Abstract
Background
Postoperative delirium is a common complication associated increased morbidity and mortality, longer hospital stays, and higher healthcare expenditures. Intraoperative electroencephalogram (EEG) slowing has previously been associated with postoperative delirium, but the relationship between intraoperative EEG suppression and postoperative delirium has not been investigated.
Methods
In this observational cohort study, 727 adult patients receiving general anesthesia with planned intensive care unit (ICU) admission were included. Duration of intraoperative EEG suppression was recorded from a frontal EEG channel (FP1 to F7). Delirium was assessed twice daily on postoperative days 1 through 5 using the Confusion Assessment Method for the ICU. Thirty days after surgery, quality of life, functional independence, and cognitive ability were measured using the VR-12 survey, the Barthel Index, and the PROMIS Applied Cognition-Abilities-Short Form 4a survey.
Results
Postoperative delirium was observed in 162 (26%) of 619 patients assessed. When comparing patients with no EEG suppression to those divided into quartiles based on duration of EEG suppression, patients with more suppression were more likely to experience delirium (χ2(4) = 25, p < 0.0001). This effect remained significant after adjusting for potential confounders (odds ratio for log(EEG suppression) 1.22 [99% CI 1.06 to 1.40, p = 0.0002] per 1-minute increase in suppression). EEG suppression may have been associated with reduced functional independence (Spearman partial correlation coefficient −0.15, p = 0.02), but not with changes in quality of life or cognitive ability. Predictors of EEG suppression included higher end-tidal volatile anesthetic concentration and lower intraoperative opioid dose.
Conclusions
EEG suppression is an independent risk factor for postoperative delirium. Future studies should investigate whether anesthesia titration to minimize EEG suppression decreases the incidence of postoperative delirium.
This is a substudy of the SATISFY-SOS surgical outcomes registry (NCT02032030).
INTRODUCTION
Delirium is an acute cognitive disorder characterized by inattention, disorganized thinking, and a fluctuating course that develops over hours to days. Delirium is a common complication after surgery, with an incidence ranging from 10% to 70%, depending on the type of procedure.1 Patients who experience postoperative delirium require longer stays in the intensive care unit (ICU), more days of mechanical ventilation, and increased hospital length of stay,2 leading to a 31% increase in hospital costs during the index admission.3 Even after hospital discharge, patients who experience postoperative delirium are at increased risk for institutionalization, death, and dementia.4 These patients have an additional $60,000 in total health care costs over the first year after discharge5 and also report decreased quality of life.6 Preventing cases of postoperative delirium would be expected to shorten the postoperative hospital stay, reduce the risk of complications after discharge, and reduce health care costs for the patient and for society.
Certain features of the intraoperative electroencephalogram (EEG) have previously been associated with poor perioperative outcomes, such as postoperative delirium. During general anesthesia with ether-derived volatile agents, the EEG often shows a dominance of delta waves (0–4 Hz) coupled with theta waves (4–8 Hz) and/or alpha (8–12 Hz) and low-beta (12–16 Hz) oscillations.7 Patients with increased low-frequency EEG activity during the rewarming phase of cardiac surgery are at increased risk for postoperative complications, including delirium.8 In response to higher effect-site concentrations of anesthetics, patients develop EEG burst suppression, characterized by periods of suppression alternating with short bursts of high amplitude activity.9 EEG suppression does not occur during physiological sleep, and it typically reflects pathology, unless deep general anesthesia or severe hypothermia are present.10–11 EEG suppression in other settings has been associated with poor outcomes, including six-month mortality in mechanically ventilated ICU patients,12 worse neurologic outcome following therapeutic hypothermia for ventricular fibrillation,13 and increased incidence of post-coma delirium in ICU patients.14 Prior studies have found a reduced incidence of delirium when anesthesia clinicians use a processed EEG monitor15–16 or when they target a higher value of the processed EEG index,17–18 but the single study19 that directly identified a relationship between intraoperative EEG suppression and postoperative delirium was relatively small, and did not adjust for potential confounding variables.
The primary aim of this study was to determine whether the duration of intraoperative EEG suppression is independently associated with postoperative delirium. Secondary aims were to determine whether duration of EEG suppression is associated with decreased quality of life, functional independence, or cognitive ability following surgery, and to identify risk factors that predict the incidence and duration of intraoperative EEG suppression.
METHODS
The Human Research Protection Office (HRPO) at Washington University approved this study. All patients provided written, informed consent for participation in the Systematic Assessment and Targeted Improvement of Services Following Yearlong Surgical Outcomes Surveys (SATISFY-SOS), which is an observational registry for which detailed data on surgical patients are obtained, and their postoperative health and well being are tracked (NCT02032030). A waiver of consent was obtained from HRPO for this sub-study of SATISFY-SOS.
Patient Population
We conducted an observational cohort study. Patients enrolled in SATISFY-SOS were eligible if they were age 18 or older, received general anesthesia for surgery with planned ICU admission at Barnes-Jewish Hospital (St. Louis, MO) between November 2012 and November 2013, and received intraoperative EEG monitoring. Patients were excluded if they underwent neurologic surgery.
Data Collection
Patients underwent anesthesia with IV induction (typically propofol) followed by maintenance with a volatile anesthetic (sevoflurane, isoflurane, desflurane, or a combination of these agents, with or without nitrous oxide). EEG suppression was obtained from a BIS Quatro® sensor (Covidien, Dublin, Ireland), which computed the suppression ratio from a single frontal EEG channel (Fp1 – F7) continuously throughout surgery. The suppression ratio describes the fraction of the preceding 63 seconds for which the EEG was electrically suppressed. Suppression ratios were captured once per minute using MetaVision® software (iMDSoft, Needham, MA). For cases where automatic data capture was not possible (141 of 727 cases), suppression ratios were captured with a five-minute sampling interval by taking photographs of the operating room monitor. Suppression ratios for the four intervening minutes were obtained by linear interpolation. Data points with a signal quality index less than 50% were excluded, and patients with valid suppression ratio values for less than half of the length of surgery were excluded. The total duration of EEG suppression, in minutes, was calculated by summing the suppression ratio values over the course of the surgery.
