Abstract
Pancreatic cancer (PC) remains a highly lethal malignancy due to its unusual chemoresistance and high aggressiveness. A subpopulation of pancreatic tumor cells, known as cancer stem cells (CSCs), is considered responsible not only for tumor-maintenance, but also for its widespread metastasis and therapeutic failure. Here we investigated the role of p-21 activated kinase 4 (PAK4) in driving PC stemness properties. Our data demonstrate that triple-positive (CD24+/CD44+/EpCAM+) subpopulation of pancreatic CSCs exhibits greater level of PAK4 as compared to triple-negative (CD24−/CD44−/EpCAM−) cells. Moreover, PAK4 silencing in PC cells leads to diminished fraction of CD24, CD44, and EpCAM positive cells. Furthermore, we show that PAK4-silenced PC cells exhibit decreased sphere-forming ability and increased chemo-sensitivity to gemcitabine toxicity. PAK4 expression is also associated with enhanced levels of stemness-associated transcription factors (Oct4/Nanog/Sox2 and KLF4). Furthermore, our data show decreased nuclear accumulation and transcriptional activity of STAT3 in PAK4-silenced PC cells and restitution of its activity leads to restoration of stem cell phenotypes. Together, our findings deliver first experimental evidence for the involvement of PAK4 in PC stemness and support its clinical utility as a novel therapeutic target in PC.
Keywords: PAK4, Pancreatic cancer, Stemness, STAT3, Sphere formation, Chemoresistance
Introduction
Pancreatic cancer (PC) is one of the most lethal malignancies and stands as the fourth leading cause of cancer-related death in the United States [1]. With continued increases in its incidence and mortality, PC is expected to take over colorectal and breast malignancies to become second leading cause by the year 2030 or even earlier [2]. High mortality in PC patients is attributed to late diagnosis and unusual resistance of the disease to currently available therapeutic modalities [3,4]. Clearly, this dire situation mandates that efforts should be made to identify novel biomarkers and therapeutic targets to enable early detection and efficient treatment based on improved mechanistic understanding of disease progression, metastasis and therapy-resistance.
A number of studies have demonstrated that a small subpopulation of cells within a tumor, referred as cancer initiating cells/cancer stem cells (CSCs), is involved in tumor initiation, development, metastasis as well as in therapy resistance and disease relapse [5–7]. Pancreatic CSCs were isolated, for the first time, based on phenotypic markers, viz. CD24, CD44 and ESA (also known as EpCAM), and demonstrated to be highly tumorigenic [8]. Subsequently, several studies attributed high rate of recurrence and chemoresistance in PC to pancreatic CSCs [9–13] suggesting that their targeting would be a logical way to find an effective cure. However, underlying molecular mechanisms and genetic drivers controlling the stemness phenotypes have remained largely undefined.
The serine/threonine kinase, p21-activated kinase 4 (PAK4), is essential for embryonic development and is a key regulator of various cellular processes including cytoskeleton dynamics, cell polarity, etc. [14–16]. In addition, aberrant expression of PAK4 is linked to a variety of human cancers [17–20]. In a sub-set of pancreatic tumor specimens, a chromosomal region 19q13.2-13.3 harboring PAK4 genetic locus was reported to be amplified [21]. Recently, we also reported overexpression of PAK4 in PC and demonstrated its role in proliferation and survival of pancreatic tumor cells [22]. The involvement of PAK4 in aggressive malignant phenotypes (EMT, invasion and metastasis) and chemoresistance of various cancers has also been reported [23–26]. However, to date there is no direct evidence associating PAK4 expression with cancer stem cell properties.
In the present study, we investigated the role of PAK4 in maintenance of the stem cell-like phenotypes in PC. The data demonstrate that PAK4 is overexpressed in pancreatic CSCs as compared to non-CSCs, and its expression is associated with increased sphere-forming potential and chemoresistance in PC. Furthermore, PAK4 was shown to activate STAT3 signaling to promote sphere formation as well as other stem-like phenotypes in PC. These findings deliver first experimental evidence for involvement of PAK4 in stemness of PC and further support its clinical utility as a therapeutic target.
Materials and methods
Cell culture
PC cell lines (MiaPaCa and T3M4) were maintained as monolayer cultures in RPMI-1640 (Life Technologies, Carlsbad, CA) with 5% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA), penicillin (100 units/mL) and streptomycin (100 μg/mL) (Life Technologies) in a humidified atmosphere (5% CO2 at 37 °C). The cells expressing high level of CD24/CD44/EpCAM surface markers were isolated from MiaPaCa and T3M4 cells and cultured in Ultra-Low attachment plate/flask (Corning Incorporated, Corning, NY) in stem cell culture medium (DMEM:F-12K, 1:1; Life Technologies) supplemented with B27, basic fibroblast growth factor (bFGF; 20 ng/mL) and epidermal growth factor (EGF; 20 ng/mL) (Life Technologies), penicillin (100 units/mL) and streptomycin (100 μg/mL) to maintain their undifferentiated status. Cells were routinely monitored for their typical morphology, and intermittently tested for mycoplasma contamination at our institutional core facility.
Antibodies, siRNAs and plasmids
Anti-PAK4 (rabbit polyclonal), -Sox2, -Nanog, -pSTAT3 (Y705) (rabbit monoclonal) and -STAT3 (mouse monoclonal) antibodies were purchased from Cell Signaling Technology (Beverly, MA). Anti-KLF4, -Oct4 (mouse monoclonal) were procured from Abcam (Cambridge, MA). Antibodies targeting Lamin A, α-tubulin (mouse mono-clonal) and respective anti-mouse or anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibodies were procured from Santa Cruz Biotechnology (Santa Cruz, CA). β-actin (mouse monoclonal) antibody was purchased from Sigma-Aldrich (St. Louis MO). For isolation of cancer stem-like cells, anti-human fluorochrome-conjugated antibodies against CD24 (Alexa Fluor-647 conjugated), CD44 (Brilliant violent-421 conjugated) and EpCAM (also known as ESA) (Phycoerythrin/cy7 conjugated) were procured from BioLegend (San Diego, CA). The construct EF.STAT3C.Ubc.GFP (Addgene plasmid #24983) was from Linzhao Cheng laboratory and the control vector FUGW (Addgene plasmid #14883) was from David Baltimore laboratory and both the plasmids were procured from Addgene. All non-target (ONTARGET plus Non-targeting pool) and target-specific (ONTARGET plus SMART pool) siRNAs were from Origene (Rockville, MD).
Isolation of cancer stem cells
MiaPaCa and T3M4 cells were trypsinized, washed with PBS and resuspended (1 × 106/mL) in PBS containing 2% FBS. Subsequently, cells were incubated with fluorochrome-conjugated anti-CD24, -CD44 and -EpCAM antibodies (5 μL/106 cells each) for 15 min on ice. After incubation, cells were washed with PBS, and subjected to sorting of triple positive (CD24+, CD44+ and EpCAM+) and negative (CD24−, CD44− and EpCAM−) subpopulation of cells using FACS AriaIITM (BD Bioscience). 7-Aminoactinomycin D (7ADD; BD Bioscience) was added to exclude non-viable cells prior to FACS analysis. Unstained cells were used as control.
