Abstract
Glioblastoma (GBM, Grade IV astrocytoma) is the most common and most aggressive of the primary malignant brain tumors in adults. Hypoxia is a distinct feature in GBM and plays a significant role in tumor progression, resistance to treatment and poor outcomes. This review considers the effects of hypoxia on astrocytic tumors and the mechanisms that contribute to tumor progression and therapeutic resistance, with a focus on the vascular changes, chemotaxic signaling pathways and metabolic alterations involved.
I. Astrocytoma
Primary CNS tumors account for approximately 1.4% of all cancers and 2.4% of all cancer-related deaths (Howlader, Noone et al. 2014). In 2015, an estimated 23,180 new cases of primary malignant brain tumors will be diagnosed and 16,570 patients will die from these tumors (Ostrom, Gittleman et al. 2014). While essentially any neuroepethelial cell in the brain can develop into a malignancy, astrocytes are the most common cell of origin for malignant brain neoplasms accounting for approximately two thirds of malignant CNS tumors. Primary brain tumors that develop from astrocytes are called astrocytomas. The World Health Organization (WHO) classifies astrocytomas into four distinct grades (I–IV) on the basis of their microscopic appearance. Collectively, the grade III and IV gliomas are referred to as high grade gliomas, with grade IV referred to as glioblastoma. With the exception of the rare grade 1 (pilocytic) astrocytoma, these tumors are considered incurable and progress in grade and aggressiveness with time from diagnosis. In fact, nearly 90% of astrocytoma present de novo as a glioblastoma. Once transformation to, or diagnosis of glioblastoma occurs, survival is quite poor, and with best available care the median survival is approximately 16 months with a 5 year survival rate of 3.3% (Ostrom, Gittleman et al. 2014).
The poor survival is attributable partly to the nature of the tumor. The infiltrative nature of GBM results in difficulty eliminating microscopic disease despite macroscopic gross-total resection, with 90% of patients having recurrence at the original tumor location (Hou, Veeravagu et al. 2006). The location of the tumor also makes drug delivery difficult with only small or lipophilic molecules able to cross the blood brain barrier to reach the tumor. Of those agents that are able to reach the tumor, GBMs have shown to be resistant to most cytotoxic agents and to quickly develop resistance when initially sensitive. Hypoxia plays a significant role in both resistance to treatment and poor outcomes. The impact of hypoxia and potential means to target hypoxia for improved outcomes in astrocytoma is the focus of this review.
The most significant advance in treatment for GBM over the last decade has come from concomitant chemoradiotherapy with temozolomide. While radiation alone is able to produce a median survival of 12.1 months, the addition of temozolomide increases median survival to 14.6 months (Stupp, Mason et al. 2005). Just as significantly, approximately 1 in 5 patients are alive in the temozolomide treated population whereas essentially no patients survive to 3 years in the absence of temozolomide. This survival benefit is predominantly driven by epigenetic modification of the methyl guanine methyl transferase (MGMT) promoter, which results in inactivation and inability to repair guanine methylation induced by temozolomide (Hegi, Diserens et al. 2005). Unfortunately, MGMT silencing only is present in 40% of GBM, suggesting that improvement in treatment by the addition of temozolomide is not realized in the approximately 60% of patients that maintain MGMT expression. No alternative is available in the newly diagnosed setting, and these patients are treated with temozolomide none the less. When combined modality therapy fails, as is universally the case, antiangiogenic treatment with the monoclonal antibody bevacizumab (Avastin) is the mainstay of salvage therapy. The median progression free survival for bevacizumab is approximately 5 months with a median overall survival of 9 months (Friedman, Prados et al. 2009). Combination therapy with either irinotecan or lomustine is often considered for patients with good performance status (Taal, Oosterkamp et al. 2014). Once patients fail salvage therapy, clinical trials are often recommended with no agents proven to impact survival.
II. Innate tumor hypoxia during tumor development
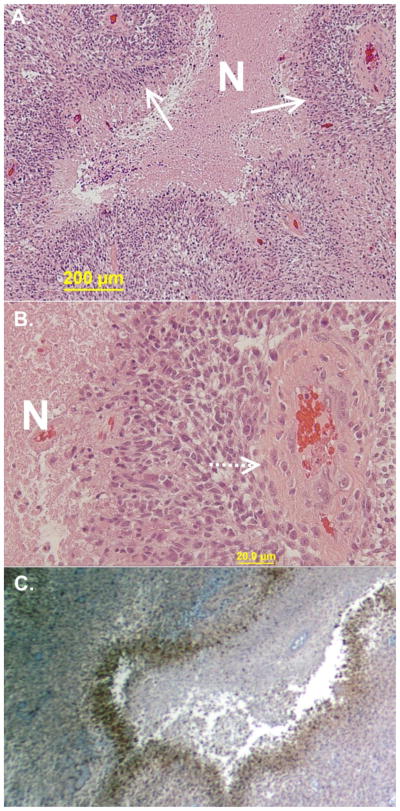
Hypoxia is a hallmark of GBM, and this is manifest in as the pathognomonic feature of pseudopalisading necrosis (Figure 1a) and vascular proliferation (Figure 1b). Pseudopalisading necrosis is the appearance of areas of hypercellularity surrounding areas of necrosis. These areas of hypercellularity have been well characterized and are not the result of increased proliferation. Rather, these areas are intensely hypoxic (Fig 1c) and have been suggested to represent two-dimensional histologic representations of tumor cells migrating away from a vaso-occlusive event with distorted, degenerating, or thrombosed blood vessels within the center (Brat, Castellano-Sanchez et al. 2004). These hypoxic foci have high levels of hypoxia induced factor 1 (HIF1) expression, resulting in proangiogenic vascular endothelial growth factor (VEGF) secretion, in turn driving vascular proliferation. However, the formed vessels in response to VEGF are severely malformed, a consequence of perturbation of the normal exquisite counterbalance of antiangiogenic growth factors (Jain 2013). The result is tortuous and chaotic vessel structure with gaps between endothelial cells and absence of pericytes. Due to malformation and inherent leakiness the interstitial pressure is increased, resulting in vascular stasis with corresponding exacerbation of hypoxia and increased microvascular thrombosis (Jain 2013). A viscious cycle of vascularization, vascular collapse, and tumor cell migration is repeated which ultimately drives the rapid expansion of cells outward from the tumor margin into the adjacent normal tissue.
