Abstract
BACKGROUND
Severe or prolonged stress can trigger psychiatric illnesses including mood and anxiety disorders. Recent work indicates that pituitary adenylate cyclase-activating polypeptide (PACAP) plays an important role in regulating stress effects. In rodents, exogenous PACAP administration can produce persistent elevations in the acoustic startle response, which may reflect anxiety-like signs including hypervigilance. Here we investigated whether PACAP causes acute or persistent alterations in behaviors that reflect other core features of mood and anxiety disorders (motivation, social interaction, and attention).
METHODS
Using male Spraque-Dawley rats, we examined if PACAP (0.25–1.0 μg, intracerebroventricular) affects motivation as measured in the intracranial self-stimulation (ICSS) test. We also examined if PACAP alters interactions with a conspecific in the social interaction (SI) test. Finally, we examined if PACAP affects performance in the 5-choice serial reaction time task (5CSRTT), which quantifies attention and error processing.
RESULTS
PACAP produced dose-dependent disruptions in motivation, social interaction, and attention, as reflected by increases in reward thresholds, decreases in social behaviors, and decreases in correct responses and alterations in post-error accuracy. Behavior normalized quickly in the ICSS and 5CSRTT tests, but remained dysregulated in the SI test. Effects on attention were attenuated by the corticotropin-releasing factor receptor-1 (CRF-R1) antagonist antalarmin but not the kappa-opioid receptor antagonist JDTic.
CONCLUSIONS
Our findings suggest that PACAP affects numerous domains often dysregulated in mood and anxiety disorders, but that individual signs depend on brain substrates that are at least partially independent. This work may help to devise therapeutics that mitigate specific signs of these disorders.
Keywords: pituitary adenylate cyclase-activating peptide OR PACAP, anhedonia, social interaction, attention, rat, model
INTRODUCTION
Severe or prolonged stress is linked to the etiology of mood and anxiety disorders such as major depressive disorder (MDD) and posttraumatic stress disorder (PTSD) (1–3). These disorders are often associated with persistent dysregulation of domains including motivation, social behavior, and attention (4,5). Despite the broad impact of these illnesses, the mechanisms by which stress induces maladaptive behavioral responses are not fully understood.
Pituitary adenylate cyclase activating polypeptide (PACAP) is a neuropeptide that plays an important role in regulating stress effects and is modified by stressful experiences (6,7). PACAP and its cognate receptor (PAC1) are widely expressed in stress- and anxiety-associated brain regions, including the amygdala and bed nucleus of the stria terminalis (BNST) (8,9). In rodents, chronic stress increases expression of PACAP mRNA within these regions (9,10), raising the possibility that neuroadaptive changes in PACAP systems alter sensitivity to subsequent stressors. Indeed, PACAP-deficient mice exhibit reduced corticosterone responses (11), anxiety-like behavior (12–14), and sensitivity to chronic social defeat stress (15). Exogenous PACAP administration produces many of the same physiological and behavioral effects of severe or chronic stress, including HPA axis activation (16), elevations in plasma corticosterone levels (17), increases in corticotropin-releasing factor expression (CRF) (17), and increases in anxiety-like behavior (9,18,19). Importantly, a single administration of PACAP produces a persistent (lasting at least one week) elevation in the acoustic startle response, a putative indicator of hypervigilence (9). PACAP has also been associated with fear responses in humans (20) and the development of affective disorders, including PTSD (21–23) and MDD (24). Thus PACAP is implicated in both the acute and long-lasting effects of stress.
Mood and anxiety disorders involve many domains, including those affecting motivational, cognitive, and social function. It was recently reported that PACAP produces acute anhedonia (reduced sensitivity to reward), and this effect is dependent on CRF systems (19). Considering that psychiatric illnesses are persistent, the present studies were designed to investigate the dose- and time-dependent effects of exogenous PACAP on motivation, social behavior, and attention as assessed by the intracranial self-stimulation (ICSS) test, social interaction (SI) test, and the 5-choice serial reaction time task (5CSRTT), respectively. Since addiction is often co-morbid with stress and anxiety disorders (25,26), we also evaluated whether PACAP exposure would affect sensitivity to the reward-related effects of cocaine. Finally, we evaluated whether CRF-receptor (CRF-R) or kappa-opioid receptor (KOR) antagonists attenuate PACAP effects on attention, focusing on this domain because our previous work suggests that it depends critically on CRF-KOR interactions (19,27).
METHODS
Animals
Male Sprague-Dawley rats (Charles River, Raleigh, NC) weighing 250–275 g at the start of experiments were pair-housed and maintained on a 12 hr light-dark cycle (lights on at 0700 h). Rats in the ICSS and SI experiments were given free access to food (Purina Rat Chow, Ralston Purina, St Louis, MO). Rats in the 5CSRTT experiments were food restricted to 85% of their free-feeding weight beginning 2 days prior to training. All rats had free access to water while in their home cages. Experiments were approved by the McLean Hospital Animal Care and Use Committee in accordance with National Institutes of Health guidelines.
Drugs
PACAP-38 (Bachem, Torrance, CA) was dissolved in artificial cerebral spinal fluid (aCSF; Harvard Apparatus, Hollister, MA). PACAP (0.25, 0.5, or 1.0 μg) or vehicle (VEH; aCSF) was infused into the lateral right ventricle with a Hamilton microsyringe (10 μl) attached to polyethylene (PE 20) tubing at a rate of 0.5 μl/min for 2 min. Because PACAP has long-lasting effects on acoustic startle (9), separate cohorts of rats were used for each dose. Antalarmin (ANT; Sigma; St. Louis, MO) was dissolved in 0.5% carboxymethylcellulose (CMC; pH 5.5; Sigma,) and injected intraperitoneally (IP) at 20 mg/kg, a dose that blocks the anxiogenic effects of CRF without producing toxicity (28). JDTic (RTI, Research Triangle, NC) was dissolved in 0.9% sterile saline (SAL) and injected at 10 mg/kg (IP), a dose that produces anxiolytic-like effects in rats (29). Cocaine HCl (Sigma) was dissolved in SAL and administered at 5.0 mg/kg (IP), a dose that produces moderate effects on ICSS (30).
Surgery
All rats tested with PACAP or VEH were anesthetized with pentobarbital (65 mg/kg, IP) and implanted with an intracerebroventricular (ICV) stainless steel guide cannula (23-ga, Plastics One, Roanoke, VA) with an obturator extending 1.5 mm beyond the cannula tip aimed at the right lateral ventricle as described previously (31) (from bregma: −0.8 mm anterior, +1.3 mm lateral, −3.6 mm ventral to dura). Rats in the ICSS experiment were simultaneously implanted with a unilateral monopolar electrode (0.25-mm diameter; Plastics One, Roanoke, VA) aimed at the right medial forebrain bundle as described (31) (from bregma: −2.8 mm anterior, −1.6 mm lateral, −7.8 mm ventral from dura). Rats were individually housed post-surgery and given 1 week of recovery.
