Abstract
Context: Severe acute malnutrition (SAM) accounts for approximately 1 million child deaths per year. High mortality is linked with comorbidities, such as diarrhea and pneumonia. Objective: The aim of this systematic review was to determine the extent to which carbohydrate malabsorption occurs in children with SAM. Data Sources: The PubMed and Embase databases were searched. Reference lists of selected articles were checked. Data Extraction: All observational and controlled intervention studies involving children with SAM in which direct or indirect measures of carbohydrate absorption were analyzed were eligible for inclusion. A total of 20 articles were selected for this review. Data Synthesis: Most studies reported carbohydrate malabsorption, particularly lactose malabsorption, and suggested an increase in diarrhea and reduced weight gain in children on a lactose-containing diet. As most studies reviewed were observational, there was no conclusive scientific evidence of a causal relationship between lactose malabsorption and a worse clinical outcome among malnourished children. Conclusion: The combined data indicate that carbohydrate malabsorption is prevalent in children with SAM. Additional well-designed intervention studies are needed to determine whether outcomes of SAM complicated by carbohydrate malabsorption could be improved by altering the carbohydrate/lactose content of therapeutic feeds and to elucidate the precise mechanisms involved.
Keywords: carbohydrate malabsorption, disaccharidase deficiency, F-75, lactose intolerance, malnutrition.
INTRODUCTION
Malnutrition in children is a great challenge in many developing nations, being directly or indirectly accountable for 3.1 million child deaths annually, or 45% of all child deaths in 2011.1 Severe acute malnutrition (SAM) is particularly problematic because of high case fatality rates.2,3 According to the joint statement of the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF), SAM includes 2 entities: severe wasting and nutritional edema.4–7 Severe wasting (marasmus) is defined as weight-for-height below −3 standard deviations (or z scores) or a mid-upper arm circumference (MUAC) of less than 115 mm.4–7 Nutritional edema (kwashiorkor) is defined by bilateral pitting edema. Severe wasting alone accounts for more than 500,000 child deaths per year, with some estimates of SAM mortality as high as 1 million per year.8
WHO treatment guidelines for SAM, first published in 19994 and recently updated in 2013,9 have standardized the treatment of SAM, and currently these guidelines play an important role in improving the outcomes of severe malnutrition.10 A major limitation of these treatment guidelines, however, is that the underpinning evidence base is sparse and is often of low or very low quality.9 One important area of uncertainty highlighted in the latest WHO guidelines surrounds optimal feeding regimens. The currently recommended diets for children with SAM include specially formulated therapeutic milks (F-75 and F-100) and an F-100–equivalent solid ready-to-use therapeutic food (RUTF). These are given at different phases of treatment in a stepwise approach. Sick children with complicated SAM are initially treated with F-75 milk, the aim of this phase being stabilization and prevention of early mortality through common complications that include hypoglycemia, diarrhea with dehydration, electrolyte imbalance, and infection. Toward these aims, F-75 has low sodium, protein, and fat contents but, as a consequence, a high carbohydrate content. It is during this stabilization phase that mortality is highest.11 Once stabilized, the child enters the rehabilitation phase of treatment, when weight gain is the aim and feeds are switched to the more nutrient-dense F-100 or RUTF, both of which contain higher amounts of protein and fat and lower amounts of carbohydrate than F-75.
SAM is associated with multiple comorbidities that may contribute to an increased risk of death, a prominent one being diarrhea.12 Several studies in children with SAM have shown that mortality is significantly higher in those with diarrhea than in those without diarrhea.12,13 In developing countries, a common cause of diarrhea is enteric infection,14 which, when associated with underlying malnutrition, could lead to villous blunting and, as a result, impaired carbohydrate absorption. In turn, significant decreases in carbohydrate absorption can lead to severe osmotic diarrhea.
There are currently few publications on absorption of different carbohydrates in SAM. To inform future modification of therapeutic feeds, it is necessary to determine the prevalence of carbohydrate malabsorption and to understand the possible impact of carbohydrate malabsorption on the recovery of malnourished children. This systematic review aims to evaluate the current research and to summarize current knowledge on carbohydrate malabsorption in children with SAM. Specifically, it aims to address the following questions: (1) Does carbohydrate malabsorption occur in children with SAM? If yes, to what extent? (2) What types of carbohydrates are malabsorbed? (3) Is carbohydrate malabsorption in children with SAM associated with osmotic diarrhea?
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standard reporting guidelines were used. A comprehensive literature search was performed in PubMed and Embase using the search terms shown in Appendix S1 in the Supporting Information online. An initial screening of title, abstract, and keywords was done with the filter “children” and using the search terms for direct or indirect evidence of carbohydrate malabsorption in malnourished children. The reference lists of the articles were further screened for additional relevant publications. Articles were thoroughly and systematically analyzed using the inclusion and exclusion criteria to identify both direct and indirect evidence to answer the research questions (Table 1). Scientific studies of all designs that investigated carbohydrate absorption in pediatric malnutrition from 1950 onward were included.
Table 1.