Figure 1.
Representative images of glioblastoma with pathognomonic features and associated hypoxia. A. Highly cellular neoplasm with areas of necrosis (N) with densely packed cells at the margins appearing to almost line up (pseudopalisading). B. Abundant vessels are seen in the tumor with many surrounded by palisades and then necrosis. C. Areas of pseudopalisades are the most hypoxic when stained for the presence of carbonic anhydrase on immunohistochemistry.
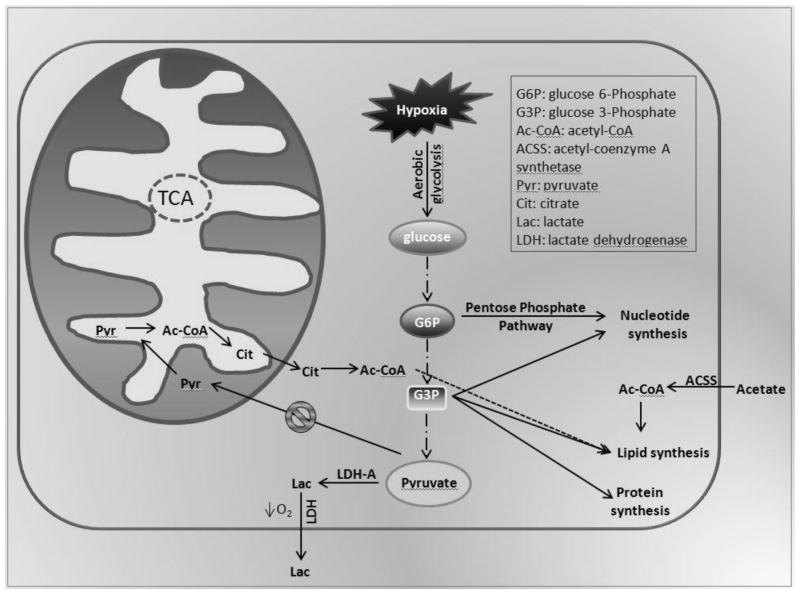
As such, intratumoral necrosis and hypoxia are both poor prognostic markers that portend progression and worse survival in patients with GBM (Burger and Green 1987, Rong, Durden et al. 2006). In fact, a developing strategy for the treatment of GBM involves measuring the volume and intensity of intratumoral hypoxia, as determined by fluoromisonidazole positron emission tomography, to quantify and appropriately target cells within hypoxic regions to improve outcome (Spence, Muzi et al. 2008). Although the underlying pathophysiologic alterations accompanying hypoxia can be understood on a biochemical basis, the impact of cellular adaptation to low levels of oxygen on tumor biology are not fully understood. The long-term sequelae of pseudopalisading necrosis may impart resistance to cytotoxic agents through a variety of mechanisms discussed herein. Because the tumor microenvironment is exceedingly heterogeneous, containing regions of variable oxygenation (Mason, Hunjan et al. 1998), stem cells residing in hypoxic pseudopalisading zones are thought to be partially buffered from the effects of chemoradiation due to vascular stasis and depletion of molecular oxygen. This topic will be further explored in section III. Additional factors that contribute to cancer stem cell drug resistance may include the metabolic reprogramming events associated with GBM. Aerobic glycolysis, also known as the Warburg effect, involves the conversion of glucose to lactate in the presence of molecular oxygen, and is commonly used by normal and malignant cells during periods of sustained rapid proliferation in order to facilitate the synthesis of nutrients into cellular biomass (Hitosugi, Kang et al. 2009, Vander Heiden, Lunt et al. 2011). Despite the inherent inefficiency of glycolysis in generating ATP independent of mitochondrial oxidative phosphorylation (OXPHOS), several groups posit that the intermediates of glycolysis, particularly glyceraldehyde 3-phosphate, are quickly diverted to anabolic pathways as substrate for biosynthesis of the lipids, DNA and protein requisite for rapid cellular replication. One consequence of this altered glycolytic pathway is that pyruvate is rapidly reduced to lactate and excreted as cellular waste, resulting in less pyruvate dehydrogenase activity and diminished production of acetyl-CoA. Acetyl-CoA is a key metabolic “hub” intermediate where carbohydrate, protein and lipid metabolism pathways converge. Even under conditions of decreased OXPHOS, acetyl-CoA plays a central role in critical metabolic pathways that balance carbohydrate and fat metabolism and is used to biosynthesize nucleotides, amino acids, fatty acids and cholesterol. In nonmalignant cells undergoing aerobic respiration, the majority of acetyl-CoA enters TCA and serves as the carbon source for production of the reducing potential and protons required for ATP synthase activity. Conversely, malignant cells predominantly forego TCA in favor of aerobic glycolysis, resulting in less glucose-derived acetyl-CoA. To compensate, a variety of cancer cell types are known to sequester and metabolize exogenous acetate as an alternative source of generating acetyl-CoA. Acetate is a byproduct of B-oxidation or ethanol metabolism but can be absorbed and conjugated to coenzyme A using one of three mammalian isoforms of short-chain acyl-CoA synthetases (ACSS).