Behavioral testing
ICSS was performed as described previously (32). ICSS thresholds were calculated using a least-squares line of best-fit analysis (33). After stable baseline thresholds were established (±10% for 3 consecutive days), rats received an infusion of VEH to ensure the infusion procedure did not affect performance. The following day, rats were infused with VEH or PACAP (0.25–1.0 μg). Testing (90 min) began immediately after infusions. Rats were tested for 7 days post-infusion to assess long-term effects of PACAP. Thresholds and maximum response rates were expressed as % mean baseline established on the 3 days that fulfilled stability criteria. We also examined if PACAP alters the reward-related effects of a cocaine challenge on day 8 following PACAP treatment. For this test, responding was first evaluated for 60 min for each rat, which served as the daily baseline. All rats then received cocaine (5.0 mg/kg, IP) and tested immediately for an additional 60 min. Data are expressed as % mean daily baselines.
Social behavior was measured using a modified version of the social interaction (SI) test (34). Rats were habituated for 10 min to the interaction arena (60 X 60 X 35 cm) one day before testing. On the test day, rats were infused with PACAP (0.25–1.0 μg) or VEH and placed in the interaction arena 60 min later with a naïve weight–matched partner rat. Partner rats were housed under identical conditions to, and had no previous contact with the treated rat. Social behavior was videotaped for 5 min in red light, and an observer blind to the treatment conditions quantified the following metrics: time spent interacting (active SI; e.g., sniffing, grooming, and play initiated or reciprocated by treated rat), time spent fleeing (social avoidance), time spent in the arena corners (anxiety-like behavior), and locomotor activity. Active SI was also quantified for the partner rat to assess whether its behavior was affected by the dose of PACAP administered to the treated rat. Rats were retested in the SI test with a novel (unfamiliar) partner rat 7 days later. A separate cohort of rats was tested only at the 1-week post-treatment time-point to control for repeated presentation effects.
The 5CSRTT was performed as described previously (35). Sessions ended after 90 trials or 30 min, whichever came first. The following performance measures, as defined previously (34), were analyzed on each day: % correct responses, % omissions, accuracy, premature responses, correct response latency, reward latency, and latency to complete the task. We also examined % correct responses post-error [=% correct/total trials following an incorrect response] and % correct responses post-correct [=% correct/total trials following a correct response], since these are affected in psychiatric illness (36). Rats were required to perform at criteria (>60% correct responses and <20% omissions, ±10% for 3 consecutive days) prior to ICV surgery and again before testing. Before the first test session, all rats received an infusion of VEH to examine the effects of the ICV infusion alone. The following day, rats received infusions of VEH or PACAP (0.25–1.0 μg) and were tested 60 min later, and on each day for 7 consecutive days.
Separate cohorts of rats were used to test whether pretreatment with the CRF-R1 antagonist antalarmin (ANT) or the KOR antagonist JDTic could attenuate PACAP effects. For ANT studies, the rats first received an IP injection of CMC (ANT vehicle) and were infused with VEH (aCSF) 10 mi later. On the next test day, they received ANT (20 mg/kg, IP) and were infused with VEH 10 min later. On the final test day they received ANT and were infused with PACAP (0.5 μg) 10 min later. For JDTic studies, the study and drug design was similar except JDTic pretreatment (10 mg/kg, IP) was given 24 hrs prior to VEH infusion to accommodate its long-lasting actions (>14 days) (29,37).
One- and two-way ANOVAs were used to examine group differences, with repeated measures when examining time effects. Significant effects were further examined with post-hoc Bonferroni tests. Post-error adjustments in the 5CSRTT experiment were analyzed with preplanned contrasts between VEH and PACAP treatment days based on a specific a priori hypothesis that PACAP would produce post-error adjustments similar to those seen in depressed humans (38).
RESULTS
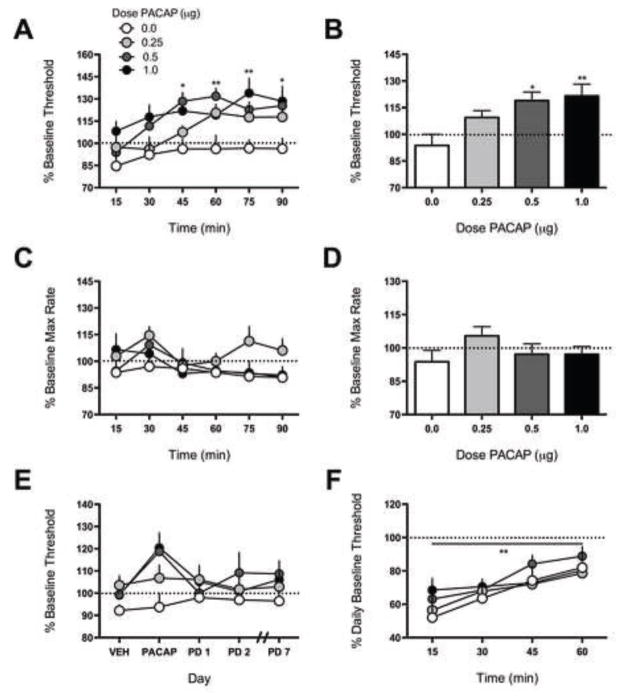
PACAP dose-dependently increased ICSS thresholds, indicating reduced sensitivity to the rewarding stimulation (anhedonia) (Fig. 1A). Effects of PACAP on ICSS thresholds depended on main effects of dose [F(3,20)=5.23, P<0.01] and time [F(5,100)=8.09, P<0.01)]. Administration of 0.5 μg PACAP elevated thresholds 45 (P<0.05), 60 (P<0.01), and 90 min (P<0.05) after treatment compared to VEH, whereas 1.0 μg PACAP elevated ICSS thresholds 75 (P<0.01) and 90 min (P<0.05) after treatment. Collapsed across time [F(3,20)=4.68, P<0.05] (Fig. 1B), PACAP elevated ICSS thresholds in rats treated with 0.5 μg (P<0.05) and 1.0 μg (P<0.01). PACAP did not affect maximum response rates (Fig. 1C), although there was a main effect of time [F(5,100)=3.862, P<0.01]. Collapsed across time, there were no significant differences among treatment groups (Fig. 1D). Rats were tested for an additional 7 days without any additional treatment; for clarity, only select days are shown (Fig. 1E). PACAP effects across this time period depended on main effects of dose [F(3,20)=3.53, P<0.05] and time [F(3,60)=4.023, P<0.05]. Administration of 0.5 and 1.0 μg PACAP increased thresholds on PACAP treatment day compared to VEH (P’s<0.05), but thresholds returned to pre-treatment baseline levels the following day. These data indicate that PACAP produces acute but transient anhedonia. Regardless of PACAP dose administered on the treatment day, all rats displayed equivalent sensitivity to the threshold-lowering effects of cocaine (Fig. 1F). There was a main effect of time [F(3,60)=12.25, P<0.01], but not of PACAP dose [F(3,20)=0.37, P=0.78].