As part of routine care, ICU nurses with structured training in delirium assessment evaluated patients for delirium using the Confusion Assessment Method for the ICU (CAM-ICU).20 Patients were assessed twice daily, unless they had been discharged from the ICU or they were sedated to a Richmond Agitation and Sedation Scale (RASS) score lower than −3. Patients were not assessed for delirium with the CAM-ICU on hospital wards. Postoperative delirium was defined as one or more positive CAM-ICU results between postoperative days 1 and 5. Cases of delirium were classified as hypoactive (RASS ≤ 0 at all positive CAM-ICU time points), hyperactive (RASS > 0 at all positive CAM-ICU time points), or mixed (RASS ≤ 0 at some time points and > 0 at other time points). Quality of life was measured using the Veterans RAND 12-item (VR-12) survey.21–22 Functional independence was measured using the Barthel Index,23 and cognitive ability was measured using the PROMIS v1.0-Applied Cognition-Abilities-Short Form 4a (available at www.nihpromis.org). As part of SATISFY-SOS, patients completed a survey 30 days after surgery that included these three patient-reported outcome measures. The VR-12 yields summary measures for both physical and mental health, each normalized such that the mean score in the United States population is 50 (standard deviation [SD] 10).22 The Barthel Index yields a score between 0 and 100. The PROMIS-Applied Cognition-Abilities tool produces T-scores with a mean of 50 (SD 10) (www.nihpromis.org). For all three tools, higher scores indicate better health or performance.
Additional predictor variables were extracted from the electronic medical record, including patient characteristics, comorbid conditions, laboratory values, surgical procedure, and perioperative medications. Comorbidities were summarized using the age-adjusted Charlson index.24 Surgical procedure was categorized as non-cardiac surgery, coronary artery bypass graft, or open cardiac surgery. Opioid medication dosages were converted to morphine equivalents using conversion factors derived from the Alberta Hospice Palliative Care Resource Manual.25 Volatile anesthetic concentrations were converted to units of age-adjusted minimum alveolar concentration (aaMAC).26
Statistical Analysis
Statistical analyses were performed using SAS v9.3 (SAS Institute, Inc., Cary, NC) unless otherwise noted. Patients who did not receive any CAM-ICU assessments were excluded from analyses of postoperative delirium. The population of patients who experienced EEG suppression was divided into quartiles based on duration of suppression. The incidence of postoperative delirium was compared across these groups using a chi-square test. For comparison, this analysis was repeated stratifying patients based on duration of bispectral index < 20. This threshold has been used in a previous study conducted by Radtke and colleagues.15 The predictive abilities of duration of EEG suppression and duration of bispectral index < 20 were compared by using the ROCCONTRAST statement within the SAS Logistic Procedure to compare the areas under the receiver operating characteristic curves.
We used logistic regression to examine the relationship between EEG suppression and postoperative delirium, adjusting for age, sex, American Society of Anesthesiologists (ASA) class, age-adjusted Charlson index, sensory impairment, alcohol use > 5 drinks per week, surgery type, surgery length, intraoperative opioid dose, intraoperative ketamine use, intraoperative packed red blood cell transfusion, and mean end-tidal anesthetic concentration. All predictor variables were entered into the regression in a single step because filtering variables based on unadjusted p values consumes degrees of freedom and can lead to an overfitted model.27 Blood transfusion was entered as both a categorical and continuous variable to account for zero-inflated values. We used generalized additive model analysis in the R statistical package to test the assumption that predictor variables were linearly associated with the logit, and we transformed variables as necessary.
We also tested for interactions between duration of EEG suppression and mean end-tidal anesthetic concentration, age, and opioid dose. Missing values for intraoperative opioid dose and mean end-tidal anesthetic concentration (fraction missing 0.6% and 3.7% respectively) were imputed using multiple (five) imputations. As a sensitivity analysis, we repeated this analysis excluding patients with a history of neuropsychiatric diseases. We also repeated this analysis excluding patients for whom automatic capture of the suppression ratio was not possible (i.e., patients for whom we interpolated some suppression ratio values). To explore whether the results differed between cardiac surgery and non-cardiac surgery patients, we repeated this analysis in each of these two subgroups.
To identify associations between duration of EEG suppression and post-discharge outcomes, we used the Spearman partial correlation coefficient. Each correlation coefficient was controlled for age, sex, age-adjusted Charlson comorbidity index, surgery type, surgery length, and postoperative delirium. In addition, the relationship between postoperative delirium and each of these outcomes was tested using a Mann-Whitney U-test.
To identify risk factors for EEG suppression, we used a two-part nonlinear mixed effects model predicting the suppression ratio at each point in time. Such an approach is appropriate when the outcome variable has a value of 0 at many time points.28 The first part of this model used a logistic likelihood function to predict the odds that the suppression ratio would take a nonzero value at a particular point in time. If the suppression ratio had a non-zero value, then the second part of the model used a generalized gamma regression to predict the value of the suppression ratio. Both parts of the model used a random intercept and adjusted for age, sex, ASA class, coronary artery disease, chronic obstructive pulmonary disease, malignancy other than skin cancer, home sedative, opioid, or alcohol use, surgical procedure, preoperative midazolam > 2 mg, intraoperative nitrous oxide use, intraoperative opioid dose, and end-tidal anesthetic concentration.
The target sample size for the chi-square test with patients stratified into quartiles based on duration of EEG suppression was 540 patients. The incidence of postoperative delirium is typically at least 25% among patients undergoing cardiac surgery.1 Assuming this overall incidence and assuming that incidence would increase linearly across the quartiles, a sample of 540 subjects would be needed to detect, with 80% power at the 0.05 level of significance, a 20% difference in the incidence of delirium between patients in the two extreme groups.
