Abstract
Over-activation of p38 is implicated in many cardiovascular diseases (CVDs), including myocardial infarction, hypertrophy, heart failure, and ischemic heart disease. Numerous therapeutic interventions for CVDs have been directed towards the inhibition of the p38 mitogen-activated protein kinase activation that contributes to the detrimental effect after ischemia/reperfusion (I/R) injuries. However, the efficacy of these treatments is far from ideal as they lack specificity and are associated with high toxicity. Previously, we demonstrated that N-acetyl cysteine (NAC) pre-treatment up-regulates DUSP4 expression in endothelial cells, regulating p38 and ERK1/2 activities, thus providing a protective effect against oxidative stress. Here, endothelial cells under hypoxia/reoxygenation (H/R) insult and isolated heart I/R injury were used to investigate the role of DUSP4 on the modulation of the p38 pathway. In rat endothelial cells, DUSP4 is time-dependently degraded with H/R (0.25 ± 0.07 fold change of control after 2 h H/R). Its degradation is closely associated with hyper-phosphorylation of p38 (2.1 ± 0.36 fold change) and cell apoptosis, as indicated by the increase in cells immunopositive for cleaved caspase-3 (12.59% ± 3.38%) or TUNEL labeling (29.46% ± 3.75%). The inhibition of p38 kinase activity with 20 µM SB203580 during H/R prevents H/R-induced apoptosis, assessed via TUNEL (12.99% ± 1.89%). Conversely, DUSP4 gene silencing in endothelial cells augments their sensitivity to H/R-induced apoptosis (45.81% ± 5.23%). This sensitivity is diminished via the inhibition of p38 activity (total apoptotic cells drop to 17.47% ± 1.45%). Interestingly, DUSP4 gene silencing contributes to the increase in superoxide generation from cells. Isolated Langendorff-perfused mouse hearts were subjected to global I/R injury. DUSP4−/− hearts had significantly larger infarct size than WT. The increase in I/R-induced infarct in DUSP4−/− mice significantly correlates with reduced functional recovery (assessed by: RPP%, LVDP%, HR%, and dP/dtmax) as well as lower CF% and a higher initial LVEDP. From immunoblotting analysis, it is evident that p38 is significantly over-activated in DUSP4−/− mice after I/R injury. The activation of cleaved caspase-3 is seen in both WT and DUSP4−/− I/R hearts. Infusion of a p38 inhibitor prior to ischemia and during the reperfusion improves both WT and DUSP4−/− cardiac function. Therefore, the identification of p38 kinase modulation by DUSP4 provides a novel therapeutic target for oxidant-induced diseases, especially myocardial infarction.
Keywords: DUSP4, p38, MAPK, oxidative stress, H/R, I/R, cardiovascular diseases
Graphical abstract
INTRODUCTION
Cardiovascular diseases (CVDs), including myocardial infarction (MI), hypertrophy, heart failure, and ischemic heart disease, are the leading cause of death worldwide [1, 2]. Ischemic heart disease is generally caused by insufficient blood flow to the myocardium [3]. Numerous interventional therapies are available for ischemic heart disease amelioration, but they do not altogether mitigate the fundamental problem: the burst of reactive oxygen species during reperfusion which contributes to the myocardial damage in ischemia-reperfusion (I/R) injury [4, 5]. Thus, understanding the mechanism leading to tissue damage with oxidative stress will help to identify improved therapeutic strategies for ischemic heart disease in the future.
p38 mitogen-activated protein kinase (MAPK) is a stress-activated and pro-inflammatory kinase. The increased activation of p38 MAPK is a major indicator of injury post-MI [6]. In I/R animal models, p38 over-activation is detrimental and, as such, the pharmacological inhibition of p38 prevents I/R induced MI and improves cardiac function after reperfusion [7, 8]. The over-activation of p38 MAPK phosphorylates MK2, promotes the activation of caspase-3 and subsequently contributes to apoptosis and cell death [9]. There are four isoforms (α, β, γ, and δ) of p38 MAPKs. p38α, a pro-inflammatory kinase, has been directly linked to myocardial injury after I/R [10-12]. It is thus reasonable to consider direct inhibition of p38 activity in the clinical setting for the treatment of ischemic heart disease. However, the major clinical trials with p38 inhibition to date have been limited to non-cardiac inflammatory diseases. None of these trials have advanced beyond phase 2 [7, 8]. The non-specificity and high toxicity of p38 inhibitors is the primary reason for the lack of progress in their clinical applications. In this study, we investigate a negative regulatory mechanism of p38 in the myocardium during ischemic heart disease, especially I/R injury. One such regulatory mechanism is via the control of p38 activity by phosphatases, such as the dual-specificity phosphatases (DUSPs).
DUSPs constitute a group of protein tyrosine phosphatases capable of dephosphorylating two residues (tyrosine and serine/threonine) within a single substrate [13-15]. DUSPs, cysteine-based phosphatases, have recently drawn a lot of attention in cardiovascular research and play critical roles in heart function, especially DUSP1 and 4 [16]. Both DUSP1 and DUSP4 constitute part of a DUSP subgroup denominated as mitogen-activated protein kinase phosphatases (MKPs) of which there are at least ten known members with a conserved C-terminus catalytic domain and a N-terminus domain that dictates MKP specificity to a member of the MAPKs: ERK (extracellular-signal-regulated kinase), p38 MAPK or JNK (c-Jun N-terminal kinase) [13]. The MAPK-specificity of DUSPs depends on the cell type and their subcellular localization. ERKs (1 and 2) are stimulated by extracellular signals and elicit pro-survival mechanisms, whereas p38 MAPKs are pro-apoptotic signaling molecules generally activated by inflammatory cytokines [17]. Moreover, MAPKAP kinase 2 (MK2) has been demonstrated to be activated by p38 MAPK [18] and it can trigger death signals via promotion of apoptosis in the mouse myocardium [19]. Therefore, duration and extent of phosphorylation of these MAPKs are critical factors in determining their physiological outcomes [14, 20]. Tight regulation of MAPK activities is pivotal for the maintenance of cellular homeostasis.
DUSP4 is an inducible nuclear phosphatase whose substrates include all three of the MAPKs (ERK, JNK and p38) and it has been demonstrated to be important for cardiovascular function [16, 21-26]. Overexpression of DUSP4 in human endothelial cells enhances adhesion molecule expression and protects against apoptosis [22]. Moreover, DUSP4 gene deletion in mouse embryonic fibroblasts revealed that DUSP4 promotes proliferation and cell survival [27]. A more recent study demonstrated that the combined disruption of DUSP1/4 promoted unrestrained p38 activity in both mouse embryonic fibroblasts and in the heart [16]. Taken together, these studies highlight the importance of DUSP4 in cell survival and in the control of p38 kinase activity.
In our recent study, we demonstrated that NAC treatment in endothelial cells up-regulates DUSP4 expression through the activation of ERK1/2 [28]. In fact, the up-regulation of DUSP4 by NAC protected against oxidant-induced cell death and apoptosis via the modulation of p38 MAPK activity. DUSP4 gene silencing resulted in an increased susceptibility to chemical-induced oxidative stress and an increase in p38 phosphorylation. These results are consistent with the earlier study in human umbilical vein endothelial cells in which induction by angiopoietin-1 led to DUSP4 up-regulation, which preferentially dephosphorylates ERK1/2, p38, and JNK and provides anti-apoptotic effects under conditions of serum deprivation [22]. However, it is still not clear how DUSP4 modulates MAPKs under various conditions of oxidative stress, especially hypoxia/re-oxygenation (H/R) and I/R. Therefore, in this study we further investigate the role of DUSP4 in the modulation of p38 MAPK under H/R and I/R using DUSP4 knockdown (siRNA) in cells and genetic knockout in mice.
EXPERIMENTAL PROCEDURES
Materials
DUSP4 (sc-1200), gp91-phox (sc-5827), Nox4 (sc-21860), and actin (sc-1616-R) antibodies were obtained from Santa Cruz (Santa Cruz, CA), p-p38 (4511S), p38 (9212S), p-ERK1/2 (9101S), ERK1/2 (9102S), p-JNK (4671S), JNK (3708S), cleaved caspase-3 (9664S), and MK-2 antibody sampler kit antibodies (9329S) from Cell Signaling (Cambridge, MA). Secondary anti-rabbit (NA934V) and anti-mouse (NXA931) IgG-HRP antibodies were purchased from GE Life Sciences (Piscataway, NJ). p38 inhibitor, SB203580, was purchased from Cayman Chemical (Ann Arbor, MI). Click-iT TUNEL Alexa Fluor 488 Imaging Assay Kit and dihydroethidium (DHE) were purchased from Life Technologies (Grand Island, NY).