Figure 1.
Effects of PACAP on ICSS. Rats received VEH (0 μg) or PACAP (0.25–1.0 μg, ICV) and were immediately tested in ICSS for 90 min; N=6–7/dose. (A) Time course of PACAP effects on ICSS thresholds. PACAP elevated ICSS thresholds approximately 45–60 min post-infusion. Data are expressed as mean % mean baseline (± SEM) from the average pre-treatment baseline threshold. *P<0.05, **P<0.01 for between-group comparison to VEH. (B) When data are expressed as mean percentage change from baseline threshold for the entire 90 min period, PACAP (0.5–1.0 μg) had significant threshold-elevating effects. *P<0.05 for between-group comparison to VEH. (C) Time course of PACAP effects on maximum (Max) rates. Response capabilities were unaltered across the 90 min test period. (D) There are no significant differences when data are expressed as mean percentage change from baseline max rates for the entire 90 min period. (E) Rats were tested for 7 days without any additional treatment (PD=post-treatment day). PACAP (0.5–1.0 μg) increased thresholds on the PACAP treatment day, but effects were not persistent. (F) Rats were given a drug challenge test on day 8 to assess sensitivity to cocaine (5 mg/kg, IP). All rats exhibited equivalent decreases in thresholds. **P<0.01 main effect of time.
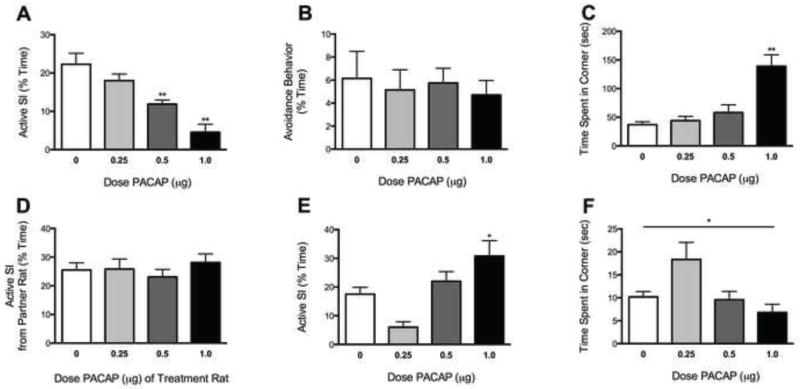
PACAP dose-dependently affected active SI behavior [F(3,25)=14.42, P<0.01] (Fig. 2A). Specifically, PACAP administration decreased active SI in rats treated with 0.5–1.0 μg (P’s<0.01) (Fig. 2A), but had no effect on social avoidance (e.g., fleeing from the partner rat) (Fig. 2B). PACAP also affected the amount of time spent in the arena corners [F(3,25)=13.06, P<0.01] (Fig. 2C); rats treated with 1.0 μg PACAP (P<0.01) spent significantly more time in arena corners than those treated with VEH. Horizontal locomotor activity was significantly decreased [F(3,25)=8.27, P<0.01] only in rats treated with 1.0 μg PACAP (P<0.01; data not shown); this likely reflects anxiety-like behavior since 1.0 μg PACAP did not affect maximum ICSS rates. Importantly, the PACAP dose administered to the treatment rat did not affect SI behaviors of the partner rat (Fig. 2D). PACAP treatment affected social behavior 1 week later [F(3,20)=8.93, P<0.01] (Fig. 2E). Interestingly, rats treated with 1.0 μg PACAP spent significantly more time engaging in active SI behaviors compared to rats treated with VEH (P<0.05). PACAP also affected the amount of time spent in the arena corners in the 1-week test (Fig. 2F). While there was a main effect of treatment [F(3,20)=4.292, P<0.05], driven largely by increased anxiety-like behavior in rats previously treated with 0.25 μg, there were no significant differences among the doses in post-hoc tests. Furthermore, there were no significant treatment differences in rats tested only at the 1-week time-point, although the data were more variable with nominal decreases in active SI at the low doses (not shown).
Figure 2.
Effects of PACAP on SI. Rats received VEH (0 μg) or PACAP (0.25–1.0 μg, ICV) and were tested for SI with a weight-matched untreated partner rat 1 hr later; N=6/dose. (A) PACAP (0.5–1.0 μg) decreased active SI. Data are expressed as % time during the 5-min test period. (B) Avoidance behavior was unaffected by PACAP. (C) PACAP (at the 1.0 μl dose) caused significant increases in time spent in the corners. (D) Effects were not due to differential behavior from the partner rats. (E) Rats that had received the 1.0 μg dose PACAP exhibited increased active SI behavior when re-tested with a new partner 7 days later, without any additional treatment. (F) PACAP treatment also affected anxiety-like behavior one week later, with nominal increases in anxiety like behavior in rats that had received the 0.25 μg dose PACAP. *P<0.05, **P<0.01, between-group comparison to VEH treatment.
