Abstract
Background & Aims
Understanding the molecular pathogenesis of hepatocellular carcinoma (HCC) is essential to identify therapeutic targets. A hepatitis B virus (HBV) related double transgenic murine model was developed.
Methods
Liver specific expression of HBV X protein (HBx) and insulin receptor substrate 1 (IRS1) was achieved and transgenic mice were followed from birth to age 21 months. Liver and tumor tissue were assessed for histologic changes as well as activation of signal transduction pathways by qRT-PCR and multiplex ELISA protein assays.
Results
Overexpression of HBx and IRS1 stimulates liver cell proliferation in the double transgenic mice. Only the male mice developed HCC starting at age 15-18 months. The IN/IGF1/IRS1/MAPK/ERK and IN/IGF1/IRS1/PI3K/AKT/GSK3β cascades were activated early (6-9 months) in the liver followed by WNT/β-catenin and Notch signaling. Aspartate β-hydroxylase (ASPH) was found to link these upstream growth factor signaling pathways to downstream Notch activation in tumor tissues.
Conclusions
Sustained overexpression of HBx and IRS1 led to constitutive activation of a tripartite growth factor signal transduction cascade in the liver and was necessary and sufficient to promote HCC development and progression.
Keywords: Hepatocellular carcinoma, transgenic mice, growth factor signaling pathways
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men and the seventh in women. Chronic hepatitis B virus (HBV) infection is the leading cause of HCC and accounts for up to 59% of cases, particularly in developing countries. The oncogenic properties of HBV have been linked to transactivation of cellular signaling pathways via the hepatitis B virus X protein (HBx). Human, woodchuck and ground squirrel hepadnaviridae members are all associated with HCC, whereas the avian hepadnavirus, lacking the HBx gene, does not develop HCC in ducks [1]. The integrated HBV DNA in most HCC tumors has been shown to contain all or part of the HBx gene [2].
In addition, the insulin (IN)/insulin-like growth factor 1 (IGF1) signaling pathway is of substantial relevance to the pathogenesis of HCC because overexpression of insulin receptor substrate 1 (IRS1) and/or aberrant activation of the downstream cascades has been detected in over 90% of human HCC samples [3]. Therefore, these two critical proteins, HBx and IRS1, appear to be involved in hepatic oncogenesis. The goal of this study was to develop an animal model for HBV-mediated HCC that accurately recapitulates the dysregulated signal transduction events observed in human disease. We hypothesized that simultaneous, constitutive expression of HBx and IRS1 as transgenes in a normal liver would be required to cause malignant transformation and it has been noted that early premalignant alternations in the liver have been observed with expression of these two transgenes [4]. Moreover, we postulated that overexpression of these two proteins in a double transgenic murine model would result in sustained and synergistic activation of IN/IGF1, WNT/β-catenin, and Notch due to the well-known and extensive crosstalk and feedback networks that exist among these three pathways. In addition, we also explored the expression of a cell surface β-hydroxylase enzyme, aspartate β-hydroxylase (ASPH), that may be central to hepatic oncogenesis through the activation of interconnected signaling cascades during tumor formation and progression [5].
2. MATERIAL AND METHODS
2.1 Transgenic Mice
The HBx gene, derived from HBV adw2 subtype and expressed under a liver-specific α1-antitrypsin (α1-AT) promoter, was introduced into an ICR/HaJ outbred background to produce the α1-AT-HBx (ATX) transgenic mice [6]. The human IRS1 gene, expressed under a liver-specific albumin promoter, was introduced into an FVB/NJ inbred background to produce the IRS1 transgenic mice [7]. Heterozygous ATX mice were crossed with heterozygous IRS1 mice to produce the ATX/IRS1 double transgenic animals. To keep the sample size in a logistically manageable yet statistically meaningful range, wild-type (WT) ICR mice, wild-type FVB mice and wild-type progeny from the cross were pooled into one group. Similarly, ATX transgenic ICR mice and ATX-positive IRS1-negative progeny were pooled into one group. The same process occurred for the IRS1 transgenic FVB mice and ATX-negative IRS1-positive progeny. The 8 groups of mice (2 genders; 4 genotypes, i.e., WT, ATX, IRS1, ATX/IRS1) were followed in a longitudinal study, in which about 20 animals from each group were sacrificed at 3, 6, 9, 12, 15, 18, and 21 months of age (Supplemental Table 1).
2.2 Histopathology (Supplementary Information)
2.3 qRT-PCR Assay (Supplementary Information)
2.4 ELISA Assay (Supplementary Information)
3. RESULTS
3.1 Transgenic Mice
As shown in Supplemental Table 1, at the end of the experiment, a total of 1282 mice were sacrificed and included in the study. Overall, they were evenly distributed with respect to gender (male-to-female ratio = 0.93:1, p = 0.1626) and age (z-critical value = 2.02), except for the 9-month-old ATX/IRS double transgenic male test group, which had a small sample size. The deviation was not statistically significant to affect analysis across genotypes (p = 0.2077) or time points (p = 0.1362). Distribution of the colony by genotype was not even (WT:ATX:IRS:ATX/IRS ratio = 1.85:1.43:1.36:1) since the ICR/HaJ and FVB/NJ transgenic mice were pooled together with the heterozygous cross progeny to simplify analysis, but it was consistent across gender and time points and was close to the theoretical ratio of 1.8:1.4:1.4:1.
3.2 Tumor Formation
Supplementary Figure 1A and B compares the histologic features in the liver between male and female ATX/IRS1 double transgenic mice. There were substantial dysplastic, steatosis and lobular architectural changes starting at 9 months of age in males compared to mild steatosis found in females under the dual expression of HBx and IRS1 genes. A representative histologic comparison at 18 months between WT, single ATX or IRS1 transgenic as well as the ATX/IRS1 double transgenic mice is presented in Supplementary Figure 2. Morphologic characteristics of severe dysplasia are apparent in the ATX/IRS1 mice.
