Abstract
Binding of natural anti-pig antibodies in humans and nonhuman primates to carbohydrate antigens expressed on the transplanted pig organ, the most important of which is galactose-α1,3-galactose (Gal), activate the complement cascade, which results in destruction of the graft within minutes or hours, known as hyperacute rejection. Even if antibody is removed from the recipient’s blood by plasmapheresis, recovery of antibody is associated with acute humoral xenograft rejection. If immunosuppressive therapy is inadequate, the development of high levels of T cell-dependent elicited anti-pig IgG similarly results in graft destruction, though classical acute cellular rejection is rarely seen. Vascular endothelial activation by low levels of anti-nonGal antibody, coupled with dysregulation of the coagulation-anticoagulation systems between pigs and primates, leads to a thrombotic microangiopathy in the graft that may be associated with a consumptive coagulopathy in the recipient. The most successful approach to overcoming these barriers is by genetically-engineering the pig to provide it with resistance to the human humoral and cellular immune responses and to correct the coagulation discrepancies between the two species. Organs and cells from pigs that (i) do not express the important Gal antigen, (ii) express a human complement-regulatory protein, and (iii) express a human coagulation-regulatory protein, when combined with an effective immunosuppressive regimen, have been associated with prolonged pig graft survival in nonhuman primates.
Keywords: Organ donation; Pig; Transplantation, islets; Transplantation, organs; Xenotransplantation
Organ xenotransplantation
When interest developed in using the pig as a potential organ source for humans, little was known about the immunology of pig-to-primate organ transplantation. Studies in the clinically-relevant (wild-type) pig-to-baboon model indicated that very rapid graft destruction occurred, usually within minutes [1–3].
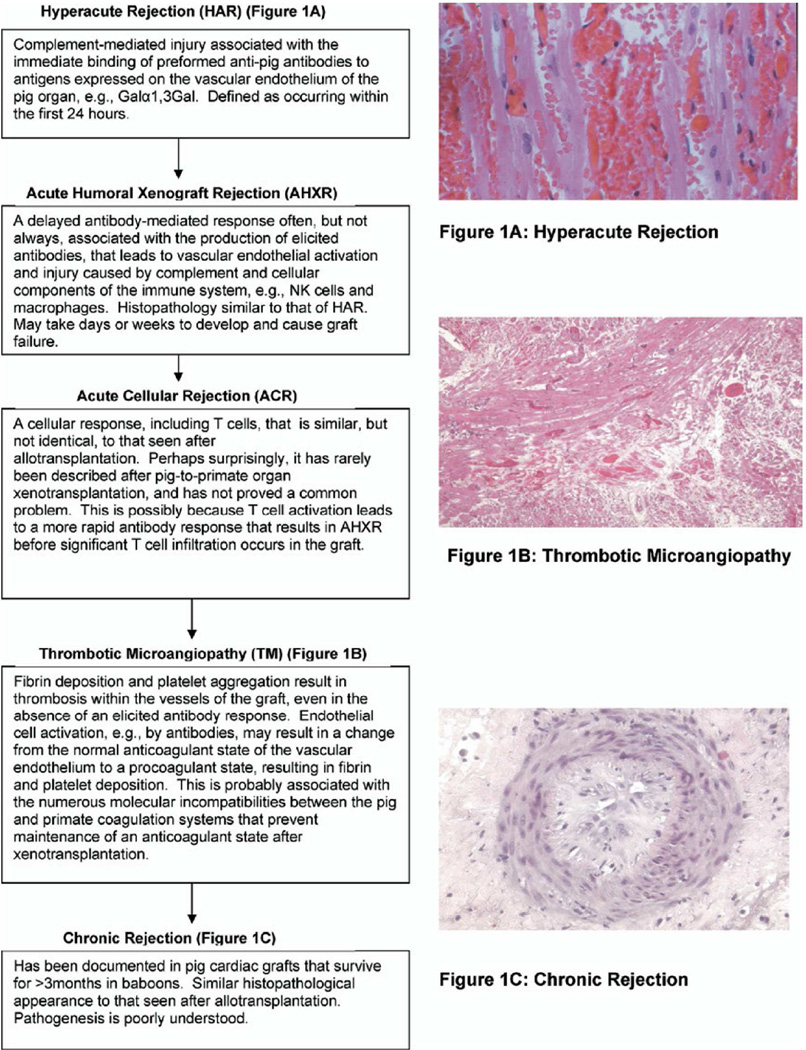
Hyperacute rejection
When a pig organ is transplanted into a human or nonhuman primate, there is an immediate immune response, known as hyperacute rejection (Figure 1A). This has been defined as destruction of the graft within 24 hours, but it frequently occurs within the first hour, and is related to binding of primate natural (preformed) anti-pig antibodies to the graft vascular endothelial cells. Antibody deposition initiates complement-mediated injury of the endothelial lining, resulting in thrombosis, interstitial hemorrhage and edema that disrupts graft function [4,5].