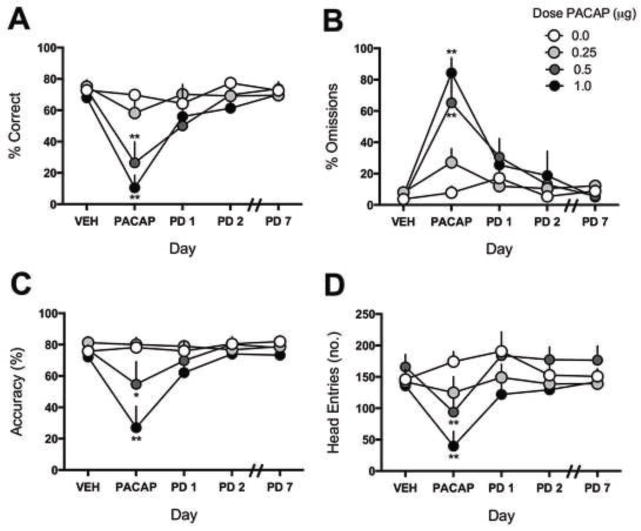
PACAP dose-dependently produced acute disruptions in performance in the 5CSRTT. Effects of PACAP depended on significant Dose x Day interactions for correct responding [F(12,76)=3.416, P<0.01], omission errors [F(12,76)=4.651, P<0.01], accuracy [F(12,76)=2.83, P<0.01], and head entries into the food magazine [F(12,76)=3.079, P<0.01] (Fig. 3A–D). Within-group comparisons to the VEH treatment day indicate that PACAP treatment reduced % correct responses at 0.5–1.0 μg (P’s<0.01) (Fig. 3A), increased % omissions at 0.5–1.0 μg (P’s<0.01) (Fig. 3B), reduced accuracy at 0.5 μg (P<0.05) and 1.0 μg (P<0.01) (Fig. 3C), and reduced the number of head entries at 0.5–1.0 μg (P’s<0.01) (Fig. 3D). Between-group comparisons to rats that received VEH indicate that PACAP reduced % correct responses at 0.5–1.0 μg (P’s<0.01) (Fig. 3A), increased % omissions at 0.5–1.0 μg (P’s<0.01) (Fig. 3B), reduced accuracy at 0.5 μg (P<0.05) and 1.0 μg (P<0.01) (Fig. 3C), and reduced the number of head entries at 0.5 μg (P<0.05) and 1.0 μg (P<0.01) (Fig. 3D). In other metrics examined (Table 1), there was a main effect of dose in latency to a correct response [F(3,19)=4.486, P<0.05], and main effects of day in the time to complete the task [F(4,76)=3.529, P<0.05], and in reward latency [F(4,76)=2.68, P<0.05]. The overall main of effect of day in the reward latency analysis was largely driven by decreases in reward latency in rats treated with 1.0 μg PACAP. PACAP did not significantly alter premature responses. PACAP effects were not persistent on any metric studied (Fig. 3A–D).
Figure 3.
Effects of PACAP on attention. Rats first received VEH to obtain baseline values. The following day, rats were infused with PACAP (0–1.0 μg, ICV) and tested 1 hr later. Rats were tested each day for 8 days without any additional treatment; N=5–7/dose. Rats treated with PACAP (0.5–1.0 μg) exhibited an acute (A) decrease in percentage correct, (B) increase in percentage omissions, (C) decrease in accuracy, and (D) decrease in the number of head entries into the food magazine, however these metrics return to VEH treatment-day levels by PD1. *P<0.05, **P<0.01, compared to VEH baseline of same treatment group.
Table 1.
Effects of PACAP on additional 5CSRTT metrics
| Metri c |
Correct Latency (sec)a |
Time to Complete (sec)b |
Reward Latency (sec)b |
Premature Responses (no.) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose PACA P (μg) |
0. 0 |
0.2 5 |
0. 0 |
0.2 5 |
0. 5 |
1. 0 |
0. 5 |
1. 0 |
0. 0 |
0.2 5 |
0. 5 |
1. 0 |
0. 0 |
0.2 5 |
0. 5 |
1. 0 |
| VEH | 0.77 ±0.04 | 0.92 ±0.04 | 12.50 ±2.9 | 8.8 ±1.6 | 12.83 ±4.3 | 14.67 ±14.8 | 0.83 ±0.08 | 0.84 ±0.08 | 1.56 ±0.08 | 2.07 ±0.53 | 1.68 ±0.16 | 1.52 ±0.11 | 12.50 ±2.9 | 8.8 ±1.6 | 12.83 ±4.3 | 14.67 ±14.8 |
| PACAP | 0.803 ±0.03 | 1.21 ±0.06 | 15.33 ±4.97 | 7.0 ±0.6 | 3.83 ±1.5 | 2.33 ±1.1 | 1.17 ±0.34 | 0.498 ±0.23 | 1.56 ±0.09 | 1.67 ±0.19 | 1.56 ±0.34 | 0.65 ±0.29 | 15.33 ±4.97 | 7.0 ±0.6 | 3.83 ±1.5 | 2.33 ±1.1 |
| PD1 | 0.99 ±0.15 | 1.13 ±0.11 | 23.5 ±10.1 | 11.0 ±3.3 | 8.17 ±1.9 | 6.5 ±1.5 | 1.16 ±0.14 | 0.83 ±0.2 | 1.65 ±0.1 | 1.84 ±0.21 | 1.79 ±0.16 | 1.51 ±0.37 | 23.5 ±10.1 | 11.0 ±3.3 | 8.17 ±1.9 | 6.5 ±1.5 |
| PD2 | 0.79 ±0.07 | 1.05 ±0.07 | 11.0±4.4 | 10.8 ±2.8 | 9.33 ±2.4 | 9.83 ±4.9 | 0.91 ±0.06 | 0.82 ±0.09 | 1.63 ±0.08 | 1.64 ±0.16 | 2.01 ±0.38 | 1.66 ±0.2 | 11.0 ±4.4 | 10.8 ±2.8 | 9.33 ±2.4 | 9.83 ±4.9 |
| PD7 | 0.95 ±0.08 | 0.89 ±0.08 | 7.33 ±3.1 | 11.6 ±3.3 | 11.5 ±3.8 | 11.0 ±2.7 | 0.79 ±0.03 | 0.91 ±0.11 | 1.60 ±0.08 | 1.67 ±0.22 | 1.57 ±0.12 | 1.58 ±0.09 | 7.33 ±3.1 | 11.6 ±3.3 | 11.5 ±3.8 | 11.0 ±2.7 |
| Statist ics (main effect s) | Dose: F(3,19)=4.486, P<0.05 Day: F(4,76)=1.03, P=0.399, n.s. |
Dose: F(3,19)=0.316, P=0.81, n.s. Day: F(4,76)=3.529, P<0.05 |
Dose: F(3,19)=1.339, P=0.29, n.s. Day: F(4,76)=2.68, P<0.05 |
Dose: F(3,19)=0.96, P=0.43, n.s. Day: F(4,76)=1.41, P=0.24, n.s. |
||||||||||||
Main effects of dose. No main effects of day or interactions.
Main effects of day (VEH, PACAP, PD 1, PD2, PD3). No main effects of dose or interactions. Values represent mean ± SEM. PD=post treatment day. n.s. = not significant.
