Abstract
Objective
To investigate the association between traumatic brain injury (TBI) related brain lesions and long-term caregiver burden in relation to dysexecutive syndrome.
Setting
National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland.
Participants
A total of 256 participants: 105 combat veterans with TBI, 23 healthy control combat veterans (HCv), and 128 caregivers.
Outcome Measure
Caregiver burden assessed by the Zarit Burden Interview (ZBI) at 40 years post-injury.
Design
Participants with penetrating TBI were compared with HCv on perceived caregiver burden and neuropsychological assessment measures. Data of Computed Tomography scans (overlay lesion maps of participants with a penetrating TBI whose caregivers have a significantly high burden) and behavioral statistical analyses were combined to identify brain lesions associated with caregiver burden.
Results
Burden was greater in caregivers of veterans with TBI than caregivers of HCv. Caregivers of participants with lesions affecting cognitive and behavioral indicators of dysexecutive syndrome (i.e., left dorsolateral prefrontal cortex and dorsal anterior cingulate cortex) showed greater long-term burden than caregivers of participants with lesions elsewhere in the brain.
Conclusion and Implication
TBI-related brain lesions have a lasting effect on long-term caregiver burden due to cognitive and behavioral factors associated with dysexecutive syndrome.
Keywords: Caregiver burden, traumatic brain injury (TBI), dorsal anterior cingulate cortex (dACC), dorsolateral prefrontal cortex (dlPFC), dysexecutive syndrome, executive
INTRODUCTION
Traumatic brain injury (TBI) is a serious health and socio-economic issue that leads to physical, cognitive and/or social limitations that may persist throughout life. These limitations also affect the family unit, particularly the primary caregiver for whom these limitations take a financial, health, and emotional toll.[1–4] The estimated economic value of the care provided by family caregivers was $450 billion in the U.S. in 2009.[5] Notably, the caregiver plays a crucial role in the rehabilitation process of an individual with TBI.[1, 6]
Caregiver burden refers to the physical, psychological, emotional, social and financial challenges one faces when providing care for patients with chronic illness.[7, 8] High levels of caregiver burden increase the risk of poor caregiver physical health, anxiety, depression, social isolation, decreased personal independence, and reduced quality of life and satisfaction.[7, 9–14] Eventually, when the caregiver is no longer able to care for the patient, assisted living or nursing home options have to be sought, leading to an increased financial burden on public services.[5]
Short-term caregiver burden has been prominently studied in individuals with fronto-temporal dementia,[15] Alzheimer disease,[16] and more recently in TBI.[3, 7, 17] These investigations demonstrated that the magnitude of burden is influenced by factors related to the patient, caregiver, and their support systems. Factors related to caregivers are: time spent caring for the patient, positive coping strategies and perceived stigma associated with caregiving.[18, 19] Furthermore, the caregiver’s mental health benefits from positive environmental factors, such as family needs being met via adequate health information as well as emotional and instrumental support.[19] Factors related to the patient consist of motor disability as well as cognitive and behavioral impairment.7,19 Although many variables affect caregiver burden, in the current study we focused on the impact of dysexecutive syndrome, a cognitive and behavioral factor, since it is a major predictor of caregiver burden.[7, 20, 21] Dysexecutive syndrome is characterized by a diverse pattern of behavioral and cognitive disorders related to impaired executive functions (EFs).[22] Dysexecutive syndrome is observed in several medical conditions including TBI, stroke and Alzheimer disease.[22] EFs encompass higher order processes such as planning, problem-solving, and abstract thinking to amend goal-directed behavior.[2, 3] Evidence exists that patients’ planning and abstract thinking deficits, disinhibition, depression, apathy, social isolation and impairment, increase caregiver burden.[2, 7, 14, 17, 23] Dysexecutive syndrome can be assessed by cognitive testing of the patient, through behavioral assessment by a clinician during an interview, and via observation by a caregiver in daily life.
The neural signatures of EFs have been widely studied in healthy individuals and participants with damage to brain areas[24, 25] including prefrontal cortex (PFC),[25–28] anterior cingulate cortex (ACC),[25, 28, 29] inferior parietal lobes,[27, 28, 30] and superior temporal lobes.[28, 30–32] The PFC and ACC play key roles in higher-level processes of EF; The ACC is subdivided in two distinct anatomic parts, the rostro-ventral ACC, i.e. subgenual and perigenual parts of the ACC, associated with affective processes, and the dorsal ACC (dACC), posterior to the rostro-ventral ACC, associated with cognitive processes.[33, 34] The PFC comprises all frontal areas anterior to the premotor cortex and is associated with cognition and behaviors related to EFs. The dACC and PFC are highly interconnected and functionally complete each other.[35] More specifically, the PFC is implicated in executive control and decision-making, whereas the dACC is involved in monitoring performance and error detection.[35, 36]
Although the effect of TBI on caregiver burden has been widely studied in the past two decades, the effect of penetrating TBI-related brain lesions on long-term burden remains unexplored. The goal of our study was two-fold. First, we investigated long-term caregiver burden in relation to dysexecutive syndrome in a group of participants with penetrating TBI and a healthy control group of Vietnam veterans 40 years after injury. We predicted that long-term burden is greater for caregivers of the TBI group than caregivers of the control group due to participants’ dysexecutive syndrome. Second, we studied the effect of TBI-related brain lesions on long-term caregiver burden and predicted that long-term burden associated with dysexecutive syndrome is greater in caregivers of participants with lesions in key areas involved in EFs than in caregivers of participants with TBI lesions not typically associated with EFs.
METHODS
Participants
Participants were drawn from Phase IV (2008–2012) of the W.F. Caveness Vietnam Head Injury Study (VHIS) registry, a longitudinal study of male veterans with mostly focal penetrating TBI. The VHIS consisted of four phases described in detail elsewhere.[37] Phase I was a recruitment period for the registry; Phase II (1981–1984) involved neuropsychological testing at the Walter Reed Army Medical Center; Phase III (2003–2006) involved neuropsychological testing, genetic testing, and computed tomography (CT) acquisition at the National Naval Medical Center in Bethesda, MD; and Phase IV (2008–2012) included neuropsychological testing at the National Institute of Neurological Disorders and Stroke (NINDS), Bethesda, MD.