Figure 1.
Summary of the known major immunologic barriers to pig-to-primate heart transplantation. (Reproduced with permission from Zhu X, et al, J Heart Lung Transplant 2007;26:210–218.)
It was subsequently determined that the most important antibodies (IgM and IgG) bind to a carbohydrate epitope, galactose-α1,3-galactose (Gal), expressed on the pig vascular endothelium (reviewed in [6]). This oligosaccharide is present in all other mammals, with the exception of humans and Old World nonhuman primates (e.g., great apes, baboons, Old World monkeys) (reviewed by [7]). These primate species lost expression of Gal several million years ago, probably from a genetic mutation, and the absence of Gal resulted in primates making antibodies against this now ‘foreign’ antigen.
These antibodies develop during neonatal life [8,9], and are almost certainly a response to Gal-expressing viruses and microorganisms that colonize the primate’s gastrointestinal tract [10]. These ‘natural’ or ‘preformed’ antibodies differ from ‘elicited’ antibodies that develop after direct exposure to an antigen, e.g., antibodies that develop after an organ transplant.
As the causative factors associated with hyperacute rejection of a xenograft were seen to be similar to those of ABO-incompatible allograft rejection [11], a similar approach was taken to prevent rejection by depleting the recipient of these anti-pig antibodies by plasmapheresis [3] or, later, by depleting specifically anti-Gal antibodies by immunoaffinity columns [12]. In addition, again based on experience with ABO-incompatibility studies, the intravenous infusion of natural or synthetic Gal oligosaccharides was tested, which were bound by anti-Gal antibody and then excreted [13,14]. Even when combined with conventional immunosuppressive therapy, these approaches were only partially successful; they delayed antibody-mediated rejection, but the graft was lost when antibody levels recovered.
An alternative or additional approach was to administer an agent that depleted or inhibited complement, e.g., cobra venom factor, which extended graft survival significantly [15,16], but again had only a temporary effect.
When genetic modification of the organ-source pig became possible, a different approach to overcoming hyperacute rejection was suggested by Dalmasso (in the USA) [17] and, independently, by White (in the UK) [18] and their respective colleagues. The cells of humans are to some extent protected from complement-mediated injury by the presence of complement-regulatory proteins on their surfaces, e.g., decay accelerating factor (DAF, CD55), or membrane cofactor protein (MCP, CD46). Although pig cells have equivalent complement-regulatory proteins, these are less able to provide protection from the effects of human complement. Dalmasso and White suggested introducing into the pig a transgene for a human complement-regulatory protein. In the mid-1990s, this was achieved by several groups, and represent the first genetically-engineered pigs directed towards xenotransplantation (reviewed by [19]).
When the importance of Gal had been established, it was suggested that the gene that produced the enzyme that attached Gal terminally on oligosaccharide chains, α1,3-galactosyltransferase, should be deleted or knocked-out [20]. The first α1,3-galactosyltransferase gene-knockout (GTKO) pig was not produced until 2003 [21,22]. Initial studies showed protection from hyperacute rejection [23,24].