PICOS criteria for inclusion of studies
| Parameter | Criteria |
|---|---|
| Patient/population | Severely malnourished children. Children meeting the current WHO criteria were included. Also included were children who met the WHO criteria but for whom no information regarding specific criteria was provided, as well as children who met historical criteria only |
| Intervention | Dietary challenge with different carbohydrates or carbohydrate mixtures |
| Comparator | Placebo or a different carbohydrate as an intervention carbohydrate |
| Outcome | Direct or indirect markers of carbohydrate absorption |
| Setting | Intervention trials (randomized and nonrandomized) were included. Since it was expected that few studies met these criteria, observational studies were also included |
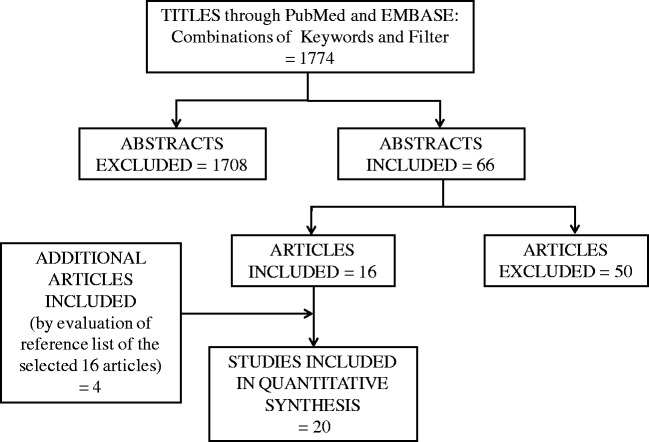
From the articles that were retrieved, studies conducted in children with SAM or that used the terms marasmus, kwashiorkor, or protein-energy malnutrition were included, since multiple definitions of SAM or protein-energy malnutrition have been used in recent decades.4–7,15 In studies in which a diagnosis of SAM was used without providing detailed information on weight-for-height or MUAC, this was noted. All observational studies in which measures of carbohydrate absorption were determined were included. Finally, for intervention studies, only interventions in which the carbohydrate content or composition was altered were included. Review articles, studies with data duplication, studies in adults, and studies not published in English were excluded (Figure 1). One reviewer performed the data extraction, which was independently checked by a second reviewer for accuracy. For controlled studies, wherever possible, numerical values for main outcome measures were extracted.
Figure 1.
PRISMA flow diagram of the literature search and selection process
RESULTS
Selection of articles
Figure 1 shows the search flowchart for the systematic review and the number of articles screened. From the 2 databases, PubMed and Embase, 1774 titles were found. After the titles were read, 1708 abstracts were excluded. The remaining 66 articles were then screened on the basis of the inclusion and exclusion criteria, and 50 articles were excluded, which left 16 articles of relevance. After evaluating the reference lists of these 16 articles, 4 additional articles were included (see Appendix S2 in the Supporting Information online). Overall, on the basis of the selection criteria, 20 articles were included in the review (Figure 1).
Dynamic tests of carbohydrate absorption
Multiple techniques were used to assess carbohydrate absorption in children with malnutrition. The blood glucose rise after carbohydrate tolerance test was the most common screening test for diagnosing carbohydrate malabsorption. For the oral tolerance test, generally 2 g of carbohydrate per kilogram of body weight dissolved in a 10% solution was given orally after a 6-hour fast, and capillary blood was then sampled every 30 minutes for 2 hours.16 Although not validated, if the blood glucose rises less than 30 mg/100 mL after the oral carbohydrate is administered, then intolerance is considered likely; increments of less than 20 mg/100 mL are generally considered diagnostic of malabsorption.17
Most studies reviewed here used a glucose response curve after an oral carbohydrate load; an overview is presented in Table 2. Children with SAM, when compared with controls, showed a decline in the average maximum glucose rise.18–20 Habte et al.21 demonstrated a significant rise in the concentration of blood glucose in malnourished children after administration of glucose + galactose, and sucrose, but not after administration of lactose. Three studies compared malnourished children before and after treatment; Viteri et al.22 reported a significant improvement in carbohydrate absorption after treatment, and Chandra et al.23 showed that 39% of children with SAM had an abnormal lactose loading test, whereas 16% had an abnormal glucose + galactose loading test before the nutritional recovery. James,17 however, showed no significant improvement in lactose or sucrose absorption after treatment.
Table 2.
Average maximum rise in blood glucose after carbohydrate administration
| Reference | Type of malnutrition | Children with diarrhea included | No. of subjects | Type of carbohydrate | Amount (g/kg) | Average maximum rise in blood glucose in mg/100 mL blood [mean (range) ± SD* or SEM**] |
P value | ||
|---|---|---|---|---|---|---|---|---|---|
| Malnourished | Controls | Treated | |||||||
| Bowie et al. (1965)24 | Kwashiorkor, SAMa | No | 3 | Lactose | 2 | 19 | – | – | – |
| Glucose + galactose | 2 | 51b | – | – | – | ||||
| Kerpel-Fronius et al. (1966)19 | SAMa | Yes | 14 | Lactose | 2–2.5 | 16.0 ± 11.0* | 66.0 ± 18.0* | – | – |
| Glucose + galactose | 2–2.5 | 49.0 ± 10.0* | 41.0 ± 15.0* | – | – | ||||
| Sucrose | 2–2.5 | 20.4 ± 13.62* | 20.4 ± 13.62* | – | – | ||||
| Chandra et al. (1968)23 | SAMa | No | 100 | Lactose | 2 | 80 | – | 125 | – |
| Glucose + galactose | 2 | 87 | – | 120 | – | ||||
| Cook et al. (1967)29 | Kwashiorkor | No | 15 | Lactose | 2 | 20.4 ± 13.62* | 24.0 ± 15.11* | – | – |
| Glucose + galactose | 1 + 1 | 39.27 ± 20.17* | 43.4 ± 15.53* | – | – | ||||
| Prinsloo et al. (1971)26 | SAMa | No | 10 | Lactose | 2 | 24.5 ± 16.5* | – | – | – |
| Glucose + galactose | 1 + 1 | 27.9 ± 23.04* | – | – | – | ||||
| James (1972)17 | MAM and SAMa | Yes | 8 | Lactose | 2 | 38.5 ± 22.61* | – | 28.75 ± 17.5* | – |
| Sucrose | 2 | 42.5 ± 29.35* | – | 51.57 ± 12.46* | – | ||||
| Bilir. (1972)18 | MAM and SAMa | Yes | 12 | Lactose | 2 | 14.5 (8–44) | 49 (48–75) | – | – |
| Glucose + galactose | 2 | 47.9 (36–68) | 53.8 (60–68) | – | – | ||||
| Viteri et al. (1973)22 | Kwashiorkor | Yes | 16 | Glucose | 1.7–2.5 | 107.5 ± 9.7** | – | 132.4 ± 10.1** | |
| Habte et al. (1973)21 | Kwashiorkor | Yes | 17 | Lactose | 2 | 78.5 ± 2.4** | – | – | |
| Sucrose | 2 | 98.5 ± 5.0** | – | – | |||||
| Glucose + galactose | 1 + 1 | 90.6 ± 4.5** | – | – | |||||
| Rothman et al. (1980)25 | Kwashiorkor | No | 12 | Lactose | 2 | 16.08 ± 9.94* | – | – | |
| Lifschitz et al. (1988)31 | SAMa | Yes | 17 | [U-13C] glucose | 0.005 | – | – | – | |
| Dextrose | 1.25 | – | – | – | |||||
| Verma & Saxena (1980)20 | Kwashiorkor | Yes | 12 | Lactose | 2 | 19 (18–20) | 36.5 (18–73) ± 10.1* | – | |
| Bandsma et al. (2011)30 | SAM | No | 15 | [U-13C] glucose | 1.75 | 79.2 ± 2.12* | 139 ± 2.26* | – | |
Abbreviations: SD, standard deviation; SEM, standard error of the mean.