Cell culture
The maintenance of rat aortic endothelial cells (RAECs) culture was the same as previously described [28-30]. For siRNA treatments, cells were seeded to a confluency of 50-60% on 6-well dishes. For H/R treatments, cells were grown on 100 mm petri dishes coated with adhesion factor to a confluency of 80-90%. Cells used for immunostaining were grown on sterile glass coverslips coated with adhesion factor.
H/R treatment
Control or DUSP4 siRNA-treated RAECs with or without 20 µM SB203580 were incubated at 37 °C for 30 min prior to H/R exposure. Following this, the cell media was removed, cells were gently washed with phenol-free Dulbecco’s Modified Eagle Medium (DMEM), and fresh phenol-free DMEM (with or without 20 µM SB203580) was added to the cells. RAECs were then either incubated in normoxic conditions or placed in a hypoxia chamber (Billups-Rothenberg, CA). The hypoxic chamber was flushed with argon for 30 min [31, 32] and the cells were incubated at 37 °C under the hypoxic environment for 1, 2, or 3 h. After hypoxia, cells were then re-exposed to normoxic conditions while they were collected (30 min reoxygenation) and processed for protein analysis via SDS-PAGE and immunoblotting. Cells exposed to only normoxic conditions served as controls and were compared to those subjected to H/R. Initially, an H/R time-exposure course of 0-3 h was conducted. Unless stated, all other H/R experiments were carried out using 2 h hypoxia exposure. The effects of p38 kinase inhibitor, SB203580, on H/R stress were analyzed.
DUSP4 gene silencing
The relationship between DUSP4 and the kinase activity of p38 in response to H/R treatment in RAECs was analyzed by using DUSP4 gene silencing. A rat DUSP4 siRNA kit (OriGene, Rockville, MD) was utilized at a dose of 10 nM, which was previously determined sufficient for silencing of at least 80% of the constitutively expressed DUSP4 72 h post transfection [28]. As a negative control, cells were transfected with siGENOME™ Non-Targeting Control siRNAs from GE Healthcare Dharmacon (Lafayette, CO). DUSP4 siRNA transfected cells were subjected to H/R experiments in conjunction with SB203580 pre-treatment and used to determine cell fate and molecular alterations in cells.
Cell fate quantification
Post H/R treatment, RAEC death was expressed as a percentage of dead cells (floating or rounded) as visualized via live cell imaging [28]. Cellular apoptosis was determined by staining for cleaved caspase-3 or terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, while cell death was determined by manually counting cells in at least 15 fields of view/treatment.
Live cell imaging microscopy
RAECs were cultured on sterile 6-well dishes and grown to 50-60% confluency. Subsequently, 72 h post DUSP4 siRNA transfection, cells underwent H/R treatment in the presence or absence of SB203580 inhibitor. After H/R, cells were then viewed with the Zeiss Axiovert 135 microscope equipped with a Tucsen TCH-5.0ICE camera at 20X magnification. Images were captured digitally and analyzed using Image J (NIH) [28].
Immunostaining of cleaved caspase-3
RAECs were cultured on sterile coverslips coated with adhesion factor to an 80-90% confluence. Cells were subjected to their treatment (H/R time course of 0-3 h), cell media was removed and coverslips were washed 2x with PBS, fixed for 20 min at RT in 4% paraformaldehyde and subsequently washed 3x with PBS containing 0.1% BSA. Cells were then blocked for 45 min at RT in PBS containing 10% FBS and 0.3% TritonX-100 and then incubated overnight at 4ºC in anti-cleaved caspase-3 diluted 1/400 in PBS with 0.3% TritonX-100. After overnight incubation, coverslips were rinsed 2x with PBS containing 0.1% BSA, and incubated with Alexa Fluor 594 secondary antibody (Cell Signaling) for 1 h at RT. Finally, coverslips were washed 3x with PBS containing 0.1% BSA, mounted onto a glass slide with ProLong Gold antifade reagent with DAPI (Life technologies, Grand Island, NY), and fluorescent images (20X) obtained using a Zeiss Axiovert 135 microscope. Cleaved caspase-3-immunopositive cells were expressed as a percentage of total cells, as determined by DAPI positive cells [28].
TUNEL assay
The role of DUSP4 on the modulation of p38 under oxidative stress was further evaluated using TUNEL assay. The TUNEL assay is a complement to the immunostaining of cleaved caspase-3 for the determination of the extent of apoptosis. Similar to the immunostaining of cleaved caspase-3 post 2h H/R, cells were fixed with 4% paraformaldehyde. The level of apoptosis was determined using Click-iT TUNEL Alexa Fluor 488 Imaging Assay Kit from Life Technologies [33]. The green fluorescent image of TUNEL positive cells was digitally taken using a Zeiss Axiovert 135 microscope. The TUNEL positive cells were expressed as a percentage of total cells, as determined by DAPI positive cells (blue).
Immunoblotting
The procedure for the immunoblotting was followed as previously described [28, 30, 34]. Samples were first separated on either an 8% or 12% Tris-glycine polyacrylamide gel, and then electrophoretically transferred to a nitrocellulose membrane. Anti-DUSP4 was used to determine the level of protein expression. The extent of p38 phosphorylation was determined using the ratio of p-p38 to total p38. Similarly, the extent of ERK1/2 phosphorylation was determined using the ratio of p-ERK1/2 to total ERK1/2. MK2 phosphorylation at T334 and T222 was used to determine the efficacy of the p38 inhibitor, SB203580. Cleaved caspase-3 was used as an apoptotic marker, and actin served as the loading control marker.
Measurement of superoxide generated from RAECs using dihydroethidium (DHE) and HLPC analysis
The level of superoxide generation from control RAECs and RAECs with DUSP4 knockdown was measured using DHE HPLC analysis as previously described [28, 35]. Three days post-transfection with DUSP4 siRNA, cells were washed once with PBS and incubated in Krebs-HEPES buffer. DHE was added to a final concentration of 25 µM and incubated at 37 °C for 30 min. The medium was then removed and replenished with fresh Krebs-HEPES buffer for additional 1 h incubation. After incubation, cells were collected and lysed in 500 µL cold methanol, and centrifuged. 2-hydroxyethidium, dihydroethidium, and ethidium were separated using a gradient HPLC system (Shimadzu LC-2010A) with a Hypersil Gold column (250 × 4.6 mm, Thermo Scientific) and detected with a fluorescence detector using an emission wavelength at 580 nm and an excitation at 480 nm. A linear gradient at a flow rate of 0.5 mL/min was developed from mobile phase A (0.1% trifluoroacetic acid) to mobile phase B (acetonitrile) over 23 min from 37% to 47% acetonitrile.
Animals
The animal protocol (2012A0000089) used in this study was approved by The Ohio State University Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by National Institute of Health (NIH Publication NO. 85-23, revised 1996). Male mice (10 weeks), of body weight > 20 g, were utilized in this study. Mice with a targeted DUSP4 gene knockout (B6;129-Dusp4tm1Jmol/J) and wild-type (WT) mice (B6129SF2/J) were purchased from Jackson Laboratory.
Genotyping
Prior to I/R experiments, the genotype of each DUSP4−/− mouse was confirmed. First, mouse ear tissue was digested overnight and DNA was purified as previously described [36]. Genotyping for DUSP4 was done according to the protocol provided by Jackson laboratory. For WT mice, only a 647 bp band was seen from WT primers reaction, while for DUSP4−/− mice, a ~620 bp band was seen from mutant primers reaction.
