Kaufman J.J., et al. . Non-invasive low-intensity pulsed ultrasound accelerates bone healing in the rabbit. J Orthop Trauma 4, 246, 1990 [DOI] [PubMed] [Google Scholar]
- 40.Angle S.R., Sena K., Sumner D.R., and Virdi A.S. Osteogenic differentiation of rat bone marrow stromal cells by various intensities of low-intensity pulsed ultrasound. Ultrasonics 51, 281, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Li L., Yang Z., Zhang H., Chen W., Chen M., and Zhu Z. Low-intensity pulsed ultrasound regulates proliferation and differentiation of osteoblasts through osteocytes. Biochem Biophys Res Commun 418, 296, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Sena K., Angle S.R., Kanaji A., Aher C., Karwo D.G., Sumner D.R., et al. . Low-intensity pulsed ultrasound (LIPUS) and cell-to-cell communication in bone marrow stromal cells. Ultrasonics 51, 639, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Unsworth J., Kaneez S., Harris S., Ridgway J., Fenwick S., Chenery D., et al. . Pulsed low intensity ultrasound enhances mineralisation in preosteoblast cells. Ultrasound Med Biol 33, 1468, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Yang Z., Ren L., Deng F., Wang Z., and Song J. Low-intensity pulsed ultrasound induces osteogenic differentiation of human periodontal ligament cells through activation of bone morphogenetic protein-smad signaling. J Ultrasound Med 33, 865, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Suzuki A., Takayama T., Suzuki N., Sato M., Fukuda T., and Ito K. Daily low-intensity pulsed ultrasound-mediated osteogenic differentiation in rat osteoblasts. Acta Biochim Biophys Sin 41, 108, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Wang X., Tokuda H., Hatakeyama D., Hirade K., Niwa M., Ito H., et al. . Mechanism of simvastatin on induction of heat shock protein in osteoblasts. Arch Biochem Biophys 415, 6, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Chen P.Y., Sun J.S., Tsuang Y.H., Chen M.H., Weng P.W., and Lin F.H. Simvastatin promotes osteoblast viability and differentiation via Ras/Smad/Erk/BMP-2 signaling pathway. Nutr Res 30, 191, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Kato K., Adachi S., Matsushima-Nishiwaki R., Minamitani C., Natsume H., Katagiri Y., et al. . Regulation by heat shock protein 27 of osteocalcin synthesis in osteoblasts. Endocrinology 152, 1872, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Erlebacher A., Filvaroff E.H., Gitelman S.E., and Derynck R. Toward a molecular understanding of skeletal development. Cell 80, 371, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Cowin S.C., Moss-Salentijn L., and Moss M.L. Candidates for the mechanosensory system in bone. J Biomech Eng 113, 191, 1991 [DOI] [PubMed] [Google Scholar]
- 51.Chung E., and Rylander M.N. Response of preosteoblasts to thermal stress conditioning and osteoinductive growth factors. Cell Stress Chaperones 17, 203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kozawa O., Niwa M., Hatakeyama D., Tokuda H., Oiso Y., Matsuno H., et al. . Specific induction of heat shock protein 27 by glucocorticoid in osteoblasts. J Cell Biochem 86, 357, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Styrkarsdottir U., Cazier J.B., Kong A., Rolfsson O., Larsen H., Bjarnadottir E., et al. . Linkage of osteoporosis to chromosome 20p12 and association to BMP2. PLoS Biol 1, E69, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benisch P., Schilling T., Klein-Hitpass L., Frey S.P., Seefried L., Raaijmakers N., et al. . The transcriptional profile of mesenchymal stem cell populations in primary osteoporosis is distinct and shows overexpression of osteogenic inhibitors. PLoS One 7, e45142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]