Abstract
Poor inhibitory control may contribute to the maintenance of binge eating (BE) among overweight and obese individuals. However, it is unknown whether deficits are general or specific to food (versus other attractive non-food stimuli), or whether observed deficits are attributable to increased depressive symptoms in BE groups. In the current study, we hypothesized that individuals with BE would display inhibitory control deficits, with more pronounced deficits occurring when food stimuli were used. Overweight or obese participants with (n=25) and without (n= 65) BE completed a Stop Signal Task (SST) with distinct task blocks featuring food-specific stimuli, positive non-food stimuli, or neutral stimuli. The BE group exhibited poorer inhibitory control across SST stimuli types (p = .003, η2p = .10), but deficits did not differ by stimuli type (p = .68, η2p <.01). Including depression as a covariate did not significantly alter results. Results suggest individuals with BE display inhibitory control deficits compared to controls; however, deficits do not appear to be specific to stimuli type. Furthermore, inhibitory control deficits do not appear to be associated with mood disturbance in the BE group. Replication and further research is needed to guide treatment targets.
Keywords: Inhibitory control, response inhibition, stop signal task, binge eating, loss-of-control eating, food responsivity
INTRODUCTION
Obesity is a major public health problem associated with a myriad of poor long-term health outcomes (Danaei et al., 2009). Obese individuals who engage in binge eating (BE; i.e., eating a large amount of food in a discrete time period, driven by a sense of loss of control [LOC]) demonstrate even poorer long-term weight outcomes and overall quality of life than their non-binge eating counterparts (Wilfley, Wilson, & Agras, 2003). Given that the majority of overweight or obese individuals do not endorse BE (Ricca et al., 2000), and that weight loss and long-term weight loss maintenance is particularly challenging for those with BE (Pagoto et al., 2007), understanding the distinct BE maintenance factors is crucial for developing tailored weight management interventions.
Reduced inhibitory control (i.e., the ability to withhold an automatic response) is one factor hypothesized to contribute to the development and maintenance of BE (Svaldi, Naumann, Trentowska, & Schmitz, 2014a) above and beyond its role in general obesity (Grucza, Przybeck, & Cloninger, 2007). Although deficits in inhibitory control have been found in those who are obese in the absence of BE (Smith, Hay, Campbell, & Trollor, 2011), even greater inhibitory deficits are theorized to contribute to the compulsive nature of BE, and the inability to stop eating once started (Balodis et al., 2013).
The extant literature on the presence of inhibitory control deficits in those with BE is somewhat mixed (Wu, Hartmann, Skunde, Herzog, & Friederich, 2013), with four studies detecting differences between BE and overweight/obese control groups (Duchesne et al., 2010; Hege et al., 2014; Manasse et al., 2014; Mobbs, Iglesias, Golay, & Van der Linden, 2011; Svaldi, Naumann, Trentowska, & Schmitz, 2014b) and three studies failing to detect such differences (Kelly, Bulik, & Mazzeo, 2013; Manasse et al., 2015; Wu, Giel, et al., 2013). One potential explanation for mixed findings is that several studies have used inhibitory control tasks that incorporate neutral stimuli (e.g., letters of the alphabet), but inhibitory deficits in those with BE may be especially pronounced when relevant stimuli are used, e.g., food (Svaldi et al., 2014b). Recent dual-process models of self-control posit that dysresgulated behavior such as BE may occur via a combination of increased appetitive drive for a stimulus (e.g., food) and deficits in self-regulatory processes such as inhibitory control (Hofmann, Friese, & Strack, 2009). Preliminary research has supported inhibitory control deficits to food-specific stimuli in overweight compared to healthy weight individuals (Houben, Nederkoorn, & Jansen, 2014; Nederkoorn, Coelho, Guerrieri, Houben, & Jansen, 2012). Moreover, differential responsivity to food stimuli has been detected in individuals with BE (Geliebter et al., 2006; Svaldi, Tuschen-Caffier, Peyk, & Blechert, 2010). Thus, utilizing both food and non-food stimuli could allow examination of the effect of appetitive drive (e.g., via pictures of food) on inhibitory control.