RESULTS
Postoperative Delirium
The cohort included 727 patients, predominantly older men undergoing cardiac surgery (Figure 1, Table 1). The median duration of EEG suppression was 4.5 (interquartile range [IQR] 0.7–17.4) minutes, and the median duration of bispectral index < 20 was 11 (IQR 5–31) minutes. Among the 619 patients assessed, 162 (26%, 95% CI 22–30%) experienced postoperative delirium. Of these, 119 patients (73%) exhibited hypoactive delirium (RASS ≤ 0 at all positive CAM-ICU time points). The remaining 43 patients developed mixed delirium; no patients experienced pure hyperactive delirium. Eighty-six patients (49%) had a single positive CAM-ICU assessment, 57 patients (33%) had two or three positive assessments, and 32 patients (18%) had four or more positive assessments. Patients who were missing delirium assessments were more likely to have higher ASA class (U = 35871, p = 0.04), undergo non-cardiac surgery (χ2(2) = 15, p = 0.02), and receive higher doses of intraoperative opioid medications (U = 44709, p = 0.007).
Figure 1.
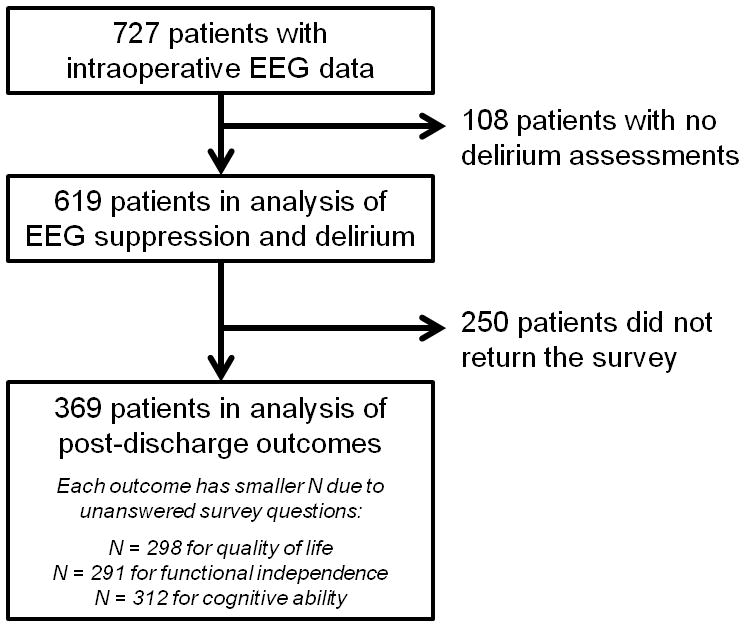
Number of patients included in the analysis. EEG = Electroencephalogram.
Table 1.
Characteristics of the Cohorta
| Variable | Full Cohort (N = 727) | Included in Analysis (N = 619) | Not Included in Analysis (N = 108) |
|---|---|---|---|
| Age (years) | 62 ± 14 | 62 ± 14 | 62 ± 14 |
| Male sex | 458 (63%) | 396 (64%) | 62 (57%) |
| American Society of Anesthesiologists class | |||
| 2 | 25 (3%) | 14 (2%) | 11 (10%) |
| 3 | 261 (36%) | 222 (36%) | 39 (36%) |
| 4 | 434 (60%) | 378 (61%) | 56 (52%) |
| 5 | 7 (1%) | 5 (1%) | 2 (2%) |
| Age-adjusted Charlson index | 3 [2, 5] | 3 [2, 5] | 4 [2, 5] |
| Dementia | 4 (0.6%) | 4 (0.7%) | 0 (0%) |
| Coronary artery disease | 350 (48%) | 299 (48%) | 51 (47%) |
| Chronic obstructive pulmonary disease | 125 (17%) | 102 (16%) | 23 (21%) |
| Malignancy, excluding skin cancer | 120 (17%) | 87 (14%) | 33 (31%) |
| Sensory impairment (hearing or vision) | 142 (23%) | 117 (22%) | 25 (27%) |
| Home sedative use | 123 (17%) | 107 (17%) | 16 (15%) |
| Home opioid use | 160 (22%) | 128 (21%) | 32 (30%) |
| Alcohol use > 5 drinks per week | 65 (9%) | 57 (9%) | 8 (8%) |
| Surgery Type | |||
| Open cardiac | 479 (66%) | 410 (66%) | 69 (64%) |
| Coronary artery bypass grafting | 144 (20%) | 132 (21%) | 12 (11%) |
| Non-cardiac | 104 (14%) | 77 (13%) | 27 (25%) |
| Midazolam dose > 2mg | 121 (17%) | 100 (16%) | 21 (19%) |
| Nitrous oxide use | 63 (9%) | 52 (8%) | 11 (10%) |
| Intraoperative ketamine use | 221 (30%) | 190 (31%) | 31 (29%) |
| Intraoperative opioid dose (morphine equivalents per kg) | 1.1 [0.8, 1.6] | 1.1 [0.8, 1.5] | 1.3 [0.9, 1.8] |
| Intraoperative blood transfusion (units) | 1 [0, 3] | 1 [0, 3] | 1 [0, 4] |
| Mean end-tidal anesthetic concentration (age-adjusted MAC units)b | 0.91 ± 0.11 | 0.91 ± 0.11 | 0.90 ± 0.13 |
Values are mean ± standard deviation, number (%), or median [lower quartile, upper quartile].
