Abstract
Genetic engineering of donor pigs to eliminate expression of the dominant xenogeneic antigen galactose α1,3 galactose (Gal) has created a sea change in the immunobiology of xenograft rejection. Antibody mediated xenograft rejection of GGTA-1 α-galactosyltransferase (GTKO) deficient organs is now directed to a combination of non-Gal pig protein and carbohydrate antigens. Glycan analysis of GTKO tissues identified no new neo-antigens but detected high levels of N-acetylneuraminic acid (Neu5Gc) modified glycoproteins and glycolipids. Humans produce anti-Neu5Gc antibody and in very limited clinical studies sometimes show an induced anti-Neu5Gc antibody response after challenge with pig tissue. The pathogenicity of anti-Neu5Gc antibody in xenotransplantation is not clear however as non-human transplant models, critical for modelling anti-Gal immunity, do not produce anti-Neu5Gc antibody. Antibody induced after xenotransplantation in non-human primates is directed to an array of pig endothelial cells proteins and to a glycan produced by the pig B4GALNT2 gene. We anticipate that immune suppression will significantly affect the T-cell dependent and independent specificity of an induced antibody response and that donor pigs deficient in synthesis of multiple xenogeneic glycans will be important to future studies.
Introduction
Xenotransplantation using pig organs has in recent years made significant advances in vascularized graft survival with median pig-to-baboon heterotopic cardiac xenograft survival beyond 6 months and individual survival in excess of 1 year (1). Increased cardiac xenograft survival is based on progressive improvements in genetically engineered donor organs and improvements in chronic immune suppression (2). Antibody mediated rejection (AMR) is the predominant form of vascularized xenograft rejection in which terminal galactose α1,3 galactose (Gal) saccharide is the dominant xenogeneic antigen. Humans and Old World non-human primates (NHP) do not make Gal but instead produce high levels of anti-Gal antibody (3) resulting in hyperacute rejection (HAR). HAR can be prevented by depletion or blocking anti-Gal antibody prior to transplant (4, 5). Pigs were engineered with a mutation in the GGTA-1 alpha-galactosyltransferase gene (GalT-KO pigs) to eliminate the Gal antigen. Extensive biochemical studies of GalT-KO porcine glycolipids and glycoproteins (6–9), the loss of tolerance and spontaneous expression of anti-Gal antibody in GalT-KO pigs (10), and the absence of an induced anti-Gal antibody response after GalT-KO organ xenotransplantation (11) all support the full elimination of the Gal antigen from GalT-KO pigs. The advent of GalT-KO pigs did not completely eliminate AMR but instead revealed the significance of a less abundant and more diverse set of antibody which mediates GalT-KO xenograft rejection by binding to “non-Gal” pig antigens. This review summarizes our current understanding of non-Gal antibodies (NGal-Ab) and antigens in NHP, the major xenotransplantation model, and in humans.
Non-Gal Antibody and Antigen: Definition
NGal-Ab has been defined based on the technologies available at the time. Lam et al (12) first identified a pathogenic role for NGal-Ab by correlating the emergence of non-Gal IgM and IgG with humoral cardiac xenograft rejection. Their analysis identified NGal-Ab by immunoabsorbing serum using Gal-coated Sepharose beads. Prior to the availability of GalT-KO pigs similar strategies of immune absorption, soluble Gal competition, or antigen depletion were commonly used to measure serum NGal-Ab (13–15). These studies were unable to fully eliminate the possibility of residual anti-Gal reactivity, however, their observations accurately presaged the role of NGal-Ab mediated graft rejection confirmed in later GalT-KO donor organ studies (11, 16–18). For this review NGal-Ab is defined as human and NHP antibody which binds to GalT-KO pig cells (19).
Preformed non-Gal Antibody: Abundance and Pathogenicity
Cytotoxic NGal-Ab is present in both human and NHP serum. Rood et al (20) surveyed human, baboon and cynomologus monkey serum for antibody binding and cytotoxicity to conventional Gal-positive (GalT+) and GalT-KO pig peripheral blood mononuclear cells (PBMNCs) and showed approximately 50% of human and baboon serum samples and 75% of cynomologus monkey serum exhibited significant cytotoxicity to GalT-KO PBMNCs. NGal-Ab cytotoxicity to porcine aortic endothelial (PAEC) and liver sinusoidal endothelial cells (LSEC) has also been reported (21, 22).
In NHPs pre-existing NGal-Ab is clearly pathogenic. In a comparison of GalT-KO and GalT-KO:CD55 donor organs Byrne et al (23) reported a case of HAR for a GalT-KO pig-to-baboon heterotopic cardiac xenograft. Rejection occurred after 90 minutes with widespread intramyocardial haemorrhage, vascular antibody and complement deposition. While HAR of GalT-KO organs is rare, early immune injury has been reported (24, 25) and interim biopsies 7 days post transplant detect vascular antibody and complement deposition presaging myocardial injury (17).