A total of 134 male veterans with braininjury and 35 male veterans without brain injury participated in Phase IV. To ensure study eligibility, a phone interview was conducted before enrollment, and a screening neurological history and examination were performed at the test site. For this study, we enrolled 128 veterans who were accompanied by their primary caregiver (or family member for the healthy control veteran (HCv) group) and whose caregiver completed the burden scale described below. The total number of participants can be divided into two groups: a group with brain injury (TBI, n=105) and a control group of veterans who had served in combat during the Vietnam era (HCv, n=23) (Note that we will also use the term “caregiver” for the HCv group instead of “family member”). Caregivers of the TBI and HCv groups were not significantly different with respect to age (TBI : mean=58.31, s.d.=8.80 ; HCv : mean=56.65, s.d.=9.24; t(126)=.81, P=.418), years of education (TBI : mean=14.31, s.d.=2.35 ; vHC : mean=14.30, s.d.=2.12; t(126)=.02, P=.985), gender (TBI : 96 females, 9 males ; HCv : 21 females, 2 males ; χ2=.00, P=.985) and type of relationship (TBI : 83 spouses, 6 children, 4 siblings and 12 others; HCv : 17 spouses, 3 children, 2 siblings, 1 other ; χ2=5.14, P=.526).
All participants gave written informed consent, and the study was approved by the Institutional Review Board of the NINDS/NIH, Bethesda, MD.
Computerized tomography (CT) acquisition
CT scans were acquired on a GE Medical Systems Light Speed Plus CT scanner in helical mode. Images were reconstructed with 1 mm overlapping slice thickness and a 1 mm interval. Location and volume of lesion were determined from CT images by manual tracing using Analysis of Brain Lesion (ABLe) software[38, 39] implemented in MEDx (Medical Numerics Inc., Sterling, VA, USA) with enhancements to support the Automated Anatomical Labeling (AAL) atlas.[40] The tracing was performed by a trained neuropsychiatrist and then reviewed by J.G., an experienced observer, who was blind to the results of the clinical evaluations. A consensus judgment determined the final lesion outline. Based on the lesion volume, we determined the percentage of volume loss (Lesion volume (cubic centimeter (cc)) × 100 / total brain volume (cc)).
Clinical assessment - Caregiver burden
We assessed perceived caregiver burden with the 22-item version of the Zarit Burden Interview (ZBI).[41] Caregivers rated statements expressing specific feelings that arose when taking care of someone else (5-point Likert scale: 0=never, 4=nearly always). Caregivers were verbally instructed to respond to this questionnaire as it pertains to the participant they were accompanying for this study. The last item measured overall burden felt from caring for someone else using the same 5-point Likert scale. A total score was obtained by summing the first 21 items; higher scores indicated greater burden. A cut-off score of 24 was determined to be clinically relevant, since it identifies caregivers who are more likely to develop depression and are thus in need of further assessment and potential interventions.[42]
Clinical assessment – Executive functions
We assessed participants’ EFs with two cognitive tasks: the phonologic verbal fluency task and the trail making test from the Delis-Kaplan executive function system (D-KEFS) battery[43]. The verbal fluency task assesses one’s capacity to produce as many words as possible that start with a given letter (F, A and S) within 60 seconds per letter (FAS). The scaled score of the total number of new words listed (repetitions not counted) (FAS-T) was used. The trail making test switching condition (TMT-Switching, TMT-S) assesses mental flexibility to connect in alternation letters and numbers, respecting alphabetic and numeric order. A second TMT condition was used to control for alphabetic order. Participants had to link letters in alphabetic order without alternating with numbers (TMT-control, TMT-C). The scaled score of the sum of sequences produced was used for each condition (TMT-S, TMT-C).
In addition, the Frontal System Behavioral (FrSBe) scale[44] and the Neurobehavioral Rating Scale (NBRS),[45] clinically relevant measures related to participants’ EFs, were collected from the caregivers and test examiner, respectively. The FrSBe consists of 46 statements assessing apathy, disinhibition and EFs. The caregivers rated the responses (5-point Likert scale: 1=almost never, 5=almost always) to reflect the care recipient’s observed behavior. Note that although each statement was rated twice during Phase IV, —once assessing dysexecutive syndrome before the injury and once assessing it at present (with higher scores indicating greater “frontal syndrome” behavior)— only the present scores were used for this study (FrSBe apathy, FrSBe disinhibition, FrSBe EF). The NBRS is based on the examiner’s observation of the care recipient’s behavior, including affect (e.g., emotional withdrawal, decreased initiative/motivation, lability of mood) and cognitive aspects (e.g., disinhibition, conceptual disorganization, poor planning) of EFs. The total pathology score on the 27 items (7-point Likert scale: 1=the symptom is not present, 7=the symptom is extremely severe) was used. The test examiner typically spent a total of 30 hours observing the participant before completing the NBRS.
Clinical assessment –Instrumental functions, PTSD and mood
We included additional neuropsychological tests as instrumental measures. We administered the Armed Forces Qualification Test (AFQT-7A), a global intelligence test of word knowledge, arithmetic word problems, object function matching, and mental imagery. Scores on this test correlate highly with performance on the Wechsler Adult Intelligence Scale.[46, 47] Participants completed the AFQT prior to military entry (pre-injury IQ) and during their visit for Phase IV (post-injury IQ). The total AFQT score was converted to a percentage score of correct answers. We assessed language abilities with the Boston Naming Test (BNT, 2nd edition),[48]on which participants viewed black and white drawings of common objects and were to name each object. We used the total score - number of correct answers.
We evaluated visual and auditory declarative memory with the Wechsler Memory Scale abbreviated (WMS-III a)[49] using the delayed memory scaled score. We assessed aspects of visual perception with the Visual Object and Space Perception battery (VOSP).[50] The VOSP consists of eight subtests: incomplete letters, silhouettes, object decision, progressive silhouettes, dot counting, position discrimination, number location, and cube analysis. For our purpose, we selected 2 of 4 tasks assessing object perception (i.e., silhouette and object decision) and 2 of 4 tasks assessing space perception (i.e., position discrimination and cube analysis), avoiding the use of letter or number as stimuli to minimize the involvement of cognitive factors’ related to letter and number knowledge (for the description of the tasks, see Schintu 2014).[51] We converted the total score of each subtest into a percentage and used the average of these 4 percentages for further statistical analyses. Finally, we used the Beck Depression Inventory (BDI-II)[52] to assess severity of depression and the Mississippi Scale (M-PTSD) to evaluate post-traumatic stress disorder.[53] The total raw scores of these self-report measures were used for our analyses. Finally, we assessed global disability using the total scaled score of the Functional Scale Questionnaire (FSQ).[54]
Statistical analysis
We used IBM© SPSS© (version 16 for Mac, www.spss.com) and applied a threshold of P<0.05 (2-tailed). To investigate long-term caregiver burden, we first compared TBI and HCv groups on demographics and clinical assessments using Mann Whitney U tests. Then partial correlations were computed between caregiver burden (ZBI total score) and EF measures (FAS, TMT-C, TMT-S, FrSBe, NBRS), while controlling for language (BNT) since our targeted brain region is located in the left hemisphere. Second, to investigate the effect of brain lesion location on long-term caregiver burden, lesion maps for the entire TBI population were overlaid to ensure coverage of regions previously identified in dysexecutive syndrome. Next, lesion maps for those participants with TBI whose caregivers had a clinically relevant high burden score (ZBI≥24) were overlaid to identify a consistent lesion pattern associated with caregiver burden. Participants whose injury was included in the identified lesion pattern were separated into a target group (TBI-T) and the remaining participants into a control group (TBI-C). In addition, three overlap maps were created, including two subtraction maps (one displaying only lesions of the TBI-T group and another only lesions of the TBI-C group) and a conjunction map (showing the overlap in both groups) (Fig. 3).