Isolated Langendorff perfused mouse heart preparation
Prior to euthanization, mice were deeply anesthetized via inhalation of isofluorane. Hearts were excised and arrested within ice-cold Kreb’s buffer. The ascending aorta was then cannulated and retrogradely perfused with oxygenated Kreb’s buffer (37 °C) consisting of 120 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2-6H2O, 25 mM NaHCO3, 16.7 mM glucose, 0.5 mM EDTA and 2.5 mM CaCl2 equilibrated with 95% O2 and 5% CO2 [37]. A fluid-filled balloon was inserted into the left ventricle (LV) across the mitral valve and connected to a pressure transducer, allowing continuous measurement of LV pressure (LVP). Hearts were submerged in a water-jacketed bath maintained at 37 °C, and the LV balloon was filled with water to yield a LV diastolic pressure of 2-4 mmHg. Coronary flow (CF) was continuously monitored via a Doppler flow probe (T206, Transonic Systems, Ithaca, NY) placed in the aortic perfusion line. LVP was recorded using an analog-to-digital converter box (Digi-Med ASA-400a, Micro-Med, Inc., Louisville, Kentucky, USA) with a Digi-Med Heart Performance Analyzer (HPA-210a, Micro-Med, Inc.). Heart rate, LV systolic pressure and end diastolic pressure were derived by computer algorithm. Left ventricular developed pressure (LVDP) was calculated as the difference between systolic and end diastolic pressures. Rate pressure product (RPP) was calculated as the product of heart rate and LVDP. dP/dtmax was derived directly from software [5].
The mouse heart was perfused at a constant pressure and equilibrated for 30 min. After equilibration, hearts underwent 30 min global ischemia followed by 30 min reperfusion (1 h for infarct size measurement). At the end of the reperfusion phase, hearts were flash frozen in liquid nitrogen for immunoblotting analysis or processed for 2,3,5-triphenyltetrazolium chloride (TTC) stain for infarct size measurement.
To further demonstrate that the activity of p38 plays a critical role on cardiovascular function after I/R injury, hearts from WT or DUSP4−/− were infused with 10 µM SB203580 10 min prior to ischemia, and continuing for 30 min during reperfusion [38]. The infusion rate (1/100 of CF) is adjusted according to the base CF in order to achieve the desired final concentration of the p38 inhibitor (10 µM).
Infarct size measurement
Control hearts, or I/R hearts were removed from their cannula at the end of their 1 h reperfusion phase and frozen at −20 °C for 30 min. The frozen heart was placed inside the mouse heart slicer (Zivic Laboratories) and cut transversely into 1 mm sections. The sections were then placed in 1% TTC at 37°C for 15 min. Following the stain, sections were fixed in 10% formalin overnight and pictures taken of the stained slices. Infarct area was calculated and expressed as a percentage of total area using ImageJ software (NIH) [39, 40].
Assessment of Nox4 expression in hearts using quantitative PCR and immunoblotting
The total RNA was isolated from WT or DUSP4−/− hearts via the Trizol-chloroform extraction procedure [28]. Extracted RNA was quantified via spectrophotometric analysis using absorption spectra at wavelengths of 230, 260 and 280 nm. A total of 1 μg of RNA was reverse-transcribed using a High-Capacity cDNA Reverse Transcription Kit according to the kit’s instructions. Gene expression was detected via quantitative real-time PCR using a Roche 480 thermal cycler. Data were calculated using the 2−ΔΔCp method and are expressed as target gene transcript fold induction normalized to β-actin. The sequence of primers used is as followed: Nox4 GAAGATTTGCCTGGAAGAACC (forward) and AGGTTT GTTGCTCCTGATGC (reverse); β-actin: GGCTGTATTCCCCTCCATCG (forward) and CCAGTTGGTAACAATGCCATGT (reverse). The level of Nox4 protein expression in WT or DUSP4−/− hearts was determined using immunoblotting against Nox4. Actin is always used as a loading control.
Statistics
Results were expressed as mean ± SEM, n ≥ 3 for cultured cells and n ≥ 7 for isolated hearts. Statistical significance of difference between results was calculated using two samples t-test. A P value (two-tailed) < 0.05 was considered statistically significant.
RESULTS
Hypoxia/reoxygenation causes molecular alterations in RAECs: DUSP4 and p38
Cells inoculated in 100 mm petri dishes and grown to a confluency of 80-90% were placed inside a modular incubator chamber (Billups-Rothenberg, Inc., Del Mar, CA) subjected to 1-3 h of hypoxia. Upon completion of the hypoxic period, cells were re-oxygenated for a total of 30 min while being processed for either imaging, immunohistochemistry or protein analysis. H/R caused time-dependent degradation of DUSP4 (Figure 1A), with significant degradation occurring with the 2 h exposure (0.25 ± 0.07 fold change of control expression level, P < 0.001). An additional hour of hypoxia, i.e. 3 h, resulted in similar DUSP4 degradation (0.29 ± 0.07 fold change of control, P < 0.001). The 2 h H/R treatment did not affect the expression of DUSP1 (0.85 ± 0.17 fold change of control; Figure 1B and C), the closest DUSP4 homologue. However, coincident with the DUSP4 degradation, phosphorylation of p38 was significantly increased with 2 h hypoxia (2.1 ± 0.36 fold change of control; Figure 1B and C, P < 0.05). There is no significant difference in ERK1/2 phosphorylation after 2 h hypoxia. No JNK phosphorylation was detected (data not shown). Thus, this is consistent with our previous study demonstrating that DUSP4 is an important modulator of p38 under oxidative stress [28].
Figure 1.
Increased cellular oxidative stress after hypoxic exposure (0-3 h) leads to a time-dependent degradation of DUSP4 in RAECs. (A) Upper panel is the immunoblot against DUSP4 and lower panel is the immunoblot against actin as the loading control. 2 and 3 hr hypoxic exposure of cells leads to significant DUSP4 degradation (0.25 ± 0.07 and 0.29 ± 0.07 fold change of control level). * and ** versus control, P < 0.001. Degradation of DUSP4 is correlated to the uncontrolled activation of p38 after H/R insult. (B) Immunoblots of RAECs exposed to either control (no H/R) or 2 h H/R. Actin was used a loading control for DUSP4 and DUSP1, whereas the ratio of p-p38/p38 was used to determine the extent of phosphorylation of p38. (C) Following 2 h H/R, only 0.25 ± 0.07 of DUSP4/actin remains, yet there is no significant change in DUSP1/actin level (0.85 ± 0.17). Phosphorylation of p38 increases more than two fold (2.1 ± 0.36). There is no significant difference in ERK1/2 phosphorylation. No JNK phosphorylation is seen (data not shown). * P < 0.001, and # P < 0.05. Data is expressed as mean ± SEM, n ≥ 3.
Hypoxia/reoxygenation leads to apoptosis of RAECs
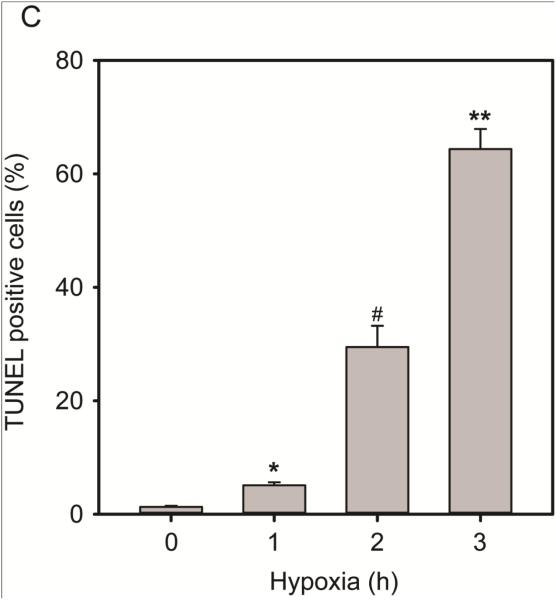
The increase in oxidative stress after H/R is the primary contributing factor for endothelial dysfunction and cell death. Live cell imaging of cells exposed to hypoxic conditions (0-3 h) (Figure 2A) showed that with extended hypoxic challenge, more cells became rounded and detached from the culture dish, and thus died. The percentage of cell death was calculated by dividing the number of rounded and detached cells over the total cells. Cells were counted in > 15 fields of view and experiments were repeated a minimum of 3 times. Cell death increased directly with H/R exposure and became significant in as little as 1 h H/R (18.49% ± 0.86%, P < 0.0001) compared to normoxic conditions (1.7% ± 0.3%). The 3 h H/R insult resulted in nearly total cell death (97.12% ± 0.70%, P < 0.0001 versus control) whereas at the 2 h time point approximately half of the cells were dead (59.85% ± 4.89%, P < 0.0001 versus control). Immunostaining of cleaved caspase-3 after H/R demonstrated that H/R-induced RAEC death is via apoptosis (Figure 2B). Cleaved caspase-3 immunoreactivity becomes significant in cells exposed to 2 and 3 h of hypoxia (12.59% ± 3.38% and 28.01% ± 5.62%, respectively P < 0.05) compared to control (0.10% ± 0.03%). TUNEL-staining of cells is significantly greater in cells exposed to the three H/R time points compared to control cells (5.09% ± 0.55% (1h); 29.46% ± 3.75% (2h); and 64.39% ± 3.53% (3h), *P ≤ 0.001). Because a minimum of 2 h exposure to hypoxia triggers significant DUSP4 degradation coupled with apoptosis (assessed by cleaved caspase-3 and TUNEL assay) and cell death, subsequent experiments were carried out using the 2 h H/R experimental protocol.
