Abstract
Earlier studies from our laboratory in MRL+/+ mice suggest that free radicals, especially overproduction of reactive nitrogen species (RNS) and lipid-derived reactive aldehydes (LDRAs), are associated with trichloroethene (TCE)-mediated autoimmune response. The current study was undertaken to further assess the contribution of RNS and LDRAs in TCE-mediated autoimmunity by using iNOS-null MRL+/+ mice. iNOS-null MRL+/+ mice were obtained by backcrossing iNOS-null mice (B6.129P2-Nos2tm1Lau/J) to MRL +/+ mice. Female MRL+/+ and iNOS-null MRL+/+ mice were given TCE (10 mmol/kg, i.p., every 4th day) for 6 weeks; their respective controls received corn oil only. TCE exposure led to significantly increased iNOS mRNA in livers, iNOS protein in livers and sera, increased nitrotyrosine (NT) formation in both livers and sera, induction of MDA-/HNE-protein adducts in livers and their respective antibodies in sera along with significant increases in serum antinuclear antibodies (ANA) and anti-dsDNA in MRL+/+ mice. Even though in iNOS-null MRL+/+ mice, the iNOS and NT levels were negligible in both TCE-treated and untreated groups, TCE treatment still led to significant increases in MDA-/HNE-protein adducts and their respective antibodies along with increases in serum ANA and anti-dsDNA compared to controls. Most remarkably, the increases in serum ANA and anti-dsDNA induced by TCE in the iNOS-null MRL+/+ mice were significantly less pronounced compared to that in MRL+/+ mice. Our results provide further evidence that both RNS and LDRAs contribute to TCE-induced autoimmunity in MRL+/+ mice, and iNOS deficiency attenuates this autoimmune response.
Keywords: Trichloroethene, autoimmunity, autoantibody, free radical, iNOS
Introduction
The harmful free radicals can cause lipid, protein or DNA damage due to their overproduction, leading to various diseases including autoimmune diseases (ADs) [1–4]. Different lines of evidence suggest the involvement of free radical-mediated reactions in the pathogenesis of ADs [1–4]. Reactive nitrogen species (RNS) are nitrogen-containing oxidants, i.e., nitric oxide (·NO), peroxynitrite (ONOO−) and nitroxyl anion (HNO−) [5]. ·NO, formed via the enzyme inducible nitric oxide synthase (iNOS), is one of the most important and widely studied RNS. The potential of ·NO in disease pathogenesis depends largely on the extent and generation of O2·− leading to the formation of peroxynitrite (ONOO−). ONOO− is a potent oxidizing agent which can oxidize tyrosine residues to nitrotyrosine (NT) [3,6–9]. In addition, ONOO−-modified endogenous proteins and DNA may become immunogenic, leading to a break in immune tolerance [3,10–12]. Accumulating evidence in murine lupus suggests an association between increasing iNOS activity and development and progression of ADs [6,8,9,13–16]. Studies using competitive inhibitors provide additional support that iNOS could play a pathogenic role in murine ADs [6,8,9,15]. Furthermore, growing observational data in humans also suggest that increased production of ONOO− via overexpression of iNOS may contribute to glomerular and vascular pathology, and to the pathogenesis of many ADs [11,14,17]. Although there is appreciable evidence that NT, the marker of nitrosative modification of proteins, is enhanced in systemic lupus erythematosus (SLE) and other ADs [12,18,19], the potential contribution of RNS establishing a cause-and-effect relationship in the pathogenesis of ADs remain largely unexplored.
Lipid-derived reactive aldehydes (LDRAs) are generally derived from unsaturated lipids. LDRAs such as malondialdehyde (MDA) and 4-hydroxynonenal (HNE) are highly reactive electrophiles and are known to bind covalently to proteins resulting in their structural modifications that may elicit an autoimmune response and contribute to disease pathogenesis [18,20–22]. Indeed higher levels of MDA-/HNE-modified proteins have been reported in patients with ADs [18,22–26], suggesting a potential role for these oxidatively modified proteins in ADs.
Trichloroethene (TCE), a common environmental contaminant and a widely used cleaning solvent, is associated with the development of ADs such as SLE, systemic sclerosis and fascitis, both in humans and animals [27–32]. Even though TCE is known to cause increased generation of RNS and LDRAs which may contribute to TCE-mediated autoimmunity [27,28,31,33–35], potential mechanisms by which TCE-induced overproduction of RNS and LDRAs lead to an autoimmune response and their contribution to disease pathogenesis need to be thoroughly investigated. Our earlier studies in MRL+/+ mice showed induction of iNOS, increased the formation of NT, and increased production of LDRA-protein adducts with concurrent production of ANA and other autoantibodies following TCE exposure [35]. To further evaluate the role of RNS and LDRAs, especially the potential of iNOS in TCE-mediated autoimmunity, we examined the markers of autoimmunity, nitrosative and oxidative stress in the sera and livers of both iNOS-null MRL+/+ mice and MRL+/+ mice treated with TCE.
Materials and methods
Animals and treatments
Both MRL+/+ (MRL/MpJ) mice and iNOS-null (B6.129P2-Nos2tm1Lau/J) mice were purchased from Jackson Laboratory (Bar Harber, ME) and housed at the University of Texas Medical Branch (UTMB) animal house facility maintained at ~ 22°C, 50–60% relative humidity, and a 12 hr light/dark cycle. The animals were provided standard lab chow and drinking water ad libitum. TCE (purity 99+ %) was purchased from Sigma chemical Co. (St. Louis, MO). The experiments were performed in accordance with the guidelines of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of UTMB. We chose to conduct our studies in female MRL+/+ mice as these mice spontaneously develop relatively mild lupus-like disease and other autoimmune disorders late in the second year of their lives [30,31,36,37]. Young MRL+/+ mice, with their propensity for autoimmunity but absence of overt disease, make a good model to test the immunostimulatory potential of TCE. Also, females are generally more susceptible to ADs than males and approximately 70–90% of SLE patients are females [18,30,31,38]. iNOS-null mice were backcrossed to MRL +/+ mice for 10 generations and then homozygous mutants (iNOS-null MRL+/+ mice) were obtained from N10 heterozygous intercrosses. Female MRL+/+ and iNOS-null MRL+/+ mice, in groups of six each, were treated with TCE (10 mmol/kg, i.p., every 4th day) [15,16,30,31,39] for 6 weeks, their respective controls received corn oil only. After 6 weeks of treatment, the animals were euthanized under nembutal (sodium pentobarbital) anesthesia, and blood was withdrawn from the inferior vena cava. Individual sera were obtained following blood clotting and centrifugation, and stored at -80°C until further analysis. At the same time, major organs were immediately removed and weighed. Portions of livers from control and TCE-treated mice were snap-frozen in liquid nitrogen and stored at −80°C for further analysis.