Figure 3. Flow chart for the criteria and triage of our groups’ constitution, as well as the number of participants.
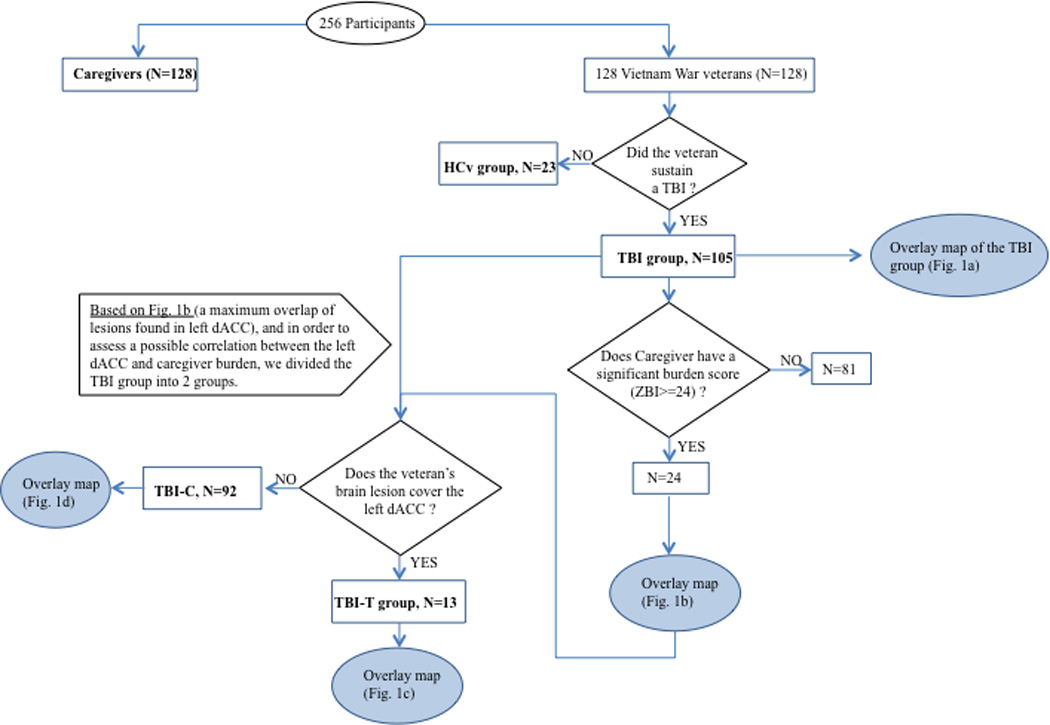
Finally, to investigate the effect of lesion location on deficits in dysexecutive syndrome that may mediate long-term caregiver burden, demographics and clinical assessment measures were compared using Kruskal-Wallis H tests among groups (TBI-T, TBI-C, HCv) and planned follow-up Mann-Whitney U tests between groups (TBI-T vs. TBI-C, TBI-T vs. HCv).
RESULTS
The groups (TBI, HCv) were matched on age, years of education, handedness and pre-injury IQ (Tab. 1). The caregivers of those in the TBI group showed a significantly higher burden than the caregivers of the HCv group (Z=−2.45, P<0.05). Participants with TBI performed significantly worse than HCv on EF tasks (FAS, TMT), post-injury IQ and visual perception. However, these groups did not differ significantly on caregiver and test examiner EFs’ assessments (respectively FrSBe and NBRS) or the remaining measures (global disability, depression, post-traumatic stress disorder, memory, language) (Tab. 1). For the TBI group, significant correlations between ZBI and all EF measures were found after controlling for language, (FAS: r=−0.19, P<0.05; TMT-S: r=−0.25, P=0.005; FrSBe executive function: r=0.65, P<0.001; FrSBe apathy: r=0.59, P<0.001; FrSBe disinhibition: r=0.60, P<0.001 and NBRS: r=0.38; P<0.001).
Table 1.
Descriptive (mean, [s.d.]) and inferential statistics of demographics and clinical data for TBI (n=105) and HCv (n=23) groups.
| TBI | HCv | Statistics | ||
|---|---|---|---|---|
| DEMOGRAPHICS | ||||
| Age (years) | 63.41 (2.99) | 62.70 (1.74) | Z=−.76; P=.448 | |
| Education (years) | 14.71 (2.17) | 15.13 (1.94) | Z=−.78; P=.437 | |
| Handedness (R:A:L) | 87:2:16 | 17:1:5 | X2 (2)=1.15 P=.562 | |
| FSQ (total score) | 95.68 (18.33) | 97.35 (20.65) | Z=−.39 P=.700 | |
| Pre-injury IQ (percentile) | 63.87 (23.66) | 73.00 (18.39) | Z=−1.57; P=.116 | |
| PERCEIVED CAREGIVER BURDEN | ||||
| ZBI (total score) | 15.51 (12.57) | 8.17 (5.18) | Z=−2.45; P=.014 | |
| EXECUTIVE FUNCTIONING MEASURES | ||||
| FAS (total scaled score) | 8.66 (3.72) | 11.09 (3.73) | Z=−2.98; P=.003 | |
| TMT control (scaled score) | 9.44 (3.63) | 11.83 (1.50) | Z=−3.10; P=.002 | |
| TMT switching (scaled score) | 9.10 (4.03) | 11.13 (2.67) | Z=−2.31; P=.021 | |
| NBRS (total pathology score) | 37.38 (13.55) | 35.17 (9.35) | Z=−.77;P=.440 | |
| FrSBe Apathy (Total score) | 63.02 (18.64) | 57.74 (18.30) | Z=−1.44;P=.151 | |
| FrSBe Disinhibition (Total score) | 58.79 (16.58) | 56.09 (15.63) | Z=−.53;P=.594 | |
| FrSBe EF (Total score) | 62.88 (17.74) | 59.57 (17.42) | Z=−.777;P=.437 | |
| CONTROL MEASURES | ||||
| Post-injury IQ (percentile) | 54.38 (25.79) | 72.65 (18.14) | Z=−3.16; P=.002 | |
| BDI-II (total raw score) | 7.55 (7.87) | 9.48 (7.91) | Z=−1.14; P=.256 | |
| M-PTSD (total score) | 78.44 (22.32) | 80.57 (22.62) | Z=−.48;P=.630 | |
| BNT (total score) | 53.02 (7.69) | 55.91 (3.87) | Z=−1.87; P=.062 | |
| WMS (delay Memory scaled score) | 100.25 (17.07) | 106.87 (17.04) | Z=−1.99; P=.077 | |
| VOSP (average percentage) | 84.45 (10.02) | 89.26 (4.19) | Z=−2.00; P=.045 | |
Bold statistics are significant. TBI-T, TBI target; TBI-C, TBI control; HCv, Healthy Control veterans; FSQ, Functional Status Questionnaire; IQ, Intelligence quotient; ZBI, Zarit Burden Interview; FAS, Verbal Fluency (letter F, A, S); TMT, Trail making test; NBRS, Neurobehavioral Rating Scale; FrSBe, Frontal System Behavioral Scale; EFs, Executive functions; BDI–II, Beck Depression Inventory; M-PTSD, Mississippi – Post-traumatic stress disorder scale; BNT, Boston Naming test; WMS, Wechsler Memory scale abbreviated; VOSP, Visual Object and Space Perception battery.