Figure 2.
Hypoxia/reoxygenation results in endothelial cell death and apoptosis. (A) 20X bright-field images of RAECs exposed to hypoxia (0-3 h), images were captured using the Zeiss Axiovert 135 microscope. Rounded, detached cells were considered dead cells and are expressed as a percentage of total cells in the graph. Cell death also time-dependently increased with all three hypoxic exposure time periods (18.49% ± 0.86% (1 h); 59.85% ± 4.89% (2 h); and 97.12% ± 0.70% (3 h)) compared to normoxic condition. *, #, and ** versus 0 h P < 0.0001. (B) RAEC immunostaining against cleaved caspase-3 and DAPI staining for cell nuclei. Immunopositive cells (red) were considered apoptotic and expressed as a percentage of total cell nuclei (blue). A significantly greater percentage of total cells become positive for apoptotic marker, cleaved caspase-3 at 2 and 3 h post-hypoxia exposure (12.59% ± 3.38% and 28.01% ± 5.62%, respectively) compared to control (0.10% ± 0.03%). * and # versus 0 h P < 0.05. (C) Quantification of TUNEL positive cells revealed a time-dependent increment in apoptotic cells. Exposure to the three H/R time points causes significant increase in TUNEL positive cells (5.09% ± 0.55% (1h); 29.46% ± 3.75% (2h); 64.39% ± 3.52% (3h) compared to control 1.28% ± 0.2%, *, #, and ** versus control P ≤ 0.001). Data is expressed as mean ± SEM, n ≥ 3.
The important role of DUSP4 is in modulation of p38 leading to H/R-induced cell death and apoptosis
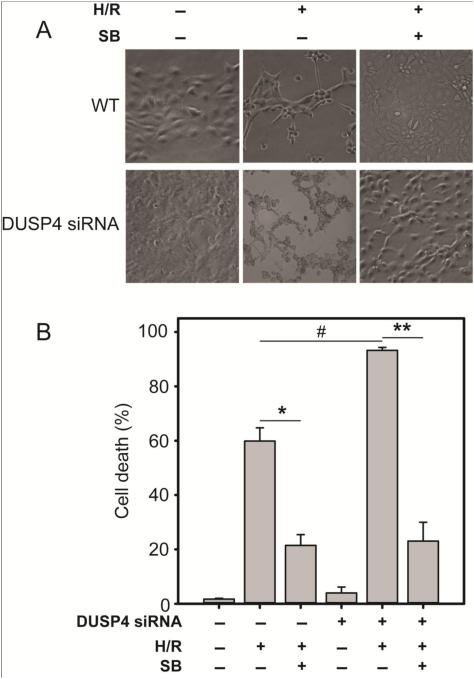
The role of DUSP4 and p38 MAPK activity on cellular response to 2 h H/R was analyzed by DUSP4 gene knockdown via siRNA treatment and/or by blocking the p38 kinase activity with an established inhibitor, SB203580 (Figures 3A-C). Quantification of live cell imaging of cells exposed to 2 h H/R (Figure 3A and B) revealed that H/R significantly increased cell death (H/R 59.85% ± 4.89% compared to control P < 0.005), and treatment with SB203580 prevented death (SB H/R 21.45% ± 3.97%, * P < 0.0005). DUSP4 gene silencing significantly enhanced H/R-induced death (93.24% ± 1.09%) compared to the H/R treatment group (# P < 0.005). This increased susceptibility was reversed by treatment with SB203580 (23.04% ± 6.93%, ** P < 0.005 compared to siRNA-silenced H/R). Quantification of TUNEL positive cells (Figure 3C) demonstrated that DUSP4 gene silencing in RAECs enhances apoptosis after H/R (45.81 ± 5.23%) compared to H/R alone (29.46% ± 3.75%, # P < 0.05). However, SB203580 treatment of transfected cells significantly lowers their susceptibility to H/R-induced apoptosis (17.47% ± 1.45%, ** P < 0.005 compared to the siRNA-silenced H/R group). Concomitant with H/R-induced DUSP4 degradation, p38 is activated and a direct downstream effector of p38 kinase, MK2, becomes phosphorylated at the T334 site (Figure 4A). MK2 phosphorylation during H/R is abolished by inhibiting the activity of p38 kinase with SB203580 (Figure 4A). DUSP4 degradation (via H/R exposure) or down-regulation (via siRNA) both results in increased p38 phosphorylation and subsequent phosphorylation of MK2 at the T334 site (Figure 4B). H/R exposure of siRNA treated cells shows a further increase in p-p38 and p-MK2 (T334). Treatment with the p38 kinase inhibitor, SB203580, abolishes the phosphorylation of MK2. A negative siRNA control group is transfected using scrambled RNA in Figure 3 and 4. No negative effect is seen from the negative control group compared to the non-transfected control cells (data not shown).
Figure 3.
Endothelial cells with DUSP4 gene silencing are more susceptible to H/R-induced cell death and apoptosis. (A) 20X bright-field images of either control or DUSP4 siRNA-transfected RAECs. (B) H/R increases cell death (H/R 59.85% ± 4.89% compared to control, P < 0.005), pre-treatment with SB203580 prevents death (SB H/R 21.45% ± 3.98%, *P<0.0005). DUSP4 gene silencing significantly enhances H/R-induced death (93.24% ± 1.09%) compared to the H/R treatment group (# P < 0.005). This increased susceptibility is reversed by treatment with SB203580 (23.04% ± 6.93%, **P < 0.005 compared to si H/R). Data is expressed as mean ± SEM, n ≥ 3. (C) DUSP4 gene silencing in RAECs enhances H/R-induced TUNEL positive cells (45.81% ± 5.23% compared to H/R alone 29.46 ± 3.75%, #P < 0.05). However, SB203580 treatment of transfected cells significantly lowers their sensitivity to H/R-induced apoptosis (17.47% ± 1.45%, ** P < 0.005 compared to the si H/R group). Similarly the inhibition of H/R-treated cells with SB203580 also significantly diminishes H/R-induced apoptosis (12.99% ± 1.89%), * P < 0.001. Data is expressed as mean ± SEM, n ≥ 3. There is no significant effect on the scrambled siRNA control group compared to control (data not shown).
Figure 4.
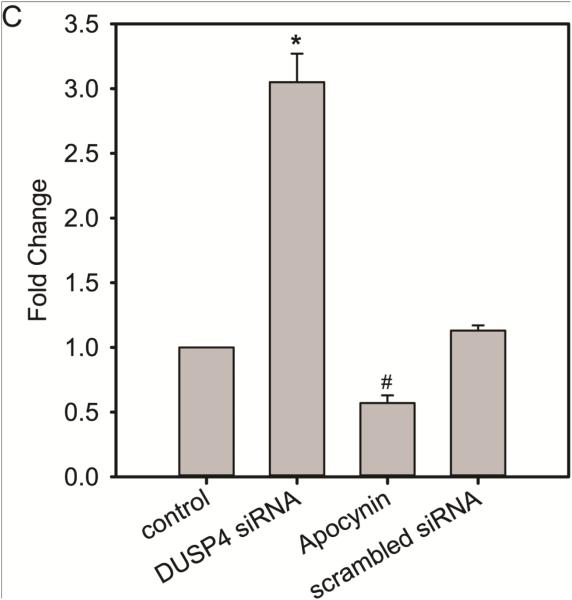
H/R-induced endothelial cell death is modulated by DUSP4 and p38 activity. (A) Immunoblotting of RAECs after H/R. DUSP4 is degraded after 2 h hypoxic treatment. The degradation of DUSP4 is correlated with the over-activation of p38. Phosphorylation of p38 subsequently activates its downstream target, MK2, which can trigger caspase-3 activation and apoptosis. Inhibition of p38 with 20 µM SB203580 prevents H/R-induced apoptosis by blocking the phosphorylation of MK2 at the T334 site. (B) DUSP4 gene silencing leads to more pronounced p38 activation and MK2 phosphorylation at the T334 site after H/R. Similarly the inhibition of p38 kinase activity with 20 µM SB203580 diminishes MK2 activation and can thus prevent H/R-induced apoptosis. * Increased p38 MAPK activity. A negative scrambled siRNA is always performed. No effect from this negative control is seen (data not shown). (C) DUSP4 gene silencing up-regulates superoxide generation from cells (3.05 ± 0.22-fold increase compared to control, * P < 0.001). This increase in superoxide generation is inhibited by the treatment of 100 µM apocynin (0.57 ± 0.06-fold change compared to control, # P < 0.05). There is no significant effect from the negative control of scrambled siRNA. Data is expressed as mean ± SEM, n ≥ 3.