aDefinition of SAM is not in accordance with the current WHO definition (which includes patients with marasmus).
bEstimated value from the curves.
Other studies, instead of comparing treated subjects with control subjects, just investigated plasma glucose increments in children with SAM to indicate malabsorption after administration of a carbohydrate load.24–26 Rothman et al.25 showed that glucose increments in 8 of 12 children with SAM fell below the cutoff value of 20 mg/100 mL, while increments in the remaining 4 were less than 30 mg/100 mL. When Bowie et al.24 carried out a glucose + galactose tolerance test and a lactose tolerance test in 3 malnourished children whose diarrhea had been controlled by a carbohydrate-free diet, lactose administration produced a much lower plasma glucose increment than a glucose + galactose bolus.
Comparison of absorption of different types of carbohydrates was also done by using the glucose + galactose combination as the reference carbohydrates and creating a ratio of other carbohydrates to this combination. Specifically, Bowie et al.27 illustrated an absorption ratio of carbohydrates against glucose + galactose in children with kwashiorkor and found that the plasma glucose increment was 143% for maltose, 124% for sucrose, and 36% for lactose, whereas James17 showed a 70% increase in glucose concentration after a lactose tolerance test in relation to glucose absorption after treatment in both moderately acute malnourished patients and SAM patients. Two studies also looked at lactose malabsorption in different types of SAM and found that the proportion of children with lactose malabsorption was highest in those with kwashiorkor, second highest in those with marasmic kwashiorkor, and lowest in those with marasmus.17,28 Overall, the above findings from the oral carbohydrate tolerance tests indicate that carbohydrate malabsorption is prevalent in children with SAM, and lactose intolerance, in particular, is a concern in these children.
Multiple studies also measured carbohydrate malabsorption by directly aspirating jejunal contents at 15-minute intervals after administration of different types of carbohydrates. This invasive technique was used by Cook et al.29 to illustrate a significant decrease in the absorption of lactose and glucose + galactose in children with SAM compared with healthy subjects. Using the same technique, James17 also observed a much lower absorption of all carbohydrates in malnourished children.
A recent study used U-13C labeled glucose for oral administration and [6,6-(2)H2]glucose for intravenous administration to calculate glucose absorption and found that the median cumulative glucose absorption was strongly decreased in children with SAM compared with controls.30 Similar findings were also seen in an earlier study by Lifschitz et al.,31 who used 13C-labeled glucose to illustrate a significantly delayed appearance of 13C in the blood of malnourished children with diarrhea compared with malnourished children without diarrhea. Torun et al.32 compared the effect of a hydrolyzed lactose diet with the effect of a regular lactose-containing diet on total energy absorption and signs of carbohydrate malabsorption using an H2 breath test during the early admission phase and during the recovery phase. No significant difference between the effects of the two diets on nutrient absorption was detected.
Fecal markers of carbohydrate malabsorption
The other most commonly used clinical methods are measurement of the fecal pH and output of water and carbohydrate. Measurement of fecal pH has been found in practice to be useful in controlling the dietary intake of carbohydrate in some malnourished children in rehabilitation centers, where rapid diagnosis of carbohydrate malabsorption is important.23,33 A pH of less than 5.5 (normal pH values range between 7 and 7.5) and the presence of reducing substances in the feces are indicative of carbohydrate intolerance and malabsorption as a result of villous atrophy. A higher mean stool weight and a higher lactic acid content are also consistent with carbohydrate malabsorption.
As shown in Table 3, reduced fecal pH was observed in children with SAM compared with controls in the studies that conducted carbohydrate tolerance tests, although the average pH was still more than 5.5 in all malnourished cohorts studied.18–20 In particular, Kerpel-Fornius et al.19 showed, in both SAM subjects and controls, a higher fecal pH in both the galactose + glucose and the sucrose tolerance tests than in the lactose tolerance test. Lower fecal pH was also found by Rothman et al.25 when a disaccharide-free diet was compared with a lactose-containing diet, and Chandra et al.23 showed that the feces of 50% of children with SAM had a pH below 6. Using a cutoff value of pH below 5.5 and the presence of reducing substances in the feces, Nyeko et al.28 showed lactose intolerance in 25.5% of children with SAM, of whom 36% had kwashiorkor and 24% marasmic-kwashiorkor. This was further supported by Beau et al.,34 who showed that 26% of malnourished children had carbohydrate malabsorption as evidenced by fecal pH below 5.5 and reducing carbohydrates in the stool. Furthermore, within this group of children, an indication of carbohydrate malabsorption was present in 47.4% of children in the 6- to 12-month age group and in 16% of children in the 13- to 24-month age group but was absent in the 25- to 36-month age group. This was further confirmed by Nyeko et al.,28 who found that the highest level of lactose intolerance was present within the 3- to 12-month age group (68%), and it is noteworthy that diarrhea worsened in 35% of these children after the therapeutic milk diet (F-75 and F-100) was initiated.