MAC = Minimum alveolar concentration
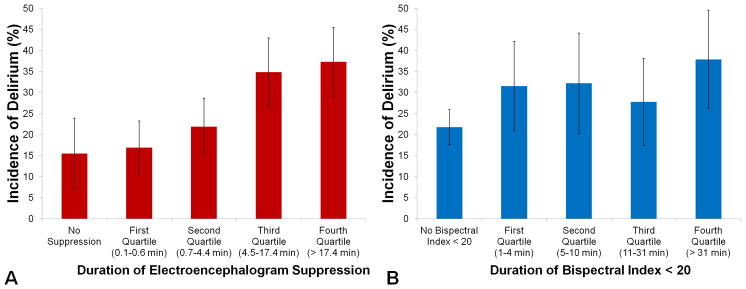
When comparing patients who had no EEG suppression and patients divided into quartiles based on duration of EEG suppression, patients who experienced more suppression were more likely to experience postoperative delirium (χ2(4) = 25, p < 0.0001, Figure 2a). By contrast, duration of bispectral index < 20 was also associated with incidence of postoperative delirium (χ2(4) = 10.8, p = 0.03), but this relationship was not monotonic (Figure 2b). Duration of EEG suppression predicted postoperative delirium with a moderate c-statistic of 0.62 (95% CI 0.57–0.67; Akaike information criterion 706.1), and duration of bispectral index < 20 predicted postoperative delirium with a moderate c-statistic of 0.57 (95% CI 0.52–0.62; Akaike information criterion 708.9). The c-statistic for duration of EEG suppression was significantly greater than the c-statistic for duration of bispectral index < 20 (χ2(1) = 8.1, p = 0.004).
Figure 2.
This descriptive figure depicts the univariable relationships between these two electroencephalogram parameters and incident delirium. There is no control for covariates in this descriptive figure. A. Incidence of delirium among patients who experienced no electroencephalogram suppression (n = 71) and among patients who experienced electroencephalogram suppression (n = 548) divided into quartiles based on duration of electroencephalogram suppression. B. Incidence of delirium among patients who never experienced bispectral index < 20 (n = 362) and among patients who experienced bispectral index < 20 (n = 257) divided into quartiles based on duration of bispectral index < 20. Error bars represent 95% confidence intervals around the incidence of delirium in each group.
After adjusting for potential confounders, duration of EEG suppression remained a significant predictor of postoperative delirium (Table 2). Based on the results of the generalized additive model analysis, duration of EEG suppression and number of blood transfusion units were log-transformed to achieve linearity with the logit. The other variables required no transformation. Increased duration of EEG suppression was associated with increased odds of postoperative delirium (odds ratio for log of minutes of suppression 1.22, 99% CI 1.06–1.40, p = 0.0002).
Table 2.
Predictors of Postoperative Delirium in a Multiple Logistic Regression (N = 619)
| Variable | Non-Transformed Model | Transformed Modela | ||
|---|---|---|---|---|
|
| ||||
| Odds Ratio (99% CI) | p | Odds Ratio (99% CI) | p | |
| Age (per year) | 1.01 (0.98, 1.03) | 0.37 | 1.00 (0.98, 1.03) | 0.69 |
| Male sex | 0.92 (0.69, 1.23) | 0.46 | 0.89 (0.67, 1.19) | 0.31 |
| American Society of Anesthesiologists class > 3 | 0.81 (0.60, 1.11) | 0.08 | 0.80 (0.58, 1.08) | 0.06 |
| Age-adjusted Charlson index (per unit) | 1.10 (0.93, 1.30) | 0.15 | 1.09 (0.92, 1.30) | 0.18 |
| Sensory impairment | 1.04 (0.63, 1.70) | 0.83 | 1.03 (0.62, 1.74) | 0.85 |
| Alcohol use > 5 drinks per week | 1.02 (0.62, 1.66) | 0.93 | 1.02 (0.62, 1.68) | 0.91 |
| Surgery Type | ||||
| Non-cardiac | reference | reference | ||
| Coronary artery bypass grafting | 1.12 (0.62, 1.66) | 0.57 | 1.26 (0.76, 2.11) | 0.24 |
| Open cardiac | 0.95 (0.60, 1.51) | 0.77 | 1.03 (0.65, 1.62) | 0.89 |
| Length of surgery (per minute) | 1.00 (1.00, 1.00) | 0.65 | 1.00 (1.00, 1.00) | 0.61 |
| Intraoperative ketamine use | 0.70 (0.38, 1.29) | 0.13 | 0.71 (0.39, 1.30) | 0.15 |
| Intraoperative opioid dose (per 1 morphine equivalent/kg increase) | 1.08 (0.71, 1.64) | 0.65 | 1.05 (0.69, 1.61) | 0.76 |
| Blood transfusion (dichotomous)a | -- | -- | 1.82 (0.83, 4.00) | 0.05 |
| Blood transfusion (per unit)a | 1.29 (1.14, 1.46) | <0.0001 | 1.77 (1.07, 2.94)a | 0.004 |
| Mean end-tidal anesthetic concentration (per 0.1 MAC unit)b | 0.66 (0.50, 0.87) | 0.0001 | 0.66 (0.50, 0.88) | 0.0002 |
| Duration of electroencephalogram suppression (in minutes)a | 1.05 (1.003, 1.103)c | 0.0065 | 1.22 (1.06, 1.40) | 0.0002 |
Natural logarithm transformation was used to obtain linearity with the logit for blood transfusion and duration of electroencephalogram suppression. In addition, a dichotomous variable for blood transfusion was added due to the large number of patients who received no blood transfusion.
MAC = Minimum alveolar concentration
In the untransformed model, odds ratio is for a 5-minute increase in duration of electroencephalogram suppression.
The interactions between EEG suppression and mean end-tidal anesthetic concentration, age, and opioid dose were not statistically significant (respective odds ratios = 0.99, p = 0.58; 1.00, p = 0.46; and 0.97, p = 0.27), and were therefore dropped from the final model. This multivariable logistic regression model had good discrimination (c-statistic of 0.77) and good calibration (Hosmer-Lemeshow test χ2(8) = 11.1, p = 0.19). Compared to patients with complete predictor variable data, patients with imputed values did not have different duration of EEG suppression, incidence of postoperative delirium, or values for other predictor variables, suggesting that these data were missing at random.