DUSP4 gene knockdown increases superoxide generation from cells
The level of superoxide generation from WT or DUSP4 gene knockdown endothelial cells was measured using DHE HPLC method. Superoxide generation from DUSP4 gene knockdown cells increases 3-fold compared to WT cells (Figure 4C). The increase in superoxide generation is inhibited by 100 µM apocynin, but not by 20 µM allopurinol (data not shown). There is no significant effect from the negative control with scrambled RNA.
DUSP4−/− mice demonstrate increased I/R myocardial damage
Langendorff ex-vivo perfusion of WT (B6129SF2/J) or DUSP4−/− (knockout, KO) mouse hearts was conducted to determine the importance of DUSP4 on the modulation of cardiovascular function under conditions of oxidative stress. TTC staining post-reperfusion was used to measure myocardial infarct area, the affected area was delineated using Image J software and expressed as a percentage of total area. DUSP4−/− hearts had significantly greater infarct size compared to WT hearts (46.75% ± 4.19% and 30.31% ± 3.33%, respectively P < 0.05) (Figure 5A). Assessment of myocardial functional recovery was calculated by the RPP, defined as the product between heart rate (HR) and LVDP. Followed 30 min of global ischemia, DUSP4−/− hearts demonstrated an accentuated impaired recovery when compared to their WT counterparts. While the RPP curve for the WT hearts recovered to 13.83% ± 2.97 % of their baseline value at the 30 min reperfusion time point, the KO RPP remained significantly less than half of the WT values throughout the 30 min reperfusion (5.13% ± 0.98% at 30 min reperfusion time point) (Figure 5B). The LVDP, also expressed as a percentage of its baseline (100%, not shown) value, followed a similar trend as the RPP, being significantly higher for the WT hearts (16.95% ± 3.48 % versus 6.70% ± 0.99% for the LVDP% at the 30 min reperfusion time point (Figure 5C). The other determinant of RPP, heart rate (HR), was significantly higher in the WT hearts up to the 20 min reperfusion period (Figure 5D). Moreover, CF tended to remain lower in the DUSP4−/− hearts compared to the WT (Figure 5E). A measure of left ventricular global contractility, the dP/dtmax, mimicked closely the trend seen in the RPP and LVDP recovery, remaining statistically greater in WT hearts throughout the entire reperfusion phase (449.80 ± 81.17 mmHg/s in WT versus 255.37 ± 32.80 mmHg/s in KO at 30 min reperfusion time point) (Figure 5F). The left ventricular end diastolic pressure (LVEDP) is a measure of chamber compliance and an elevated LVEDP is the result of impaired relaxation [41]. We observed that the LVEDP of DUSP4−/− hearts remains more elevated than that of WT during the entire reperfusion phase, being significantly different during the initial 5 min and then decreasing similarly with reperfusion time for both the WT and the KO hearts (Figure 5G).
Figure 5.
DUSP4−/− mice are more prone to I/R-induced myocardial damage. (A) TTC-stained Langendorff-perfused heart slices from DUSP4−/− versus WT hearts subjected to 30 min global ischemia and 60 min reperfusion demonstrated a significantly greater infarct size (46.75% ± 4.19%) compared to WT (30.31% ± 3.33%). P < 0.05. n = 6 (B-G) Post-ischemic myocardial recovery following 30 min global ischemia and 30 min reperfusion for WT and DUSP4−/− hearts. (B) Rate pressure product (RPP), (C) Left ventricular developed pressure (LVDP), (D) Heart rate (HR), and (E) Coronary flow (CF) are expressed as a percentage of pre-ischemic (PI) value (100 %). (F) dP/dtmax is expressed in mmHg/s. The pre-ischemic (PI) value of dP/dtmax of WT is 4078.4 ± 183.5 mmHg/s, and PI value of dP/dtmax of DUSP4 KO is 5236.8 ± 270.4 mmHg/s. (G) Left ventricular end diastolic pressure (LVEDP) is expressed in mmHg. * P ≤ 0.05, and ** P ≤ 0.01, n > 10.
DUSP4 gene deletion up-regulates Nox4 expression in hearts
Nox4 and Nox2 are the two major isoforms of NADPH oxidase in cardiomyocytes. Via immunoblotting analysis, we found that Nox4 expression is up-regulated in DUSP4−/− hearts (Figure 6A), but not Nox2 (data not shown). It has been demonstrated that Nox4 activity is modulated at the mRNA level, thus we used quantitative PCR to measure the level of mRNA for both WT and DUSP4−/− hearts. The level of Nox4 mRNA from DUSP4−/− hearts increases by almost 5-fold (Figure 6B). This result helps to explain why DUSP4−/− hearts are more susceptible to I/R-induced oxidant stress and tissue injury.
Figure 6.
DUSP4 gene deletion up-regulates Nox4 expression. (A) Immunoblotting against Nox4 antibody reveals that DUSP4 gene deletion promotes Nox4 protein expression (11.3 ± 3.64-fold change versus control, * P < 0.05) under basal conditions. (B) Quantitative PCR analysis shows that DUSP4 gene deletion increases Nox4 mRNA expression (4.9 ± 1.17-fold change versus control, * P < 0.01) under basal conditions. Data is expressed as mean ± SEM, n ≥ 3.
I/R-induced molecular alterations in the DUSP4−/− myocardium
WT or DUSP4−/− mice were confirmed by genotyping. The extent of p38 phosphorylation (p-p38/p38) is augmented post I/R and this is more pronounced in DUSP4−/− hearts (1.35 ± 0.06 versus WT I/R hearts set as 1; #, * P < 0.0001, Figure 7A). The uncontrolled p38 activation is correlated to an increase in MK2 phosphorylation at T334 and cleaved caspase-3 expression (Figure 7B) for both WT and DUSP4−/− I/R hearts. No significant change was seen on MK2 phosphorylation at the T222 site. This is consistent with previous reports of I/R –induced tissue injuries via the apoptotic pathway [42, 43]. This further supports our hypothesis that DUSP4 plays an important role in regulating p38 MAPK activity under oxidative stress.
Figure 7.
Molecular alterations in DUSP4−/− hearts after I/R injury. (A) Increased p38 phosphorylation is evident in hearts subjected to I/R. This effect is more pronounced in DUSP4−/− mice. The ratio of p-p38/p38 of WT I/R hearts is set at 1. * versus WT I/R hearts, P < 0.001 n = 5. (B) The increase in p38 activity leads to MK2 phosphorylation at T334 and cleaved caspase-3 activation. Immunoblotting of cleaved caspase-3 is an indicator of apoptosis. WT and DUSP4−/− hearts both show an increase in caspase-3 and cleaved caspase-3 expression. * Increased p38 MAPK activity. (C) Direct inhibition of p38 activity by infusing with 10 µM SB203580 improves cardiac function at the end of 30 min reperfusion. RPP (%), LVDP (%), and CF (%) are dramatically improved for both WT and DUSP4−/− hearts. For (dP/dt)max, only DUSP4−/− hearts show significant recovery after the treatment of p38 inhibitor. There is no significant difference for heart rate and LVEDP at the end of 30 min reperfusion for both WT and DUSP4−/− hearts. * P < 0.05, and ** P < 0.01. n ≥ 7.
p38 inhibition improves both WT and DUSP4−/− heart function after I/R
To demonstrate that p38 activation during I/R is a major effector contributing to cell death or tissue injury, WT or DUSP4−/− hearts were infused with 10 µM SB203580. Indeed, infusion with p38 inhibitor dramatically improves cardiac function, including RRP (%), LVDP (%), CF (%), and dP/dtmax at the end of 30 min reperfusion for both WT and DUSP4−/− hearts (Figure 7C). These results further enforce our hypothesis that p38 activity plays a critical role in modulating cardiac function post-ischemia, and the proper regulation of p38 activity by DUSP4 is required in order to maintain its function.