Acute humoral xenograft rejection
Even when hyperacute rejection was prevented, a similar form of rejection occurred, generally within a few days or weeks - acute humoral xenograft rejection (AHXR). It is also related to the deposition of antibody and complement, which activate the endothelium [25], and the effect of graft infiltration by innate immune cells (e.g., polymorphonuclear leukocytes, macrophages, NK cells) that together destroy the graft. When a GTKO pig organ is transplanted, the antibodies involved are natural antibodies directed against nonGal antigens, the exact nature of which remains uncertain, although two have been identified (see below).
The combination of GTKO and a human complement-regulatory protein was even more successful in preventing early graft failure of a transplanted pig organ [26,27].
The adaptive immune response
If both hyperacute and AHXR are prevented, but immunosuppressive therapy is inadequate, a T-cell dependent elicited antibody response develops, resulting in high levels of anti-pig IgG [28]. Binding of these antibodies to the vascular endothelium initiates histopathological changes indistinguishable from AHXR. Surprisingly, acute cellular rejection, as seen in the majority of allotransplants, has virtually never been recorded after pig-to-nonhuman primate organ xenotransplantation. This is most likely because the humoral response is more rapid and overwhelms the cellular response, though T and B cells may well be seen in the graft.
Although high doses of conventional immunosuppressive therapy, e.g., tacrolimus, mycophenolate mofetil, and corticosteroids, have delayed graft failure [29,30], they have been associated with a relatively high incidence of infectious complications. A potentially more successful approach has been with the administration of agents that are directed to costimulation blockade, first introduced into xenotransplantation by Buhler in 2000 ([28]; reviewed in [31]).
Here again, genetic engineering of the organ-source pigs may prove beneficial (reviewed in [31]) Pigs transgenic for the T cell costimulation blockade agent, CTLA4-Ig, have been produced successfully [32], but, because of the high levels of soluble CTLA4-Ig produced, proved to immunocompromised, prohibiting their long-term survival. Subsequent efforts have been directed towards expressing CTLA4-Ig in only selected tissues, e.g., the vascular endothelium or the islets.
A second approach has been to express a mutant human Major Histocompatibility Complex (MHC) class II transactivator transgene in the pig. This mutation results in downregulation of swine leukocyte class II expression, and inhibits upregulation of expression after activation of the pig endothelial cells, thus reducing the human T cell response [33]. Recently, MHC class I-knockout pigs have been produced [34], and their effect as donors of xenografts is being investigated.
Coagulation dysfunction
Even when hyperacute rejection, AHXR, and the T cell response were successfully controlled, it became increasingly clear that abnormal coagulation within the vessels of the graft played a significant role in graft failure (reviewed by [35,36]). Coagulation dysregulation can result in the development of a thrombotic microangiopathy, in which the vasculature of the organ is steadily occluded by thrombus, resulting in ischemic necrosis of the tissues (Figure 1B) [37,38].
Small vessels in the graft become occluded by fibrin and platelet aggregation. When advanced, the loss of platelets and clotting factors in the graft may result in a consumptive coagulopathy in the recipient primate [39]. The initiating cause of the thrombotic microangiopathy almost certainly results from activation of the vascular endothelial cells by antibody, complement, and/or innate immune cells, changing the phenotype of the cells from an anticoagulant state to a procoagulant state. However, there are several known incompatibilities in the coagulation/anticoagulation factors between pig and primate that almost certainly contribute to coagulation dysregulation between graft and host [35,36]. Consumptive coagulopathy appears to develop much more rapidly in nonhuman primates with pig kidney grafts whereas a thrombotic microangiopathy predominates after pig heart transplantation, followed terminally by a consumptive coagulopathy [23,39,40].
Efforts were made to control this problem by the administration of anticoagulants and/or anti-thrombotic agents to the recipient nonhuman primate, with partial success [37], but evidence was presented indicating that increased immunosuppression was more effective than anticoagulant agents [41]. This problem is also steadily being overcome by further genetic manipulation of the organ source pig, e.g., by the insertion of an ‘anticoagulant’ or ‘anti-thrombotic’ gene, such as thrombomodulin, endothelial protein C receptor (EPCR), tissue factor pathway inhibitor (TFPI), or CD39 [35,36], resulting in extended graft survival [42].
