Abstract
The use of growth factors, such as glial cell line-derived neurotrophic factor (GDNF), for the treatment of peripheral nerve injury has been useful in promoting axon survival and regeneration. Unfortunately, finding a method that delivers the appropriate spatial and temporal release profile to promote functional recovery has proven difficult. Some release methods result in burst release profiles too short to remain effective over the regeneration period; however, prolonged exposure to GDNF can result in axonal entrapment at the site of release. Thus, GDNF was delivered in both a spatially and temporally controlled manner using a two-phase system comprised of an affinity-based release system and conditional lentiviral GDNF overexpression from Schwann cells (SCs). Briefly, SCs were transduced with a tetracycline-inducible (Tet-On) GDNF overexpressing lentivirus before transplantation. Three-centimeter acellular nerve allografts (ANAs) were modified by injection of a GDNF-releasing fibrin scaffold under the epineurium and then used to bridge a 3 cm sciatic nerve defect. To encourage growth past the ANA, GDNF-SCs were transplanted into the distal nerve and doxycycline was administered for 4, 6, or 8 weeks to determine the optimal duration of GDNF expression in the distal nerve. Live imaging and histomorphometric analysis determined that 6 weeks of doxycycline treatment resulted in enhanced regeneration compared to 4 or 8 weeks. This enhanced regeneration resulted in increased gastrocnemius and tibialis anterior muscle mass for animals receiving doxycycline for 6 weeks. The results of this study demonstrate that strategies providing spatial and temporal control of delivery can improve axonal regeneration and functional muscle reinnervation.
Introduction
Peripheral nerve injury (PNI) often results in significant loss of motor and sensory function, even after surgical intervention. For example, only 25% of patients regain proper motor function, and less than 3% regain sensation.1 For injuries that result in significant damage, substantial tissue may need to be trimmed for easier repair.2 The gold standard for surgical repair is an autograft, in which minor sensory nerves are sacrificed to bridge the nerve defect site. Due to several negative side effects of using autografts (donor site morbidity, multiple surgeries, risk of infection), research has turned to alternative grafting strategies.2 A current clinical option is acellular nerve allografts (ANAs), cadaveric donor nerves that undergo decellularization to remove immunogenic cellular components leaving the basal lamina and extracellular matrix (ECM) proteins largely intact.2–4 The native nerve architecture of ANAs has proven effective in promoting axon regeneration, yet functional outcomes still remain inferior to that of autografts in long gap models.5 Thus ANAs may need to be modified using cell transplantation and/or growth factor delivery to improve their regenerative capacity.
Glial cell line-derived neurotrophic factor (GDNF), a member of the transforming growth factor-β superfamily, has been found to promote survival of midbrain dopaminergic neurons that are damaged in degenerative diseases, such as Parkinson's, and shown to improve motoneuron survival.6,7 It has also been used to target sensory neurons to alleviate pain in cases of chronic denervation.8–10 Signaling through the GDNF family receptor α1 and its coreceptor Ret tyrosine kinase, GDNF increases neurite extension and survival of motor and sensory neurons both in vitro and in vivo.11,12 Unfortunately, endogenous GDNF levels can decrease with chronic denervation, which impedes regeneration.13 Thus, it is important to develop effective delivery strategies that target the site of injury and distal nerve to improve regeneration and functional recovery.
Various strategies have been employed to deliver GDNF, including diffusion-based, vehicle-based, gene-based, and cell-based delivery. The simplest method to locally deliver GDNF is passive diffusion from saline injections or growth factor-soaked materials.14,15 However, protein drugs can lose their efficacy over long regeneration periods due to denaturation or degradation. Affinity-based delivery systems (ABDS) temporarily sequester growth factors within implanted matrices.16,17 For example, heparin-based delivery systems (HBDS) use the ability of heparin to temporarily bind to growth factors through electrostatic interactions17–20 and protect them from degradation. As the scaffold is enzymatically degraded by infiltrating cells and regenerating axon growth cones, GDNF can be released and bound to receptors. Delivery of GDNF from fibrin-filled silicone conduits using an HBDS resulted in increased regeneration and functional recovery compared to fibrin-alone.21,22 However, the HBDS provides controlled release of GDNF over ∼2 weeks, which is relatively short compared to the time required for regeneration across long nerve defects. Vehicle-based GDNF delivery using poly (lactic-co-glycolic acid) microspheres have shown promise in nerve regeneration,23–25 yet this system acts on a similar time scale as the ABDS.
Methods for long-term delivery include gene- or cell-based delivery. Dorsal root ganglia (DRG) treated with cells transfected with a GDNF lentiviral vector resulted in an increased neurite outgrowth in vitro.26 Yet, when this GDNF lentivirus was injected into injured nerves in vivo, regenerating axons did not reach their target organs.26,27 Upon further inspection, it was found that prolonged overexpression of GDNF entrapped axons caused axonal swirling and coils and abnormal formation of myelin.26,28 The entrapment of axons was termed the “candy-store effect,” as regenerating axons are entrapped at the site of GDNF production. Similar results were observed when Schwann cells (SCs) constitutively overexpressing GDNF were transplanted within ANAs.29
The goal of this study was to improve the regenerative capacity of ANAs by optimizing the spatial and temporal delivery of GDNF. The GDNF release was regulated through transduction of SCs with a tetracycline-inducible GDNF, overexpressing lentiviral vector. Through this mechanism, doxycycline administration controls the duration of GDNF expression by SCs. The bioactivity of GDNF released by SCs was confirmed through an in vitro assay, and then the cells were transplanted in an in vivo injury model. A 3-cm nerve gap was bridged using ANAs injected with fibrin containing GDNF and HBDS, and GDNF-SCs were transplanted into the distal nerve segment. Rats were given doxycycline for 4, 6, or 8 weeks, and the optimal duration of GDNF expression was determined through live imaging and histomorphometry of regenerating nerves. An intermediate time point of 6 weeks reduced the candy-store effect resulting in a total axon number in the distal nerve similar to an isograft, indicating that temporal and spatial regulation of delivery can improve regeneration.
Materials and Methods
All materials were purchased from Sigma Aldrich unless otherwise noted.
Construction and production of lentiviral vector
The GDNF Tet-on and tdTomato (in vivo control vector) vectors were made using similar established protocols.30 Briefly, DsRed cDNA sequence was amplified and inserted into FCIV-GDNF (containing GDNF-IRES-Venus, from Milbrandt Lab at Washington University) resulting in FCIV-GDNF-IRES-DsRed. The GDNF-IRES-DsRed fragment was excised and inserted into pEN-TRE-DsRed-PL (derived from pEN_TRmiRc2, ATCC Catalog No. MBA-250), resulting in pEN-TRE-GDNF-IRES-DsRed. The Hope Center Viral Core at the Washington University School of Medicine performed all viral vector design and production using a standard 293T packing cell line.
SC isolation and viral vector transduction
SCs were isolated from adult male Sprague-Dawley sciatic nerves as previously described.30 SCs were seeded at 1.5 × 104 cells/cm2 on poly-l-lysine-coated wells in a 24-well plate and allowed to attach overnight. Cells were incubated with 2 μg/mL polybrene (Santa Cruz Biotechnology) in culture media for 1 h and then the Tet-on GDNF-IRES-DsRed viral vector was added at a multiplicity of infection (MOI) of 20 for 20 h.30 The DsRed fluorescent reporter was inserted into the conditional GDNF vector to allow visualization of transfected cells. SCs were washed in fresh culture media. To confirm transduction, cells were cultured in 5 μg/mL doxycycline and imaged for DsRed fluorescence after 72–96 h. To ensure similarity of fluorescence in transplanted cells, SCs were sorted using fluorescence-activated cell sorting with a MoFlo sorter (Beckman Coulter, Inc.) at the Siteman Flow Cytometry Core (data not shown).
