Abstract
Significant progress in life-supporting kidney xenograft survival in nonhuman primates (NHPs) has been associated largely with the increasing availability of pigs with genetic modifications that protect the pig tissues from the primate immune response and/or correct molecular incompatibilities between pig and primate. Blockade of the CD40/CD154 costimulation pathway with anti-CD154 mAb therapy has contributed to prolongation of kidney xenograft survival, although this agent may not be clinically available. An anti-CD40 mAb-based regimen is proving equally successful, but blockade of the CD28/B7 pathway is inadequate. Severe proteinuria were uniformly documented in the early studies of pig kidney xenotransplantation, but whether this resulted from immune injury or from physiological incompatibilities between the species, or both, remained uncertain. Recent experiments suggest it was related to a continuing immune response. Before 2014, the longest survival of a pig kidney graft in a NHP was 90 days, though graft survival >30 days was unusual. Recently this has been extended to >125 days, without features of a consumptive coagulopathy or a protein-losing nephropathy. In conclusion, overcoming the immune, coagulation, and inflammatory responses by the development of precise genetic modifications in donor pigs, along with effective immunosuppressive and anticoagulant/anti-inflammatory therapy is advancing the field towards clinical trials.
Introduction
Despite advances in medicine and technology, and increased awareness of organ donation and transplantation, the gap between supply and demand continues to widen. There are currently, 109,533 people waiting for kidney transplants in the U.S (as of June 5, 2015). Although 17,106 kidney transplants took place in 2014, 4,589 patients died while waiting for a kidney. Xenotransplantation offers the possibility of overcoming the chronic shortage of deceased human donors.
History of kidney xenotransplantation
