Abstract
Objective
We examined whether nonadherence to hydroxychloroquine (HCQ) or immunosuppressive medications (IS) was associated with higher subsequent acute care utilization among Medicaid beneficiaries with systemic lupus erythematosus (SLE).
Methods
We utilized U.S. Medicaid data from 2000–2006 to identify adults 18–64 years with SLE who were new users of HCQ or IS. We defined the index date as receipt of HCQ or IS without use in the prior six months. We measured adherence using the medication possession ratio (MPR), the proportion of days covered by total days supply dispensed, for one-year post-index date. Our outcomes were all-cause and SLE-related emergency department (ED) visits and hospitalizations in the subsequent year. We used multivariable Poisson regression models to examine the association between nonadherence (MPR<80%) and acute care utilization adjusting for sociodemographics and comorbidities.
Results
We identified 9,600 HCQ new users and 3,829 IS new users with SLE. The mean MPR for HCQ was 47.8% (SD 30.3) and for IS, 42.7% (SD 30.7). 79% of HCQ users and 83% of IS users were nonadherent (MPR<80%). In multivariable models, among HCQ users, the incidence rate ratio (IRR) of ED visits was 1.55 (95% CI 1.43–1.69) and the IRR of hospitalizations was 1.37 (95% CI 1.25–1.50), comparing nonadherers to adherers. For IS users, the IRR of ED visits was 1.64 (95% CI 1.42–1.89) and of hospitalizations was 1.67 (95% CI 1.41–1.96) for nonadherers versus adherers.
Conclusion
In this cohort, nonadherence to HCQ and IS was common and was associated with significantly higher subsequent acute care utilization.
Keywords: systemic lupus erythematosus, medication adherence, health services research, disparities, outcome measures, access to care
Medication nonadherence, the failure to take medications as prescribed, is a widespread problem accounting for over $100 billion in preventable healthcare costs in the U.S. annually.(1, 2) Data from systemic lupus erythematosus (SLE) clinical trials demonstrate reduced disease activity and morbidity from use of hydroxychloroquine (HCQ) and immunosuppressive drugs (IS) (3–5). Despite this, studies suggest nonadherence to be a particularly pervasive problem with only 30 to 60 percent of SLE patients taking medications as prescribed.(6–10) Characteristics unique to SLE may render adherence particularly challenging, including frequent disease activity fluctuations, the complexity and toxicity of medication regimens, a high disease burden among lower socioeconomic status groups, and cognitive and psychological manifestations.(8, 10, 11) Adverse outcomes, notably end-stage renal disease, may be more frequent among nonadherent SLE patients.(12, 13)
A number of studies demonstrate high costs of SLE patient care that are in large part due to high health care utilization.(14, 15) Each year, one in four SLE patients are hospitalized, one in six hospitalized patients are readmitted within 30 days of discharge, and one in two SLE patients visit the emergency department (ED).(16–18) Among cardiovascular disease patients, adherence has been shown to significantly impact health care utilization, costs and mortality.(19, 20) In SLE, one cross-sectional study found that patients who reported forgetting to take their medications some of the time had increased odds of ED visits compared to patients who did not report forgetting.(10) A second SLE study demonstrated a potential relationship between poor adherence and increased hospitalizations, however the sample size was small.(21)
To our knowledge, there are no studies to date that examine whether there is a temporal relationship between SLE medication nonadherence and subsequent acute care use. We studied patterns of medication nonadherence in a large, nationwide, racially and ethnically diverse cohort and investigated whether medication nonadherence is associated with high-cost health care utilization. We hypothesized that nonadherers would have a greater number of ED visits and hospitalizations compared to adherers even after adjusting for sociodemographic factors and comorbidities.