The very limited number of clinical xeno-studies, performed several years ago, have all used GalT+ pig kidneys (26, 27), livers (28–30) or porcine hepatocytes (31). Therefore, no information regarding the contribution of NGal-Ab to the extensive tissue injury is available.
Baboon non-Gal antibodies
Two general approaches have been used to identify potential non-Gal antigens, profiling serum antibody reactivity to identify immunoreactive porcine antigens and biochemical studies comparing the antigenic profile of GalT-KO porcine and human tissues. Biochemical studies have largely focused on identifying porcine specific carbohydrate antigens.
Sensitized baboon sera, obtained after xenotransplantation of various pig GalT+ or GalT-KO organs, has been used to profile the specificity of NGal-Ab (6, 15, 25, 32–34). An early study used Gal-specific immune absorption to measure NGal-Ab binding to GalT+ pig red blood cells (RBCs). The induced NGal-Ab level was about 4% the level of induced anti-Gal antibody and non-Gal binding to RBCs was not diminished after treating cells with α-galactosidase or other exoglycosidases. This suggested that the NGal-Ab was mainly directed to pig proteins. Yeh et. al. (32) used an ELISA assay to measure antibody reactivity to a series of neo-glycoconjugates representing suspected glycan antigens (Forssman, A/B-blood group tri-saccharides, Lewis antigens, P1, Pk, Gal, α and β lactosamine and sulphated lactosamine). Naïve human and baboon serum reacted primarily with blood group A/B antigens, Gal, α-lactosamine, Forssman, P1 and Pk antigens. Highly sensitized baboon serum showed significantly higher IgG binding to GalT-KO cells, but did not show increased reactivity to any of the neo-glycoconjugates. These results demonstrated that some prospective glycans (Forssman, and lactosamine) were unlikely NGal-Ab targets and further supported a predominantly protein directed NGal-Ab response in baboons.
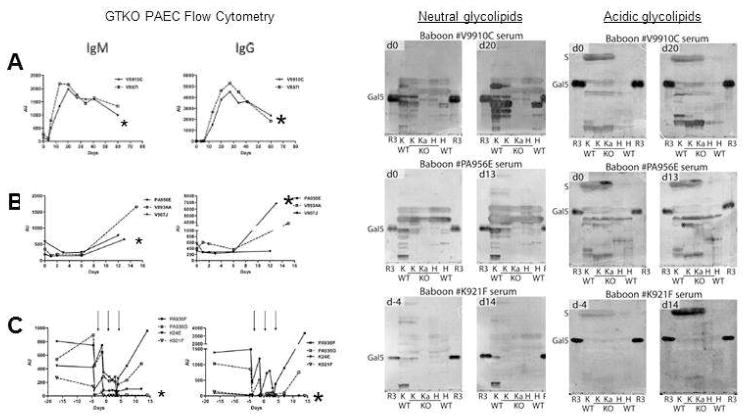
A glycan reactive NGal-Ab response was initially reported by Diswall et al (6) after GalT-KO cardiac xenotransplantation. Post transplant baboon serum showed increased binding to a trace, acidic glycolipid extracted from GalT-KO heart tissue. In a recent study a complex induced NGal-Ab response to both neutral and acidic glycolipids was reported after GalT-KO kidney xenotransplantation (Figure 1) (25). Control non-immunosuppressed recipients showed the strongest induction of NGal-Ab evidenced by binding to GalT-KO PAECs and to neutral and acidic glycolipids. While NGal-Ab from these recipients appeared to show proportionate reactivity to GalT-KO cells and glycolipids, there remained a high level of individual variability in the diversity and intensity of antibody response. This limits the general conclusions about immunogenicity that can be drawn from a limited number of cases.
Figure 1.
Antibody responses to GalT-KO PAECs and glycolipids after pig-to-baboon GalT-KO:CD55/59/39/H-transferase kidney xenotransplantation. Left: Binding of serum IgM and IgG to GalT-KO donor PAECs (left) A; Control not immune suppressed recipients. B; Immune suppressed recipients. C; Immune suppressed recipient further treated with plasmapheresis (arrows). Right: The corresponding antibody (IgG + IgM) reactivity for each of these recipients to neutral and acidic glycolipids from GalT+) and GalT-KO (KO) pig individuals separated on thin-layer chromatography plates. Glycolipids from different pig kidneys were applied (lanes K and Ka) and pig hearts (lanes H) together with reference Galα3nLc4 glycolipid (lane R3). Anti-PAEC profiles for the individual animals tested in the TLC immmunostainings are indicated by asterix to the left. Recipients V9910C (A) and PA956E (B) show a strong to moderate increase in nonGal IgM and IgG while recipient K921F (C) shows minimal induction of anti-nonGal antibody. Adapted from Le Bas-Bernardet et al. (25).