The first clinical organ xenotransplants were of pig and sheep kidneys to patients with renal failure, but all failed rapidly (Table1), resulting in no further reports. However, several attempts were made to provide humans with organs from closely-related species [1]. In the 1960s, Reemtsma transplanted chimpanzee kidneys into 13 patients, with survival ranging from 11 days to 2 months, except for one patient who survived for almost 9 months. The majority of deaths were related to rejection or infection. During the same period, Starzl performed six baboon kidney xenotransplants in patients with end-stage renal failure, with survival ranging from 19 to 60 days.
Table 1.
Early clinical attempts to transplant kidneys from non-primate mammals
| Surgeon | Year | Donor species | Patient survival (days) |
|---|---|---|---|
| Princeteau | 1905 | Rabbit (slices) | 16 |
| Jaboulay | 1906 | Pig / Goat | 3 |
| Neuhof | 1923 | Sheep | 9 |
| Kuss | 1966 | Pig | 2 |
Subsequently, efforts were directed to clinical allotransplantation, with more successful results.
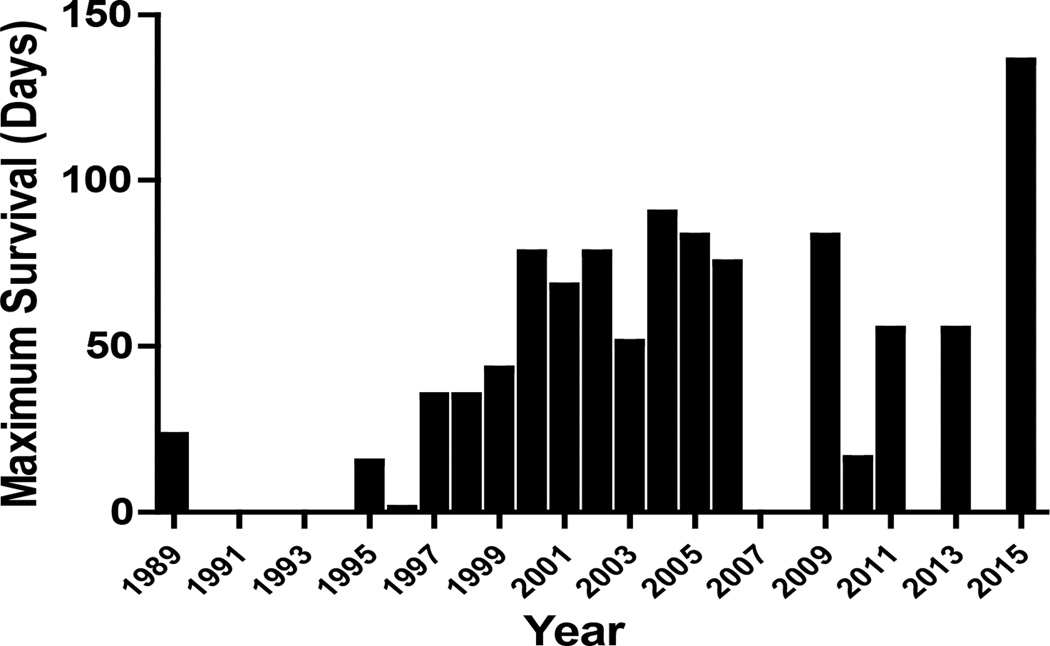
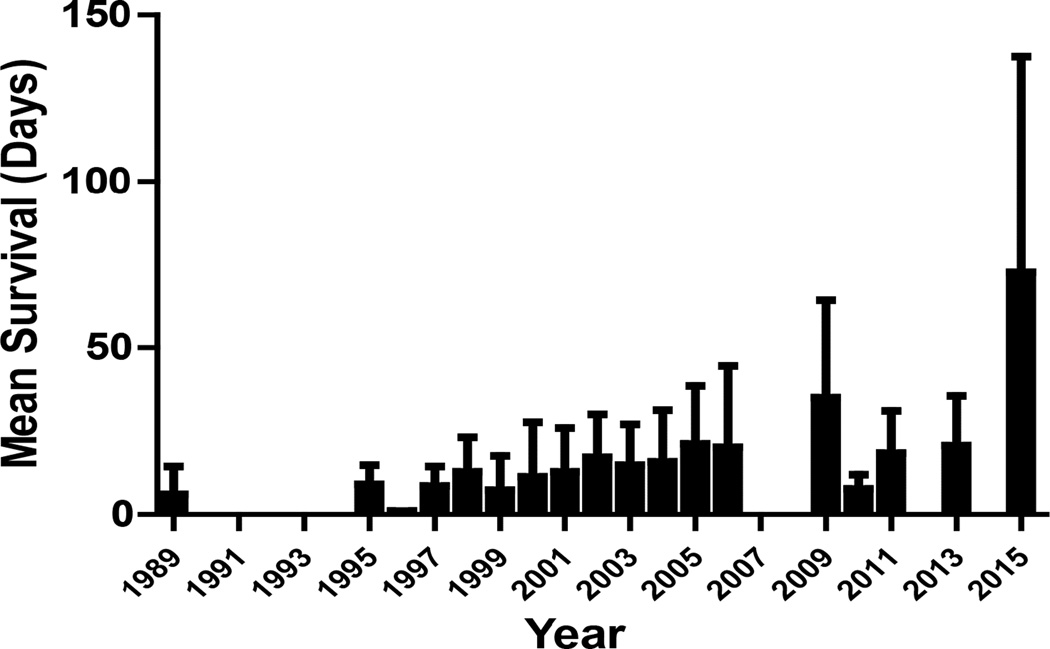
However, since human organs remained scarce because of increasing demand, researchers turned again to the possibility of xenotransplantation. For a number of reasons, the pig has been identified as the most likely potential source of organs for humans. The pig-to-nonhuman primate (NHP) model was introduced into xenotransplantation research in the 1980s and has become the standard experimental model [2–4]. Since then, in studies of pig kidney xenotransplantation in NHPs, graft survival has increased significantly [5,6] (Figures 1 and 2). This has been associated largely with the increasing availability of pigs with genetic modifications that protect the pig tissues from the primate immune response and correct molecular incompatibilities between pig and primate [7–9].
Figure 1.
Maximum pig kidney graft survival in NHPs (worldwide data)
Figure 2.
