Abstract
Significant progress in understanding and overcoming cardiac xenograft rejection using a clinically relevant large animal pig-to-baboon model has accelerated in recent years. This advancement is based on improved immune suppression, which attained more effective regulation of B lymphocytes and possibly newer donor genetics. These improvements have enhanced heterotopic cardiac xenograft survival from a few weeks to over 2 years, achieved intrathoracic heterotopic cardiac xenograft survival of 50 days and orthotopic survival of 57 days. This encouraging progress has rekindled interest in xenotransplantation research and refocused efforts on preclinical orthotopic cardiac xenotransplantation.
1. Introduction
Transplantation is an effective therapy for patients with end stage cardiac disease but is limited by the chronic shortage of donor organs. Mechanical circulatory support (MCS) devices have been approved as destination therapy as a result of new pump designs and greatly improved patient survival [1]. Device related gastrointestinal bleeding, thrombosis, infection, power supply limitations, and quality of life questions remain limitations for MCS [2]. The use of MCS has increased significantly and is synergistic with heart transplantation. There remains however a significant group of patients who would benefit from organ transplantation if donor organs had greater availability. Recent advances in heterotopic cardiac xenotransplantation (hCXTx) survival suggest that cardiac xenotransplantation (CXTx) offers a viable solution to donor organ shortages if hCXTx results can be replicated in preclinical life-supporting pig-to-non human primate transplants.
In this report the authors, representing three major international CXTx research programs, will summarize the insights and advances instrumental in bringing the field to its present state and define the challenges which remain on the path to clinical application. We refer the reader to previous reviews [3–5] for additional background related to these recent advances.
2. Heterotopic Abdominal Cardiac Xenotransplantation and Xenograft Survival
The predominant CXTx model (pig-to-nonhuman primate (NHP)) has been the abdominal hCXTx procedure in which the pig pulmonary artery is anastomosed to the recipient inferior vena cava and the pig aorta to the recipient abdominal aorta (Figure 1A). [6] The graft is contractile and perfused but does not support the recipient circulation. The hCXTx is used to define immune suppression methods and pig genetic modifications, which prolong graft contractility. The outcomes from notable hCXTx studies are summarized in Table 1 and discussed below.
Figure 1.
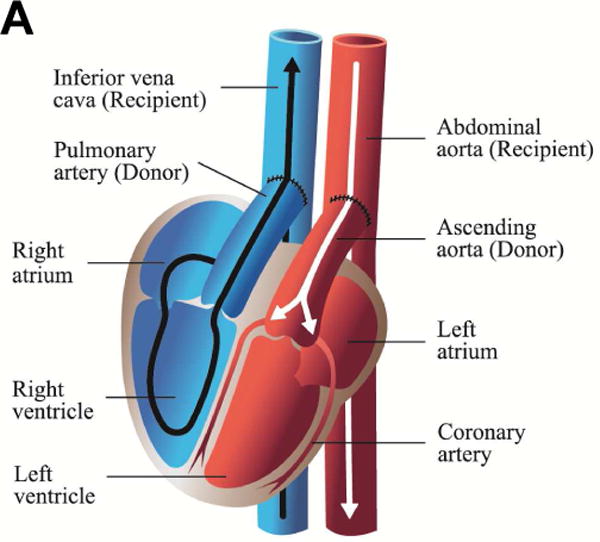
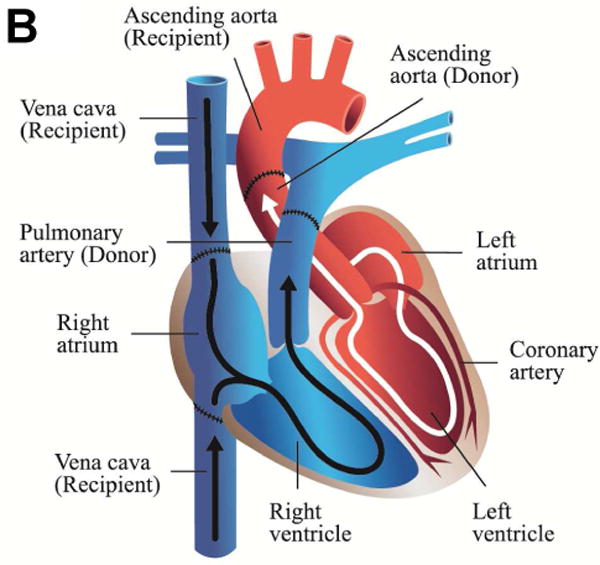
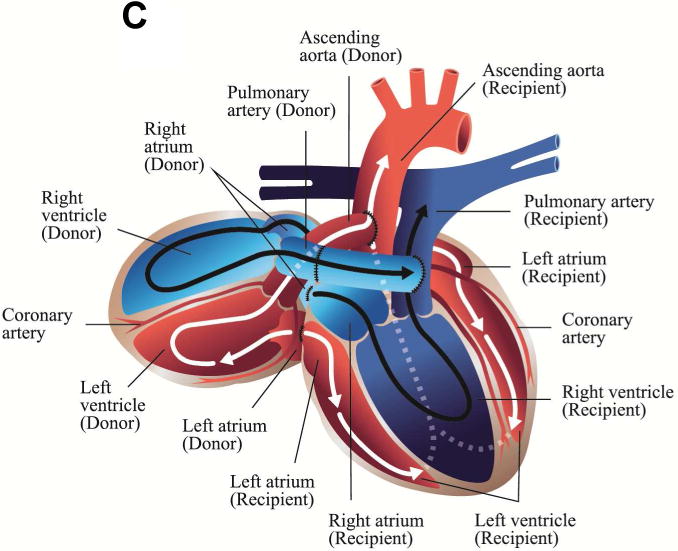
Illustrations of cardiac xenotransplantation surgical techniques. A. Heterotopic abdominal transplantation. Only the coronary arteries are perfused (via the abdominal aorta), the coronary venous blood enters the right atrium, then the ventricle. It is ejected (via the pulmonary trunk) into the inferior cava. The heart is beating, but non-working. B. Orthotopic cardiac transplantation. The recipient heart is removed at the atrial level; ascending aorta and the pulmonary trunk are cut. The donor organ is then connected