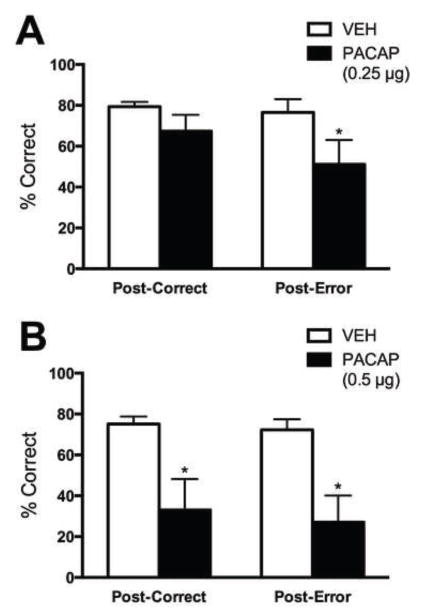
Humans normally adjust their response rates in decision-making tasks after an incorrect response, but this behavior is altered in depression (38). To determine if PACAP causes dysregulation of decision-making, we analyzed post-error and post-correct responses (as reflected by % correct) (Fig. 4). A pre-planned Bonferonni analysis revealed that 0.25 μg PACAP produced decreases in % correct responses following an error (post-error; P<0.05; main effect of condition [F(1,16)=5.55, P<0.05]) without affecting % correct responses following a correct response (post-correct) (Fig. 4A). A higher (0.5 μg) dose produced disruptions in both post-error and post-correct responses (P’s<0.05; main effect of condition [F(1,10)=16.14, P<0.01]) (Fig. 4B). These results are consistent with our hypothesis that PACAP causes disruptions in post-error adjustments that are similar to those seen in humans with depression.
Figure 4.
Effects of PACAP on error processing. (A) Rats treated with 0.5 μg PACAP exhibited significant decreases in correct responding following both an incorrect and a correct response. (B) Rats treated with 0.25 μg PACAP exhibited significant decreases in correct responding only following an incorrect response. *P<0.05 post-hoc Bonferroni tests compared to VEH.
Previous work has established interactions between CRF and KOR systems in regulating attention (39). The CRF antagonist ANT attenuated, but did not completely reverse, the effects of PACAP (0.5 μg) and did not have any effects on its own (Table 2). There were no significant differences in performance among rats treated with CMC plus VEH, ANT plus VEH, or ANT plus PACAP. Rats that received ANT pretreatment plus PACAP still displayed nominal deficits, but ANT pretreatment reduced the magnitude of impairment. Pretreatment with JDTic had no effect on PACAP-induced performance deficits in the 5CSRTT (Table 2), although it attenuated the effects of PACAP on post-correct and post-error processing.
Table 2.
Effects of KOR antagonist (JDTic) and CRF-R1 antagonist (Antalarmin) pretreatment on 5CSRTT metrics
| Metric | Corre ct (%) |
Omissi ons (%) |
Accura cy |
Head Entri es |
Rewa rd Laten cy |
Laten cy to Corre ct |
Time to Compl ete Task |
Premat ure Respon ses |
Post- Corre ct |
Post- Erro r |
|---|---|---|---|---|---|---|---|---|---|---|
| PACAP Alone (0.5 μg) | ||||||||||
| PACAP | 26.48 ±13.23 | 65.19 ±15.69 | 54.74 ±14.48 | 93.83 ±39.89 | 1.56 ±0.34 | 1.17 ±0.35 | 1249 ±60.4 | 3.83 ±1.49 | 33.07 ±15.13 | 27.03 ±13.09 |
| Antalarmin (ANT) Pretreatment | ||||||||||
| CMC +VEH | 77.59 ±2.30 | 4.81 ±1.1 | 81.62 ±2.81 | 160.5 ±8.99 | 1.89 ±0.41 | 0.81 ±0.06 | 1042 ±37.53 | 11.0 ±3.49 | 75.41 ±3.32 | 72.71 ±5.56 |
| ANT+VEH | 73.89 ±1.9 | 4.07 ±1.02 | 77.03 ±1.81 | 152.0 ±13.97 | 1.48 ±0.11 | 081 ±0.06 | 1055 ±31.85 | 15.0 ±3.69 | 72.67 ±3.51 | 75.12 ±5.21 |
| ANT+PACAP | 51.67 ±14.31 | 34.07 ±17.04 | 62.43 ±13.29 | 118.3 ±25.47 | 1.89 ±0.28 | 1.25 ±0.20 | 1154 ±72.69 | 7.33 ±2.77 | 56.85 ±13.92 | 45.09 ±15.4 |
| Statistics | F(2,10) = 2.75, P=0.11 n.s. | F(2,10)=3.13, P=0.09 n.s. | F(2,10)= 158, P=0.25 n.s. | F(2,10) = 1.77, P=0.22 n.s. | F(2,10) = 1.88, P=0.21 n.s. | F(2,10) = 3.39, P=0.08 n.s. | F(2,10)= 1.22, P=0.34 n.s. | F(2,10)= 1.07, P=0.38 n.s. | F(2,20)=0.28 P=0.76, n.s. |
|
| JDTic Pretreatment | ||||||||||
| SAL+VEH | 72.78±4.12 | 5.74 ±1.14 | 77.50 ±4.12 | 145.8 ±13.91 | 1.56 ±0.15 | 0.87 ±0.087 | 1032 ±14.21 | 10.33 ±2.39 | 66.55 ±4.5 | 76.95 ±8.45 |
| JDTic+VEH | 70.56 ±3.86 | 6.48 ±2.14 | 77.07 ±2.84 | 157.0 ±17.35 | 1.47 ±0.104 | 0.94 ±0.08 | 1034 ±17.98 | 10.17 ±2.14 | 66.23 ±5.93 | 70.77 ±2.99 |
| JDTic+PACAP | 30.09 ±12.35 ** | 55.0 ±18.06* | 53.34 ±13.64 | 61.17 ±23.84 ** | 1.54 ±0.156 | 0.93 ±0.22 | 1221 ±65.0* | 7.33 ±2.65 | 53.63 ±11.69 | 43.55 ±17.23 |
| Statistics | F(2,10) = 10.93, P<0.01 | F(2,10)= 8.31, P<0.01 | F(2,10)= 0.11, P=0.151 n.s. | F(2,10) = 13.25, P<0.01 | F(2,10) = 0.88, P=0.45 n.s. | F(2,10) = 0.14, P=0.87 n.s. | F(2,10)= 8.0, P<0.01 | F(2,10)= 1.16, P=0.35 n.s. | F(2,20)=0.53 P=0.59, n.s. |
|
P<0.05,
P<0.01 compared to JDTic+VEH group. Pretreatment groups represented as IP pretreatment + ICV treatment. VEH= Vehicle (aCSF), SAL=saline vehicle, CMC= carboxymethylcellulose vehicle. Values represent mean ± SEM, n.s. = not significant.
DISCUSSION
PACAP disrupts motivation, social interaction, and attention, thereby affecting multiple domains disrupted in mood and anxiety disorders. Specifically, we found that PACAP produces anhedonia (as reflected by increased reward thresholds) in the ICSS test, decreases social behaviors and increases anxiety-like behaviors in the SI test, and disrupts performance in the 5CSRTT. Whereas PACAP-induced increases in startle are reportedly persistent (9), we demonstrate that PACAP effects on motivation and attention recover by the following day. In contrast, PACAP dysregulation of SI behaviors was long-lasting, suggesting that the neural substrates that regulate this behavior are at least partially independent of those regulating motivation and attention. These findings are broadly consistent with previous work demonstrating the role of PACAP in stress-related behaviors, and extend our understanding of how this peptide influences aspects of complex cognitive behaviors.
