Abstract
A gap in our understanding of the beneficial systemic responses to dietary constituents nitrate (NO3−), nitrite (NO2−) and conjugated linoleic acid (cLA) is the identification of the downstream metabolites that mediate their actions. To examine these reactions in a clinical context, investigational drug preparations of 15N-labeled NO3− and NO2− were orally administered to healthy humans with and without cLA. Mass spectrometry analysis of plasma and urine indicated that the nitrating species nitrogen dioxide was formed and reacted with the olefinic carbons of unsaturated fatty acids to yield the electrophilic fatty acid, nitro-cLA (NO2-cLA). These species mediate the post-translational modification (PTM) of proteins via reversible Michael addition with nucleophilic amino acids. The PTM of critical target proteins by electrophilic lipids has been described as a sensing mechanism that regulates adaptive cellular responses, but little is known about the endogenous generation of fatty acid nitroalkenes and their metabolites. We report that healthy humans consuming 15N-labeled NO3− or NO2−, with and without cLA supplementation, produce 15NO2-cLA and corresponding metabolites that are detected in plasma and urine. These data support that the dietary constituents NO3−, NO2− and cLA promote the further generation of secondary electrophilic lipid products that are absorbed into the circulation at concentrations sufficient to exert systemic effects before being catabolized or excreted.
Keywords: nitrogen metabolism, diet, redox signaling, nitro-fatty acid, conjugated linoleic acid, nitrate, nitrite
Graphical abstract
Introduction
Fatty acid nitroalkene derivatives (NO2-FA) are detected in species as diverse as plants, insects and humans [1–3], but little is known of how NO2-FA are endogenously generated. NO2-FA display signaling actions that expands both the pharmacokinetics and scope of nitric oxide (·NO) signaling events to include actions beyond the activation of guanylate cyclase-dependent cGMP production [4–7]. Thus, it is important to better understand both the mechanisms of formation and actions of these lipid signaling mediators. In addition to previously described biomolecule nitration mechanisms [8, 9], metal center-independent reactions of ·NO and nitrite (NO2−) at neutral pH yield both symmetric and asymmetric dinitrogen trioxide (N2O3), that in turn mediates the concerted S-nitrosation of thiols and nitration of unsaturated fatty acid conjugated dienes [10]. We now report that the reactions of these oxides of nitrogen support substantial NO2-FA generation in humans, the levels of which can be altered by dietary supplementation.
The unique electrophilic character of fatty acid nitroalkenes promotes the reversible Michael addition of susceptible cysteines in a limited number of transcriptional regulatory factors and enzymes [11]. This post-translational modification of functionally-significant hyperreactive thiols induces pleiotropic responses that include the cGMP-independent activation of transcriptional programs regulated by nuclear factor (erythroid-derived-2)-like 2 (Nrf2) and nuclear lipid receptors, as well as inhibition of pro-inflammatory signaling by nuclear factor kappa-β (NF-kβ) [12–15]. Herein, the mass spectrometry analysis of healthy human urine and plasma at different times after oral consumption of 15N-labeled stable isotopes of NO2− and NO3− and conjugated linoleic acid (cLA) reveals substantial NO2-FA generation at concentrations capable of mediating metabolic and inflammatory signaling responses.
Materials and Methods
Study Population
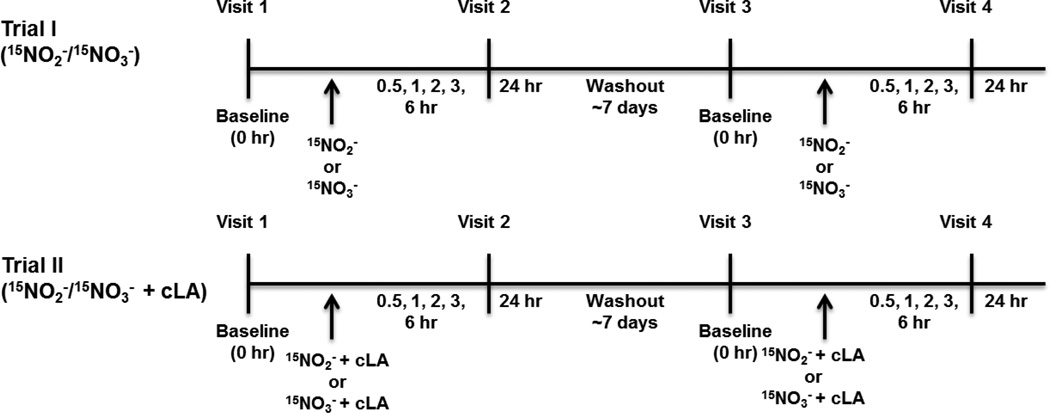
This study was performed with University of Pittsburgh IRB (PRO11120134) and FDA IND (#115926) approval. Informed consent was obtained from all subjects before participation. Subjects who had a normal blood pressure (BP) defined as systolic BP≤130 and diastolic BP≤85 mm Hg were recruited from university/community advertisements. Subjects meeting any of the following were excluded: 1) Positive urine pregnancy test; 2) Recent addition/change in birth control dosing (pills, shot or intrauterine device); 3) Concurrent use of medications affecting glucose or lipid metabolism; 4) Current use of BP medications regardless of BP control; 5) Current use of PD5 inhibitors or organic nitrates; 6) Not stable on treatments for the prior three months or not planning to remain on current dose of medications for birth control, etc.; 7) Chronic mental health or medical conditions including diabetes, obesity syndromes, liver or kidney disease; 8) Smoker. Healthy volunteers fasted overnight (10–12 h) and were randomized to receive either Na15NO2 (20 mg) or Na15NO3 (1 gm). Following ~7 day washout period, volunteers were crossed over to receive the other nitrogen oxide (NOx) (Trial I). In Trial II, cLA (3 gm) was given concurrently with both NOx (Fig. 1). Blood was drawn at baseline, 0.5, 1, 2, 3, 6, and 24 h after oral administration of NOx ± cLA. Urine samples were collected at baseline, 6, and 24 h for Trial II only. Vital signs including blood and mean arterial pressure, respiratory rate, heart rate, and methemoglobin % (noninvasively by co-oximetry) were monitored as a safety precaution and remained within normal ranges for the duration of the trials.
Figure 1.
Trial I and II study design. Trial I: volunteers were randomized to receive either 15NO2− (20 mg) or 15NO3− (1 gm) and blood samples were collected at t = 0, 0.5, 1, 2, 3, 6, and 24 h. After a 7 day washout period, volunteers returned to receive the other oxide of nitrogen. In Trial II, the same 15NO2− or 15NO3− administration and blood collection protocol was implemented, along with cLA (3 gm) supplementation and urine collection at t = 0, 6, and 24 h.
Materials
Total Lean™ cLA capsules (1 gm) were purchased from General Nutrition Company (Pittsburgh, PA). The safflower oil-derived capsules are a racemic mixture of (9Z,11E)-cLA and (10E,12Z)-cLA. 2,3-diaminonaphthalene (DAN) and 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) were purchased from Sigma-Aldrich (St. Louis, MO). (9Z,11E)-cLA was purchased from Nu-Chek Prep (Elysian, MN) and (11Z,13E)-cLA acid standard was purchased from Matreya (Pleasant Gap, PA). [13C18]-NO2-OA internal standard and NO2-cLA standard were synthesized as described previously [3, 16]. MS grade solvents for liquid chromatography electrospray ionization tandem mass spectrometry (LC-MS/MS) were purchased from Burdick and Jackson (Muskegon, MI). Solvents for extractions and solid phase extraction (SPE) columns (C18 reversed phase; 500 mg, 6 mL capacity) were purchased from Thermo Scientific (Pittsburgh, PA).