The baboon NGal-Ab response was also analysed using proteomic technologies and by expression library screening. Sensitized baboon IgG exhibited variable but proportionate binding to GalT-KO PAECs in flow cytometry and to a limited set of immunodominant antigens in 1D western blots suggesting IgG binding was to a similar or overlapping set of GalT-KO PAEC membrane antigens.(34). Furthermore, 2D- western blots identified 40 immunoreactive proteins of which 16, directed to fibronectin, stress response and inflammation associated proteins, were considered likely candidates to contribute to graft rejection. Subsequently this group screened a retrovirus encoded PAEC cDNA expression library which could only identify porcine cDNA encoded antigens expressed on the surface of library transduced humans embryonic kidney (HEK) cells (33). They identified 5 pig endothelial cell membrane proteins involved in complement regulation and haemostasis (Table 1). Antibody reactivity to these pig proteins, expressed on HEK cells, was most likely primarily directed to immunogenic polypeptides and not to carbohydrate epitopes. The exception to this was the recovery of the pig β1,4 N-acetylgalactosaminyltransferase (B4GALNT2) gene. B4GALNT2 is a glycosyltransferase which catalyzes the terminal addition of N-acetylgalactosamine to a sialic acid modified lactosamine to produce GalNAcβ4[Neu5Acα2,3]Gal β4GlcNAcβ3Gal, the Sda blood group antigen. This terminal trisaccharide is also present on the GM2 gangliosides found in human organs. Porcine B4GALNT2 expression in human HEK cells (HEK-B4T) resulted in increased reactivity with anti-Sda antibody and increased sensitivity to complement mediated lysis (35). Induced NGal-Ab, detected by binding to GalT-KO PAECs correlates with NGal-Ab which preferentially bound to HEK-B4T cells suggesting that induced antibody to this glycan is frequent in pig-to-baboon cardiac xenotransplantation (Table 2). Most humans produce low levels of antibody to Sda (36). It remains to be determined if the porcine B4GALNT2 produced antigen will be immunogenic in humans.
Table 1.
Amino acid diversity of endothelial cell proteins
| Specific non-Gal antigens* | Gene name | Amino acid identity (%) |
|---|---|---|
| NM_214006.1 | CD9*; tetraspanin | 89.5 |
| NM_213888.1 | CD46*; MCP | 47.4 |
| NM_214170.1 | CD59*; protectin | 48.0 |
| NM_001163406 | PROCR*; EC protein C receptor | 71.2 |
| NM_001005726 | ANAX2* | 98.2 |
| NM_001244330.1 | B4GALNT2 | 75.8 |
| Common endothelial cell proteins | ||
| NM_213816 | CD54; ICAM-1 | 53.6 |
| NM_213891 | CD106; VCAM | 54.9 |
| NM_001001631 | CD102; ICAM-2 | 56.1 |
| NM_214268 | CD62E; E-selectin | 58.2 |
| NM_214086 | CD34 | 64.7 |
| NM_213907 | CD31; PECAM | 71.5 |
| NM_001001649 | CDH5; VE-Cadherin | 78.9 |
| XM_003358192 | SCARF1; acetylated-LDL receptor | 79.3 |
| XM_005667691 | CD36 | 83.0 |
| NM_001083932 | CD51; ITGAV, Vitronectin receptor | 95.0 |
The percent amino acid identity of the porcine protein compared to the human sequence was calculated using the European Molecular Biology Laboratory EMBOSS WATER pairwise protein sequence alignment algorithm. On average these porcine genes show only 71% amino acid identity to the human homolog.
An induced antibody response has been reported to these endothelial cell membrane products after pig-to-baboon heterotopic cardiac xenotransplantation (33).
Table 2.
Induced NGal-Ab after pig-to-baboon cardiac xenotransplantation.
| Donor | Survival (days) | HEK-B4T IgM | HEK-B4T IgG | GalT-KO PAEC IgM | GalT-KO PAEC IgG |
|---|---|---|---|---|---|
| GalT-KO:CD55 | 71 | − | + | 0.5 | 0.6 |
| GalT-KO:CD55 | 31 | − | − | 0.5 | 0.4 |
| GalT-KO:CD55 | 28 | +++ | +++ | 4.8 | 2.5 |
| GalT-KO:CD55 | 27 | +++ | + | 2.2 | 8.5 |
| GalT-KO | 22 | +++ | ++++ | 1.9 | 7.3 |
| GalT-KO | 21 | − | − | 1.3 | 0.9 |
| GalT-KO:CD55 | 18 | − | − | 0.4 | 0.4 |
Antibody reactivity to HEK-B4T cells is estimated as the ratio of specific antibody binding to HEK-B4T relative to HEK cells and represented in a semi-quantitative scale with ratios less then 2 scored as negative, 2.0–2.5 +, 2.5–5.0 ++, 5.0–10.0 +++ and greater then 10 ++++. Antibody to GalT-KO PAECs is the fold increase in MFI in post explant serum compared to pretransplant serum. There is a positive and significant (p<0.05) Spearman rank correlation coefficient between the anti-PAEC and anti-HEK-B4T IgM (r = 0.8929) and IgG (r = 0.8571) immune response. Adapted from Byrne et al (35).