Table 3.
Average stool pH after different carbohydrate-based diets
| Reference | Type of malnutrition | Children with diarrhea included | No. of subjects | Average stool pH with different carbohydrate-based diets (mean ± SD* or SEM**) |
P value | |||
|---|---|---|---|---|---|---|---|---|
| Lactose | Galactose + glucose | Sucrose | Disaccharide free | |||||
| Kerpel-Fornius et al. (1966)19 | SAMa | Yes | 4 | 5.0 ± 0.8* | 6.1 ± 1.0* | 6.1 ± 1.0* | – | – |
| Controls | 10 | 6.8 ± 1.0* | 7.3 ± 1.0* | 7.5 ± 0.9* | – | – | ||
| Rothman et al. (1980)25 | Kwashiorkor | No | 12 | 5.8 ± 0.2** | – | – | 6.9 ± 0.2** | <0.01 |
| Verma & Saxena (1980)20 | Kwashiorkor | Yes | 2 | 5.7 | – | – | – | – |
| Controls | 10 | 6.9 | – | – | – | – | ||
Abbreviations: SD, standard deviation; SEM, standard error of the mean.
aDefinition of SAM is not in accordance with the current WHO definition (which includes patients with marasmus).
Four studies demonstrated a significant reduction in mean stool weight in children on a disaccharide-free diet compared with children on a lactose-containing diet (Table 4).24,25,27,35 Solomons et al.36 found a trend of lower average stool weight in subjects on a hydrolyzed-lactose diet compared with those on a lactose diet. Maclean and Graham37 demonstrated that children with SAM on a low-lactose diet had a mean stool weight nearly 3 times lower than that of convalescent children. In a large study performed in 120 children with kwashiorkor, Prinsloo et al.35 compared 6 different diets: 5 that contained different disaccharides and 1 that contained no disaccharide. They found that children on the disaccharide-free diet had the lowest average stool weight.
Table 4.
Stool weight and lactic acid content after different carbohydrate-based diets or tolerance tests
| Reference | Type of malnutrition | Children with diarrhea included | No. of subjects | Mean (range) ± SD* or SEM** |
P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactose |
Galactose + glucose |
Sucrose |
Disaccharide free |
|||||||||
| Average stool weight (g/24 h) | Average stool LA (mg/24 h)) | Average stool weight (g/24 h) | Average stool LA content (mg/24 h | Average stool weight (g/24 h) | Average stool LA content (mg/24 h) | Average stool weight (g/24 h) | Average stool LA content (mg/24 h) | |||||
| Bowie et al. (1965)24 | Kwashiorkor | No | 16 | 486 ± 308** | 2.91 ± 3.63** | – | – | – | – | 174 ± 64** | 0.12 ± 0.09** | <0.01 |
| 11 | 373 ± 345** | 1.49 ± 1.87** | 136 ± 91** | 0.07 ± 0.02** | <0.05 | |||||||
| Cook et al. (1967)29 | Kwashiorkor | No | 5 | 86.8 (0–325)^ | 0.054 (0–0.031)^ | 126.9 (0–410)^ | 0.082 (0–0.062)^ | – | – | – | – | |
| Bowie et al. (1967)27 | Kwashiorkor | No | 20 | 374 ± 449* | 1.58 ± 2.35* | – | – | – | – | 1084 ± 69* | 0.12 ± 0.15* | |
| Prinsloo et al. (1969)35 | Kwashiorkor | No | 120 | 375 | 1.8 | 164 | 0.075 | 144 | 0.075 | 116 | 0.01 | |
| Rothman et al. (1980)25 | Kwashiorkor | No | 12 | 307 ± 43** | – | – | – | – | – | 142 ± 25** | – | <0.01 |
Abbreviations: LA, lactic acid; SD, standard deviation; SEM, standard error of the mean.
Overall, the data from fecal examination conducted in the above studies suggest the prevalence of carbohydrate malabsorption in children with SAM, as determined by increased mean stool mass, the presence of reducing substances, and an acidic fecal pH.
Metabolic enzymes
The other indirect method used for assessing carbohydrate absorption is the measurement of lactase, sucrase, and maltase activities in jejunal mucosal biopsy samples.38,39 Mucosal disaccharidases, specifically, are essential for disaccharide absorption.
Based on the results of small-bowel biopsies, different studies observed reduced levels of disaccharidases in malnourished children, as summarized in Table 5.17,26,27,35 James17 further illustrated a rise in disaccharidase levels after treatment of both children with moderately acute malnutrition and children with SAM. In another study, 2 children with SAM showed normal lactase, sucrase, and maltase activities; 1 child with SAM had low sucrase and maltase activities and borderline low lactase activity, and 8 children with SAM had low lactase and sucrase activities, 6 of whom also had low maltase activity.27
Table 5.
Comparison of disaccharidase levels in malnourished children
| Reference | Type of malnutrition | Children with diarrhea included | No. of subjects | Mean ± SD |
P value | |||
|---|---|---|---|---|---|---|---|---|
| Lactase | Sucrase | Isomaltase | Maltase | |||||
| Bowie et al. (1967)27 | Kwashiorkor | No | 11 | 12.57 ± 24.62a 0.355 ± 0.69b | 57.63 ± 78.74a 1.89 ± 2.71b | ND | 297.06 ± 498.90a 10.01 ± 17.74b | – |
| Prinsloo et al. (1969)35 | Kwashiorkor | No | 120 | 7a | 35a | 51a | 100a | – |
| Prinsloo et al. (1971)26 | SAMc | No | 10 | 24.5 ± 7.69a | 50.08 ± 29.05a | 67.9 ± 32.15a | 185.7 ± 97.34a | – |
| James (1972)17 | MAM and SAMc | Yes | 8 | 3.2 ± 4.58b | 2.8 ± 2.64b | ND | ND | – |
| Treated | 8 | 3.15 ± 2.98b | 5.19 ± 1.67b | ND | ND | – | ||
Abbreviations: MAM, moderately acute malnutrition; ND, not determined; SAM, severe acute malnutrition.