Patients and Methods
Patient Population
We utilized the Medicaid Analytic eXtract (MAX), an administrative database that includes billing claims and demographic information for all Medicaid enrollees from 47 states and Washington, D.C. Arizona, Tennessee and Maine do not contribute to MAX. Medicaid is the largest source of health coverage overall in the U.S. and the public health insurance program for low-income individuals and families.(22) It is jointly funded by states and the federal government and covers over 60 million Americans. We included all adults, aged 18–<65 years, continuously enrolled in Medicaid for ≥6 months between January 1, 2000 and December 31, 2006. As more than 90 percent of U.S. adults 65 years and older are enrolled in Medicare, we excluded this group given the potential for incomplete Medicaid claims, and because SLE disproportionately affects younger age groups.(23) We identified all adults with prevalent SLE, defined as ≥3 International Classification of Diseases, ninth revision (ICD-9) codes for SLE (710.0), separated by at least 30 days, from hospital discharge diagnoses or physician visit claims. (24, 25)
Medication New User Identification
In this cohort of SLE patients, we identified new users of an oral immunosuppressive drug (IS) or hydroxychloroquine (HCQ) utilizing the date of first receipt (index date) with no outpatient pharmacy claims for the drug of interest during the prior 6 months of continuous enrollment (Figure 1). We restricted our analyses to incident users in order to minimize differences that may be observed between long-time users of medications and initiators.(26) An incident user cohort also allows for clearly defined temporality of baseline characteristics, medication use and outcomes reducing the potential for reverse causation. Of the new users identified, we included those with ≥2 years of follow-up after the index date. The first 365-day period following the index date was the adherence assessment period and the second 365-day period was the outcome (acute care utilization) assessment period.
Figure 1.
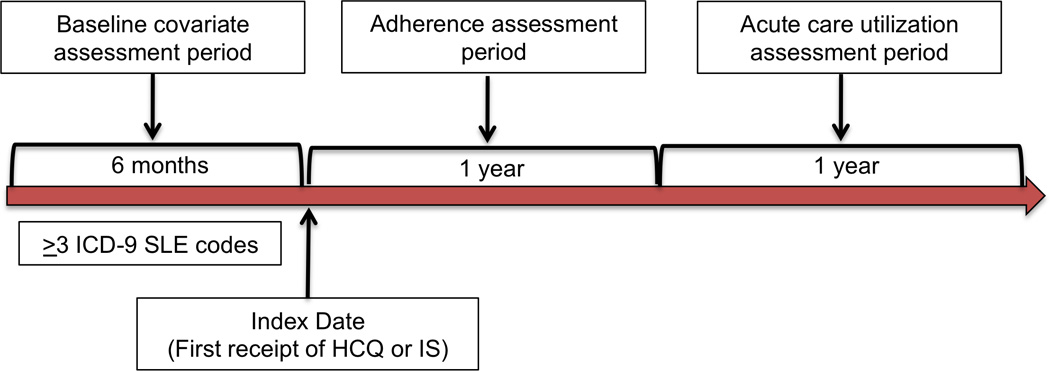
New users of hydroxychloroquine (HCQ) or immunosuppressive medications (IS) were identified among SLE patients with six months of prior continuous enrollment in Medicaid and no use of these drugs during that time. Adherence was assessed using the Medication Possession Ratio (MPR) during the year following the index date and the outcome (acute care utilization) was measured during the subsequent year.
Oral IS included mycophenolate mofetil, mycophenolic acid, azathioprine, leflunomide, methotrexate, tacrolimus and oral cyclophosphamide. We restricted our analysis to oral immunosuppressives for a number of reasons. First, a limitation of Medicaid claims data is that inpatient intravenous medications are billed differently depending on the state and thus we might not be able to account for all infusions given during hospitalization. Second, while there is a standard of care associated with dosing schedules of oral medications for SLE, protocols for intravenous therapy, for example cyclophosphamide, differ and therefore adherence assessment using pharmacy refill claims data becomes challenging. Studies in rheumatoid arthritis have assigned days’ supply of medications such as infliximab based on a recommended dosing schedule.(27) However for SLE patients, recommended dosing schedules for cyclophosphamide and rituximab vary significantly and therefore we would not be able to follow this strategy without biasing our adherence estimate. Third, we hypothesize that adherence to oral medications may be influenced by different factors than adherence to hospital-based or outpatient appointments for intravenous therapy. Issues such as lack of transportation, inability to miss work, or lack of childcare, may be obstacles specific to adherence to intravenous therapy that are especially relevant in this low-income vulnerable population. Due to the use of claims data, we would not have been able to appropriately incorporate these factors in our analyses.