DISCUSSION
The central idea of this study is to determine the interrelationship between DUSP4 and p38 MAPK, and the role of DUSP4 on the modulation of cardiovascular function after H/R and I/R-induced oxidative stress. From endothelial cells subjected to H/R insult, we identified that DUSP4 is degraded after H/R, which is correlated to the increased activation of p38 MAPK. This uncontrolled p38 MAPK activation is a major indicator of apoptosis and cell death [28]. Endothelial cells with DUSP4 gene silencing are more susceptible to H/R-mediated death than WT cells. In mouse hearts subjected to global I/R injury, DUSP4 KO mice have a more severe heart injury and worse physiological recovery than their WT counterparts. Thus, in this study we demonstrate for the first time that DUSP4 is of critical importance in regulating p38 activity under oxidative stress, especially I/R injury.
Mitogen-activated protein kinases regulate cellular proliferation, survival, apoptosis, and death. The duration, extent, and subcellular localization of MAPK phosphorylation are critical in deciding cell fate and, thus, the tight regulation of these kinases is required to maintain proper cell function [44]. Although the underlying mechanism leading to p38 over-activation is still unclear, it is known that MAPKs are negatively modulated by DUSPs via the process of dephosphorylation. There is extensive evidence that uncontrolled activation of p38 MAPK is detrimental to cells under oxidative stress, such as in I/R injury. However, all of these studies primarily focus on p38 MAPK activation and its downstream effectors [7, 45]. The role of DUSPs on the modulation of p38 MAPK under oxidative stress has been consistently overlooked. Using immunoblotting analysis, we demonstrate that endothelial DUSP4 degradation is dependent on H/R exposure time (Figure 1A). DUSP4 is one of the DUSPs that can preferentially dephosphorylate p38 MAPK [46]. A recently published study utilizing DUSP1−/−/DUSP4−/− mice suggested that both DUSP1 and DUSP4 are important modulators of p38 activity and that their absence affects cardiovascular function [16]. However, in cultured endothelial cells, we hereby demonstrate that, under H/R, DUSP4 is the main regulator of p38 activity and its degradation dictates cell fate. No significant DUSP1 degradation was seen after 2 h H/R exposure. The degradation of DUSP4, not DUSP1, is correlated to the over-activation of p38 MAPK (Figure 1B and C). There is no significant difference in ERK1/2 phosphorylation after H/R. Moreover, no JNK phosphorylation is detected. With increasing H/R exposure, more endothelial cells die via the apoptotic pathway, as demonstrated by immunostaining of cleaved caspase-3 (Figure 2B) and TUNEL assay (Figure 2C). Taken together, this data strongly suggests that as the endothelial DUSP4 becomes depleted with H/R, cells are unable to properly regulate p38 activity. This leads to the uncontrolled activation of p38 MAPK, which contributes to cellular dysfunction and ultimately apoptosis. This result supports our previous report of DUSP4 as an antioxidant gene, as we have also showed it plays a critical role against Cd2+-induced oxidative stress via p38 MAPK regulation [28]. Thus, identifying the modulatory mechanism of DUSP4 on p38 function under H/R provides an alternative target in oxidant-derived diseases.
Furthermore, using the RNA silencing technique to knock down DUSP4 expression in endothelial cells, we demonstrate that DUSP4 plays a protective role against oxidative stress via the modulation of p38 MAPK activity. Endothelial cells with DUSP4 gene silencing are more sensitive to H/R-induced death and apoptosis compared to cells expressing native amounts of DUSP4 (Figure 3A-C). It is interesting to note that DUSP4 gene silencing leads to increased superoxide generation from cells, and is inhibited by the treatment of apocynin, but not allopurinol (Figure 4C). This further corroborates our hypothesis of DUSP4 as an antioxidant and stress-regulated gene. Indeed, a recent study showed that over-expression of DUSP4 in endothelial cells protects against tumor necrosis factor-a mediated apoptosis [25]. To further delineate the negative regulatory relationship between DUSP4 and p38 MAPK, we treated cells with a well-characterized p38 inhibitor, SB203580, before and during H/R. Treatment with 20µM SB203580 rescued cells, both native and DUSP4 knockdown, from H/R-induced apoptosis (Figure 3A-C). This strongly suggests that, under oxidative stress, DUSP4 becomes degraded and is thus unable to modulate p38 activity, a scenario that culminates in cell death via the apoptotic pathway (Figure 3C). Inhibition of p38 kinase activity is able to prevent H/R-induced cell death, even in cells lacking DUSP4, the main negative regulator of this kinase. Using immunoblotting analysis of the p38 downstream effector, MK2, we have been able to further dissect the signaling pathway controlled by DUSP4. It is evident that in cells with knocked down DUSP4 expression, p38 becomes hyper-phosphorylated and it, in turn, phosphorylates MK2 at the T334 site (Figure 4). Phosphorylation at this site has been demonstrated to be important, not only for MK2 activity, but also for MK2 nuclear translocation [47]. Therefore, it is clear that DUSP4 expression in endothelial cells prevents p38 hyper-phosphorylation and over-activation of MK2, thereby improving cell survival during H/R stress. Thus, as p38 MAPK plays a critical role in modulating cell function under oxidative stress and DUSP4 is a direct regulator of p38, controlling DUSP4 expression in endothelial cells can have beneficial physiological effects.
The activity of MAPKs is critical in deciding cellular biological outcomes, such as differentiation, proliferation, survival, and apoptosis. In response to a stimulus, the signal amplification of MAPKs is modulated by a series of phosphorylation events. Thus, the uncontrolled modulation of this signal cascade can lead to catastrophic consequences [20, 48]. It has been argued whether the activation of p38 in the myocardium is beneficial or detrimental to the organ’s function [38, 49, 50]. We have demonstrated that H/R-induced DUSP4 degradation in endothelial cells contributes to the uncontrolled activation of p38 and that this leads to apoptosis. Not only have we now looked into cell DUSP4 gene silencing, but upon also examining the response of DUSP4−/− mouse myocardium to oxidative stress, our results further support the concept that DUSP4 is required to regulate p38 MAPK under oxidative stress and prevents it from over-activation and subsequent triggering of apoptosis and tissue injury.
DUSP4−/− mouse hearts subjected to global I/R are more prone to I/R-induced injury (Figure 5A). Myocardial oxygen consumption is a good indicator of the response of coronary circulation to increased myocardial oxygen demand, and can be indirectly measured by myocardial rate pressure product (RRP %) [51, 52]. We observed that DUSP4 KO hearts have poor recovery in parameters such as RRP% and LVDP% (Figure 5B and C), compared to WT hearts after I/R injury. Genetic deletion of DUSP4 also affects the myocardial contractility as determined by (dP/dt)max (Figure 5F). The LVEDP of DUSP4 KO hearts after 30 min ischemia is significantly higher in the first 5 min of reperfusion than that of WT hearts (Figure 5G). This is correlated with the increase in the I/R-induced infarct seen in DUSP4 KO hearts. Even though there is no significant difference in CF (%) after I/R injury, the overall flow recovery is lower for DUSP4 KO hearts (Figure 5E). Interestingly, the level of Nox4 protein and mRNA expression is up-regulated in DUSP4−/− hearts (Figure 6). This data strongly suggests that DUSP4 is of critical importance in modulating myocardial function after I/R injury and the reason why DUSP4−/− hearts are more susceptible to I/R injury. Several previous reports have shown that p38 is over-activated after I/R [53, 54]. In our current study, we observe that there is a dramatic increase in p38 phosphorylation (Figure 7A) in WT hearts after I/R-induced oxidative stress and it is known that the over-activation of p38 MAPK is the primary cause for the cardiac dysfunction observed post-myocardial infarction [55]. The phosphorylation of p38 MAPK is further enhanced in DUSP4 KO hearts (Figure 7A). Moreover, there is an increase in caspase-3 and cleaved caspase-3 expression in both WT and DUSP4 KO hearts subjected to I/R (Figure 7B), an observation that is consistent with previous reports of I/R –induced tissue injuries via the apoptotic pathway [42, 43]. However, infusion of p38 inhibitor improves both WT and DUSP4−/− cardiac functional recovery post-ischemia (Figure 7C). This further supports our hypothesis that DUSP4 plays an important role in regulating p38 MAPK activity under oxidative stress.