Mean pig kidney graft survival in NHPs (worldwide data)
Recent progress in kidney xenotransplantation
Owing to the availability of genetically-engineered donor pigs, e.g., α1,3-galactosyltransferase gene-knockout (GTKO) pigs [7,10] expressing one or more human complement-regulatory proteins (hCRPs), e.g., CD46, CD55 [hDAF], or CD59) [11–15], the transplantation of pig organs into NHPs is now no longer limited by antibody-mediated (hyperacute or acute vascular) rejection. In 1989, using wild-type (genetically-unmodified) pig kidneys, the longest life-supporting kidney graft survival was 23 days [4,5] (Figure 1). By 2004, CD55 kidney graft survival had been extended to 90 days [16]. With cotransplantation of donor-specific thymic tissue, GTKO pig kidney graft survival reached 83 days in 2005 [10]. In 2015, two groups reported survival of GTKO/hCRP pig kidneys longer than 125 days [17,18].
With the Gal problem resolved, other issues became more prominent, in particular dysregulated coagulation, such as the development of thrombotic microangiopathy in the grafts [19] and/or systemic consumptive coagulopathy in the recipient [12,20], which can be fatal once established, and appears to develop more rapidly when the kidney is transplanted than the heart [15,21]. Although the cause of coagulation dysfunction is yet to be fully understood, it is likely that several factors contribute to excessive coagulation.
First, the vascular endothelial cells of the graft are activated by antibody, complement, and interaction with recipient immune cells and platelets, and consequently express tissue factor, the primary physiological initiator of coagulation [22]. Second, it has been proposed that recipient tissue factor is expressed on platelets and monocytes following their activation by inflammation in the presence of xenograft endothelium [23]. Third, pig endothelial cells express direct prothrombinase (fgl2) induced by proinflammatory cytokines that converts human prothrombin to thrombin in a tissue factor-independent manner [24]. Fourth, the regulatory mechanisms of the coagulation-anticoagulation system are compromised by molecular incompatibilities between pig and primate [25,26]. Finally, endothelial cell activation in the graft leads to the downregulation or shedding of anticoagulant molecules [27–29].
Attempts are being made to overcome coagulation dysfunction by the introduction into GTKO/hCRP pigs of human coagulation-regulatory genes, e.g., thrombomodulin [TBM], endothelial protein C receptor [EPCR], tissue factor pathway inhibitor [TFPI], CD39 [30,31]. Various combinations of genetically-engineered pigs are now available. Treatment with anticoagulant and/or anti-platelet agents may provide further benefit [32]. Administration of recombinant human antithrombin was shown to be protective in the first week after pig-to-baboon kidney xenotransplantation [33], but had no apparent long-term benefit in the pig-to-macaque renal model [34], even when combined with human activated protein C [35]. It would be reasonable to conclude that both genetic modification of the pig and pharmacotherapy may be necessary to fully correct coagulation dysfunction in kidney xenotransplantation.
Coagulation, inflammation, and the immune response have a complex inter-relationship that needs further investigation [36]. Further genetic modifications may protect the graft from the recipient’s inflammatory response (e.g., expression of human A20 and/or hemeoxygenase-1 [37,38]), or from the immune response (e.g., expression of a pig dominant-negative MHC class II transactivator [39,40]. There may also be a need for treatment with anti-inflammatory agents, e.g., anti-cytokine agents (anti-TNF-α, anti-IL-6R) and/or statins [41,42].
Improved immunosuppressive regimens have contributed to progress in pig kidney transplantation in NHPs. Initially, induction therapy with cyclophosphamide (although this agent is rarely used today) and a combination of conventional maintenance immunosuppressive agents (e.g., cyclosporine, mycophenolate mofetil [MMF], and corticosteroids) proved successful in prolonging kidney xenograft survival [43]. There has been increasing interest in costimulation blockade, introduced into xenotransplantation by Buhler et al [44], using agents that inhibit the CD28/B7 and/or CD40/CD154 pathways.