accordingly. C. Intrathoracic heterotopic cardiac transplantation. The donor organ is to the right of the recipient heart, within the right chest. There are two common atria, since they are end-to-end anastomosed. The blood volume is ejected by both hearts via two end-to-side connections at the levels of the ascending aortas, the pulmonary artery trunks (the latter needs a graft interposition). Both organs work non-synchronously. Their shares of the cardiac output depend on the respective preloads, which in turn are related to the parts of the cycles of the two hearts.(illustrations by Nina Bantschow)
Table 1.
Progressive improvements in heterotopic cardiac xenograft survival
| Immune suppression | Donor Genetics | Median survival (days) | Min-Max. survival (days) | Reference |
|---|---|---|---|---|
| 1 approved drugs | CD46 | 96 | 15 – 137 | [21] |
| 2 anti-CD154 | CD55 | 27 | 4 – 139 | [18] |
| 2 anti-CD154 | GTKO | 78 | 16 – 179 | [24] |
| 3 anti-CD154 | GTKO:CD46 | 71 | 36 – 236 | [29] |
| 4 anti-CD40 | GTKO:CD46 | 84 | 30 – 149 | [32] |
| 5 anti-CD40 | GTKO:CD46:hTM | 200 | 146–550 | [33] |
Immune suppression lists the key immune suppressive agent. Further details of each protocol are given below.
Induction with ATG and Rituximab, pre-transplant splenectomy. Pre and post-transplant Galpolymer, steroid taper, Tacrolimus, and Sirolimus. No post-transplant rescue therapy or anticoagulation.
Induction with ATG and thymic irradiation. Pre and post-transplant Gal polymer, MMF, cobra venom factor (CVF), steroid taper, and anti-CD154. Post transplant LoCD2b used for high T-cell counts. Heparin for anticoagulation and prostacyclin for inflammation.
Induction with ATG, Rituximab and anti-CD154. Pre and post transplant CVF, and post-transplant anti-CD154, MMF, and steroid taper. Anticoagulation with aspirin and heparin. Post-transplant rescue therapy of methyl prednisolone and heparin.
Induction with ATG, Rituximab, anti-CD40 (20mg/kg), and CVF. Post-transplant anti-CD40 (5–20 mg/kg), MMF and steroid taper. Anticoagulation with aspirin and heparin.
Induction with ATG, Rituximab, anti-CD40 (50mg/kg), and CVF. Post-transplant anti-CD40 (25 – 50 mg/kg), MMF and steroid taper. Anticoagulation with heparin.
2.1 Complement Regulation
Pig-to-NHP hCXTx was initially limited by hyperacute xenograft rejection (HAR) resulting from complement mediated vascular injury caused by anti-Gal antibody in the recipient and high levels of Gal antigen on the endothelium of the graft [3]. HAR can be prevented with systemic complement inhibition [7, 8], but such treatments significantly enhanced the risk of infection. This led to the central strategy of xenotransplantation, namely to genetically engineer donor organs for resistance to rejection and thereby reduce the immune suppressive burden. Transgenic pigs expressing human complement regulatory proteins (hCRP) CD46, CD55 or CD59 were produced to inhibit complement mediated graft injury [9–11]. The hCRP transgenic donors were largely resistant to hyperacute rejection, validating the genetic engineering approach. Without immune suppression hCRP hearts succumbed to delayed xenograft rejection usually within one week, due to intense induction of anti-Gal antibody whereas with immune suppression survival averaged about 3 weeks with occasional exceptions [4, 12].