In a sensitivity analysis excluding patients with depression (n = 67), bipolar disorder (n = 9), and preexisting dementia (n = 4), the results were qualitatively unchanged. The logarithm of EEG suppression was associated with increased odds of postoperative delirium (adjusted odds ratio 1.29, 99% CI 1.10–1.50, p < 0.0001). In a sensitivity analysis excluding patients (n = 141) for whom some suppression ratio values were interpolated, the results were qualitatively unchanged. The logarithm of EEG suppression was associated with increased odds of postoperative delirium (adjusted odds ratio 1.24, 99% CI 1.06–1.44, p = 0.0004). In subgroup analyses, longer duration of EEG suppression was associated with increased odds of postoperative delirium in cardiac surgery patients (n = 542, odds ratio 1.19, 99% CI 1.03–1.38, p = 0.002) and may also have been associated in non-cardiac surgery patients (n = 77, odds ratio 1.70, 99% CI 0.96–3.03, p = 0.02). The subgroup results are qualitatively the same as the results of the primary analysis.
Post-Discharge Outcomes
The mean VR-12 physical health summary measure was 36 (SD 10), the median mental health summary measure was 54 (IQR 44–60), the median Barthel Index was 100 (IQR 95–100), and the median PROMIS-Applied Cognition-Abilities T-score was 48 (IQR 42–58). Survey response rates were similar among patients who experienced EEG suppression (45%) and among those who did not (49%). However, patients who experienced postoperative delirium were less likely to return the survey than those who did not (response rates 40% versus 55%).
Duration of EEG suppression was not correlated with VR-12 physical health summary measure (Spearman partial ρ = −0.05, p = 0.47), VR-12 mental health summary measure (Spearman partial ρ = −0.04, p = 0.56), or PROMIS-Applied Cognition score (Spearman partial ρ = −0.04, p = 0.47). After controlling for potential confounders, longer duration of EEG suppression may have been correlated with lower Barthel Index score (Spearman partial ρ = −0.15, p = 0.02).
Patients who experienced postoperative delirium had lower Barthel Index values (median 95, IQR 85–100) than those who did not (median 100, IQR 95–100): Mann-Whitney U = 4117, p = 0.0004. The VR-12 physical health summary measure, VR-12 mental health summary measure, and PROMIS-Applied Cognition score did not differ between patients with and without postoperative delirium.
Predictors of EEG Suppression
In the two-part nonlinear mixed effects model (Table 3), patients who received less intraoperative opioid medication were more likely to experience EEG suppression at any particular time (odds ratio 0.5 per morphine equivalent/kilogram increase, 95% CI 0.4–0.6). Patients with higher end-tidal anesthetic concentration were more likely to experience EEG suppression (odds ratio 1.5, 95% CI 1.5–1.6) and were also more likely to experience greater amounts of EEG suppression (gamma regression location coefficient 0.45, 95% CI 0.41–0.47).
Table 3.
Predictors of Suppression Ratio (N = 672)a
| Variable | Odds of Non-Zero Suppression Ratio | Value of Suppression Ratio (Gamma Regression) | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p | Location Coefficient (95% CI) | p | |
| Age (per year) | 1.0 (1.0,1.0) | 0.05 | −0.01 (−0.02,0.01) | 0.23 |
| Male sex | 0.8 (0.6,1.0) | 0.08 | −0.04 (−0.33,0.24) | 0.77 |
| American Society of Anesthesiologists class > 3 | 1.2 (0.8,1.6) | 0.39 | −0.01 (−0.29,0.28) | 0.96 |
| Coronary artery disease | 1.2 (0.9,1.6) | 0.32 | −0.24 (−0.53,0.05) | 0.11 |
| Chronic obstructive pulmonary disease | 1.1 (0.8,1.7) | 0.52 | 0.05 (−0.31,0.41) | 0.77 |
| Malignancy, excluding skin cancer | 0.9 (0.6,1.4) | 0.71 | 0.03 (−0.36,0.43) | 0.88 |
| Home sedative, opioid, or alcohol use | 1.0 (0.7,1.3) | 0.80 | 0.01 (−0.27,0.28) | 0.97 |
| Midazolam dose > 2 mg | 1.1 (0.8,1.6) | 0.58 | 0.09 (−0.26,0.44) | 0.61 |
| Intraoperative opioid dose (per 1 morphine equivalent/kg increase) | 0.5 (0.4,0.6) | <0.0001 | 0.16 (−0.03,0.36) | 0.11 |
| Nitrous oxide use | 1.0 (0.6,1.6) | 0.89 | 0.01 (−0.47,0.47) | 0.99 |
| Cardiac surgery | 0.8 (0.5,1.2) | 0.28 | 0.21 (−0.22,0.64) | 0.33 |
| End-tidal anesthetic concentration (per 0.5 MAC unit)b | 1.5 (1.5,1.6) | <0.0001 | 0.45 (0.41,0.47) | <0.0001 |
Suppression ratio (SR) was predicted using a two-part nonlinear mixed effects model. The first part used a logistic likelihood function to predict the odds of a non-zero SR. The second part used a generalized gamma regression to predict the value of the SR.
MAC = Minimum alveolar concentration
DISCUSSION
This study demonstrated that longer duration of intraoperative EEG suppression was associated with an increased incidence of postoperative delirium. Patients who experienced intraoperative EEG suppression also had lower functional independence scores 30 days after surgery compared to patients who experienced no EEG suppression. Furthermore, higher concentrations of volatile anesthetic and lower doses of opioid medications were risk factors for EEG suppression.
Our results are consistent with a recent study by Soehle and colleagues, in which patients who experienced postoperative delirium spent more time in burst suppression during surgery than patients who did not.19 Our results are also consistent with a recent study by Radtke and colleagues, in which patients who spent a greater fraction of surgery with a bispectral index value less than 20 had increased odds of postoperative delirium.15 A similar study from our institution failed to replicate Radtke’s finding.16 The bispectral index monitor uses real-time EEG data to produce a numeric index, with lower values intended to indicate deeper anesthesia. Although the bispectral index algorithm is proprietary, suppression ratio is known to be one of the components of the algorithm.29 In our study, duration of EEG suppression predicted postoperative delirium with a c-statistic of 0.62, while duration of bispectral index < 20 predicted delirium with a c-statistic of 0.57. These results suggest that EEG suppression predicts postoperative delirium just as well as bispectral index values < 20, if not slightly better. This finding is important because EEG suppression, unlike the bispectral index, is a nonproprietary measure that can be freely and inexpensively incorporated into any brain monitoring device.