ALDEFLUOR assay
ALDEFLUOR assay was performed using ALDEFLOUR™ kit (Stem Cell Technologies, Vancouver, BC, Canada) according to the manufacturer’s protocol. Briefly, MiaPaCa and T3M4 cells (2.5 × 105) were incubated in ALDH1 assay buffer containing ALDH1 substrate (Bodipy Aminoacetaldehyde; BAAA) at 37 °C for 45 min with intermittent mixing. After incubation, cells were centrifuged at 250 g for 5 min and resuspended in 0.5 mL of ALDH1 assay buffer for analysis using FACS AriaIITM. For control, cells were treated with the ALDH1 enzyme inhibitor [diethylaminobenzaldehyde (DEAB)]. 7-AAD was added to the cell suspension prior to FACS analysis for the exclusion of dead cells.
RNA isolation and reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent (Invitrogen) and reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) following manufacturer’s instructions as described earlier [27]. Quantitative real-time PCR was performed in 96-well plates using cDNA and SYBR Green Master Mix on an iCycler system (Bio-Rad, Hercules, CA) using specific sets of primer pairs (Table S1). The following thermal conditions were used: cycle 1: 95 °C for 10 min, cycle 2 (×40): 95 °C for 10 sec and 58 °C for 45 sec. Threshold cycle (CT) value for each gene was separately normalized against CT value for GAPDH, and a relative fold-change in expression with respect to a reference sample was calculated by the 2−ΔΔCt method.
Transfections and treatments
Stable PAK4 knockdown cell lines (MiaPaCa-shPAK4 and T3M4-shPAK4) were generated previously [22]. To dissect the role of the STAT3 signaling pathway, PAK4-expressing PC (MiaPaCa- and T3M4-NTScr) cells were cultured in 6-well plates and transfected with 30 nM of human STAT3 specific siRNAs, while in another approach PAK4-silenced PC cells (MiaPaCa- and T3M4-shPAK4) were transduced with a lentivirus expressing a constitutively active mutant form of STAT3 (EF.STAT3C.Ubc.GFP) and respective control vector (FUGW). For deciphering the role of PAK4 in chemoresistance, cells were treated with increasing concentration of gemcitabine (0–10 μM) for 72 h and cell viability was calculated using WST-1 assay kit (Roche, Indianapolis, IN) as described earlier [28].
Immunoblot analysis
Immunoblotting was performed as described earlier [29] using specific antibodies against various proteins. β-actin, α-tubulin and Lamin A were used as loading controls for total, cytoplasmic and nuclear proteins, respectively. All the primary antibodies were used at 1:1000 dilution whereas all the secondary antibodies were used at 1:2500 dilution. β-actin was used at 1:20,000 dilution. The signal was detected using ECL plus Western Blotting substrate (Thermo Scientific, Logan, UT) and LAS-3000 image analyzer (Fuji Photo Film Co., Tokyo, Japan).
Nuclear and cytoplasmic fractionation
The preparation of cytoplasmic and nuclear extracts was performed using the nuclear extract kit (Active Motif, Carlsbad, CA) as described previously [22,30].
STAT3 transcriptional activity assay
The transcriptional activity of STAT3 was measured by Cignal STAT3 Reporter (Luc) kit (SA Bioscience, Valencia, CA) according to manufacturer’s instructions. Briefly, PC cells were grown in 6-well plate and transiently transfected with 1.0 μg of STAT3 reporter along with negative control. STAT3 reporter is a mixture of inducible STAT3-responsive firefly luciferase construct and constitutively expressing Renilla luciferase construct (40:1). Negative control is a mixture of non-inducible firefly luciferase construct and constitutively expressing Renilla luciferase construct (40:1). After 48 h of transfection, cells were harvested in passive lysis buffer and luciferase activity was measured using the Dual Luciferase Assay System (Promega, Madison, WI).
Clonogenicity and sphere formation assay
The clonogenicity and sphere formation assays were performed as described previously [31,32]. Briefly, for sphere formation assay, cells (1 × 103/well) were seeded in the 6-well ultralow attachment plate in 2 mL of stem cell culture medium and on every alternate day the medium was supplemented with 2% B27, 20 ng/mL EGF and 20 ng/mL FGF-b. After 10 days, the pancreatospheres larger than 50 μm were counted and photographed using phase contrast microscope as described previously [32].
Statistical analysis
All the experiments were performed at least three times independently, and all data are expressed as “mean ± SD”. Wherever appropriate, the data were also subjected to unpaired two tailed Student’s t-test. p < 0.05 was considered statistically significant.
Results
PAK4 is highly expressed in pancreatic cancer stem cells
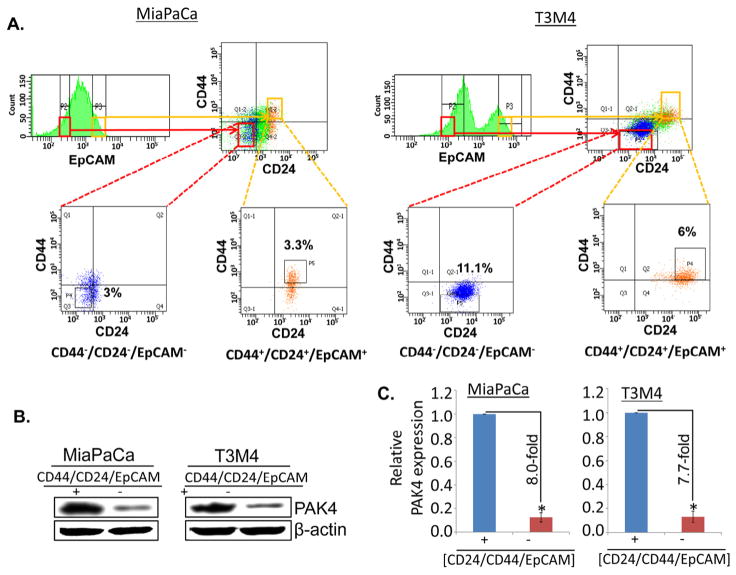
To investigate the role of PAK4 in stemness of PC cells, we isolated cancer stem cells (CSCs) from two PAK4 expressing highly aggressive PC cell lines (MiaPaCa and T3M4) by flow-cytometry using a set of previously characterized phenotypic biomarkers, viz. CD24, CD44 and EpCAM [8]. Sub-set of PC cells with high expression of CD24, CD44 and EpCAM was designated as CSCs (CD24+/CD44+/EpCAM+) while cells with no or negligible expression were designated as non-CSCs (CD24−/CD44−/EpCAM−). Apparently, ~3.3% (MiaPaCa) and ~6.0% (T3M4) cells were identified as CSC population while the fraction of non-CSCs was ~3.0 and ~11.1% in MiaPaCa and T3M4 cells, respectively (Fig. 1A). Furthermore, we also examined the expression of PAK4 in CSCs (CD24+/CD44+/EpCAM+) and non-CSCs (CD24−/ CD44−/EpCAM−) by immunoblot analysis. We observed that the expression level of PAK4 is remarkably higher in CSCs (CD24+/ CD44+/EpCAM+) as compared to non-CSCs (CD24−/CD44−/EpCAM−) in both the cell lines (MiaPaCa and T3M4) (Fig. 1B). The densitometry analyses show that the expression of PAK4 is 8.0- and 7.7-fold higher in CSCs of MiaPaCa and T3M4 cells, respectively (Fig. 1C). Together, these findings suggest that PAK4 is highly expressed in pancreatic cancer stem cells.