2.2 Anti-Gal Antibody Therapies
Intransigent Gal-mediated rejection prompted the search for methods to block anti-Gal antibody by ex vivo removal [13] or in vivo blocking with infused carbohydrate [14] or non-antigenic Gal-polymers [15, 16]. Immunoapheresis effectively reduce anti-Gal antibody levels but its use had a negative impact on survival [17]. Enduring reduction of preformed and induced anti-Gal antibody was achieved by infusion of non-antigenic Gal-polymers [18–21] culminating in a median 96 day survival of CD46 heterotopic transplants (Table 1) [21]. This study showed that, with the exception of the Gal-polymer, cardiac xenograft survival could be maintained for 3 months using only approved immune suppressive agents. Postoperative control of induced anti-Gal antibody was also achieved using Gal-polymers and immune suppression based on T-cell induction with chronic anti-CD154 co-stimulation blockage with maximal survival of 137 days [18]. Together these transplants demonstrated that by blocking the effects of anti-Gal antibody hCXTx could consistently exceed graft contractility beyond the 3 weeks without anti-Gal therapy.
2.3 Gal knockout Donor Pigs
Gal-free genetically modified pigs (GTKO) were produced by somatic cell nuclear transfer in 2003 [22, 23] and the first GTKO hCXTx results were reported in 2005 [24]. This landmark study used a similar T-cell and anti-CD154 antibody immune suppression, which previously coupled to Gal inhibition, had extended CD55 pig heart survival (Table 1). Median GTKO graft survival was 78 days with maximal survival of 179 days. There was no hyperacute rejection, no evidence of any effect on anti-Gal antibody and graft rejection was commensurate with non-Gal antibody and complement deposition, microvascular thrombosis, and evidence of endothelial cell activation [25]. GTKO transplants by other groups did not uniformly show comparably good survival [26]. This variation is due in part to the type of immune suppression and the status of the recipient, with specific pathogen free baboons with low levels of anti-pig antibody showing the best organ survival [27–29].
2.4 Improvements in Immune Suppression
An improvement in hCXTx survival came by combining ATG induction therapy and B-cell depletion with chronic anti-CD154 immune suppression [29]. This regimen resulted in median GTKO:CD46 donor heart survival of 71 days, with 3 of 9 xenografts showing good contractility for over 100 days (Table 1). One recipient in this study was electively explanted with a beating xenograft at 236 days. Bleeding associated with anti-CD154 antibody was a significant complication in this study.
Immune suppression using anti-CD154 antibody is effective in xenotransplantation however is known to contribute to systemic thromboembolism [30] and hCXTx studies using this reagent often reported a high frequency of perioperative thrombocytopenia and post transplant consumptive coagulopathy [24, 30, 31]. While these pathologies cannot solely be attributed to anti-CD154, there has been growing interest in finding alternative co-stimulation blocking therapies. Two anti-CD40 clones were tested as substitutes for anti-CD154 in pig to baboon hCXTx [32]. One clone (2C10R4) was effective resulting in median hCXTx graft survival of 84 days when administered at 20 mg/kg for first 60 days only (Table 1). Higher dosage (50 mg/kg) and continued administration increased median hCXTx graft survival to more then 200 days with 3 of 5 grafts showing good contractility 200 to 500 days after transplant [33]. The graft survival was further extended to 945 days in the same group (unpublished data, Mohiuddin et al) In this latter study anti-CD40 was used in conjunction with GTKO:CD46 donor hearts additionally expressing human thrombomodulin (hTM) which may also have contributed to graft survival.
Significantly improved hCXTx survival associated with anti-CD40 therapy shows that antibody mediated xenograft rejection can be prevented for a clinically significant period of time with manageable infectious complications. This result casts an intense focus on replicating this level of graft survival in functional life-support transplants.
3. Life-Supporting Functional Cardiac Xenotransplantation
There are two clinical cardiac transplantation procedures which have been used in CXTx, orthotopic cardiac xenotransplantation (oCXTx) (Figure 1B) where the xenograft replaces the recipient heart and intrathoracic heterotopic cardiac xenotransplantation (ITHCXTx) (Figure 1C) where the graft “piggy-backs” to the native heart and supplements the recipient’s circulation [6].