Other studies that have investigated the association between the bispectral index and postoperative delirium have not examined the effect of extremely low bispectral index values (< 20). In patients receiving spinal anesthesia for hip fracture repair, Sieber and colleagues found that the incidence of postoperative delirium was lower among patients randomized to receive light sedation (target bispectral index 80) than among those who received deep sedation (target bispectral index 50).17 In the CODA trial, bispectral-index-guided anesthesia was associated with reduced postoperative delirium compared to routine care.18 The average bispectral index values in this trial were 53 in the bispectral index-guided group and 39 in the routine care group.18 Patients in the ongoing Balanced Anesthesia Study (ACTRN12612000632897) are randomized to a target bispectral index of either 50 or 35. In a pilot study, the actual average bispectral index values were 48 (95% CI 46–49) and 39 (95% CI 38–41) in the two groups.30 Because the bispectral index only becomes linearly associated with suppression ratio when the bispectral index is in the mid-20s or lower,29 the primary analyses from these published and ongoing studies do not provide the same information as the present study examining burst suppression.
EEG burst suppression is likely caused by increased cortical excitability, with extracellular calcium depletion and activity of the adenosine triophosphate-gated potassium channel contributing to the suppression.31–32 There are several ways to interpret the association between EEG suppression and postoperative delirium. One explanation is that EEG suppression indicates excessive depth of anesthesia, with excess exposure to potent volatile agents, leading to an increased incidence of postoperative delirium. The observation that higher concentrations of volatile anesthetic were associated with higher suppression ratios supports this hypothesis. Another interpretation is that EEG suppression occurs more often in patients with preoperative subclinical neural pathology. Many cognitive disorders are associated with pathologic findings, such as amyloid plaques in Alzheimer’s disease, that precede the onset of clinically-apparent cognitive decline by years,33 and these cognitive disorders are known risk factors for delirium.34 Exposure to anesthesia may serve as a sort of neural “stress test,” driving patients with subclinical brain pathology to develop acute confusion.
Our research group is currently conducting the ENGAGES clinical trial (NCT02241655), which may shed further light on the association between intraoperative burst suppression and postoperative delirium. In the ENGAGES trial, patients are randomized to EEG-guided anesthesia or EEG-blinded anesthesia. All participating anesthesia clinicians have been trained in the interpretation of raw EEG waveforms. In the EEG-guided arm, clinicians view the raw EEG during surgery and attempt to maintain slow wave anesthesia, avoiding burst suppression. In the EEG-blinded arm, the raw EEG and all derived parameters are hidden from the clinician. If patients in the EEG-guided arm have less burst suppression and less postoperative delirium than patients in the EEG-blinded arm, then those results would support the hypothesis that avoiding burst suppression during surgery can prevent postoperative delirium.
Even after the resolution of postoperative delirium, patients who experienced greater amounts of EEG suppression reported poorer functional independence than those who experienced less.. Because we did not measure preoperative Barthel Index, we cannot tell whether reduced functional independence was present before surgery as well. This association was not entirely mediated via postoperative delirium, as the correlation remained statistically significant after controlling for postoperative delirium. However, delirium may have played a role, as patients who experienced postoperative delirium had poorer functional independence than those who did not. While it is true that the median Barthel Index score was only five points lower in the group with postoperative delirium, the difference between a score of 100 and 95 is clinically significant, because this represents the difference between complete independence and partial dependence in completing tasks of daily living. The true effect may be even greater than observed, as the reduced survey response rate among patients who experienced postoperative delirium could, in part, be due to limited functional independence. Past work by our group has demonstrated that delirium measured by the same methods used in this study was associated with additional adverse outcomes, including longer ICU and hospital stay and increased mortality.16
To our knowledge, only two studies have previously examined risk factors for EEG suppression during general anesthesia.35–36 During propofol-remifentanil anesthesia, older age, history of coronary artery disease, and male sex were associated with an increased probability of having an elevated suppression ratio.35 None of these characteristics was a risk factor in our study, perhaps because we used inhaled agents rather than total IV anesthesia. In a study of general anesthesia with volatile agents, many risk factors for EEG suppression were identified, including high end-tidal anesthetic concentration and high intraoperative opioid dose.35 We have replicated the finding regarding anesthetic concentration, but we observed the opposite relationship between opioid dose and EEG suppression. One explanation for the opioid association we observed is that opioid medications protect patients from the development of EEG suppression. Another possibility is that patients who developed EEG suppression were deeply anesthetized, and did not display signs of nociception, so practitioners administered less opioid medication. Either explanation is compatible with our results, as our data do not distinguish whether reduced opioid medication or EEG suppression came first temporally.
This study has important limitations. Because this was an observational study, our findings cannot indicate whether the relationship between EEG suppression and delirium is causal. Delirium was assessed as part of routine clinical care, and such assessments have limited sensitivity despite high specificity.37 Although some cases of delirium may have been missed, any bias in delirium measurement was likely non-differential, because nurses did not know which patients had experienced EEG suppression. Another limitation is that some patients either left the ICU prior to the first delirium assessment or were sedated at all assessment time points. However, this was unlikely to bias the main result of our study because, compared to patients who were assessed for delirium, patients who were not assessed did not differ with respect to any of the statistically significant predictors from our logistic regression, including EEG suppression. The post-discharge outcomes may be limited due to incomplete survey responses, particularly because patients who experienced postoperative delirium were less likely to return the survey. Furthermore, the Barthel Index was not performed preoperatively, and thus it is not possible to distinguish whether patients who experienced EEG suppression had reduced functional independence before surgery as well, although we think this is unlikely. This study also restricted its focus to patients with planned ICU admission after surgery, so care should be taken when applying these results to a broader surgical patient population.
