Abstract
Inflammation is a complex response that involves interactions between multiple proteins in the human body. The interaction between inflammation and coagulation is well-recognized, but its role in the dysregulation of coagulation in xenograft recipients is not well-understood. Additionally, inflammation is known to prevent the development of T cell tolerance after transplantation.
Recent evidence indicates that systemic inflammation precedes and may be promoting activation of coagulation after pig-to-primate xenotransplantation. Activated recipient innate immune cells expressing tissue factor are increased after xenotransplantation, irrespective of immunosuppressive therapy. With immunosuppression, C-reactive protein (C-RP), fibrinogen, and interleukin-6 levels are significantly increased in pig artery patch recipients. In pig organ recipients, increased C-RP levels are observed prior to the development of features of consumptive coagulopathy. Systemic inflammation in xenograft recipients (SIXR) may be a key factor in the development of dysregulation of coagulation, as well as in resistance to immunosuppressive therapy.
While genetic modification of the donor pigs provides protection against humoral responses and the development of thrombotic microangiopathy, therapeutic prevention of SIXR may be essential in order to prevent systemic dysregulation of coagulation in xenograft recipients without the use of intensive immunosuppression.
Keywords: Coagulation, Innate immune cells, Inflammation, Pig, Primate, Xenotransplantation
Introduction
Inflammation is a natural and essential response in the human body to infection, cell stress, and tissue damage. It is frequently associated with the development of pathological conditions as diverse as diabetes, ischemia, and atherosclerosis. At sites of inflammation, a wide range of cytokines and chemokines are produced. Chemokines are required for recruiting innate immune cells to the site of inflammation. Cytokines modulate maturation, growth and responsiveness of various immune cells that are responsible for elimination of pathogens, removal of dead cells, and stimulation of tissue repair.
Following an inflammatory response, effective resolution of inflammation requires the cessation of pro-inflammatory signals, followed by the disappearance of inflammatory cellular infiltrates. However, under certain conditions, inflammation persists. Prolonged (or chronic) inflammatory responses are associated with various pathological conditions, such as rheumatoid arthritis and atherosclerosis. Similarly, intensified inflammatory responses can be associated adverse effects.
In the last two decades, a substantial network of interactions has been revealed between inflammation, the coagulation system, and the innate immune system [1]. Multiple cellular and molecular mechanisms have been identified linking inflammatory responses and homeostasis. The pro-inflammatory roles of coagulation proteins and the role of cytokines in promoting activation of coagulation have been well-characterized. Accordingly, the interaction between inflammation and coagulation results in an amplification circuit [2] promoting the production of both inflammatory mediators as well as coagulation factors.
Dysregulation of the coagulation system is considered a hallmark associated with failure of organ xenografts. Thrombotic microangiopathy (TM) in the graft and/or consumptive coagulopathy (CC) in the recipient are characteristic features associated with xenograft failure or rejection, and are major barriers to prolonged xenograft survival in nonhuman primates [3–6].
The roles of inflammatory signals in promoting T cell activation after allotransplantation have been recognized as key elements in the loss of T cell tolerance and prevention of long-term allograft survival [7], associated with B cell activation and antibody production. Recent studies suggest that the inflammatory response in pig-to-nonhuman primate xenotransplantation may have been underappreciated. Systemic inflammation in xenograft recipients (SIXR) may play a key role in the perpetuation of activation of coagulation (and therefore of coagulation dysregulation), as well as in increased resistance to the effects of immunosuppressive therapy (IS) after xenotransplantation [8].
Role of innate immune cells in inflammation and activation of coagulation
Innate immune cells are considered a barrier to prolonged xenograft survival in nonhuman primates [9,10]. In addition to their role in the process of inflammation and production of pro-inflammatory cytokines, innate immune cells, e.g., dendritic cells, are known to promote activation of coagulation through multiple mechanisms, providing an additional link between inflammation and activation of coagulation [11].