In vitro bioactivity assay
GDNF expression from GDNF-SCs and tdTomato-SCs (in vivo control vector) was analyzed using a GDNF ELISA Duoset kit (R&D Systems) according to previous methods.31 To confirm the GDNF bioactivity, Tet-on GDNF-SCs were cocultured with dissociated E8 chick DRG in Transwell plates (Corning) similar to previously described methods30 and neurite growth was compared to wild-type (WT) and YFP-SC (in vitro bioactivity control vector) cocultures.
Fibrin matrix preparation
Fibrinogen solutions were made by dialyzing 8 mg/mL human plasminogen-free fibrinogen (EMD Bioscience) as previously described.20 Fibrin matrices were prepared by mixing the following components (at final concentration): 4 mg/mL fibrinogen, 2.5 mM CaCl2, 2 NIH U/mL thrombin, 0.25 mM antithrombin III peptide,16,32 62.5 μM heparin, and 100 ng/mL recombinant human GDNF (Peprotech).
ANA decellularization and injection
Three-centimeter sciatic nerve grafts were harvested from 27 male Lewis rats for ANA processing. Sciatic nerves were decellularized using previously established protocols.3,33 ANAs were injected with the fibrin-based HBDS for the controlled release of GDNF adjacent to regenerating fibers in the proximal nerve stump. All solutions were kept on ice during injection to prevent premature gelation within the syringe. For each nerve graft, ∼80 μL of GDNF-HBDS fibrin was injected beneath the epineurium in 10 μL volumes using a 27-gauge Hamilton syringe as previously described.34 Grafts were held immobilized during the injection using drosophila fly pins, and the distal end was clamped shut with a microvessel clamp to prevent leakage. Once injected, nerves were placed in a 37°C incubator for 30 min to allow fibrin polymerization before implantation.
Experimental design and surgical procedures
Adult male and female Thy1-GFP Sprague-Dawley rats (Harlan Laboratories), weighing between 250–300 g, were used.35 All surgical procedures and perioperative care measures were performed in strict accordance with the National Institutes of Health guidelines and were approved by the Washington University Animal Studies Committee (IACUC). All animals were housed in a central animal facility. After surgical procedures, animals recovered in a warm environment and were closely monitored.
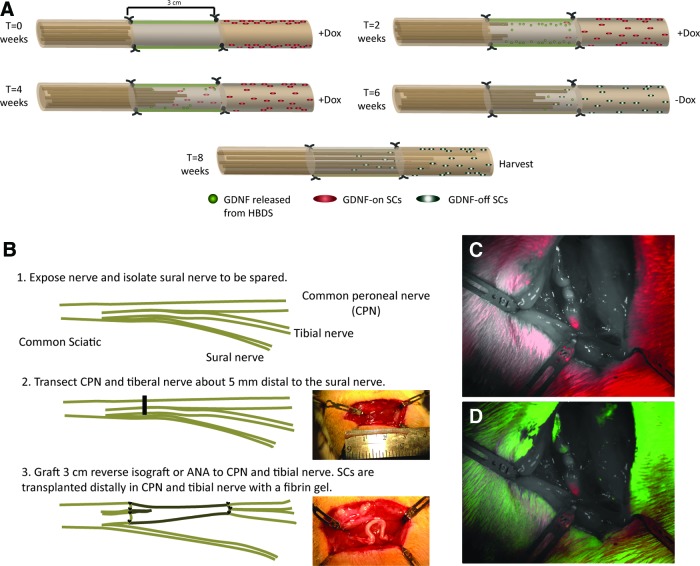
Fifty-one Thy1-GFP rats were randomized into eight groups as shown in Table 1, and additional three rats were used as donors for isograft controls. To administer doxycycline, fresh water supply was supplemented with 1 mg/mL doxycycline every other day, and the rats drank ad libitum. A representative schematic of GDNF delivery though the GDNF HBDS and Tet-on GDNF SCs using doxycycline administration for 6 weeks is shown in Figure 1A. Surgical procedures were performed using the aseptic technique for microsurgical dissection and repairs. Rats were anesthetized with subcutaneous delivery of ketamine (75 mg/kg; Fort Dodge Animal Health) and dexmedetomidine (0.5 mg/kg; Pfizer Animal Health). Briefly, the hind leg was shaved and prepped, and the sciatic nerve was exposed using a dorsolateral gluteal muscle-splitting incision. In all experimental groups, the sciatic nerve was transected through the common peroneal and tibial nerves ∼5 mm distal to the trifurcation, to spare the sural nerve and prevent autophagia. A schematic of the surgical procedure is shown in Figure 1B. In groups with transplanted SCs (tdTomato control vector or GDNF-expressing), 1 × 106 SCs were injected beneath the epineurium of the distal nerve stumps with 20 μL fibrin to improve cell engraftment. Representative images of SCs, 2 weeks after transplantation in the distal nerve, are shown in Figure 1C and D. Suturing reversed nerve grafts to the proximal and distal nerve stumps using three to four 9-0 nylon sutures through the epineurium created a 3-cm critical nerve gap. Wounds were closed with 5-0 vicryl sutures through the muscle fascia and 4-0 nylon skin sutures. Over the entire recovery period, the sciatic nerve was reexposed every 2 weeks for live imaging.35 Once exposed, the rats were placed under a mounted upright fluorescent microscope and imaged using Micromanager software.36 After imaging, the wounds were closed.
Table 1.
Experimental Design for Tet-On GDNF Overexpression In Vivo Study
| Group no. | Group name | GDNF-SCs | HBDS+ GDNF | Time of doxycycline treatment (weeks) | Live image at 2, 4, 6, 8 weeks | Harvest (weeks) | No. of rats |
|---|---|---|---|---|---|---|---|
| I | Isograft | − | − | − | + | 8 | 6 |
| II | ANA | − | − | − | + | 8 | 6 |
| III | GDNF delivery system (DS) | − | + | − | + | 8 | 6 |
| IV | Control vector-SCs+GDNF DS | − | + | 8 | + | 8 | 6 |
| V | GDNF-SCs Dox8 no GDNF DS | + | − | 8 | + | 8 | 6 |
| VI | GDNF-SCs Dox4+GDNF DS | + | + | 4 | + | 8 | 7 |
| VII | GDNF-SCs Dox6+GDNF DS | + | + | 6 | + | 8 | 7 |
| VIII | GDNF-SCs Dox8+GDNF DS | + | + | 8 | + | 8 | 7 |
ANA, acellular nerve allograft; GDNF, glial cell line-derived neurotrophic factor; HBDS, heparin-based delivery systems; SC, Schwann cells.
FIG. 1.
Representation of isograft and acellular nerve allograft (ANA) surgical procedure. (A) A representative schematic of an intermediate glial cell line-derived neurotrophic factor (GDNF) delivery time point in which GDNF is released by both heparin-based delivery systems (HBDS) and Tet-on GDNF Schwann cells (SCs) under 6 weeks of doxycycline induction. (B) The sciatic nerve is exposed and separated after the trifurcation to spare the sural nerve. The common peroneal and tibial nerves are transected ∼5 mm distal to the trifurcation and a 3-cm reverse isograft or ANA is used to bridge the proximal and distal nerve stumps. Three to four sutures were used to coapt the grafts to the host tissue. For animals receiving SCs, SCs were injected beneath the epineurium distal to the graft area. Representative images are shown of the 3-cm GDNF-DS modified ANA before grafting and immediately after. (C) tdTomato control vector and (D) merged GFP and tdTomato expression is observed 2 weeks after injection into distal nerve stumps. Images are taken at 0.63× magnification. Red indicates control vector-labeled SCs, green represents Thy1-GFP axons. Color images available online at www.liebertpub.com/tea
After 8 weeks of recovery, animals were again placed under anesthesia, and the sciatic nerve was reexposed. The nerve grafts were harvested with an additional 10-mm segment of native nerve taken proximally and distally. Midgraft and distal tissue sections were removed and placed in 3% glutaraldehyde solution in 0.1 M phosphate buffer at 4°C until histomorphometric analysis. Animals were euthanized with an intracardiac injection of Euthasol (150 mg/kg) (Delmarva Laboratories).