aDisaccharidase levels: micromoles of substrate split per minute at 37°C/g of protein.
bDisaccharidase levels: micromoles of substrate split per minute at 37°C/g of wet weight.
cDefinition of SAM is not in accordance with the current WHO definition (which includes patients with marasmus)
Anthropometric markers
Measurement of anthropometric markers can be indirectly related to carbohydrate absorption and is harder to control for influencing factors. A study conducted in a cohort of 20 male children with SAM indicated that, despite the increased incidence of diarrhea in the cohort on a lactose-containing diet compared with the cohort on a lactose-free diet, both cohorts recovered well and in a similar fashion with regard to anthropometric characteristics.35 In contrast, 2 studies showed a decreased weight gain in children on a lactose-containing diet compared with children on a lactose-free diet.37,40 In 1 study in 20 malnourished children placed on a semielemental diet containing glucose and maltodextrin as the carbohydrates, the average weight gain after 21 days was 420 g, while in the 18 malnourished children on the cow’s milk–based diet, the average weight gain after 21 days was 110 g.40 The second study compared convalescent malnourished children with SAM children on a low-lactose diet and found that the weight change on average was 3.28 g/kg/day in convalescent malnourished children and 2.48 g/kg/day in SAM children.37
DISCUSSION
The current study is the first review to systematically evaluate carbohydrate absorption in severely malnourished children. Most of the publications included in this systematic review suggest different levels of carbohydrate malabsorption in children with SAM. Establishing a consistent overview of the current scientific research is helpful for designing intervention studies that can contribute data to improve upon the current WHO guidelines for treatment of SAM.
Of the 20 articles in this review, 19 reported some form of carbohydrate malabsorption in children with SAM. Most studies, using different approaches, showed decreased absorption of disaccharides, especially decreased absorption of lactose.17–30,34 Several studies reported that all lactose-containing diets significantly increased stool weight compared with disaccharide-free diets, which is indicative of lactose malabsorption and intolerance. Sucrose- and glucose + galactose–based diets resulted in slightly higher stool weights than disaccharide-free diets but much lower stool weights than lactose-based diets.35 A trend of a decrease in fecal pH and an increase in fecal lactic acid content was observed in malnourished children compared with controls, and the lowest pH values were seen in malnourished children on a lactose-containing diet.19,20,25 The combined findings provide consistent evidence that lactose malabsorption is widely present in children with SAM, with potentially more severely impaired absorption in children with kwashiorkor than in those with marasmus.21,26
Several studies showed decreased absorption of monosaccharides in malnourished children.18–20,22,23,29–32 As such, infants with acquired monosaccharide intolerance develop chronic acidic diarrhea secondary to carbohydrate malabsorption.41 Importantly, Lifschitz et al.31 observed that the monosaccharide absorption rates in children with SAM and diarrhea are as low as those in children with proven congenital glucose + galactose malabsorption, indicating that glucose or galactose may not be an effective therapeutic agent for malnutrition with coexisting diarrhea.
Diarrhea in children with SAM greatly increases the risk of mortality,11,13 and carbohydrate malabsorption can induce osmotic diarrhea. Few studies, however, have evaluated the possible relation between carbohydrate malabsorption and clinical outcome. In 2 studies, the anthropometric markers demonstrated a reduced weight gain in children with SAM on lactose-containing diets compared with those on lactose-free diets.37,40 In support, a study by Kukuruzovic and Brewster42 that included both malnourished and nonmalnourished children found better weight gain and a lower incidence of diarrhea after a trial with a low osmolarity lactose-free formulation compared with a partially hydrolyzed formulation. However, the reduced weight gain after the lactose-containing diet was not always observed.37 A study by Brewster et al.43 found lower rates of diarrhea in children with SAM who were fed a maize-based diet or a lactose-containing diet. However, the maize-based diet was associated with higher mortality rates, indicating certain advantages of a lactose-containing diet. Notwithstanding, the most consistent findings in the reviewed articles were those of lactose malabsorption in severely malnourished children and increased prevalence of diarrhea, especially in infants.17–19,23 Upon checking whether the children included in these studies were admitted with or without diarrhea and whether they were co-treated with antibiotics, it was found that, in 10 studies (50% of all reviewed studies), the children had diarrhea at admission. Only 4 of these 10 studies (20% of all reviewed studies), however, indicated the presence of culture-proven bacterial intestinal infection in these children.
Although findings across studies were broadly similar, the methodology of the studies and the validity of the results differed. The blood glucose rise after carbohydrate tolerance test has been used extensively as a screening test for diagnosing carbohydrate malabsorption,16 but the result of this test can be influenced by gastric emptying, factors affecting insulin release, disaccharidase availability, and the absorption of glucose.44 Since there is no clear clinical guideline for glucose increment reference values after an oral tolerance test to diagnose carbohydrate malabsorption, different values were used; for instance, 2 studies used higher values for malabsorption, which influenced the results.19,23 A low pH with a high stool weight per 24 hours indicates carbohydrate malabsorption in children,44 and Robayo-Torres et al.16 stated that a pH below 5.5 indicates glucose in the stool. The lowest fecal pH reported in the studies in this review was 5.7, and thus the findings based on fecal pH values may not be entirely consistent with carbohydrate malabsorption; nevertheless, all findings presented here indicated below-normal pH values (7–7.5) in all malnourished children. The breath hydrogen test is another tool widely used to diagnose carbohydrate intolerance, as the undigested carbohydrates are fermented by the gut flora and release hydrogen.16 However, this test is not a quantitative method and can have false negative results in patients with mucosal damage or those on antimicrobial therapy; occasional non-hydrogen production can also cause false negative results. To obtain a more quantitative evaluation, the method of isotope-labeled carbohydrate loading followed by breath analyses can be used.16 Future studies need to focus on the fecal osmotic gap to differentiate between osmotic and secretory diarrhea, thereby facilitating differential diagnosis of the origin of diarrhea.