Human non-Gal antibodies
Human serum contains autoreactive antibody which may contribute to immune surveillance, regulation and clearance of cellular debris (37). These autoreactive B-cells may be a reservoir of potential NGal-Ab reactivity to the cognate pig antigens. Human serum also contains antibody which binds to a complex array of sialic acid modified glycans carrying N-glycolylneuraminic acid (Neu5Gc) (38). Neu5Gc and the related N-acetylneuraminic acid (Neu5Ac) are the most common form of sialic acid in mammalian tissue, however humans are unique in that they are deficient in cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) required to synthesize Neu5Gc. As a consequence humans do not make Neu5Gc, although they do incorporate it from dietary sources, but they do make anti-Neu5Gc antibody. Anti-Neu5Gc antibody is responsible for inducing serum sickness in patients treated with multiple doses of animal serum (39) but its pathogenicity in xenotransplantation is less clear as baboons do not produce this antibody. CMAH−/− knockout mice make anti-Neu5Gc antibody, and inhibit engraftment of low doses of isogenic CMAH+/+ islets. Inhibition is overcome however with a larger dose of islets and isogenic CMAH+/+ hearts are not rejected by CMAH−/− recipients.(40) Under similar conditions GalT-KO mice reject isogenic GalT+ mouse hearts (41) suggesting that the pathogenicity of anti-Neu5Gc antibody may be less then anti-Gal antibody.
The clinical antibody response to GalT+ pig tissues has been examined for evidence of NGal-Abs. Induced antibody from diabetic patients treated with porcine fetal islets was initially reported to be only anti-Gal antibody (42). Subsequent studies initially failed to find evidence for a general induced anti-Neu5Gc response (43) but more recently have detected induced anti-Neu5Gc specific antibody in some patients (44). A dominant anti-Gal immune response was also seen in the two patients participating in an ex vivo pig kidney perfusion trial (45) and one individual produced anti-ganglioside antibodies (46). Burn patients treated with whole pig skin showed a moderate increased in anti-Gal antibody but a sustained increase in anti-Neu5Gc antibody reactivity (47). These limited studies suggest that the human immune response is primarily directed to oligosaccharide antigens. However, direct exposure of humans to GalT-KO tissues has not been reported and the duration of exposure in these studies, especially for vascularized organs, was limited.
Burlak et al (48) have reported a detailed proteomic study of naïve human antibody reactivity to GalT-KO porcine LSEC membrane antigens. They identified 19 NGal-Ab targeted LSEC membrane proteins. Some of these, α-enolase, CD9, and fibronectin were previously identified as non-Gal antigens recognized by baboon (33, 34). Naïve human antibody binding to GalT-KO porcine fibronectin is in part due to anti-Neu5Gc antibody reactivity (49).
Non-Gal Pig Antigens
An alternative method to identify putative non-Gal pig antigens is to compare the antigenic profile of pig and human tissues to identify unique porcine antigens. At the protein level 80 million years of separate evolution has led to a high level of polypeptide diversity between pigs and humans (Table 2). Non-Gal antigens identified in pig-to-baboon transplants and common endothelial cell proteins show variable amino acid identity to their cognate human proteins. This high degree of peptide diversity is not likely to be alleviated by gene replacement so will require chronic immune suppression or systemic tolerance induction to prevent an induced NGal-Ab response.
The GalT-KO glycome has been screened for unique antigens which may have arisen as a result of the GGTA-1 mutation. Detailed comparisons of glycolipids from GalT+ and GalT-KO pig tissues (6, 7) find no evidence of the Gal antigen in GalT-KO samples but do detect increased amounts of lactosamine (Galβ4GlcNAc) the immediate precursor for both Gal and blood group H (O) antigen. Increased levels of P1 and x2 glycolipids have also been detected but these are also normal human glycans. Mass spectroscopy analysis of N-linked kidney glycans confirmed the abundance of Gal, present in up to 50% of complex glycans, and detected Neu5Gc containing oligosaccharides (50). Neu5Gc was also reported on O-linked pig heart glycans (51). Lectin microarrays have been used to capture fluorescent labelled glycoproteins derived from GalT+ and GalT-KO pig as well as human tissues (52). In addition to elimination of the Gal antigen from GalT-KO cells, GalT-KO EC showed reduced expression of terminal αGalNAc and Galβ3GalNAc containing oligosaccharides and modest increases in α2,3 and α2,6 silaic acid containing glycans. Similar results were reported for porcine islets (53). In no instance was a new oligosaccharide detected in GalT-KO samples (6, 7), suggesting the GGTA-1 mutation resulted in only moderate quantitative changes in the pig glycome.