15NO3− and 15NO2− Capsule Formulation
Stable isotope 15N-labeled sodium nitrite (10 mg) and sodium nitrate (500 mg) capsules were prepared by the NIH Pharmaceutical Development Section. The 15N isotopes were purchased from Cambridge Isotope Laboratories (Andover, MA). Each isotope was milled separately to provide uniformity in particle size with the needed excipients to ensure uniform powder mixture content and controlled disintegration and dissolution characteristics.
15Nitrite was especially difficult because of the variation in particle size and difficulty in milling. A mini blender was used initially followed by passage through a #40 mesh screen. After this, microcrystalline cellulose, pregelatinized starch, sodium starch glycolate, crospovidone and colloidal silicon dioxide were added sequentially. The mixture was milled on a Waring blender at low speed for 2 min before passing the through the #40 mesh screen 4 times. This produced an approximate particle size of 425 micrometers. 15Nitrate was mixed with all the same excipients used for the nitrites, but it did not require the same degree of mechanical milling and were passed through a #60 mesh screen. This produced an approximate particle size of 250 micrometers. Uniformity of capsule strength was affected by use of a mini cap-100 capsule filler making full fill capsules of each salt form. The quality and stability of the capsules were measured by stability indicating HPLC assays. All operations were performed in a GMP pharmaceutical manufacturing facility with chemical analysis preformed on site.
Lipid extraction
Plasma samples collected at each time point were extracted in triplicate using a modified Bligh-Dyer with interim vortexing. Samples were centrifuged at 2800 × g for 5 min. The top layer (aqueous) was transferred to a clean vial, [13C18]-NO2-OA internal standard (10 µL of 1 µM) was added, samples (5.5 mL) were vortexed, and placed on ice for 15 min. HgCl2 (10 mM) was added and the samples were incubated for 30 min at 37°C to release adducted nitro-fatty acids. A 10% w/v sulfanilamide solution (100 µL) was then added to inhibit artifactual nitration. The aqueous layer was extracted with 1 M formic acid:isopropanol:hexanes (2:20:30, vol/vol/vol) followed by an additional 1 mL of hexanes, vortexing, and centrifugation. The upper organic phase was dried and reconstituted in 100 µL methanol before MS analysis. This extraction procedure allowed for measurement of NO2-cLA that was non-covalently bound to albumin and other proteins as well as the NO2-cLA that was covalently adducted to small thiols and proteins. These species were found in the protein precipitate interface and aqueous layer that form during the extraction procedure. The extraction of NO2-cLA and its metabolites, including Cys-NO2-cLA, from urine was performed and normalized to creatinine (Cre) as previously described [3, 17].
LC-MS/MS
A CTC PAL autosampler (Leap Technologies, Carrboro, NC) and a Shimadzu LC-20AD pump (Columbia, MD) coupled to an AB Sciex (Framingham, MA) 5000 triple quadrupole mass spectrometer was used for the quantification of modified fatty acids. Samples (10 µL) were separated on a reversed phase HPLC column (Luna C18(2), 5µ, 2 × 100 mm, Phenomenex, Torrence, CA) at a 0.75 mL/min flow rate using a gradient solvent system consisting of water containing 0.1% acetic acid (solvent A) and acetonitrile containing 0.1% acetic acid (solvent B). Samples were applied to the column at 40% B (0.5 min) and eluted with a linear increase in solvent B (40–100%) over 10 min. This was followed by a wash using 100% B for 2 min. The gradient then returned to starting conditions at 40% B for 3 min. MS analyses used electrospray ionization in the negative ion mode with the collision gas set at 5 units, curtain gas 50 units, ion source gas #1 55 units and #2 50 units, ion spray voltage −4500 V, and temperature 650°C. The declustering potential was −60, entrance potential −10, collision energy −35, and the collision exit potential −3. MRM was used for sample analysis and quantification of lipids showing loss of a nitro group (m/z 46) upon collision-induced dissociation. A standard curve using synthetic NO2-cLA and [13C18]-NO2-OA internal standard allowed for the quantification of endogenous products.
Nitrite determination
Total plasma and urine NO2− was measured using the triiodide method as previously described [18]. To determine the contribution of 14NO2− and 15NO2− to these measurements, plasma and urine was reacted with diaminonaphthalene (DAN) and the products, 14N-napthotriazole (14NT) and 15N-napthotriazole (15NT) were measured using LC-MS/MS. A calibration curve using a Na14NO2 standard undergoing the same sample preparation was used for quantification. Additionally, the “bleed over” of 14N into the 15N transition has been accounted for in the calculations of the 15N contribution to total nitrite. For samples, 100 µL of plasma or urine was deproteinized with 1 vol of cold acetonitrile and centrifuged (5000×g, 5 min). Supernatants were adjusted to pH 6 with 5 µL of 0.62 M HCl, then 10 µL of 0.5 mg ml−1 DAN in 0.62 M HCl was added, samples incubated at RT for 15 min, and reactions were neutralized with 10 µL of 1.4 M NaOH. The samples (10 µL injection) containing 14NT and 15NT were separated on a reversed phase HPLC column (Phenomenex Gemini C18, 3 µ, 2 × 20 mm) at a 0.65 mL min−1 flow rate. Samples were applied to the column at 5% B (0.3 min) and eluted with a linear increase in solvent B (5–100%) over 3.5 min. This was followed by a wash using 100% B for 1 min. The gradient was returned to starting conditions with 5% B for 0.5 min. MS analyses were conducted using electrospray ionization in positive ion mode with the collision gas set at 5 units, curtain gas 40 units, ion source gas #1 at 55 units and 2 at 50 units, ion spray voltage −5500 V, and temperature at 650°C. The declustering potential was set to 70, entrance potential 5, collision energy 35, and the collision exit potential 5. MRM transitions for 14NT (170→115) and 15NT (171→115) were used for analyses.
cLA determination
cLA detection and characterization was performed by LC-MS/MS in negative ion mode after derivatization with 10 mM 4-phenyl-1,2,4 triazoline-3,5 dione (PTAD) to form the respective Diels-Alder adducts. As an internal standard, 1 µM of “non-natural” (11Z,13E)-cLA was added. The following transitions were used for detecting derivatized cLA positional isomers: (10E,12Z)-cLA (454→182, 454→205, 454→220, 454→238) and (9Z,11E)-cLA (454→168, 454→191, 454→206, 454→224). Quantification was performed using standard curves of PTAD-cLA (0–10 µM) and (11Z,13E)-cLA (400 nM).
Statistical analysis
Plasma and urine extractions were performed on samples collected from 5 subjects, with analyses done in duplicate or triplicate for each subject per time point. Data is represented as mean ± SEM for plasma cLA and overall 14NO2-cLA levels after 15NO2− and 15NO3− supplementation. The 14N/15N contributions to total nitrite were determined as percentages of the total nitrite concentration for each time point in plasma and urine. Box plots represent the concentration of 14NO2-cLA and 15NO2-cLA in plasma and urine over time; with median and range reported. Plasma levels are reported as concentration and urine levels are reported as pmol mg−1 creatinine.