Dual expression of HBx and IRS1 transgenes constitutively promotes liver cell proliferation over 3 to 21 months characterized by enhanced proliferating cell nuclear antigen (PCNA) expression (Figure 1A). This proliferative stimulus was accentuated in tumor tissue from ATX/IRS1 mice as compared to other genotypes at 18 months as shown in Figure 1A (middle panel) (p<0.01). Tumor development was exclusive to the male ATX/IRS1 transgenic mice starting at about 15 months as demonstrated in Figure 1A (right hand panel).
Figure 1.
Phenotypic features of tumor development in the ATX/IRS1 double transgenic mice. (A) Proliferative stimulus provided by expression of the HBx and IRS1 transgenes. Measurement of PCNA expression by qRT-PCR in WT, ATX, IRS1, and ATX/IRS1 livers. Note the comparison to the PCNA level found in ATX/IRS1 generated HCC tumors compared to WT (p=0.00005). Incidence of HCC development in female and male ATX/IRS1 transgenic mice. In males tumor development and progression starts after 15 months of age. (B) Morphologic appearance of HCC found in ATX/IRS1 male transgenic mice at 18 months of age. (C) Histologic features of HCC where approximately 70% of tumors was surrounded by a fibrous capsule (arrows).
A total of 31 mice developed histologic evidence of hepatic tumor formation and 19 mice developed gross hepatic tumors from 3 to 21 months (WT: 3/182, 1.6%; ATX: 2/143, 1.4%; IRS1: 3/148, 2.0%; ATX/IRS1: 13/111, 12% (p<0.0001 compared to the other 3 genotypes). As shown in Figure 1B, the tumors varied in appearance, size and location, from multiple tiny nodules covering the entire liver to a single 50 mm mass. The tumors were exclusively found in male, in older animals (15-month or older), and predominately in ATX/IRS1 double transgenic mice. Gross and microscopic tumor development occurred earlier and in higher frequency across all time points in the male ATX/IRS1 mice than their wild-type or single transgenic littermates.
Definitive histological evidence of neoplastic changes was first observed among the 15-month-old ATX/IRS1 samples; the percentage of tumor-positive samples increased even further at later time points to yield an overall rate of 25%. A wide range of histological appearances was observed, as shown in Figure 1C, from well-differentiated cells arranged in trabeculae to highly anaplastic cells in a disorganized pattern. Furthermore, many lesions (70%) were found to be partially surrounded by a fibrous capsule.
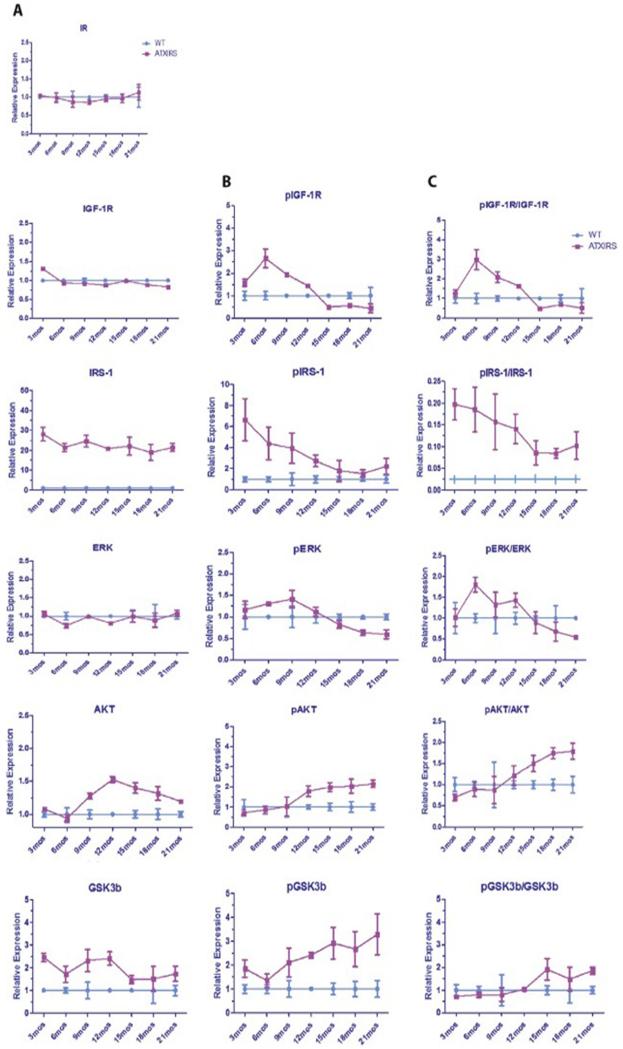
Since the tumor formation rate was very low and no different between WT and single ATX or IRS1 transgenic mice, comparisons were made between WT and ATX/IRS1 double transgenic mice with respect to the activation of the IN/IGF1/IRS1/RAS/RAF/MAPK/ERK and IN/IGF1/IRS1/PI3K/AKT/GSK3β growth factor signal transduction cascades in livers collected from animals between 3 and 21 months of age using multiplex ELISA analysis. As shown in Figure 2A, B and C, there was early evidence of enhanced phosphorylation of both the IGF1 receptor (IGF1R) as well as the overexpressed human IRS1 in the ATX/IRS1 double transgenic mice between 3 to 12 months. This was followed by activation of Erk, Akt and GSK3β, suggesting that these two growth factor signaling cascades were involved in early hepatic oncogenesis. Supplemental Figures 3 and 4 (A, B, C and D) further demonstrates that activation of the two growth factor signaling cascades was exaggerated in tumors generated from the ATX/IRS1 double transgenic male mice at age 18 months, compared to the surrounding uninvolved double transgenic livers or WT livers, indicating that these pathways actively promoted and sustained tumor development.