In response to pro-inflammatory signals, activated monocytes and dendritic cells upregulate tissue factor (TF) expression, and potentiate activation of coagulation [11–13]. Activated dendritic cells express thrombin receptors [14], while thrombin is also known to influence dendritic cell functions [15]. Furthermore, activated dendritic cells have been shown to express and release functional TF [13].
Expression of porcine TF by activated endothelial cells is an expected initial mechanism in the development of TM and CC. It is also likely that upregulation of recipient TF expression may further augment systemic activation of coagulation [16], particularly with prolonged xenograft survival. Pig aortic endothelial cells (pAECs) are known to stimulate human CD14+ monocytes associated with the production of pro-inflammatory cytokines [17] and the upregulation of TF expression [18]. Inflammatory CD11c+ dendritic cells have been shown to be monocyte-derived [19,20]. Our previous studies have shown that macrophages and peripheral blood mononuclear cells upregulate TF expression after pig organ xenotransplantation [10,21]. In pig artery patch recipient baboons, we have observed a significant increase in the percentage of peripheral blood CD14+CD11c+ cells, in the presence or absence of IS. This was associated with significant upregulation of TF expression on monocytes and dendritic cells in the recipient baboons [8].
While transgenic expression of human coagulation-regulatory proteins in pig xenografts provides protection against the development of TM, it will be essential to prevent long-term activation and upregulation of TF expression by innate immune cells in xenograft recipients in order to prevent dysregulation of coagulation.
Pro-inflammatory cytokines after xenotransplantation
Pro-inflammatory cytokines are essential for protection against infections. However, excessive cytokine production can promote inflammation [22]. Pro-inflammatory cytokines are known to promote activation of coagulation. Tumor necrosis factor-alpha (TNF-α) [23] and interleukin (IL)-6 [24] promote TF expression by various cell types, which can lead to activation of coagulation [23,25]. The potential roles of pro-inflammatory cytokines and chemokines in the development of dysregulation of coagulation in xenograft recipients are not well understood, despite their recognized contribution to xenograft rejection [26–29].
After organ xenotransplantation, IS may not efficiently reduce pro-inflammatory cytokines despite efficient blockade of adaptive immune responses. Detectable levels of interferon gamma (IFN-γ), IL-12 and IL-8 can still be observed after pig organ xenotransplantation in baboons. Significantly high levels of TNF-α, monocyte chemotactic protein 1 (MCP1), and IL-6 are also detected [8]. In pig artery patch recipient baboons, costimulation-based IS efficiently reduced pro-inflammatory cytokines except IL-6 [8], which has been linked to both inflammatory and thrombotic complications [30] and promotion of TF expression [31] on innate immune cells [25]. This observation suggests that blockade of IL-6 activity may be beneficial after xenotransplantation
C-reactive protein (C-RP) in xenograft recipients
C-RP is a long-studied acute-phase protein recognized to be produced in humans and nonhuman primates during episodes of inflammation, e.g. infection [32,33]. C-RP has been considered a sensitive, but not specific, marker for graft-related complications after organ allotransplantation in humans [34,35]. Notably, C-RP can also promote TF expression [36].