There are several limitations to this study. As with all studies, there is a risk of publication bias, leading to an overrepresentation of studies in which significant changes in carbohydrate absorption were found. For this review, scientific studies that investigated carbohydrate absorption in pediatric malnutrition from 1950 onward were included. As the case definitions have changed over the years,4–7,15 the older studies may not necessarily be comparable with the most recent ones. In addition, for a number of studies, it was not clear what definition was used in the different tables, and this could have led to the inclusion of subjects with either moderately acute malnutrition or very severe acute malnutrition, i.e., selection bias. Historically, various classifications were used to diagnose severe malnutrition, i.e., severe wasting and edematous malnutrition. The Wellcome classification, introduced in 1970,45 was based on weight-for-age and was widely used. In light of the obvious disadvantages of using age and not accounting for stunting, Waterlow46 proposed a new classification in 1972 that was based on weight-for-height using the Harvard reference growth curves. The WHO introduced standardized criteria to interpret anthropometric indicators of nutritional statues only in 1986.47 In 1999, the WHO defined severe malnutrition in children as a weight-for-height below −3 standard deviations, which was based on the National Center for Health Statistics reference data, and/or the presence of edema to diagnose kwashiorkor.4 Mid-upper arm circumference was added as an independent criterion in 2005. The only recent study in the present review that included children on the basis of the current definition was published in 2011.30 Nevertheless, the data observed from all the other studies were consistent, regardless of whether a clear definition of severe malnutrition was provided.
Another limitation was that “uncomplicated” and “complicated” categories of SAM, with “complicated” meaning SAM accompanied by poor appetite or other danger signs such as fever, pneumonia, or hypothermia, could not be analyzed separately. As most of the studies included in this review were observational and, therefore, inconclusive, additional interventional and definitive studies are required to more accurately estimate the prevalence of lactose intolerance in children with SAM. Few studies used appropriate statistical techniques to evaluate findings, and there was limited direct evidence of how different types of carbohydrate malabsorption would affect the prognosis of the children with SAM. There have been a number of studies, including the one by Torun et al.,32 that evaluated carbohydrate absorption in the recovery phase, which was not the main aim of the current review. Leslie et al.48 studied lactose absorption after a prolonged period of admission for malnutrition and showed an abnormal response in 61% of children. A Peruvian study on lactose malabsorption that included children who had been previously malnourished indicated lactose malabsorption in a substantial proportion of these children, though not all of them.49
As most studies indicated some degree of disaccharide malabsorption, it is possible that a disaccharide-based treatment, particularly a lactose-based formula feeding, is not the optimal treatment in malnourished children, at least in the initial phase of treatment of SAM children with diarrhea. These findings are important because the therapeutic diets for malnutrition recommended in the WHO manual typically have high carbohydrate contents. These milk-based diets have high lactose levels (7.3 mg/L and 21 g/L–23 g/L for F-75 and F-100, respectively) as well as high osmolality (333 mOsmol/L and 419 mOsmol/L, respectively).50 Although there is no specific published data available on tolerability of F-75 or F-100, recently obtained data (authors’ unpublished data, June 2015) from an ongoing clinical trial in children with SAM in Malawi (the TranSAM study) showed that 9 of 74 children with SAM (12%) failed to transition to F-100 or an RUTF diet.51 Thus, the lower lactose content in F-75 during the initial phase of severe malnutrition (particularly for cases of SAM or kwashiorkor with persistent diarrhea), along with the use of a lactose-free milk, could possibly benefit selected cases of SAM.
FUTURE DIRECTIONS
Based on the conclusions from this study that carbohydrate malabsorption, in general, and lactose intolerance, in particular, are prevalent in children with SAM, multicenter observational studies are needed to confirm the link between SAM, carbohydrate malabsorption, and clinical outcomes, especially diarrhea. In addition, clinical trials are essential to determine whether reformulation of the nutritional stabilization diet, i.e., F-75, leads to a lower incidence of diarrhea and an overall improved clinical outcome. Two trials are currently ongoing to address the role of carbohydrates in diarrhea and clinical recovery in severe malnutrition (NCT02246296 [https://clinicaltrials.gov/ct2/show/NCT02246296] and ISRCTN13916953 [http://www.isrctn.com/ISRCTN13916953]). In 1 of these trials, the standard F-75 formula that provides approximately 63% of total energy from carbohydrates, including 10% from lactose, is replaced with a reformulated F-75 formula with a carbohydrate content reduced to 43% of total energy, without any lactose. Ready-to-use therapeutic food also has a relatively high carbohydrate content and often a high sucrose content of more than 20% by weight. Although patients generally have stabilized by the time RUTF is introduced, it is likely that enteropathy persists, especially in sicker children with SAM who require inpatient management. These patients might therefore be more vulnerable to the development of diarrhea when they receive formulations that contain a high amount of mono- and disaccharides. Specific studies aimed at determining whether the composition of RUTF can be optimized are urgently needed. If results from trials with F-75 or RUTF indeed indicate that a high carbohydrate content in these formulations has clinical relevance, this may have implications for post-discharge home-based nutrition. Nutritional counseling could then potentially include the advice to at least limit the amount of table sugar added to the diet. In addition, preclinical research is needed to study the underlying mechanisms of disturbed intestinal function and to test novel interventions in a model system, both in vitro and in vivo. The results from these studies can subsequently guide the design of new clinical interventions to help restore intestinal function in general and absorption of carbohydrates in particular. As there might be significant geographical differences with respect to the specific impairments in intestinal function, clinical trials should ideally be multinational, including patients from different geographical regions.