We used ICSS to assess whether PACAP produces anhedonia, a core feature of mood and anxiety disorders including MDD and PTSD (4). PACAP treatment dose-dependently increased ICSS thresholds 45–60 min post-infusion, indicating a reduction in reward sensitivity. Threshold elevations are produced by other manipulations that cause depressive-like behaviors including chronic social defeat stress (40,41), drug withdrawal (42), exogenous CRF (43) or KOR agonists (32); these elevations are thought to reflect decreased motivation and/or anhedonia (33). Our findings corroborate a recent report (19) that PACAP produces similar effects in the ICSS test, although at a dose (5.0 μg) that we find produces non-specific disruptions in behavior (e.g., gross motor impairments that produce paradoxical decreases in the acoustic startle test and non-specific freezing in the homecage) (unpublished observations). Here, we demonstrate that PACAP induces anhedonia at much lower doses (0.5–1.0 μg), without affecting motor performance (maximum response rates). We also show that ICSS thresholds had normalized within 24 hr of treatment, which is important considering the persistence and intractability of psychiatric illnesses such as MDD and PTSD. We also demonstrate that prior treatment with PACAP does not alter sensitivity to the reward-related effects of cocaine when rats were tested 1 week after treatment, although PACAP may alter cocaine sensitivity or reinstatement of drug seeking under other conditions (44).
Social withdrawal (diminished interest or participation in social activities) is another core feature of mood and anxiety disorders (4). We show that rats treated with 0.5 μg or 1.0 μg PACAP exhibit decreases in active SI (approach, reciprocal behaviors), which is consistent with other studies showing that exposure to an aversive stimulus (predatory order, CRF infusion, fear conditioning) disrupts SI behaviors in rats (45,46). Our results are broadly consistent with a report showing PAC1 receptor deficient mice exhibit increases in affiliative behaviors, implicating endogenous PACAP systems in regulating social behavior (47). Importantly, the SI behaviors of the untreated partner rats were not correlated with the PACAP dose administered to the treatment rats. PACAP had no effect on avoidance (fleeing) behavior, suggesting that the decreases in SI are driven by increases in impassive behaviors. Rats treated with the high dose of PACAP (1.0 μg) also exhibited increases in anxiety-like behaviors in the SI test, as reflected by increased time spent in corners. This is broadly consistent with the anxiogenic effects of PACAP reported in other tests (6,7,19). Notably, SI deficits were evident at a dose (0.5 μg) that did not increase overt anxiety-like behavior, suggesting that these behaviors represent distinct domains regulated by separate, yet overlapping, neural circuits (40). Rats that received PACAP continued to exhibit dysregulated social behavior when tested 1 week later with a new partner. Surprisingly, the dose-response pattern observed at the acute test was reversed at the 1 week test; rats that had received the highest dose of PACAP (1.0 μg) showed increases in direct SI, whereas rats that received the lowest dose of PACAP (0.25 μg) showed nominal decreases in SI behaviors and increases in anxiogenic behaviors. The mechanism of this effect is unclear, although biphasic effects of PACAP on fear expression have been described in a preliminary report (48). In that study, PACAP treatment prior to fear conditioning produced initial reductions in fear expression followed by progressive increases in freezing across test days. While the pattern of effects on fear is opposite to those observed in the SI test (i.e., long-term increased anxiety versus decreased anxiety), these data demonstrate that biphasic responses may be a consequence of PACAP-induced neuroadaptations. The observation that PACAP-treated rats tested only at the 1-week time-point did not exhibit significant differences in active SI or anxiety-related behavior suggests that an initial SI exposure is necessary for the development of the long-term pattern observed. However, it remains unclear why the pattern of these effects is not uniform across behaviors and why PACAP produces persistent effects in some tests (acoustic startle, SI, fear conditioning) but not others (ICSS, 5CSRTT).
We also demonstrate that PACAP dose-dependently disrupts performance in the 5CSRTT, suggestive of attentional deficits, another core feature of mood and anxiety disorders. PACAP (0.5–1.0 μg) decreased the percentage of correct responses, increased the percentage of trials in which the rats failed to respond (omission errors), and decreased accuracy (commission errors). This pattern of effects is broadly consistent with gross impairments in attention (49). PACAP has been shown to affect feeding and weight (19,50–53) and locomotor activity (13), raising the possibility that effects on 5CSRTT performance reflect a reduced motivation for the sugar pellets or a motor impairment. However, it was reported recently that ICV administration of 1.0 μg PACAP does not reduce food intake or weight (53), suggesting that the peptide does not alter motivation for food within the dose range used for the current studies. Moreover, PACAP did not increase the latency to collect food reward, a metric that reflects both motor performance and motivation to obtain food reinforcement (34,49,54); if anything, latency was reduced at the highest dose. Further, PACAP did not affect maximum rates of responding in the ICSS test, suggesting minimal effects on motor capabilities at the doses tested here.
While 0.5 μg PACAP produced deficits in correct responding following both correct and incorrect responses, we found that 0.25 μg PACAP reduced accuracy only after incorrect responses. Interestingly, there were no statistically significant impairments in any of the traditional metrics at this dose, suggesting that the error-processing analysis is exceptionally sensitive to depressive-like effects. The deficit in post-error behavior adjustments mimics deficits observed in depressed humans and in rats treated with CRF (36), and thus may be broadly useful for translational studies.
PACAP-immunoreactive fibers innervate CRF-expressing neurons in the PVN and BNST (55,56), and CRF receptor antagonism blocks the anxiogenic and anhedonic effects of PACAP (19). Additionally, PACAP-deficient mice have reduced hypothalamic CRF mRNA activation in response to emotional stressors (11). We investigated whether the CRF-R1 antagonist antalarmin could mitigate the attention deficits induced by PACAP, using a PACAP dose (0.5 μg) that produced intermediate performance deficits in 5CSRTT. Antalarmin attenuated the effects of PACAP, reaching an intermediate level of performance that was lower than—but not statistically different from—performance after VEH. While this is consistent with the hypothesized interactions between PACAP and CRF systems, others have reported that CRF antagonism completely reverses the anhedonic effects of a much higher dose of PACAP (5.0 μg) in the ICSS test (19). This discrepancy may reflect that PACAP affects motivational and cognitive behaviors through separate neural mechanisms. It is also possible that PACAP effects are dependent on both CRF-R1 and CRF-R2 activation. We used a CRF-1R specific antagonist because CRF-1 receptors have been found to mediate the anxiety-like effects of CRF (57). Accumulating evidence suggests that stress-related effects of CRF are also mediated by KORs (58), and that JDTic attenuates CRF effects in the 5CSRTT (39). Here we show that JDTic does not block the effects of PACAP on attention, but may partially block the effects of PACAP on post-error adjustments in the 5CSRTT, a potentially more sensitive measure of depressive-like behavior. These data suggest that stress-related PACAP and CRF circuitries may overlap more completely in the regulation of specific domains (e.g., anhedonia). A more detailed understanding of this interaction awaits the development of small molecule PACAP antagonists that can be given systemically.