In addition to its importance in energy metabolism, acetyl-CoA also contributes to epigenetic regulation of gene expression and posttranslational protein modification through the reversible addition of acetyl groups onto histones or enzymes (Kaelin and McKnight 2013). Recent studies demonstrate that human glioma stem-like cells exhibit global hypomethylation and that supplementation with Triacetin, an FDA approved food additive, reduced proliferation in a panel of 6 human primary GBM-derived glioma stem-like cell lines (Long, Tighe et al. 2015). Parallel studies also show that human GBM cells grown in the brain of mice are capable of capturing and metabolizing exogenous acetate to maintain adequate pools of acetyl-CoA, with as much as 50% being derived from exogenous acetate (Mashimo, Pichumani et al. 2014). Under normal conditions, only a small percent of the acetyl-CoA is produced from scavenged acetate (Lyssiotis and Cantley 2014). Considering the importance of acetate scavenging in GBM on energy metabolism and epigenetic gene regulation, further studies regarding the role of acetate metabolism are warranted.
It is generally accepted that alterations in energy metabolism may impact the function of cellular organelles involved in drug detoxification. Peroxisomes are intracellular organelles derived from the smooth endoplasmic reticulum that are required for debranching of very long-chain fatty acids prior to L-carnitine mediated transport into the mitochondrial matrix for B-oxidation (Poulos, Beckman et al. 1992). In the liver, peroxisomes are the site of ethanol detoxification to acetate and water by a series of enzymes including alchohol dehydrogenase, aldehyde dehydrogenase and catalase. In the brain, ethanol is detoxified by cytochrome P450 and catalase. Due to the known perturbations in lipid metabolism found in GBM that may affect peroxisome function, Laurenti et al. clearly demonstrates that hypoxia regulates the expression of peroxisomal enzymes involved in lipid metabolism, resulting in accumulation of lipids in GBM specimens (Laurenti, Benedetti et al. 2011). This confirms original findings by Benedetti et al. in which the authors observe a significant accumulation of lipid droplets within human glioma specimens and conclude that these inclusions are positively correlated with higher grade malignancy (Benedetti, Galzio et al. 2010). As peroxisomes also play an important role in cellular detoxification, where reactive oxygen species such as hydrogen peroxide are reduced by catalase (Singh 1996), an important question yet to be satisfactorily addressed is the degree to which impaired peroxisome function contributes to chemotherapy resistance.
III. Treatment Induced Hypoxia and Resistance
Under normoxic conditions an oxygen enhancement effect is observed whereby radiation reacts with intracellular water in the presence of molecular oxygen resulting in the formation of free oxygen radicals (Amberger-Murphy 2009). These hydroxyl radicals formed by hydrolysis can attack the deoxyribose DNA backbone and bases, with formation of a multitude of lesions which cannot be easily repaired. In fact, nearly two thirds of the DNA damage is caused by hydroxyl radicals (Ward 1988). In a hypoxic environment, DNA damage is reduced because fewer free radicals are generated and they are more likely to react with H+ ions, returning to their original form. Damage is then limited to direct effects of radiation on base pairing, and 3-fold higher levels are needed to induce the same effect. Interestingly, pre-existing hypoxia is in fact exacerbated by radiation itself, as radiotherapy can damage tumor vascular function, promote activation of the coagulation system, and lead to secondary ischemic tumor stress (Denham and Hauer-Jensen 2002). Since the efficacy of ionizing radiation relies directly on adequate oxygen tensions, tumor hypoxia is a major constraint in tumor treatment by radiotherapy. Further, tumor cells which survive hypoxic stress are selected for reduced apoptotic potential, increased angiogenic signaling, and greater resistance to radiotherapy and cytotoxic chemotherapy. It appears that the population of cells that is particularly radioresistant consists of “stem-like” cells or cells with tumor initiating capacity. It also has been shown that hypoxia promotes persistence of the undifferentiated state of these stem-like or tumor initiating cells. However, published data suggests that radioresistance is not just a characteristic of glioma stem-like cells but a result of a heterotypic interaction between these cells and the tumor microenvironment; in other words, a property of the “microenvironment-stem cell unit” (Mannino and Chalmers 2011).
Hypoxia-inducible factor 1-alpha (HIF-1 alpha) is an integral component of hypoxic response, and is the major focus of numerous reviews. As such, and because few HIF-1 inhibitors have progressed through clinical development as successful anticancer therapeutics (Onnis, Rapisarda et al. 2009), this review will briefly describe the role of HIF-1 in modulating the hypoxic response. Under conditions of low oxygen, transcription of HIF1A is induced in an NF-κB dependent manner and HIF-1 alpha regulates the expression of over 60 genes involved in glycolysis, angiogenesis, invasion and epithelial-mesenchymal transition. Cumulatively, HIF-1 alpha may facilitate the hypoxic response, in part, through upregulation of glucose transporter 1 (glut1), thereby altering cellular metabolism to survive the unfavorable conditions encountered during hypoxia and radiation therapy. Recently, Chen et al. described that during oncogenic transformation, H-Ras overexpression is sufficient to induce HIF-1 alpha mediated expression of glut1 mRNA (Chen, Pore et al. 2001). Tumor initiating or cancer stem-like cells that undergo HIF-1 mediated adaptation to low oxygen may subsequently modulate other properties of the hypoxic response. Mannino et al. reported higher levels of various HIF-regulated genes in these cells compared to non-stem-like cells providing evidence for a differential response of glioma stem-like cells to hypoxia (Mannino and Chalmers 2011). Mendez et al. showed that reduction in HIF-1 alpha expression and glioma response to hypoxia results in reduced migration ability and overall less invasive tumors (Mendez, Zavadil et al. 2010). Clinically, the net effect of hypoxia on survival in GBM is unquestionable. Spence et al. (Spence, Muzi et al. 2008) have shown that survival in patients whose tumors contained hypoxic volume ratios greater than the median survived only approximately 4 months as compared to more than 12 months for those with less than median hypoxic burden (P<0.001); multivariate analyses against the covariates for enhancing tumor volume, age, and performance score reached significance only for hypoxic volume.