Inflammatory response
Ezzelarab has presented evidence that an inflammatory response precedes the development of coagulation dysfunction, emphasizing the inter-relationships between the immune, coagulation and inflammatory responses [43]. In particular, the potential role of interleukin-6 (IL-6) has been highlighted [43,44], (and the beneficial effect of an IL-6R antagonist is being explored [44].
Graft vasculopathy (chronic rejection)
Chronic rejection (graft vasculopathy) has been documented in some pig cardiac xenografts that have functioned for >3 months in baboons, and has a similar histopathological appearance to that seen after allotransplantation (Figure 1C) [45]. Some encouragement, however, is provided by the ongoing studies of Mohiuddin et al in which graft vasculopathy has not been reported in heart grafts from GTKO/hCD46 pigs expressing human thrombomodulin, with follow-up in one case for more than two years [42].
Remaining barriers
The immunobiological problems associated with transplantation of pig livers [46] and lungs [47] are more complex, and to date have limited graft survival to days rather than weeks, months or years (reviewed in [48]). Humans are known to have natural antibodies to at least two other antigens on pig cells, namely N-glycolylneuraminic acid (NeuGc) (reviewed in [49]) and β1,4 N-acetylgalactosaminyltransferase [50]. As most nonhuman mammals express NeuGc, including Old World nonhuman primates (even chimpanzees), it is difficult to investigate its effect in in vivo experimental models, e.g., pig-to-baboon organ transplantation. Most information to date, therefore, has been obtained from staining of pig tissues for expression of NeuGc, and laboratory assays. There is increasing evidence that this antibody-antigen interaction will need to be prevented if a pig organ or cells are transplanted into a human patient. The recent production of pigs in which the gene responsible for producing NeuGc has been deleted (as well as that for Gal) is a major step towards this goal [51].
Cell and tissue xenotransplantation
Pigs could also act as a source of other tissues, such as pancreatic islets (for the treatment of diabetes), neuronal cells (for conditions such as Parkinson’s disease), corneas (for patients with corneal blindness), and red blood cells (for clinical transfusion). The immunobiology of graft injury is similar to that seen in pig organs, but a T cell-induced response appears to play a more important role, as in allografts.
Considerable attention has been paid to the transplantation of porcine islets of Langerhans as a means of providing a source of insulin in patients with Type 1 diabetes (reviewed in [52]). Techniques are available, e.g., by the use of an insulin-specific promoter, that enable a desired transgene to be expressed in the islets alone, thus negating any potential detrimental effects (e.g., systemic anticoagulation) that might result from widespread expression in the pig [53]. However, success has not yet been consistent, and further study is required.
The cellular immune response can be inhibited by currently-available immunosuppressive agents, particularly by those that result in blockade of T cell costimulation. Both wild-type and genetically-engineered adult porcine islets have been demonstrated to maintain normoglycemia in diabetic monkeys for periods in excess of 1 year [53–55]. Wild-type neonatal pig islets have also maintained normoglycemia in diabetic monkeys for >6 months [56,57].
Induction of tolerance to pig organs and cells
The ideal goal in both allotransplantation and xenotransplantation is the induction of immunological ‘tolerance’, in which the immune system of the recipient is manipulated so that the transplanted organ or cells are accepted as ‘self’ with no effort made to reject them. The identification and availability of the ‘donor’ prior to the transplant allows the procedure to be ‘timed’, and may facilitate the induction of tolerance to the transplanted organs of cells. Efforts in this respect have been made through inducing hematopoietic cell chimerism [58,59] or by pig thymic transplantation [24], but without complete success to date.
Conclusion
With the increasing variety of genetically-engineered pigs (Table 1) and immunosuppressive and anti-inflammatory agents now becoming available, it is likely the remaining immunobiological problems will be resolved.
Table 1.