Quantification of nitrotyrosine and iNOS in serum
Nitrotyrosine (NT) levels in the mouse serum were quantitated by an ELISA kit (Cell Sciences, Norwood, MA), whereas iNOS was measured by an ELISA established in our laboratory [15,27].
Western blot detection of iNOS in the livers
iNOS in the livers of mice was also detected by Western blot analysis as described previously [27]. Briefly, liver proteins from control, TCE-treated mice were obtained using a lysis buffer (Pierce, Rockford, IL), and protein concentration in the lysates was determined by Bio-Rad Protein Assay method (Bio-Rad Laboratories, Inc., Hercules, CA). Fifty µg of protein dissolved in sample buffer was loaded onto a 12% Novex Tris-Glycine Gel (Invitrogen, Carlsbad, CA), resolved by electrophoresis, and subsequently transferred to a nitrocellulose membrane. The membrane was incubated with TBS with 0.1% Tween-20 and 5% non-fat dry milk at room temperature for 2 h and subsequently probed with rabbit polyclonal anti-iNOS antibody (1:3000; Alpha Diagnostic Int’l, San Antonio, TX) for 2 h. Blots were washed thoroughly and incubated with horseradish peroxidase-conjugated goat anti-rabbit antibody (1:5000; Upstate) for 1 h. iNOS bands were detected by using enhanced chemiluminescence (ECL) system (Amersham, Piscataway, NJ). The density of iNOS bands was analyzed with Eagle Eye II software (Stratagene, La Jolla, CA).
Real-time PCR analysis for iNOS gene expression in liver
Real-time RT-PCR was performed essentially as described earlier [15,16]. Briefly, total RNA was isolated from livers using a RiboPure kit (Ambion, Austin, TX) following the manufacturer's instructions. cDNA was prepared from total RNA by using the SuperScript first-strand synthesis kit (Invitrogen, Carlsbad, CA) as per the manufacturer's manual. Real-time PCR employing a two-step cycling protocol (denaturation and annealing/extension) was carried out using a Mastercycler Realplex (Eppendorf, Westbury, NY) and the primer pairs used in the real-time PCR process were 5’-TGTCTGCAGCACTTGGATCA (forward) and 5’-AACTTCGGAAGGGAGCAATG (reverse). For each cDNA sample, parallel reactions were performed in triplicate for the detection of 18S and iNOS. The reaction samples in a final volume of 25 µl contained 2 µl cDNA templates, 2 µl primer pair, 12.5 µl iQ SYBR Green Supermix, and 8.5 µl water. Amplification conditions were identical for all reactions: 95°C for 2 min for template denaturation and hot-start before PCR cycling. A typical cycling protocol consisted of three stages, 15 s at 95°C for denaturation, 30 s at 60°C for annealing, and 30 s at 72°C for extension and an additional 20-s hold for fluorescent signal acquisition.
Quantitation of nitrotyrosine in the livers
Nitrated proteins were quantitated in the livers of control and TCE-treated mice. Liver homogenates (10%, w/v) were made in PBS (pH 7.4) containing protease inhibitor cocktail (Sigma), centrifuged at 10,000 g at 4 °C for 15 min and NT in the supernatants was quantitated by an ELISA (Cell Sciences) [15,27].
Anti-MDA- and anti-HNE-protein adduct specific antibodies in the serum
ELISAs to analyze anti-MDA- and -HNE-protein adduct-specific antibodies in the mouse serum were performed as described earlier [18,28,31,35]. The samples with net OD values (the difference between OD for MDA-/HNE-ovalbumin and ovalbumin) greater than 0.2, 0.4, or 0.6 were graded as moderately positive (+), highly positive (++), or strongly positive (+++), respectively [28,31].
Quantitation of MDA- and HNE-protein adducts in livers
Quantitative competitive ELISAs for MDA- and HNE-protein adducts in the liver homogenates of control and TCE-treated mice was performed according to Wang et al. [18,35]. Flat bottomed 96-well microtiter plates were coated with MDA-/HNE-ovalbumin adducts or ovalbumin (0.5 µg/well) overnight at 4 °C. For the competitive ELISA, rabbit antisera (1:2000 diluted anti-MDA or 1:3000 diluted anti-HNE) were incubated with test samples (standards or unknown) at 4 °C overnight. The coated plates were blocked with a blocking buffer (50 mM Tris-buffered saline with 1% BSA, pH 8.0; Bio-Rad Laboratories) for 2 h at room temperature (RT), then a 50 µl aliquot of each of the above mentioned incubation mixtures was added to duplicate wells and incubated for 2 h at RT. After washing, 50 µl of goat anti-rabbit IgG-HRP (1:2000 dilution, Millipore, Billerica, MA) was added and incubated for 1 h at RT. After washing, 100 µl of TMB peroxidase substrate (KPL, Gaithersburg, MD) was added to each well. The reaction was stopped after 10 min by adding 100 µl 2M H2SO4 and the absorbance was read at 450 nm on a Bio-Rad Benchmark plus microplate spectrophotometer (Bio-Rad Laboratories, Hercules, CA).
Anti-nuclear and anti-double stranded DNA antibodies in the serum
Serum levels of anti-nuclear antibodies (ANA) and anti-double stranded DNA antibodies (anti-dsDNA) were determined by using mouse-specific ELISA kits (Alpha Diagnostic Int’l) as described earlier [27,28,30,35].
Statistical analyses
The values presented are means ± SD. Two-way ANOVA followed by Tukey’s multiple comparison test (GraphPad Prism software, La Jolla, CA) was performed for the statistical analysis. A p value < 0.05 was considered to be statistically significant.