While targeting of VEGF has proven to be clinically relevant as discussed above, given the key importance of VEGF and its receptor VEGFR2 in tumor angiogenesis, hopes were once raised that blocking this pathway would eradicate the tumor vasculature and heal cancer. This clearly has not been the case as clinical practice reveals that therapy with angiogenesis inhibitors generally does not prolong survival of cancer patients for more than months. Increasing evidence points to the root cause of angiogenesis, hypoxia, as a driving force for resistance to anti-angiogenics. In a prospective clinical trial (Sathornsumetee, Cao et al. 2008) of bevacizumab and irinotecan in which biomarkers were assessed, the most significant predictor (negative) of both response to therapy and overall survival was the presence of hypoxia induced carbonic anhydrase (CA9) [P(χ2)=0.020, HR 2.72, CI 1.17 to 6.36]. Second to this was hypoxia induced factor 2α (HIF-2α). Not only is hypoxia as measured by carbonic anhydrase IX (CA9) predictive of resistance, but hypoxia as measured by CA9 and HIF-1α has also been found increased at the time of progression for patients that initially responded to bevacizumab (Iwamoto, Abrey et al. 2009). In a prospective clinical study in which the pan-VEGF receptor inhibitor AZD2171 was given to GBM patients (Batchelor, Sorensen et al. 2007), the most predictive blood biomarker of tumor progression was stromal derived factor 1α (SDF1α, p=0.058). It has previously been shown that SDF1α is strongly induced by HIF-1α under conditions of hypoxia (Ceradini, Kulkarni et al. 2004), suggesting that the correlation seen between progression on AZD2171 and SDF1α was driven by hypoxia. In animal models, bevacizumab treatment results in an accumulation of metabolic products including lactate, choline, and creatine, a combination that is associated with increased hypoxia in human brain tumor spectra, with an associated increase in HIF1α, matrix metalloproteinases, chemokines and a more invasive phenotype (Kunkel, Ulbricht et al. 2001, Lucio-Eterovic, Piao et al. 2009, Keunen, Johansson et al. 2011). Thus, antiangiogenic therapy exacerbates tumoral hypoxia and in doing so exacerbates the hypoxia resistant phenotype.
IV. Targeting Hypoxia in Astrocytoma
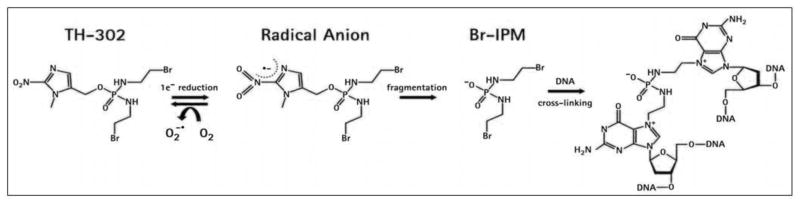
Given the impact of hypoxia on therapeutic resistance, poor outcomes associated with high tumor hypoxic burden, and the high degree of hypoxia encountered in astrocytoma, our recent efforts have focused on reversing the impact of hypoxia in astrocytoma. This can be achieved either directly or indirectly. A direct approach is via a hypoxia activated prodrug such as TH-302. TH-302 is a nitroimidazole prodrug of the cytotoxin, bromo-isophosphoramide mustard (Br-IPM). When exposed to hypoxic conditions, TH-302 is reduced at the nitroimadazole site of the prodrug by intracellular reductases leading to the release of the alkylating agent Br-IPM (Figure 4). Br-IPM can then act as a DNA crosslinking agent. It may also diffuse to adjacent cells in normoxic regions and thus act as a cytotoxic agent outside of the hypoxic activation zone. TH-302 has little activity under normoxic conditions but is highly cytotoxic under hypoxic conditions (Meng, Evans et al.), and shows broad activity in proliferation and clonogenic assays using a number of human cancer cell lines. TH-302 is currently under development for soft tissue sarcomas, pancreatic cancer, and lung cancer.
While the safety and preliminary efficacy of TH-302 has been evaluated as monotherapy (Weiss, Infante et al. 2011), as well as in combination with multiple conventional agents (Sankhala, Chiorean et al. 2010, Schelman 2010, Vlahovic, Infante et al. 2010, Ganjoo, Cranmer et al. 2011), it’s safety in patients with GBM or in combination with bevacizumab had not previously been evaluated. Therefore, we embarked upon an investigator initiated study of TH-302 with bevacizumab in patients with glioblastoma, with a surgical component to allow for tissue analysis. In order to characterize the hypoxic burden, TH-302 was followed by the exogenous hypoxia marker pimonidazole on the day of surgical debulking. Pimonidazole is a 2-nitroimidazole compound which forms protein adducts in the presence of reductases that can be detected via immunohistochemistry and provide a reliable estimate of biologically relevant hypoxia (Raleigh and Koch 1990, Raleigh, Chou et al. 2001). Excised tumor tissue and serum was collected for further analysis. Following a 4 week period of post-surgical recovery, patients were started on combination therapy of bevacizumab at the standard presurgical dose of 10mg/kg with TH-302 every 2 weeks. TH-302 was well tolerated in this patient population, and early results have been very promising with a combined clinical benefit rate of 62% (Brenner, Floyd et al. 2014). Additionally, we were able to confirm the marked hypoxia in these tumors by the use of pimonidozole, with concordance between both the exogenous marker (pimonidazole) and endogenous marker (CA9) of hypoxia was observed throughout (r2=0.79, p=0.0001), suggesting both may be suitable for future clinical study, and merit further investigation for predictive potential. Further, DNA damage as measured by γH2AX was observed in tumors from patients receiving a pre-surgical dose of TH-302, supporting that this agent is able to access the tumor across the blood brain barrier. A multicenter trial of TH-302 with bevacizumab in recurrent glioblastoma is ongoing as part of an FDA sponsored study.