Genetically-engineered pigs produced for xenotransplantation research
| Complement regulation by human complement-regulatory gene expression: |
| CD46 (membrane cofactor protein) |
| CD55 (decay-accelerating factor) |
| CD59 (protectin or membrane inhibitor of reactive lysis) |
| Gal or nonGal antigen ‘masking’ or deletion: |
| human H-transferase gene expression (expression of blood type O antigen) |
| endo-beta-galactosidase C (reduction of Gal antigen expression) |
| α1,3-galactosyltransferase gene-knockout (GTKO) |
| Cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) gene-knockout (NeuGc-KO) |
| β4GalNT2 (β1,4 N-acetylgalactosaminyltransferase) gene-knockout (β4GalNT2-KO) |
| Suppression of cellular immune response by gene expression or downregulation |
| CIITA-DN (MHC class II transactivator knockdown, resulting in swine leukocyte antigen class II knockdown) |
| Class I MHC-knockout (MHC-I-KO) |
| HLA-E/human β2-microglobulin (inhibits human natural killer cell cytotoxicity) |
| human FAS ligand (CD95L) |
| human GnT-III (N-acetylglucosaminyltransferase III) gene |
| porcine CTLA4-Ig (Cytotoxic T-Lymphocyte Antigen 4 or CD152) |
| human TRAIL (tumor necrosis factor-alpha-related apoptosis-inducing ligand) |
| Anticoagulation and anti-inflammatory gene expression or deletion |
| von Willebrand factor (vWF)-deficient (natural mutant) |
| human tissue factor pathway inhibitor (TFPI) |
| human thrombomodulin |
| human endothelial protein C receptor (EPCR) |
| human CD39 (ectonucleoside triphosphate diphosphohydrolase-1) |
| Anticoagulation, anti-inflammatory, and anti-apoptotic gene expression |
| human A20 (tumor necrosis factor-alpha-induced protein 3) |
| human heme oxygenase-1 (HO-1) |
| Porcine asialoglycoprotein receptor 1 gene-knockout (ASGR1-KO) (decreases platelet phagocytosis) |
| Human signal regulatory protein α (SIRPα) (decreases platelet phagocytosis by ‘self’ recognition) |
| Prevention of porcine endogenous retrovirus (PERV) activation |
| PERV siRNA |
HIGHLIGHTS.
-
–
Several barriers need overcoming if pig organs are to be transplanted successfully
-
–
Both the innate and adaptive immune responses need to be overcome
-
–
Additional barriers include coagulation dysfunction and an inflammatory response Most barriers can be overcome by the genetic engineering of the pigs
ACKNOWLEDGEMENTS
Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute at the University of Pittsburgh has been supported in part by NIH grants U01 AI068642, R21 AI074844, and U19 AI090959, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA.
ABBREVIATIONS
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- NeuGc
N-glycolylneuraminic acid
- NeuGcKO
N-glycolylneuraminic acid gene-knockout
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Ethical approval
None.
Research Registry
Not required for the review.
Author Contribution
DKCC, BE, AJT – all participated in review of the literature, writing of the manuscript, and final approval of the manuscript.