3.1 Orthotopic Cardiac Xenotransplantation
Median hCXTx survival is now more then 2-fold longer then that recommended by the ISHLT committee on Xenotransplantation for the duration of life-supporting preclinical studies as a criterion to consider clinical testing [34]. Replicating this level of survival in a life-supporting oCXTx model is now clearly the primary objective in the field. There have been relatively few life supporting oCXTx reports [35–42] as this is a far more complex and demanding procedure. These studies have used GT+:hCRP donor hearts, with and without Gal-polymers to block anti-Gal antibody, and GTKO:hCRP donor hearts. Orthotopic cardiac xenograft survival ranged from 1 to 57 days. In most instances recipient deaths occurred due to postoperative complications and not graft rejection. Grafts explanted between 9 and 57 days showed limited histologic evidence of rejection although gene expression analysis suggests the grafts were subject to ongoing immune challenge and endothelial cell activation [43]. These earlier studies, which did not utilize the more recent hCXTx immune suppression regimens, suggest that the efficacy of oCXTx is limited not by cardiac function but by the challenges of immune rejection and postoperative management.
A high level of perioperative mortality within the first 48 hours post transplant is reported in oCXTx studies [5]. This phenomenon, termed perioperative cardiac xenograft dysfunction (PCXD), has been seen at frequencies ranging from 40 to 60% by all research groups. PCXD is not observed in hCXTx and the histology of PCXD, showing vascular antibody deposition but otherwise normal myocardium, is not consistent with hyperacute rejection. Early graft failure appears to be more similar to ischemic reperfusion injury or cardiac stunning. While PCXD is evident to lesser degrees even in recipients which survive beyond 48 hours echocardiographic analysis indicates that PCXD is reversible within 2 weeks of transplantation [39]. Improved methods of organ preservation and minimization of early xenogeneic inflammation can decrease the frequency of PCXD [44]. Transgenic expression of CD39 in donor pigs enhances resistance to ischemia-reperfusion [45] and may also improve perioperative orthotopic graft survival.
3.2 Intrathoracic Heterotopic Cardiac Xenotransplantation
Intrathoracic heterotopic cardiac allotransplantation was clinically introduced by Christian Barnard over 50 years ago [46]. The donor organ is placed on the right side of the native heart and four anastomoses are made between the left and right atria, the respective ascending aortas and the pulmonary artery trunks using an interposition graft (Figure 1C). The original clinical results with this technique were good with 63%, 54% and 43% survival after one, two and five postoperative years [47]. This ITHCTx technique while surgically complex is advantageous as the native recipient’s heart can act as a back-up support for the graft during the perioperative period or during rejection. In xenotransplantation this transplant model might be used clinically as a bridge to transplant or experimentally as a method to study PCXD and xenograft rejection.
In a preliminary report a GTKO:CD46 donor heart survived for 50 days after ITHCXTx [48]. Notably a rejection episode was diagnosed in this recipient, based on increased serum troponin and rising anti-pig antibody levels. This presumptive rejection, not confirmed by histology, was treated and reversed using a combination of ATG, immunapheresis and Bortezomib proteosome inhibition. The unique properties of ITHCXTx may make this model ideal for developing essential methods to diagnose and treat antibody mediated cardiac xenograft rejection. Additional ITHCXTx and orthotopic studies using GTKO, hCD46, ±hTBM and adopting a modified version of the anti-CD40 immune suppression described by Mohiuddin et al (33) are ongoing.
4. Genetic modifications of donors
An advantage of xenotransplantation is the ability to use genetic engineering to create donor pig organs which resist rejection and a diverse array of genetic modifications involving complement regulation, control of haemostasis, T-cell and NK-cell activation, and antigen reduction have been reported [49]. In CXTx the majority of transplants have used GT+:hCRP, GTKO or GTKO:hCRP donors. Expression of an hCRP transgene is clearly advantageous as discussed previously. No formal studies have been done and many variables impact xenograft survival, but, the aggregate of evidence suggests that there is no clear advantage of one hCRP (CD46, CD55 or CD59) over any other so long as the level of endothelial cell gene expression is high. Expression of multiple hCRPs increases in vitro resistance to complement mediated lysis assays, however, in hCXTx a prospective comparison of single (CD55) and double (CD55:CD46) transgenic donors found no additional survival benefit from the double transgenic donor hearts [50]. In the GTKO background a head-to-head comparisons of GTKO and GTKO:CD55 donors transplanted under identical immune suppression regimens shows GTKO:CD55 donor hearts have improved complement regulation and prevent early graft failure but do not prolong graft survival [51]. The early protective effects of hCRP expression in hCXTx was recently confirmed by an aggregate analysis of GTKO, GTKO:CD55 or GTKO:CD46 transplants from three transplant centers [26].