This study has identified EEG suppression as a novel, independent risk factor for postoperative delirium in surgical patients after anesthesia with inhaled agents. EEG suppression was also correlated with reduced functional independence one month after surgery. Furthermore, patients experience more suppression when they are exposed to higher concentrations of inhaled agents. Because EEG suppression can be quantified in real-time using suppression ratio values displayed by any EEG monitor, it may be possible to reduce the amount of suppression that patients experience by using the suppression ratio as a guide while titrating anesthesia. The next step would be to investigate whether such an intervention leads to decreased incidence of postoperative delirium.
Acknowledgments
Funding: This work was supported by the Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and TL1 TR000449 from the National Center for Advancing Translational Sciences. This work was also supported by grant 1UH2AG050312-01 from the National Institute on Aging and grant BJHF#7937-77from the Barnes-Jewish Hospital Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was also supported by the Washington University Department of Anesthesiology and the Barnes-Jewish Hospital Foundation.
Analytical and informatics resources as well as research mentorship for this study were provided by the Institute of Quality Improvement, Research and Informatics (INQUIRI) at Washington University. The authors wish to thank Lewis E. Kazis, Ph.D., Professor of Health Policy and Management, Boston University School of Public Health (Boston, MA) for permission to use the VR-12 scoring algorithm.
Footnotes
The authors declare no conflict of interests.
Reprints will not be available from the authors.
DISCLOSURES
Name: Bradley A. Fritz, M.D.
Contribution: This author helped design the study, collect data, analyze data, and prepare the manuscript.
Attestation: Bradley A. Fritz approved the final manuscript, attests to the integrity of the original data and the analysis reported in this manuscript, and is the archival author.
Name: Philip L. Kalarickal, M.D.
Contribution: This author helped design the study, enroll patients, collect data, and critically revise the manuscript.
Attestation: Philip L. Kalarickal approved the final manuscript.
Name: Hannah R. Maybrier, B.S.
Contribution: This author helped enroll patients, conduct the study, collect data, and critically revise the manuscript.
Attestation: Hannah R. Maybrier approved the final manuscript.
Name: Maxwell R. Muench, B.S.
Contribution: This author helped enroll patients, conduct the study, collect data, and critically revise the manuscript.
Attestation: Maxwell R. Muench approved the final manuscript.
Name: Doug Dearth, M.D.
Contribution: This author helped enroll patients, conduct the study, collect data, and critically revise the manuscript.
Attestation: Doug Dearth approved the final manuscript.
Name: Yulong Chen, B.A.
Contribution: This author helped interpret data and critically revise the manuscript.
Attestation: Yulong Chen approved the final manuscript.
Name: Krisztina E. Escallier, M.D.
Contribution: This author helped interpret data and critically revise the manuscript.
Attestation: Krisztina E. Escallier approved the final manuscript.
Name: Arbi Ben Abdallah, Ph.D.
Contribution: This author helped design the study, analyze the data, and critically revise the manuscript.
Attestation: Arbi Ben Abdallah approved the final manuscript.
Name: Nan Lin, Ph.D.
Contribution: This author helped analyze the data, and critically revise the manuscript.
Attestation: Nan Lin approved the final manuscript.
Name: Michael S. Avidan, M.B.B.Ch.
Contribution: This author helped design the study, interpret data, and critically revise the manuscript.
Attestation: Michael S. Avidan approved the final manuscript and attests to the integrity of the original data and the analysis reported in this manuscript.
This manuscript was handled by: Gregory Crosby, MD
Contributor Information
Bradley A. Fritz, Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri
Philip L. Kalarickal, Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri
Hannah R. Maybrier, Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri
Maxwell R. Muench, Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri
Doug Dearth, Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri
Yulong Chen, Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri
Krisztina E. Escallier, Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri
Arbi Ben Abdallah, Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri
Nan Lin, Department of Mathematics, Washington University, St. Louis, Missouri
Michael S. Avidan, Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri
References
- 1.Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis and treatment. Crit Care Clin. 2008;24:657–722. doi: 10.1016/j.ccc.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Lat I, McMillian W, Taylor S, Janzen JM, Papadopoulos S, Korth L, Ehtisham A, Nold J, Agarwal S, Azocar R, Burke P. The impact of delirium on clinical outcomes in mechanically ventilated surgical and trauma patients. Crit Care Med. 2009;37:1898–905. doi: 10.1097/CCM.0b013e31819ffe38. [DOI] [PubMed] [Google Scholar]
- 3.Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, Truman B, Bernard GR, Dittus RS, Ely EW. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–62. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 4.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: A meta-analysis. J Am Med Assoc. 2010;304:443–51. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 5.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168:27–32. doi: 10.1001/archinternmed.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koster S, Hensens AG, Schuurmans MJ, van der Palen J. Consequences of delirium after cardiac operations. Ann Thorac Surg. 2012;93:705–11. doi: 10.1016/j.athoracsur.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Chander D, Garcia PS, MacColl JN, Illing S, Sleigh JW. Electroencephalographic variation during end maintenance and emergence from surgical anesthesia. PLoS One. 2014;9:e106291. doi: 10.1371/journal.pone.0106291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofste WJ, Linssen CA, Boezeman EH, Hengeveld JS, Leusink JA, de-Boer A. Delirium and cognitive disorders after cardiac operations: Relationship to pre- and intraoperative quantitative electroencephalogram. Int J Clin Monit Comput. 1997;14:29–36. doi: 10.1007/BF03356576. [DOI] [PubMed] [Google Scholar]