CONCLUSION
This review finds a consistently reported reduced capacity for carbohydrate absorption in severely malnourished children. The extent of carbohydrate malabsorption, the impact of malabsoprtion on severe diarrhea, dehydration, and other adverse clinical outcomes, and the relationship between malabsorption and infection are unclear, owing to the lack of conclusive studies. Most of the observational studies reviewed here suggested a prevalence of lactose malabsorption, while other studies suggested glucose + galactose malabsorption. The consistent observation of malabsorption of both monosaccharides and disaccharides could have profound implications for current treatment of severe malnutrition, since the therapeutic foods in most treatment protocols have a relatively high carbohydrate content.
Acknowledgment
The authors express their appreciation to Noortje Roerdink and Agnes Oosting for conducting the initial literature search for this study.
Financial disclosures. There were no external funding sources for this work.
Declaration of interest. The authors have no relevant interests to declare.
SUPPORTING INFORMATION
The following Supporting Information is available through the online version of this article at the publisher’s website.
Appendix S1. Search terms used to identify studies for potential inclusion in the review.
Appendix S2. List of the 20 articles included in the review.
References
- 1.Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. [DOI] [PubMed] [Google Scholar]
- 2.Schofield C, Ashworth A. Severe malnutrition in children: high case-fatality rates can be reduced. Afr Health. 1997;19:17–18. [PubMed] [Google Scholar]
- 3.Heikens GT, Bunn J, Amadi B, et al. Case management of HIV-infected severely malnourished children: challenges in the area of highest prevalence. Lancet. 2008;371:1305–1307. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Management of severe malnutrition: a manual for physicians and other senior health workers. http://www.who.int/nutrition/publications/en/manage_severe_malnutrition_eng.pdf. Published 1999. Accessed September 19, 2010. [Google Scholar]
- 5.World Health Organization–United Nations Children’s Fund. WHO child growth standards and the identification of severe acute malnutrition in infants and children: a joint statement by the World Health Organization and the United Nations Children’s Fund. http://www.who.int/nutrition/publications/severemalnutrition/9789241598163_eng.pdf. Published 2009. Accessed December 5, 2012. [Google Scholar]
- 6.Prudhon C, Prinzo ZW, Briend A, et al. Proceedings of the WHO, UNICEF, and SCN informal consultation on community-based management of severe malnutrition in children. Food Nutr Bull. 2006;27(3 suppl):S99–S104. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization–United Nations Children’s Fund. WHO, UNICEF, and SCN informal consultation on community-based management of severe malnutrition in children. http://www.who.int/child_adolescent_health/documents/fnb_v27n3_suppl/en/. Published 2006. Accessed July 2, 2012. [Google Scholar]
- 8.World Health Organization. Community-based management of severe acute malnutrition. A Joint Statement by the WHO, the World Food Programme, the United Nations System Standing Committee on Nutrition, and the United Nations Children’s Fund. http://www.who.int/nutrition/topics/Statement_community_based_man_sev_acute_mal_eng.pdf. Published May 2007. Accessed July 2, 2012. [Google Scholar]
- 9.World Health Organization. Updates on the management of severe acute malnutrition in infants and children (guideline). http://www.who.int/nutrition/publications/guidelines/updates_management_SAM_infantandchildren/en/index.html. Published 2013. Accessed January 27, 2014. [Google Scholar]
- 10.Ashworth A, Chopra M, McCoy D, et al. WHO guidelines for management of severe malnutrition in rural South African hospitals: effect on case fatality and the influence of operational factors. Lancet. 2004;363:1110–1115. [DOI] [PubMed] [Google Scholar]
- 11.Maitland K, Berkley JA, Shebbe M, et al. Children with severe malnutrition: can those at highest risk of death be identified with the WHO protocol? PLoS Med. 2006;3:e500 doi:10.1371/journal.pmed.0030500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brewster DR. Critical appraisal of the management of severe malnutrition: 3. Complications. J Paediatr Child Health. 2006;42:583–593. [DOI] [PubMed] [Google Scholar]
- 13.Talbert A, Thuo N, Karisa J, et al. Diarrhoea complicating severe acute malnutrition in Kenyan children: a prospective descriptive study of risk factors and outcome. PLoS One. 2012;7:e38321 doi:10.1371/journal.pone.0038321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhutta ZA, Ghishan F, Lindley K, et al. Persistent and chronic diarrhea and malabsorption: working group report of the Second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39 (suppl 2):S711–S716. [DOI] [PubMed] [Google Scholar]
- 15.Wellcome Trust Working Party. Classification of infantile manutrition. Lancet. 1970;ii:302–303. [Google Scholar]
- 16.Robayo-Torres CC, Quezada-Calvillo R, Nichols BL. Disaccharide digestion: clinical and molecular aspects. Clin Gastroenterol Hepatol. 2006;4:276–287. [DOI] [PubMed] [Google Scholar]
- 17.James WP. Comparison of three methods used in assessment of carbohydrate absorption in malnourished children. Arch Dis Child. 1972;47:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilir S. Acquired disaccharide intolerance in children with malnutrition. Am J Clin Nutr. 1972;25:664–671. [DOI] [PubMed] [Google Scholar]
- 19.Kerpel-Fronius E, Jani L, Fekete M. Disaccharide malabsorption in different types of malnutrition. Ann Paediatr. 1966;206:245–257. [PubMed] [Google Scholar]
- 20.Verma M, Saxena S. Lactose intolerance in children with protein-energy malnutrition. Indian J Pediatr. 1980;47:273–277. [DOI] [PubMed] [Google Scholar]