Histomorphometry
After distal and midgraft tissues were harvested and fixed, they were postfixed with 1% osmium tetroxide, ethanol dehydrated, and embedded in Araldite 502 (Polyscience, Inc.) as previously described.37 Cross-sections were imaged midgraft and distally at 1000× and analyzed by a blinded observer for total axon count, axon density, percent neural tissue, percent myelin debris, fiber width, and myelin width.
Quantitative real-time polymerase chain reaction
Distal nerve segments were stored in RNAlater, and then mRNA was collected and purified using an RNeasy mini prep kit (Qiagen). The purity and concentration of mRNA were determined using a NanoPhotometer (Implen), and mRNA was converted to cDNA using the High Capacity mRNA-to-cDNA kit (Life Technologies). Gene expression of GDNF and the housekeeping gene β-actin was determined using Taqman gene expression assays (Life Technologies) and Fast Master Mix (Life Technologies) on the Step One Plus Real-Time PCR system.
Statistical analysis
All results are reported as mean ± standard error of the mean. The number of animals analyzed within each group is indicated by “n” values. Statistical analysis was performed using Statistica version 6 (Statsoft, Inc.). All data were evaluated for differences between groups using the analysis of variance test with least-squared difference post hoc tests with statistical significance set at α = 0.05 (p < 0.05).
Results
Bioactivity of GDNF released from Tet-on GDNF SCs
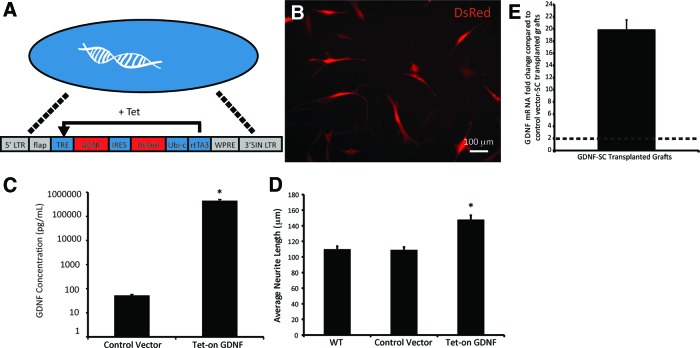
For conditional long-term delivery of GDNF, SCs were transduced with a doxycycline-inducible GDNF overexpressing lentiviral vector (Fig. 2A). Transduction was confirmed through image analysis of DsRed fluorescence after activation of the tetracycline response element by doxycycline (Fig. 2B). With an MOI of 20, the efficiency of transduction was ∼100%. To test whether the GDNF expression was elevated compared to normal SCs, GDNF expression in GDNF-SCs were compared to tdTomato-SCs (in vivo control vector) using an ELISA. The GDNF expression in GDNF-SCs was significantly higher than the control even after 2 days of doxycycline treatment (Fig. 2C). To confirm the biological activity, GDNF-SCs, control vector-SCs, and WT-SCs were cultured in Transwell plates with dissociated DRG, and the neurite extension was measured after 18 h. As shown in Figure 2D, transduction of SCs with the control vector had no effect on the neurite extension compared to WT-SCs, demonstrating that the transfection process alone does not affect neurite outgrowth. When neurons were cultured with GDNF-SCs, the neurite extension was increased by ∼32% versus WT-SCs. These results indicate that the level of biologically active GDNF expressed by the SCs after transduction with the Tet-on GDNF lentiviral vector increased in the presence of doxycycline.
FIG. 2.
SCs were successfully transduced with a Tet-on GDNF LV vector. (A) A cartoon depiction of the Tet-on GDNF LV vector shows GDNF expression induced with tetracycline (or its analog doxycycline). Tetracycline binds to the reverse transcriptase activator (rtTA3), and then, together the tetracycline and rtTA3 activate the tetracycline response element (TRE) driving GDNF and DsRed expression. (B) Merged bright-field and fluorescent image is shown of Tet-on GDNF-SCs just before injection. (C) GDNF released from control vector-SCs and GDNF-SCs after doxycycline induction. (D) Average neurite length showed no difference between wild-type (WT) and control vector-SCs. Neurite length significantly increased in cultures with GDNF overexpressing SCs. Data are represented by the mean ± SEM. * denotes statistical significance from WT-SCs (p < 0.05). (E) mRNA was extracted from the distal nerve segments of GDNF-SC and control vector-SC-transplanted animals, and GDNF mRNA levels were examined. Compared to control vector-SC animals, GDNF mRNA was almost 20-fold higher in the distal nerve segment where SCs overexpressed GDNF. Dotted line marks upregulation. Color images available online at www.liebertpub.com/tea
In addition to in vitro bioactivity, we also wanted to ensure that transplanted Tet-on GDNF-SCs continued to overexpress GDNF in the distal nerve during doxycycline administration. We performed quantitative real-time polymerase chain reaction on the distal nerve segments of control vector-SC and GDNF-SC transplanted groups at 4 weeks posttransplantation. GDNF mRNA levels are significantly upregulated in the distal nerve with Tet-on GDNF-SCs compared to control vector-SCs (Fig. 2E). This result demonstrates that with doxycycline, GDNF expression remains elevated for at least 4 weeks in vivo.
Live tracking of regenerating axons
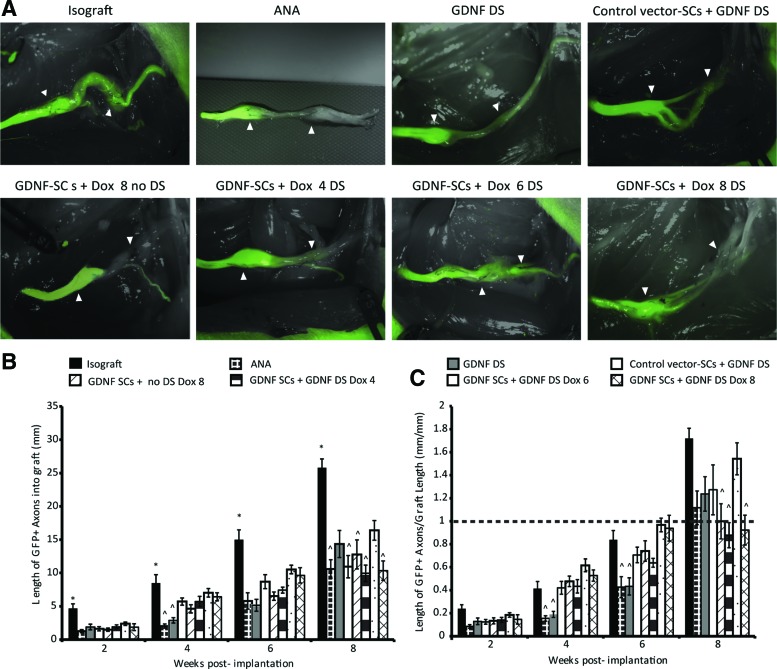
Live fluorescence imaging allows us to monitor regenerating axons during the recovery period to see differences in the rate of regeneration and when axons become entrapped. It also allows us to determine when the axon front has reached the distal nerve so that we can shut off GDNF expression to prevent trapping. As shown in Figure 3A, differences in GFP+ axon growth through the grafts can be visualized at 8 weeks, indicating various regeneration rates depending on the treatment group.