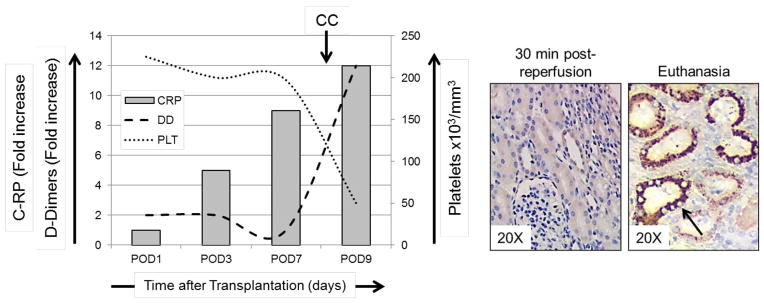
C-RP levels are increased in pig organ xenograft recipients within days after transplantation [8]. Importantly, this rise in C-RP levels occurred prior to signs of activation of coagulation and the development of CC in the recipients (Figure 1). Of note, higher C-RP levels were associated with rapid development of dysregulation of coagulation and earlier failure of kidney xenografts compared to heart xenografts [8]. The variable incidence of systemic complications in organ xenograft recipients may be attributed to organ xenograft heterogeneity and organ-specific vascular gene expression [37]. At the time of euthanasia, C-RP deposition was detected in the heart and kidney xenografts. Furthermore, C-RP-positive immune cells were observed in the native lungs of xenograft recipients, indicating a systemic inflammatory response.
Figure 1. Increased C-RP levels in xenograft recipient baboon and deposition of C-RP in the pig xenografts.
Left: Platelet counts, and fold increases in C-RP and D-Dimer were calculated in a pig kidney xenograft recipient. High levels of C-RP were detected as early as 3 days after kidney xenotransplantation, prior to the development of consumptive coagulopathy (CC), as indicated by reduced platelet counts and elevated D-Dimer levels. Right: At 30 min after reperfusion, no C-RP was detected, while at the time of euthanasia C-RP deposition was detected in the kidney tubules (arrow).
In addition to its pro-inflammatory effects, IL-6 is known to induce C-RP production by hepatocytes [38] and smooth muscle cells [39]. High IL-6 levels are associated with high C-RP levels in humans [40]. In pig artery patch recipient baboons, we have documented a significant positive correlation between IL-6 and C-RP levels, as well as between C-RP and fibrinogen levels in the blood [8]. Both C-RP and fibrinogen are known to be acute-phase reactant proteins produced in response to acute inflammation.
Dysregulation of coagulation does not develop in pig artery patch recipients, where high levels of both C-RP and fibrinogen are maintained. In contrast, while C-RP levels continue to increase in organ xenograft recipients, fibrinogen levels gradually decline in concomitance with the onset of activation of coagulation due to consumption of coagulation factors and the development of CC. Hence, steps that to initially prevent the increase in C-RP and fibrinogen levels in pig organ recipients should be beneficial in preventing dysregulation of coagulation and in promoting long-term xenograft survival.
The effect of IS on the inflammatory responses and activation of coagulation in xenograft recipients
Activation of coagulation and fibrin deposition remain central to pig organ xenograft failure in nonhuman primates. Upregulation of pro-coagulant proteins by pig endothelial cells is thought to be critical for the development of TM in organ xenografts, where induction of a pro-coagulant phenotype by pig endothelial cells can be a result of increased binding of natural and elicited anti-pig antibodies [3]. Prevention of production of elicited antibodies is thought to be essential for prevention of pig endothelial cell activation and upregulation of pro-coagulant proteins. Accordingly, efficient IS is critical to achieve prolonged xenograft survival, not only through the prevention of the adaptive immune response and elicited anti-pig antibodies, but also through the prevention of consequent activation of coagulation.
While dysregulation of the coagulation system in nonhuman primates is a characteristic feature of xenograft failure, anticoagulation has been shown to be relatively inefficient in prolonging pig kidney xenograft survival in monkeys receiving IS [41]. Furthermore, anticoagulation was shown to be inefficient in promoting pig heart xenograft survival in baboons [42]. These observations indicated that IS - but not anticoagulation - promotes long-term xenograft survival in nonhuman primates. It is important to note that these studies were performed using wild-type pigs expressing human complement-regulatory proteins CD55 (DAF) or CD46, respectively.