Fig. 1.
PAK4 expression analysis in pancreatic cancer stem cells (CD44+/CD24+/EpCAM+) and non-CSCs (CD44−/CD24−/EpCAM−). (A) Pancreatic cancer stem cells (CSCs) were isolated from PAK4 expressing MiaPaCa and T3M4 PC parent cells on the basis of triple positive and negative CD24, CD44, and EpCAM surface markers using flow-cytometry. (B) PAK4 expression was analyzed in pancreatic CSCs (CD44+/CD24+/EpCAM+) and non-CSCs (CD44−/CD24−/EpCAM−) by immunoblot assay using PAK4 specific antibody. β-actin was used as an internal control. (C) Relative expression of PAK4 in CSCs and non-CSCs isolated from MiaPaCa and T3M4 cells estimated by densitometry. Error bars represent the mean ± SD; n = 3; *p < 0.05.
Silencing of PAK4 reduces the expression of stemness-associated markers in PC cells
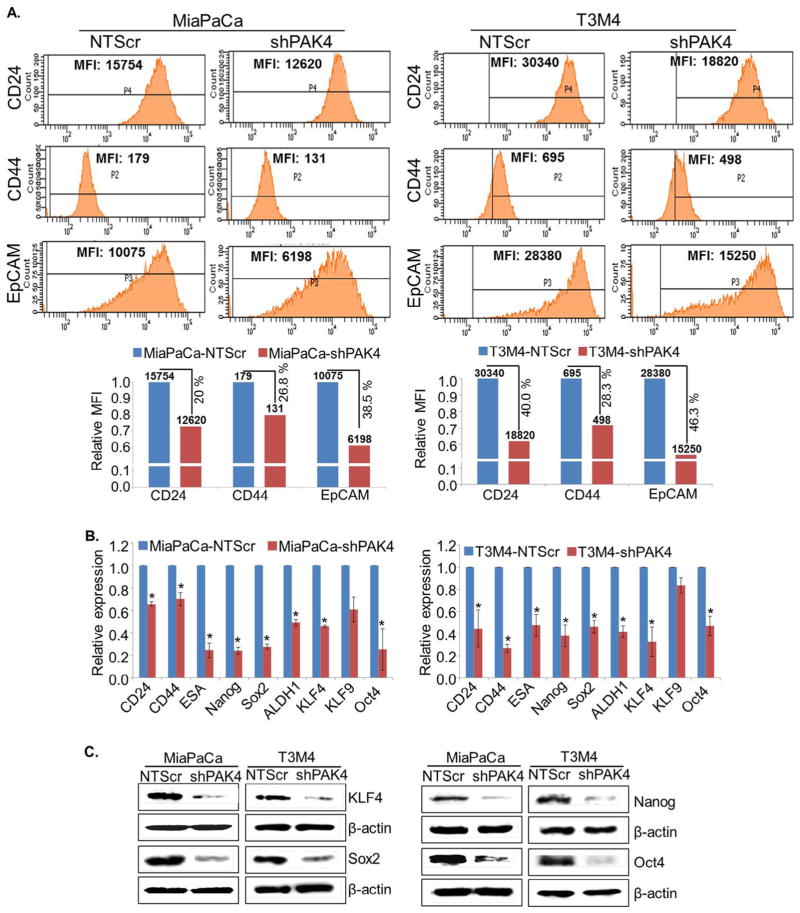
Having observed the elevated expression of PAK4 in CSCs, we sought to investigate its significance in PC stemness. For this, we used PAK4-silenced (-shPAK4) stable sublines of MiaPaCa and T3M4 cells (Fig. S1) and examined the expression of various stemness-associated markers (CD24, CD44 and EpCAM) by flow-cytometry. We observed that the individual geometric mean fluorescence intensity (MFI) of CD24, CD44 and EpCAM was considerably decreased in PAK4-silenced PC cells (Fig. 2A). The relative MFI of CD24, CD44 and EpCAM is reduced by 20.0%, 26.8% and 38.5%, respectively, in MiaPaCa-shPAK4 cells, while it was decreased by 40.0%, 28.3% and 46.3%, respectively, in PAK4-silenced T3M4 cells (Fig. 2A). Since pancreatic CSCs are also known to be enriched with aldehyde dehydrogenase (ALDH1) [33], we explored whether PAK4 silencing has any effect on ALDH1 level. Data from flow-cytometry analysis demonstrate that the number of ALDH1 positive cells is remarkably decreased ~2.5 fold (MiaPaCa) and ~1.7 fold (T3M4) upon silencing of PAK4 (Fig. S2). Furthermore, we confirmed all these findings by performing quantitative RT-PCR assays. Similar to our flow cytometry data, we found that the expression level of CD24, CD44, EpCAM and ALDH1 was significantly decreased in low PAK4 expressing (MiaPaCa- and T3M4-shPAK4) cells in comparison to their control cells (MiaPaCa- and T3M4-NTScr) at mRNA level (Fig. 2B). In addition, we also examined the effect of PAK4 silencing on the expression of stemness-associated transcription factors (KLF4, Sox2, Nanog and Oct4) at mRNA level. Data demonstrate a drastic decrease in the level of KLF4, Sox2, Nanog and Oct4 in PAK4 silenced MiaPaCa and T3M4 cells (Fig. 2B). PAK4-silencing induced downregulation of KLF4, Sox2, Nanog and Oct4 was further validated at protein level by immunoblot assay (Fig. 2C).
Fig. 2.
PAK4 induces expression of stemness-associated markers in PC cells. (A) PAK4-expressing and silenced cells were incubated with fluorochrome-conjugated anti-CD24, -CD44 and -EpCAM antibodies for 15 min on ice, followed by washing with PBS and subjected to FACS analysis. The data are presented as geometric mean of fluorescence intensity (MFI). The expression of other stemness associated markers was examined (B) at mRNA level by quantitative RT-PCR (mean ± SD; n = 3; *p < 0.05) and (C) protein level by immunoblot assay. β-actin was used as an internal control.