To date, only one study has compared non-food and food-specific inhibitory control in individuals with BE (Svaldi et al., 2014b), although another utilized a solely food-specific go/no-go paradigm (Hege et al., 2014). Both investigations detected food-specific inhibitory control deficits in BE individuals. However, no studies have included food-specific, neutral, and positively-valenced non-food stimuli (e.g., sunsets) in the same task. Inclusion of the non-food stimuli blocks provides information regarding general inhibitory control deficits, and using positively-valenced non-food stimuli serves as a method of controlling for the “attractiveness” of food stimuli. In addition, the extant literature is inconsistent in terms of the facent of inhibitory control being measured (e.g., conflict monitoring using a Stroop task vs. late-stage inhibition using a Stop Signal Task; SST). Given that hedonic drive, as described above, generates a strong automatic response, the most theoretically consistent form of inhibitory control to measure may be the ability to withhold an already-initiated motor response, as in the SST. Thus, utilizing all three stimuli in a SST may help specify whether response inhibition deficits in BE are limited to food-based, generally attractive, and/or neutral stimuli.
Another limitation of the extant literature is the lack of inclusion of relevant mediating variables in analyses. In particular, depressive symptoms, which are associated with poor inhibitory control (Kaiser et al., 2003), are often significantly elevated in individuals with BE (Grucza et al., 2007; Telch & Stice, 1998). In one study, controlling for depressive symptoms essentially eliminated differences in inhibitory control (measured by a Stroop task) between BE and control groups (Manasse et al., 2015). It is possible that mood disturbance could differentially impact performance on tasks measuring information processing and monitoring as in the Stroop task (van Veen, Cohen, Botvinick, Stenger, & Carter, 2001) and late stage motor inhibition (i.e., withholding an already initiated response) as in the SST. To extend current research on inhibitory control deficits among individuals with BE, it is necessary to (1) compare inhibitory control performance of BE and overweight controls across stimulus types (non-food, positively valenced non-food, food); and (2) control for depressive symptoms between groups.
Current study
In the current study, we sought to examine the main and interaction effects of BE status and type of stimuli used in a SST task on inhibitory control performance. We modified a standard SST to include three distinct stimulus types: food stimuli, positively-valenced non-food stimuli, and neutral stimuli. We hypothesized that the BE group would perform worse than the overweight control group (OWC) on all stimulus types of the SST, but that there would be a BE status x SST stimulus type interaction such that the differences between groups would be most pronounced on the food stimuli of the SST. In addition, we also hypothesized that inhibitory control deficits in individuals with BE are not attributable to mood disturbance and thus that differences would persist when statistically controlling for depressive symptoms.
METHODS
Participants and procedure
We included treatment-seeking overweight and obese (BMI = 26-50 kg/m2) females who endorsed regular BE (BE group) and a group of overweight or obese women without any past or present BE (OWC group). Participants were recruited via treatment studies for weight loss or binge eating, and were assessed prior to receiving any treatment.
Participants in the OWC group (n = 65) had no LOC eating episodes in the past 3 months and no current or past history of BE. Participants in the BE group (n = 25) endorsed at least 12 objectively or subjectively large binge episodes over the past 3 months, and did not meet criteria for bulimia nervosa. We included individuals with subjectively large binge episodes given evidence that neurocognitive factors (Manasse et al., 2014) and functional impairment associated with BE is most associated with presence of LOC, not size of binge episodes (Latner, Hildebrandt, Rosewall, Chisholm, & Hayashi, 2007; Mond et al., 2006).
Measures
Binge Eating
The Eating Disorders Examination (EDE) is the gold-standard semi-structured interview for assessing for BE (Grilo, Masheb, Lozano-Blanco, & Barry, 2004; Wilfley, Schwartz, Spurrell, & Fairburn, 1997). The Overeating section was administered to all participants to examine for presence of BE.
Eating disorder symptoms
The Eating Disorders Examination Questionnaire (EDE-Q; (Fairburn & Beglin, 1994) is a reliable and valid short-form, self-report version of the EDE that has demonstrated reliability for the EDE (Peterson et al., 2007). Internal consistency of the EDE-Q in the current study was adequate (α =.83).