Results
iNOS in the sera and livers
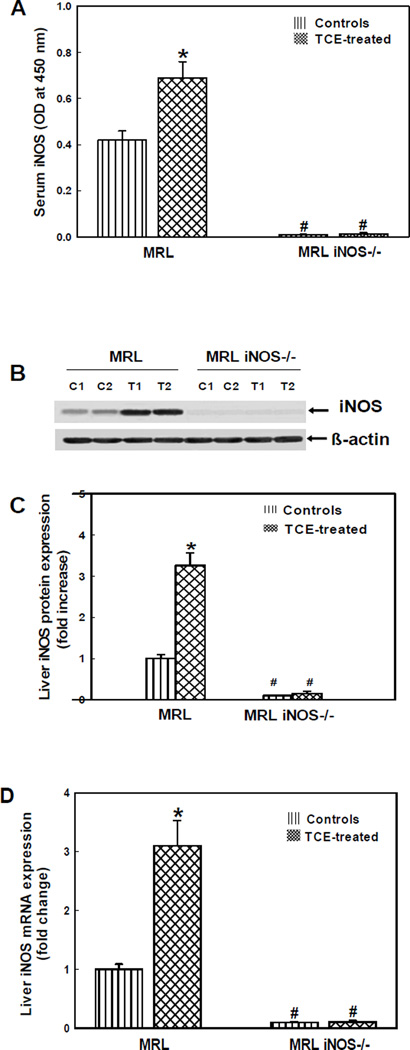
iNOS that generates ·NO, the most important RNS, is associated with the development and progression of ADs [5,6,14]. The levels of iNOS in the sera, determined by ELISA, in the MRL+/+ and iNOS-null MRL+/+ mice are presented in Fig. 1A. iNOS levels in the TCE-treated group were significantly higher in comparison to their controls in MRL+/+ mice, but no iNOS was detectable in both TCE-treated and control iNOS-null MRL+/+ mice. The iNOS protein expression in the livers (Western blot analysis) showed significantly increased expression in the livers of TCE-treated MRL+/+ mice (~3.2 fold, Fig. 1B,C) compared to their respective controls, whereas iNOS expression was negligible in both TCE-treated or control iNOS-null MRL+/+ mice.
Fig. 1.
iNOS content in the sera (A), iNOS protein expression in the livers (B, C) and iNOS mRNA expression in the livers (D) of MRL+/+ (MRL) and iNOS-null MRL+/+ (MRL iNOS−/−) mice treated with TCE. Values are means ± SD. * p < 0.05 vs. controls.
To further determine the impact of TCE exposure on iNOS regulation, the iNOS mRNA expression was analyzed using real-time PCR in the livers of MRL+/+ and iNOS-null MRL+/+ mice. The mRNA levels in livers of TCE-treated MRL+/+ mice increased significantly (~3.1 fold) in comparison to their respective controls (Fig. 1D). As expected, iNOS mRNA expression was also negligible in both TCE-treated and control iNOS-null MRL+/+ mice. Interestingly, the changes in liver mRNA expression matched well with increases in protein expression as determined by Western blot (Fig. 1B,C).
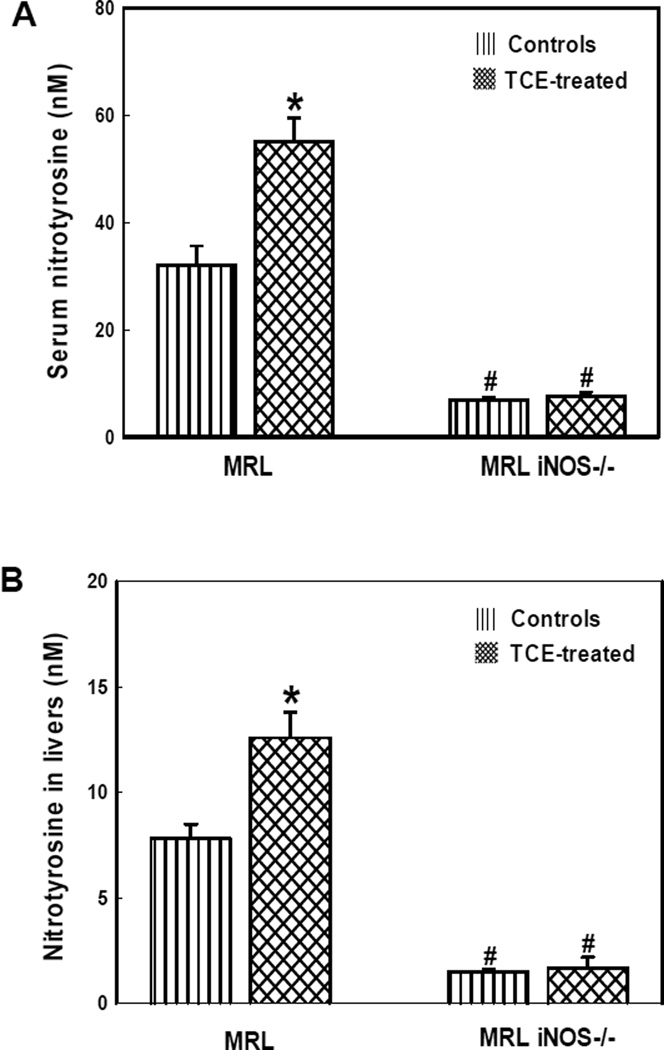
Nitrotyrosine levels in the serum and livers
Nitrotyrosine, a biomarker of RNS-modified proteins, is implicated in the pathogenesis of ADs [6,12,18,40]. The role of nitrosative stress in TCE-mediated autoimmune response was further assessed by measuring serum levels of NT in the MRL+/+ and iNOS-null MRL+/+ mice. Fig. 2A shows that TCE exposure led to significant increases in serum NT formation in MRL+/+ mice, whereas significantly low levels of NT were detected in iNOS-null MRL+/+ mice (Fig. 2A). The NT levels in liver, a major organ where TCE is known to generate free radicals and lead to autoimmune damages [28,29,33,35], were also analyzed. The NT levels in livers were also significantly higher in TCE-treated MRL+/+ mice compared to their respective controls, whereas the levels of NT were very low in both TCE-treated or control iNOS-null MRL+/+ mice (Fig. 2B).