Two additional classes of donor genetics have been reported, transgenes expressing human regulators of hemostasis (CD39 and hTM) and pigs with further genetic deletions to depleted additional xenogeneic carbohydrate antigens [52]. Anticoagulation genes are primarily expected to rectify well documented molecular incompatibilities in porcine thrombomodulin which may enhance the thrombogenic potential of transplanted pig organs. Secondarily, increased graft specific hemostatic regulation has been proposed to suppress the incidence of thrombocytopenia and consumptive coagulopathies reported from some transplant centers. As these findings have been absent in some large hCXTx studies, it is unclear if they result from ongoing rejection, an inherent thrombogenic potential of the pig organ, or more likely from the use of CVF and anti-CD154 therapies. Prospective studies of systemic anticoagulation or anti-platelet therapies have failed to prevent microvascular thrombosis brought on by rejection or shown improved xenograft survival [53–55]. While in vitro study clearly show improved TM function of cells from hTM transgenic pigs [56], the limited hCXTx experience with hTM donor organs, and variable immune suppression strategies without appropriate controls makes it impossible to clearly define a benefit of to hTM expression. Studies using hTM hearts however, have shown clearly the best graft survival to date after hCXTx with minimal thrombocytopenia and bleeding compared to anti-CD154 treated hCXTx recipients and minimal rejection (thrombotic microangiopathy) [33]. Dissecting the contribution of anti-CD40 and hTM to these landmark results will require further experimentation.
Pig with deletions in the enzymes required to produce Neu5Gc modified glycans and an SDa related GalNAc antigen show the lowest level of antibody reactivity to human and nonhuman primate antibody [52]. There has been no transplant experience with antigen reduced donor pigs.
5. Conclusion
Cardiac xenotransplantation research has made great progress secondary to improvements in B-cell immunosuppression and genetically engineered donor pigs. The most recent results suggest that acceptable immune suppressive regimens are available, at least in baboons, to suppress antibody mediated rejection of the graft for a clinically meaningful period of time. Preclinical translation of these hCXTx results to life-supporting orthotopic pig-to-baboon transplants however faces significant challenges. A prospective preclinical program with any chance of success is a substantial undertaking which requires assembling an expert experienced team of surgeons, scientists, veterinarians, reagents, and experimental animals. Uniform donor genetics, likely to be useful in clinical testing, will need to be agreed and reliably produced. This may be problematic as the data showing beneficial effects of CD39 or hTM expression in the absence of anti CD40 treatment is not available, and triple knockout donors with minimal antigenicity, which would likely need to incorporate strong hCRP gene expression, have never been tested in transplants. Combining these genetics from disparate sources may represent a significant intellectual property challenge. Production of pigs expressing 7 human genes (CD46, TM, EPCR, CIITA, CD39, CD55, CD47) has been achieved (personal communication with Dave Ayares, Revivicor, Inc.). Immune suppression would likely be based on anti-CD40 co-stimulation blockage. Additionally median survival will be compromised if PCXD is not modulated to improve perioperative graft function.
There is little question that with most recent immune suppression regimen and donor genetics the results of CXTx have improved to a level where the near to medium term clinical potential of cardiac xenotransplantation is real. The principal barrier is now obtaining the resources to provide clinical or better levels of postoperative care for extremely demanding pig-to-non human primate preclinical studies.
Highlights.
There has been significant improvement in long term pig-to-baboon heterotopic cardiac xenograft survival.
Immune suppression using an anti-CD40 antibody appears to have improved xenograft survival.
The value of further donor genetic modification remains to be proven.
The future of experimental cardiac xenotransplantation is transitioning to preclinical life sustaining cardiac xenotransplantation studies.
Acknowledgments
Funding:
NHLBI / NIH (Mohiuddin)
Revivicor Inc (Mohiuddin)
German Research Foundation (Bruno Reichart)
NIAID (AI066310) (Guerard Byrne and Christopher McGregor)
National Institute for Health Research University College London Hospitals Biomedical Research Centre.) (Guerard Byrne and Christopher McGregor)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: none
Ethical approval:
NHLBI/NIH animal care and use committee
Protocol #s H0152 and H0153
Government of Upper Bavaria, Germany
55.2-1-54-2532-65-06
55.2-1-54-2532-70-11
Author contribution:
Drs. Muhammad Mohiuddin, Bruno Reichart, Guerard Byrne and Christopher McGregor equally participated in writing this review.