Conclusions
The use of GalT-KO donor pigs has shifted the immune biology of xenograft rejection from a single dominant antigen to potentially many different non-Gal antigens. The baboon is now a less effective immune model since baboons do not produce a major human NGal-Ab specificity, anti-Neu5Gc. None-the-less the baboon shows NGal-Ab responses to both protein and glycan antigens and similar induced NGal-Ab responses are likely in humans. It is essential however to define the precise human NGal-Ab response in future clinical trials using GalT-KO tissues. Further, we expect that induction of T-dependent antibody to polypeptides would be diminished or blocked with co-stimulation based immune suppression (1) while synthesis of heterophile germline encoded antibody to glycans may not. In that regard it is notable that pigs engineered with mutations that block synthesis of the known xenogeneic glycans, GGTA-1 (Gal), CMAH (Neu5Gc) and B4GALNT2 (Sda) show the lowest level of antibody reactivity in both human and baboon serum (54). Future preclinical studies should incorporate transgenes for complement and haemostasis regulation into this minimally antigenic triple KO background to minimize donor immunogenicity and the potential chronic effects of heterophile xenoreactive antibody.
Highlights.
Pigs deficient in the synthesis of the major xenogeneic antigen Gal (GTKO) have significantly altered antibody mediated xenograft rejection which is now directed to non-Gal antigens.
Data evaluating clinical human non-Gal antibody responses to pig tissue is rare, but, where available shows variable induction of anti-Neu5Gc antibody.
Nonhuman primates do not make anti-Neu5Gc antibody so the pathogenicity of this specificity remains unknown.
Nonhuman primates induce antibody to pig EC proteins and an Sda-like glycan made by porcine B4GALNT2.
Acknowledgments
Funding
Funding for this work was provided to Dr. Byrne and McGregor by an Immunobiology of Xenotransplantation cooperative research grant (AI066310) from the National Institute of Allergy and Infectious Disease at the National Institute of Health, by Comprehensive Biomedical Research Centre funds from the National Institute of Health Research, and supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. Dr. Breimer was supported by governmental grants to the Sahlgrenska University Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML, 3rd, Lewis BG, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014 Feb;14(2):488–489. doi: 10.1111/ajt.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrne GW, Azimzadeh AM, Ezzelarab M, Tazelaar HD, Ekser B, Pierson RN, et al. Histopathologic insights into the mechanism of anti-non-Gal antibody-mediated pig cardiac xenograft rejection. Xenotransplantation. 2013 Sep-Oct;20(5):292–307. doi: 10.1111/xen.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galili U, Shohet SB, Kobrin E, Stults CM, Macher BA. Man, apes, and old world monkeys differ from other mammals in the expression of alpha-Galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263(33):17755–17762. [PubMed] [Google Scholar]
- 4.Leventhal JR, Sakiyalak P, Witson J, Simone P, Matas AJ, Bolman RM, et al. The synergistic effect of combined antibody and complement depletion on discordant cardiac xenograft survival in nonhuman primates. Transplantation. 1993;57(6):974–978. [PubMed] [Google Scholar]
- 5.Diamond LE, Byrne GW, Schwarz A, Davis TA, Adams DH, Logan JS. Analysis of the control of the anti-Gal immune response in a non-human primate by galactose alpha-1-3 galactose trisaccharide-polyethylene glycol conjugate. Transplantation. 2002 Jun 15;73(11):1780–1787. doi: 10.1097/00007890-200206150-00014. [DOI] [PubMed] [Google Scholar]
- 6.Diswall M, Angstrom J, Karlsson H, Phelps CJ, Ayares D, Teneberg S, et al. Structural characterization of alpha1,3-galactosyltransferase knockout pig heart and kidney glycolipids and their reactivity with human and baboon antibodies. Xenotransplantation. 2010 Jan;17(1):48–60. doi: 10.1111/j.1399-3089.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- 7.Diswall M, Angstrom J, Schuurman HJ, Dor FJ, Rydberg L, Breimer ME. Studies on glycolipid antigens in small intestine and pancreas from alpha1,3-galactosyltransferase knockout miniature swine. Transplantation. 2007 Nov 27;84(10):1348–1356. doi: 10.1097/01.tp.0000287599.46165.15. [DOI] [PubMed] [Google Scholar]