Fig. 2.
Nitrotyrosine content in the sera (A) and livers (B) of MRL+/+ (MRL) and iNOS-null MRL+/+ (MRL iNOS−/−) mice treated with TCE. Values are means ± SD. * p < 0.05 vs. controls.
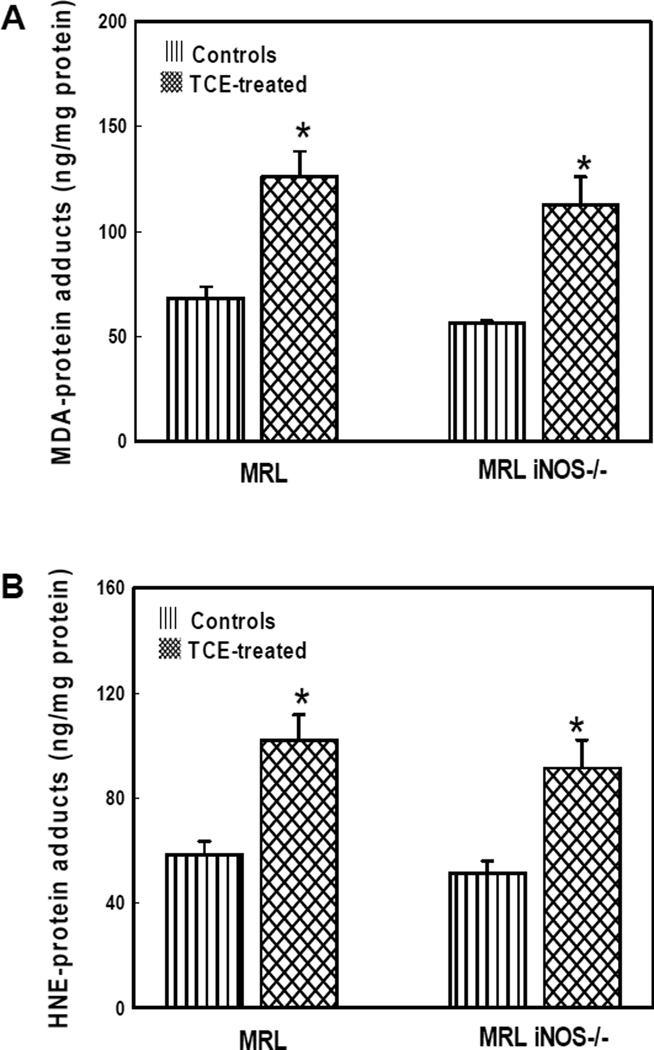
MDA- and HNE-protein adducts in the livers
Increasing evidence suggests that LDRAs such as MDA and HNE play a potential role in the pathogenesis of ADs [18,21,22,24,25,35]. To obtain more evidence for the involvement of LDRAs in TCE-mediated autoimmunity, we quantified MDA-/HNE-protein adducts in the liver homogenates from both MRL+/+ and iNOS-null MRL+/+ mice (Fig. 3). TCE treatment in both MRL+/+ and iNOS-null MRL+/+ mice led to significantly increased formation of MDA-protein adducts in the livers in comparison to their corresponding controls. Increases in these adducts induced by TCE in MRL+/+ mice were similar as that in iNOS-null MRL+/+ mice (Fig. 3A). Similarly, the HNE-protein adduct levels were also significantly higher in the livers of both MRL+/+ and iNOS-null MRL+/+ mice treated with TCE compared to their corresponding controls (Fig. 3B), and the increased formation of HNE-protein adducts in the two groups showed no significant difference.
Fig. 3.
MDA-protein adducts (A) and HNE-protein adducts (B) in the livers of MRL+/+ (MRL) and iNOS-null MRL+/+ (MRL iNOS−/−) mice treated with TCE. Values are means ± SD. * p < 0.05 vs. controls.
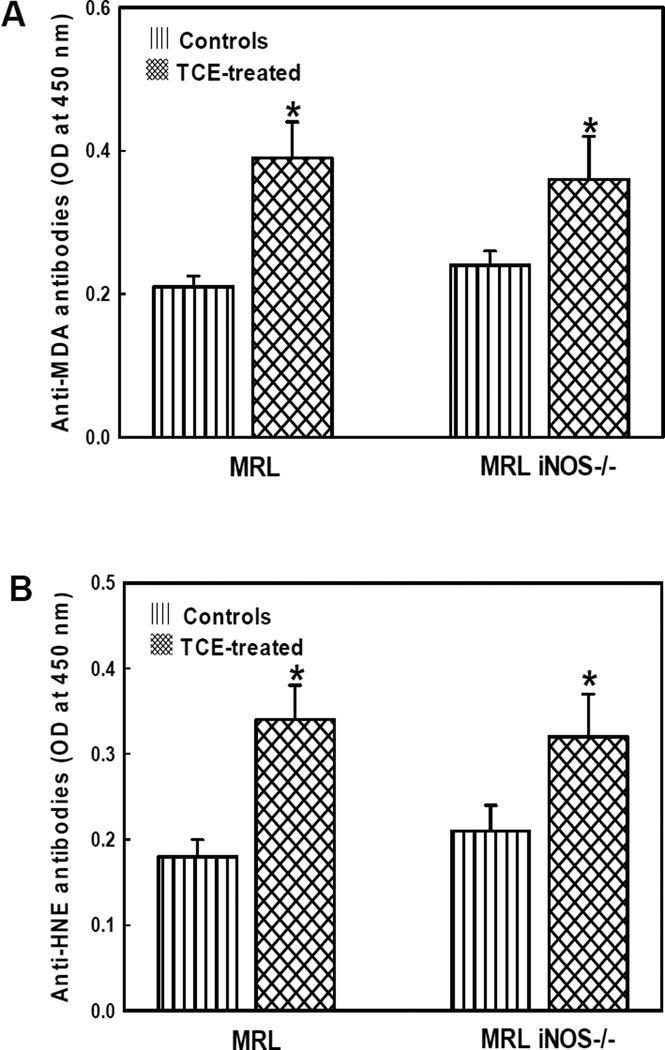
Anti-MDA- and anti-HNE-protein adduct antibodies in the serum
Since TCE exposure caused increases in MDA/HNE-protein adducts in the livers of both MRL+/+ and iNOS-null MRL+/+ mice, it was necessary to examine the potential role of MDA-and HNE-adducts in TCE-mediated autoimmunity. Therefore, we were evaluated the autoimmunogenicity of these adducts by determining serum anti-MDA-/HNE-protein adduct antibodies in MRL+/+ and iNOS-null MRL+/+ mice (Fig. 4). As evident from Fig. 4A, the levels of anti-MDA-protein adduct antibodies in both MRL+/+ and iNOS-null MRL+/+ mice treated with TCE for 6 weeks were significantly increased compared to their corresponding controls. Moreover, the number and percentage of samples positive (+), highly positive (++) and strongly positive (+++) for these antibodies were also higher in the TCE-treated mice compared to their respective controls (data not shown). There was no significant difference in the levels of anti-MDA-protein adduct antibodies in TCE-treated MRL+/+ mice compared to that in TCE-treated iNOS-null MRL+/+ mice.