Silencing of PAK4 suppresses sphere formation ability and chemo-resistance in PC cells
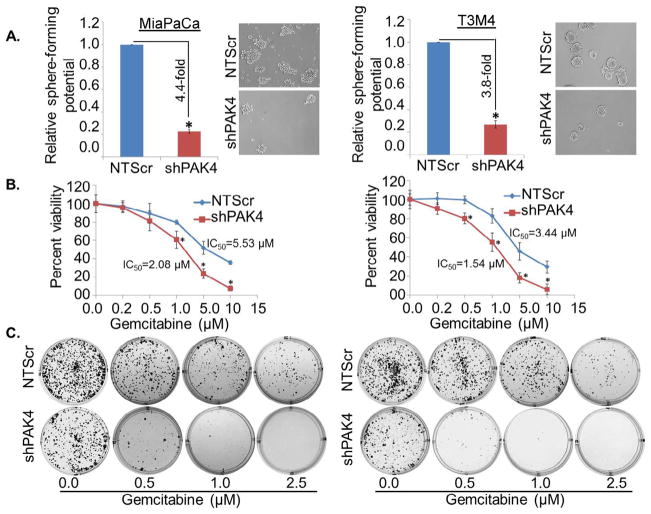
Unlimited self-renewal potential to maintain their undifferentiated state is a hallmark of CSCs [6]. Therefore, we next sought to investigate the role of PAK4 in the self-renewal ability of pancreatic CSCs by sphere formation assay. For this, PAK4-silenced sublines (-shPAK4) of MiaPaCa and T3M4 cells were seeded at low density (1 × 103/well) along with their high PAK4-expressing (MiaPaCa- and T3M4-NTScr) counterparts in low attachment plate. The cells were allowed to grow in stem cell culture medium for 10 days and the number of spheres formed was counted. We observed lesser sphere forming ability in PAK4-silenced PC cells as compared to PAK4 expressing cells (Fig. 3A). The data demonstrate 4.4-fold and 3.8-fold greater sphere formation in MiaPaCa-NTScr and T3M4-NTScr cells, respectively, as compared to MiaPaCa-shPAK4 and T3M4-shPAK4 cells (Fig. 3A).
Fig. 3.
PAK4 facilitates sphere formation and drug resistance in PC cells. PAK4 expressing or silenced MiaPaCa and T3M4 cells (1 × 103) were seeded in ultra-low attachment plate and allowed to grow for 10 days in stem cell culture medium. (A) Images were taken under light microscope at 200× magnification and the number of spheres (size > 50 μm) was counted. Error bars represent the mean ± SD; n = 3; *p < 0.05. (B) PAK4 expressing or silenced PC cells were treated with indicated doses of gemcitabine (0–10 μM) for 72 h. Cell viability was analyzed by WST-1 assay. OD value of untreated cells was taken as 100% viable. Data represent the mean ± SD; n = 3; *p < 0.01. (C) Representative images of colonies formed by PAK4-expressing and -silenced cells treated with various concentrations of gemcitabine (0, 0.5, 1.0 and 2.5 μM) for 24 h and further allowed to form colonies for 2 weeks.
Resistance to chemo-therapeutic agents is another key characteristic of CSCs [6,7]. To examine the significance of PAK4 in chemoresistance, PAK4-expressing and -silenced cells were treated with increasing doses of gemcitabine and the effect on growth was examined by short-term (WST1 assay) and long-term (colony-formation) growth assays. Our data showed that PAK4-silenced cells (MiaPaCa- and T3M4-shPAK4) were more sensitive to gemcitabine-cytotoxicity as compared to respective control cells in both short-term (Fig. 3B) and long-term (Fig. 3C) growth assays. Data show significantly (p ≤ 0.01) lower IC50 values of gemcitabine ~2.08 μM in MiaPaCa-shPAK4 and 1.54 μM in T3M4-shPAK4 cells as compared to MiaPaCa-NTScr (~5.53 μM) and T3M4-NTScr (~3.44 μM) cells (Fig. 3B). Importantly, the chemosensitizing effect of PAK4 silencing is more prominent in long-term assay as evidenced by very few or no visible colonies in PAK4 silenced cells at 1.0 and 2.5 μM gemcitabine doses. Taken together, our findings suggest that PAK4 enhances stem cell-associated phenotypes in PC cells.
PAK4 enhances stemness potential via activation of STAT3 signaling in PC cells
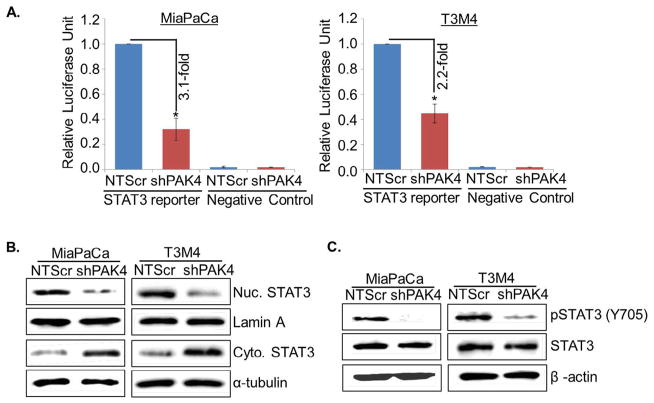
Signal transducer and activator of transcription 3 (STAT3) remains constitutively active in various cancer types including PC and plays pivotal role in proliferation and self-renewal of CSCs [34,35]. Therefore, we investigated the involvement of STAT3 in PAK4-induced stemness in PC cells. For this, we first examined the effect of PAK4 silencing on the transcriptional activity of STAT3 by luciferase-based promoter reporter assay. Data show that the transcriptional activity of STAT3 responsive promoter is decreased by ~3.1 fold (MiaPaCa) and ~2.2 fold (T3M4) upon silencing of PAK4 (Fig. 4A). These findings were further confirmed by analyzing the cellular localization and activation status of STAT3 in low and high PAK4-expressing PC cells by immunoblot assay. An enhanced nuclear level with concomitant decreased cytoplasmic level of STAT3 is observed in high PAK4 expressing (MiaPaCa-NTScr and T3M4-NTScr) cells as compared to low PAK4 expressing (MiaPaCa-shPAK4 and T3M4-shPAK4) cells (Fig. 4B). In addition, significantly decreased phosphorylation of STAT3 in PAK4-silenced MiaPaCa as well as T3M4 cells is observed (Fig. 4C).
Fig. 4.