In conclusion, we demonstrate for the first time that the modulation of p38 MAPK activity by DUSP4 in endothelial cells and hearts under oxidative stress is critical in deciding their physiological outcome. Moreover, cells with DUSP4 gene knockdown and DUSP4 KO hearts are more susceptible to oxidant-induced death and tissue injury. Thus, this work further supports that DUSP4 provides a critical defense against oxidative stress, and that the proper modulation of p38 MAPK by DUSP4 is required to maintain cellular homeostasis under oxidative stress. Continuing molecular and cellular studies in DUSP4 KO myocardium subjected to oxidant stress should aid in the determination of the precise role of DUSP4 on the redox modulation of the MAPK signal pathways and its effects on cardiovascular function.
Highlights.
DUSP4 is degraded under oxidative stress.
The degradation of DUSP4 under oxidative stress is associated with the over-activation of p38 MAPK.
The over-activation of p38 MAPK contributes to cell death via the apoptotic pathway.
DUSP4 gene silencing in endothelial cells and gene deletion in hearts enhances their sensitivity to oxidant stress.
The modulation of p38 MAPK by DUSP4 is of critical importance against oxidative stress.
Acknowledgments
This work was supported by R00 Grant HL103846 (C.-A. C.) from the National Institutes of Health.
List of Abbreviations
- CF
Coronary flow
- CVDs
Cardiovascular diseases
- DUSP
Dual-specificity phosphatase
- ERK
Extracellular-signal-regulated kinase
- H/R
Hypoxia/reoxygenation
- HR
Heart rate
- I/R
Ischemia/reoxygenation
- JNK
c-Jun N-terminal kinase
- KO
Knockout, refers to DUSP4 knock-out
- LVEDP
Left ventricular end diastolic pressure
- LVDP
Left ventricular developed pressure
- MAPK
Mitogen-activated protein kinase
- MI
Myocardial infarction
- MK2
MAPKAP kinase 2
- NAC
N-acetyl cysteine
- PI
Pre-ischemia
- RAECs
Rat aorta endothelial cells
- RPP
Rate pressure product
- siRNA
Small interfering RNA
- TTC
2,3,5-triphenyltetrazolium chloride
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- WT
Wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014. [DOI] [PMC free article] [PubMed]
- [2].Mendis S, Puska P, Norrving B, World Health Organization. World Heart Federation. World Stroke Organization . Global atlas on cardiovascular disease prevention and control. World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization; Geneva: 2011. [Google Scholar]
- [3].Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth. 2012;16:123–132. doi: 10.1177/1089253211436350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- [5].Aune SE, Yeh ST, Zelinski DP, Angelos MG. Measurement of hydrogen peroxide and oxidant stress in a recirculating whole blood-perfused rat heart model. Resuscitation. 2011;82:222–227. doi: 10.1016/j.resuscitation.2010.10.027. [DOI] [PubMed] [Google Scholar]
- [6].Cook SA, Sugden PH, Clerk A. Activation of c-Jun N-terminal kinases and p38-mitogen-activated protein kinases in human heart failure secondary to ischaemic heart disease. Journal of molecular and cellular cardiology. 1999;31:1429–1434. doi: 10.1006/jmcc.1999.0979. [DOI] [PubMed] [Google Scholar]
- [7].Marber MS, Rose B, Wang Y. The p38 mitogen-activated protein kinase pathway--a potential target for intervention in infarction, hypertrophy, and heart failure. Journal of molecular and cellular cardiology. 2011;51:485–490. doi: 10.1016/j.yjmcc.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kumphune S, Chattipakorn S, Chattipakorn N. Role of p38 inhibition in cardiac ischemia/reperfusion injury. Eur J Clin Pharmacol. 2012;68:513–524. doi: 10.1007/s00228-011-1193-2. [DOI] [PubMed] [Google Scholar]
- [9].Damarla M, Parniani AR, Johnston L, Maredia H, Serebreni L, Hamdan O, Sidhaye VK, Shimoda LA, Myers AC, Crow MT, Schmidt EP, Machamer CE, Gaestel M, Rane MJ, Kolb TM, Kim BS, Damico RL, Hassoun PM. Mitogen-Activated Protein Kinase-Activated Protein Kinase 2 Mediates Apoptosis during Lung Vascular Permeability by Regulating Movement of Cleaved Caspase 3. American journal of respiratory cell and molecular biology. 2014;50:932–941. doi: 10.1165/rcmb.2013-0361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Martin JL, Avkiran M, Quinlan RA, Cohen P, Marber MS. Antiischemic effects of SB203580 are mediated through the inhibition of p38 alpha mitogen-activated protein kinase - Evidence from ectopic expression of an inhibition-resistant kinase. Circulation research. 2001;89:750–752. doi: 10.1161/hh2101.099504. [DOI] [PubMed] [Google Scholar]
- [11].Kaiser RA, Bueno OF, Lips DJ, Doevendans PA, Jones F, Kimball TF, Molkentin JD. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. Journal of Biological Chemistry. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
- [12].Kumphune S, Bassi R, Jacquet S, Sicard P, Clark JE, Verma S, Avkiran M, O'Keefe SJ, Marber MS. A chemical genetic approach reveals that p38αlpha MAPK activation by diphosphorylation aggravates myocardial infarction and is prevented by the direct binding of SB203580. The Journal of biological chemistry. 2010;285:2968–2975. doi: 10.1074/jbc.M109.079228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Patterson KI, Brummer T, O'Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochemical Journal. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- [14].Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci. 2006;119:4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- [15].Jeffrey KL, Camps M, Rommel C, Mackay CR. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov. 2007;6:391–403. doi: 10.1038/nrd2289. [DOI] [PubMed] [Google Scholar]
- [16].Auger-Messier M, Accornero F, Goonasekera SA, Bueno OF, Lorenz JN, van Berlo JH, Willette RN, Molkentin JD. Unrestrained p38 MAPK activation in Dusp1/4 double-null mice induces cardiomyopathy. Circulation research. 2013;112:48–56. doi: 10.1161/CIRCRESAHA.112.272963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen G, Hitomi M, Han J, Stacey DW. The p38 pathway provides negative feedback for Ras proliferative signaling. The Journal of biological chemistry. 2000;275:38973–38980. doi: 10.1074/jbc.M002856200. [DOI] [PubMed] [Google Scholar]
- [19].Ashraf MI, Ebner M, Wallner C, Haller M, Khalid S, Schwelberger H, Koziel K, Enthammer M, Hermann M, Sickinger S, Soleiman A, Steger C, Vallant S, Sucher R, Brandacher G, Santer P, Dragun D, Troppmair J. A p38MAPK/MK2 signaling pathway leading to redox stress, cell death and ischemia/reperfusion injury. Cell Commun Signal. 2014;12:6. doi: 10.1186/1478-811X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiological reviews. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wadgaonkar R, Pierce JW, Somnay K, Damico RL, Crow MT, Collins T, Garcia JG. Regulation of c-Jun N-terminal kinase and p38 kinase pathways in endothelial cells. American journal of respiratory cell and molecular biology. 2004;31:423–431. doi: 10.1165/rcmb.2003-0384OC. [DOI] [PubMed] [Google Scholar]