Results
NO2-cLA formation upon oral ingestion of NO3− and NO2−
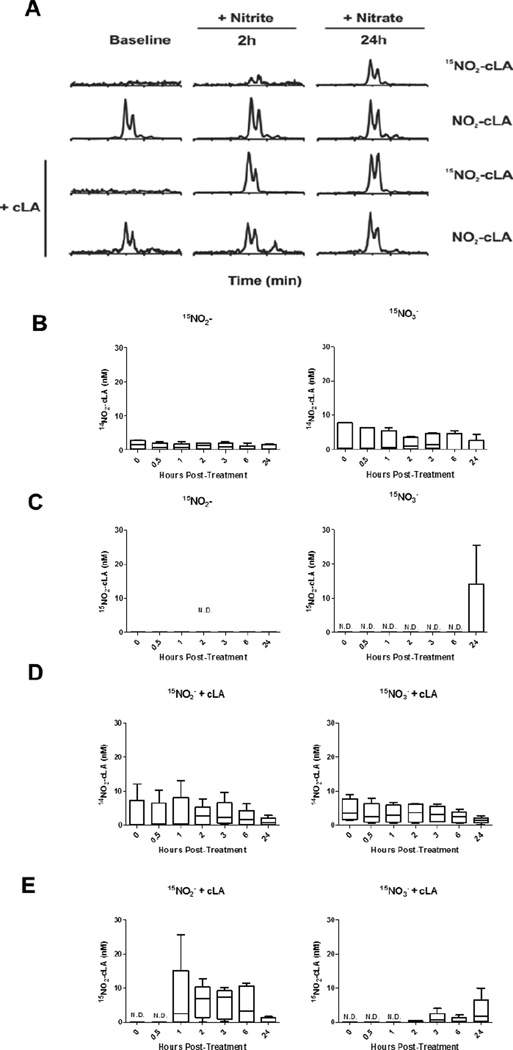
In Trial I, healthy volunteers (3 males, 2 females, mean age 29 ± 7 yr, other clinical indices in Supplementary Table 1) fasted overnight prior to supplement randomization with either oral 15NO2− (20 mg) or 15NO3− (1 gm). Blood was drawn at baseline, 0.5, 1, 2, 3, 6, and 24 h post-supplementation (Fig. 1). After a 7 day washout, the same subjects received the opposite NOx and blood was drawn as before. Plasma lipids were extracted and both 14NO2-cLA (endogenous) and 15NO2-cLA (formed from 15NOx) were measured and quantified by LC-MS/MS (Fig. 2A). Endogenous 14NO2-cLA was present in the plasma of all volunteers and did not vary significantly over the 24 h sampling period after both 15NO2− and 15NO3− supplementation (15NO2−: 1.0 ± 0.3 nM and 15NO3−: 2.1 ± 0.7 nM, Fig. 2B). 15NO2-cLA was not detectable over the sampling period after 15NO2− supplementation and was only detectable 24 h after 15NO3− consumption (0.14 nM median, range = 0 to 25.3 nM, Fig. 2C).
Figure 2.
Dietary supplementation of nitrite, nitrate and conjugated linoleic acid supports NO2-cLA formation detected in plasma. Oral 15NO2− (20 mg) or 15NO3− (1 gm) was ingested ± cLA (3 gm). (A) LC-MS/MS chromatograms of plasma lipid extract show endogenous 14NO2-cLA (MRM, 326/46) present at each time point and 15NO2-cLA (MRM, 327/47) formed following 15NOx supplementation for 15NO2− at 2 h and 15NO3− at 24 h. (B) 14NO2-cLA remains at basal levels over 24 h after 15NO2− (1.0 ± 0.3 nM) or 15NO3− supplementation (2.1 ± 0.7 nM). (C) Only 15NO3− administration led to detectable 15NO2-cLA at 24 h (range = 0 to 25.3 nM, 0.14 nM median). (D) 14NO2-cLA remained at basal levels after cLA supplementation (+15NO2−, 2.6 ± 0.8 nM) and (+15NO3−, 3.1 ± 0.9 nM). (E) 15NO2− + cLA resulted in early and significant increases from 1–6 h in 15NO2-cLA (1 h range = 0 to 25.7 nM, 2.5 nM median and 6 h range = 0 to 11.5 nM, 3.3 nM median). Following 15NO3− + cLA, 15NO2-cLA was detectable at 2 h and highest at 24 h (range = 0 to 9.9 nM, 1.6 nM median). n=5 volunteers.
NO2-cLA generation is promoted by cLA consumption
The same 5 healthy volunteers returned 3 months later for Trial II, identical in design with respect to oral 15NOx administration and the timing of blood sampling. The protocol also included a) the additional consumption of cLA (3 gm) in concert with each 15NOx and b) the collection of urine at baseline, 6 and 24 h after 15NOx + cLA administration. Similar concentrations of endogenous 14NO2-cLA were detectable over the 24 h sampling period and not affected by 15NOx + cLA supplementation (15NO2− + cLA : 2.6 ± 0.8 nM and 15NO3− + cLA L 3.1 ± 0.9 nM, Fig. 2D). The modest decrease over time in 14NO2-cLA may have been due to volunteers fasting prior to 15NOx + cLA supplementation during the first 6 h of blood collection. Furthermore, volunteers were instructed to consume an evening meal and beverage low in NOx and cLA before overnight fasting prior to 24 h sampling. 15NO2-cLA generation became detectable at 1 h (2.5 nM median, range = 0 to 25.7 nM) after 15NO2− + cLA supplementation and remained elevated until 6 h (3.3 nM median, range = 0 to 11.5 nM). In contrast, 15NO3− + cLA mediated 15NO2-cLA generation became detectable at 2 h and was the greatest at 24 h (1.6 nM median, range = 0 to 9.9 nM), compared to baseline (Fig. 2E).
Plasma levels of free cLA and NO2−
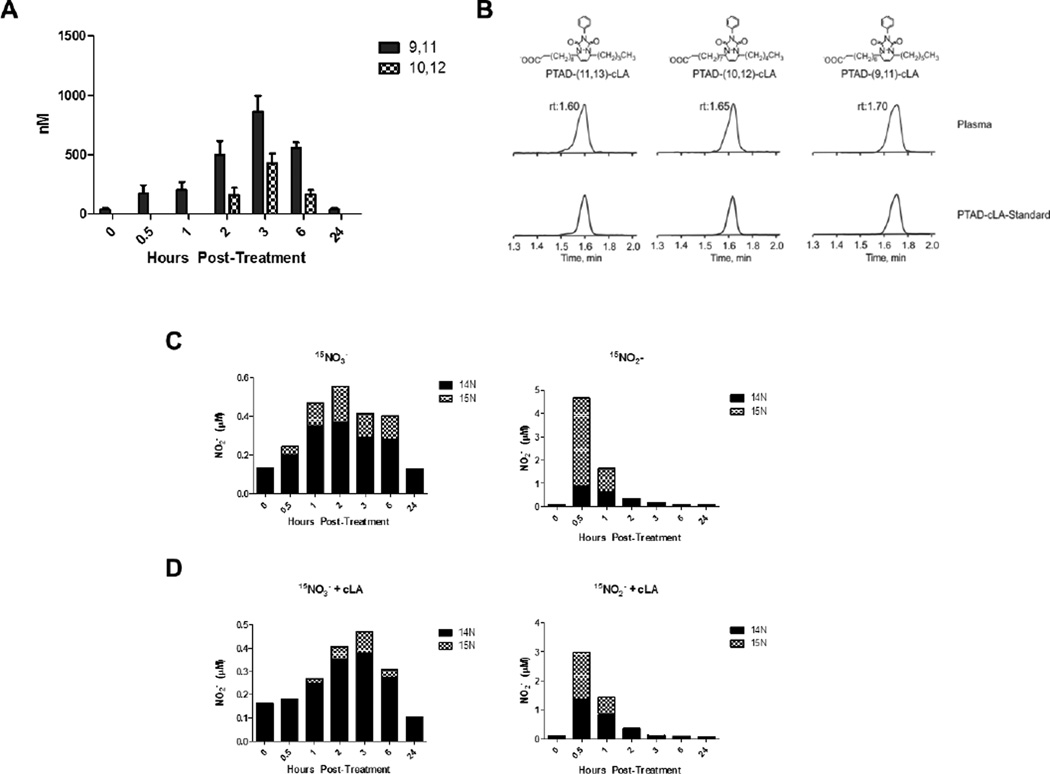
The consumption of cLA in Trial II supported significant increases in plasma 15NO2-cLA levels from 1 to 6 h following 15NO2− + cLA administration (Fig. 2E). These changes corresponded with the timing of peak plasma concentrations of both (9Z,11E)-cLA and (10E,12Z)-cLA (Fig. 3A). The (9Z,11E)-cLA and (10E,12Z)-cLA isomers were quantified by LC-MS using a PTAD derivatization and the non-naturally occurring 11,13-cLA isomer as an internal standard (Fig. 3B). The (9Z,11E)-cLA isomer remained elevated above baseline between 0.5 and 6 h before decreasing back to baseline while the (10E,12Z)-cLA isomer, derived from the cLA supplement, was only detected from 2 to 6 h (Fig. 3A). The concentration of both isomers peaked at 3 h after 15NO2− + cLA administration with (9Z,11E)-cLA ranging from 530 to 1350 nM with an average of 858 ± 138 nM and (10E,12Z)-cLA ranging from 271 to 710 nM, with an average of 430.3 ± 77.8 nM (Fig. 3A). The levels of 15NO2-cLA after 15NO3− + cLA administration were lower and detectable at later time points compared to those after 15NO2− + cLA supplementation. The lower levels of cLA at later time points may have contributed to the decreased 15NO3−-derived 15NO2-cLA formation (Fig. 2D and E).