Inhibitory Control
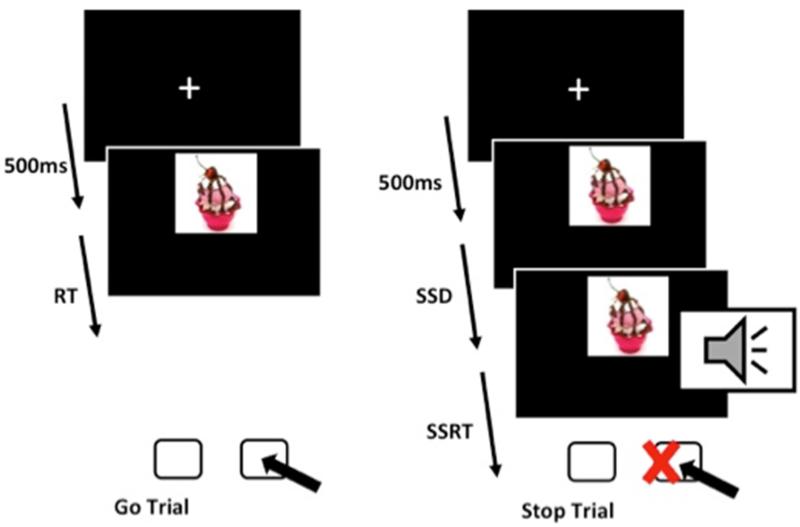
A modified version of the traditional computer-based SST (See Figure 1) was used to measure inhibitory control. During this task, an image presented on the screen for 1,000ms, preceded by a 500ms fixation cross. For “go” trials, participants were instructed to respond as fast as possible to categorize the images located on the top or bottom of the screen using left or right shift keys (i.e., left for top, right for bottom). Using top or bottom screen location is consistent with other investigations, and allowed us to 1) maintain the categorization element of the original SST (Logan, 1994); and 2) counterbalance instructions within subjects (Houben, 2011; Houben et al., 2014). The screen cleared after 1,500ms without a response. During “stop” trials, participants were asked to inhibit responses to stimuli upon hearing a discrete (5ms) auditory tone through headphones. The delay between the presented stimuli and stop signal was initially set at 250ms, and could not exceed 1,050ms. Depending on the performance, the stop signal delay was either increased or decreased by 50ms such that the task becomes more or less difficult (i.e., success at inhibition will prompt a decreased interval between stop times). Using this method, each participant should be able to achieve inhibited responses on approximately 50% of all stop trials (Logan, Schachar, & Tannock, 1997).
Figure 1.
Schematic of the stop signal task (SST)
The task began with two practice blocks using six randomly selected neutral images (e.g., paperclips). The subsequent six test blocks consisted of 60 trials each (20 of which were “stop” trials) from a pool of six images per stimulus category (e.g., neutral, pleasant, and highly palatable foods). On average, participants rated the taste of the food stimuli to be between “fairly good” and “very good” (M = 3.19, SD = .44, on a 4-point Likert scale), suggesting that food the stimuli were strong enough to trigger an appetitive response. We counterbalanced for the order of task blocks, thereby controlling for order effects. Test blocks consisted of two blocks of neutral image stimuli (e.g. scissors), then two blocks of pleasant stimuli (e.g. flowers), then two blocks of highly palatable food stimuli (e.g. pizza). We reversed the stimuli block order such that the food stimuli were administered first and the neutral stimuli were administered last for approximately half of the participants.
The outcome measure used for the current study is the stop signal reaction time (SSRT). SSRT was calculated for each set of stimuli (i.e., SSRT stimulus type) for each subject by subtracting average stop signal delay from the average reaction time on “go” trials (Verbruggen & Logan, 2008). The recording accuracy of reaction time and stop signal delay measurement was to the millisecond. A smaller SSRT is indicative of greater inhibitory control and a larger SSRT reflects poorer inhibitory control.
Depressive symptoms
The Beck Depression Inventory-II (BDI-II; (Beck, Steer, & Brown, 1996) is a reliable and valid self-report measure of depressive symptomatology in the previous two weeks. (Dozois, Dobson, & Ahnberg, 1998; Steer, Ball, Ranieri, & Beck, 1997). Internal consistency in the current sample was good (α =.91).