- [22].Al-Mutairi M, Al-Harthi S, Cadalbert L, Plevin R. Over-expression of mitogen-activated protein kinase phosphatase-2 enhances adhesion molecule expression and protects against apoptosis in human endothelial cells. British journal of pharmacology. 2010;161:782–798. doi: 10.1111/j.1476-5381.2010.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lubos E, Kelly NJ, Oldebeken SR, Leopold JA, Zhang YY, Loscalzo J, Handy DE. Glutathione peroxidase-1 deficiency augments proinflammatory cytokine-induced redox signaling and human endothelial cell activation. The Journal of biological chemistry. 2011;286:35407–35417. doi: 10.1074/jbc.M110.205708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Choi JC, Wu W, Muchir A, Iwata S, Homma S, Worman HJ. Dual specificity phosphatase 4 mediates cardiomyopathy caused by lamin A/C (LMNA) gene mutation. The Journal of biological chemistry. 2012;287:40513–40524. doi: 10.1074/jbc.M112.404541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kao DD, Oldebeken SR, Rai A, Lubos E, Leopold JA, Loscalzo J, Handy DE. Tumor necrosis factor-alpha-mediated suppression of dual-specificity phosphatase 4: crosstalk between NFkappaB and MAPK regulates endothelial cell survival. Molecular and cellular biochemistry. 2013;382:153–162. doi: 10.1007/s11010-013-1730-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xu T, Wu X, Chen Q, Zhu S, Liu Y, Pan D, Chen X, Li D. The anti-apoptotic and cardioprotective effects of salvianolic acid a on rat cardiomyocytes following ischemia/reperfusion by DUSP-mediated regulation of the ERK1/2/JNK pathway. PloS one. 2014;9:e102292. doi: 10.1371/journal.pone.0102292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lawan A, Al-Harthi S, Cadalbert L, McCluskey AG, Shweash M, Grassia G, Grant A, Boyd M, Currie S, Plevin R. Deletion of the dual specific phosphatase-4 (DUSP-4) gene reveals an essential non-redundant role for MAP kinase phosphatase-2 (MKP-2) in proliferation and cell survival. The Journal of biological chemistry. 2011;286:12933–12943. doi: 10.1074/jbc.M110.181370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Barajas-Espinosa A, Basye A, Jesse E, Yan H, Quan D, Chen CA. Redox activation of DUSP4 by N-acetylcysteine protects endothelial cells from Cd(2)(+)-induced apoptosis. Free Radic Biol Med. 2014;74:188–199. doi: 10.1016/j.freeradbiomed.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen CA, Lin CH, Druhan LJ, Wang TY, Chen YR, Zweier JL. Superoxide induces endothelial nitric-oxide synthase protein thiyl radical formation, a novel mechanism regulating eNOS function and coupling. The Journal of biological chemistry. 2011;286:29098–29107. doi: 10.1074/jbc.M111.240127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].De Pascali F, Hemann C, Samons K, Chen CA, Zweier JL. Hypoxia and Reoxygenation Induce Endothelial Nitric Oxide Synthase Uncoupling in Endothelial Cells through Tetrahydrobiopterin Depletion and S-Glutathionylation. Biochemistry. 2014;53:3679–3688. doi: 10.1021/bi500076r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Denes L, Szilagyi G, Gal A, Bori Z, Nagy Z. Cytoprotective effect of two synthetic enhancer substances, (−)-BPAP and (−)-deprenyl, on human brain capillary endothelial cells and rat PC12 cells. Life sciences. 2006;79:1034–1039. doi: 10.1016/j.lfs.2006.03.005. [DOI] [PubMed] [Google Scholar]
- [33].Wilhelm M, Kukekov NV, Schmit TL, Biagas KV, Sproul AA, Gire S, Maes ME, Xu Z, Greene LA. Sh3rf2/POSHER protein promotes cell survival by ring-mediated proteasomal degradation of the c-Jun N-terminal kinase scaffold POSH (Plenty of SH3s) protein. The Journal of biological chemistry. 2012;287:2247–2256. doi: 10.1074/jbc.M111.269431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen CA, De Pascali F, Basye A, Hemann C, Zweier JL. Redox modulation of endothelial nitric oxide synthase by glutaredoxin-1 through reversible oxidative post-translational modification. Biochemistry. 2013;52:6712–6723. doi: 10.1021/bi400404s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang Z, Storm DR. Extraction of DNA from mouse tails. Biotechniques. 2006;41:410–412. doi: 10.2144/000112255. [DOI] [PubMed] [Google Scholar]
- [37].Talukder MAH, Kalyanasundaram A, Zhao X, Zuo L, Bhupathy P, Babu GJ, Cardounel AJ, Periasamy M, Zweier JL. Expression of SERCA isoform with faster Ca2+ transport properties improves postischemic cardiac function and Ca2+ handling and decreases myocardial infarction. American Journal of Physiology-Heart and Circulatory Physiology. 2007;293:H2418–H2428. doi: 10.1152/ajpheart.00663.2007. [DOI] [PubMed] [Google Scholar]
- [38].Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, Wang C, Lee JC, Feuerstein GZ, Yue TL. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99:1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- [39].Ojha N, Roy S, Radtke J, Simonetti O, Gnyawali S, Zweier JL, Kuppusamy P, Sen CK. Characterization of the structural and functional changes in the myocardium following focal ischemia-reperfusion injury. American journal of physiology. Heart and circulatory physiology. 2008;294:H2435–2443. doi: 10.1152/ajpheart.01190.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ni NC, Yan D, Ballantyne LL, Barajas-Espinosa A, St Amand T, Pratt DA, Funk CD. A Selective Cysteinyl Leukotriene Receptor 2 Antagonist Blocks Myocardial Ischemia/Reperfusion Injury and Vascular Permeability in Mice. Journal of Pharmacology and Experimental Therapeutics. 2011;339:768–778. doi: 10.1124/jpet.111.186031. [DOI] [PubMed] [Google Scholar]
- [41].Mielniczuk LM, Lamas GA, Flaker GC, Mitchell G, Smith SC, Gersh BJ, Solomon SD, Moye LA, Rouleau JL, Rutherford JD, Pfeffer MA. Left ventricular end-diastolic pressure and risk of subsequent heart failure in patients following an acute myocardial infarction. Congest Heart Fail. 2007;13:209–214. doi: 10.1111/j.1527-5299.2007.06624.x. [DOI] [PubMed] [Google Scholar]
- [42].Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gottlieb RA, Engler RL. Apoptosis in myocardial ischemia-reperfusion. Ann N Y Acad Sci. 1999;874:412–426. doi: 10.1111/j.1749-6632.1999.tb09255.x. [DOI] [PubMed] [Google Scholar]
- [44].Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- [45].Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis. Journal of molecular and cellular cardiology. 2005;38:47–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- [46].Echavarria R, Hussain SN. Regulation of angiopoietin-1/Tie-2 receptor signaling in endothelial cells by dual-specificity phosphatases 1, 4, and 5. J Am Heart Assoc. 2013;2:e000571. doi: 10.1161/JAHA.113.000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Meng W, Swenson LL, Fitzgibbon MJ, Hayakawa K, Ter Haar E, Behrens AE, Fulghum JR, Lippke JA. Structure of mitogen-activated protein kinase-activated protein (MAPKAP) kinase 2 suggests a bifunctional switch that couples kinase activation with nuclear export. The Journal of biological chemistry. 2002;277:37401–37405. doi: 10.1074/jbc.C200418200. [DOI] [PubMed] [Google Scholar]
- [48].Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends in biochemical sciences. 2006;31:268–275. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- [49].Barancik M, Htun P, Strohm C, Kilian S, Schaper W. Inhibition of the cardiac p38-MAPK pathway by SB203580 delays ischemic cell death. J Cardiovasc Pharmacol. 2000;35:474–483. doi: 10.1097/00005344-200003000-00019. [DOI] [PubMed] [Google Scholar]
- [50].Mocanu MM, Baxter GF, Yue Y, Critz SD, Yellon DM. The p38 MAPK inhibitor, SB203580, abrogates ischaemic preconditioning in rat heart but timing of administration is critical. Basic Res Cardiol. 2000;95:472–478. doi: 10.1007/s003950070023. [DOI] [PubMed] [Google Scholar]
- [51].Stoner JD, Angelos MG, Clanton TL. Myocardial contractile function during postischemic low-flow reperfusion: critical thresholds of NADH and O2 delivery. American journal of physiology. Heart and circulatory physiology. 2004;286:H375–380. doi: 10.1152/ajpheart.00436.2003. [DOI] [PubMed] [Google Scholar]
- [52].Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation. 1978;57:549–556. doi: 10.1161/01.cir.57.3.549. [DOI] [PubMed] [Google Scholar]
- [53].Shimizu N, Yoshiyama M, Omura T, Hanatani A, Kim S, Takeuchi K, Iwao H, Yoshikawa J. Activation of mitogen-activated protein kinases and activator protein-1 in myocardial infarction in rats. Cardiovasc Res. 1998;38:116–124. doi: 10.1016/s0008-6363(97)00327-1. [DOI] [PubMed] [Google Scholar]
- [54].Yin T, Sandhu G, Wolfgang CD, Burrier A, Webb RL, Rigel DF, Hai T, Whelan J. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. The Journal of biological chemistry. 1997;272:19943–19950. doi: 10.1074/jbc.272.32.19943. [DOI] [PubMed] [Google Scholar]
- [55].Sy JC, Seshadri G, Yang SC, Brown M, Oh T, Dikalov S, Murthy N, Davis ME. Sustained release of a p38 inhibitor from non-inflammatory microspheres inhibits cardiac dysfunction. Nat Mater. 2008;7:863–868. doi: 10.1038/nmat2299. [DOI] [PMC free article] [PubMed] [Google Scholar]