To investigate the effect of lesion location on long-term caregiver burden, an overlay lesion map for the entire TBI sample was examined and showed coverage of brain regions previously identified in dysexecutive syndrome (Fig. 1a). Another overlay lesion map was created using the 24 participants with TBI whose caregivers had a clinically relevant higher burden (ZBI total score ≥24) (Fig. 1b). For these, a maximum overlap of lesions (6 participants) was found in the left dACC (peak coordinate: x=−14; y=16; z=43, in Montreal Neurological Institute space). Participants with injury to the left dACC were placed into a TBI-T subgroup (n=13) (Fig. 1c) and the remaining participants into a TBI-C subgroup (n=92) (Fig. 1d). Subtraction maps between groups showed that the TBI-T group had lesions covering predominantly the left dACC and dorsolateral PFC (dlPFC) (left frontal superior and middle gyri), but also the left frontal inferior gyrus, left precentral gyrus, left supplementary motor area, left insula and, to a lesser extent, the right inferior gyrus, right supplementary motor area, right ACC and right olfactory bulb (Fig. 2a). The TBI-C group had lesions that cover most of the right frontal lobe (except for the middle and superior orbital gyri), a limited portion of the left frontal lobe (precentral gyrus), most of the left and right parietal, temporal and occipital lobes (Fig. 2b). The conjunction map showed that the groups had common lesions in the left and right anterior parts of the frontal superior, middle and inferior gyri and, to a lesser extent, the left and right rostro-ventral ACC (Fig. 2c).
Figure 1. Lesion overlay map. Z: superior-inferior coordinate in the Talairach space.
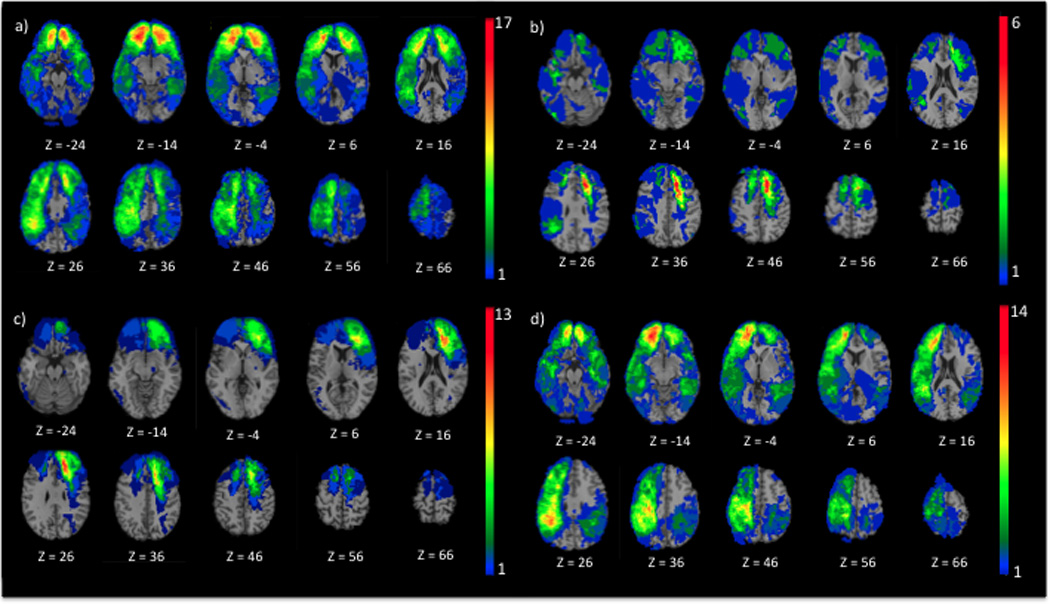
a) Entire TBI sample (n=105), b) participants with TBI (n=24) whose caregiver had significant caregiver burden (ZBI total score ≥ 24), c) TBI-T, participants whose lesion comprised left dACC and dlPFC (n=13), and d) TBI-C, participants whose lesion does not comprise left dACC and dlPFC (n=92). The color bar represents the number of overlapping lesions at each voxel. Red indicates a greater number of participants with TBI who have a lesion on a particular voxel. In each image, the right hemisphere is on the reader’s left.
Figure 2. Subtraction and conjunction maps.