Figure 2.
Serial changes in IN/IGF1/IRS1/RAS/RAF/MAPK/ERK and IN/IGF1/IRS1/PI3K/AKT/GSK3β signaling cascades in the livers of male WT vs. ATX/IRS1 double transgenic mice as measured by Multiplex ELISA assays. (A, B and C) Left panel represents total protein expression, middle panel represents phosphorylated proteins and right panel represents the ratio of protein/phosphoprotein serially studied from 3 to 21 months of age. Insulin receptor (IR) showing no change in expression. Early (3 month) increase in pIGF1R ratio compared to WT (p<0.003). Enhanced phosphorylation of IRS1 in transgenic liver at 3 months compared to WT (p<0.0001). Early (3-6 months) enhanced phosphorylation of ERK (p<0.01). Elevated expression and phosphorylation of AKT at 9-12 month of age and persistence from 12-21 months (p<0.003). Enhanced expression and phosphorylation of GSK3β that persisted for 21 months compared to WT (p<0.001). The findings suggest that activation of these two growth factor signaling pathways occur early during the spontaneous development and growth of HCC due to constitutive overexpression of HBx and IRS1 transgenes and they persist in the liver up to 21 months of age (characterized by pAKT and pGSK3β).
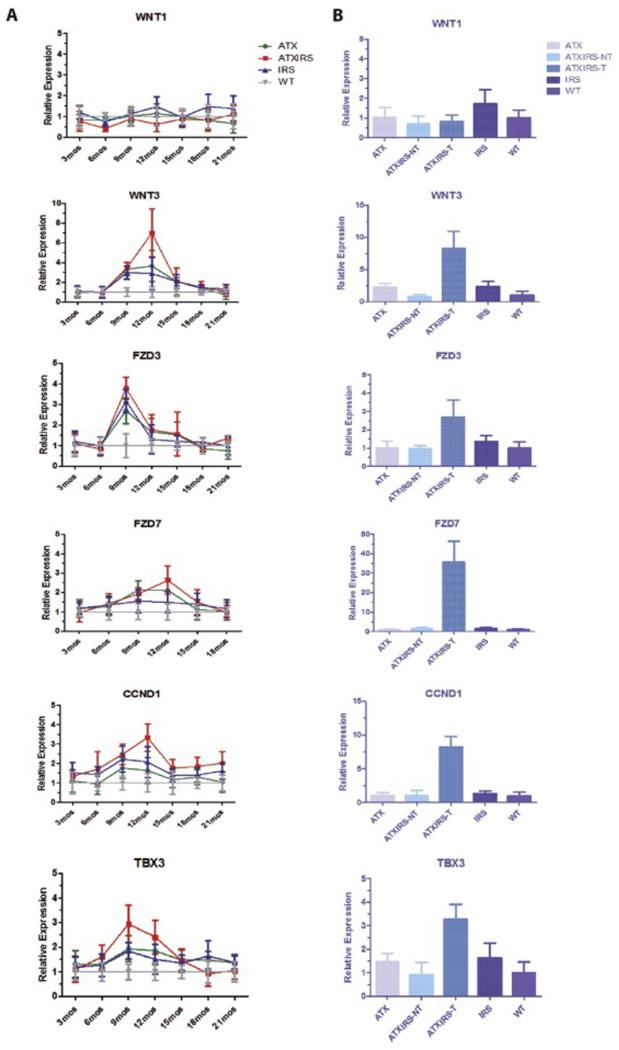
The Wnt/β-catenin signaling cascade plays a major role in the pathogenesis of HCC [8]. It was of interest, therefore, to determine if this pathway was activated during tumor development and progression in our animal model. As shown in Figure 3A and B, there was increased expression of WNT3 ligand, Frizzled (FZD) 3, and FZD7 receptors by qRT-PCR, particularly between 9 to 15 months, in the ATX/IRS1 double transgenic line, and to a lesser extent, in the ATX or IRS1 single transgenic mice. As expected, activation of downstream genes regulated by WNT/β-catenin signaling, such as cyclin D1 and transcription factor TBX3, was also observed. A constitutive high expression of this signaling transduction cascade induced by HBx and IRS1 was demonstrated by overexpression of WNT3, FZD7, FZD3, cyclin D1 and TBX3 genes in tumors derived from the ATX/IRS1 double transgenic mice at 18 months compared to normal livers from other age-matched male animals.
Figure 3.
Serial studies in single and double transgenic mice liver demonstrating activation of the WNT/β-catenin pathway during tumorigenesis. The left panel (A) represents RT-PCR results in the liver from different time points and the right panel (B), a direct comparison to HCC tumor tissue. WNT1 expression was minimally elevated (p>0.05) in IRS1 transgenic liver at 18 months. WNT3 expression was highly upregulated in ATX/IRS1, to a less extent in ATX or IRS1 single transgenic mice at 12 months as compared to WT (p<0.0001, p<0.001, p<0.01) respectively. Tumor tissue exhibited a high level WNT3 expression in single and double transgenic mice, compared to liver tissue derived from WT (p<0.00005). FZD3 expression was substantially upregulated in transgenic mice at 9 months compared to WT and overexpressed in tumor tissue as well (p<0.008). FZD7 was overexpressed at 9-12 months in transgenic liver (p<0.0006) and strikingly upregulated in tumor tissue (p<0.0004). The downstream WNT/β-catenin regulated gene (cyclin D1) was upregulated between 9-12 months (p<0.001) and highly overexpressed in tumor tissue (p<0.00007). The transcription factor TBX3 as a representative downstream target of WNT/β-catenin was substantially upregulated in the ATX/IRS1 mice (p<0.003) as well as tumors derived from these animals (p<0.001).