PAK4 induces transcriptional activity of STAT3 in human PC cells by promoting its nuclear translocation. The cells were co-transfected with Cignal STAT3 reporter (mixture of STAT3-responsive firefly luciferase reporter and Renilla luciferase plasmids) and negative control plasmids. (A) After 48 h, cells were harvested in passive lysis buffer and luciferase activity was assessed using a dual-luciferase assay system. Data are presented as normalized relative luciferase activity (mean ± SD; n = 3, *p < 0.05). Total, nuclear and cytoplasmic extracts were prepared and (B) cellular localization of STAT3 and (C) expression level of pSTAT3 or total were analyzed by immunoblot analysis. Lamin A (for nuclear fraction), α-tubulin (for cytoplasmic fraction) and β-actin (for total fraction) were used as loading controls.
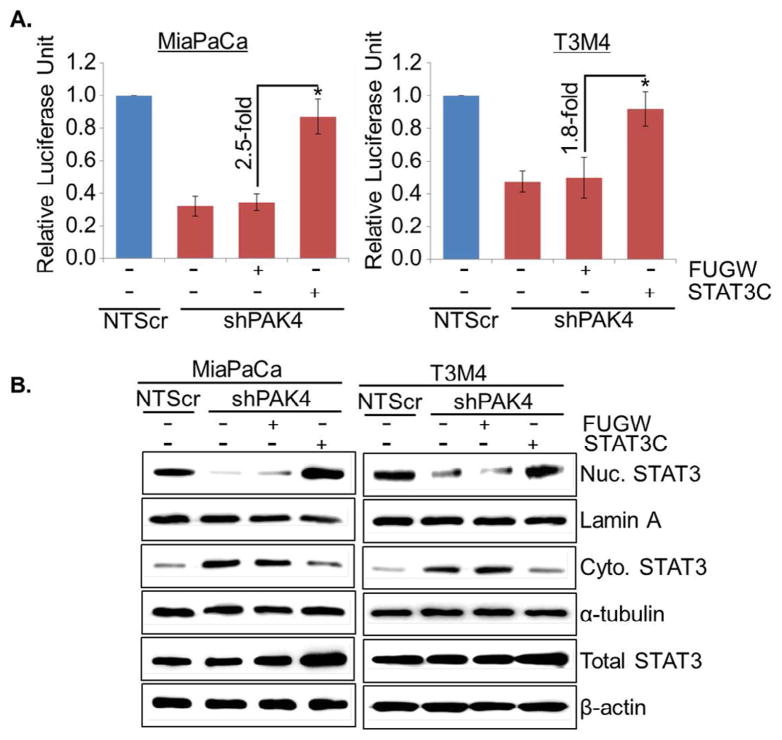
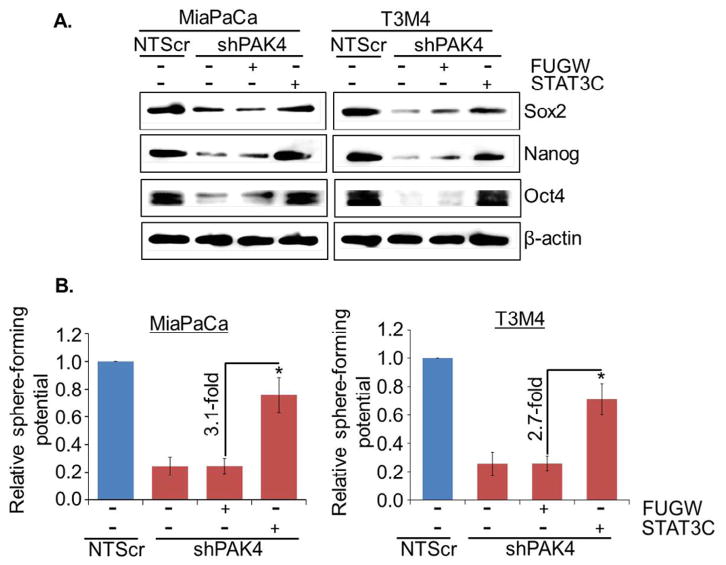
To validate whether STAT3 is involved in PAK4-induced stemness in PC cells, low PAK4 expressing (MiaPaCa-shPAK4 and T3M4-shPAK4) cells were transiently transfected with constitutively active mutant of STAT3 (STAT3C) and the effect on transcriptional activity of STAT3, stemness-associated markers and sphere-forming potential was examined. Our data demonstrate that transcriptional activity (Fig. 5A) is restored in PAK4-silenced MiaPaCa and T3M4 cells transfected with active mutant of STAT3, which is associated with increased nuclear and decreased cytoplasmic level of STAT3 in these cells (Fig. 5B). Notably, we observed regain of stemness-associated markers (Oct4, Sox2 and Nanog) in PAK4-silenced PC cells upon transfection of active mutant of STAT3 (Fig. 6A). Moreover, sphere-forming ability of PAK4-silenced cells is also restored after forced activation of STAT3 in these cells (Fig. 6B). These findings were further confirmed by reverse approach where STAT3 expression was silenced in MiaPaCa-NTScr and T3M4-NTScr cells (Fig. S3). Taken together, our findings suggest that PAK4-induces stemness potential in PC cells through activation of STAT3.
Fig. 5.
Forced expression of STAT3 active mutant restores transcriptional activity and localization of STAT3 in PAK4-silenced PC cells. In this approach, PAK4 silenced cells were transfected with constitutively active STAT3 mutant (EF.STAT3C.Ubc.GFP) or empty vector (FUGW) and (A) after 48 h transfection, cells were again transfected with STAT3 luciferase promoter-reporter plasmid and transcriptional activity of STAT3 was examined. Data are presented as normalized relative luciferase activity (mean ± SD; n = 3, *p < 0.05). (B) Nuclear and cytoplasmic fractions were prepared after 24 h of transfection and expression level of STAT3 was examined by immunoblot analysis. Lamin A (for nuclear fraction), α-tubulin (for cytoplasmic fraction) and β-actin (for total fraction) were used as loading controls.
Fig. 6.
STAT3-mediates PAK4-induced stemness in PC cells. (A) The expression of stem cell markers was analyzed by immunoblot analysis. β-actin was used as an internal control. (B) After transfection, cells were cultured in ultra-low attachment plate to analyze the effect of STAT3 on sphere-forming potential of these cells by sphere forming capacity. Sphere >50 μm in diameter was counted at 200× magnification (mean ± SD; n = 3, *p < 0.05).
Discussion
In spite of advances in surgery and chemotherapy, the mortality rate of PC remains very high due to lack of effective therapy and late diagnosis. Chemoresistance imposes one of the major clinical problems in PC treatment. Recent studies have shown that CSCs, characterized by high expression of ESA, CD24 and CD44 surface proteins in PC [8], are the main entity responsible for therapy resistance and tumor recurrence [5,9,11–13]. Therefore, therapeutic targeting of CSCs along with bulk epithelial tumor cells would have profound clinical implications for better management of this disease. In this study, we demonstrated, for the first time, that PAK4 expression is associated with increased stemness characteristics like high expression of stemness-associated markers, sphere-forming ability as well as chemoresistance.