Anti-CD154 monoclonal antibody (mAb) therapy contributed significantly to prolongation of GTKO renal xenograft survival [10], but is unlikely to be available for clinical use because of its thrombogenic properties [21,45]. Attention is now being directed toward alternative costimulation blockade agents, such as anti-CTLA4-Ig and anti-CD40 mAb. Recent studies suggest that an anti-CD40 mAb-based regimen is likely to be successful [46], but blockade of the CD28/B7 pathway alone would appear to be inadequate [17,40]. Overall, the current experimental data suggest that costimulation blockade may be the preferred immunosuppressive therapy.
Attention has now shifted from Gal to other carbohydrate potential targets that are expressed on pig cells, particularly N-glycolylneuraminic acid (NeuGc) [47]. In 2013, the first NeuGcKO pigs became available [48]. Although the expression of NeuGc on pig vascular endothelial cells will play no part in rejection in NHP models (as Old World NHPs also express NeuGc), it will be important in clinical xenotransplantation (as humans do not express NeuGc and therefore produce anti-NeuGc antibodies).
Physiological barriers
Although pig kidneys are remarkably similar in structure and relative size to human kidneys, there are physiological discrepancies between pig and primate that were also initially found to be problematic [12,49,50], though whether these were secondary to immune activation in the graft remains uncertain.
In humans, uric acid is produced as an end-product of purine metabolism, but in lower mammals it is further oxidized by urate oxidase [51]. Although humans cannot oxidize uric acid, pig kidneys can eliminate uric acid so hyperuricemia should not be a major problem [52].
With regard to the renin-angiotensin-aldosterone system, pig renin has been shown to be unable to cleave human angiotensinogen [50]. However, as NHPs with a well-functioning pig kidney graft have maintained normal fluid balance and body weight for several months, there is possibly an alternative regulatory mechanism that can maintain fluid balance despite decreased renin activity.
Anemia was originally reported and was attributed to a molecular incompatibility of pig erythropoietin with the primate Epo receptor [49]. However, in recent experience, it may have been associated with drugs -induced myelosuppression (e.g., cyclophosphamide) and/or frequent blood sampling. Furthermore, since erythropoietin is clinically beneficial, it does not seem to be so problematic.
Although most serum electrolytes (e.g., sodium, chloride, potassium) remained within the normal range after pig kidney transplantation in NHPs, phosphate levels progressively decreased during the period of stable xenograft function [49]. The cause of hypophosphatemia was not established. Furthermore, hypoalbuminemia and moderate-to-severe proteinuria was uniformly documented, necessitating frequent infusions of human albumin [10,49], although whether this phenomenon was due to the immune response (activation or injury of glomerular and/or tubular cells) or to an inherent physiological incompatibility between pigs and primates, or both, was uncertain.
Recently, two groups reported minimal proteinuria with no accompanying hypoalbuminemia in NHPs with pig kidney grafts, probably because the immune response had been more fully controlled by the genetic modification of the donor pig and/or pharmacological intervention [17,18]. This was associated with graft survival for >125 days in both studies. In another study of pig to NHP kidney transplantation, treatment with rituximab successfully delayed the development of proteinuria, possibly due to prevention of pig podocyte disruption [53].
Higginbotham et al. exhibited prolonged kidney recipient survival following the transplantation of GTKO/CD55 pig kidneys in rhesus monkeys selected for their low titers of anti-pig antibodies [17]. The immunosuppressive regimen included T cell depletion followed by maintenance therapy with anti-CD154 mAb, MMF, and corticosteroids. In two monkeys, there was no evidence of rejection or other pathology on renal biopsies on day 100. This group also reported that a recipient with a high titer of anti-pig antibody rejected the kidney graft within the first week, and two low-titer monkeys treated with belatacept rejected their grafts within three weeks.
A kidney graft from a GTKO/CD46/CD55/TBM/EPCR/CD39 pig (though TBM and CD39 were very poorly expressed in the kidney) in a baboon (with very high titers of anti-pig antibodies) functioned for 136 days [18]. Immunosuppressive therapy included induction with ATG, anti-CD20mAb, and cobra venom factor, and maintenance with anti CD40mAb, rapamycin, and corticosteroids. In addition, anti-TNF-α and anti-IL-6R therapy was administered. Serum creatinine was generally stable (0.6–1.6mg/dL) and there were no features of a consumptive coagulopathy or a protein-losing nephropathy. Histology of a biopsy on day 103 was normal, but by day 136 the kidney showed features of glomerular enlargement, thrombi, and mesangial expansion. The combination of (i) a graft from a specific genetically-engineered pig, (ii) an effective immunosuppressive regimen, and (iii) anti-inflammatory therapy appeared to prevent immune injury and a protein-losing nephropathy, and delayed coagulation dysfunction.