Guarantor:
Muhammad Mohiuddin, Bruno Reichart, Guerard Byrne and Christopher McGregor
References
- 1.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Patel CB, Cowger JA, Zuckermann A. A contemporary review of mechanical circulatory support. J Heart Lung Transplant. 2014;33:667–674. doi: 10.1016/j.healun.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Buhler L, Friedman T, Iacomini J, Cooper DK. Xenotransplantation–state of the art–update 1999. Front Biosci. 1999;4:D416–432. doi: 10.2741/A438. [DOI] [PubMed] [Google Scholar]
- 4.Ekser B, Rigotti P, Gridelli B, Cooper DK. Xenotransplantation of solid organs in the pig-to-primate model. Transpl Immunol. 2009;21:87–92. doi: 10.1016/j.trim.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Byrne GW, McGregor CG. Cardiac xenotransplantation: progress and challenges. Curr Opin Organ Transplant. 2012;17:148–154. doi: 10.1097/MOT.0b013e3283509120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postrach J, Bauer A, Schmoeckel M, Reichart B, Brenner P. Heart xenotransplantation in primate models. Methods Mol Biol. 2012;885:155–168. doi: 10.1007/978-1-61779-845-0_10. [DOI] [PubMed] [Google Scholar]
- 7.Leventhal JR, Sakiyalak P, Witson J, Simone P, Matas AJ, Bolman RM, et al. The synergistic effect of combined antibody and complement depletion on discordant cardiac xenograft survival in nonhuman primates. Transplantation. 1993;57:974–978. [PubMed] [Google Scholar]
- 8.Kobayashi T, Taniguchi S, Neethling FA, Rose AG, Hancock WW, Ye Y, et al. Delayed xenograft rejection of pig-to-baboon cardiac transplants after cobra venom factor therapy. Transplantation. 1997;64:1255–1261. doi: 10.1097/00007890-199711150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Cozzi E, White DJG. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995;1:964–966. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 10.Byrne GW, McCurry KR, Martin MJ, McClellan SM, Platt JL, Logan JS. Transgenic pigs expressing human CD59 and decay-accelerating factor produce an intrinsic barrier to complement-mediated damage. Transplantation. 1997;63:149–155. doi: 10.1097/00007890-199701150-00027. [DOI] [PubMed] [Google Scholar]
- 11.Thorley BR, Milland J, Christiansen D, Lanteri MB, McInnes B, Moeller I, et al. Transgenic expression of a CD46 (membrance cofactor protein) minigene: studies of xenotransplantation and measles virus infection. Eur J Immunol. 1997;27:726–734. doi: 10.1002/eji.1830270322. [DOI] [PubMed] [Google Scholar]
- 12.Goddard MJ, Dunning JJ, Horsley J, Atkinson C, Pino-Chavez G, Wallwork J. Histopathology of cardiac xenograft rejection in the pig-to-baboon model. J Heart Lung Transplant. 2002;21:474–484. doi: 10.1016/s1053-2498(01)00402-8. [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi S, Neethling FA, Korchagina EY, Bovin N, Ye Y, Kobayashi T, et al. In vivo immunoadsorption of antipig antibodies in baboons using a specific galα1-3gal column. Transplantation. 1996;62:1379–1384. doi: 10.1097/00007890-199611270-00001. [DOI] [PubMed] [Google Scholar]
- 14.Ye Y, Neethling FA, Niekrasz M, Koren E, Richards SV, Martin M, et al. Evidence that intravenously administered α-Galactosyl carbohydrates reduce baboon serum cytotoxicity to pig kidney cells (PK15) and transplanted pig hearts. Transplantation. 1994;58:330–337. [PubMed] [Google Scholar]
- 15.Nagasaka T, Kobayashi T, Muramatsu H, Fujimoto H, Matsuo I, Ajisaka K, et al. Alpha Galactosyl oligosaccharides conjugated with polyethylene glycol as potential inhibitors of hyperacute rejection upon xenotransplantation. Biochem Biophysic Res Com. 1997;232:731–736. doi: 10.1006/bbrc.1997.6360. [DOI] [PubMed] [Google Scholar]
- 16.Byrne GW, Schwarz A, Fesi JR, Birch P, Nepomich A, Bakaj I, et al. Evaluation of different alpha-Galactosyl glycoconjugates for use in xenotransplantation. Bioconjugate Chem. 2002;13:571–581. doi: 10.1021/bc015565e. [DOI] [PubMed] [Google Scholar]
- 17.Lin SS, Weidner BC, Byrne GW, Diamond LE, Lawson JH, Hoopes CW, et al. The role of antibodies in acute vascular rejection of pig-to-baboon cardiac transplants. J Clin Invest. 1998;101:1745–1756. doi: 10.1172/JCI2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuwaki K, Knosalla C, Dor FJMF, Gollackner B, Tseng Y-L, Houser S, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004;4:363–372. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 19.Lam TT, Paniagua R, Shivaram G, Schuurman HJ, Borie DC, Morris RE. Anti-non-Gal porcine endothelial cell antibodies in acute humoral xenograft rejection of hDAF-transgenic porcine hearts in cynomolgus monkeys. Xenotransplantation. 2004;11:531–535. doi: 10.1111/j.1399-3089.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 20.McGregor CGA, Teotia SS, Byrne GW, Michaels MG, Risdahl JM, Schirmer JM, et al. Cardiac xenotransplantation: Progress toward the clinic. Transplantation. 2004;78:1569–1575. doi: 10.1097/01.tp.0000147302.64947.43. [DOI] [PubMed] [Google Scholar]