An indirect solution to hypoxia and therapeutic resistance was suggested by findings in patients progressing on antiangiogenics and data from stroke models. As mentioned previously, stromal derived factor 1α (CXCL12) increases at the time of progression on antiangiogenics and appears to be tied to tumoral hypoxia (Batchelor, Sorensen et al. 2007). The most well established role of CXCL12 is through interaction with its G protein couple receptor CXCR4 to induce chemotaxis of progenitor cells in numerous normal physiologic and developmental processes (Murdoch 2000) including lymphopoesis, vascularization, and embryogenesis. In the injured CNS, CXCL12 regulates inflammatory processes (Cartier, Hartley et al. 2005) and promotes tissue regeneration by recruiting neural progenitor cells to the site of injury (Tran, Banisadr et al. 2007). Analogous roles are observed in multiple models of tumorigenesis, suggesting that the normal functions of CXCL12 are abrogated for the purposes of tumor progression. In orthotopic mouse models of glioblastoma, CXCL12 induces recruitment of bone marrow-derived CD45+ myeloid cells as well as endothelial and pericyte progenitor cells to promote neovascularization (Du, Lu et al. 2008). Newcomb et al. (Zagzag, Esencay et al. 2008) have shown that changes in VEGF are able to increase CXCL12 within the tumor microenvironment and that the formation of perivascular structures in glioblastoma is in fact dependent upon an intact CXCL12/CXCR4 signaling. In breast cancer, carcinoma associated fibroblasts have high expression of CXCL12 not seen in normal fibroblasts (Muller, Homey et al. 2001). A resulting recruitment of endothelial progenitor cells ensues with tumor neovascularization. Indeed, the organs representing the first destinations of breast cancer metastasis are the ones with the highest expression of CXCL12. Interference of CXCL12/CXCR4 interaction by anti-CXCR4 antibodies results in decreased metastasis in in vivo murine studies. While much of the research in malignancy has previously focused on the CXCR4 dependent effects of CXCL12, CXCR7 was more recently identified as a second receptor for CXCL12 with ability to modulate many of the aforementioned functions as well as having novel functions independent of CXCR4. Along these lines, it was found by Bakondi et al. that bone marrow derived CD133+ multipotent stem cells which migrate to areas of stroke induced ischemia dramatically upregulate expression and secretion of CXCL12, that CXCL12 was protective of the surrounding neural progenitor cells under conditions of stroke induced hypoxia, and that only CXCR7 activity was functionally linked to survival signaling in neuro-progenitor cells during hypoxia exposure (Bakondi, Shimada et al. 2010). In glioma models, Hatterman et al. have shown that CXCR7 is more highly expressed than CXCR4, that treatment with the alkylator temozolomide induced CXCL12 expression, and that CXCL12 protected glioma cells in a CXCR7 dependent manner from temozolomide induced apoptosis (Hattermann, Mentlein et al. 2012). In multiple other tumor types, critical roles were confirmed for CXCL12 signaling through CXCR7 and not CXCR4 in tumor vascular maintenance, angiogenesis, and promotion of neoplastic growth in vivo (Miao, Luker et al. 2007, Wang, Shiozawa et al. 2008, Boimel, Smirnova et al. 2012). In animals bearing breast tumors, increases in CXCL12 are observed with increasing dose of the anitangiogenic VEGF receptor (VEGFR) inhibitor sunitinib (Ebos, Lee et al. 2007). In a phase 2 study of sunitinib in patients with hepatocellular carcinoma, Jain et al. investigated a number of potential biomarkers to predict response to treatment. In a time dependent proportional hazards model they observed elevation of plasma CXCL12 levels to be associated with a higher hazard of immediate progression of sunitinib (Zhu, Sahani et al. 2009). In patients with colorectal cancer that receive bevacizumab in a neoadjuvant fashion, elevated CXCL12 levels in the plasma are associated with an increased risk of progression and can be directly attributed to the tumor (Xu, Duda et al. 2009). In patients with glioblastoma who experienced tumor progression while on the pan-VEGFR inhibitor cedirinib, increases in tumor enhancement volume were associated with significant increases in plasma levels of CXCL12 (Batchelor, Sorensen et al. 2007).
Moreover, there was a statistically significant positive correlation between CXCL12 levels and vessel size measured by MRI. In summary, CXCL12 plasma levels appear to rise contemporaneously with neoplastic disease progression while receiving anitangiogenic therapy regardless of the anitangiogenic agent or histologic type. Given the emerging roles of CXCL12 in recruitment of progenitor cells, formation of perivascular structures, tumor cell migration, and protection from hypoxia, the association of CXCL12 elevation with progression on antiangiogenics seems hardly coincidental. In preliminary experiments performed in our laboratory, we have observed a 22% improvement in median survival of nude rats bearing glioblastoma neurosphere orthotopic xenografts when the CXCR7 inhibitor CCX777 was combined with anti-VEGF therapy. While, further evaluation of CXCL12’s role in evasion from antiangiogenics and the receptors that mediate this role is ongoing, additional data supporting CXC7 inhibition in radiation is available. In a GBM intracranial model whereby animals received cranial irradiation with or without CXCR7 inhibition, the addition of CXCR7 inhibition improved animal survival over radiation alone (Walters, Ebsworth et al. 2014). No benefit was seen with CXCR7 inhibition in the absence of radiation. Even more intriguing, this benefit was seen independent of CXCR7 expression within the tumor, further supporting a role for CXCR7 in survival of cancer stem cells under conditions of radiation induced hypoxia.