Statistical Analysis
A 2 × 3 (BE status by SST stimulus type) factorial analysis of covariance (ANCOVA), with SST stimulus type as the within-subjects factor and group (BE or OWC) as the between-subjects factor, was conducted to examine main effects and interaction effects of these variables on SSRT scores. Statistical Package for the Social Sciences v. 20.0 (IBM, 2013) was used to analyze data. The BE group was younger than the OW group, thus, we included age as a covariate in analyses. We first ran the ANCOVA controlling only for age. We repeated the model with depressive symptoms (BDI-II) added as a covariate in order to examine whether differences in depressive symptoms could explain differences between groups. SSRT scores were positively skewed; thus, we conducted a square root transformation, which successfully normalized the distributions. Use of the transformed variables in analyses did not significantly alter results; thus, we report statistics using non-transformed variables. Mauchly's test of sphericity was violated in ANCOVA analyses; thus, we utilized a Greenhouse-Geisser correction to decrease the odds of Type I error.
RESULTS
Descriptive statistics
Sample demographics and clinical characteristics are presented in Table 1. BMI showed non-significant associations with SST on all conditions of the task (Pearson's rs = .06-.14, ps = .31-.67). Of the OWC group, 12.0% (n=7) were on a psychiatric medication, while 48% (n=12) of the BE group were on a psychiatric medication (χ2 = 15.35, p < .01). Taking a psychiatric medication did not significantly impact SST performance (ts = .47-.83, ps = .41-.64). Depressive symptoms trended towards being higher in those taking a psychiatric medication (t=1.96, p = .05). Descriptive statistics of performance on the SST are included in Table 2.
Table 1.
Descriptive and clinical characteristics by group
| BE Group (n=25), M(SD) | OWC Group (n=65), M(SD) | t | p | Cohen's d | |
|---|---|---|---|---|---|
| Age (yrs) | 45.06 (14.86) | 52.40 (9.17) | 2.50 | .02 | 0.59 |
| Objective binge episodesa | 11.08 (9.69) | -- | -- | -- | -- |
| Subjective binge episodesa | 6.08 (12.48) | -- | -- | -- | -- |
| Body Mass Index (kg/m2) | 35.23 (7.69) | 36.72 (5.54) | .96 | .34 | 0.22 |
| BDI-II | 17.54 (10.13) | 8.49 (7.62) | 4.53 | < .01 | 1.00 |
| EDE-Q Restraint | 1.65 (1.46) | 1.42 (1.25) | .56 | .55 | 0.17 |
| EDE-Q Eating Concern | 2.47 (1.29) | 1.49 (1.20) | 3.10 | < .01 | .79 |
| EDE-Q Shape Concern | 3.93 (1.60) | 3.67 (1.25) | 0.96 | .34 | 0.18 |
| EDE-Q Weight Concern | 3.72 (1.23) | 3.27 (1.02) | 1.59 | .12 | 0.40 |
| EDE-Q Global Score | 2.97 (1.12) | 2.44 (.82) | 2.27 | < .05 | 0.54 |
BE = binge eating, OWC = overweight control BDI-II = Beck Depression Inventory – II, EDE-Q = Eating Disorders Examination Questionnaire;
In the past 30 days
Table 2.