Li et al. used CD24, CD44 and ESA markers alone or in combination for isolating the CSC population from pancreatic tumors [8]. Their study revealed that pancreatic tumor cells that were positive for all three (CD24, CD44 and ESA) surface molecules exhibited greater self-renewal capacity and were highly tumorigenic in nature as compared to pancreatic tumor cells that were positive for single or double markers [8]. Ohara et al. investigated the clinical significance of CD44+/CD24+/EpCAM+ cells in pancreatic adenocarcinoma. Their study suggested that the presence of CD44+/CD24+/EpCAM+ cells correlates with poor differentiation [36]. Moreover, CD44+/ CD24+ cells are associated with poor prognosis of pancreatic cancer patients [36]. Additionally, high rate of chemoresistance in PC is suggested to be attributed to CD44, CD24, and EpCAM positive stem cells [37]. The present study demonstrated that the level of PAK4 was considerably high in triple positive (CD24+/CD44+/EpCAM+) pancreatic CSCs as compared to non-CSCs (CD24−/CD44−/EpCAM−). Moreover, silencing of PAK4 in high PAK4 expressing (MiaPaCa and T3M4) PC cells led to reduction in expression of CD24, CD44 and EpCAM. These complementary sets of data provide a direct support for the association of high PAK4 levels with pancreatic CSCs.
In addition to the CD44, CD24 and EpCAM, we also observed decreased expression of other stemness-associated markers including ALDH1, KLF4, Oct4, Sox2 and Nanog in PAK4-silenced PC cells. Enormous studies are available to support the role of ALDH1, KLF4, Oct4, Sox2 and Nanog in stemness potential of different cancer types including PC [38–42]. Wei et al. reported that KLF4 is up-regulated in PC, and its expression inversely correlates with the survival of PC patients [43]. Overexpression of Oct4 and Nanog in PDAC is associated with early state carcinogenesis [44] and worst prognosis [45]. These studies indicate that KLF4, Oct4, and Nanog may contribute to PC pathobiology via enhancing the stemness potential of pancreatic tumor cells. Sox2 expression has been associated with increased levels of the stem cell markers, viz. ALDH1, EpCAM and CD44, in pancreatic tumor and CD44/EpCAM positive CSCs isolated from pancreatic tumor specimens have enhanced expression of Sox2 [46]. Rasheed et al. established the role of ALDH1 in the maintenance of stem cells potential of PC [33]. Together, these findings add further support for the association of PAK4 with pancreatic CSCs.
Enhanced sphere-forming potential and resistance to therapy are the main characteristics of CSCs [6,9]. It is widely accepted that the resistance to chemotherapeutic agents is largely attributed to self-renewing subpopulations of CSCs present in the bulk tumor. Earlier studies have shown that pancreatic CSCs confer increased resistance towards 5-fluorouracil (5-FU) and gemcitabine [47,48]. We demonstrated that PAK4-silencing not only reduced the expression of stemness markers, but also decreased the sphere formation ability of PC cells. Moreover, PAK4 silencing caused sensitization of PC cells against gemcitabine-induced toxicity. Enhanced chemoresistance ability of CSCs is majorly due to their enhanced survival mechanisms [49] as compared to the other cells of the tumor bulk. In this regard, our earlier findings supporting a direct role of PAK4 with increased survival of PC cells are highly relevant [22]. We observed that PAK4-overexpressing PC cells exhibited enhanced expression of anti-apoptotic proteins (Bcl-2 and Bcl-xL) [22], which are shown to confer apoptosis-resistance to CSCs [50,51]. PAK4 has been shown to promote chemoresistance in other cancer types as well [52,53] suggesting its universal association with CSC phenotypes.
Using various experimental approaches, we demonstrated a role of STAT3, an oncogenic transcription factor, in PAK4-induced expression of stem cell-associated markers and sphere-forming potential of pancreatic tumor cells. In the aspect of pancreatic car-cinogenesis, it has been suggested that STAT3 is frequently overexpressed in PC [35,54] and plays a vital role in PC pathogenesis by regulating genes involved in proliferation, survival, and metastasis of pancreatic tumor cells [55–57]. In addition, a role of STAT3 in the maintenance of stemness behavior in various tumor types, including PC, has also been well documented [34,58–60]. Recently, Guha et al. revealed the important role of STAT3 in nicotine-induced apoptosis resistance and CSC characteristics in breast cancer cells [61]. Furthermore, the important role of STAT3/Sox2 axis in maintaining stem cell phenotypes in PC cells has also been documented [62]. Notably, apart from maintaining the stemness phenotypes in tumor cells, STAT3 activation is also suggested to convert non-CSCs to CSCs [63]. The precise molecular mechanism(s) responsible for the activation of STAT3 in PC is not well understood. In this regard, our findings suggesting PAK4 as an upstream activator of STAT3 are highly innovative. However, the signaling axis/molecules involved in PAK4-mediated STAT3 activation need to be explored in future studies.
In conclusion, the present study establishes a direct association of PAK4 with pancreatic cancer stemness, an effect that is mediated through STAT3 activation. Thus, targeting of PAK4 could be of great significance for not only managing the bulk tumor cells, but CSCs as well. Therefore, development of novel approaches for inhibiting PAK4 activity in PC should be the next logical step that could lead to an effective cure for this devastating malignancy.
Supplementary Material
Acknowledgments
We would like to acknowledge the funding support from NIH/NCI [CA169829 (to SS) and CA167137, CA175772 (to APS)] and USAMCI.
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.canlet.2015.10.028.
Footnotes
Conflict of interest
None.