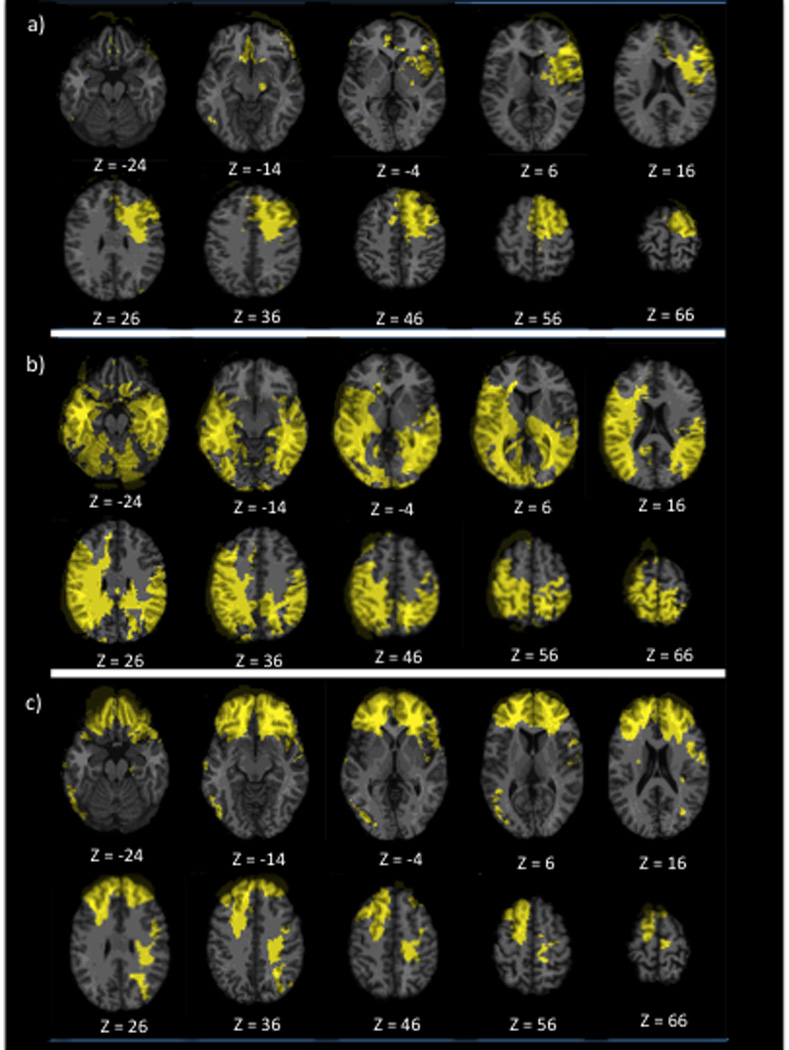
a) Subtraction overlay map showing lesions present only in the TBI-T group, b) Subtraction overlay map showing lesions present only in the TBI-C group, c) Conjunction overlay map showing lesions present in both TBI groups. In each slice, the right hemisphere is on the reader’s left and the lesions are represented in yellow.
To investigate the effect of brain lesion location on deficits in EF affecting long-term caregiver burden, we compared groups (TBI-T, TBI-C, HCv) on age, years of education, handedness, global disability, TBI severity and pre-injury IQ, and found no significant differences, but the TBI-T group had a significantly larger percentage of volume loss than the TBI-C group (Tab. 2). As expected, the caregivers of those in the TBI-T group had a significantly higher burden than the caregivers of participants in the TBI-C group (Z=−2.08; P<0.037, r=0.20) and HCv (ZBI, Z=−3.25; P<0.001, r=0.541). Since TBI-T and TBI-C groups differed significantly on percentage of brain volume loss, a Spearman’s coefficient correlation was applied between percentage of brain volume loss and ZBI total score, but the correlation was not significant (rs=0.45; P=0.121).
Table 2.
Descriptive and inferential statistics for demographics and clinical data for TBI-T (n=13), TBI-C (n=92), and HCv (n=23) groups.
| TBI-T Mean (s.d.) [Min;Max] |
TBI-C Mean (s.d.) [Min;Max] |
HCv Mean (s.d.) [Min;Max] |
Statistics | ||
|---|---|---|---|---|---|
| DEMOGRAPHICS | |||||
| Age (years) | 62.15 (1.35) | 63.59 (3.12) | 62.70 (1.74) | H(2)=3.68; P=.159 | |
| Education (years) | 14.31 (2.02) | 14.77 (2.19) | 15.13 (1.94) | H(2)=1.35; P=.508 | |
| Handedness (R:A:L) | 10:0:3 | 77:2:13 | 17:1:5 | X2 (4)=2.00 P=.735 | |
| Pre-injury IQ (percentile) | 62.92 (27.52) | 64.01 (23.23) | 73.00 (18.39) | H(2)=2.58; P=.275 | |
| TBI severity Mild Moderate Severe |
N=6 N=0 N= 7 |
N=52 N=3 N= 22 |
X2 (2)=3.50 P=.174 | ||
| FSQ (total scaled score) | 89.77 (17.30) | 96.51 (18.41) | 97.35 (20.65) | H=2.22; P=.329 | |
| Brain volume loss (percentage) | 6.00 (3.64) | 2.29 (3.05) | - | Z=−4.10; P<.001 | |
| PERCEIVED CAREGIVER BURDEN | |||||
| ZBI (total score) | 22.38 (10.83) [2;39] | 14.54 (12.55) [0;49] | 8.17 (5.18) [0;18] | H(2)=12.89; P=.002 | |
| EXECUTIVE FUNCTIONING MEASURES | |||||
| FAS (total scaled score) | 5.54 (2.60) | 9.10 (3.65) | 11.09 (3.73) | H(2)=18.73; P<.001 | |
| TMT control (scaled score) | 8.38 (3.73) | 9.60 (3.61) | 11.83 (1.50) | H(2)=12.49; P=.002 | |
| TMT switching (scaled score) | 6.92 (3.43) | 9.42 (4.03) | 11.13 (2.67) | H(2)=13.13; P=.001 | |
| NBRS (total pathology score) | 47.23 (22.65) | 35.99 (11.24) | 35.17 (9.35) | H(2)=9.13; P=.009 | |
| FrSBe Apathy (Total score) | 77.31 (20.28) | 60.98 (17.58) | 57.74 (18.30) | H(2)=9.84; P=.007 | |
| FrSBe Disinhibition (Total score) | 71.46 (17.20) | 56.98 (15.77) | 56.09 (15.63) | H(2)=7.97; P=0.019 | |
| FrSBe EF (Total score) | 76.08 (18.73) | 60.99 (16.87) | 60.17 (19.79) | H(2)=7.55; P=.023 | |
| CONTROL MEASURES | |||||
| Post-injury IQ (percentile) | 44.23 (27.08) | 55.87 (25.42) | 72.65 (18.14) | H(2)=12.29; P=.002 | |
| BDI-II (total raw score) | 12.69 (11.76) | 6.83 (6.95) | 9.48 (7.91) | H(2)=4.31; P=.116 | |
| M-PTSD (total score) | 87.00 (21.04) | 77.23 (22.34) | 80.57 (22.62) | H(2)=3.57; P=.168 | |
| BNT (total score) | 49.15 (13.74) | 53.58 (6.30) | 55.91 (3.87) | H(2)=3.65; P=.161 | |
| WMS (delay Memory scaled score) | 93.23 (21.32) | 101.28 (16.25) | 106.87 (17.04) | H(2)=5.12; P=.077 | |
| VOSP (average percentage) | 81.89 (10.11) | 84.83 (10.01) | 89.26 (4.19) | H(2)=5.37; P=.068 | |
Bold statistics are significant. TBI-T, TBI target; TBI-C, TBI control; HCv, Healthy Control veterans; IQ, Intelligence quotient; FSQ, Functional Status Questionnaire; ZBI, Zarit Burden Interview; FAS, Verbal Fluency (letter F, A, S); TMT, Trail making test; NBRS, Neurobehavioral Rating Scale; FrSBe, Frontal System Behavioral Scale; EF, Executive function; BDI-II, Beck Depression Inventory; M-PTSD, Mississippi – Post-traumatic stress disorder scale; BNT, Boston Naming test; WMS, Wechsler Memory scale abbreviated; VOSP, Visual Object and Space Perception battery.