- 8.Nottle MB, Beebe LF, Harrison SJ, McIlfatrick SM, Ashman RJ, O’Connell PJ, et al. Production of homozygous alpha-1,3-galactosyltransferase knockout pigs by breeding and somatic cell nuclear transfer. Xenotransplantation. 2007 Jul;14(4):339–344. doi: 10.1111/j.1399-3089.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 9.Diswall M, Gustafsson A, Holgersson J, Sandrin MS, Breimer ME. Antigen-binding specificity of anti-alphaGal reagents determined by solid-phase glycolipid-binding assays. A complete lack of alphaGal glycolipid reactivity in alpha1,3GalT-KO pig small intestine. Xenotransplantation. 2011 Jan-Feb;18(1):28–39. doi: 10.1111/j.1399-3089.2011.00623.x. [DOI] [PubMed] [Google Scholar]
- 10.Dor FJ, Tseng YL, Cheng J, Moran K, Sanderson TM, Lancos CJ, et al. alpha1,3-Galactosyltransferase gene-knockout miniature swine produce natural cytotoxic anti-Gal antibodies. Transplantation. 2004 Jul 15;78(1):15–20. doi: 10.1097/01.tp.0000130487.68051.eb. [DOI] [PubMed] [Google Scholar]
- 11.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005 Jan;11(1):29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 12.Lam TT, Paniagua R, Shivaram G, Schuurman HJ, Borie DC, Morris RE. Anti-non-Gal porcine endothelial cell antibodies in acute humoral xenograft rejection of hDAF-transgenic porcine hearts in cynomolgus monkeys. Xenotransplantation. 2004;11(6):531–535. doi: 10.1111/j.1399-3089.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 13.Wu G, Pfeiffer S, Schroder C, Zhang T, Nguyen BN, Lea W, et al. Co-stimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation. 2005;12(3):197–208. doi: 10.1111/j.1399-3089.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 14.Byrne GW, Schirmer JM, Fass DN, Teotia SS, Kremers WK, Xu H, et al. Warfarin or low-molecular-weight heparin therapy does not prolong pig-to-primate cardiac xenograft function. Am J Transplant. 2005;5(5):1011–1020. doi: 10.1111/j.1600-6143.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 15.Buhler L, Xu Y, Li W, Zhu A, Cooper DK. An investigation of the specificity of induced anti-pig antibodies in baboons. Xenotransplantation. 2003;10(1):88–93. doi: 10.1034/j.1399-3089.2003.01122.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005 Dec;11(12):1295–1298. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu A, Hisashi Y, Kuwaki K, Tseng YL, Dor FJ, Houser SL, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Path. 2008 Jun;172(6):1471–1481. doi: 10.2353/ajpath.2008.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tazelaar HD, Byrne GW, McGregor CG. Comparison of Gal and non-Gal-mediated cardiac xenograft rejection. Transplantation. 2011 May 15;91(9):968–975. doi: 10.1097/TP.0b013e318212c7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azimzadeh AM, Byrne GW, Ezzelarab M, Welty E, Braileanu G, Cheng X, et al. Development of a consensus protocol to quantify primate anti-non-Gal xenoreactive antibodies using pig aortic endothelial cells. Xenotransplantation. 2014 Nov;21(6):555–566. doi: 10.1111/xen.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rood PP, Hara H, Ezzelarab M, Busch J, Zhu X, Ibrahim Z, et al. Preformed antibodies to alpha1,3-galactosyltransferase gene-knockout (GT-KO) pig cells in humans, baboons, and monkeys: implications for xenotransplantation. Transplant Proc. 2005;37(8):3514–3515. doi: 10.1016/j.transproceed.2005.09.082. [DOI] [PubMed] [Google Scholar]
- 21.Saethre M, Baumann BC, Fung M, Seebach JD, Mollnes TE. Characterization of natural human anti-non-gal antibodies and their effect on activation of porcine gal-deficient endothelial cells. Transplantation. 2007 Jul 27;84(2):244–250. doi: 10.1097/01.tp.0000268815.90675.d5. [DOI] [PubMed] [Google Scholar]