Descriptive statistics regarding performance on the stop signal task (SST)
| BE Group M(SD) | OWC Group M(SD) | t | p | Cohen's d | |
|---|---|---|---|---|---|
| SST neutral condition | |||||
| Stop signal delay (ms) | 618 (188) | 678 (230) | 1.17 | .25 | 0.28 |
| Accuracy* | .76 (.08) | .78 (.10) | .93 | .36 | 0.02 |
| Go reaction time (ms) | 914 (165) | 909 (173) | .12 | .90 | 0.03 |
| Correctly inhibited† | .52 (.06) | .56 (.14) | 1.17 | .24 | 0.37 |
| SST positive non-food condition | |||||
| Stop signal delay (ms) | 692 (201) | 742 (237) | .92 | .36 | 0.23 |
| Accuracy* | .76 (.07) | .76 (.09) | −.11 | .91 | 0.00 |
| Go reaction time (ms) | 948 (185) | 936 (189) | .28 | .78 | 0.07 |
| Correctly inhibited† | .55(.05) | .53 (.10) | .89 | .38 | 0.25 |
| SST food condition | |||||
| Stop signal delay (ms) | 681 (242) | 724 (224) | .80 | .43 | 0.19 |
| Accuracy* | .72 (.08) | .75 (.08) | 1.31 | .20 | 0.38 |
| Go reaction time (ms) | 955 (177) | 925 (193) | .61 | .54 | 0.14 |
| Correctly inhibited† | .52 (.07) | .54 (.06) | .62 | .54 | 0.31 |
Accuracy = proportion of participant correct responses on go trials (categorization) to total go trials in block
Correctly inhibited = proportion of participant correct responses on stop trials (no response) to total stop trials in block
Outcome analyses
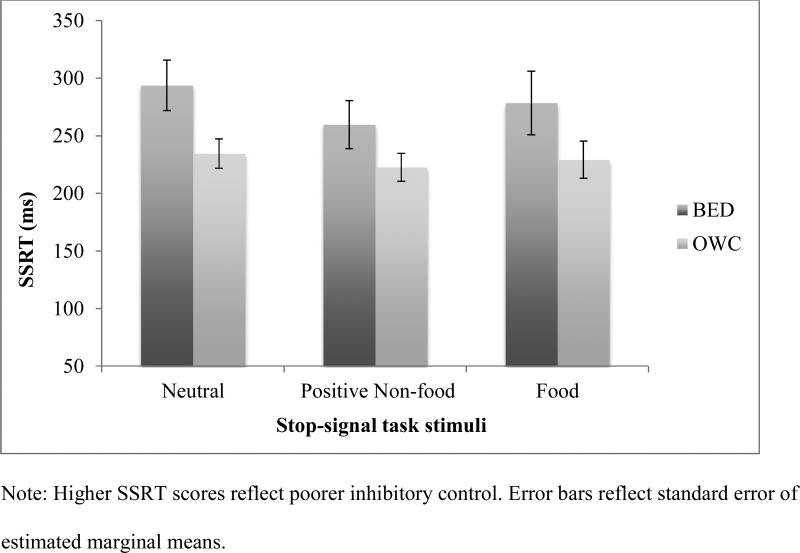
When controlling only for age, the ANCOVA revealed a small and statistically non-significant main effect of SST stimulus type (F (1.63, 142.06) = .84, p = .36, η2p = .01). Consistent with hypotheses, there was a statistically significant effect of BE status on SSRT (F (1, 88) = 7.97, p = .003, η2p = .10). The BE status x SST stimulus type interaction effect was, however, negligible (F(1.63, 142.06) = .32, p = .68, η2p < .01). When depressive symptoms were added as a covariate, results were not meaningfully changed (See Figure 2). Specifically, the main effect of SST stimulus type was small and not statistically significant (F (1.63, 140.40) = 1.51, p = .23, η2p =.02), the main effect of BE status on SSRT remained significant (F(1,87) = 5.70, p = .02, η2p = .06), and the BE status x SST stimulus type interaction effect remained statistically non-significant (F(1.63, 140.40) = .16, p = .81, η2p < .01). BDI-II as a covariate was not significant (η2p < .01) in the model.
Figure 2.
Estimated marginal means of SSRT performance by group and SSRT stimulus type, controlling for age
DISCUSSION
The results of the current study partially support our initial hypotheses. As predicted, and consistent with previous research, the BE group exhibited poorer inhibitory control when compared to BMI-matched controls on all stimulus categories of the SST. This result highlights the potentially important role of inhibitory control in the development and/or maintenance of BE (Duchesne et al., 2010; Hege et al., 2014; Manasse et al., 2014; Mobbs et al., 2011; Svaldi et al., 2014b). Importantly, the deficits were observed relative to a BMI-equivalent non-BE sample, adding to the evidence base that inhibitory control is implicated specifically in the maintenance of BE in the context of obesity. Individuals with BE may have weaker inhibitory control, even in the absence of food stimuli, that may contribute to the development of a compelled drive (i.e., LOC) to eat that distinguishes BE episodes from general overeating episodes. More research is required to establish temporal precedence between inhibitory control deficits and onset of BE. However, extant evidence indicates directly improving inhibitory control could be an important treatment target, such as using a SST paradigm to enhance inhibitory control (Houben, 2011).