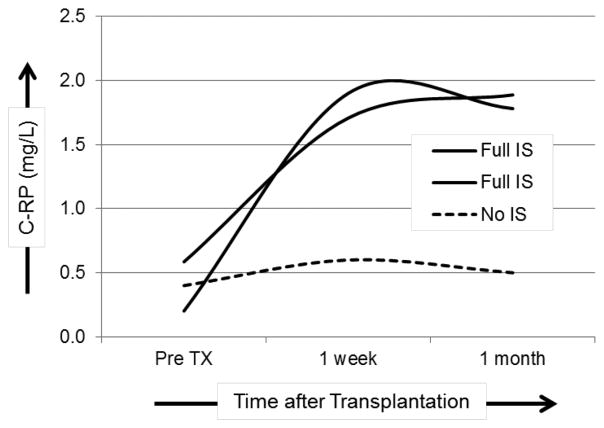
Therefore, while it might be expected that anticoagulants would ameliorate dysregulation of coagulation after xenotransplantation, effective IS may be more critical in preventing and/or delaying activation of coagulation. Prevention of T cell responses and depletion of T cells in the blood is associated with reduced pro-inflammatory cytokines [8]. Costimulation-based IS prevented the production of pro-inflammatory cytokines in pig artery patch recipients [8,43]. However, the level of IL-6 in the blood was higher when IS was administered, compared to the level seen in baboons not receiving IS. Similarly, C-RP levels were continuously elevated when IS was administered, but remained low in baboons without IS (Figure 2). Interestingly, there was a significant positive correlation between the levels of IL-6, fibrinogen, and C-RP in those recipients. However, when a similar IS regimen was administered to pig kidney and heart recipients, it did not have a comparable effect (i.e., it did not efficiently reduce the production of pro-inflammatory cytokines) indicating that more intensive IS and/or anti-inflammatory agents may be required to reduce SIXR after organ xenotransplantation.
Figure 2. Immunosuppressive therapy (IS) is associated with increased C-RP levels in baboon recipients of pig artery patch grafts.
C-RP levels were measured in baboons receiving full IS (n=2) or without IS (n=1), before, and one week and one month after pig artery patch xenotransplantation.
These observations suggest that SIXR and the effect of IS on the inflammatory response may vary based on the type of xenograft, the extent of the antigenic load, and the intensity of IS. The IS regimens adopted by different groups may have variable effects on the inflammatory milieu in the xenograft recipient. The effect of IS on both pro- and anti-inflammatory cytokine levels in xenograft recipients is not clear and requires further investigation.
Observations reported in humans suggest that IS can on occasion stimulate an inflammatory response [44]. Also, a systemic inflammatory response syndrome in humans is well-described and involves dysregulation of coagulation [45]. In contrast, under certain conditions, prolonged inflammation may lead to reduced immune responses [46–48]. The long-term effects of IS on SIXR as well as the effects of SIXR on immune responses are yet to be determined. This may be critical in order to avoid complications associated with prolonged and intensive IS.
Conclusions
The underlying mechanisms of the inflammatory response in xenograft recipients are poorly understood. It is yet to be determined whether the inflammatory response varies dependent on the nature of the xenograft and the type of the administered IS. Recent studies suggest that SIXR may be playing a crucial role in the outcome of organ and cell xenotransplantation. Also, it will be important to determine the effect of SIXR on the adaptive immune response and on activation of coagulation. Defining both pro- and anti-inflammatory mechanisms may be critical to develop effective therapies after xenotransplantation. Clinically-relevant IS may be easier to achieve by also targeting pro-inflammatory factors in xenograft recipients. Anti-inflammatory therapy may enable a reduction in the intensity of the IS required to maintain pig xenograft survival.
Highlights.
Systemic inflammation in xenograft recipients (SIXR) precedes activation of coagulation
Increased inflammation may promote activation of coagulation in xenograft recipients
Innate immune cells upregulate tissue factor expression after xeno-Tx
Immunosuppression does not consistently reduce pro-inflammatory cytokines after xeno-Tx
Prevention of SIXR may be essential to reduce dysregulation of coagulation after xeno-Tx
Acknowledgments
Funding Acknowledgements
MBE was supported in part by the Joseph A. Patrick Fellowship of the Thomas E. Starzl Transplantation Institute. Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute is, or has been, supported by NIH grants U19 AI090959, U01 AI068642, and R21 A1074844, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA.