- 22.van Poll D, Nahmias Y, Soto-Gutierrez A, Ghasemi M, Yagi H, Kobayashi N, et al. Human immune reactivity against liver sinusoidal endothelial cells from GalTalpha(1,3)GalT-deficient pigs. Cell transplantation. 2010;19(6):783–789. doi: 10.3727/096368910X508898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGregor CG, Ricci D, Miyagi N, Stalboerger PG, Du Z, Oehler EA, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation. 2012 Apr 15;93(7):686–692. doi: 10.1097/TP.0b013e3182472850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ezzelarab M, Garcia B, Azimzadeh A, Sun H, Lin CC, Hara H, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009 Mar 27;87(6):805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Bas-Bernardet S, Tillou X, Branchereau J, Dilek N, Poirier N, Chatelais M, et al. Bortezomib, C1-inhibitor and plasma exchange do not prolong the survival of multi-transgenic GalT-KO pig kidney xenografts in baboons. Am J Transplant. 2015 Feb;15(2):358–370. doi: 10.1111/ajt.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cairns TDH, Taube DH, Stevens N, Binns R, Welsh KI. Xenografts - future prospects for clinical transplantation. Immunology Letters. 1991;29:167–170. doi: 10.1016/0165-2478(91)90221-u. [DOI] [PubMed] [Google Scholar]
- 27.Breimer ME, Bjorck S, Svalandr CT, Bengtsson A, Rydberg L, Lie-Karlsen K, et al. Extracorporeal (“ex vivo”) connection of pig kidneys to humans. I. Clinical data and studies of platelet destruction. Xenotransplantation. 1996;3:328–339. doi: 10.1111/j.1399-3089.1996.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 28.Horslen SP, Hammel JM, Fristoe LW, Kangas JA, Collier DS, Sudan DL, et al. Extracorporeal liver perfusion using human and pig livers for acute liver failure. Transplantation. 2000 Nov 27;70(10):1472–1478. doi: 10.1097/00007890-200011270-00014. [DOI] [PubMed] [Google Scholar]
- 29.Levy MF, Crippin J, Sutton S, Netto G, McCormack J, Curiel T, et al. Liver allotransplantation after extracorporeal hepatic support with transgenic (hCD55/hCD59) porcine livers. Transplantation. 2000;69(2):272–280. doi: 10.1097/00007890-200001270-00013. [DOI] [PubMed] [Google Scholar]
- 30.Chari RS, Collins BH, Magee JC, DiMaio M, Kirk AD, Harland RC, et al. Brief Report: Treatment of hepatic failure with ex vivo pig-liver perfusion followed by liver transplantation. The New England Journal of Medicine. 1994;331(4):234–237. doi: 10.1056/NEJM199407283310404. [DOI] [PubMed] [Google Scholar]
- 31.Demetriou AA, Brown RS, Jr, Busuttil RW, Fair J, McGuire BM, Rosenthal P, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004 May;239(5):660–667. doi: 10.1097/01.sla.0000124298.74199.e5. discussion 667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh P, Ezzelarab M, Bovin N, Hara H, Long C, Tomiyama K, et al. Investigation of potential carbohydrate antigen targets for human and baboon antibodies. Xenotransplantation. 2010 May-Jun;17(3):197–206. doi: 10.1111/j.1399-3089.2010.00579.x. [DOI] [PubMed] [Google Scholar]
- 33.Byrne GW, Stalboerger PG, Du Z, Davis TR, McGregor CG. Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation. 2011 Feb 15;91(3):287–292. doi: 10.1097/TP.0b013e318203c27d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byrne GW, Stalboerger PG, Davila E, Heppelmann CJ, Gazi MH, McGregor HC, et al. Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation. Xenotransplantation. 2008 Jul;15(4):268–276. doi: 10.1111/j.1399-3089.2008.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrne GW, Du Z, Stalboerger P, Kogelberg H, McGregor CGA. Cloning and expression of porcine β1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation. 2014;21:543–554. doi: 10.1111/xen.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bird GW, Wingham J. Cad(super Sda) in a British family with eastern connections: a note on the specificity of the Dolichos biflorus lectin. Journal of immunogenetics. 1976 Oct;3(5):297–302. doi: 10.1111/j.1744-313x.1976.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 37.Merbl Y, Zucker-Toledano M, Quintana FJ, Cohen IR. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. The Journal of clinical investigation. 2007 Mar;117(3):712–718. doi: 10.1172/JCI29943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padler-Karavani V, Yu H, Cao H, Chokhawala H, Karp F, Varki N, et al. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology. 2008 Oct;18(10):818–830. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higashi H, Naiki M, Matuo S, Okouchi K. Antigen of “serum sickness” type of heterophile antibodies in human sera: indentification as gangliosides with N-glycolylneuraminic acid. Biochem Biophys Res Commun. 1977 Nov 21;79(2):388–395. doi: 10.1016/0006-291x(77)90169-3. [DOI] [PubMed] [Google Scholar]