- 21.McGregor CG, Davies WR, Oi K, Teotia SS, Schirmer JM, Risdahl JM, et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg. 2005;130:844–851. doi: 10.1016/j.jtcvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Lai L, Kolber-Simonds D, Park K-W, Cheong H-T, Greenstein JL, Im G-S, et al. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 23.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu A, Hisashi Y, Kuwaki K, Tseng YL, Dor FJ, Houser SL, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Path. 2008;172:1471–1481. doi: 10.2353/ajpath.2008.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azimzadeh AM, Kelishadi SS, Ezzelarab MB, Singh AK, Stoddard T, Iwase H, et al. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein. Xenotransplantation. 2015 doi: 10.1111/xen.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada K, Tasaki M, Sekijima M, Wilkinson RA, Villani V, Moran SG, et al. Porcine cytomegalovirus infection is associated with early rejection of kidney grafts in a pig to baboon xenotransplantation model. Transplantation. 2014;98:411–418. doi: 10.1097/TP.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higginbotham L, Mathews D, Breeden CA, Song M, Farris AB, 3rd, Larsen CP, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015 doi: 10.1111/xen.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohiuddin MM, Corcoran PC, Singh AK, Azimzadeh A, Hoyt RF, Jr, Thomas ML, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012;12:763–771. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buhler L, Basker M, Alwayn IPJ, Goepfert C, Kitamura H, Kawai T, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70:1323–1331. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 31.Kuwaki K, Knosalla C, Dor FJ, Gollackner B, Tseng YL, Houser S, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004;4:363–372. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 32.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML, 3rd, Lewis BG, et al. Role of anti-CD40 antibody-mediated costimulation blockade on non-Gal antibody production and heterotopic cardiac xenograft survival in a GTKO.hCD46Tg pig-to-baboon model. Xenotransplantation. 2013 doi: 10.1111/xen.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML, 3rd, Ayares D, et al. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J Thorac Cardiovasc Surg. 2014;148:1106–1113. doi: 10.1016/j.jtcvs.2014.06.002. discussion 1113–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper DK, Keogh AM, Brink J, Corris PA, Klepetko W, Pierson RN, et al. Report of the Xenotransplantation Advisory Committee of the International Society for Heart and Lung Transplantation: the present status of xenotransplantation and its potential role in the treatment of end-stage cardiac and pulmonary diseases. J Heart Lung Transplant. 2000;19:1125–1165. doi: 10.1016/s1053-2498(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 35.Waterworth PD, Dunning J, Tolan M, Cozzi E, Langford G, Chavez G, et al. Life-supporting pig-to-baboon heart xenotransplantation. J Heart Lung Transplant. 1998;17:1201–1207. [PubMed] [Google Scholar]
- 36.Vial CM, Ostlie DJ, Bhatti FNK, Cozzi E, Goddard M, Chavez GP, et al. Life supporting function for over one month of a transgenic porcine heart in a baboon. J Heart Lung Transplant. 2000;19:224–229. doi: 10.1016/s1053-2498(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 37.Schmoeckel M, Bhatti FNK, Zaidi A, Cozzi E, Waterworth PD, Tolan MJ, Goddard M, Warner RG, Langford GA, Dunning JJ, Wallwork J, White DJG. Orthotopic heart transplantation in a transgenic pig-tp-primate model. Transplantation. 1998;65:1570–1577. doi: 10.1097/00007890-199806270-00006. [DOI] [PubMed] [Google Scholar]
- 38.Xu H, Gundry Sr, Hancock WW, Matsumiya G, Zuppan CW, Morimoto T, et al. Prolonged discordant xenograft survival and delayed xenograft rejection in a pig-to-baboon orthotopic cardiac xenograft model. J Thorac Cardiovasc Surg. 1998;115:1342–1349. doi: 10.1016/S0022-5223(98)70218-1. [DOI] [PubMed] [Google Scholar]
- 39.McGregor CGA, Davies WR, Oi K, Tazelaar HD, Walker RC, Chandrasekaran K, et al. Recovery of cardiac function after pig-to-primate orthotopic heart transplant. (Abstr 98) Am J Transplant. 2008;8:205–206. [Google Scholar]