Fig. 4.
Anti-MDA-protein adduct antibodies (A) and Anti-HNE-protein adduct antibodies (B) in the sera of MRL+/+ (MRL) and iNOS-null MRL+/+ (MRL iNOS−/−) mice treated with TCE. Values are means ± SD. * p < 0.05 vs. controls.
Serum levels of anti-HNE-protein adduct antibodies, and the number and percentage of serum samples positive for these antibodies in TCE-treated MRL+/+ and iNOS-null MRL+/+mice showed a pattern similar to that of anti-MDA-protein adduct antibodies. No significant difference in the levels of anti-HNE-protein adduct antibodies between TCE-treated MRL+/+ mice and TCE-treated iNOS-null MRL+/+ mice was observed (Fig. 4B).
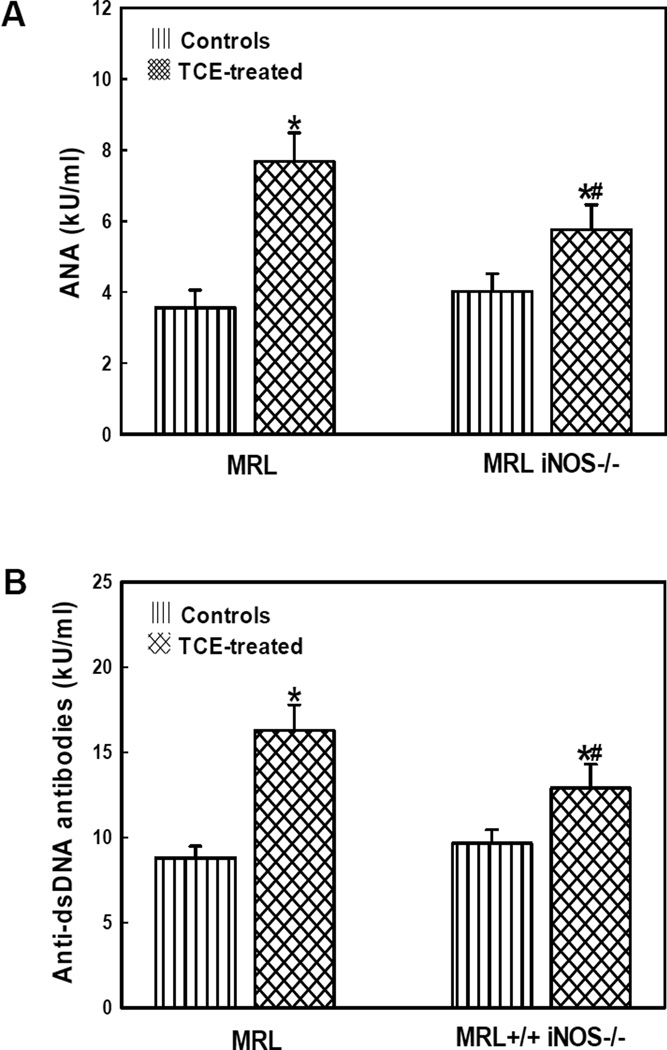
Serum autoantibodies in the serum
Autoantibodies including ANA and dsDNA, are considered important indices and biomarkers of ADs [41,42] and hence were analyzed in the sera of MRL+/+ and iNOS-null MRL+/+ mice (Fig. 5). In comparison to respective controls, there were significant increases in serum ANA and dsDNA levels in both MRL+/+ and iNOS-null mice treated with TCE. Most importantly, the increases in serum ANA and anti-dsDNA antibodies induced by TCE in the iNOS-null mice were significantly less pronounced compared to that in MRL+/+ mice (Fig. 5).
Fig. 5.
Serum levels of anti-nuclear antibodies (A) and anti-dsDNA antibodies (B) in the MRL+/+ (MRL) and iNOS-null MRL+/+ (MRL iNOS−/−) mice treated with TCE. Values are means ± SD. * p < 0.05 vs. controls, # p < 0.05 vs. TCE- treated MRL+/+ mice.
Discussion
In recent years, increasing evidence is presented to suggest that free radical-mediated reactions could potentially be involved in the pathogenesis of ADs [18,24,28,31,43,44]. This is well-supported by reports showing increased oxidative/nitrosative stress in ADs [14,18,23,24,43,45,46]. Earlier studies in our laboratory showed that increased formation of RNS and LDRAs was associated with increased levels of autoantibodies in autoimmune-prone MRL+/+ mice, suggesting a potential role of oxidative/nitrosative stress in TCE-mediated autoimmune response [27,28,31,35]. However, the molecular mechanisms have not been clearly elucidated. The present study was, therefore, aimed to provide new mechanistic evidence for the role of RNS/ROS in TCE-mediated autoimmune response by knocking out iNOS gene from MRL +/+ mice and treating both MRL +/+ and iNOS-null MRL+/+ mice with TCE.