Contrary to our hypotheses, however, we did not detect a BE status x SST stimulus type interaction effect, suggesting that the inhibitory control performance was equivalently impaired for BE participants across stimuli. The absence of an interaction effect between stands in conflict with a previous finding (Svaldi et al., 2014b). However, Svaldi and colleagues used commission errors for each stimuli type as the outcome variable in the stimuli type x group interaction analysis, possibly explaining the disparate findings. We chose to use the SSRT variable because it provides a comprehensive picture of inhibitory control performance, taking into account individual differences in task difficulty adjusted based on an individual's reaction time. Thus, more consistency in outcome variables used from tasks such as the SST is necessary in order to further clarify whether inhibitory control deficits in individuals with BE are more pronounced when food stimuli are used.
Lastly, results indicate that deficits in inhibitory control were not mediated by the mood disturbance in the BE group. This finding appears to conflict with one study suggesting response inhibition deficits (as measured by the Stroop task) among BE individuals could be largely attributed to depressive symptoms (Manasse et al., 2015). It is possible that deficits in late-stage response inhibition (e.g., SSRT), could be specific to BE and poor conflict-monitoring (e.g., Stroop performance) could be attributable to depressive symptoms, although replication is needed to test this claim. Poor conflict monitoring might contribute to the frequent initiation of eating episodes (and association with obesity generally) whereas poor ability to inhibit an already-initiated motor response (e.g., eating), might contribute to the development of LOC over an eating episode (a unique feature of BE).
Findings in this study should be considered in light of several limitations. For example, the current study featured a relatively small, all-female, overweight or obese, treatment-seeking BE sample. In addition, lack of a healthy weight control group may have precluded detecting differences in inhibitory control by stimulus type (e.g., food-specific) that could be present both in OWC and BE groups. We also did not standardize hunger state prior to the SST, which may have introduced error into the analyses (e.g., it is feasible that one group was more likely to eat prior to the assessment session). Additionally, we did not control for psychiatric diagnoses apart from depressive symptoms measured by the BDI-II. It should also be noted that we observed relatively slower reaction times in our sample compared to other studies, which could be attributable to a higher mean age of our sample. Finally, the SST uses visual stimuli; it could be that other types of food-related stimuli (e.g., olfactory, gustatory) may affect inhibitory control.
In sum, results from the current study support extant research reporting a relative inhibitory control deficit in overweight individuals with BE pathology. However, our hypothesis of a more pronounced inhibitory deficit in response to food stimuli was not supported. Future research will benefit from replication in order to provide directions for treatment development, particularly for enhancing weight outcomes in those with BE.
Highlights.