ABBREVIATIONS
- CC
Consumptive coagulopathy
- C-RP
C-reactive protein
- IS
immunosuppressive therapy
- SIXR
systemic inflammation in xenograft recipients
- TF
tissue factor
- TM
Thrombotic microangiopathy
Footnotes
Conflicts of Interest - None
Ethical Approval – Not required
Author contribution
M.B.E. and D.K.C.C – participated in review of the literature, writing of the manuscript and final approval of the manuscript.
Guarantor – Mohamed B. Ezzelarab
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Esmon CT, Xu J, Lupu F. Innate immunity and coagulation. J Thromb Haemost. 2011;9(Suppl 1):182–188. doi: 10.1111/j.1538-7836.2011.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strukova S. Blood coagulation-dependent inflammation. Coagulation-dependent inflammation and inflammation-dependent thrombosis. Front Biosci. 2006;11:59–80. doi: 10.2741/1780. [DOI] [PubMed] [Google Scholar]
- 3.Cowan PJ, Robson SC, d’Apice AJ. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant. 2011;16:214–221. doi: 10.1097/MOT.0b013e3283446c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 5.Buhler L, Basker M, Alwayn IP, Goepfert C, Kitamura H, Kawai T, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70:1323–1331. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 6.Robson SC, Cooper DK, d’Apice AJ. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation. 2000;7:166–176. doi: 10.1034/j.1399-3089.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 7.Chong AS, Alegre ML. The impact of infection and tissue damage in solid-organ transplantation. Nat Rev Immunol. 2012;12:459–471. doi: 10.1038/nri3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezzelarab MB, Ekser B, Azimzadeh A, Lin CC, Zhao Y, Rodriguez R, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22:32–47. doi: 10.1111/xen.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider MK, Seebach JD. Current cellular innate immune hurdles in pig-to-primate xenotransplantation. Curr Opin Organ Transplant. 2008;13:171–177. doi: 10.1097/MOT.0b013e3282f88a30. [DOI] [PubMed] [Google Scholar]
- 10.Ezzelarab M, Garcia B, Azimzadeh A, Sun H, Lin CC, Hara H, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, et al. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 12.van den Eijnden MM, Steenhauer SI, Reitsma PH, Bertina RM. Tissue factor expression during monocyte-macrophage differentiation. Thromb Haemost. 1997;77:1129–1136. [PubMed] [Google Scholar]
- 13.Baroni M, Pizzirani C, Pinotti M, Ferrari D, Adinolfi E, Calzavarini S, et al. Stimulation of P2 (P2X7) receptors in human dendritic cells induces the release of tissue factor-bearing microparticles. FASEB J. 2007;21:1926–1933. doi: 10.1096/fj.06-7238com. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Syrovets T, Paskas S, Laumonnier Y, Simmet T. Mature dendritic cells express functional thrombin receptors triggering chemotaxis and CCL18/pulmonary and activation-regulated chemokine induction. J Immunol. 2008;181:1215–1223. doi: 10.4049/jimmunol.181.2.1215. [DOI] [PubMed] [Google Scholar]
- 15.Yanagita M, Kobayashi R, Kashiwagi Y, Shimabukuro Y, Murakami S. Thrombin regulates the function of human blood dendritic cells. Biochem Biophys Res Commun. 2007;364:318–324. doi: 10.1016/j.bbrc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Nagayasu T, Saadi S, Holzknecht RA, Plummer TB, Platt JL. Expression of tissue factor mRNA in cardiac xenografts: clues to the pathogenesis of acute vascular rejection. Transplantation. 2000;69:475–482. doi: 10.1097/00007890-200002270-00003. [DOI] [PubMed] [Google Scholar]