- 40.Brandl U, Michel S, Erhardt M, Brenner P, Burdorf L, Jockle H, et al. Transgenic animals in experimental xenotransplantation models: orthotopic heart transplantation in the pig-to-baboon model. Transplant Proc. 2007;39:577–578. doi: 10.1016/j.transproceed.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 41.Brandl U, Michel S, Erhardt M, Brenner P, Bittmann I, Rossle M, et al. Administration of GAS914 in an orthotopic pig-to-baboon heart transplantation model. Xenotransplantation. 2005;12:134–141. doi: 10.1111/j.1399-3089.2005.00208.x. [DOI] [PubMed] [Google Scholar]
- 42.McGregor CGA, Byrne GW, Vlasin M, Walker RC, Tazelaar HD, Davies WR, Chandrasekaran K, Oehler EA, Boilson BA, Wiseman BS, Logan JS. Cardiac function after preclinical orthotopic cardiac xenotransplanation. Am J Transplant. 2009;9:380. [Google Scholar]
- 43.Byrne GW, Du Z, Sun Z, Asmann YW, McGregor CG. Changes in cardiac gene expression after pig-to-primate orthotopic xenotransplantation. Xenotransplantation. 2011;18:14–27. doi: 10.1111/j.1399-3089.2010.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Byrne G. Strategy to Overcome Non-Gal NAb in Xenotransplantation. Xenotransplantation. 2013;20:324–325. [Google Scholar]
- 45.Wheeler DG, Joseph ME, Mahamud SD, Aurand WL, Mohler PJ, Pompili VJ, et al. Transgenic swine: expression of human CD39 protects against myocardial injury. Journal of molecular and cellular cardiology. 2012;52:958–961. doi: 10.1016/j.yjmcc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnard CN, Losman JG, Curcio CA, Sanchez HE, Wolpowitz A, Barnard MS. The advantage of heterotopic cardiac transplantation over orthotopic cardiac transplantation in the management of severe acute rejection. J Thorac Cardiovasc Surg. 1977;74:918–924. [PubMed] [Google Scholar]
- 47.Reichenspurner H, Odell JA, Cooper DK, Novitzky D, Human PA, Von Oppell U, et al. Twenty years of heart transplantation at Groote Schuur Hospital. J Heart Transplant. 1987;6:317–323. [PubMed] [Google Scholar]
- 48.Bauer A, Postrach J, Thormann M, Blanck S, Faber C, Wintersperger B, et al. First experience with heterotopic thoracic pig-to-baboon cardiac xenotransplantation. Xenotransplantation. 2010;17:243–249. doi: 10.1111/j.1399-3089.2010.00587.x. [DOI] [PubMed] [Google Scholar]
- 49.Klymiuk N, Aigner B, Brem G, Wolf E. Genetic modification of pigs as organ donors for xenotransplantation. Mol Reprod Dev. 2010;77:209–221. doi: 10.1002/mrd.21127. [DOI] [PubMed] [Google Scholar]
- 50.Manez R, Lopez-Pelaez E, Centeno A, Herrera JM, Juffe A, Domenech N, et al. Transgenic expression in pig hearts of both human decay-accelerating factor and human membrane cofactor protein does not provide an additional benefit to that of human decay-accelerating factor alone in pig-to-baboon xenotransplantation. Transplantation. 2004;78:930–933. doi: 10.1097/01.tp.0000133309.82387.8c. [DOI] [PubMed] [Google Scholar]
- 51.McGregor CG, Ricci D, Miyagi N, Stalboerger PG, Du Z, Oehler EA, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation. 2012;93:686–692. doi: 10.1097/TP.0b013e3182472850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Estrada JL, Martens G, Li P, Adams A, Newell KA, Ford ML, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 2015;22:203–210. doi: 10.1111/xen.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byrne GW, Schirmer JM, Fass DN, Teotia SS, Kremers WK, Xu H, et al. Warfarin or low-molecular-weight heparin therapy does not prolong pig-to-primate cardiac xenograft function. Am J Transplant. 2005;5:1011–1020. doi: 10.1111/j.1600-6143.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 54.Schirmer JM, Fass DN, Byrne GW, Tazelaar HD, Logan JS, McGregor CG. Effective antiplatelet therapy does not prolong transgenic pig to baboon cardiac xenograft survival. Xenotransplantation. 2004;11:436–443. doi: 10.1111/j.1399-3089.2004.00159.x. [DOI] [PubMed] [Google Scholar]
- 55.Cozzi E, Simioni P, Boldrin M, Seveso M, Calabrese F, Baldan N, et al. Effects of long-term administration of high-dose recombinant human antithrombin in immunosuppressed primate recipients of porcine xenografts. Transplantation. 2005;80:1501–1510. doi: 10.1097/01.tp.0000178377.55615.8b. [DOI] [PubMed] [Google Scholar]
- 56.Petersen B, Ramackers W, Tiede A, Lucas-Hahn A, Herrmann D, Barg-Kues B, et al. Pigs transgenic for human thrombomodulin have elevated production of activated protein C. Xenotransplantation. 2009;16:486–495. doi: 10.1111/j.1399-3089.2009.00537.x. [DOI] [PubMed] [Google Scholar]