Non-parametric analyses (Kruskal-Wallis H test) demonstrated that groups differed significantly on EF measures (FAS, TMT, NBRS, FrSBe apathy, FrSBe disinhibtion, FrSBe EFs), memory (WMS), and post-injury IQ (AFQT total score) (Tab. 2). Follow-up planned non-parametric analyses (Mann-Whitney U tests, Bonferroni corrected) between lesion groups revealed that the TBI-T participants performed significantly worse than those in the TBI-C group on verbal fluency (FAS: Z=−2.85, P<0.012), mental flexibility (TMT-S: Z=−2.72, P<0.021), caregiver assessment (FrSBe apathy: Z=−2.82; P=0.015, FrSBe disinhibition Z=−2.79; P=0.015, FrSBe EFs: Z=−2.67; P=0.024), and examiner assessment (NBRS: Z=−3.00, P<0.009), but not on memory (WMS: Z=−1.72, P=0.258), a control task (TMT-C: Z=−1.65, P=0.100), or post-injury IQ (AFQT total score: Z=−1.98, P=0.141). Comparing the TBI-T group with the HCv group, non-parametric tests (Mann-Whitney U tests, Bonferroni corrected) demonstrated that the TBI-T group performed significantly worse on EF —verbal fluency (FAS: Z=−3.44, P<0.005), mental flexibility (TMT-S: Z=−3.83, P<0.001), caregiver assessments (FrSBe apathy: Z=−2.90; P=0.009, FrSBe disinhibition: Z=−2.34; P=0.048, FrSBe EF: Z=−2.34; P=0.048), and examiner assessment (NBRS: Z=−2.51, P<0.05)— but also on post-injury IQ (AFQT total score: Z=−3.20; P<0.001). TBI-T and HCv did not differ significantly on memory (WMS: Z=−1.87, P=0.195).
DISCUSSION
Our study investigated the association between brain lesion location in penetrating TBI and long-term perceived burden in caregivers as it related to dysexecutive syndrome in participants with TBI. Our first goal was to assess caregiver burden in a cohort of participants with TBI who sustained their injury 40 years earlier. As predicted, we found that burden was significantly higher in caregivers of participants with TBI than in caregivers of matched healthy controls. Our reported measure of burden was lower than previously reported in studies with severe TBI,[7, 55] probably due to the time since injury in our investigation. Previous studies focused on a time frame ranging from six months to five years post-injury and demonstrated that caregiver burden is already substantially decreased from six months to one year post-injury.[2, 12] While our study involved a cross-sectional sample approximately 40 years after injury, those in the TBI group still exhibited cognitive deficits, and their caregivers demonstrated greater burden compared to the control group.
Our second goal was to identify any relation between TBI-related brain lesions and long-term caregiver burden. As hypothesized, we found that long-term caregiver burden was associated with impaired EF from lesions to the left dACC and dlPFC, two highly interconnected key regions involved in executive control and decision-making, particularly in novel situations, as well as monitoring of performance and error detection, respectively.[26, 56–59] Participants with lesions in these areas demonstrated deficits in cognitive and behavioral indicators associated with dysexecutive syndrome but not in other control measures (i.e., language abilities, space and object perception, memory, depression, post-traumatic stress). These participants were impaired on EF laboratory tasks such as verbal fluency and mental flexibility. Given that verbal fluency requires inhibition of inappropriate responses, error detection, and monitoring of conflicting responses[56, 60], previous imaging studies demonstrated consistent activation in the left dACC and dlPFC for this task in healthy controls.[56, 60–62] Furthermore, although bilateral PFC has been implicated in mental flexibility, some evidence points to a more dominant role of the left PFC (i.e., dlPFC)[58, 63] and functionally connected regions such as ACC in mental flexibility.[64]
Moreover, these participants also showed deficits in behaviors associated with dysexecutive syndrome reported by the caregiver (evaluation of apathy, disinhibition, and EFs in daily life) and the examiner (evaluation of cognitive and affective aspects of EFs).
We found that participants with a TBI in the left dlPFC and dACC have greater brain volume loss than in those with TBI without a lesion in these areas. This can be explained by the location of the ACC deep in the brain; since they were all penetrating brain injuries, in order to reach a midline structure like the ACC, the lesion needed to penetrate deeply, hence affecting on average more cortical tissue than a lesion restricted to the lateral surface of the cortex. However, further correlation analysis between brain volume loss and caregiver burden did not show an association.
We argue that the lesion localization we identified has clinical significance. Indeed, clinicians should be aware that a caregiver whose spouse/offspring/parent has a left dACC/dlPFC lesion is at higher risk of feeling burdened and developing mental health issues. This specific risk may relate not only to caregivers of TBI patients, but possibly also caregivers of patients affected by any disease involving dysfunctional left dACC/dlPFC, such as some forms of multiple sclerosis, stroke, fronto-temporal or vascular dementias. Paying special attention to caregivers of these patients is important not only at the individual level but also at the societal level, since the health care system does not offer comprehensive services and private caregivers often serve a complementary role in providing such care. Social and instrumental support reduce caregiver burden. Psychological therapy that teaches coping strategies would be beneficial for reducing caregiver burden. Providing caregivers with information on TBI (including expectations for progression) is also recommended so that they can develop an appropriate care plan[16, 55].
It is important to take care of caregivers of individuals suffering from TBI in the left dACC/dlPFC brain, probably associated with dysexecutive syndrome. Nevertheless, one has to be aware that patients’ outcomes are sometimes dissociated from imaging results. For example, in individuals with dementia, it is not rare to notice a double dissociation between brain atrophy and cognitive outcomes, and even more specifically, autonomy in daily life.[65, 66]
Our study had some limitations. Given the homogeneity of our all male TBI sample (uniqueness of the injury, time frame since the injury) and the relatively few subjects with a left dACC/dlPFC lesion, path analysis was not a suitable option. An interesting study direction would be to select a larger group of participants with left dACC/dlPFC lesions and run path analyses to replicate our work and better determine the links among brain lesion, executive functions and caregiver burden. CT scans were used as an imaging technique in this study rather than more optimal techniques such as magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI). Since penetrating injuries often resulted in the participant retaining metallic fragments or shrapnel at the site of injury, MRI scans were not feasible. The use of CT scans may have limited our ability to see more detailed structures within the brain, particularly fiber tracks and brain connectivity that can be demonstrated with DTI techniques. Note that there was approximately a 6-year gap between the acquisition of CT scans and of the administration of the cognitive measures used in this study. However, clinical evaluation of the Phase 4 CT scans done currently with the cognitive measures did not reveal any additional pathology besides the pTBI. Nonetheless, we can’t exclude additional incidental, non-traumatic and ischemic lesions in the white matter due to aging that may have been detected had the participants received MRI scans more recently.