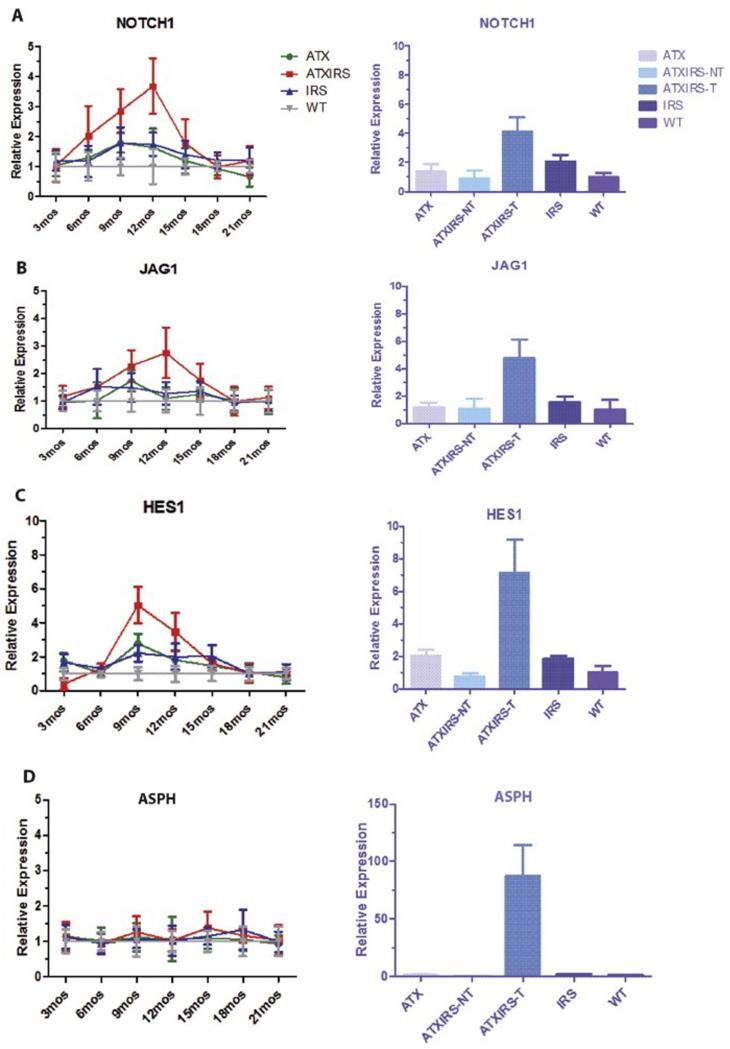
Notch activation may play an important role in promoting tumor cell migration, invasion and metastases in HCC [5]. The link between coupling of upstream growth factor signal cascades with downstream Notch activation may be through an intermediate protein such as aspartate beta-hydroxylase (ASPH) [5], which is transcriptionally regulated by IN/IGF1 signaling. Therefore, we determined if both Notch and ASPH were activated during HCC development and growth in this HBV-related double transgenic model. As shown in Figure 4, a substantial upregulation of Notch1, Jagged 1 (JAG1) and downstream HES1 gene was observed, principally in the ATX/IRS1 double transgenic line; ASPH levels in the liver of the 4 genotypes were entirely normal. However, in HCC tumor tissue at 18 months, there was constitutive activation of Notch1, JAG1, HES1 and ASPH. ASPH expression was increased over 70-fold in the tumors compared to that in the normal livers from other age-matched male animals [9].
Figure 4.
Serial studies on Notch activation during hepatic oncogenesis in transgenic mice liver at different time points and among different genotypes as measured by qRT-PCR. (A) Upregulation of Notch1 gene expression in ATX/IRS1 mice that peaked at 12 months (p<0.009) with overexpression in tumor tissue (p<0.00001). (B) Enhanced expression of the Jag1 ligand both in the liver of double transgenic male mice (p<0.01) and their derived HCC tumors (p<0.00003). (C) Downstream Notch regulated Hes1 gene was also upregulated (9 months) in the ATX/IRS1 transgenic male mice (p<0.0007) and tumor tissue (p<0.00004). (D) ASPH levels were unchanged in the liver of WT and transgenic animals but was elevated 70 fold in HCC tumors (p<0.00001).
4. DISCUSSION
This transgenic murine HCC model was based on the previous findings that HBx and IRS1 are constitutively expressed in HBV-related liver tumors from patients and activate signaling cascades. Figure 5 depicts how these pathways may interact with and activate each other; as well as emphasizes the key role of a novel cell surface enzyme ASPH. As a regulatory protein, ASPH links the upstream IN/IGF1 growth factor signaling cascade to downstream Notch activation necessary to produce a malignant phenotype characterized by enhanced proliferation, migration, invasion and metastasis. We also attempted to define the sequential changes in signaling cascades activated in the liver prior to and during tumor development (Figure 5).
Figure 5.
A. Crosstalk of HBx with IN/IGF and Wnt/β-catenin signaling cascades. HBx can upregulate IN/IGF signaling by activating Ras and upregulate Wnt/β-catenin signaling by suppressing GSK3β. An extensive network of crosstalk and feedback circuit exists between the two pathways, the PI3K/AKT cascade downstream of IN/IGF can suppress GSK3β activity through an inhibitory phosphorylation event. B. Hypothesized network of pathways/molecules involved in HCC oncogenesis in ATX/IRS1 transgenic mice. ASPH acts as a link between the IN/IGF growth factors and the downstream pathways, especially activation of Notch signaling. ASPH expression can be upregulated by IN or IGF1/2 stimulation leading to activation of Notch to promote cell migration, invasion and metastasis of HCC. A small molecule inhibitor (SMI) that inhibits β-hydroxylase activity by 80% demonstrates anti-tumor effects on HCC growth and progression [5].