Figure 3.
Plasma levels of free cLA and NO2−. (A) Free (10E,12Z)-cLA and (9Z,11E)-cLA levels measured from plasma over time in Trial II. The mean represents a combination of values from 15NO3− and 15NO2− supplementation. (9Z,11E)-cLA is found endogenously whereas (10E, 12Z)-cLA is solely derived from the supplement. Both isomers of free cLA peaked at 3 h with (9Z,11E)-cLA levels ranging from 530 to 1350 nM with a mean of 858 ± 138 nM. (10E,12Z)-cLA ranging from 270 to 710 nM with a mean of 430 ± 77.8 nM. (B) Representative chromatograms of the PTAD-derivatized (9Z,11E)-cLA, (10E,12Z)-cLA, and (11Z,13E)-cLA internal standard in plasma. (C) The 14N and 15N contribution to total plasma NO2− concentration after 15NO2− or 15NO3− supplementation in Trial I based on the mean NO2− value (Fig S1A). (D) The 14N and 15N contribution to total plasma NO2− concentration after 15NO2− + cLA or 15NO3− + cLA supplementation in Trial II based on the mean NO2− value (Fig S1B). LC-MS/MS was used to differentiate between the contributions of 14NOx and 15NOx species to overall NO2− levels and the mean concentration for each time point was used for both (B) and (C). The mean NO2− concentration ± SEM can be found in Supplementary Fig. S1 for n=5 volunteers.
Plasma NO2− levels after 15NOx supplementation were determined for both trials using both chemiluminescence and tri-iodide-based reactions (Supplementary Fig S1A and B) [18, 19]. To distinguish between the contributions of endogenous and exogenously-administered NOx species to total NO2−, 2,3-diaminonaphthalene (DAN) was reacted with NO2− and the reaction products 14N- and 15N-napthotriazole (NT) were differentiated using LC-MS/MS (Fig. 3C and D) [20]. The mean NO2− concentration for each time point (Supplementary Fig S1A and B) was used to calculate relative 14N and 15N contributions to total NO2− concentration. Plasma NO2− concentrations were greatest at 0.5 h post-15NO2− supplementation in both trials; however, total NO2− concentrations were greatest at 0.5 h in Trial I, where there was a larger contribution of 15NO2− to total NO2− (Fig. 3C). The peak plasma 15NO2− concentration at 0.5 h preceded the highest 15NO2− + cLA-dependent 15NO2-cLA level, seen at 2 h (Fig. 2E). The NO2− concentrations after 15NO3− supplementation in Trials I and II peaked between 2 and 3 h with a slightly longer lag time when cLA was added (Fig. 3C and D). As expected, 15NO3− did not contribute as much to the net NO2− concentration as direct administration of 15NO2− (Fig. 3C and D). Notably, dietary supplementation with 15NO3− and 15NO2− caused an increase of all detectable endogenous NOx species, thereby increasing 14NO2− levels that paralleled concentrations of 15NOx species. This is most evident with 15NO3− supplementation in both Trial I and II (Fig. 3C ad D).
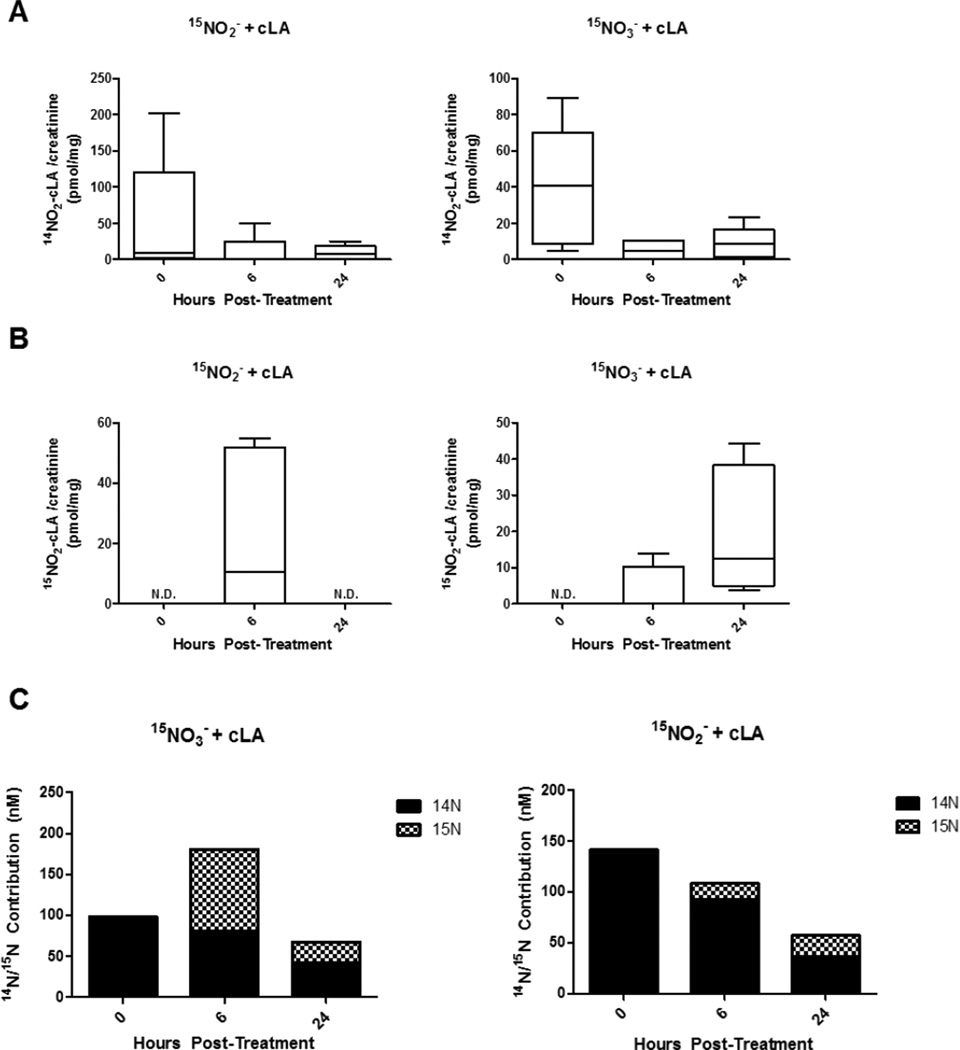
Urinary NO2-cLA and NO2− concentrations
Urine was collected in Trial II at baseline, 6 and 24 h following 15NO2− or 15NO3− + cLA supplementation. Endogenous urinary mean 14NO2-cLA concentrations modestly decreased after 15NO2− + cLA or 15NO3− + cLA supplementation over the 24 h period (Fig. 4A). The concentration of urinary 15NO2-cLA was greatest 6 h after 15NO2− + cLA consumption (10.7 pmol/mg creatinine (Cre) median, range = 0 to 55.0 pmol/mg Cre) and 24 h after 15NO3− + cLA consumption (12.5 pmol/mg Cre median, range = 3.8 to 44.3 pmol/mg Cre, Fig. 4B). Urinary NO2− was also measured via triiodide chemiluminescence (Supplementary Fig S1C) [18, 19] and the mean NO2− concentration for each time point was used to calculate relative 14N and 15N contributions to total NO2− concentration (Fig. 4C and Supplementary Fig. S1C). This paralleled 15NO2-cLA levels, peaking at 6 h with an equimolar contribution from 14NOx and 15NOx species (Fig. 4C). Total urinary NO2− concentrations decreased after 15NO3− + cLA administration over the 24 h time period, with little contribution from 15NO3− (Fig. 4C). Urinary β-oxidation products and cysteine-adducts (Supplementary Fig. S2 and S3) of 14NO2-cLA and 15NO2-cLA were also measured, confirming the electrophilic reactivity of the parent molecules and metabolites [3]. Cysteine-NO2-cLA adducts were detected in urine, with the ion intensity of Cys-15NO2-cLA metabolites being ~10-fold greater than Cys-14NO2-cLA metabolites at 24 h (Supplementary Fig. S3).