- 9.Bennett C, Voss LJ, Barnard JP, Sleigh JW. Practical use of the raw electroencephalogram waveform during general anesthesia: The art and science. Anesth Analg. 2009;109:539–50. doi: 10.1213/ane.0b013e3181a9fc38. [DOI] [PubMed] [Google Scholar]
- 10.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–50. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashida M, Sekiyama H, Orii R, Chinzei M, Ogawa M, Arita H, Hanaoka K, Takamoto S. Effects of deep hypothermic circulatory arrest with retrograde cerebral perfusion on electroencephalographic bispectral index and suppression ratio. J Cardiothorac Vasc Anesth. 2007;21:61–7. doi: 10.1053/j.jvca.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Watson PL, Shintani AK, Tyson R, Pandharipande PP, Pun BT, Ely EW. Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit Care Med. 2008;36:3171–7. doi: 10.1097/CCM.0b013e318186b9ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wennervirta JE, Ermes MJ, Tiainen SM, Salmi TK, Hynninen MS, Sarkela MO, Hynynen MJ, Stenman UH, Viertio-Oja HE, Saastamoinen KP, Pettila VY, Vakkuri AP. Hypothermia-treated cardiac arrest patients with good neurological outcome differ early in quantitative variables of EEG suppression and epileptiform activity. Crit Care Med. 2009;37:2427–35. doi: 10.1097/CCM.0b013e3181a0ff84. [DOI] [PubMed] [Google Scholar]
- 14.Andresen JM, Girard TD, Pandharipande PP, Davidson MA, Ely EW, Watson PL. Burst suppression on processed electroencephalography as a predictor of postcoma delirium in mechanically ventilated ICU patients. Crit Care Med. 2014;42:2244–51. doi: 10.1097/CCM.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radtke FM, Franck M, Lendner J, Kruger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110(Suppl 1):i98–105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 16.Whitlock EL, Torres BA, Lin N, Helsten DL, Nadelson MR, Mashour GA, Avidan MS. Postoperative Delirium in a Substudy of Cardiothoracic Surgical Patients in the BAG-RECALL Clinical Trial. Anesth Analg. 2014;118:809–17. doi: 10.1213/ANE.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieber FE, Zakryia KJ, Gottschalk A, Blute MR, Lee HB, Rosenberg PB, Mears SC. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan MT, Cheng BC, Lee TM, Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 19.Soehle M, Dittmann A, Ellerkmann RK, Baumgarten G, Putensen C, Guenther U. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: A prospective, observational study. BMC Anesthesiol. 2015 doi: 10.1186/s12871-015-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK. Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–9. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Kazis LE, Miller DR, Clark JA, Skinner KM, Lee A, Ren XS, Spiro A, 3rd, Rogers WH, Ware JE., Jr Improving the response choices on the veterans SF-36 health survey role functioning scales: Results from the Veterans Health Study. J Ambul Care Manage. 2004;27:263–80. doi: 10.1097/00004479-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Jones D, Kazis L, Lee A, Rogers W, Skinner K, Cassar L, Wilson N, Hendricks A. Health status assessments using the Veterans SF-12 and SF-36: Methods for evaluating otucomes in the Veterans Health Administration. J Ambul Care Manage. 2001;24:68–86. doi: 10.1097/00004479-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J. 1965;14:61–5. [PubMed] [Google Scholar]
- 24.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 25.Pereira J, Berry Otfinowski P, Hagen N, Bruera E, Fainsinger R, Summers N. Alberta Hospice Palliative Care Resource Manual. 2. Calgary, AB Canada: Alberta Cancer Board; 2001. [Google Scholar]
- 26.Nickalls RW, Mapleson WW. Age-related iso-MAC charts for isoflurane, sevoflurane and desflurane in man. Br J Anaesth. 2003;91:170–4. doi: 10.1093/bja/aeg132. [DOI] [PubMed] [Google Scholar]
- 27.Babyak MA. What you see may not be what you get: A brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66:411–21. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Strawderman RL, Johnson BA, O’Quigley JM. Analyzing repeated measures semi-continuous data, with application to an alcohol dependence study. Stat Methods Med Res. 2012 doi: 10.1177/0962280212443324. [DOI] [PubMed] [Google Scholar]
- 29.Bruhn J, Bouillon TW, Shafer SL. Bispectral index (BIS) and burst suppression: revealing a part of the BIS algorithm. J Clin Monit Comput. 2000;16:593–6. doi: 10.1023/A:1012216600170. [DOI] [PubMed] [Google Scholar]
- 30.Short TG, Leslie K, Campbell D, Chan MT, Corcoran T, O’Loughlin E, Frampton C, Myles P. A pilot study for a prospective, randomized, double-blind trial of the influence of anesthetic depth on long-term outcome. Anesth Analg. 2014;118:981–6. doi: 10.1213/ANE.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 31.Kroeger D, Amzica F. Hypersensitivity of the anesthesia-induced comatose brain. J Neurosci. 2007;27:10597–607. doi: 10.1523/JNEUROSCI.3440-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ching S, Purdon PL, Vijayan S, Kopell NJ, Brown EN. A neurophysiological-metabolic model for burst suppression. Proc Natl Acad Sci USA. 2012;109:3095–100. doi: 10.1073/pnas.1121461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J Mol Neurosci. 2001;17:101–18. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 34.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–22. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Besch G, Liu N, Samain E, Pericard C, Boichut N, Mercier M, Chazot T, Pili-Floury S. Occurrence of and risk factors for electroencephalogram burst suppression during propofol-remifentanil anaesthesia. Br J Anaesth. 2011;107:749–56. doi: 10.1093/bja/aer235. [DOI] [PubMed] [Google Scholar]
- 36.Willingham M, Ben Abdallah A, Gradwohl S, Helsten D, Lin N, Villafranca A, Jacobsohn E, Avidan M, Kaiser H. Association between intraoperative electroencephalographic suppression and postoperative mortality. Br J Anaesth. 2014;113:1001–8. doi: 10.1093/bja/aeu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM., Jr Nurses’ recognition of delirium and its symptoms: Comparison of nurse and researcher ratings. Arch Intern Med. 2001;161:2467–73. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]