This review considers the effects of hypoxia on astrocytic tumors and the mechanisms that contribute to tumor progression and therapuetic resistance, with a focus on the vascular changes, chemotaxic signaling pathways and metabolic consequences involved. Emerging evidence suggests that pseudopalisading GBM cells undergo a complex metabolic adaptation to low oxygenation. Progenitor stem-like cells residing in these hypoxic zones are thought to be buffered from vascular perfusion, hence further shielded from drug delivery in addition to those already imposed by the blood brain barrier. A further challenge in targeting pseudopalisading cells is that most effective therapeutics require sufficient oxygen tension to generate ROS and initiate a cascade of cytotoxic and genotoxic events. To overcome these inherent limitations, new hypoxia-activated DNA alkylating prodrugs are currently in clinical trials to evaluate the effects on overall patient survival and are the focus of continued interest by our lab and others. Related areas of interest include the development of inhibitors that target CXCL-4/7 receptor signaling to attenuate CXCL-12 mediated chemoattraction of peripheral progenitor stem-like cells to the tumor under conditions of hypoxia. Because CXCL12 is the most predictive blood biomarker of tumor progression, and as early preclinical studies suggest its efficacy in blocking CXCL12-mediated signaling, we are currently evaluating a panel of inhibitors to improve treatment. Other areas of ongoing research interest include the evaluation of metabolic inhibitors that exhibit specificity to those biochemical pathways deregulated under conditions of hypoxia, including aerobic glyolysis, acetate metabolism and de novo lipid biogenesis. Because the majority of these pathways have been well characterized since the early 20th century, and given that numerous FDA approved drugs are known to inhibit many of these pathways, the potential to discover new nontoxic drugs for alternate use that synergize with current treatment modalities is limitless. Considering the poor survival associated with GBM in the newly diagnosed setting, new therapeutics that target energy metabolism should be considered. Thus, a more refined knowledge of the mechanisms by which hypoxia modulates key metabolic enzymes and the accompanying shift in cellular bioenergetics may dramatically improve patient outcome.
Figure 2.
Model of TH-302 activation. Due to the high degree of hypoxia associated with GBM, a novel hypoxia-activated prodrug that selectively targets cells within pseudopalisading regions is in clinical development. Under conditions of hypoxia, TH-302 is activated by reactive oxygen species to release the bis-alkylating agent Br-IPM, resulting in DNA crosslinks that may be processed by various DNA repair mechanisms into lethal DNA breaks or render cells unable to replicate their DNA for cellular division. Once activated in hypoxic tissues, Br-IPM can also diffuse into surrounding oxygenated regions of the tumor and kill cells there via a “bystander effect”.
Figure 3.
Illustration of key biochemical pathways that undergo metabolic derangement in GBM. Cancer cells are known to undergo a metabolic switch toward aerobic glycolysis. Under conditions of hypoxia, glucose consumption and lactate secretion are further enhanced with a concomitant decrease in OXPHOS. Although inherently less efficient than aerobic respiration, cancer cells utilize the biochemical intermediates of aerobic glycolysis, including glucose 6-phosphate and glucose 3-phosphate to produce the nucleotides, proteins and lipids required for rapid cellular proliferation. To maintain adequate pools of precursors involved in lipid and cholesterol biosynthesis, intermediates from TCA may be converted to acetyl-CoA. Due to the high demand for acetyl-CoA during lipogenesis, coupled with the decreased supply from glycolytic sources, exogenous acetate may be incorporated and converted to acetyl-CoA by acetyl-conenzyme A synthetase. This compensatory use of acetate to maintain acetyl-CoA reserves has renewed interest in ACSS inhibitors as a treatment for GBM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amberger-Murphy V. Hypoxia helps glioma to fight therapy. Curr Cancer Drug Targets. 2009;9(3):381–390. doi: 10.2174/156800909788166637. [DOI] [PubMed] [Google Scholar]
- Bakondi B, Shimada IS, Peterson BM, Spees JL. Stromal-Derived Factor 1 Alpha Secreted by Human CD133-Derived Multipotent Stromal Cells Promotes Neural Progenitor Cell Survival Through CXCR7. Stem Cells Dev. 2010 doi: 10.1089/scd.2010.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti E, Galzio R, Laurenti G, D’Angelo B, Melchiorre E, Cifone MG, Fanelli F, Muzi P, Coletti G, Alecci M, Sotgiu A, Ceru MP, Cimini A. Lipid metabolism impairment in human gliomas: expression of peroxisomal proteins in human gliomas at different grades of malignancy. Int J Immunopathol Pharmacol. 2010;23(1):235–246. doi: 10.1177/039463201002300121. [DOI] [PubMed] [Google Scholar]
- Boimel PJ, Smirnova T, Zhou ZN, Wyckoff J, Park H, Coniglio SJ, Qian BZ, Stanley ER, Cox D, Pollard JW, Muller WJ, Condeelis J, Segall JE. fContribution of CXCL12 secretion to invasion of breast cancer cells. Breast Cancer Res. 2012;14(1):R23. doi: 10.1186/bcr3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brat DJ, Castellano-Sanchez AA, Hunter SB, Pecot M, Cohen C, Hammond EH, Devi SN, Kaur B, Van Meir EG. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64(3):920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- Brenner AJ, Floyd J, Eng C, Kroll S, Fichtel L, Gruslova A, Lodi A, Tiziani S. Phase 1/2 study of TH-302, investigational hypoxia-activated prodrug, and bevacizumab in patients with bevacizumab-refractory recurrent glioblastoma “. Neuro Oncol. 2014;16(S5) [Google Scholar]