·NO, the most widely studied RNS, plays an important role in SLE and other ADs [8,9,11,47]. ·NO, formed in excessive amounts via iNOS activation, is a short-lived radical that contributes to the pathogenesis largely by reacting with superoxide to yield ONOO− which can react with tyrosine residues to form NT in a given protein [3,8,11,13,47]. It has been shown that increases in iNOS activity is associated with increased formation of NT and plays a pathogenic role in the development and progression of ADs [6,8,9,13]. This led to our interest in exploring the potential contributions of nitrosative stress, especially the iNOS gene to TCE-mediated autoimmunity by generating iNOS null MRL+/+ mice. The observed increases in iNOS protein and mRNA expression were accompanied by an enhanced formation of NT in MRL+/+ mice treated with TCE. On the other hand, iNOS-null MRL+/+ mice treated with TCE or their controls showed almost no iNOS and NT formation, suggesting TCE induces an increased nitrosative stress in MRL +/+ mice, but not in iNOS-null MRL +/+ mice.
MDA and HNE, two widely studied LDRAs, could be involved in the pathogenesis of ADs [18,21,22,24,25,35]. Studies suggest that RNS such as ·NO may be an intermediary in the initiation of lipid peroxidation through peroxynitrite [9,48], and inhibition of iNOS prevented lipid peroxidation [9,49,50]. Earlier studies from our laboratory have shown the involvement of MDA and HNE in TCE-mediated autoimmune response in MRL+/+ mice [27,28,31,35]. To further assess the possible contribution of iNOS gene in MDA and HNE formation, we also measured the liver MDA-/HNE-protein adducts as well as serum anti-MDA-/HNE-protein adduct antibodies in both MRL+/+ and iNOS-null MRL+/+ mice treated with TCE. TCE treatment led to significant increases in liver MDA- and anti-HNE-protein adducts and serum levels of anti-MDA- and anti-HNE-protein adduct antibodies in both MRL +/+ and iNOS-null MRL +/+ mice. Even though TCE exposure also led to increased formation of MDA- and HNE-protein adducts and their respective antibodies in iNOS-null MRL +/+ mice, their levels were apparently less pronounced compared to that in MRL+/+ mice. However, there was no statistically significant difference either in the levels of adducts or their antibodies in the two mouse types.
TCE exposure is known to induce/exacerbate autoimmune response both in humans and animals [27,28,30–32,35,51–53]. Our results in this study showed that TCE treatment led to significant increases in serum ANA and anti-dsDNA, the established markers of autoimmunity in both MRL +/+ and iNOS-null MRL +/+ mice. Remarkably, the increases in these autoantibodies induced by TCE in the iNOS-null mice were significantly less pronounced compared to that in MRL+/+ mice, suggesting iNOS is also a contributor of TCE-induced autoimmune response. To our knowledge, this is the first study to dissect the role of iNOS in TCE-mediated autoimmunity.
Taken together, our data clearly show that TCE treatment leads to significant increases in iNOS and NT formation, induction of MDA-/HNE-protein adducts and their respective antibodies along with significant increases in serum ANA and anti-dsDNA in MRL+/+ mice. Even though in iNOS-null MRL+/+ mice, the iNOS and NT levels were negligible in both TCE-treated and untreated groups, TCE treatment still led to significant increases in MDA-/HNE-protein adducts and their respective antibodies along with increases in serum ANA and anti-dsDNA compared to controls. Most remarkably, the increases in serum ANA and anti-dsDNA induced by TCE in the iNOS-null MRL+/+ mice were significantly less pronounced compared to that in MRL+/+ mice. Our results clearly demonstrated that iNOS contributes to TCE-induced autoimmunity in MRL+/+ mice.
Highlights.
TCE led to significantly increased RNS/LDRAs along with autoimmunity in MRL+/+ mice.
TCE-induced autoimmune response in iNOS-null MRL+/+ mice was less pronounced.
Both RNS and LDRAs contribute to TCE-induced autoimmunity in MRL +/+ mice.
iNOS deficiency attenuated the autoimmune response in this animal model.
Acknowledgements
This work was supported by Grant ES016302 from the National Institute of Environmental Health Sciences (NIEHS), NIH, and by P30 ES06676 (NIEHS center grant). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that no competing interests exist.
References
- 1.Al-Shobaili HA, Al Robaee AA, Alzolibani AA, Rasheed Z. Immunological studies of reactive oxygen species damaged catalase in patients with systemic lupus erythematosus: correlation with disease activity index. Immunol. Invest. 2013;42:191–203. doi: 10.3109/08820139.2012.751396. [DOI] [PubMed] [Google Scholar]
- 2.Oates JC. The biology of reactive intermediates in systemic lupus erythematosus. Autoimmunity. 2010;43:56–63. doi: 10.3109/08916930903374683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths HR. Is the generation of neo-antigenic determinants by free radicals central to the development of autoimmune rheumatoid disease? Autoimmun. Rev. 2008;7:544–549. doi: 10.1016/j.autrev.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Hill BG, Dranka BP, Bailey SM, Lancaster JR, Jr, Darley-Usmar VM. What part of NO don't you understand? Some answers to the cardinal questions in nitric oxide biology. J. Biol. Chem. 2010;285:19699–19704. doi: 10.1074/jbc.R110.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karpuzoglu E, Ahmed SA. Estrogen regulation of nitric oxide and inducible nitric oxide synthase (iNOS) in immune cells: implications for immunity, autoimmune diseases, and apoptosis. Nitric Oxide. 2006;15:177–186. doi: 10.1016/j.niox.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Phillips DC, Dias HK, Kitas GD, Griffiths HR. Aberrant reactive oxygen and nitrogen species generation in rheumatoid arthritis (RA): causes and consequences for immune function, cell survival, and therapeutic intervention. Antioxid. Redox Signal. 