The insulin receptor (IR) subfamily is a member of the receptor tyrosine kinase (RTK) superfamily of evolutionarily conserved membrane spanning cell surface receptors. They affect many fundamental cellular processes, such as differentiation, proliferation, migration, and apoptosis, in almost every organ [10]. The IR subfamily plays a crucial role in the liver. Both IGF1 and IGF2 are strong mitogens that exert proliferative and antiapoptotic effects via two downstream signaling pathways, MAPK/ERK and PI3K/AKT cascades [11].
The IN/IGF signaling pathway is involved in the pathogenesis of HCC because overexpression of IRS1 and aberrant activation of the downstream cascades have been detected in >90% of tumors [3]. Inhibition of the signaling cascade by a dominant-negative IRS1 mutant can reverse the malignant phenotype of HCC cells [12]. Overexpression of IRS1 in NIH-3T3 cells has been shown to cause malignant transformation by promoting proliferation, anti-apoptosis, and tumor formation in nude mice [13]. Dysregulation of IN/IGF signaling pathway in HCC occurs frequently at the level of IGF2. Overexpression of IGF2, as a result of loss of promoter-specific imprinting and/or reactivation of fetal promoters, has been detected in HCC samples (40%) [14] HCC cell lines [15] and animal models [16]. IGF2 bioavailability can be affected by downregulation or deletion of IGF2R, which has been reported in >60% of human HCC [17]. Finally, IRS1 promoter sequences contain β-catenin binding sites that upregulate the expression of IRS1 through activation of Wnt/β-catenin signaling. These pathways crosstalk and directly interact with each other as shown in Figure 5A and B [18].
It has been proposed that HBx can upregulate IN/IGF signaling [19]. Furthermore, HBx has been shown to upregulate expressions of IGF2 and IGF1R through phosphorylation of the transcription factor Sp1, leading to direct activation of the IN/IGF signaling pathway [20].
The canonical Wnt/β-catenin pathway is highly conserved and involved in early liver development and liver regeneration after partial hepatectomy [21]. When Wnt ligands bind to the FZD-LRP5/6-Dishevelled receptor complex, phosphorylation of β-catenin at specific serine/threonine residues by GSK3β is inhibited to allow for its accumulation in the cytoplasm where it translocates to the nucleus and binds to T-cell factor (TCF) to upregulate downstream target genes such as cyclin D1 and TBX3 among others.
Mutations of β-catenin are found in 13-43% of human HCC samples, while the frequency of β-catenin nuclear accumulation varies between 17 to 75% as determined by immunohistochemistry [22-24]. Clinically, β-catenin accumulation is associated with poorly differentiated tumor morphology, highly proliferative activities, and aggressive vascular invasion [25, 26]. These findings suggest that cytoplasmic and nuclear accumulation of β-catenin is an excellent biomarker for activation of canonical Wnt/β-catenin signaling in HCC, although such activation may be caused by overexpression of upstream components, such as Wnt ligands and FZD receptors [27-30]. Indeed, upregulation of Wnt-3 and FZD-7 is observed in 95% of HCCs and 68% of peritumor tissues [27]. Accumulation of Wnt ligand/receptor in both HBV and HCV-related human HCC as well as various animal models of HCC suggests that Wnt/β-catenin pathway activation is essential to the development of HCC and is likely to occur at an early stage of the hepatocarinogenesis [31-33].
HBx can upregulate Wnt/β-catenin signaling in vitro [19, 34, 35] via activation of Src kinase, which phosphorylates GSK3β at Thr43 and thus suppresses its activity [19]. Inactivation of GSK3β inhibits the action of the GSK3β/Axin/APC destruction complex, resulting in nuclear accumulation of β-catenin and activation of downstream transcription factors as shown in Figure 5.
The Notch signaling components include four large transmembrane receptors (Notch1, 2, 3 and 4) coupled with ligands [JAG1 and 2; and Delta-like (DLL) 1, 3 and 4]. It is activated upon cell-cell interaction. A key molecular event is the cleavage of full-length receptors and the release of the intracellular domain (NICD) where it translocates to the nucleus to form a transcriptional complex that upregulates Notch responsive genes, such as HES and HEY family members. Consistently elevated levels of NCID were present in over 80% of human HCC samples [36] and JAG expression was significantly upregulated in cirrhosis. Thus, Notch signaling may play a critical role in HCC development [37]. Constitutive activation of Notch by conditional overexpression at the NICD produced HCC in 88-100% of the animals [38, 39]. Notch activation has been described in 30-90% of human HCC tumors [37, 39, 40] although its exact role and mechanisms of activation that contribute to HCC development and progression are controversial [37-39, 41, 42]. However, it is a major regulator of cell proliferation, migration, invasion and apoptosis.
Accumulating evidence supports that HBx expression can influence the Notch signaling pathway. HBx co-localizes with JAG1 in HBx-expressing HCC cell lines and HCC tumors [43]. Furthermore, HBx stable-transfected cell lines show elevated mRNA levels of Notch1, JAG1, and Hes1 [44]. It is uncertain, however, whether HBx interacts directly with components of the Notch pathway or acts through crosstalk with other signaling pathways.
Although the IN/IGF, Wnt/β-catenin and Notch pathways are often considered as independent signaling cascades, each having its own complements of ligands, receptors, transducers, and effectors, it has been indicated that these three pathways can interact with one another (Figure 5). IN/IGF and Wnt/β-catenin form a positive feedback loop, via TCF-mediated upregulation of IRS1, which activates PI3K/AKT and MAPK/ERK [18, 45]. Furthermore, Wnt/β-catenin and Notch signaling may cooperate functionally to regulate hepatocyte proliferation and biliary differentiation [46]. IN/IGF and Notch signaling may behave synergistically to induce hepatocyte transformation [47].