Figure 4.
Urinary NO2-cLA and NO2− concentrations. Urine was collected at 0, 6, and 24 h following oral 15NO2−+ cLA or 15NO3− + cLA administration. (A) 14NO2-cLA levels in urine are highest at baseline and decrease over time. (B) Urinary 15NO2-cLA was greatest 6 h after 15NO2− + cLA consumption (range = 0 to 55.0 pmol/mg Cre, 10.7 pmol/mg Cre median) and 24 h after 15NO3− + cLA consumption (range = 3.8 to 44.3 pmol/mg Cre, 12.5 pmol/mg Cre median). (C) The 14N and 15N contribution to total urinary NO2− concentration after 15NO2− + cLA or 15NO3− + cLA supplementation in Trial II based on the mean NO2− value (Supplementary Fig. S1C). n=5 volunteers.
Discussion
Intrinsic to the metabolism of NO3− and NO2− is the formation of the nitrogen dioxide radical (·NO2). This species is formed by metalloprotein-catalyzed oxidation of NO2−, the autoxidation of ·NO, the protonation of NO2− to N2O3 followed by its disproportionation, decomposition of nitrous acid, and the homolytic scission of two key products of ·NO and superoxide (O2 ·−) reaction, peroxynitrous acid and nitrosoperoxocarbonate [21–23]. Unsaturated fatty acids having conjugated double bonds, such as cLA, are highly reactive with ·NO2, a reaction that occurs much more readily than tyrosine nitration [24]. ·NO2-induced nitration is also several orders of magnitude greater for the conjugated diene system of cLA compared to the bis-allylic diene system found in linoleic and oleic acids due to resonance stabilization of the radical product formed during ·NO2 radical addition [25]. This makes cLA the preferable substrate for nitration despite the fact that conjugated dienes are present in ~100-fold lower concentrations than bis-allylic unsaturated fatty acids in plasma [26]. Dietary sources of cLA include dairy products and meat and cLA is also a product of enterosalivary bacterial Δ9-desaturase activity [27]. The predominant endogenous cLA isomer is 9-cis, 11-trans-cLA, whereas plant-derived oils contain a racemic mixture of 9-cis, 11-trans and 10-trans, 12-cis-cLA [28]. The consumption of cLA is linked with beneficial clinical responses, including the reduction of obesity-related body mass index, improved insulin sensitivity and attenuation of airway hyperactivity, all of which we propose are due in part to the formation and signaling actions of NO2-cLA [29–32].
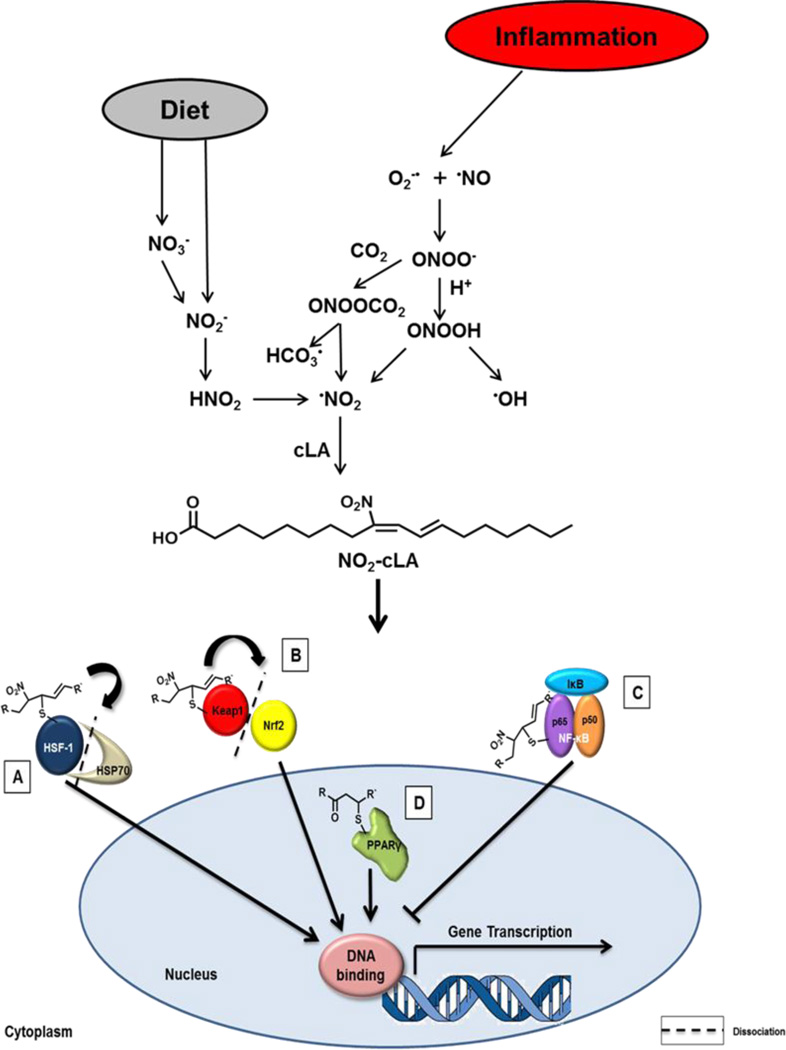
NO2-cLA is found basally in the plasma and urine of healthy humans and its concentrations increase in organelles and tissues undergoing metabolic and inflammatory stress [3, 16] (Fig. 5). Electrophilic nitroalkenes such as NO2-cLA induce kinetically rapid posttranslational modification of nucleophilic cysteines of key transcriptional regulatory proteins and inhibit the activity of functionally-significant electrophile-sensitive enzymes [11, 33, 34]. Previously reported targets of nitroalkylation include the Cys285 of PPARγ (peroxisome proliferator activator receptor γ) [35], Cys273 and Cys288 of the Nrf2 regulatory protein Keap1 [Kelch-like ECH-associated protein 1 (Keap1)/regulator of nuclear factor (erythroid-derived-2)-like 2 (Nrf2)] [12], heat shock proteins[12], and Cys38 of the p65 subunit of nuclear factor κB (NF-κB) [36] (Fig. 5). Consequently, synthetic homologs of endogenous nitroalkenes induce human coronary artery endothelium to alter the expression of over 400 genes related to metabolism, tissue repair and inflammatory responses [12]. Nitroalkylation also directly inhibits the catalytic activity of enzymes including xanthine oxidoreductase [33], cyclooxygenase [37] and soluble epoxide hydrolase (Cys521) [34], reactions that result in significant beneficial physiological responses. These alkylation reactions are reversible, with the exception of xanthine oxidoreductase, and can be modulated by competing tissue nucleophiles such as cysteine, glutathione (GSH) and hydrogen sulfide [11, 38]. Thus, lipid nitroalkene derivatives represent salutary signaling molecules that both modulate inflammation and serve as sensitive biomarkers for tissue nitrative and nitrosative reactions, much like 8-iso-prostaglandin F2α is a marker for oxidative reactions.