These outcomes provide encouragement for further studies and potential clinical translation.
Comment
The consistent demonstration of life-supporting renal function for at least 6 months in the pig-to-NHP model, with a clinically-applicable immunosuppressive regimen, without evidence of infectious or other complications would be sufficient to consider a clinical trial. The most appropriate patients for an initial trial might include those highly allo-sensitized with broadly reactive alloantibodies who are unlikely to receive a human kidney graft. In vitro studies indicate that these patients would be at no greater risk of rejecting a pig kidney graft than others [54–56]. In addition, patients who no longer have access sites to allow dialysis might be considered. (Present evidence, though very limited, suggests that rejection of a pig kidney would not increase the patient’s sensitization to a potential kidney allograft [54,57,58]. We suggest that the potential benefit to the patient, and the relatively low risk of the procedure, would make such a limited trial ethical.
In summary, recent progress in the transplantation of kidneys from genetically-engineered pigs into NHPs has indicated that clinical trials of pig kidney xenotransplantation may be possible within the next few years. To date, no safety concerns that would definitively prohibit such clinical trials have been identified.
HIGHLIGHTS.
History of kidney xenotransplantation
Progress in pig-to-nonhuman primate kidney xenotransplantation
Life-supporting pig kidney survival in nonhuman primates up to 136 days
Development of transgenic pigs, immunosuppressive and anti-inflammatory therapy
Vision for future clinical kidney xenotransplantation trials
Acknowledgments
Funding:
Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh has been supported in part by NIH grants #U19 AI090959, #U01 AI068642, and # R21 A1074844, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA. The baboon used in the study was from the Oklahoma University Health Sciences Center, Division of Animal Resources, which is supported by NIH P40 sponsored grant RR012317-09.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
none
Ethical approval:
None
Research Registration Unique Identifying Number (UIN):
None required for the review.
Trial registry number – ISRCTN:
None
Author contribution:
HI, TK – all participated in review of the literature, writing of the manuscript, and final approval of the manuscript.
Guarantor:
Hayato Iwase, MD, PhD, and Takaaki Kobayashi, MD, PhD.
References
- 1.Cooper DK. A brief history of cross-species organ transplantation. Proc (Bayl Univ Med Cent) 2012;25:49–57. doi: 10.1080/08998280.2012.11928783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lexer G, Cooper DK, Rose AG, Wicomb WN, Rees J, Keraan M, et al. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J Heart Transplant. 1986;5:411–418. [PubMed] [Google Scholar]
- 3.Cooper DK, Human PA, Lexer G, Rose AG, Rees J, Keraan M, et al. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant. 1988;7:238–246. [PubMed] [Google Scholar]
- 4.Alexandre GPJ, Gianello P, Latinne D, Carlier M, Dewaele A, van Obbergh L. Plasmapheresis and splenectomy in experimental renal xenotransplantation. In: Hardy M, editor. Xenograft. Vol. 25. Amsterdam, New York, Oxford: Excerpta Medica; 1989. pp. 259–266. [Google Scholar]