We sought to distinguish food-specific from general inhibitory control deficits in overweight women with binge eating
Treatment-seeking overweight and obese women (with and without binge eating) were assessed
Women with binge eating displayed deficits in inhibitory control
Deficits did not appear to be more pronounced when using a food-specific inhibitory control task
Acknowledgments
The current study was funded by a grant from the National Institutes of Digestive and Kidney Diseases awarded to Dr. Forman (R01DK095069), and two grants from the Psi Chi Honors society and the American Psychological Association of Graduate Students, awarded to Ms. Manasse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Balodis IM, Molina ND, Kober H, Worhunsky PD, White MA, Sinha R, Potenza MN. Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity. 2013;21(2):367–377. doi: 10.1002/oby.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. The Psychological Corporation; San Antonio. TX: 1996. [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJL, Ezzati M. The Preventable Causes of Death in the United States: Comparative Risk Assessment of Dietary, Lifestyle, and Metabolic Risk Factors. PLoS Medicine. 2009;6(4):e1000058. doi: 10.1371/journal.pmed.1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory - II. Psychological Assessment. 1998;10(2):83–89. doi: Doi 10.1037/1040-3590.10.2.83. [Google Scholar]
- Duchesne M, Mattos P, Appolinário J, de Freitas S, Coutinho G, Santos C, Coutinho W. Revista brasileira de psiquiatria. 4. Vol. 32. São Paulo; Brazil: 2010. Assessment of executive functions in obese individuals with binge eating disorder. p. 381. 1999. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Beglin SJ. Assessment of Eating Disorders - Interview or Self-Report Questionnaire. International journal of eating disorders. 1994;16(4):363–370. [PubMed] [Google Scholar]
- Geliebter A, Ladell T, Logan M, Schweider T, Sharafi M, Hirsch J. Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite. 2006;46(1):31–35. doi: 10.1016/j.appet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Lozano-Blanco C, Barry DT. Reliability of the Eating Disorder Examination in patients with binge eating disorder. Int J Eat Disord. 2004;35(1):80–85. doi: 10.1002/eat.10238. doi: 10.1002/eat.10238. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Przybeck TR, Cloninger CR. Prevalence and correlates of binge eating disorder in a community sample. Comprehensive psychiatry. 2007;48(2):124–131. doi: 10.1016/j.comppsych.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hege M, Stingl K, Kullmann S, Schag K, Giel K, Zipfel S, Preissl H. Attentional impulsivity in binge eating disorder modulates response inhibition performance and frontal brain networks. International Journal of Obesity. 2014 doi: 10.1038/ijo.2014.99. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Friese M, Strack F. Impulse and self-control from a dual-systems perspective. Perspectives on Psychological Science. 2009;4(2):162–176. doi: 10.1111/j.1745-6924.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- Houben K. Overcoming the urge to splurge: Influencing eating behavior by manipulating inhibitory control. Journal of behavior therapy and experimental psychiatry. 2011;42(3):384–388. doi: 10.1016/j.jbtep.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Houben K, Nederkoorn C, Jansen A. Eating on impulse: The relation between overweight and food - specific inhibitory control. Obesity. 2014;22(5):E6–E8. doi: 10.1002/oby.20670. [DOI] [PubMed] [Google Scholar]
- IBM. SPSS Statistics for Macintosh, Version 22.0. 2013 [Google Scholar]
- Armonk NY, IBM Corp. Kaiser S, Unger J, Kiefer M, Markela J, Mundt C, Weisbrod M. Executive control deficit in depression: event-related potentials in a Go/Nogo task. Psychiatry Research: Neuroimaging. 2003;122(3):169–184. doi: 10.1016/s0925-4927(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Kelly NR, Bulik CM, Mazzeo SE. Executive functioning and behavioral impulsivity of young women who binge eat. International journal of eating disorders. 2013;46(2):127–139. doi: 10.1002/eat.22096. [DOI] [PubMed] [Google Scholar]
- Latner JD, Hildebrandt T, Rosewall JK, Chisholm AM, Hayashi K. Loss of control over eating reflects eating disturbances and general psychopathology. Behaviour research and therapy. 2007;45(9):2203–2211. doi: 10.1016/j.brat.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Logan G. On the ability to inhibit thought and action: A users' guide to the stop signal paradigm. 1994 [Google Scholar]
- Logan G, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8(1):60–64. [Google Scholar]