FIG. 3.
Live imaging of GFP axons highlights differences in regeneration. (A) Fluorescent images of Thy1-GFP axons at the end of the 8-week recovery period for the control and experimental conditions are shown. By the end of the recovery period, regeneration of GFP+ axons past the graft area can be observed clearly in the isograft control group. The GDNF-DS group also indicates distal growth of GFP+ axons, but regeneration appears greatly reduced in the ANA group and slightly reduced in the control vector-SC group. When doxycycline was removed too early at 4 weeks or continued for the entire 8 weeks (with or without the GDNF-DS), GFP+ axons become entrapped within the graft. Only 6 weeks of doxycycline show GFP+ axons past the distal suture line. White arrowheads denote start and end of graft region. Scale bar = 3 mm. *denotes statistical significance from all other groups. (B) Average length of GFP+ axons over the 8-week recovery period is shown. Isografts had significantly greater length of GFP fluorescence over time. Only at 8 weeks does is stand out that DS ANA and GDNF-SCs DS Dox6 promoted greater GFP+ axon extension into the grafts. Data are represented by the mean ± SEM. ^ denotes significance from GDNF-SCs DS Dox6 (p < 0.05). (C) Length of GFP+ axons was normalized to the length of the grafts as the grafts can shrink over time. The dotted line at 1 marks the growth of GFP+ axons at graft length and clearly identifies groups in which axons become entrapped within the graft. Color images available online at www.liebertpub.com/tea
The length of GFP+ axon growth into the grafts for each group is shown in Figure 3B. In addition, we examined the length of GFP+ axons normalized to graft length as the grafts can shrink with time (Fig. 3C). Early, there is little difference between the experimental groups, although groups with GDNF trend slightly higher than the (negative) ANA control. Isograft controls (positive) have a significantly increased rate and length of regeneration at all points; however, when the GFP length was normalized to graft length, there was no difference between isografts and animals with 6 weeks of GDNF overexpression. It is interesting to note that the length of axons for GDNF-SCs given doxycycline for 4 weeks (“Dox4”) stops increasing at the same rate after 4 weeks, whereas doxycycline treatment for 6 (“Dox6”) and 8 weeks (“Dox8”) continued to increase. It is not until week 8 that we observe axons with GDNF-SCs given doxycycline the entire 8-week recovery period stop regenerating. As indicated by Figure 3C, groups that remained entrapped within the graft could be identified as falling below the graft normalized length.
Histology
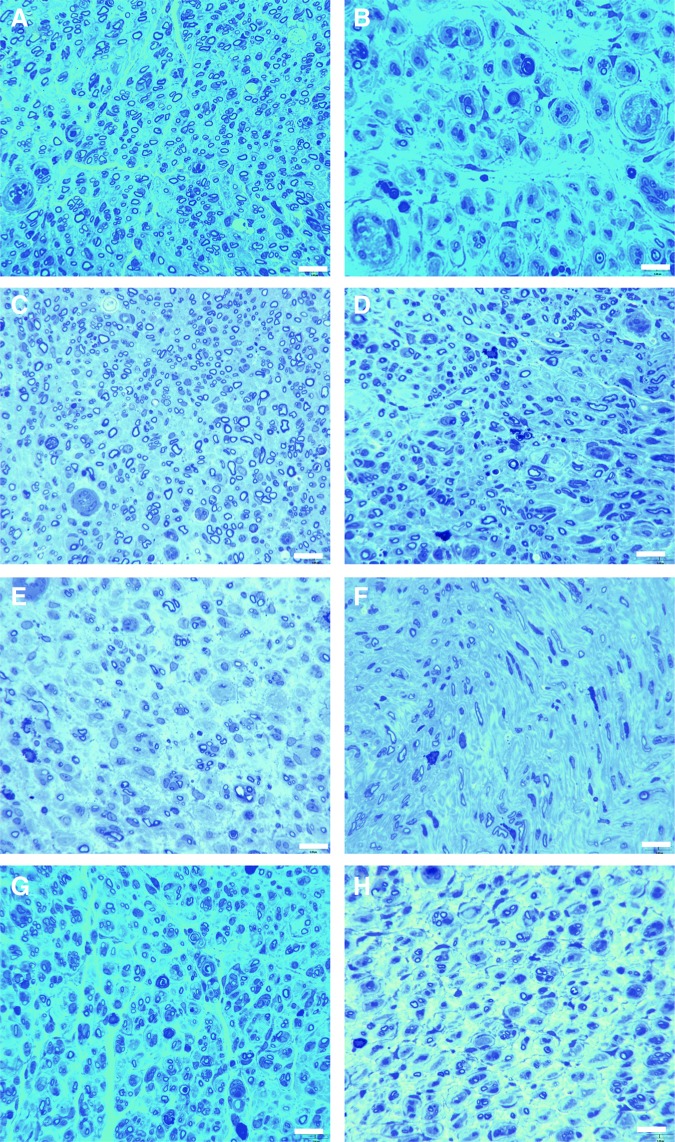
Qualitative assessment of nerve tissue distal to the grafts was performed by light microscopy. Nerve organization and architecture of the regenerating fibers can be observed and differentiated between the different experimental and control groups (Fig. 4). Normal, uninjured nerve organization can be classified as having compact uniformly arranged fibers that are similar in size and shape. GDNF-DS and GDNF-SCs-Dox6 had the most organized nerve architecture similar to isograft-positive controls (Fig. 4C, G). A more disorganized architecture was observed in ANA and control vector-SCs groups. Groups with doxycycline removed too early (4 weeks) or too late (8 weeks) had severely disorganized nerve architecture with few axons randomly arranged.
FIG. 4.