Authors’ contributions
SS, APS, NT, AB: study design; NT, SM, SMc, SKD: acquisition of data; SS, APS, NT, SM, AB, JEC: analysis and interpretation of data; SS, APS, NT, SM, AB, SKS: manuscript preparation; APS, SS: obtained funding.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Ansari D, Gustafsson A, Andersson R. Update on the management of pancreatic cancer: surgery is not enough. World J Gastroenterol. 2015;21:3157–3165. doi: 10.3748/wjg.v21.i11.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mysliwiec P, Kedra B. Causes of delayed diagnosis of pancreatic cancer. Own study and proposed algorithm. Przegl Lek. 2008;65:345–348. [PubMed] [Google Scholar]
- 5.Raj D, Aicher A, Heeschen C. Concise reviews: stem cells in pancreatic cancer: from concept to translation. Stem Cells. 2015;33:2893–2902. doi: 10.1002/stem.2114. [DOI] [PubMed] [Google Scholar]
- 6.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 7.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 9.Bednar F, Simeone DM. Pancreatic cancer stem cells and relevance to cancer treatments. J Cell Biochem. 2009;107:40–45. doi: 10.1002/jcb.22093. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Kong D, Ahmad A, Bao B, Sarkar FH. Pancreatic cancer stem cells: emerging target for designing novel therapy. Cancer Lett. 2013;338:94–100. doi: 10.1016/j.canlet.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasheed ZA, Matsui W. Biological and clinical relevance of stem cells in pancreatic adenocarcinoma. J Gastroenterol Hepatol. 2012;27(Suppl 2):15–18. doi: 10.1111/j.1440-1746.2011.07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarkar FH, Li Y, Wang Z, Kong D. Pancreatic cancer stem cells and EMT in drug resistance and metastasis. Minerva Chir. 2009;64:489–500. [PMC free article] [PubMed] [Google Scholar]
- 13.Tanase CP, Neagu AI, Necula LG, Mambet C, Enciu AM, Calenic B, et al. Cancer stem cells: involvement in pancreatic cancer pathogenesis and perspectives on cancer therapeutics. World J Gastroenterol. 2014;20:10790–10801. doi: 10.3748/wjg.v20.i31.10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abo A, Qu J, Cammarano MS, Dan C, Fritsch A, Baud V, et al. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 1998;17:6527–6540. doi: 10.1093/emboj/17.22.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Zhang H, Lundin L, Thullberg M, Liu Y, Wang Y, et al. p21-activated kinase 4 phosphorylation of integrin beta5 Ser-759 and Ser-762 regulates cell migration. J Biol Chem. 2010;285:23699–23710. doi: 10.1074/jbc.M110.123497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selamat W, Tay PL, Baskaran Y, Manser E. The Cdc42 effector kinase PAK4 localizes to cell-cell junctions and contributes to establishing cell polarity. PLoS ONE. 2015;10:e0129634. doi: 10.1371/journal.pone.0129634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn HK, Jang J, Lee J, Se HP, Park JO, Park YS, et al. P21-activated kinase 4 overexpression in metastatic gastric cancer patients. Transl Oncol. 2011;4:345–349. doi: 10.1593/tlo.11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai S, Ye Z, Wang X, Pan Y, Weng Y, Lao S, et al. Overexpression of P21-activated kinase 4 is associated with poor prognosis in non-small cell lung cancer and promotes migration and invasion. J Exp Clin Cancer Res. 2015;34:48. doi: 10.1186/s13046-015-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mak GW, Chan MM, Leong VY, Lee JM, Yau TO, Ng IO, et al. Overexpression of a novel activator of PAK4, the CDK5 kinase-associated protein CDK5RAP3, promotes hepatocellular carcinoma metastasis. Cancer Res. 2011;71:2949–2958. doi: 10.1158/0008-5472.CAN-10-4046. [DOI] [PubMed] [Google Scholar]
- 20.Siu MK, Chan HY, Kong DS, Wong ES, Wong OG, Ngan HY, et al. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients. Proc Natl Acad Sci USA. 2010;107:18622–18627. doi: 10.1073/pnas.0907481107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Auletta T, Dovirak O, Hutter C, Kuntz K, El-ftesi S, et al. Copy number alterations in pancreatic cancer identify recurrent PAK4 amplification. Cancer Biol Ther. 2008;7:1793–1802. doi: 10.4161/cbt.7.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyagi N, Bhardwaj A, Singh AP, McClellan S, Carter JE, Singh S. p-21 activated kinase 4 promotes proliferation and survival of pancreatic cancer cells through AKT- and ERK-dependent activation of NF-kappaB pathway. Oncotarget. 2014;5:8778–8789. doi: 10.18632/oncotarget.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Q, Su N, Zhang J, Li X, Miao Z, Wang G, et al. PAK4 kinase-mediated SCG10 phosphorylation involved in gastric cancer metastasis. Oncogene. 2014;33:3277–3287. doi: 10.1038/onc.2013.296. [DOI] [PubMed] [Google Scholar]
- 24.Minden A. The pak4 protein kinase in breast cancer. ISRN Oncol. 2012;2012:694201. doi: 10.5402/2012/694201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu BJ, Lee H, Kim SH, Heo JN, Choi SW, Yeon JT, et al. PF-3758309, p21-activated kinase 4 inhibitor, suppresses migration and invasion of A549 human lung cancer cells via regulation of CREB, NF-kappaB, and beta-catenin signalings. Mol Cell Biochem. 2014;389:69–77. doi: 10.1007/s11010-013-1928-8. [DOI] [PubMed] [Google Scholar]
- 26.Song B, Wang W, Zheng Y, Yang J, Xu Z. P21-activated kinase 1 and 4 were associated with colorectal cancer metastasis and infiltration. J Surg Res. 2015;196:130–135. doi: 10.1016/j.jss.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava SK, Bhardwaj A, Arora S, Tyagi N, Singh S, Andrews J, et al. MicroRNA-345 induces apoptosis in pancreatic cancer cells through potentiation of caspase-dependent and -independent pathways. Br J Cancer. 2015;113:660–668. doi: 10.1038/bjc.2015.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palanki R, Arora S, Tyagi N, Rusu L, Singh AP, Palanki S, et al. Size is an essential parameter in governing the UVB-protective efficacy of silver nanoparticles in human keratinocytes. BMC Cancer. 2015;15:636. doi: 10.1186/s12885-015-1644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arora S, Tyagi N, Bhardwaj A, Rusu L, Palanki R, Vig K, et al. Silver nanoparticles protect human keratinocytes against UVB radiation-induced DNA damage and apoptosis: potential for prevention of skin carcinogenesis. Nanomedicine. 2015;11:1265–1275. doi: 10.1016/j.nano.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srivastava SK, Bhardwaj A, Arora S, Tyagi N, Singh AP, Carter JE, et al. Interleukin-8 is a key mediator of FKBP51-induced melanoma growth, angiogenesis and metastasis. Br J Cancer. 2015;112:1772–1781. doi: 10.1038/bjc.2015.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deshmukh SK, Srivastava SK, Bhardwaj A, Singh AP, Tyagi N, Marimuthu S, et al. Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation. Oncotarget. 2015;6:11231–11241. doi: 10.18632/oncotarget.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyagi N, Bhardwaj A, Srivastava SK, Arora S, Marimuthu S, Deshmukh SK, et al. Development and characterization of a novel in vitro progression model for UVB-induced skin carcinogenesis. Sci Rep. 2015;5:13894. doi: 10.1038/srep13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, Murter C, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panni RZ, Sanford DE, Belt BA, Mitchem JB, Worley LA, Goetz BD, et al. Tumor-induced STAT3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer Immunol Immunother. 2014;63:513–528. doi: 10.1007/s00262-014-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scholz A, Heinze S, Detjen KM, Peters M, Welzel M, Hauff P, et al. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology. 2003;125:891–905. doi: 10.1016/s0016-5085(03)01064-3. [DOI] [PubMed] [Google Scholar]