- 17.Manna PP, Steward N, Lowell J, Mohanakumar T. Differentiation and functional maturation of human CD14(+) adherent peripheral blood monocytes by xenogeneic endothelial cells: up-regulation of costimulation, cytokine generation, and toll-like receptors. Transplantation. 2002;74:243–252. doi: 10.1097/00007890-200207270-00016. [DOI] [PubMed] [Google Scholar]
- 18.Lin CC, Chen D, McVey JH, Cooper DK, Dorling A. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008;86:702–709. doi: 10.1097/TP.0b013e31818410a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 20.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 21.Lin CC, Ezzelarab M, Shapiro R, Ekser B, Long C, Hara H, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10:1556–1568. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Kambas K, Markiewski MM, Pneumatikos IA, Rafail SS, Theodorou V, Konstantonis D, et al. C5a and TNF-alpha up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J Immunol. 2008;180:7368–7375. doi: 10.4049/jimmunol.180.11.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruithof EK, Mestries JC, Gascon MP, Ythier A. The coagulation and fibrinolytic responses of baboons after in vivo thrombin generation--effect of interleukin 6. Thromb Haemost. 1997;77:905–910. [PubMed] [Google Scholar]
- 25.Kotloff RM, Little J, Elias JA. Human alveolar macrophage and blood monocyte interleukin-6 production. Am J Respir Cell Mol Biol. 1990;3:497–505. doi: 10.1165/ajrcmb/3.5.497. [DOI] [PubMed] [Google Scholar]
- 26.Solomon MF, Kuziel WA, Mann DA, Simeonovic CJ. The role of chemokines and their receptors in the rejection of pig islet tissue xenografts. Xenotransplantation. 2003;10:164–177. doi: 10.1034/j.1399-3089.2003.01146.x. [DOI] [PubMed] [Google Scholar]
- 27.Chandra AP, Ouyang L, Yi S, Wong JK, Ha H, Walters SN, et al. Chemokine and toll-like receptor signaling in macrophage mediated islet xenograft rejection. Xenotransplantation. 2007;14:48–59. doi: 10.1111/j.1399-3089.2006.00363.x. [DOI] [PubMed] [Google Scholar]
- 28.Yi S, Ouyang L, Ha H, O’Hara JM, Chandra AP, Akima S, et al. Involvement of CCR5 signaling in macrophage recruitment to porcine islet xenografts. Transplantation. 2005;80:1468–1475. doi: 10.1097/01.tp.0000183398.82878.47. [DOI] [PubMed] [Google Scholar]
- 29.Remy S, Canova C, Daguin-Nerriere V, Martin C, Melchior B, Neveu I, et al. Different mechanisms mediate the rejection of porcine neurons and endothelial cells transplanted into the rat brain. Xenotransplantation. 2001;8:136–148. doi: 10.1034/j.1399-3089.2001.00076.x. [DOI] [PubMed] [Google Scholar]
- 30.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 31.Levi M, van der Poll T, ten Cate H, van Deventer SJ. The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Invest. 1997;27:3–9. doi: 10.1046/j.1365-2362.1997.570614.x. [DOI] [PubMed] [Google Scholar]
- 32.Abernethy TJ, Avery OT. The Occurrence during Acute Infections of a Protein Not Normally Present in the Blood : I. Distribution of the Reactive Protein in Patients’ Sera and the Effect of Calcium on the Flocculation Reaction with C Polysaccharide of Pneumococcus. J Exp Med. 1941;73:173–182. doi: 10.1084/jem.73.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tillett WS, Francis T. Serological Reactions in Pneumonia with a Non-Protein Somatic Fraction of Pneumococcus. J Exp Med. 1930;52:561–571. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wullstein C, Drognitz O, Woeste G, Schareck WD, Bechstein WO, Hopt UT, et al. High levels of C-reactive protein after simultaneous pancreas-kidney transplantation predict pancreas graft-related complications and graft survival. Transplantation. 2004;77:60–64. doi: 10.1097/01.TP.0000100683.92689.27. [DOI] [PubMed] [Google Scholar]