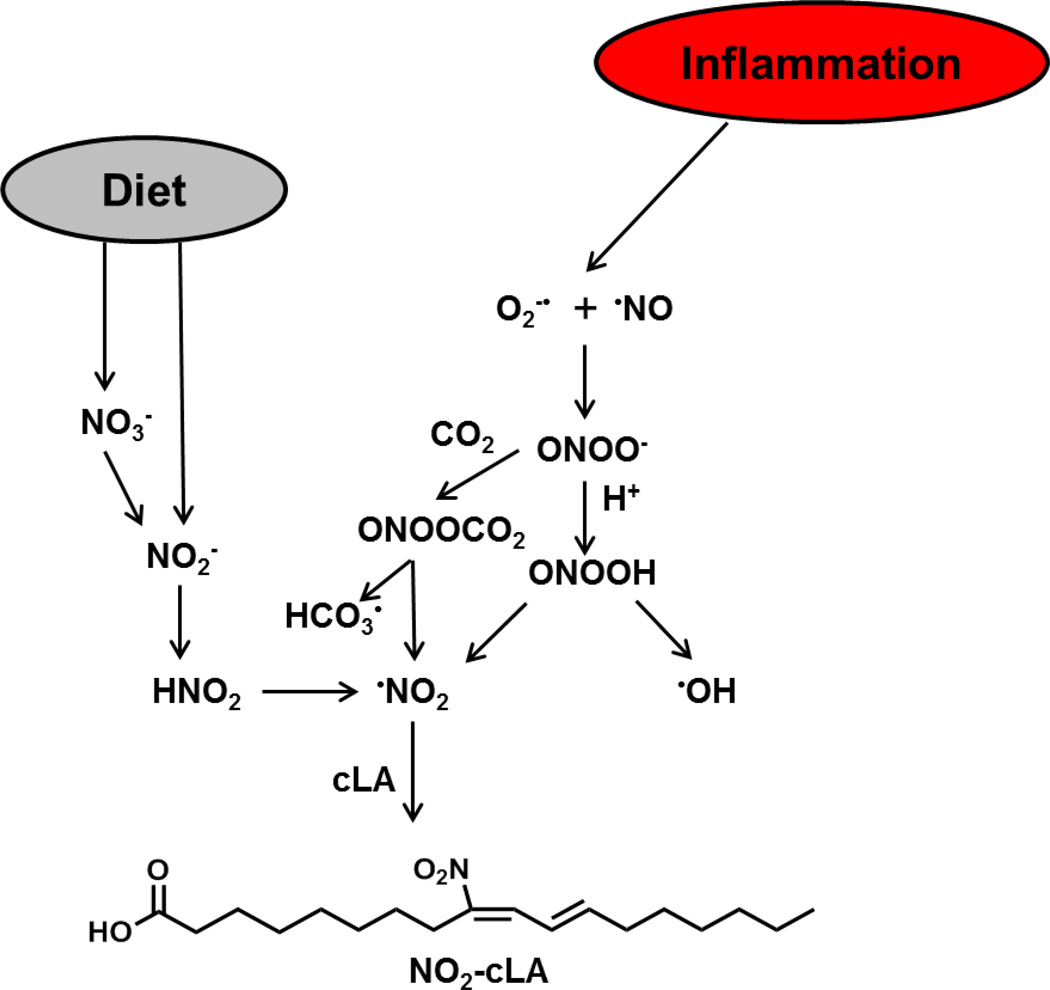
Figure 5.
Formation and signaling of NO2-cLA. Nitrate (NO3−) and nitrite (NO2−) are dietary sources of nitrogen dioxide (•NO2). Nitrate is reduced to nitrite by entero-salivary bacteria. Nitrite, combined with the low pH of the stomach favors •NO2 formation via nitrous acid (HNO2) generation. Various oxides of nitrogen can form from the decomposition of HNO2 in the gut, including •NO2. In inflammation, •NO2 can arise from the protonation of NO2− to nitrous acid (HNO2) or NO2− oxidation by heme peroxidases. Another significant mechanism of •NO2 formation involves peroxynitrite (ONOO−), peroxynitrous acid (ONOOH), which are formed through a reaction of •NO and superoxide (O2•−). These species can readily diffuse through the membrane to mediate unsaturated fatty acid nitration and oxidation via homolysis of ONOOH to •NO2 and •OH. Peroxynitrite also reacts with CO2 to form nitrosoperoxocarbonate (ONOOCO2) and like HNO2, this compound can undergo homolytic scission to form •NO2. Nitrogen dioxide reacts with the π electrons of alkenes via an addition reaction and a reaction with a second •NO2 results in the reformation of the double bond. Conjugated diene containing PUFAs, such as cLA, are especially susceptible to nitration, as opposed to methylene-interrupted species. The endogenous production and exogenous administration of electrophilic fatty acids targets multiple redox-sensing transcriptional regulators. It has been previously demonstrated that nitroalkenes (a) putatively bind HSP70, releasing HSF-1 and thus driving HSF-1-dependent gene transcription; (b) covalently adduct Keap1, causing dissociation from and translocation of Nrf2 to induce ARE gene transcription, and (c) modify the p65 subunit of NF-κB, sustaining inhibition by IκB and blocking p50/p65-dependent gene transcription. In the nucleus, (d) nitroalkenes covalently bind and act as partial PPARγ agonists, stimulating gene transcription.
It was hypothesized that dietary NOx would be limiting in NO2-cLA formation; however, in the absence of cLA supplementation in combination with overnight fasting, plasma 15NO2-cLA concentrations were barely detectable and only became significantly elevated 24 h after 15NO3− consumption (Fig. 2C). With cLA supplementation, greater concentrations of 15NO2-cLA were detected earlier after both 15NO2− and 15NO3− supplementation (Fig. 2E). Plasma 15NO2-cLA concentrations in the 15NO2−-supplemented cohort paralleled plasma cLA concentrations (Fig. 2E and 3A). Maximum levels of plasma 15NO2− after dietary intake occurred after 30 min (Fig. 3C), thus preceding 15NO2-cLA formation. The plasma half-life of NO2− is 5–6 h, also tracking with the decrease of 15NO2-cLA in plasma between 6 and 24 h [39, 40]. The 14NO2− levels were increased in plasma after 15NOx supplementation (Fig. 3C and D). It is possible that the 1 gm of orally-consumed 15NO3− exchanged with and increased concentrations of endogenous 14NOx counterions available for forming nitrating species.
The detection and quantification of NO2-cLA and other electrophilic fatty acids is challenged by their facile reaction with biological nucleophiles (e.g., cysteine, GSH and protein thiols and histidine). Also, electrophilic fatty acids become esterified in complex lipids [2], are enzymatically reduced to non-electrophilic nitroalkane derivatives by prostaglandin reductase 1 [41], can be further oxidized and readily undergo mitochondrial β-oxidation [11, 42]. Evidence for these reactions occurring in humans was reflected by the detection of urinary 15NO2-cLA β-oxidation products and corresponding cysteine-NO2-cLA and cysteine-NO2-cLA β-oxidation products detected after 15NOx + cLA supplementation (Supplementary Fig S2 and S3). From a pharmacological perspective, some of these transformations retain intrinsic signaling competency via preservation of their electrophilic moiety and others do not. The relative signaling activities of electrophilic β-oxidation products, the extents of NO2-fatty acid esterification into glycerolipids and the half-lives of nitro-fatty acids esterified into various complex lipid and tissue compartments remain to be fully characterized.