2010;12:743–785. doi: 10.1089/ars.2009.2607. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg JB, Granger DL, Pisetsky DS, Seldin MF, Misukonis MA, Mason SN, Pippen AM, Ruiz P, Wood ER, Gilkeson GS. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L-arginine. J. Exp. Med. 1994;179:651–660. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc. Natl. Acad. Sci. USA. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurien BT, Scofield RH. Autoimmunity and oxidatively modified autoantigens. Autoimmun. Rev. 2008;7:567–573. doi: 10.1016/j.autrev.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagy G, Clark JM, Buzás EI, Gorman CL, Cope AP. Nitric oxide, chronic inflammation and autoimmunity. Immunol. Lett. 2007;111:1–5. doi: 10.1016/j.imlet.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Ohmori H, Kanayama N. Immunogenicity of an inflammation-associated product, tyrosine nitrated self-proteins. Autoimmun. Rev. 2005;4:224–229. doi: 10.1016/j.autrev.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad R, Ahsan H. Role of peroxynitrite-modified biomolecules in the etiopathogenesis of systemic lupus erythematosus. Clin. Exp. Med. 2014;14:1–11. doi: 10.1007/s10238-012-0222-5. [DOI] [PubMed] [Google Scholar]
- 14.Morgan PE, Sturgess AD, Davies MJ. Evidence for chronically elevated serum protein oxidation in systemic lupus erythematosus patients. Free Radic. Res. 2009;43:117–127. doi: 10.1080/10715760802623896. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Wang J, Ma H, Khan MF. Increased nitration and carbonylation of proteins in MRL+/+ mice exposed to trichloroethene: potential role of protein oxidation in autoimmunity. Toxicol. Appl. Pharmacol. 2009;237:188–195. doi: 10.1016/j.taap.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang G, Wang J, Luo X, Ansari GA, Khan MF. Nitrosative stress and nitrated proteins in trichloroethene-mediated autoimmunity. PLoS One. 2014;9:e98660. doi: 10.1371/journal.pone.0098660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanchu A, Khullar M, Deodhar SD, Bambery P, Sud A. Nitric oxide synthesis is increased in patients with systemic lupus erythematosus. Rheumatol. Int. 1998;18:41–43. doi: 10.1007/s002960050055. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Pierangeli SS, Papalardo E, Ansari GA, Khan MF. Markers of oxidative and nitrosative stress in systemic lupus erythematosus: correlation with disease activity. Arthritis Rheum. 2010;62:2064–2072. doi: 10.1002/art.27442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oates JC, Christensen EF, Reilly CM, Self SE, Gilkeson GS. Prospective measure of serum 3-nitrotyrosine levels in systemic lupus erythematosus: correlation with disease activity. Proc. Assoc. Am. Physicians. 1999;111:611–621. doi: 10.1046/j.1525-1381.1999.99110.x. [DOI] [PubMed] [Google Scholar]
- 20.Januszewski AS, Alderson NL, Jenkins AJ, Thorpe SR, Baynes JW. Chemical modification of proteins during peroxidation of phospholipids. J. Lipid Res. 2005;46:1440–1449. doi: 10.1194/jlr.M400442-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Reed TT, Pierce WM, Markesbery WR, Butterfield DA. Proteomic identification of HNE-bound proteins in early Alzheimer disease: Insights into the role of lipid peroxidation in the progression of AD. Brain Res. 2009;1274:66–76. doi: 10.1016/j.brainres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Ben Mansour R, Lassoued S, Elgaied A, Haddouk S, Marzouk S, Bahloul Z, Masmoudi H, Attia H, Aïfa MS, Fakhfakh F. Enhanced reactivity to malondialdehyde-modified proteins by systemic lupus erythematosus autoantibodies. Scand. J. Rheumatol. 2010;39:247–253. doi: 10.3109/03009740903362511. [DOI] [PubMed] [Google Scholar]
- 23.Grune T, Michel P, Sitte N, Eggert W, Albrecht-Nebe H, Esterbauer H, Siems WG. Increased levels of 4-hydroxynonenal modified proteins in plasma of children with autoimmune diseases. Free Radic. Biol. Med. 1997;23:357–360. doi: 10.1016/s0891-5849(96)00586-2. [DOI] [PubMed] [Google Scholar]
- 24.Frostegard J, Svenungsson E, Wu R, Gunnarsson I, Lundberg IE, Klareskog L, Hörkkö S, Witztum JL. Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Rheum. 2005;52:192–200. doi: 10.1002/art.20780. [DOI] [PubMed] [Google Scholar]
- 25.D'souza A, Kurien BT, Rodgers R, Shenoi J, Kurono S, Matsumoto H, Hensley K, Nath SK, Scofield RH. Detection of catalase as a major protein target of the lipid peroxidation product 4-HNE and the lack of its genetic association as a risk factor in SLE. BMC Med. Genet. 2008;9:62–69. doi: 10.1186/1471-2350-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurien BT, Scofield RH. Free radical mediated peroxidative damage in systemic lupus erythematosus. Life Sci. 2003;73:1655–1666. doi: 10.1016/s0024-3205(03)00475-2. [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Cai P, Ansari GA, Khan MF. Oxidative and nitrosative stress in trichloroethene-mediated autoimmune response. Toxicology. 2007;229:186–193. doi: 10.1016/j.tox.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang G, König R, Ansari GAS, Khan MF. Lipid peroxidation-derived aldehyde-protein adducts contribute to trichloroethene-mediated autoimmunity via activation of CD4+ T cells. Free Radic. Biol. Med. 2008;44:1475–1482. doi: 10.1016/j.freeradbiomed.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin JM, Blossom SJ, Jackson SK, Gilbert KM, Pumford NR. Trichloroethylene accelerates an autoimmune response by Th1 T cell activation in MRL +/+ mice. Immunopharmacology. 2000;46:123–137. doi: 10.1016/s0162-3109(99)00164-2. [DOI] [PubMed] [Google Scholar]
- 30.Khan MF, Kaphalia BS, Prabhakar BS, Kanz MF, Ansari GA. Trichloroethene-induced autoimmune response in female MRL +/+ mice. Toxicol. Appl. Pharmacol. 1995;134:155–160. doi: 10.1006/taap.1995.1179. [DOI] [PubMed] [Google Scholar]