Guarantor
David K.C. Cooper, MD, PhD
References
- 1.Lexer G, Cooper DK, Rose AG, Wicomb WN, Rees J, Keraan M, et al. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J Heart Transplant. 1986;5:411–418. [PubMed] [Google Scholar]
- 2.Cooper DK, Human PA, Lexer G, Rose AG, Rees J, Keraan M, et al. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant. 1988;7:238–246. [PubMed] [Google Scholar]
- 3.Alexandre GPJ, Gianello P, Latinne D, Carlier M, Dewaele A, van Obbergh L, Hardy M. Xenograft. Vol. 25. Amsterdam, New York, Oxford: Excerpta Medica; 1989. Plasmapheresis and splenectomy in experimental renal xenotransplantation; pp. 259–266. [Google Scholar]
- 4.Rose AG, Cooper DK, Human PA, Reichenspurner H, Reichart B. Histopathology of hyperacute rejection of the heart: experimental and clinical observations in allografts and xenografts. J Heart Lung Transplant. 1991;10:223–234. [PubMed] [Google Scholar]
- 5.Rose AG, Cooper DK. Venular thrombosis is the key event in the pathogenesis of antibody-mediated cardiac rejection. Xenotransplantation. 2000;7:31–41. doi: 10.1034/j.1399-3089.2000.00042.x. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi T, Cooper DK. Anti-Gal, alpha-Gal epitopes, and xenotransplantation. Subcell Biochem. 1999;32:229–257. doi: 10.1007/978-1-4615-4771-6_10. [DOI] [PubMed] [Google Scholar]
- 7.Galili U. Evolution of alpha 1,3galactosyltransferase and of the alpha-Gal epitope. Subcell Biochem. 1999;32:1–23. doi: 10.1007/978-1-4615-4771-6_1. [DOI] [PubMed] [Google Scholar]
- 8.Rood PP, Tai HC, Hara H, Long C, Ezzelarab M, Lin YJ, et al. Late onset of development of natural anti-nonGal antibodies in infant humans and baboons: implications for xenotransplantation in infants. Transpl Int. 2007;20:1050–1058. doi: 10.1111/j.1432-2277.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 9.Dons EM, Montoya C, Long CE, Hara H, Echeverri GJ, Ekser B, et al. Tcell- based immunosuppressive therapy inhibits the development of natural antibodies in infant baboons. Transplantation. 2012;93:769–776. doi: 10.1097/TP.0b013e3182481168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988;56:1730–1737. doi: 10.1128/iai.56.7.1730-1737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stussi G, West L, Cooper DK, Seebach JD. ABO-incompatible allotransplantation as a basis for clinical xenotransplantation. Xenotransplantation. 2006;13:390–399. doi: 10.1111/j.1399-3089.2006.00324.x. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi S, Neethling FA, Korchagina EY, Bovin N, Ye Y, Kobayashi T, et al. In vivo immunoadsorption of antipig antibodies in baboons using a specific Gal(alpha)1-3Gal column. Transplantation. 1996;62:1379–1384. doi: 10.1097/00007890-199611270-00001. [DOI] [PubMed] [Google Scholar]
- 13.Ye Y, Neethling FA, Niekrasz M, Koren E, Richards SV, Martin M, et al. Evidence that intravenously administered alpha-galactosyl carbohydrates reduce baboon serum cytotoxicity to pig kidney cells (PK15) and transplanted pig hearts. Transplantation. 1994;58:330–337. [PubMed] [Google Scholar]
- 14.Simon PM, Neethling FA, Taniguchi S, Goode PL, Zopf D, Hancock WW, et al. Intravenous infusion of Galalpha1-3Gal oligosaccharides in baboons delays hyperacute rejection of porcine heart xenografts. Transplantation. 1998;65:346–353. doi: 10.1097/00007890-199802150-00009. [DOI] [PubMed] [Google Scholar]
- 15.Leventhal JR, Sakiyalak P, Witson J, Simone P, Matas AJ, Bolman RM, et al. The synergistic effect of combined antibody and complement depletion on discordant cardiac xenograft survival in nonhuman primates. Transplantation. 1994;57:974–978. [PubMed] [Google Scholar]
- 16.Kobayashi T, Taniguchi S, Neethling FA, Rose AG, Hancock WW, Ye Y, et al. Delayed xenograft rejection of pig-to-baboon cardiac transplants after cobra venom factor therapy. Transplantation. 1997;64:1255–1261. doi: 10.1097/00007890-199711150-00005. [DOI] [PubMed] [Google Scholar]
- 17.Dalmasso AP, Vercellotti GM, Platt JL, Bach FH. Inhibition of complement-mediated endothelial cell cytotoxicity by decay-accelerating factor. Potential for prevention of xenograft hyperacute rejection. Transplantation. 1991;52:530–533. doi: 10.1097/00007890-199109000-00029. [DOI] [PubMed] [Google Scholar]