Improved regeneration of nerves by gross histology is observed in DS ANA and GDNF-SC-Dox6 groups. Gross histology of sectioned nerves indicates differences in regeneration between groups. Representative images for each group are shown for (A) Isograft, (B) ANA, (C) GDNF-DS, (D) control vector-SCs + GDNF-DS, (E) GDNF-SC-Dox8 no GDNF-DS, (F) GDNF-SCs GDNF-DS Dox4, (G) GDNF-SCs GDNF-DS Dox6, and (H) GDNF-SCs GDNF-DS Dox8. Organized nerve architecture of compact uniformly sized fibers can be observed clearly in the isograft control. Similar morphology can be seen in DS ANA and GDNF-SCs DS Dox6. Delivery of GDNF for too short (4 weeks) or too long (8 weeks) a period results in poor organization and tissue “swirling.” Scale bar = 5 μm. Color images available online at www.liebertpub.com/tea
Histomorphometry
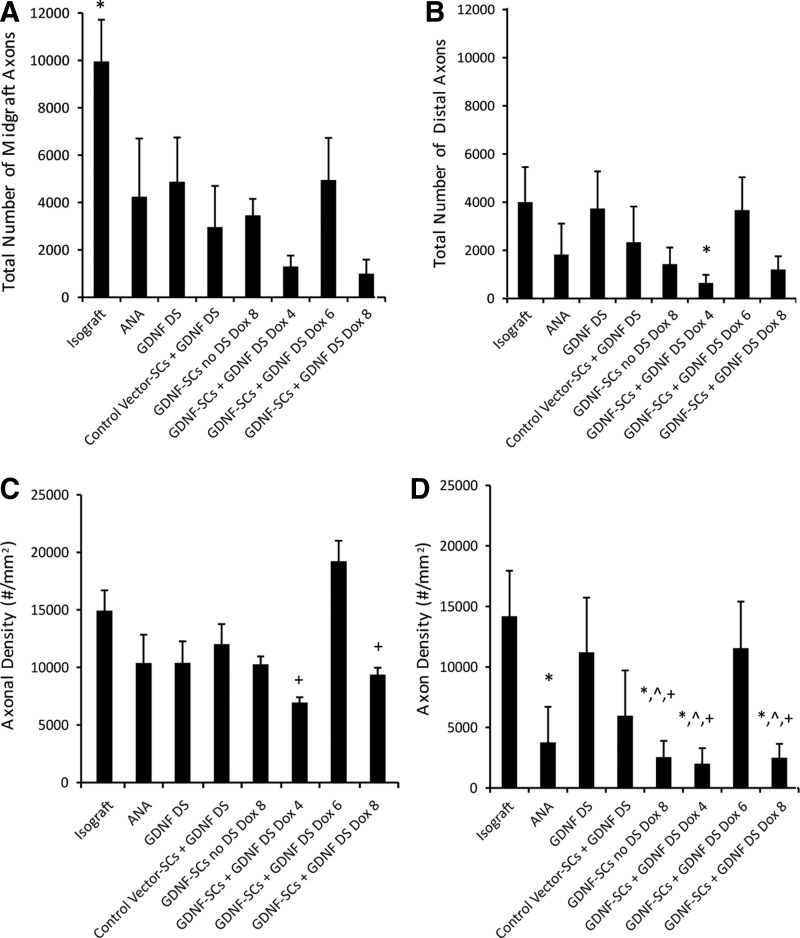
At the end of the 8-week recovery period, the average total axon fiber count for midgraft and distal nerve segments was measured for each group, and isografts were found to have the greatest fiber number (Fig. 5A, B). As predicted by the live imaging data, the experimental group with GDNF-SCs given doxycycline for 6 weeks had a total number of axon fibers most similar to isografts. On the other hand, when doxycycline was removed too early (4 weeks), or maintained for the entire recovery period (8 weeks), regenerating axons stopped before reaching the distal nerve. GDNF-SC-Dox4+GDNF-DS, GDNF-SCs no GDNF-DS Dox8, and GDNF-SC-Dox8+GDNF-DS groups had significantly lower fiber counts in the midgraft and distal nerve segments compared to isografts.
FIG. 5.
GDNF delivery from the DS-modified ANA and 6 weeks of GDNF overexpression improves total axon number and axon density. Total number of axons in the (A) midgraft and (B) distal nerve sections for each group was quantified. Axon density was quantified in the (C) midgraft and (D) distal nerve segments. Data are represented by the mean ± SEM (n ≥ 6 animals per group). * denotes statistical significance from isograft (p < 0.05), ^ denotes significance from DS ANA (p < 0.05), and + denotes significance from GDNF-SCs DS Dox6 (p < 0.05).
Of the control groups, ANAs modified with the GDNF-DS were most similar to the isografts fiber number, whereas the negative control (ANA alone) was significantly lower (Fig. 5A, B). The addition of control vector-SCs to the distal nerve in general appeared to improve the frequency of regeneration, but the total axon number was only slightly higher than the ANA alone. Overall, these results identify treatment strategies for improving axon regeneration in long critical nerve gaps. Clearly, the time and method of GDNF delivery can have a significant effect on axons.
The axon fiber density was also measured for each group in the midgraft and distal nerve and was significantly affected by the duration of GDNF delivery (Fig. 5C, D). Isograft controls had the highest nerve fiber density in the distal nerve, although the GDNF-DS and GDNF-SC-Dox6+GDNF-DS groups were not significantly different (Fig. 5D). Interestingly, in the midgraft, axon density was highest when GDNF was overexpressed by GDNF-SCs for 6 weeks (Fig. 5C). ANAs alone had significantly lower fiber density compared to isografts in the distal nerve. GDNF expression for too short or too long a time led to decreased fiber density distally.
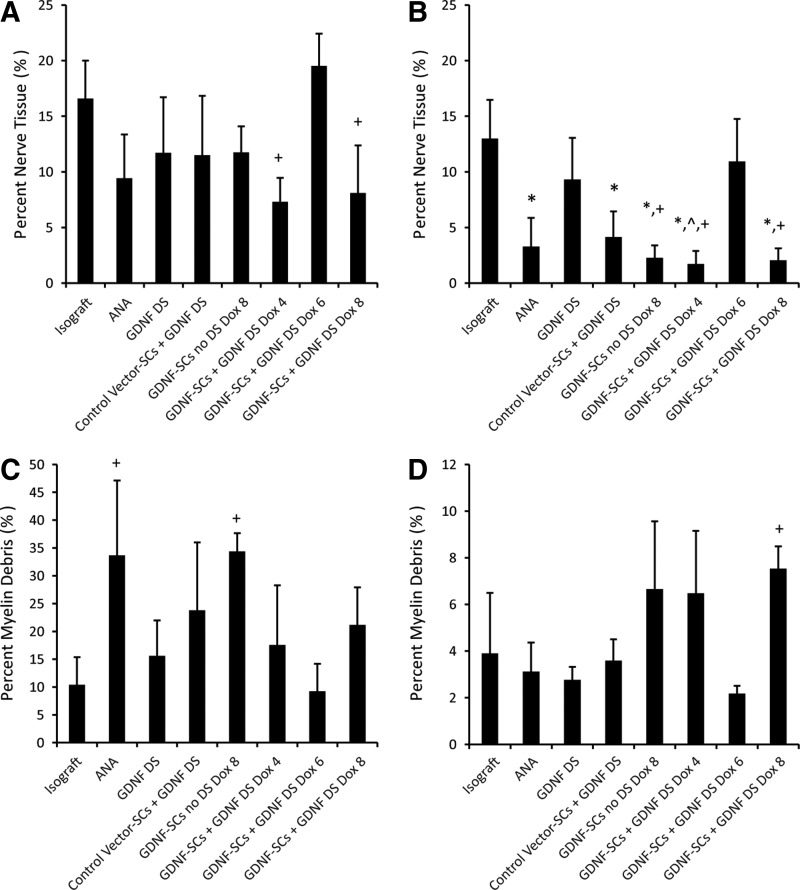
To assess the quality of the regenerating nerve, we also examined the percent neural tissue and percent myelin debris (Fig. 6). There was little change in percent nerve tissue in the midgraft nerve segment between groups; however, the highest percent nerve was found in GDNF-SC Dox6 with ∼19.5% (Fig. 6A). In the distal nerve, the percent nerve tissue was highest for isografts, with GDNF-DS and GDNF-SC-Dox6+GDNF-DS not significantly different from isografts (Fig. 6B). ANAs alone and those with control vector-SCs were significantly lower, but it was the groups with under- or over-GDNF expression that contain the lowest percent of neural tissue. Normal uninjured nerves typically contain around 61% nerve tissue. These results inversely relate to the percent myelin debris found within the distal nerve segment. The groups with the lowest percent neural tissue (GDNF-SC-Dox4 + GDNFDS and GDNF-SC-Dox8+/−GDNFDS) had the highest amount of myelin debris (Fig. 6C, D). The high amount of myelin debris is likely not the result of cell transplantation, as both groups control vector-SCs and GDNF-SC-Dox6 had myelin debris levels similar to that of isografts.
FIG. 6.
The quality of nerve regeneration was improved for DS-modified ANA and 6 weeks of GDNF overexpression. The percent neural tissue in the (A) midgraft and (B) distal nerve for each group was quantified. The percent myelin debris was quantified in the (C) midgraft and (D) distal nerve segments. Data are represented by the mean ± SEM (n ≥ 6 animals per group). * denotes statistical significance from isograft (p < 0.05), ^ denotes significance from DS (p < 0.05), and + denotes significance from GDNF-SCs DS Dox6 (p < 0.05).