- 21.Habte D, Hyvarinen A, Sterky G. Carbohydrate malabsorption in kwashiorkor. Ethiop Med J. 1973;11:33–40. [PubMed] [Google Scholar]
- 22.Viteri FE, Flores JM, Alvarado J, et al. Intestinal malabsorption in malnourished children before and during recovery. Relation between severity of protein deficiency and the malabsorption process. Am J Dig Dis. 1973;18:201–211. [DOI] [PubMed] [Google Scholar]
- 23.Chandra RK, Pawa RR, Ghai OP. Sugar intolerance in malnourished infants and children. Br Med J. 1968;4:611–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowie MD, Brinkman GL, Hansen JD. Acquired disaccharide intolerance in malnutrition. J Pediatr. 1965;66:1083–1091. [DOI] [PubMed] [Google Scholar]
- 25.Rothman D, Habte D, Latham M. The effect of lactose on diarrhoea in the treatment of kwashiorkor. J Trop Pediatr. 1980;26:193–197. [DOI] [PubMed] [Google Scholar]
- 26.Prinsloo JG, Wittmann W, Kruger H, et al. Lactose absorption and mucosal disaccharidases in convalescent pellagra and kwashiorkor children. Arch Dis Child. 1971;46:474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowie MD, Barbezat GO, Hansen JD. Carbohydrate absorption in malnourished children. Am J Clin Nutr. 1967;20:89–97. [DOI] [PubMed] [Google Scholar]
- 28.Nyeko R, Kalyesubula I, Mworozi E, et al. Lactose intolerance among severely malnourished children with diarrhoea admitted to the nutrition unit, Mulago hospital, Uganda. BMC Pediatr. 2010;10:31 doi:10.1186/1471-2431-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook GC, Lakin A, Whitehead RG. Absorption of lactose and its digestion products in the normal and malnourished Ugandan. Gut. 1967;8:622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandsma RH, Spoelstra MN, Mari A, et al. Impaired glucose absorption in children with severe malnutrition [published online September 15, 2010]. J Pediatr. 2011;158:282–287.e1. [DOI] [PubMed] [Google Scholar]
- 31.Lifschitz CH, Boutton TW, Carrazza F, et al. A carbon-13 breath test to characterize glucose absorption and utilization in children. J Pediatr Gastroenterol Nutr. 1988;7:842–847. [DOI] [PubMed] [Google Scholar]
- 32.Torun B, Solomons NW, Caballero B, et al. The effect of dietary lactose on the early recovery from protein-energy malnutrition. II. Indices of nutrient absorption. Am J Clin Nutr. 1984;40:601–610. [DOI] [PubMed] [Google Scholar]
- 33.Wharton B, Howells G, Phillips I. Diarrhoea in kwashiorkor. Brit Med J. 1968;4:608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beau JP, Fontaine O, Garenne M. Management of malnourished children with acute diarrhoea and sugar intolerance. J Trop Pediatr. 1989;35:281–284. [DOI] [PubMed] [Google Scholar]
- 35.Prinsloo JG, Wittmann W, Pretorius PJ, et al. Effect of different sugars on diarrhoea of acute kwashiorkor. Arch Dis Child. 1969;44:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solomons NW, Torun B, Caballero B, et al. The effect of dietary lactose on the early recovery from protein-energy malnutrition. I. Clinical and anthropometric indices. Am J Clin Nutr. 1984;40:591–600. [DOI] [PubMed] [Google Scholar]
- 37.MacLean WC, Jr, Graham GC. Evaluation of a low-lactose nutritional supplement in malnourished children. J Am Diet Assoc. 1975;67:558–564. [PubMed] [Google Scholar]
- 38.Dahlqvist A. Method for assay of intestinal disaccharidases. Analyt Biochem. 1964;7:18–25. [DOI] [PubMed] [Google Scholar]
- 39.Burke V, Kerry KR, Anderson CM. The relationship of dietary lactose to refractory diarrhoea in infancy. J Paediatr Child Health. 1965;1:147–160. [Google Scholar]
- 40.Eichenberger JR, Hadorn B, Schmidt BJ. A semi-elemental diet with low osmolarity and high content of hydrolyzed lactalbumin in the treatment of acute diarrhea in malnourished children. Arq Gastroenterol. 1984;21:130–135. [PubMed] [Google Scholar]
- 41.Klish WJ, Rodriguez JT, Soriano H, et al. Morphologic basis for glucose malabsorption in infants with acquired monosaccharide intolerance (AMI) [abstract]. Pediatr Res. 1974;8:382 doi:10.1203/00006450-197404000-00255. [Google Scholar]
- 42.Kukuruzovic RH, Brewster DR. Milk formulas in acute gastroenteritis and malnutrition: a randomized trial. J Paediatr Child Health. 2002;38:571–577. [DOI] [PubMed] [Google Scholar]
- 43.Brewster DR, Manary MJ, Menzies IS, et al. Comparison of milk and maize based diets in kwashiorkor. Arch Dis Child. 1997;76:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMichael HB. Disorders of carbohydrate digestion and absorption. Clin Endocrinol Metab. 1976;5:627–649. [DOI] [PubMed] [Google Scholar]
- 45.Wellcome Trust Working Party. Classification of infantile malnutrition. Lancet. 1970;ii:302–303. [Google Scholar]
- 46.Waterlow JC. Classification and definition of protein-calorie malnutrition. Br Med J. 1972;3:566–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.WHO Working Group. Use and interpretation of anthropometric indicators of nutritional status. Bull World Health Organ. 1986;64:929–941. [PMC free article] [PubMed] [Google Scholar]
- 48.Leslie J, MacLean WC, Jr, Graham GG. Effect of an episode of severe malnutrition and age on lactose absorption by recovered infants and children. Am J Clin Nutr. 1979;32:971–974. [DOI] [PubMed] [Google Scholar]
- 49.Paige DM, Leonardo E, Cordano A, et al. Lactose intolerance in Peruvian children: effect of age and early nutrition. Am J Clin Nutr. 1972;25:297–301. [DOI] [PubMed] [Google Scholar]
- 50.Gracey M, Burke V. Sugar-induced diarrhoea in children. Arch Dis Child. 1973;48:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laboratory of Paediatrics, University Medical Center Groningen (The Netherlands). Evaluation of three standard dietary regimes in the treatment of severe malnutrition – a randomized control trial: The ‘TranSAM study'. ISRCTN. 2013. http://www.isrctn.com/ISRCTN13916953. Accessed April 16, 2015. [Google Scholar]