In this study, a transgenic murine model of HBV-related HCC was created by overexpressing HBx and IRS1 under the control of liver-specific promoters in an otherwise tumor resistant background. The ATX or IRS1 single transgenic line had a very low incidence of spontaneous neoplasm. The ATX/IRS1 double transgenic line, in contrast, developed microscopic and gross tumors at a significantly higher frequency, particularly in older male mice. Biochemical analysis have demonstrated that the IN/IGF1/IRS1/MAPK/ERK and IN/IGF1/IRS1/PI3K/AKT cascades were activated early in hepatic oncogenesis followed by sustained activation of WNT/β-catenin and Notch signaling, which was maintained in fully developed tumors. The activation status of these pathways also correlated with histological evidence of accelerated proliferation and tumor formation. We have observed markedly overexpression of ASPH, an enzyme that bridges upstream growth factor signaling pathways to downstream Notch activation, in tumor tissues derived from the double transgenic animals similar to that found in human HCC [5]. It is of interest that HCC development was observed only in male mice. Male predominance has been well described in rodent models of HCC growth and progression induced by chemical carcinogens [48]. A novel mechanism has been suggested that involves estrogen-mediated reduction of interleukin-6 production by Kupffer’s cells as directed through MyD88 signaling which reduces the risk of liver cancer in female mice [49]. The male dominance was particularly striking in the double-transgenic murine model presented here and may be due, in part, to additional epigenetic factors such as down-regulation of transgene expression through methylation of the promoter regions in female mice (unpublished observations).
In summary, our observations in over 1,000 mice that demonstrates 1) a potentially direct relationship of HBV through HBx expression to HCC development that involves sequential activation of signaling pathways in the liver; 2) a long latency from transgene expressions and activation of the tripartite signaling cascade exists prior to the development of HCC (>15 months) which mimics the natural progress from chronic hepatitis B to HCC in human; 3) a prominent male over female predominance with respect to tumor formation similar to HBV-related HCC in human [50]; 4) a high level activation of the IN/IGF1 signaling with enhanced expression (>70 fold) of IGF2 in tumor tissues; 5) common activation of Wnt/β-catenin signaling cascade due to overexpression of upstream components such as WNT3 and FZD7 was common; 6) overexpression of ASPH as an activator and modulator of Notch signaling with a consistent finding in tumor tissue; 7) histopathology simulating HBV-related HCC tumors encapsulated by fibrous tissues; 8) accelerated hepatocyte proliferation and characteristic dysplastic changes in the liver preceded malignant transformation; and 9) multifocal microscopic dysplastic foci that occurred prior to the development of adenomas, which progressed to HCC without the presence of cirrhosis. Taken together, this study emphasizes the importance of interactions between signaling cascades and a viral non-structural protein in the development of HBV related HCC tumors. This model system provides an opportunity to identify new targets for therapy as exemplified by the proposed role for ASPH in tumor growth and progression; indeed, anti-tumor effects have now been demonstrated by small molecule inhibitors of it β-hydroxylase enzymatic activity [5].
Supplementary Material
HIGHLIGHTS.
Demonstrates the role of viral-cellular protein interactions during oncogenesis.
Sequential activation of tripartite signaling cascades in the development of HCC.
Describes the similarity of a transgenic murine model to human HCC.
Links upstream growth factor pathways to Notch activation in HCC.
Acknowledgments
Financial Support:
This work was supported by grants from Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training (IT-32 DK60415), NIH/NCI (R01CA123544), National Institute for General Medical Sciences (NIGMS), National Institutes of Health (NIH) RI-INBRE Faculty Development Research Project Grant (8 P20GM103430-12).
Abbreviations
- ASPH
Aspartate β-hydroxylase
- ATX
α1-AT-HBx
- ELISA
enzyme-linked immunosorbent assay
- FZD
Frizzled
- JAG1
Jagged 1
- HBV
hepatitis B virus
- HBx
hepatitis B virus X protein
- HCC
hepatocellular carcinoma
- IGF1
insulin-like growth factor 1
- IGF1R
insulin-like growth factor 1 receptor
- IN
insulin
- IR
insulin receptor
- IRS1
insulin receptor substrate 1
- RTK
receptor tyrosine kinase
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
REFERENCES
- [1].Hollinger FB, Purcell RH, Gerin JL, Ganem DE, Feinstone SM. Viral Hepatitis. 1st Edition Lippincott Williams & Wilkins; 2002. [Google Scholar]
- [2].Schluter V, Meyer M, Hofschneider PH, Koshy R, Caselmann WH. Integrated hepatitis B virus X and 3′ truncated preS/S sequences derived from human hepatomas encode functionally active transactivators. Oncogene. 1994;9:3335–3344. [PubMed] [Google Scholar]
- [3].Nishiyama M, Wands JR. Cloning and increased expression of an insulin receptor substrate-1-like gene in human hepatocellular carcinoma. Biochem Biophys Res Commun. 1992;183:280–285. doi: 10.1016/0006-291x(92)91640-c. [DOI] [PubMed] [Google Scholar]