In summary, the formation of NO2-cLA and its metabolites after dietary nitrogen oxide and cLA consumption reveals that diet, digestion and metabolism yield a spectrum of absorbable electrophilic metabolites that are detectable in both plasma and urine. Model systems show that these products mediate distinctive downstream post-translational protein modifications, altered gene expression and signaling responses at nM concentrations. Phase 1 safety studies using FDA-approved nitro-oleic acid reveal a wide therapeutic window for NO2-fatty acids (clinicaltrials.gov ID: NCT02127190). Significant metabolic and anti-inflammatory responses are induced by exogenously-administered fatty acid nitroalkenes in murine models after administration of doses giving 5–10 nM plasma concentrations [43, 44]. Herein, human plasma NO2-cLA concentrations after 15NOx + cLA supplementation increased from an average basal level of 3 nM to 10 nM. These data support the concept that the consumption of dietary constituents high in NO3− and cLA, such as leafy vegetables, dairy products and meat, can mediate physiological responses via the generation of NO2-cLA.
Supplementary Material
Highlights.
15N-labeled NO3−/NO2− supplementation results in 15NO2-cLA formation
NO2-cLA formation is dependent on levels of free cLA
NO2-cLA concentration can be modulated by dietary components
Detectable NO2-cLA concentrations are sufficient to exert systemic effects
Acknowledgments
The authors thank Ram Agarwal, Deborah Sperling and Judith Starling for formulating 15NOx, Nydia Chien and Suchitra Barge for their IRB and regulatory assistance and Nicole Helbling and Alex Despines for trial implementation. This work was supported by NIH grants R01-HL058115, R01-HL64937 (BF), P01-HL103455 (MG, BF), AT00682 (FJS), K12-HD052892 (KSH), the Cochrane Weber Fund of Children’s Hospital of Pittsburgh of UPMC and the Vascular Medicine Institute/CTSI Pilot Project Program in Hemostasis & Vascular Biology. FJS and BAF acknowledge interest in Complexa, Inc. MTG is co-inventor on a US Government Patent for the use of nitrite salts for cardiovascular indications (IND # 70,411) and has received royalties from the US Government.
Abbreviations
- PTAD
4-phenyl-1,2,4 triazoline-3,5 dione
- [13C18]-NO2-OA
[13C18]-nitro-oleic acid
- 14NO2-cLA
14nitro-conjugated linoleic acid
- 15NO2-cLA
15nitro-conjugated linoleic acid
- cLA
conjugated linoleic acid
- cGMP
cyclic GMP
- DAN
diaminonaphthalene
- GSH
glutathione
- HPLC
high performance liquid chromatography
- KEAP1
Kelch-like ECH-associated protein 1
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- NT
N-napthotriazole
- NO2-FA
nitro-fatty acid
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PPARγ
Peroxisome proliferator-activated receptor gamma
- PTM
post-translational modification
- RT
retention time
- SPE
solid phase extraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Piskorski R, Hanus R, Vasickova S, Cvacka J, Sobotnik J, Svatos A, Valterova I. Nitroalkenes and sesquiterpene hydrocarbons from the frontal gland of three prorhinotermes termite species. Journal of chemical ecology. 2007;33:1787–1794. doi: 10.1007/s10886-007-9341-y. [DOI] [PubMed] [Google Scholar]
- 2.Fazzari M, Trostchansky A, Schopfer FJ, Salvatore SR, Sanchez-Calvo B, Vitturi D, Valderrama R, Barroso JB, Radi R, Freeman BA, Rubbo H. Olives and olive oil are sources of electrophilic fatty acid nitroalkenes. PLoS One. 2014;9:e84884. doi: 10.1371/journal.pone.0084884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salvatore SR, Vitturi DA, Baker PR, Bonacci G, Koenitzer JR, Woodcock SR, Freeman BA, Schopfer FJ. Characterization and quantification of endogenous fatty acid nitroalkene metabolites in human urine. Journal of lipid research. 2013;54:1998–2009. doi: 10.1194/jlr.M037804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Villacorta L, Chang L, Fan Z, Hamblin M, Zhu T, Chen CS, Cole MP, Schopfer FJ, Deng CX, Garcia-Barrio MT, Feng YH, Freeman BA, Chen YE. Nitro-oleic acid inhibits angiotensin II-induced hypertension. Circ Res. 2010;107:540–548. doi: 10.1161/CIRCRESAHA.110.218404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoo NK, Rudolph V, Cole MP, Golin-Bisello F, Schopfer FJ, Woodcock SR, Batthyany C, Freeman BA. Activation of vascular endothelial nitric oxide synthase and heme oxygenase-1 expression by electrophilic nitro-fatty acids. Free Radic Biol Med. 2010;48:230–239. doi: 10.1016/j.freeradbiomed.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley EE, Baust J, Bonacci G, Golin-Bisello F, Devlin JE, St Croix CM, Watkins SC, Gor S, Cantu-Medellin N, Weidert ER, Frisbee JC, Gladwin MT, Champion HC, Freeman BA, Khoo NK. Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity. Cardiovasc Res. 2014;101:352–363. doi: 10.1093/cvr/cvt341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy AT, Lakshmi SP, Dornadula S, Pinni S, Rampa DR, Reddy RC. The nitrated fatty acid 10-nitro-oleate attenuates allergic airway disease. J Immunol. 2013;191:2053–2063. doi: 10.4049/jimmunol.1300730. [DOI] [PubMed] [Google Scholar]
- 8.Radi R. Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Accounts of chemical research. 2013;46:550–559. doi: 10.1021/ar300234c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR, Jr, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitturi DA, Minarrieta L, Salvatore Sonia R, Postlethwait Edward M, Fazzari Marco, Ferrer-Sueta Gerardo, Lancaster Jack R, Freeman Bruce A, Schopfer Francisco J. Convergence of Biological Nitration and Nitrosation Reactions via Symmetrical Nitrous Anhydride (N2O3) Nature Chemical Biology. 2015 doi: 10.1038/nchembio.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schopfer FJ, Cipollina C, Freeman BA. Formation and signaling actions of electrophilic lipids. Chem Rev. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kansanen E, Jyrkkanen HK, Volger OL, Leinonen H, Kivela AM, Hakkinen SK, Woodcock SR, Schopfer FJ, Horrevoets AJ, Yla-Herttuala S, Freeman BA, Levonen AL. Nrf2-dependent and - independent responses to nitro-fatty acids in human endothelial cells: identification of heat shock response as the major pathway activated by nitro-oleic acid. J Biol Chem. 2009;284:33233–33241. doi: 10.1074/jbc.M109.064873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Yla-Herttuala S, Freeman BA, Levonen AL. Electrophilic Nitro-fatty Acids Activate NRF2 by a KEAP1 Cysteine 151-independent Mechanism. J Biol Chem. 2011;286:14019–14027. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schopfer F, Cipollina C, Freeman BA. Formation and signaling actions of electrophilic lipids. Chem Rev. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J. Biol. Chem. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trapasso E, Cosco D, Celia C, Fresta M, Paolino D. Retinoids: new use by innovative drug-delivery systems. Expert opinion on drug delivery. 2009;6:465–483. doi: 10.1517/17425240902832827. [DOI] [PubMed] [Google Scholar]