- 31.Khan MF, Wu X, Ansari GA. Anti-malondialdehyde antibodies in MRL+/+ mice treated with trichloroethene and dichloroacetyl chloride: possible role of lipid peroxidation in autoimmunity. Toxicol. Appl. Pharmacol. 2001;170:88–92. doi: 10.1006/taap.2000.9086. [DOI] [PubMed] [Google Scholar]
- 32.Kilburn KH, Warshaw RH. Prevalence of symptoms of systemic lupus erythematosus (SLE) and of fluorescent antinuclear antibodies associated with chronic exposure to trichloroethylene and other chemicals in well water. Environ. Res. 1992;57:1–9. doi: 10.1016/s0013-9351(05)80014-3. [DOI] [PubMed] [Google Scholar]
- 33.Channel SR, Latendresse JR, Kidney JK, Grabau JH, Lane JW, Steel-Goodwin L, Gothaus MC. A subchronic exposure to trichloroethylene causes lipid peroxidation and hepatocellular proliferation in male B6C3F1 mouse liver. Toxicol. Sci. 1998;43:145–154. doi: 10.1006/toxs.1998.2456. [DOI] [PubMed] [Google Scholar]
- 34.Zhu QX, Shen T, Ding R, Liang ZZ, Zhang XJ. Cytotoxicity of trichloroethylene and perchloroethylene on normal human epidermal keratinocytes and protective role of vitamin E. Toxicology. 2005;209:55–67. doi: 10.1016/j.tox.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Wang G, Wang J, Fan X, Ansari GA, Khan MF. Protein adducts of malondialdehyde and 4-hydroxynonenal contribute to trichloroethene-mediated autoimmunity via activating Th17 cells: dose- and time-response studies in female MRL+/+ mice. Toxicology. 2012;292:113–122. doi: 10.1016/j.tox.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang G, Li H, Khan FM. Differential oxidative modification of proteins in MRL+/+ and MRL/lpr mice: Increased formation of lipid peroxidation-derived aldehyde-protein adducts may contribute to accelerated onset of autoimmune response. Free Radic. Res. 2012;46:1472–1481. doi: 10.3109/10715762.2012.727209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anam K, Amare M, Naik S, Szabo KA, Davis TA. Severe tissue trauma triggers the autoimmune state systemic lupus erythematosus in the MRL/++ lupus-prone mouse. Lupus. 2009;18:318–331. doi: 10.1177/0961203308097479. [DOI] [PubMed] [Google Scholar]
- 38.Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15:308–318. doi: 10.1191/0961203306lu2305xx. [DOI] [PubMed] [Google Scholar]
- 39.Wang G, Wang J, Ma H, Ansari GA, Khan MF. N-Acetylcysteine protects against trichloroethene-mediated autoimmunity by attenuating oxidative stress. Toxicol. Appl. Pharmacol. 2013;273:189–195. doi: 10.1016/j.taap.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahsan H. 3-Nitrotyrosine: A biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum. Immunol. 2013;74:1392–1399. doi: 10.1016/j.humimm.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Reveille JD. Predictive value of autoantibodies for activity of systemic lupus erythematosus. Lupus. 2004;13:290–297. doi: 10.1191/0961203303lu1015oa. [DOI] [PubMed] [Google Scholar]
- 42.Egner W. The use of laboratory tests in the diagnosis of SLE. J. Clin. Pathol. 2000;53:424–432. doi: 10.1136/jcp.53.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasanthi P, Nalini G, Rajasekhar G. Status of oxidative stress in rheumatoid arthritis. Int. J. Rheum. Dis. 2009;12:29–33. doi: 10.1111/j.1756-185X.2009.01375.x. [DOI] [PubMed] [Google Scholar]
- 44.Iuchi Y, Kibe N, Tsunoda S, Suzuki S, Mikami T, Okada F, Uchida K, Fujii J. Implication of oxidative stress as a cause of autoimmune hemolytic anemia in NZB mice. Free Radic. Biol. Med. 2010;48:935–944. doi: 10.1016/j.freeradbiomed.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Shah D, Kiran R, Wanchu A, Bhatnagar A. Oxidative stress in systemic lupus erythematosus: relationship to Th1 cytokine and disease activity. Immunol. Lett. 2010;129:7–12. doi: 10.1016/j.imlet.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Tam LS, Li EK, Leung VY, Griffith JF, Benzie IF, Lim PL, Whitney B, Lee VW, Lee KK, Thomas GN, Tomlinson B. Effects of vitamins C and E on oxidative stress markers and endothelial function in patients with systemic lupus erythematosus: a double blind, placebo controlled pilot study. J. Rheumatol. 2005;32:275–282. [PubMed] [Google Scholar]
- 47.Nagy G, Koncz A, Telarico T, Fernandez D, Ersek B, Buzás E, Perl A. Central role of nitric oxide in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res. Ther. 2010;12:210–2015. doi: 10.1186/ar3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadiiska MB, Bonini MG, Ruggiero C, Cleland E, Wicks S, Stadler K. Thiazolidinedione treatment decreases oxidative stress in spontaneously hypertensive heart failure rats through attenuation of inducible nitric oxide synthase-mediated lipid radical formation. Diabetes. 2012;61:586–596. doi: 10.2337/db11-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bentz M, Zaouter C, Shi Q, Fahmi H, Moldovan F, Fernandes JC, Benderdour M. Inhibition of inducible nitric oxide synthase prevents lipid peroxidation in osteoarthritic chondrocytes. J. Cell. Biochem. 2012;113:2256–2267. doi: 10.1002/jcb.24096. [DOI] [PubMed] [Google Scholar]
- 50.Hogg N, Kalyanaraman B. Nitric oxide and lipid peroxidation. Biochim. Biophys. Acta. 1999;1411:378–384. doi: 10.1016/s0005-2728(99)00027-4. [DOI] [PubMed] [Google Scholar]
- 51.Cai P, König R, Boor PJ, Kondraganti S, Kaphalia BS, Khan MF, Ansari GA. Chronic exposure to trichloroethene causes early onset of SLE-like disease in female MRL +/+ mice. Toxicol. Appl. Pharmacol. 2008;228:68–75. doi: 10.1016/j.taap.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooper GS, Makris SL, Nietert PJ, Jinot J. Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ. Health Perspect. 2009;117:696–702. doi: 10.1289/ehp.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flindt-Hansen H, Isager H. Scleroderma after occupational exposure to trichlorethylene and trichlorethane. Acta Derm. Venereol. 1987;67:263–264. [PubMed] [Google Scholar]