- [4].Longato L, de la Monte S, Kuzushita N, Horimoto M, Rogers AB, Slagle BL, Wands JR. Overexpression of insulin receptor substrate-1 and hepatitis Bx genes causes premalignant alterations in the liver. Hepatology. 2009;49:1935–1943. doi: 10.1002/hep.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aihara A, Huang CK, Olsen MJ, Lin Q, Chung W, Tang Q, Dong X, Wands JR. A Cell surface beta-Hydroxylase is a biomarker and therapeutic target for hepatocellular carcinoma. Hepatology. 2014 doi: 10.1002/hep.27275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee TH, Finegold MJ, Shen RF, DeMayo JL, Woo SL, Butel JS. Hepatitis B virus transactivator X protein is not tumorigenic in transgenic mice. J Virol. 1990;64:5939–5947. doi: 10.1128/jvi.64.12.5939-5947.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tanaka S, Mohr L, Schmidt EV, Sugimachi K, Wands JR. Biological effects of human insulin receptor substrate-1 overexpression in hepatocytes. Hepatology. 1997;26:598–604. doi: 10.1002/hep.510260310. [DOI] [PubMed] [Google Scholar]
- [8].Wands JR, Kim M. WNT/beta-catenin signaling and hepatocellular carcinoma. Hepatology. 2014;60:452–454. doi: 10.1002/hep.27081. [DOI] [PubMed] [Google Scholar]
- [9].Wang K, Liu J, Yan ZL, Li J, Shi LH, Cong WM, Xia Y, Zou QF, Xi T, Shen F, Wang HY, Wu MC. Overexpression of aspartyl-(asparaginyl)-beta-hydroxylase in hepatocellular carcinoma is associated with worse surgical outcome. Hepatology. 2010;52:164–173. doi: 10.1002/hep.23650. [DOI] [PubMed] [Google Scholar]
- [10].Stewart CE, Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiological reviews. 1996;76:1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- [11].Le Roith D. The insulin-like growth factor system. Experimental diabesity research. 2003;4:205–212. doi: 10.1155/EDR.2003.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tanaka S, Wands JR. A carboxy-terminal truncated insulin receptor substrate-1 dominant negative protein reverses the human hepatocellular carcinoma malignant phenotype. J Clin Invest. 1996;98:2100–2108. doi: 10.1172/JCI119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ito T, Sasaki Y, Wands JR. Overexpression of human insulin receptor substrate 1 induces cellular transformation with activation of mitogen-activated protein kinases. Mol Cell Biol. 1996;16:943–951. doi: 10.1128/mcb.16.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cariani E, Lasserre C, Seurin D, Hamelin B, Kemeny F, Franco D, Czech MP, Ullrich A, Brechot C. Differential expression of insulin-like growth factor II mRNA in human primary liver cancers, benign liver tumors, and liver cirrhosis. Cancer Res. 1988;48:6844–6849. [PubMed] [Google Scholar]
- [15].Li X, Nong Z, Ekstrom C, Larsson E, Nordlinder H, Hofmann WJ, Trautwein C, Odenthal M, Dienes HP, Ekstrom TJ, Schirmacher P. Disrupted IGF2 promoter control by silencing of promoter P1 in human hepatocellular carcinoma. Cancer Res. 1997;57:2048–2054. [PubMed] [Google Scholar]
- [16].Harris TM, Rogler LE, Rogler CE. Reactivation of the maternally imprinted IGF2 allele in TGFalpha induced hepatocellular carcinomas in mice. Oncogene. 1998;16:203–209. doi: 10.1038/sj.onc.1201519. [DOI] [PubMed] [Google Scholar]
- [17].Sue SR, Chari RS, Kong FM, Mills JJ, Fine RL, Jirtle RL, Meyers WC. Transforming growth factor-beta receptors and mannose 6-phosphate/insulin-like growth factor-II receptor expression in human hepatocellular carcinoma. Annals of surgery. 1995;222:171–178. doi: 10.1097/00000658-199508000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bommer GT, Feng Y, Iura A, Giordano TJ, Kuick R, Kadikoy H, Sikorski D, Wu R, Cho KR, Fearon ER. IRS1 regulation by Wnt/beta-catenin signaling and varied contribution of IRS1 to the neoplastic phenotype. The Journal of biological chemistry. 2010;285:1928–1938. doi: 10.1074/jbc.M109.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Molecular cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- [20].Kim SO, Park JG, Lee YI. Increased expression of the insulin-like growth factor I (IGF-I) receptor gene in hepatocellular carcinoma cell lines: implications of IGF-I receptor gene activation by hepatitis B virus X gene product. Cancer Res. 1996;56:3831–3836. [PubMed] [Google Scholar]
- [21].Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33:1098–1109. doi: 10.1053/jhep.2001.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Inagawa S, Itabashi M, Adachi S, Kawamoto T, Hori M, Shimazaki J, Yoshimi F, Fukao K. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: correlation with tumor progression and postoperative survival. Clin Cancer Res. 2002;8:450–456. [PubMed] [Google Scholar]
- [23].Laurent-Puig P, Zucman-Rossi J. Genetics of hepatocellular tumors. Oncogene. 2006;25:3778–3786. doi: 10.1038/sj.onc.1209547. [DOI] [PubMed] [Google Scholar]
- [24].Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92:136–145. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [25].Devereux TR, Stern MC, Flake GP, Yu MC, Zhang ZQ, London SJ, Taylor JA. CTNNB1 mutations and beta-catenin protein accumulation in human hepatocellular carcinomas associated with high exposure to aflatoxin B1. Molecular carcinogenesis. 2001;31:68–73. doi: 10.1002/mc.1041. [DOI] [PubMed] [Google Scholar]