- Burger PC, Green SB. Patient age, histologic features, and length of survival in patients with glioblastoma multiforme. Cancer. 1987;59(9):1617–1625. doi: 10.1002/1097-0142(19870501)59:9<1617::aid-cncr2820590916>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48(1):16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10(8):858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem. 2001;276(12):9519–9525. doi: 10.1074/jbc.M010144200. [DOI] [PubMed] [Google Scholar]
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury--a complex ‘wound’. Radiother Oncol. 2002;63(2):129–145. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci U S A. 2007;104(43):17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- Ganjoo KN, Cranmer LD, Butrynski JE, Rushing D, Adkins D, Okuno SH, Lorente G, Kroll S, Langmuir VK, Chawla SP. A phase I study of the safety and pharmacokinetics of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. Oncology. 2011;80(1–2):50–56. doi: 10.1159/000327739. [DOI] [PubMed] [Google Scholar]
- Hattermann K, Mentlein R, Held-Feindt J. CXCL12 mediates apoptosis resistance in rat C6 glioma cells. Oncol Rep. 2012;27(5):1348–1352. doi: 10.3892/or.2012.1674. [DOI] [PubMed] [Google Scholar]
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu TL, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, Chen J. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2(97):ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou LC, Veeravagu A, Hsu AR, Tse VC. Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus. 2006;20(4):E5. doi: 10.3171/foc.2006.20.4.2. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone A, Krapcho M, Garshel lJ, Miller D, Altekruse S, Kosary C, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis D, Chen H, Feuer E, Cronin K. SEER Cancer Statistics Review, 1975–2011 2014 [Google Scholar]
- Iwamoto FM, Abrey LE, Beal K, Gutin PH, Rosenblum MK, Reuter VE, DeAngelis LM, Lassman AB. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73(15):1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Jr, McKnight SL. Influence of metabolism on epigenetics and disease. Cell. 2013;153(1):56–69. doi: 10.1016/j.cell.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SA, Fack F, Thorsen F, Taxt T, Bartos M, Jirik R, Miletic H, Wang J, Stieber D, Stuhr L, Moen I, Rygh CB, Bjerkvig R, Niclou SP. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A. 2011;108(9):3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel P, Ulbricht U, Bohlen P, Brockmann MA, Fillbrandt R, Stavrou D, Westphal M, Lamszus K. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61(18):6624–6628. [PubMed] [Google Scholar]
- Laurenti G, Benedetti E, D’Angelo B, Cristiano L, Cinque B, Raysi S, Alecci M, Ceru MP, Cifone MG, Galzio R, Giordano A, Cimini A. Hypoxia induces peroxisome proliferator-activated receptor alpha (PPARalpha) and lipid metabolism peroxisomal enzymes in human glioblastoma cells. J Cell Biochem. 2011;112(12):3891–3901. doi: 10.1002/jcb.23323. [DOI] [PubMed] [Google Scholar]
- Long PM, Tighe SW, Driscoll HE, Fortner KA, Viapiano MS, Jaworski DM. Acetate supplementation as a means of inducing glioblastoma stem-like cell growth arrest. J Cell Physiol. 2015 doi: 10.1002/jcp.24927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15(14):4589–4599. doi: 10.1158/1078-0432.CCR-09-0575. [DOI] [PubMed] [Google Scholar]
- Lyssiotis CA, Cantley LC. Acetate fuels the cancer engine. Cell. 2014;159(7):1492–1494. doi: 10.1016/j.cell.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Mannino M, Chalmers AJ. Radioresistance of glioma stem cells: intrinsic characteristic or property of the ‘microenvironment-stem cell unit’? Mol Oncol. 2011;5(4):374–386. doi: 10.1016/j.molonc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, Nannepaga S, Piccirillo SG, Kovacs Z, Foong C, Huang Z, Barnett S, Mickey BE, DeBerardinis RJ, Tu BP, Maher EA, Bachoo RM. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159(7):1603–1614. doi: 10.1016/j.cell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RP, Hunjan S, Le D, Constantinescu A, Barker BR, Wong PS, Peschke P, Hahn EW, Antich PP. Regional tumor oxygen tension: fluorine echo planar imaging of hexafluorobenzene reveals heterogeneity of dynamics. Int J Radiat Oncol Biol Phys. 1998;42(4):747–750. doi: 10.1016/s0360-3016(98)00306-x. [DOI] [PubMed] [Google Scholar]
- Mendez O, Zavadil J, Esencay M, Lukyanov Y, Santovasi D, Wang SC, Newcomb EW, Zagzag D. Knock down of HIF-1alpha in glioma cells reduces migration in vitro and invasion in vivo and impairs their ability to form tumor spheres. Mol Cancer. 2010;9:133. doi: 10.1186/1476-4598-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Evans JW, Bhupathi D, Banica M, Lan L, Lorente G, Duan JX, Cai X, Mowday AM, Guise CP, Maroz A, Anderson RF, Patterson AV, Stachelek GC, Glazer PM, Matteucci MD, Hart CP. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol Cancer Ther. doi: 10.1158/1535-7163.MCT-11-0634. [DOI] [PubMed] [Google Scholar]
- Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, Kleer CG, Essner JJ, Nasevicius A, Luker GD, Howard MC, Schall TJ. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci U S A. 2007;104(40):15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Murdoch C. CXCR4: chemokine receptor extraordinaire. Immunol Rev. 2000;177:175–184. doi: 10.1034/j.1600-065x.2000.17715.x. [DOI] [PubMed] [Google Scholar]