In conclusion, we showed that some caregivers of participants with TBI still perceived burden 40 years after injury. We also showed an association between brain lesion location and long-term caregiver burden likely due to the cognitive and behavioral factors associated with dysexecutive syndrome.
Footnotes
Conflicts of Interest and Source of Funding: None declared
References
- 1.Taylor HG, et al. Bidirectional child-family influences on outcomes of traumatic brain injury in children. J Int Neuropsychol Soc. 2001;7(6):755–767. doi: 10.1017/s1355617701766118. [DOI] [PubMed] [Google Scholar]
- 2.Marsh NV, et al. Caregiver burden during the year following severe traumatic brain injury. J Clin Exp Neuropsychol. 2002;24(4):434–447. doi: 10.1076/jcen.24.4.434.1030. [DOI] [PubMed] [Google Scholar]
- 3.Draper K, Ponsford J. Long-term outcome following traumatic brain injury: A comparison of subjective reports by those injured and their relatives. Neuropsychological Rehabilitation. 2009;19(5):645–661. doi: 10.1080/17405620802613935. [DOI] [PubMed] [Google Scholar]
- 4.Draper K, Ponsford J, Schonberger M. Psychosocial and emotional outcomes 10 years following traumatic brain injury. J Head Trauma Rehabil. 2007;22(5):278–287. doi: 10.1097/01.HTR.0000290972.63753.a7. [DOI] [PubMed] [Google Scholar]
- 5.Feinberg L, et al. Insight on the issues 51. Washington, DC: AARP Public Policy Institute; 2011. Valuing the Invaluable: 2011 Update. The growing contributions and costs of family caregiving. [Google Scholar]
- 6.Brioschi Guevara A, et al. Association Between Long-Term Cognitive Decline in Vietnam Veterans With TBI and Caregiver Attachment Style. J Head Trauma Rehabil. 2014 doi: 10.1097/HTR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayen E, et al. Predictors of Informal Care Burden 1 Year After a Severe Traumatic Brain Injury: Results From the PariS-TBI study. J Head Trauma Rehabil. 2012 doi: 10.1097/HTR.0b013e31825413cf. [DOI] [PubMed] [Google Scholar]
- 8.Leggett AN, et al. Stress and Burden Among Caregivers of Patients with Lewy Body Dementia. Gerontologist. 2011;51(1):76–85. doi: 10.1093/geront/gnq055. [DOI] [PubMed] [Google Scholar]
- 9.Moules S, Chandler BJ. A study of the health and social needs of carers of traumatically brain injured individuals served by one community rehabilitation team. Brain Inj. 1999;13(12):983–993. doi: 10.1080/026990599120990. [DOI] [PubMed] [Google Scholar]
- 10.Kolakowsky-Hayner SA, Miner KD, Kreutzer JS. Long-term life quality and family needs after traumatic brain injury. J Head Trauma Rehabil. 2001;16(4):374–385. doi: 10.1097/00001199-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Livingston LA, et al. Predictors of family caregivers' life satisfaction after traumatic brain injury at one and two years post-injury: A longitudinal multi-center investigation. NeuroRehabilitation. 2010;27(1):73–81. doi: 10.3233/NRE-2010-0582. [DOI] [PubMed] [Google Scholar]
- 12.Ponsford J, Schonberger M. Family functioning and emotional state two and five years after traumatic brain injury. Journal of the International Neuropsychological Society. 2010;16(2):306–317. doi: 10.1017/S1355617709991342. [DOI] [PubMed] [Google Scholar]
- 13.Marsh NV, et al. Caregiver burden at 1 year following severe traumatic brain injury. Brain Injury. 1998;12(12):1045–1059. doi: 10.1080/026990598121954. [DOI] [PubMed] [Google Scholar]
- 14.Rivera P, et al. Predictors of caregiver depression among community-residing families living with traumatic brain injury. NeuroRehabilitation. 2007;22(1):3–8. [PubMed] [Google Scholar]
- 15.Knutson KM, et al. Neural correlates of caregiver burden in cortical basal syndrome and frontotemporal dementia. Dement Geriatr Cogn Disord. 2008;26(5):467–474. doi: 10.1159/000167268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson D, et al. Acquired brain injury and dementia: a comparison of carer experiences. Brain Inj. 2009;23(5):433–44. doi: 10.1080/02699050902788451. [DOI] [PubMed] [Google Scholar]
- 17.Nonterah CW, et al. The influence of TBI impairments on family caregiver mental health in Mexico. Brain Inj. 2013;27(11):1287–1293. doi: 10.3109/02699052.2013.812243. [DOI] [PubMed] [Google Scholar]
- 18.Papastavrou E, et al. Caring and coping: the dementia caregivers. Aging Ment Health. 2011;15(6):702–711. doi: 10.1080/13607863.2011.562178. [DOI] [PubMed] [Google Scholar]
- 19.Phelan SM, et al. Perceived stigma, strain, and mental health among caregivers of veterans with traumatic brain injury. Disabil Health J. 2011;4(3):177–184. doi: 10.1016/j.dhjo.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Anderson MI, Parmenter TR, Mok M. The relationship between neurobehavioural problems of severe traumatic brain injury (TBI), family functioning and the psychological well-being of the spouse/caregiver: path model analysis. Brain Inj. 2002;16(9):743–757. doi: 10.1080/02699050210128906. [DOI] [PubMed] [Google Scholar]
- 21.Cosseddu M, et al. The other face of the coin: the caregiver burden in frontotemporal lobar degeneration. International Journal of Geriatric Psychiatry. 2013;28(6):655–657. doi: 10.1002/gps.3892. [DOI] [PubMed] [Google Scholar]
- 22.Godefroy O, et al. Dysexecutive syndrome: diagnostic criteria and validation study. Ann Neurol. 2010;68(6):855–864. doi: 10.1002/ana.22117. [DOI] [PubMed] [Google Scholar]
- 23.de Vugt ME, et al. Impact of behavioural problems on spousal caregivers: a comparison between Alzheimer's disease and frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;22(1):35–41. doi: 10.1159/000093102. [DOI] [PubMed] [Google Scholar]
- 24.Su YL, et al. The effects of morphine on basal neuronal activities in the lateral and medial pain pathways. Neurosci Lett. 2012;525(2):173–178. doi: 10.1016/j.neulet.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16(1):17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 26.Hagen K, et al. Activation during the Trail Making Test measured with functional near-infrared spectroscopy in healthy elderly subjects. Neuroimage. 2014;85 Pt 1:583–591. doi: 10.1016/j.neuroimage.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neurosci Biobehav Rev. 2014;42C:180–192. doi: 10.1016/j.neubiorev.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchsbaum BR, et al. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25(1):35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laird AR, et al. A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp. 2005;25(1):6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson SC, et al. An fMRI investigation of a novel analogue to the Trail-Making Test. Brain Cogn. 2011;77(1):60–70. doi: 10.1016/j.bandc.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Merkley TL, et al. Structural and functional changes of the cingulate gyrus following traumatic brain injury: relation to attention and executive skills. J Int Neuropsychol Soc. 2013;19(8):899–910. doi: 10.1017/S135561771300074X. [DOI] [PubMed] [Google Scholar]