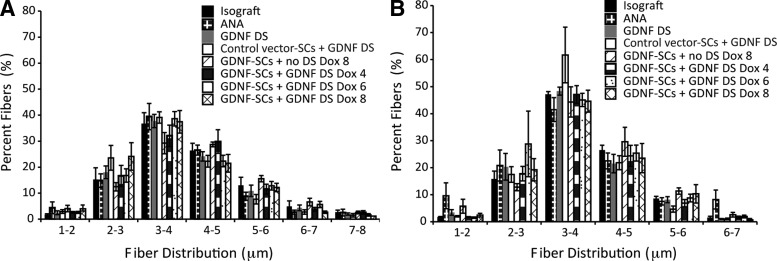
Another measurement to assess the maturation of regenerating fibers is the myelinated nerve fiber width. Mature myelinated nerve fibers in an uninjured sciatic nerve are ∼6.5 μm in width. The myelinated fiber distribution varied little between groups in both the midgraft and distal nerve segments (Fig. 7A, B); however, the spread of fiber width was influenced by whether midgraft or distal axons were analyzed. Midgraft, there was a larger spread of fiber widths, whereas in the distal nerve there were fewer large diameter fibers and the percentage of fibers falling between 3–4 μm increased. In this study, it is important to note that only animals that had measurable fibers were analyzed. Myelin width was also measured and found to be similar between groups.
FIG. 7.
The timing or method of GDNF delivery did not affect fiber width distribution. Distribution of the average fiber width of regenerated fibers in the (A) midgraft and (B) distal nerve was quantified. Data are represented by the mean ± SEM (n ≥ 3 animals per group, animals with 0 regenerated fibers were not included).
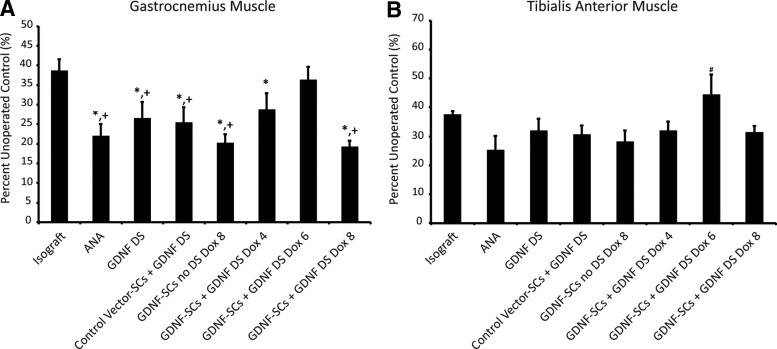
Muscle mass analysis
Muscle atrophy occurs in cases of long-term denervation due to lack of neurotrophic activity and results in decreased muscle fiber organization, muscle mass, and contractility.38 The degree of reinnervation correlates to the increase in mass of the muscles affected by injury. In this study, we measured the tibialis anterior (TA) and gastrocnemius (GA) muscle mass at the end of the 8-week recovery period and normalized these values to the unoperated contralateral muscle. The isograft group showed a 60% decrease in GA muscle mass compared to the unoperated control (Fig. 8A). Only the group with GDNF-SCs and 6-week doxycycline treatment was not significantly lower than the isograft, suggesting a similar degree of reinnervation. Groups with only 4 weeks of doxycycline or the entire 8 weeks of doxycycline resulted in a 75–80% muscle mass decrease. As with the GA muscle, only the isograft group was not significantly different than GDNF-SC-Dox6 for the TA muscle, for operated muscles were ∼38% and 42% of their unoperated control muscles, respectively (Fig. 8B). The differences observed between the muscles are likely attributed to differences in regeneration of the common peroneal and tibial nerves.
FIG. 8.
Six weeks of GDNF overexpression from distally transplanted SCs leads to enhanced muscle mass recovery. Percent muscle mass recovery of the (A) gastrocnemius muscle and (B) tibialis anterior muscle compared to the unoperated control. Data are represented by the mean ± SEM (n ≥ 6 animals per group). * denotes statistical significance from isograft (p < 0.05), + denotes significance from GDNF-SCs DS Dox6 (p < 0.05), and # denotes significance from all groups except isograft (p < 0.05).
Discussion
The focus on repairing PNI has shifted in recent years from a single mechanism of treatment toward combinatorial approaches that seek to recapitulate aspects of native nerves to obtain similar regeneration to autografts. Many groups have designed nerve guidance conduits as bridging support structures, yet they lack the microstructure found in native tissue.39–41 One must take into account the physical structure of nerves, as well as any biochemical or cellular components to fully mimic autograft regeneration. ANAs have proven to be a clinically viable option because they provide both the structure of endoneurial tubes and native ECM components.33 Unfortunately, neurotrophic factors are lost during decellularization, and poor results are seen in long nerve defects (>3 cm).42 Cell transplantation therapies have been shown to improve the regenerative capacity of ANAs, yet remain inferior to that of isografts.5 Therefore, the addition of cells and/or growth factors, such as GDNF, may improve the outcomes for ANAs in long gap nerve repairs.
In this study, we carefully designed a cell transplantation and drug delivery platform to deliver GDNF to regenerating axons in a spatially and temporally controlled manner. First, GDNF was released adjacent to the proximal nerve stump from a HBDS injected beneath the epineurium of a 3 cm ANA to help jump start regenerating axons in the first 2 weeks of regeneration, as well as promote migration of endogenous SCs into the graft. Second, sustained delivery of GDNF from virally transduced SCs modified to conditionally overexpress GDNF in the distal nerve segment was used to promote regeneration of axons into the distal nerve. The main goal of this study was to determine how the timing of GDNF overexpression from transplanted SCs could significantly impact the level of axonal regeneration.
Regulating the timing of GDNF delivery is critical to developing an effective therapy for functional regeneration. Constitutive expression of GDNF through gene- or cell-based delivery has led to the entrapment of axons.27,28 The term “candy-store effect” was coined due to the contentment of axons to remain at the site of GDNF overexpression, where there is a concentrated neurotrophic support. Tannemaat et al. showed that the direct injection of GDNF lentiviral vectors led to constant GDNF overexpression, and regenerating motor axons never reached their target end organs.27,43 Others have shown that GDNF-expressing SCs transplanted at the site of injury failed to promote regeneration past the midgraft line and limited functional recovery was observed.29,44 Transient overexpression of GDNF from nonvirally transduced adipose-derived stem cells in an injured ventral root model has shown improved regeneration, however, the lack of a controlled temporal expression mechanism prevents specific tuning for various applications.45 Consequently, we wanted to design a system in which GDNF expression can be turned off as regenerating axons approach the site of GDNF production, allowing axons growth through the site and reinnervating target organs.
In this study, SCs were transduced with a conditional tetracycline-inducible GDNF expressing lentiviral vector. Upon doxycycline treatment, GDNF is overexpressed, but can be turned off with the removal of doxycycline. The results of the study demonstrated that fine-tuning the timing of delivery significantly affects regeneration. When doxycycline was removed too early (4 weeks), regenerating axons effectively stopped in their tracks. Only four animals out of seven had any regeneration, and of those, the total number of axons was very low. The exact mechanism of this abrupt stop is unknown, but it could be due to the sudden loss of a GDNF gradient. Conversely, 8 weeks of GDNF overexpression led to poor outcomes, suggesting that axons may be entrapped at or just before the site of GDNF production. It was only with 6 weeks of GDNF overexpression that axons were able to reach the distal nerve segment and then continue on. Shakhbazau et al. reported a similar result, in which constant GDNF expression led to regeneration failure.46 However, after 1 week of GDNF expression, axons with normal morphology were capable of extending past the site of production. This shorter time window for optimal expression was due to the difference in transplantation site (midgraft in a 1.5-cm graft vs. distal to a 3-cm graft in our study). Unfortunately, the former study only looked qualitatively at the regenerating axons and did not quantify how well the grafts performed in histomorphometric analysis.