Our results show that ICV PACAP administration has profound effects on cognitive behaviors that represent domains often dysregulated in mood and anxiety disorders. It is currently not known which brain regions mediate the specific drug effects observed; indeed, characterization of PACAP effects on these individual behaviors provides an important first step in the process of identifying the brain circuits involved. In addition to PACAP’s well-characterized actions in the BNST, the behavioral effects observed in the present studies provide rationale for studying PACAP effects in regions broadly implicated in motivation, emotion, and cognition (e.g., ventral tegmental area, nucleus accumbens, prefrontal cortex, amygdala). While more work is needed to determine whether the effects of PACAP described here are attributable to different sites or mechanisms, our studies enable a more comprehensive understanding of the degree to which PACAP contributes to core signs of mood disorders. A better understanding of the impact and persistence of PACAP effects, and its ability to regulate CRF systems (59), may facilitate the development of improved treatments for stress-related illness.
Acknowledgments
This research was funded by a National Institutes of Health grant (MH097860 to WC). RJD received support from the Sackler Scholar Programme in Psychobiology.
Footnotes
FINANCIAL DISCLOSURES
Dr. Carlezon discloses that he is inventor on a patent that claims the use of kappa-opioid receptor antagonists for the treatment of depression (Assignee: McLean Hospital) and over the past two years has received compensation for editorial duties from the American College of Neuropsychopharmacology. Dr. Carroll is an inventor of US patents claiming the composition of JDTic owned by the Research Triangle Institute. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keane TM, Marshall AD, Taft CT. Posttraumatic Stress Disorder: Etiology, Epidemiology, and Treatment Outcome. Annu Rev Clin Psychol. 2006;2:161–197. doi: 10.1146/annurev.clinpsy.2.022305.095305. [DOI] [PubMed] [Google Scholar]
- 2.Kessler R, Ruscio A, Shear K, Wittchen HU. Epidemiology of Anxiety Disorders. In: Stein MB, Steckler T, editors. Behavioral Neurobiology of Anxiety and its Treatment. Current Topics in Behavioral Neurosciences. Vol. 2. Springer; Berlin Heidelberg: 2010. pp. 21–35. [PubMed] [Google Scholar]
- 3.Saveanu RV, Nemeroff CB. Etiology of Depression: Genetic and Environmental Factors. Depression. 2012;35:51–71. doi: 10.1016/j.psc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 2013. [Google Scholar]
- 5.Sachinvala N, von Scotti H, McGuire M, Fairbanks L, Bakst K, McGuire M, Brown N. Memory, Attention, Function, and Mood among Patients with Chronic Posttraumatic Stress Disorder. J Nerv Ment Dis. 2000;188:818–823. doi: 10.1097/00005053-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Hammack SE, May V. Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.12.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto H, Shintani N, Tanida M, Hayata A, Hashimoto R, Baba A. PACAP is Implicated in the Stress Axes. Curr Pharm Des. 2011;17:985–989. doi: 10.2174/138161211795589382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K, et al. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J Comp Neurol. 1996;371:567–577. doi: 10.1002/(SICI)1096-9861(19960805)371:4<567::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, et al. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST) roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lezak KR, Roelke E, Harris OM, Choi I, Edwards S, Gick N, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) in the bed nucleus of the stria terminalis (BNST) increases corticosterone in male and female rats. Psychoneuroendocrinology. 2014;45:11–20. doi: 10.1016/j.psyneuen.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsukiyama N, Saida Y, Kakuda M, Shintani N, Hayata A, Morita Y, et al. PACAP centrally mediates emotional stress-induced corticosterone responses in mice. Stress Amst Neth. 2011;14:368–375. doi: 10.3109/10253890.2010.544345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto H, Shintani N, Tanaka K, Mori W, Hirose M, Matsuda T, et al. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP) Proc Natl Acad Sci USA. 2001;98:13355–13360. doi: 10.1073/pnas.231094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schütz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res. 2001;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- 14.Gaszner B, Kormos V, Kozicz T, Hashimoto H, Reglodi D, Helyes Z. The behavioral phenotype of pituitary adenylate-cyclase activating polypeptide-deficient mice in anxiety and depression tests is accompanied by blunted c-Fos expression in the bed nucleus of the stria terminalis, central projecting Edinger-Westphal nucleus, ventral lateral septum, and dorsal raphe nucleus. Neuroscience. 2012;202:283–299. doi: 10.1016/j.neuroscience.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann ML, Mustafa T, Eiden AM, Herkenham M, Eiden LE. PACAP-deficient mice show attenuated corticosterone secretion and fail to develop depressive behavior during chronic social defeat stress. Psychoneuroendocrinology. 2013;38:702–715. doi: 10.1016/j.psyneuen.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nussdorfer GG, Malendowicz LK. Role of VIP, PACAP, and related peptides in the regulation of the hypothalamo-pituitary-adrenal axis. Peptides. 1998;19:1443–1467. doi: 10.1016/s0196-9781(98)00102-8. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res. 2005;138:45–57. doi: 10.1016/j.molbrainres.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legradi G, Das M, Giunta B, Hirani K, Mitchell EA, Diamond DM. Microinfusion of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural Plast. 2007;2007:79102. doi: 10.1155/2007/79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dore R, Iemolo A, Smith KL, Wang X, Cottone P, Sabino V. CRF mediates the anxiogenic and anti-rewarding, but not the anorectic effects of PACAP. Neuropsychopharmacology. 2013;38:2160–2169. doi: 10.1038/npp.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD, et al. PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proc Natl Acad Sci USA. 2014;111:3158–3163. doi: 10.1073/pnas.1318954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Cao C, Wang R, Qing Y, Zhang J, Zhang XY. PAC1 receptor (ADCYAP1R1) genotype is associated with PTSD’s emotional numbing symptoms in Chinese earthquake survivors. J Affect Disord. 2013;150:156–159. doi: 10.1016/j.jad.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Uddin M, Chang S-C, Zhang C, Ressler K, Mercer KB, Galea S, et al. Adcyap1r1 genotype, posttraumatic stress disorder, and depression among women exposed to childhood maltreatment. Depress Anxiety. 2013;30:251–258. doi: 10.1002/da.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto R, Hashimoto H, Shintani N, Ohi K, Hori H, Saitoh O, et al. Possible association between the pituitary adenylate cyclase-activating polypeptide (PACAP) gene and major depressive disorder. Neurosci Lett. 2010;468:300–302. doi: 10.1016/j.neulet.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse: Results from the epidemiologic catchment area (eca) study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 26.Brown PJ, Wolfe J. Substance abuse and post-traumatic stress disorder comorbidity. Drug Alcohol Depend. 1994;35:51–59. doi: 10.1016/0376-8716(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 27.Van’t Veer A, Carlezon WA. Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl) 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]