- 32.Spitz G, et al. Regional cortical volume and cognitive functioning following traumatic brain injury. Brain Cogn. 2013;83(1):34–44. doi: 10.1016/j.bandc.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 34.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 35.Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29(4):1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mars RB, et al. Neural Basis of Motivational and Cognitive Control. Boston, MA: Massachusetts Institute of Technology; 2011. [Google Scholar]
- 37.Raymont V, et al. “Studying injured minds”–theVietnam head injury study and 40 years of brain injury research. Frontiers in Neurotrauma. 2011;2:1–15. doi: 10.3389/fneur.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makale M, et al. Quantification of brain lesions using interactive automated software. Behav Res Methods Instrum Comput. 2002;34(1):6–18. doi: 10.3758/bf03195419. [DOI] [PubMed] [Google Scholar]
- 39.Solomon J, et al. User-friendly software for the analysis of brain lesions (ABLe) Comput Methods Programs Biomed. 2007;86(3):245–254. doi: 10.1016/j.cmpb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 41.Zarit SH, Todd PA, Zarit JM. Subjective burden of husbands and wives as caregivers: a longitudinal study. Gerontologist. 1986;26(3):260–266. doi: 10.1093/geront/26.3.260. [DOI] [PubMed] [Google Scholar]
- 42.Schreiner AS, et al. Assessing family caregiver's mental health using a statistically derived cut-off score for the Zarit Burden Interview. Aging Ment Health. 2006;10(2):107–111. doi: 10.1080/13607860500312142. [DOI] [PubMed] [Google Scholar]
- 43.Delis DC, Kaplan AS, Kramer A. Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological corporation; 2001. [Google Scholar]
- 44.Grace J, Malloy P. Frontal System Behavior Scale. Lutz, FL: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- 45.Levin HS, et al. The neurobehavioural rating scale: assessment of the behavioural sequelae of head injury by the clinician. J Neurol Neurosurg Psychiatry. 1987;50(2):183–193. doi: 10.1136/jnnp.50.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grafman J, et al. Intellectual function following penetrating head injury in Vietnam veterans. Brain. 1988;111(Pt 1):169–184. doi: 10.1093/brain/111.1.169. [DOI] [PubMed] [Google Scholar]
- 47.U.S, D.o.D.A test manual for the Armed Services Vocational Aptitude Battery 1984Chicago, IL: U.S. Military Entrance Processing Command [Google Scholar]
- 48.Kaplan E, Goodglass H, Weintraub S. Boston naming test. 2nd ed. Philadelphia PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 49.Wechsler D. Wechsler Memory Scale. San Antonio, TX: the Psychological Corporation; 1997. [Google Scholar]
- 50.Warrington EK, James M. Visual Object and Space Perception Battery (VOSP) Suffolk, England: Thames Valley Test Company; 1991. [Google Scholar]
- 51.Schintu S, et al. Object and space perception- Is it a matter of hemisphere. Cortex. 2014 doi: 10.1016/j.cortex.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Beck AT, et al. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 53.Keane TM, Caddell JM, Taylor KL. Mississippi Scale for Combat-Related Posttraumatic Stress Disorder: three studies in reliability and validity. J Consult Clin Psychol. 1988;56(1):85–90. doi: 10.1037//0022-006x.56.1.85. [DOI] [PubMed] [Google Scholar]
- 54.Jette AM, et al. The Functional Status Questionnaire: reliability and validity when used in primary care. J Gen Intern Med. 1986;1(3):143–149. doi: 10.1007/BF02602324. [DOI] [PubMed] [Google Scholar]
- 55.Doyle ST, et al. Connecting family needs and TBI caregiver mental health in Mexico City, Mexico. Brain Inj. 2013 doi: 10.3109/02699052.2013.826505. [DOI] [PubMed] [Google Scholar]
- 56.Senhorini MC, et al. Brain activity patterns during phonological verbal fluency performance with varying levels of difficulty: a functional magnetic resonance imaging study in Portuguese-speaking healthy individuals. J Clin Exp Neuropsychol. 2011;33(8):864–873. doi: 10.1080/13803395.2011.561299. [DOI] [PubMed] [Google Scholar]
- 57.Badre D. Opening the gate to working memory. Proc Natl Acad Sci U S A. 2012;109(49):19878–19879. doi: 10.1073/pnas.1216902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the Trail Making Test. Neuropsychologia. 2005;43(13):1878–1886. doi: 10.1016/j.neuropsychologia.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 59.Pa J, et al. Gray matter correlates of set-shifting among neurodegenerative disease, mild cognitive impairment, and healthy older adults. J Int Neuropsychol Soc. 2010;16(4):640–650. doi: 10.1017/S1355617710000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Audenaert K, et al. Verbal fluency as a prefrontal activation probe: a validation study using 99mTc-ECD brain SPET. Eur J Nucl Med. 2000;27(12):1800–1808. doi: 10.1007/s002590000351. [DOI] [PubMed] [Google Scholar]
- 61.Barch DM, et al. Anterior cingulate and the monitoriing of response conflict: evidence from an fMRI study of overt verb generation. J Cogn Neurosci. 2000;12(2):298–309. doi: 10.1162/089892900562110. [DOI] [PubMed] [Google Scholar]
- 62.Gauthier CT, et al. Sex and performance level effects on brain activation during a verbal fluency task: a functional magnetic resonance imaging study. Cortex. 2009;45(2):164–176. doi: 10.1016/j.cortex.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Kramer JH, et al. Magnetic resonance imaging correlates of set shifting. J Int Neuropsychol Soc. 2007;13(3):386–392. doi: 10.1017/S1355617707070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shafritz KM, Kartheiser P, Belger A. Dissociation of neural systems mediating shifts in behavioral response and cognitive set. Neuroimage. 2005;25(2):600–606. doi: 10.1016/j.neuroimage.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 65.Scarmeas N, Stern Y. Cognitive reserve: implications for diagnosis and prevention of Alzheimer's disease. Curr Neurol Neurosci Rep. 2004;4(5):374–380. doi: 10.1007/s11910-004-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stern Y. Cognitive reserve: implications for assessment and intervention. Folia Phoniatr Logop. 2013;65(2):49–54. doi: 10.1159/000353443. [DOI] [PMC free article] [PubMed] [Google Scholar]