- 5.Lambrigts D, Sachs DH, Cooper DK. Discordant organ xenotransplantation in primates: world experience and current status. Transplantation. 1998;66:547–561. doi: 10.1097/00007890-199809150-00001. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DK, Satyananda V, Ekser B, Van der Windt DJ, Hara H, Ezzelarab M, et al. Progress in pig-to-nonhuman primate transplantation models (1998–2013): a comprehensive review of the literature. Xenotransplantation. 2014;21:397–419. doi: 10.1111/xen.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierson RN, 3rd, Dorling A, Ayares D, Rees MA, Seebach JD, Fishman JA, et al. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16:263–280. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yazaki S, Iwamoto M, Onishi A, Miwa Y, Hashimoto M, Oishi T, et al. Production of cloned pigs expressing human thrombomodulin in endothelial cells. Xenotransplantation. 2012;19:82–91. doi: 10.1111/j.1399-3089.2012.00696.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 11.Cozzi E, White DJ. Xenotransplantation. Curr Opin Nephrol Hypertens. 1996;5:514–518. doi: 10.1097/00041552-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Cowan PJ, Aminian A, Barlow H, Brown AA, Chen CG, Fisicaro N, et al. Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in non-immunosuppressed baboons. Transplantation. 2000;69:2504–2515. doi: 10.1097/00007890-200006270-00008. [DOI] [PubMed] [Google Scholar]
- 13.Loveland BE, Milland J, Kyriakou P, Thorley BR, Christiansen D, Lanteri MB, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation. 2004;11:171–183. doi: 10.1046/j.1399-3089.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 14.Menoret S, Plat M, Blancho G, Martinat-Botte F, Bernard P, Karam G, et al. Characterization of human CD55 and CD59 transgenic pigs and kidney xenotransplantation in the pig-to-baboon combination. Transplantation. 2004;77:1468–1471. doi: 10.1097/01.tp.0000111758.35048.ea. [DOI] [PubMed] [Google Scholar]
- 15.Lin CC, Ezzelarab M, Shapiro R, Ekser B, Long C, Hara H, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10:1556–1568. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldan N, Rigotti P, Calabrese F, Cadrobbi R, Dedja A, Iacopetti I, et al. Ureteral stenosis in HDAF pig-to-primate renal xenotransplantation: a phenomenon related to immunological events? Am J Transplant. 2004;4:475–481. doi: 10.1111/j.1600-6143.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- 17.Higginbotham L, Mathews D, Breeden CA, Song M, Farris AB, 3rd, Larsen CP, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22:221–230. doi: 10.1111/xen.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwase H, Liu H, Wijkstrom M, Zhou H, Singh J, Hara H, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015 doi: 10.1111/xen.12174. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu A, Yamada K, Yamamoto S, Lavelle JM, Barth RN, Robson SC, et al. Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenografts. J Am Soc Nephrol. 2005;16:2732–2745. doi: 10.1681/ASN.2004121148. [DOI] [PubMed] [Google Scholar]
- 20.Ierino FL, Kozlowski T, Siegel JB, Shimizu A, Colvin RB, Banerjee PT, et al. Disseminated intravascular coagulation in association with the delayed rejection of pig-to-baboon renal xenografts. Transplantation. 1998;66:1439–1450. doi: 10.1097/00007890-199812150-00006. [DOI] [PubMed] [Google Scholar]
- 21.Knosalla C, Yazawa K, Behdad A, Bodyak N, Shang H, Buhler L, et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am J Transplant. 2009;9:1006–1016. doi: 10.1111/j.1600-6143.2009.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gollackner B, Goh SK, Qawi I, Buhler L, Knosalla C, Daniel S, et al. Acute vascular rejection of xenografts: roles of natural and elicited xenoreactive antibodies in activation of vascular endothelial cells and induction of procoagulant activity. Transplantation. 2004;77:1735–1741. doi: 10.1097/01.tp.0000131167.21930.b8. [DOI] [PubMed] [Google Scholar]
- 23.Lin CC, Chen D, McVey JH, Cooper DK, Dorling A. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008;86:702–709. doi: 10.1097/TP.0b013e31818410a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghanekar A, Mendicino M, Liu H, He W, Liu M, Zhong R, et al. Endothelial induction of fgl2 contributes to thrombosis during acute vascular xenograft rejection. J Immunol. 2004;172:5693–5701. doi: 10.4049/jimmunol.172.9.5693. [DOI] [PubMed] [Google Scholar]