- 29.Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA. Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- 30.Mague SD, Andersen SL, Carlezon WA. Early developmental exposure to methylphenidate reduces cocaine-induced potentiation of brain stimulation reward in rats. Biol Psychiatry. 2005;57:120–125. doi: 10.1016/j.biopsych.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 31.Meloni EG, Reedy CL, Cohen BM, Carlezon WA. Activation of raphe efferents to the medial prefrontal cortex by corticotropin-releasing factor: correlation with anxiety-like behavior. Biol Psychiatry. 2008;63:832–839. doi: 10.1016/j.biopsych.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA. Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- 33.Carlezon WA, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 34.Gehlert DR, Shekhar A, Morin SM, Hipskind PA, Zink C, Gackenheimer SL, et al. Stress and central Urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur J Pharmacol. 2005;509:145–153. doi: 10.1016/j.ejphar.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 35.Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA. Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biol Psychiatry. 2007;62:687–693. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Beard C, Donahue RJ, Dillon DG, Van’t Veer A, Webber C, Lee J, et al. Abnormal error processing in depressive states: a translational examination in humans and rats. Transl Psychiatry. 2015;5:e564. doi: 10.1038/tp.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carroll I, Thomas JB, Dykstra LA, Granger AL, Allen RM, Howard JL, et al. Pharmacological properties of JDTic: a novel kappa-opioid receptor antagonist. Eur J Pharmacol. 2004;501:111–119. doi: 10.1016/j.ejphar.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 38.Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry. 2008;65:179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van’t Veer A, Yano JM, Carroll FI, Cohen BM, Carlezon WA. Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the μ-opioid receptor antagonist JDTic. Neuropsychopharmacology. 2012;37:2809–2816. doi: 10.1038/npp.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA., Jr Effects of striatal ΔFosB overexpression and ketamine on social defeat stress–induced anhedonia in mice. Biol Psychiatry. 2014;76:550–558. doi: 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry. 2014;76:542–549. doi: 10.1016/j.biopsych.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- 43.Macey DJ, Koob GF, Markou A. CRF and urocortin decreased brain stimulation reward in the rat: reversal by a CRF receptor antagonist. Brain Res. 2000;866:82–91. doi: 10.1016/s0006-8993(00)02229-0. [DOI] [PubMed] [Google Scholar]
- 44.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adamec RE, Blundell J, Burton P. Relationship of the predatory attack experience to neural plasticity, pCREB expression and neuroendocrine response. Neurosci Biobehav Rev. 2006;30:356–375. doi: 10.1016/j.neubiorev.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Dunn AJ, File SE. Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Horm Behav. 1987;21:193–202. doi: 10.1016/0018-506x(87)90044-4. [DOI] [PubMed] [Google Scholar]
- 47.Nicot A, Otto T, Brabet P, Dicicco-Bloom EM. Altered social behavior in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. J Neurosci. 2004;24:8786–8795. doi: 10.1523/JNEUROSCI.1910-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meloni EG, Venkatarman A, Donahue RJ, Carlezon WA., Jr . Neuroscience 2014 Abstracts. Washington, DC: Society for Neuroscience; 2014. PACAP promotes a PTSD-like phenotype in fear conditioned rats. Program No. 350.02. [Google Scholar]
- 49.Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 50.Matsuda K, Maruyama K, Nakamachi T, Miura T, Uchiyama M, Shioda S. Inhibitory effects of pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) on food intake in the goldfish, Carassius auratus. Peptides. 2005;26:1611–1616. doi: 10.1016/j.peptides.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 51.Morley JE, Horowitz M, Morley PM, Flood JF. Pituitary adenylate cyclase activating polypeptide (PACAP) reduces food intake in mice. Peptides. 1992;13:1133–1135. doi: 10.1016/0196-9781(92)90019-y. [DOI] [PubMed] [Google Scholar]
- 52.Mizuno Y, Kondo K, Terashima Y, Arima H, Murase T, Oiso Y. Anorectic effect of pituitary adenylate cyclase activating polypeptide (PACAP) in rats: lack of evidence for involvement of hypothalamic neuropeptide gene expression. J Neuroendocrinol. 1998;10:611–616. doi: 10.1046/j.1365-2826.1998.00244.x. [DOI] [PubMed] [Google Scholar]
- 53.Kocho-Schellenberg M, Lezak KR, Harris OM, Roelke E, Gick N, Choi I, et al. PACAP in the BNST produces anorexia and weight loss in male and female rats. Neuropsychopharmacology. 2014;39:1614–1623. doi: 10.1038/npp.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nemeth CL, Paine TA, Rittiner JE, Béguin C, Carroll FI, Roth BL, et al. Role of kappa-opioid receptors in the effects of salvinorin A and ketamine on attention in rats. Psychopharmacology (Berl) 2010;210:263–274. doi: 10.1007/s00213-010-1834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hannibal J, Mikkelsen JD, Fahrenkrug J, Larsen PJ. Pituitary adenylate cyclase-activating peptide gene expression in corticotropin-releasing factor-containing parvicellular neurons of the rat hypothalamic paraventricular nucleus is induced by colchicine, but not by adrenalectomy, acute osmotic, ether, or restraint stress. Endocrinology. 1995;136:4116–4124. doi: 10.1210/endo.136.9.7649120. [DOI] [PubMed] [Google Scholar]
- 56.Légrádi G, Hannibal J, Lechan RM. Pituitary adenylate cyclase-activating polypeptide-nerve terminals densely innervate corticotropin-releasing hormone-neurons in the hypothalamic paraventricular nucleus of the rat. Neurosci Lett. 1998;246:145–148. doi: 10.1016/s0304-3940(98)00255-9. [DOI] [PubMed] [Google Scholar]
- 57.Sahuque LL, Kullberg EF, Mcgeehan AJ, Kinder JR, Hicks MP, Blanton MG, et al. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology (Berl) 2006;186:122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knoll AT, Carlezon WA. Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grillon C, Hale E, Lieberman L, Davis A, Pine DS, Ernst M. The CRH1 Antagonist GSK561679 Increases Human Fear But Not Anxiety as Assessed by Startle. Neuropsychopharmacology. 2015;40:1064–1071. doi: 10.1038/npp.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]