The transplantation of GDNF-expressing SCs also played a role in the quality of nerve regeneration. The delivery of GDNF from SCs for 6 weeks led to an improved percent neural tissue and decreased myelin debris, while any other duration of GDNF delivery led to an inferior quality of regeneration. The percent neural tissue when GDNF was released for 4 or 8 weeks was significantly lower than all other groups. Furthermore, the percent myelin debris was much higher in these groups compared to the other groups. Transplantation of exogenous SCs alone does not lead to these results, as control vector-expressing SCs and GDNF-SC-Dox6 were comparable to isografts in percent myelin debris. Thus, these results are likely due to the breakdown of regeneration caused by either abrupt removal of GDNF or persistent GDNF expression. In a study by Hoyng et al., they found that the entrapment of axons at the site of continual GDNF overexpression led to a breakdown in nerve structure with a high degree of abnormal SC morphology, myelin debris, and axon swirling, similar to Figure 4.28,43
The axon regeneration results indicated by both live imaging and histomorphometry coincided with trends observed in muscle mass.38 Specifically, the GDNF-SC-Dox6 group had the highest percentage of muscle mass compared to all of the groups for the TA muscle and was the most similar to isograft controls in the GA muscle. While this test is not a true quantification of functional muscle force recovery, the increase in operated muscle mass indicates a high degree of reinnervation and function as increased use leads to increased mass. The significant improvement in muscle mass of the GDNF-SC-Dox6 group is also impressive due to the relatively short time of 8 weeks. Previous work has shown improvement of muscle mass and grid grip tests from isografts at 8 weeks, but it took up to 12 weeks for improvement in groups with GDNF released from the HBDS within 15-mm silicone conduits.21 It is also interesting to note that while the control ANAs modified with the GDNF-DS had a significant number of total regenerated axons, this did not translate to an increased muscle mass. This could be due to incorrect reinnervation (sensory axons innervating muscle end organs) or the increase of sensory axon regeneration over motor axons. GDNF is a known stimulant of sensory neuron survival and regeneration, thus the early release from the ANA may tend to target sensory axons.11,27,47
Conclusions
Previous work has shown that ANAs serving as an effective platform for regeneration and transplantation of SCs further improves their regenerative capacity, but they still lack the neurotrophic support, such as GDNF, necessary to promote functional regeneration across long nerve gaps. However, as uncontrolled GDNF expression can actually impair regeneration, careful spatial and temporal control of GDNF delivery may help in developing a successful treatment strategy. Therefore, we have designed and implemented a cell transplantation and growth factor delivery paradigm where GDNF is released in both a spatially and temporally controlled manner from an affinity-based system and inducible SC overexpression system. We have found that a finely tuned window of time for GDNF overexpression results in increased axonal regeneration and muscle mass recovery, whereas delivery for too short a time arrests extending axons, while prolonged expression entraps axons. Live imaging analysis aided in identifying the position and time point at which GDNF overexpression should be removed to encourage axons to reach and reinnervate their target end organs.
Acknowledgments
This research was funded, in part, by NIH R01NS051706 and NSF DGE-1143954 (L.M.M.). The Hope Center Viral Vectors Core is supported by a Neuroscience Blueprint Core grant (P30 NS057105) from NIH to Washington University. We would like to thank Dr. Mingjie Li (Washington University) for producing the lentiviruses and technical assistance.
Disclosure Statement
S.S.E. is an inventor on patents covering the heparin-binding delivery systems used for growth factor delivery in this study and may receive royalties from these patents. These patents are licensed by Kuros Therapeutics. No funding for this study was provided by Kuros.
References
- 1.Mackinnon S.E., and Dellon A.L. Median nerve entrapment in the proximal forearm and brachium: results of surgery. In: Mackinnon S.E., and Dellon A.L., eds. Surgery of the Peripheral Nerve. New York: Thieme, 1988, p. 192 [Google Scholar]
- 2.Siemionow M., and Sonmez E. Nerve allograft transplantation: a review. J Reconstruct Microsurg 23, 511, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Hudson T.W., Liu S.Y., and Schmidt C.E. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng 10, 1346, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Ide C., Tohyama K., Yokota R., Nitatori T., and Onodera S. Schwann cell basal lamina and nerve regeneration. Brain Res 288, 61, 1983 [DOI] [PubMed] [Google Scholar]
- 5.Fox I.K., Schwetye K.E., Keune J.D., Brenner M.J., Yu J.W., Hunter D.A., Wood P.M., and Mackinnon S.E. Schwann-cell injection of cold-preserved nerve allografts. Microsurgery 25, 502, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Herrán E., Ruiz-Ortega J.Á., Aristieta A., Igartua M., Requejo C., Lafuente J.V., Ugedo L., Pedraz J.L., and Hernández R.M. In vivo administration of VEGF- and GDNF-releasing biodegradable polymeric microspheres in a severe lesion model of Parkinson's disease. Eur J Pharm Biopharm 85, 1183, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Sakamoto T., Kawazoe Y., Shen J.S., Takeda Y., Arakawa Y., Ogawa J., Oyanagi K., Ohashi T., Watanabe K., Inoue K., Eto Y., and Watabe K. Adenoviral gene transfer of GDNF, BDNF and TGF beta 2, but not CNTF, cardiotrophin-1 or IGF1, protects injured adult motoneurons after facial nerve avulsion. J Neurosci Res 72, 54, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Nagano M., Sakai A., Takahashi N., Umino M., Yoshioka K., and Suzuki H. Decreased expression of glial cell line-derived neurotrophic factor signaling in rat models of neuropathic pain. Br J Pharmacol 140, 1252, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoke A. Augmenting glial cell-line derived neurotrophic factor signaling to treat painful neuropathies. Proc Natl Acad Sci U S A 111, 2060, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedstrom K.L., Murtie J.C., Albers K., Calcutt N.A., and Corfas G. Treating small fiber neuropathy by topical application of a small molecule modulator of ligand-induced GFRalpha/RET receptor signaling. Proc Natl Acad Sci U S A 111, 2325, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Airaksinen M.S., and Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3, 383, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Robertson K., and Mason I. The GDNF-RET signalling partnership. Trends Genet 13, 1, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Hoke A., Gordon T., Zochodne D.W., and Sulaiman O.A.R. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol 173, 77, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Fine E.G., Decosterd I., Papaloizos M., Zurn A.D., and Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci 15, 589, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Fon D., Al-Abboodi A., Chan P.P., Zhou K., Crack P., Finkelstein D.I., and Forsythe J.S. Effects of GDNF-loaded injectable gelatin-based hydrogels on endogenous neural progenitor cell migration. Adv Healthc Mater 3, 761, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Sakiyama S.E., Schense J.C., and Hubbell J.A. Incorporation of heparin-binding peptides into fibrin gels enhances neurite extension: an example of designer matrices in tissue engineering. FASEB J 13, 2214, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Sakiyama-Elbert S.E., and Hubbell J.A. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Controlled Release 65, 389, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Salek-Ardakani S., Arrand J.R., Shaw D., and Mackett M. Heparin and heparan sulfate bind interleukin-10 and modulate its activity. Blood 96, 1879, 2000 [PubMed] [Google Scholar]