- Onnis B, Rapisarda A, Melillo G. Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med. 2009;13(9A):2780–2786. doi: 10.1111/j.1582-4934.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-oncology. 2014;16(4) doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(Suppl 4):iv1–63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos A, Beckman K, Johnson DW, Paton BC, Robinson BS, Sharp P, Usher S, Singh H. Very long-chain fatty acids in peroxisomal disease. Adv Exp Med Biol. 1992;318:331–340. doi: 10.1007/978-1-4615-3426-6_30. [DOI] [PubMed] [Google Scholar]
- Raleigh JA, Chou SC, Bono EL, Thrall DE, Varia MA. Semiquantitative immunohistochemical analysis for hypoxia in human tumors. Int J Radiat Oncol Biol Phys. 2001;49(2):569–574. doi: 10.1016/s0360-3016(00)01505-4. [DOI] [PubMed] [Google Scholar]
- Raleigh JA, Koch CJ. Importance of thiols in the reductive binding of 2-nitroimidazoles to macromolecules. Biochem Pharmacol. 1990;40(11):2457–2464. doi: 10.1016/0006-2952(90)90086-z. [DOI] [PubMed] [Google Scholar]
- Rong Y, Durden DL, Van Meir EG, Brat DJ. ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol. 2006;65(6):529–539. doi: 10.1097/00005072-200606000-00001. [DOI] [PubMed] [Google Scholar]
- Sankhala KK, Chiorean EG, Armstrong AJ, Borad MJ, Traynor AM, Gadgeel SM, Langmuir VK, Eng C, Kroll S, Burris H. Phase I/II study of TH-302 in combination with docetaxel in patients with solid tumors including NSCLC and castrate-resistant prostate cancer (CRPC). 2010 ASCO Annual Meeting J Clin Oncol.2010. [Google Scholar]
- Sathornsumetee S, Cao Y, Marcello JE, Herndon JE, 2nd, McLendon RE, Desjardins A, Friedman HS, Dewhirst MW, Vredenburgh JJ, Rich JN. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26(2):271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelman WR. Phase I/II study of TH-302 in combination with gemcitabine in patients with solid tumors including advanced pancreatic cancer. 2010 ASCO Annual Meeting J Clin Oncol.2010. [Google Scholar]
- Singh I. Mammalian peroxisomes: metabolism of oxygen and reactive oxygen species. Ann N Y Acad Sci. 1996;804:612–627. doi: 10.1111/j.1749-6632.1996.tb18648.x. [DOI] [PubMed] [Google Scholar]
- Spence AM, Muzi M, Swanson KR, O’Sullivan F, Rockhill JK, Rajendran JG, Adamsen TC, Link JM, Swanson PE, Yagle KJ, Rostomily RC, Silbergeld DL, Krohn KA. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin Cancer Res. 2008;14(9):2623–2630. doi: 10.1158/1078-0432.CCR-07-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY, Dinjens WN, Enting RH, Taphoorn MJ, van den Berkmortel FW, Jansen RL, Brandsma D, Bromberg JE, van Heuvel I, Vernhout RM, van der Holt B, van den Bent MJ. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. doi: 10.1016/S1470-2045(14)70314-6. [DOI] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500(6):1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Lunt SY, Dayton TL, Fiske BP, Israelsen WJ, Mattaini KR, Vokes NI, Stephanopoulos G, Cantley LC, Metallo CM, Locasale JW. Metabolic pathway alterations that support cell proliferation. Cold Spring Harb Symp Quant Biol. 2011;76:325–334. doi: 10.1101/sqb.2012.76.010900. [DOI] [PubMed] [Google Scholar]
- Vlahovic G, Infante JR, Mita AC, Traynor AM, Molina JR, Lacouture ME, Langmuir VK, Eng C, Kroll S, Borad MJ. Phase I/II study of TH-302 in combination with pemetrexed in patients with solid tumors including NSCLC. 2010 ASCO Annual Meeting..2010. [Google Scholar]
- Walters MJ, Ebsworth K, Berahovich RD, Penfold ME, Liu SC, Al Omran R, Kioi M, Chernikova SB, Tseng D, Mulkearns-Hubert EE, Sinyuk M, Ransohoff RM, Lathia JD, Karamchandani J, Kohrt HE, Zhang P, Powers JP, Jaen JC, Schall TJ, Merchant M, Recht L, Brown JM. Inhibition of CXCR7 extends survival following irradiation of brain tumours in mice and rats. Br J Cancer. 2014;110(5):1179–1188. doi: 10.1038/bjc.2013.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283(7):4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- Ward JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- Weiss GJ, Infante JR, Chiorean EG, Borad MJ, Bendell JC, Molina JR, Tibes R, Ramanathan RK, Lewandowski K, Jones SF, Lacouture ME, Langmuir VK, Lee H, Kroll S, Burris HA., 3rd Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin Cancer Res. 2011;17(9):2997–3004. doi: 10.1158/1078-0432.CCR-10-3425. [DOI] [PubMed] [Google Scholar]
- Xu L, Duda DG, di Tomaso E, Ancukiewicz M, Chung DC, Lauwers GY, Samuel R, Shellito P, Czito BG, Lin PC, Poleski M, Bentley R, Clark JW, Willett CG, Jain RK. Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1alpha, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res. 2009;69(20):7905–7910. doi: 10.1158/0008-5472.CAN-09-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagzag D, Esencay M, Mendez O, Yee H, Smirnova I, Huang Y, Chiriboga L, Lukyanov E, Liu M, Newcomb EW. Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1alpha/CXCR4 expression in glioblastomas: one plausible explanation of Scherer’s structures. Am J Pathol. 2008;173(2):545–560. doi: 10.2353/ajpath.2008.071197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS, Lahdenranta J, Bhargava P, Meyerhardt J, Clark JW, Kwak EL, Hezel AF, Miksad R, Abrams TA, Enzinger PC, Fuchs CS, Ryan DP, Jain RK. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27(18):3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]