- Manasse SM, Forman EM, Ruocco AC, Butryn ML, Juarascio AS, Fitzpatrick KK. Do executive functioning deficits underpin binge eating disorder? A comparison of overweight women with and without binge eating pathology. International journal of eating disorders. 2015 doi: 10.1002/eat.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasse SM, Juarascio AS, Forman EM, Berner LA, Butryn ML, Ruocco AC. Executive Functioning in Overweight Individuals with and without Loss - of - Control Eating. European Eating Disorders Review. 2014;22(5):373–377. doi: 10.1002/erv.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs O, Iglesias K, Golay A, Van der Linden M. Cognitive deficits in obese persons with and without binge eating disorder. Investigation using a mental flexibility task. Appetite. 2011;57(1):263–271. doi: 10.1016/j.appet.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Mond J, Hay P, Rodgers B, Owen C, Crosby R, Mitchell J. Use of extreme weight control behaviors with and without binge eating in a community sample: Implications for the classification of bulimic - type eating disorders. International journal of eating disorders. 2006;39(4):294–302. doi: 10.1002/eat.20265. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Coelho JS, Guerrieri R, Houben K, Jansen A. Specificity of the failure to inhibit responses in overweight children. Appetite. 2012;59(2):409–413. doi: 10.1016/j.appet.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Pagoto S, Bodenlos JS, Kantor L, Gitkind M, Curtin C, Ma Y. Association of Major Depression and Binge Eating Disorder with Weight Loss in a Clinical Setting. Obesity. 2007;15(11):2557–2559. doi: 10.1038/oby.2007.307. doi: 10.1038/oby.2007.307. [DOI] [PubMed] [Google Scholar]
- Peterson CB, Crosby RD, Wonderlich SA, Joiner T, Crow SJ, Mitchell JE, le Grange D. Psychometric properties of the eating disorder examination-questionnaire: factor structure and internal consistency. Int J Eat Disord. 2007;40(4):386–389. doi: 10.1002/eat.20373. doi: 10.1002/eat.20373. [DOI] [PubMed] [Google Scholar]
- Ricca V, Mannucci E, Moretti S, Di Bernardo M, Zucchi T, Cabras P, Rotella C. Screening for binge eating disorder in obese outpatients. Comprehensive psychiatry. 2000;41(2):111–115. doi: 10.1016/s0010-440x(00)90143-3. [DOI] [PubMed] [Google Scholar]
- Smith E, Hay P, Campbell L, Trollor J. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obesity Reviews. 2011;12(9):740–755. doi: 10.1111/j.1467-789X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- Steer RA, Ball R, Ranieri WF, Beck AT. Further evidence for the construct validity of the Beck Depression Inventory-II with psychiatric outpatients. Psychological Reports. 1997;80(2):443–446. doi: 10.2466/pr0.1997.80.2.443. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Naumann E, Trentowska M, Schmitz F. General and food-specific inhibitory deficits in binge eating disorder. International journal of eating disorders. 2014a;47(5):534–542. doi: 10.1002/eat.22260. doi: 10.1002/eat.22260. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Naumann E, Trentowska M, Schmitz F. General and food - specific inhibitory deficits in binge eating disorder. International journal of eating disorders. 2014b;47(5):534–542. doi: 10.1002/eat.22260. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Tuschen-Caffier B, Peyk P, Blechert J. Information processing of food pictures in binge eating disorder. Appetite. 2010;55(3):685–694. doi: 10.1016/j.appet.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Telch CF, Stice E. Psychiatric comorbidity in women with binge eating disorder: Prevalence rates from a non-treatment-seeking sample. Journal of Consulting and Clinical Psychology. 1998;66(5):768. doi: 10.1037//0022-006x.66.5.768. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14(6):1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends in cognitive sciences. 2008;12(11):418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfley DE, Schwartz MB, Spurrell EB, Fairburn CG. Assessing the specific psychopathology of binge eating disorder patients: interview or self-report? Behav Res Ther. 1997;35(12):1151–1159. [PubMed] [Google Scholar]
- Wilfley DE, Wilson GT, Agras WS. The clinical significance of binge eating disorder. International journal of eating disorders. 2003;34(S1):S96–S106. doi: 10.1002/eat.10209. [DOI] [PubMed] [Google Scholar]
- Wu M, Giel KE, Skunde M, Schag K, Rudofsky G, Zwaan M, Friederich HC. Inhibitory control and decision making under risk in bulimia nervosa and binge - eating disorder. International journal of eating disorders. 2013;46(7):721–728. doi: 10.1002/eat.22143. [DOI] [PubMed] [Google Scholar]
- Wu M, Hartmann M, Skunde M, Herzog W, Friederich H-C. Inhibitory control in bulimic-type eating disorders: a systematic review and meta-analysis. PloS one. 2013;8(12):e83412. doi: 10.1371/journal.pone.0083412. [DOI] [PMC free article] [PubMed] [Google Scholar]