- 18.White DJ, Oglesby T, Liszewski MK, Tedja I, Hourcade D, Wang MW, et al. Expression of human decay accelerating factor or membrane cofactor protein genes on mouse cells inhibits lysis by human complement. Transpl Int. 1992;5(Suppl 1):S648–S650. doi: 10.1007/978-3-642-77423-2_190. [DOI] [PubMed] [Google Scholar]
- 19.Lambrigts D, Sachs DH, Cooper DK. Discordant organ xenotransplantation in primates: world experience and current status. Transplantation. 1998;66:547–561. doi: 10.1097/00007890-199809150-00001. [DOI] [PubMed] [Google Scholar]
- 20.Cooper DK, Koren E, Oriol R. Genetically engineered pigs. Lancet. 1993;342:682–683. doi: 10.1016/0140-6736(93)91791-j. [DOI] [PubMed] [Google Scholar]
- 21.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 24.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 25.Gollackner B, Goh SK, Qawi I, Buhler L, Knosalla C, Daniel S, et al. Acute vascular rejection of xenografts: roles of natural and elicited xenoreactive antibodies in activation of vascular endothelial cells and induction of procoagulant activity. Transplantation. 2004;77:1735–1741. doi: 10.1097/01.tp.0000131167.21930.b8. [DOI] [PubMed] [Google Scholar]
- 26.Hara H, Long C, Lin YJ, Tai HC, Ezzelarab M, Ayares D, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–1174. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 27.McGregor CG, Ricci D, Miyagi N, Stalboerger PG, Du Z, Oehler EA, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation. 2012;93:686–692. doi: 10.1097/TP.0b013e3182472850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buhler L, Awwad M, Basker M, Gojo S, Watts A, Treter S, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69:2296–2304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 29.Cozzi E, Vial C, Ostlie D, Farah B, Chavez G, Smith KG, et al. Maintenance triple immunosuppression with cyclosporin A, mycophenolate sodium and steroids allows prolonged survival of primate recipients of hDAF porcine renal xenografts. Xenotransplantation. 2003;10:300–310. doi: 10.1034/j.1399-3089.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 30.McGregor CG, Davies WR, Oi K, Teotia SS, Schirmer JM, Risdahl JM, et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg. 2005;130:844–851. doi: 10.1016/j.jtcvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Satyananda V, Hara H, Ezzelarab MB, Phelps C, Ayares D, Cooper DK. New concepts of immune modulation in xenotransplantation. Transplantation. 2013;96:937–945. doi: 10.1097/TP.0b013e31829bbcb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phelps CJ, Ball SF, Vaught TD, Vance AM, Mendicino M, Monahan JA, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009;16:477–485. doi: 10.1111/j.1399-3089.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- 33.Hara H, Witt W, Crossley T, Long C, Isse K, Fan L, et al. Human dominant-negative class II transactivator transgenic pigs - effect on the human anti-pig T-cell immune response and immune status. Immunology. 2013;140:39–46. doi: 10.1111/imm.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reyes LM, Estrada JL, Wang ZY, Blosser RJ, Smith RF, Sidner RA, et al. Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J Immunol. 2014;193:5751–5757. doi: 10.4049/jimmunol.1402059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robson SC, Cooper DK, d'Apice AJ. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation. 2000;7:166–176. doi: 10.1034/j.1399-3089.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 36.Cowan PJ, Robson SC, d'Apice AJ. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant. 2011;16:214–221. doi: 10.1097/MOT.0b013e3283446c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buhler L, Basker M, Alwayn IP, Goepfert C, Kitamura H, Kawai T, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70:1323–1331. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 38.Houser SL, Kuwaki K, Knosalla C, Dor FJ, Gollackner B, Cheng J, et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004;11:416–425. doi: 10.1111/j.1399-3089.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 39.Lin CC, Ezzelarab M, Shapiro R, Ekser B, Long C, Hara H, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10:1556–1568. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knosalla C, Yazawa K, Behdad A, Bodyak N, Shang H, Buhler L, et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am J Transplant. 2009;9:1006–1016. doi: 10.1111/j.1600-6143.2009.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byrne GW, Davies WR, Oi K, Rao VP, Teotia SS, Ricci D, et al. Increased immunosuppression, not anticoagulation, extends cardiac xenograft survival. Transplantation. 2006;82:1787–1791. doi: 10.1097/01.tp.0000251387.40499.0f. [DOI] [PubMed] [Google Scholar]