- 25.Schulte Am Esch J, 2nd, Robson SC, Knoefel WT, Hosch SB, Rogiers X. O-linked glycosylation and functional incompatibility of porcine von Willebrand factor for human platelet GPIb receptors. Xenotransplantation. 2005;12:30–37. doi: 10.1111/j.1399-3089.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 26.Cowan PJ, Robson SC, d'Apice AJ. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant. 2011;16:214–221. doi: 10.1097/MOT.0b013e3283446c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platt JL, Vercellotti GM, Lindman BJ, Oegema TR, Jr, Bach FH, Dalmasso AP. Release of heparan sulfate from endothelial cells. Implications for pathogenesis of hyperacute rejection. J Exp Med. 1990;171:1363–1368. doi: 10.1084/jem.171.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robson SC, Kaczmarek E, Siegel JB, Candinas D, Koziak K, Millan M, et al. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med. 1997;185:153–163. doi: 10.1084/jem.185.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menschikowski M, Hagelgans A, Eisenhofer G, Siegert G. Regulation of endothelial protein C receptor shedding by cytokines is mediated through differential activation of MAP kinase signaling pathways. Exp Cell Res. 2009;315:2673–2682. doi: 10.1016/j.yexcr.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Miwa Y, Yamamoto K, Onishi A, Iwamoto M, Yazaki S, Haneda M, et al. Potential value of human thrombomodulin and DAF expression for coagulation control in pig-to-human xenotransplantation. Xenotransplantation. 2010;17:26–37. doi: 10.1111/j.1399-3089.2009.00555.x. [DOI] [PubMed] [Google Scholar]
- 31.Ekser B, Ezzelarab M, Hara H, van der Windt DJ, Wijkstrom M, Bottino R, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 32.Iwase H, Ezzelarab MB, Ekser B, Cooper DK. The role of platelets in coagulation dysfunction in xenotransplantation, and therapeutic options. Xenotransplantation. 2014;21:201–220. doi: 10.1111/xen.12085. [DOI] [PubMed] [Google Scholar]
- 33.Cowan PJ, Aminian A, Barlow H, Brown AA, Dwyer K, Filshie RJ, et al. Protective effects of recombinant human antithrombin III in pig-to-primate renal xenotransplantation. Am J Transplant. 2002;2:520–525. doi: 10.1034/j.1600-6143.2002.20605.x. [DOI] [PubMed] [Google Scholar]
- 34.Cozzi E, Simioni P, Boldrin M, Seveso M, Calabrese F, Baldan N, et al. Effects of long-term administration of high-dose recombinant human antithrombin in immunosuppressed primate recipients of porcine xenografts. Transplantation. 2005;80:1501–1510. doi: 10.1097/01.tp.0000178377.55615.8b. [DOI] [PubMed] [Google Scholar]
- 35.Simioni P, Boldrin M, Gavasso S, Seveso M, Radu C, Bulato C, et al. Effects of long-term administration of recombinant human protein C in xenografted primates. Transplantation. 2011;91:161–168. doi: 10.1097/TP.0b013e318200ba0e. [DOI] [PubMed] [Google Scholar]
- 36.Esmon CT, Xu J, Lupu F. Innate immunity and coagulation. J Thromb Haemost. 2011;9(Suppl 1):182–188. doi: 10.1111/j.1538-7836.2011.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oropeza M, Petersen B, Carnwath JW, Lucas-Hahn A, Lemme E, Hassel P, et al. Transgenic expression of the human A20 gene in cloned pigs provides protection against apoptotic and inflammatory stimuli. Xenotransplantation. 2009;16:522–534. doi: 10.1111/j.1399-3089.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 38.Petersen B, Lucas-Hahn A, Lemme E, Queisser AL, Oropeza M, Herrmann D, et al. Generation and characterization of pigs transgenic for human hemeoxygenase-1 (hHO-1) Xenotransplantation. 2010;17:102–103. [Google Scholar]