- 35.Their M, Ronnholm K, Sairanen H, Holmberg C, Jalanko H. Serum C-reactive protein in pediatric kidney and liver transplant patients. Pediatr Transplant. 2002;6:153–160. doi: 10.1034/j.1399-3046.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- 36.Wu J, Stevenson MJ, Brown JM, Grunz EA, Strawn TL, Fay WP. C-reactive protein enhances tissue factor expression by vascular smooth muscle cells: mechanisms and in vivo significance. Arterioscler Thromb Vasc Biol. 2008;28:698–704. doi: 10.1161/ATVBAHA.107.160903. [DOI] [PubMed] [Google Scholar]
- 37.Knosalla C, Yazawa K, Behdad A, Bodyak N, Shang H, Buhler L, et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am J Transplant. 2009;9:1006–1016. doi: 10.1111/j.1600-6143.2009.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moshage HJ, Roelofs HM, van Pelt JF, Hazenberg BP, van Leeuwen MA, Limburg PC, et al. The effect of interleukin-1, interleukin-6 and its interrelationship on the synthesis of serum amyloid A and C-reactive protein in primary cultures of adult human hepatocytes. Biochem Biophys Res Commun. 1988;155:112–117. doi: 10.1016/s0006-291x(88)81056-8. [DOI] [PubMed] [Google Scholar]
- 39.Calabro P, Willerson JT, Yeh ET. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108:1930–1932. doi: 10.1161/01.CIR.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]
- 40.Marcucci R, Gori AM, Giannotti F, Baldi M, Verdiani V, Del Pace S, et al. Markers of hypercoagulability and inflammation predict mortality in patients with heart failure. J Thromb Haemost. 2006;4:1017–1022. doi: 10.1111/j.1538-7836.2006.01916.x. [DOI] [PubMed] [Google Scholar]
- 41.Cozzi E, Simioni P, Boldrin M, Seveso M, Calabrese F, Baldan N, et al. Effects of long-term administration of high-dose recombinant human antithrombin in immunosuppressed primate recipients of porcine xenografts. Transplantation. 2005;80:1501–1510. doi: 10.1097/01.tp.0000178377.55615.8b. [DOI] [PubMed] [Google Scholar]
- 42.Byrne GW, Davies WR, Oi K, Rao VP, Teotia SS, Ricci D, et al. Increased immunosuppression, not anticoagulation, extends cardiac xenograft survival. Transplantation. 2006;82:1787–1791. doi: 10.1097/01.tp.0000251387.40499.0f. [DOI] [PubMed] [Google Scholar]
- 43.Ezzelarab MB, Ekser B, Echeverri G, Hara H, Ezzelarab C, Long C, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012;19:221–232. doi: 10.1111/j.1399-3089.2012.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34:43–62. doi: 10.1007/s00281-011-0290-8. [DOI] [PubMed] [Google Scholar]
- 45.Matsuda N, Hattori Y. Systemic inflammatory response syndrome (SIRS): molecular pathophysiology and gene therapy. J Pharmacol Sci. 2006;101:189–198. doi: 10.1254/jphs.crj06010x. [DOI] [PubMed] [Google Scholar]
- 46.Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, Ungaro R, Davis R, Cuenca AG, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. 2014;76:21–29. doi: 10.1097/TA.0b013e3182ab1ab5. discussion 29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanterman J, Sade-Feldman M, Baniyash M. New insights into chronic inflammation-induced immunosuppression. Semin Cancer Biol. 2012;22:307–318. doi: 10.1016/j.semcancer.2012.02.008. [DOI] [PubMed] [Google Scholar]