- 42.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML, 3rd, Ayares D, et al. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J Thorac Cardiovasc Surg. 2014;148:1106–1113. doi: 10.1016/j.jtcvs.2014.06.002. discussion 1113-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ezzelarab MB, Ekser B, Azimzadeh A, Lin CC, Zhao Y, Rodriguez R, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22:32–47. doi: 10.1111/xen.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwase H, Ekser B, Zhou H, Liu H, Satyananda V, Humar R, et al. Further evidence for a sustained systemic inflammatory response in xenograft recipients (SIXR) 2015 doi: 10.1111/xen.12182. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuwaki K, Knosalla C, Dor FJ, Gollackner B, Tseng YL, Houser S, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004;4:363–372. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 46.Ekser B, Burlak C, Waldman JP, Lutz AJ, Paris LL, Veroux M, et al. Immunobiology of liver xenotransplantation. Expert Rev Clin Immunol. 2012;8:621–634. doi: 10.1586/eci.12.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris DG, Quinn KJ, French BM, Schwartz E, Kang E, Dahi S, et al. Meta-analysis of the independent and cumulative effects of multiple genetic modifications on pig lung xenograft performance during ex vivo perfusion with human blood. Xenotransplantation. 2015;22:102–111. doi: 10.1111/xen.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper DK, Satyananda V, Ekser B, Van der Windt DJ, Hara H, Ezzelarab M, et al. Progress in pig-to-nonhuman primate transplantation models (1998–2013): a comprehensive review of the literature. Xenotransplantation. 2014;21:397–419. doi: 10.1111/xen.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padler-Karavani V, Varki A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. 2011;18:1–5. doi: 10.1111/j.1399-3089.2011.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Byrne GW, Du Z, Stalboerger P, Kogelberg H, McGregor CG. Cloning and expression of porcine beta1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation. 2014;21:543–554. doi: 10.1111/xen.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 52.van der Windt DJ, Bottino R, Kumar G, Wijkstrom M, Hara H, Ezzelarab M, et al. Clinical islet xenotransplantation: how close are we? Diabetes. 2012;61:3046–3055. doi: 10.2337/db12-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bottino R, Wijkstrom M, van der Windt DJ, Hara H, Ezzelarab M, Murase N, et al. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am J Transplant. 2014;14:2275–2287. doi: 10.1111/ajt.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9:2716–2726. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 55.Shin JS, Kim JM, Kim JS, Min BH, Kim YH, Kim HJ, et al. Long-term control of diabetes in imunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant. 1915 doi: 10.1111/ajt.13345. [Epub ahead or Print] [DOI] [PubMed] [Google Scholar]
- 56.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 57.Thompson P, Badell IR, Lowe M, Cano J, Song M, Leopardi F, et al. Islet xenotransplantation using gal-deficient neonatal donors improves engraftment and function. Am J Transplant. 2011;11:2593–2602. doi: 10.1111/j.1600-6143.2011.03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tseng YL, Sachs DH, Cooper DK. Porcine hematopoietic progenitor cell transplantation in nonhuman primates: a review of progress. Transplantation. 2005;79:1–9. doi: 10.1097/01.tp.0000146504.73727.13. [DOI] [PubMed] [Google Scholar]
- 59.Tasaki M, Wamala I, Tena A, Villani V, Sekijima M, Pathiraja V, et al. High Incidence of Xenogenic Bone Marrow Engraftment in Pig-to-Baboon Intra-Bone Bone Marrow Transplantation. Am J Transplant. 2015;15:974–983. doi: 10.1111/ajt.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]