- 17.Chromy V, Rozkosna K, Sedlak P. Determination of serum creatinine by Jaffe method and how to calibrate to eliminate matrix interference problems. Clin Chem Lab Med. 2008;46:1127–1133. doi: 10.1515/CCLM.2008.224. [DOI] [PubMed] [Google Scholar]
- 18.MacArthur PH, Shiva S, Gladwin MT. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Curtis E, Hsu LL, Noguchi AC, Geary L, Shiva S. Oxygen regulates tissue nitrite metabolism. Antioxidants & redox signaling. 2012;17:951–961. doi: 10.1089/ars.2011.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin S, Fung HL. Evaluation of an LC-MS/MS assay for 15N-nitrite for cellular studies of L-arginine action. Journal of pharmaceutical and biomedical analysis. 2011;56:1127–1131. doi: 10.1016/j.jpba.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suarez HG, Daya-Grosjean L, Schlaifer D, Nardeux P, Renault G, Bos JL, Sarasin A. Activated oncogenes in human skin tumors from a repair-deficient syndrome, xeroderma pigmentosum. Cancer research. 1989;49:1223–1228. [PubMed] [Google Scholar]
- 22.Leach SA, Thompson M, Hill M. Bacterially catalysed N-nitrosation reactions and their relative importance in the human stomach. Carcinogenesis. 1987;8:1907–1912. doi: 10.1093/carcin/8.12.1907. [DOI] [PubMed] [Google Scholar]
- 23.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, Van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 24.Bonacci G, Baker PR, Salvatore SR, Shores D, Khoo NK, Koenitzer JR, Vitturi DA, Woodcock SR, Golin-Bisello F, Cole MP, Watkins S, St Croix C, Batthyany CI, Freeman BA, Schopfer FJ. Conjugated linoleic acid is a preferential substrate for fatty acid nitration. J Biol Chem. 2012;287:44071–44082. doi: 10.1074/jbc.M112.401356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claridge RP, Deeming AJ, Paul N, Tocher DA, Ridd JH. The reactions of nitrogen dioxide with dienes. Journal of the Chemical Society - Perkin Transactions. 1998;1:3523–3528. [Google Scholar]
- 26.Zlatanos* Spiros N, KLaAS Conjugated linoleic acid content of human plasma. Lipids in health and disease. 2008;7 doi: 10.1186/1476-511X-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Churruca I, Fernandez-Quintela A, Portillo MP. Conjugated linoleic acid isomers: differences in metabolism and biological effects. BioFactors. 2009;35:105–111. doi: 10.1002/biof.13. [DOI] [PubMed] [Google Scholar]
- 28.Kitz R, Rose MA, Schubert R, Beermann C, Kaufmann A, Bohles HJ, Schulze J, Zielen S. Omega-3 polyunsaturated fatty acids and bronchial inflammation in grass pollen allergy after allergen challenge. Respiratory medicine. 2010;104:1793–1798. doi: 10.1016/j.rmed.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Tricon S, Burdge GC, Kew S, Banerjee T, Russell JJ, Grimble RF, Williams CM, Calder PC, Yaqoob P. Effects of cis-9,trans-11 and trans-10,cis-12 conjugated linoleic acid on immune cell function in healthy humans. The American journal of clinical nutrition. 2004;80:1626–1633. doi: 10.1093/ajcn/80.6.1626. [DOI] [PubMed] [Google Scholar]
- 30.Tricon S, Burdge GC, Kew S, Banerjee T, Russell JJ, Jones EL, Grimble RF, Williams CM, Yaqoob P, Calder PC. Opposing effects of cis-9,trans-11 and trans-10,cis-12 conjugated linoleic acid on blood lipids in healthy humans. The American journal of clinical nutrition. 2004;80:614–620. doi: 10.1093/ajcn/80.3.614. [DOI] [PubMed] [Google Scholar]
- 31.MacRedmond R, Singhera G, Attridge S, Bahzad M, Fava C, Lai Y, Hallstrand TS, Dorscheid DR. Conjugated linoleic acid improves airway hyper-reactivity in overweight mild asthmatics. Clin Exp Allergy. 2010;40:1071–1078. doi: 10.1111/j.1365-2222.2010.03531.x. [DOI] [PubMed] [Google Scholar]
- 32.Zulet MA, Marti A, Parra MD, Martinez JA. Inflammation and conjugated linoleic acid: mechanisms of action and implications for human health. J Physiol Biochem. 2005;61:483–494. doi: 10.1007/BF03168454. [DOI] [PubMed] [Google Scholar]
- 33.Kelley EE, Batthyany CI, Hundley NJ, Woodcock SR, Bonacci G, Del Rio JM, Schopfer FJ, Lancaster JR, Jr, Freeman BA, Tarpey MM. Nitro-oleic Acid, a Novel and Irreversible Inhibitor of Xanthine Oxidoreductase. J Biol Chem. 2008;283:36176–36184. doi: 10.1074/jbc.M802402200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bianchini A, Santoni F, Paolini J, Bernardini AF, Mouillot D, Costa J. Partitioning the relative contributions of inorganic plant composition and soil characteristics to the quality of Helichrysum italicum subsp. italicum (Roth) G. Don fil. essential oil. Chemistry & biodiversity. 2009;6:1014–1033. doi: 10.1002/cbdv.200800328. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira AM, Minarrieta L, Lamas Bervejillo M, Rubbo H. Nitro-fatty acids as novel electrophilic ligands for peroxisome proliferator-activated receptors. Free radical biology & medicine. 2012;53:1654–1663. doi: 10.1016/j.freeradbiomed.2012.08.572. [DOI] [PubMed] [Google Scholar]
- 36.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. The Journal of biological chemistry. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trostchansky A, Bonilla L, Thomas CP, O'Donnell VB, Marnett LJ, Radi R, Rubbo H. Nitroarachidonic acid, a novel peroxidase inhibitor of prostaglandin endoperoxide H synthases 1 and 2. J Biol Chem. 2011;286:12891–12900. doi: 10.1074/jbc.M110.154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishida M, Sawa T, Kitajima N, Ono K, Inoue H, Ihara H, Motohashi H, Yamamoto M, Suematsu M, Kurose H, van der Vliet A, Freeman BA, Shibata T, Uchida K, Kumagai Y, Akaike T. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat Chem Biol. 2012;8:714–724. doi: 10.1038/nchembio.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner DA, Schultz DS, Deen WM, Young VR, Tannenbaum SR. Metabolic fate of an oral dose of 15N-labeled nitrate in humans: effect of diet supplementation with ascorbic acid. Cancer research. 1983;43:1921–1925. [PubMed] [Google Scholar]
- 40.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 41.Vitturi DA, Chen CS, Woodcock SR, Salvatore SR, Bonacci G, Koenitzer JR, Stewart NA, Wakabayashi N, Kensler TW, Freeman BA, Schopfer FJ. Modulation of nitro-fatty acid signaling: prostaglandin reductase-1 is a nitroalkene reductase. J Biol Chem. 2013;288:25626–25637. doi: 10.1074/jbc.M113.486282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudolph V, Schopfer FJ, Khoo NK, Rudolph TK, Cole MP, Woodcock SR, Bonacci G, Groeger AL, Golin-Bisello F, Chen CS, Baker PR, Freeman BA. Nitro-fatty acid metabolome: saturation, desaturation, beta-oxidation, and protein adduction. J. Biol. Chem. 2009;284:1461–1473. doi: 10.1074/jbc.M802298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villacorta L, Chang L, Salvatore SR, Ichikawa T, Zhang J, Petrovic-Djergovic D, Jia L, Carlsen H, Schopfer FJ, Freeman BA, Chen YE. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovasc Res. 2013;98:116–124. doi: 10.1093/cvr/cvt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borniquel S, Jansson EA, Cole MP, Freeman BA, Lundberg JO. Nitrated oleic acid up-regulates PPARgamma and attenuates experimental inflammatory bowel disease. Free Radic Biol Med. 2010;48:499–505. doi: 10.1016/j.freeradbiomed.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.