- [26].Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL, Peng SY. Beta-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol. 2000;157:763–770. doi: 10.1016/s0002-9440(10)64590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bengochea A, de Souza MM, Lefrancois L, Le Roux E, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P, Scoazec JY, Vitvitski L, Merle P. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143–150. doi: 10.1038/sj.bjc.6604422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, Merle P, Wands JR. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J Hepatol. 2008;48:780–791. doi: 10.1016/j.jhep.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, Kew MC, Trepo C, Wands JR. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004;127:1110–1122. doi: 10.1053/j.gastro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- [30].Merle P, Kim M, Herrmann M, Gupte A, Lefrancois L, Califano S, Trepo C, Tanaka S, Vitvitski L, de la Monte S, Wands JR. Oncogenic role of the frizzled-7/beta-catenin pathway in hepatocellular carcinoma. J Hepatol. 2005;43:854–862. doi: 10.1016/j.jhep.2005.05.018. [DOI] [PubMed] [Google Scholar]
- [31].Calvisi DF, Factor VM, Ladu S, Conner EA, Thorgeirsson SS. Disruption of beta-catenin pathway or genomic instability define two distinct categories of liver cancer in transgenic mice. Gastroenterology. 2004;126:1374–1386. doi: 10.1053/j.gastro.2004.02.014. [DOI] [PubMed] [Google Scholar]
- [32].Calvisi DF, Factor VM, Loi R, Thorgeirsson SS. Activation of beta-catenin during hepatocarcinogenesis in transgenic mouse models: relationship to phenotype and tumor grade. Cancer Res. 2001;61:2085–2091. [PubMed] [Google Scholar]
- [33].Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- [34].Cha MY, Kim CM, Park YM, Ryu WS. Hepatitis B virus X protein is essential for the activation of Wnt/beta-catenin signaling in hepatoma cells. Hepatology. 2004;39:1683–1693. doi: 10.1002/hep.20245. [DOI] [PubMed] [Google Scholar]
- [35].Hsieh A, Kim H-S, Lim S-O, Yu D-Y, Jung G. Hepatitis B viral X protein interacts with tumor suppressor adenomatous polyposis coli to activate Wnt/[beta]-catenin signaling. Cancer Letters. 2011;300:162–172. doi: 10.1016/j.canlet.2010.09.018. [DOI] [PubMed] [Google Scholar]
- [36].Ning L, Wentworth L, Chen H, Weber SM. Down-regulation of Notch1 signaling inhibits tumor growth in human hepatocellular carcinoma. American journal of translational research. 2009;1:358–366. [PMC free article] [PubMed] [Google Scholar]
- [37].Strazzabosco M. Notch signaling in hepatocellular carcinoma: guilty in association. Gastroenterology. 2012;143:1430–1434. doi: 10.1053/j.gastro.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dill MT, Tornillo L, Fritzius T, Terracciano L, Semela D, Bettler B, Heim MH, Tchorz JS. Constitutive Notch2 signaling induces hepatic tumors in mice. Hepatology. 2013;57:1607–1619. doi: 10.1002/hep.26165. [DOI] [PubMed] [Google Scholar]
- [39].Villanueva A, Alsinet C, Yanger K, Hoshida Y, Zong Y, Toffanin S, Rodriguez-Carunchio L, Sole M, Thung S, Stanger BZ, Llovet JM. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology. 2012;143:1660–1669. e1667. doi: 10.1053/j.gastro.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cantarini MC, de la Monte SM, Pang M, Tong M, D’Errico A, Trevisani F, Wands JR. Aspartyl-asparagyl beta hydroxylase over-expression in human hepatoma is linked to activation of insulin-like growth factor and notch signaling mechanisms. Hepatology. 2006;44:446–457. doi: 10.1002/hep.21272. [DOI] [PubMed] [Google Scholar]
- [41].Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, Sticht C, Tomasi ML, Delogu S, Evert M, Fan B, Ribback S, Jiang L, Brozzetti S, Bergmann F, Dombrowski F, Schirmacher P, Calvisi DF, Breuhahn K. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530–1542. e1512. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhou L, Zhang N, Song W, You N, Li Q, Sun W, Zhang Y, Wang D, Dou K. The significance of Notch1 compared with Notch3 in high metastasis and poor overall survival in hepatocellular carcinoma. PLoS One. 2013;8:e57382. doi: 10.1371/journal.pone.0057382. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [43].Gao J, Chen C, Hong L, Wang J, Du Y, Song J, Shao X, Zhang J, Han H, Liu J, Fan D. Expression of Jagged1 and its association with hepatitis B virus X protein in hepatocellular carcinoma. Biochem Biophys Res Commun. 2007;356:341–347. doi: 10.1016/j.bbrc.2007.02.130. [DOI] [PubMed] [Google Scholar]
- [44].Wang F, Zhou H, Xia X, Sun Q, Wang Y, Cheng B. Activated Notch signaling is required for hepatitis B virus X protein to promote proliferation and survival of human hepatic cells. Cancer Letters. 2010;298:64–73. doi: 10.1016/j.canlet.2010.06.003. [DOI] [PubMed] [Google Scholar]
- [45].Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. The Journal of biological chemistry. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- [46].Spee B, Carpino G, Schotanus BA, Katoonizadeh A, Vander Borght S, Gaudio E, Roskams T. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut. 2010;59:247–257. doi: 10.1136/gut.2009.188367. [DOI] [PubMed] [Google Scholar]
- [47].Fan R, Chen P, Zhao D, Tong JL, Li J, Liu F. Cooperation of deregulated Notch signaling and Ras pathway in human hepatocarcinogenesis. Journal of molecular histology. 2011;42:473–481. doi: 10.1007/s10735-011-9353-3. [DOI] [PubMed] [Google Scholar]
- [48].Wands J. Hepatocellular carcinoma and sex. The New England journal of medicine. 2007;357:1974–1976. doi: 10.1056/NEJMcibr075652. [DOI] [PubMed] [Google Scholar]
- [49].Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- [50].Lee CM, Lu SN, Changchien CS, Yeh CT, Hsu TT, Tang JH, Wang JH, Lin DY, Chen CL, Chen WJ. Age, gender, and local geographic variations of viral etiology of hepatocellular carcinoma in a hyperendemic area for hepatitis B virus infection. Cancer. 1999;86:1143–1150. doi: 10.1002/(sici)1097-0142(19991001)86:7<1143::aid-cncr7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.