- 36.Ohara Y, Oda T, Sugano M, Hashimoto S, Enomoto T, Yamada K, et al. Histological and prognostic importance of CD44(+)/CD24(+)/EpCAM(+) expression in clinical pancreatic cancer. Cancer Sci. 2013;104:1127–1134. doi: 10.1111/cas.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 38.Evans PM, Liu C. Roles of Krupel-like factor 4 in normal homeostasis, cancer and stem cells. Acta Biochim Biophys Sin (Shanghai) 2008;40:554–564. doi: 10.1111/j.1745-7270.2008.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu K, Lin B, Zhao M, Yang X, Chen M, Gao A, et al. The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cell Signal. 2013;25:1264–1271. doi: 10.1016/j.cellsig.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma I, Allan AL. The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Rev. 2011;7:292–306. doi: 10.1007/s12015-010-9208-4. [DOI] [PubMed] [Google Scholar]
- 41.Rao CV, Mohammed A. New insights into pancreatic cancer stem cells. World J Stem Cells. 2015;7:547–555. doi: 10.4252/wjsc.v7.i3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D, Lu P, Zhang H, Luo M, Zhang X, Wei X, et al. Oct-4 and Nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patients. Oncotarget. 2014;5:10803–10815. doi: 10.18632/oncotarget.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei D, Wang L, Kanai M, Jia Z, Le X, Li Q, et al. KLF4alpha up-regulation promotes cell cycle progression and reduces survival time of patients with pancreatic cancer. Gastroenterology. 2010;139:2135–2145. doi: 10.1053/j.gastro.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen J, Park JY, Park KH, Chung HW, Bang S, Park SW, et al. Oct4 and Nanog expression is associated with early stages of pancreatic carcinogenesis. Pancreas. 2010;39:622–626. doi: 10.1097/MPA.0b013e3181c75f5e. [DOI] [PubMed] [Google Scholar]
- 45.Lu Y, Zhu H, Shan H, Lu J, Chang X, Li X, et al. Knockdown of Oct4 and Nanog expression inhibits the stemness of pancreatic cancer cells. Cancer Lett. 2013;340:113–123. doi: 10.1016/j.canlet.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Herreros-Villanueva M, Zhang JS, Koenig A, Abel EV, Smyrk TC, Bamlet WR, et al. SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis. 2013;2:e61. doi: 10.1038/oncsis.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izumiya M, Kabashima A, Higuchi H, Igarashi T, Sakai G, Iizuka H, et al. Chemoresistance is associated with cancer stem cell-like properties and epithelial-to-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2012;32:3847–3853. [PubMed] [Google Scholar]
- 48.Hong SP, Wen J, Bang S, Park S, Song SY. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125:2323–2331. doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 49.Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2:3. doi: 10.1186/2001-1326-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konopleva M, Zhao S, Hu W, Jiang S, Snell V, Weidner D, et al. The anti-apoptotic genes Bcl-X(L) and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br J Haematol. 2002;118:521–534. doi: 10.1046/j.1365-2141.2002.03637.x. [DOI] [PubMed] [Google Scholar]
- 51.Madjd Z, Mehrjerdi AZ, Sharifi AM, Molanaei S, Shahzadi SZ, Asadi-Lari M. CD44+ cancer cells express higher levels of the anti-apoptotic protein Bcl-2 in breast tumours. Cancer Immun. 2009;9:4. [PMC free article] [PubMed] [Google Scholar]
- 52.Bhardwaj A, Srivastava SK, Singh S, Arora S, Tyagi N, Andrews J, et al. CXCL12/CXCR4 signaling counteracts docetaxel-induced microtubule stabilization via p21-activated kinase 4-dependent activation of LIM domain kinase 1. Oncotarget. 2014;5:11490–11500. doi: 10.18632/oncotarget.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu X, Feng J, Zeng D, Ding Y, Yu C, Yang B. PAK4 confers cisplatin resistance in gastric cancer cells via PI3K/Akt- and MEK/Erk-dependent pathways. Biosci Rep. 2014;34(2):e00094. doi: 10.1042/BSR20130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toyonaga T, Nakano K, Nagano M, Zhao G, Yamaguchi K, Kuroki S, et al. Blockade of constitutively activated Janus kinase/signal transducer and activator of transcription-3 pathway inhibits growth of human pancreatic cancer. Cancer Lett. 2003;201:107–116. doi: 10.1016/s0304-3835(03)00482-8. [DOI] [PubMed] [Google Scholar]
- 55.Fofaria NM, Srivastava SK. STAT3 induces anoikis resistance, promotes cell invasion and metastatic potential in pancreatic cancer cells. Carcinogenesis. 2015;36:142–150. doi: 10.1093/carcin/bgu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiu Z, Huang C, Sun J, Qiu W, Zhang J, Li H, et al. RNA interference-mediated signal transducers and activators of transcription 3 gene silencing inhibits invasion and metastasis of human pancreatic cancer cells. Cancer Sci. 2007;98:1099–1106. doi: 10.1111/j.1349-7006.2007.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 58.Lin L, Fuchs J, Li C, Olson V, Bekaii-Saab T, Lin J. STAT3 signaling pathway is necessary for cell survival and tumorsphere forming capacity in ALDH(+)/CD133(+) stem cell-like human colon cancer cells. Biochem Biophys Res Commun. 2011;416:246–251. doi: 10.1016/j.bbrc.2011.10.112. [DOI] [PubMed] [Google Scholar]
- 59.Lin L, Hutzen B, Lee HF, Peng Z, Wang W, Zhao C, et al. Evaluation of STAT3 signaling in ALDH+ and ALDH+/CD44+/CD24− subpopulations of breast cancer cells. PLoS ONE. 2013;8:e82821. doi: 10.1371/journal.pone.0082821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yin X, Zhang BH, Zheng SS, Gao DM, Qiu SJ, Wu WZ, et al. Coexpression of gene Oct4 and Nanog initiates stem cell characteristics in hepatocellular carcinoma and promotes epithelial-mesenchymal transition through activation of Stat3/Snail signaling. J Hematol Oncol. 2015;8:23. doi: 10.1186/s\-015-0119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guha P, Bandyopadhyaya G, Polumuri SK, Chumsri S, Gade P, Kalvakolanu DV, et al. Nicotine promotes apoptosis resistance of breast cancer cells and enrichment of side population cells with cancer stem cell-like properties via a signaling cascade involving galectin-3, alpha9 nicotinic acetylcholine receptor and STAT3. Breast Cancer Res Treat. 2014;145:5–22. doi: 10.1007/s10549-014-2912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang J, Li Z, Yu C, Chen M, Tian S, Sun C. MiR-1181 inhibits stem cell-like phenotypes and suppresses SOX2 and STAT3 in human pancreatic cancer. Cancer Lett. 2015;356:962–970. doi: 10.1016/j.canlet.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Kim SY, Kang JW, Song X, Kim BK, Yoo YD, Kwon YT, et al. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signal. 2013;25:961–969. doi: 10.1016/j.cellsig.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.