- 39.Hara H, Witt W, Crossley T, Long C, Isse K, Fan L, et al. Human dominant-negative class II transactivator transgenic pigs - effect on the human anti-pig T-cell immune response and immune status. Immunology. 2013;140:39–46. doi: 10.1111/imm.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwase H, Satyananda V, Zhou H, Hara H, Bajona P, Wijkstrom M, et al. Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs. Transpl Immunol. 2015;32:99–108. doi: 10.1016/j.trim.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ezzelarab M, Cooper DK. The potential of statins in xenotransplantation. Xenotransplantation. 2007;14:100–103. doi: 10.1111/j.1399-3089.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 42.Iwase H, Ekser B, Zhou H, Liu H, Satyananda V, Humar R, et al. Further evidence for a sustained systemic inflammatory response in xenograft recipients (SIXR) Submitted. 2015 doi: 10.1111/xen.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cozzi E, Bhatti F, Schmoeckel M, Chavez G, Smith KG, Zaidi A, et al. Long-term survival of nonhuman primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation. 2000;70:15–21. [PubMed] [Google Scholar]
- 44.Buhler L, Awwad M, Basker M, Gojo S, Watts A, Treter S, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69:2296–2304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 45.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 46.Iwase H, Ekser B, Satyananda V, Bhama JK, Hara H, Ezzelarab M, et al. Pig-to-baboon heart transplantation - first experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation. 2015;22:211–220. doi: 10.1111/xen.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miwa Y, Kobayashi T, Nagasaka T, Liu D, Yu M, Yokoyama I, et al. Are N-glycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation? Xenotransplantation. 2004;11:247–253. doi: 10.1111/j.1399-3089.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 48.Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 49.Soin B, Smith KG, Zaidi A, Cozzi E, Bradley JR, Ostlie DJ, et al. Physiological aspects of pig-to-primate renal xenotransplantation. Kidney Int. 2001;60:1592–1597. doi: 10.1046/j.1523-1755.2001.00973.x. [DOI] [PubMed] [Google Scholar]
- 50.Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DK. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006;13:488–499. doi: 10.1111/j.1399-3089.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 51.Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19:640–653. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- 52.Simmonds HA, Hatfield PJ, Cameron JS, Cadenhead A. Uric acid excretion by the pig kidney. Am J Physiol. 1976;230:1654–1661. doi: 10.1152/ajplegacy.1976.230.6.1654. [DOI] [PubMed] [Google Scholar]
- 53.Tasaki M, Shimizu A, Hanekamp I, Torabi R, Villani V, Yamada K. Rituximab treatment prevents the early development of proteinuria following pig-to-baboon xeno-kidney transplantation. J Am Soc Nephrol. 2014;25:737–744. doi: 10.1681/ASN.2013040363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper DK, Tseng YL, Saidman SL. Alloantibody and xenoantibody cross-reactivity in transplantation. Transplantation. 2004;77:1–5. doi: 10.1097/01.TP.0000105116.74032.63. [DOI] [PubMed] [Google Scholar]
- 55.Hara H, Ezzelarab M, Rood PP, Lin YJ, Busch J, Ibrahim Z, et al. Allosensitized humans are at no greater risk of humoral rejection of GT-KO pig organs than other humans. Xenotransplantation. 2006;13:357–365. doi: 10.1111/j.1399-3089.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 56.Wong BS, Yamada K, Okumi M, Weiner J, O'Malley PE, Tseng YL, et al. Allosensitization does not increase the risk of xenoreactivity to alpha1,3-galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation. 2006;82:314–319. doi: 10.1097/01.tp.0000228907.12073.0b. [DOI] [PubMed] [Google Scholar]
- 57.Ye Y, Luo Y, Kobayashi T, Taniguchi S, Li S, Niekrasz M, et al. Secondary organ allografting after a primary "bridging" xenotransplant. Transplantation. 1995;60:19–22. doi: 10.1097/00007890-199507150-00004. [DOI] [PubMed] [Google Scholar]
- 58.Baertschiger RM, Dor FJ, Prabharasuth D, Kuwaki K, Cooper DK. Absence of humoral and cellular alloreactivity in baboons sensitized to pig antigens. Xenotransplantation. 2004;11:27–32. doi: 10.1111/j.1399-3089.2004.00075.x. [DOI] [PubMed] [Google Scholar]