- 19.Taylor S.J., McDonald J.W., 3rd, and Sakiyama-Elbert S.E. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J Controlled Release 98, 281, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Wood M.D., Borschel G.H., and Sakiyama-Elbert S.E. Controlled release of glial-derived neurotrophic factor from fibrin matrices containing an affinity-based delivery system. J Biomed Mater Res Part A 89A, 909, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Wood M.D., MacEwan M.R., French A.R., Moore A.M., Hunter D.A., Mackinnon S.E., Moran D.W., Borschel G.H., and Sakiyama-Elbert S.E. Fibrin matrices with affinity-based delivery systems and neurotrophic factors promote functional nerve regeneration. Biotechnol Bioeng 106, 970, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Wood M.D., Moore A.M., Hunter D.A., Tuffaha S., Borschel G.H., Mackinnon S.E., and Sakiyama-Elbert S.E. Affinity-based release of glial-derived neurotrophic factor from fibrin matrices enhances sciatic nerve regeneration. Acta Biomater 5, 959, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokai L.E., Bourbeau D., Weber D., McAtee J., and Marra K.G. Sustained growth factor delivery promotes axonal regeneration in long gap peripheral nerve repair. Tissue Eng Part A 17, 1263, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y.C., Ramadan M., Hronik-Tupaj M., Kaplan D.L., Philips B.J., Sivak W., Rubin J.P., and Marra K.G. Spatially controlled delivery of neurotrophic factors in silk fibroin-based nerve conduits for peripheral nerve repair. Ann Plast Surg 67, 147, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Wood M.D., Gordon T., Kim H., Szynkaruk M., Phua P., Lafontaine C., Kemp S.W., Shoichet M.S., and Borschel G.H. Fibrin gels containing GDNF microspheres increase axonal regeneration after delayed peripheral nerve repair. Regen Med 8, 27, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Eggers R., Hendriks W.T., Tannemaat M.R., van Heerikhuize J.J., Pool C.W., Carlstedt T.P., Zaldumbide A., Hoeben R.C., Boer G.J., and Verhaagen J. Neuroregenerative effects of lentiviral vector-mediated GDNF expression in reimplanted ventral roots. Mol Cell Neurosci 39, 105, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Tannemaat M.R., Eggers R., Hendriks W.T., de Ruiter G.C., van Heerikhuize J.J., Pool C.W., Malessy M.J., Boer G.J., and Verhaagen J. Differential effects of lentiviral vector-mediated overexpression of nerve growth factor and glial cell line-derived neurotrophic factor on regenerating sensory and motor axons in the transected peripheral nerve. Eur J Neurosci 28, 1467, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Eggers R., de Winter F., Hoyng S.A., Roet K.C., Ehlert E.M., Malessy M.J., Verhaagen J., and Tannemaat M.R. Lentiviral vector-mediated gradients of GDNF in the injured peripheral nerve: effects on nerve coil formation, Schwann cell maturation and myelination. PLoS One 8, e71076, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santosa K.B., Jesuraj N.J., Viader A., MacEwan M., Newton P., Hunter D.A., Mackinnon S.E., and Johnson P.J. Nerve allografts supplemented with Schwann cells overexpressing glial-cell-line–derived neurotrophic factor. Muscle Nerve 47, 213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu-Fienberg Y., Moore A.M., Marquardt L.M., Newton P., Johnson P.J., Mackinnon S.E., Sakiyama-Elbert S.E., and Wood M.D. Viral transduction of primary Schwann cells using a Cre-lox system to regulate GDNF expression. Biotechnol Bioeng 111, 1886, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquardt L.M., and Sakiyama-Elbert S.E. GDNF preconditioning can overcome Schwann cell phenotypic memory. Exp Neurol 265, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood M.D., and Sakiyama-Elbert S.E. Release rate controls biological activity of nerve growth factor released from fibrin matrices containing affinity-based delivery systems. J Biomed Mater Res A 84, 300, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Hudson T.W., Zawko S., Deister C., Lundy S., Hu C.Y., Lee K., and Schmidt C.E. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng 10, 1641, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Jesuraj N.J., Santosa K.B., Newton P., Liu Z., Hunter D.A., Mackinnon S.E., Sakiyama-Elbert S.E., and Johnson P.J. A systematic evaluation of Schwann cell injection into acellular cold-preserved nerve grafts. J Neurosci Methods 197, 209, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore A.M., Borschel G.H., Santosa K.A., Flagg E.R., Tong A.Y., Kasukurthi R., Newton P., Yan Y., Hunter D.A., Johnson P.J., and Mackinnon S.E. A transgenic rat expressing green fluorescent protein (GFP) in peripheral nerves provides a new hindlimb model for the study of nerve injury and regeneration. J Neurosci Methods 204, 19, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Edelstein A., Amodaj N., Hoover K., Vale R., and Stuurman N. Computer Control of Microscopes Using μManager. John Wiley & Sons, Inc., Hoboken, NJ, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter D.A., Moradzadeh A., Whitlock E.L., Brenner M.J., Myckatyn T.M., Wei C.H., Tung T.H., and Mackinnon S.E. Binary imaging analysis for comprehensive quantitative histomorphometry of peripheral nerve. J Neurosci Methods 166, 116, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boncompagni S., Kern H., Rossini K., Hofer C., Mayr W., Carraro U., and Protasi F. Structural differentiation of skeletal muscle fibers in the absence of innervation in humans. Proc Natl Acad Sci U S A 104, 19339, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao J.I., Sun C.K., Zhao H., Xiao Z.F., Chen B., Gao J., Zheng T.Z., Wu W., Wu S., Wang J.Y., and Dai J.W. The use of laminin modified linear ordered collagen scaffolds loaded with laminin-binding ciliary neurotrophic factor for sciatic nerve regeneration in rats. Biomaterials 32, 3939, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Daly W.T., Yao L., Abu-rub M.T., O'Connell C., Zeugolis D.I., Windebank A.J., and Pandit A.S. The effect of intraluminal contact mediated guidance signals on axonal mismatch during peripheral nerve repair. Biomaterials 33, 6660, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Hadlock T., Sundback C., Hunter D., Cheney M., and Vacanti J.P. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng 6, 119, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Saheb-Al-Zamani M., Yan Y., Farber S.J., Hunter D.A., Newton P., Wood M.D., Stewart S.A., Johnson P.J., and Mackinnon S.E. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp Neurol 247, 165, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoyng S.A., de Winter F., Gnavi S., de Boer R., Boon L.I., Korvers L.M., Tannemaat M.R., Malessy M.J.A., and Verhaagen J. A comparative morphological, electrophysiological and functional analysis of axon regeneration through peripheral nerve autografts genetically modified to overexpress BDNF, CNTF, GDNF, NGF, NT3 or VEGF. Exp Neurol 261, 578, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Yan Y., Sun H.H., Mackinnon S.E., and Johnson P.J. Evaluation of peripheral nerve regeneration via in vivo serial transcutaneous imaging using transgenic Thy1-YFP mice. Exp Neurol 232, 7, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Pajenda G., Hercher D., Marton G., Pajer K., Feichtinger G.A., Maleth J., Redl H., and Nogradi A. Spatiotemporally limited BDNF and GDNF overexpression rescues motoneurons destined to die and induces elongative axon growth. Exp Neurol 261, 367, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Shakhbazau A., Mohanty C., Shcharbin D., Bryszewska M., Caminade A.-M., Majoral J.-P., Alant J., and Midha R. Doxycycline-regulated GDNF expression promotes axonal regeneration and functional recovery in transected peripheral nerve. J Controlled Release 172, 841, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Bennett D.L., Michael G.J., Ramachandran N., Munson J.B., Averill S., Yan Q., McMahon S.B., and Priestley J.V. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci 18, 3059, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]