- 40.Tahara H, Ide K, Basnet NB, Tanaka Y, Matsuda H, Takematsu H, et al. Immunological property of antibodies against N-glycolylneuraminic acid epitopes in cytidine monophospho-N-acetylneuraminic acid hydroxylase-deficient mice. J Immunol. 2010 Mar 15;184(6):3269–3275. doi: 10.4049/jimmunol.0902857. [DOI] [PubMed] [Google Scholar]
- 41.Ohdan H, Yang YG, Shimizu A, Swenson KG, Sykes M. Mixed chimerism induced without lethal conditioning prevents T cell- and anti-Gal alpha 1,3Gal-mediated graft rejection. J Clin Invest. 1999 Aug;104(3):281–290. doi: 10.1172/JCI6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Satake M, Kawagishi N, Kumagai-Braesch M, Samuelsson BE, Rydberg L, Tibell A, et al. Specificity of Human Xenoantibodies Formed in Response to Fetal Porcine Isletlike Cell Clusters. Transplantation Proceedings. 1994 Jun;26(3):1122. [PubMed] [Google Scholar]
- 43.Kobayashi T, Yokoyama I, Suzuki A, Abe M, Hayashi S, Matsuda H, et al. Lack of antibody production against hanganutziu-deicher (H-D) antigens with N-glycolylneuraminic acid in patients with porcine exposure history. Xenotransplantation. 2000;7:177–180. doi: 10.1034/j.1399-3089.2000.00056.x. [DOI] [PubMed] [Google Scholar]
- 44.Blixt O, Kumagai-Braesch M, Tibell A, Groth CG, Holgersson J. Anticarbohydrate antibody repertoires in patients transplanted with fetal pig islets revealed by glycan arrays. Am J Transplant. 2009;9(1):83–90. [Google Scholar]
- 45.Rydberg L, Bjorck S, Hallberg E, Magnusson S, Sumitran S, Samuelsson BE, et al. Extracorporeal (“ex vivo”) connection of pig kidneys to humans. II. The anti-pig antibody response. Xenotransplantation. 1996;3:340–353. doi: 10.1111/j.1399-3089.1996.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 46.Magnusson S, Mansson JE, Strokan V, Jussila R, Kobayashi T, Rydberg L, et al. Release of pig leukocytes during pig kidney perfusion and characterization of pig lymphocyte carbohydrate xenoantigens. Xenotransplantation. 2003 Sep;10(5):432–445. doi: 10.1034/j.1399-3089.2003.02052.x. [DOI] [PubMed] [Google Scholar]
- 47.Scobie L, Padler-Karavani V, Le Bas-Bernardet S, Crossan C, Blaha J, Matouskova M, et al. Long-term IgG response to porcine Neu5Gc antigens without transmission of PERV in burn patients treated with porcine skin xenografts. J Immunol. 2013 Sep 15;191(6):2907–2915. doi: 10.4049/jimmunol.1301195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burlak C, Wang ZY, Chihara RK, Lutz AJ, Wang Y, Estrada JL, et al. Identification of human preformed antibody targets in GTKO pigs. Xenotransplantation. 2012 Mar;19(2):92–101. doi: 10.1111/j.1399-3089.2012.00695.x. [DOI] [PubMed] [Google Scholar]
- 49.Chihara RK, Lutz AJ, Paris LL, Wang ZY, Sidner RA, Heyrman AT, et al. Fibronectin from alpha 1,3-galactosyltransferase knockout pigs is a xenoantigen. The Journal of surgical research. 2013 Oct;184(2):1123–1133. doi: 10.1016/j.jss.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Kim YG, Gil GC, Harvey DJ, Kim BG. Structural analysis of alpha-Gal and new non-Gal carbohydrate epitopes from specific pathogen-free miniature pig kidney. Proteomics. 2008 Jul;8(13):2596–2610. doi: 10.1002/pmic.200700972. [DOI] [PubMed] [Google Scholar]
- 51.Jeong HJ, Adhya M, Park HM, Kim YG, Kim BG. Detection of Hanganutziu-Deicher antigens in O-glycans from pig heart tissues by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Xenotransplantation. 2013 Nov-Dec;20(6):407–417. doi: 10.1111/xen.12045. [DOI] [PubMed] [Google Scholar]
- 52.Miyagawa S, Takeishi S, Yamamoto A, Ikeda K, Matsunari H, Yamada M, et al. Survey of glycoantigens in cells from alpha1-3galactosyltransferase knockout pig using a lectin microarray. Xenotransplantation. 2010 Jan-Feb;17(1):61–70. doi: 10.1111/j.1399-3089.2009.00565.x. [DOI] [PubMed] [Google Scholar]
- 53.Miyagawa S, Maeda A, Takeishi S, Ueno T, Usui N, Matsumoto S, et al. Lectin array analysis for wild-type and alpha-Gal-knockout pig islets versus healthy human islets. Surg Today. 2013 Dec;43(12):1439–1447. doi: 10.1007/s00595-013-0569-6. [DOI] [PubMed] [Google Scholar]
- 54.Estrada JL, Martens G, Li P, Adams A, Newell KA, Ford ML, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 2015 May;22(3):203–210. doi: 10.1111/xen.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]