Medication Adherence: Medication Possession Ratio
We used prescription refill claims to assess medication adherence for a one-year period beginning at the index date. We calculated the mean medication possession ratio (MPR) separately for HCQ and IS. We defined MPR as the total medication days, according to the prescription fill date and the days supplied, divided by the 365-day adherence assessment period beginning on the index date (date of first prescription). We excluded any hospitalization days from both the numerator and the denominator. We dichotomized the MPR as <80% and ≥80% (28) and MPR≥80% was considered adherent.(29) This standard, dichotomized measure has been used to assess medication adherence among Medicaid beneficiaries in prior studies.(20, 30) We conducted secondary analyses utilizing three MPR thresholds, 0–49%, 50–79% and ≥80%, to determine whether there was a relationship between degree of nonadherence and the outcomes of interest. These cutoffs have also been previously used to evaluate adherence behavior among Medicaid beneficiaries.(31, 32)
Outcome: Acute Care Utilization
We assessed our primary outcome, acute care utilization, by examining the number of ED visits and the number of hospitalizations during the one-year follow-up period (Figure 1). We assessed all-cause hospitalizations and ED visits, and SLE-related ED visits and hospitalizations utilizing SLE discharge diagnosis codes (ICD-9 710.0).
Covariates
We collected all covariates during the six months of continuous enrollment prior to the index date (Figure 1). We determined age at the index date, sex, U.S. geographic region (Northeast, Midwest, South, West) and race/ethnicity. We used Medicaid’s categorizations of race/ethnicity based on self-report. Due to small numbers that would prevent reporting in accordance with Centers for Medicare and Medicaid Services (CMS) policies, we described combined categories: White, Black or African American, Hispanic or Latino (including Hispanic or Latino and one or more races), Asian (including Native Hawaiian or other Pacific Islander), Native American (including American Indian or Alaskan Native) and Other (including unknown).(33)
Area-level socioeconomic status (SES) was determined using a previously validated composite score of seven U.S. Census variables: median household income, proportion with income below 200% of federal poverty level, median home value, median monthly rent, mean education level, proportion of people age ≥25 who were college graduates, and proportion of employed persons with a professional occupation.(34) We obtained U.S. Census data by ZIP code for each patient and aggregated this to the county level and then divided it into binary categories of higher versus lower area-level SES.
We used a previously developed SLE-specific risk adjustment index to characterize comorbidities. This index utilizes ICD-9 codes for comorbidities particularly relevant to SLE patients and was shown to account for more variation in the risk of mortality among SLE patients than the traditionally used Charlson comorbidity index.(35) We used the median score in our population to divide patients into higher or lower risk categories. We also determined the number of different medication prescriptions and overall health care utilization (number of outpatient visits, hospitalizations and ED visits) during the six month baseline period.
Statistical Analysis
We used Chi-square tests and Student’s t-tests to compare sociodemographic characteristics of adherent (MPR≥80%) and nonadherent (MPR<80%) patients with SLE, separately for HCQ and IS. We determined the number of HCQ and IS users with zero, one or two or more hospitalizations or ED visits, all-cause and SLE-related, during the follow-up period, and compared them by prior adherence category using Chi-square tests. To assess the association between nonadherence (MPR<80% versus MPR ≥80%) and acute care utilization, we used multivariable Poisson regression adjusting for calendar year, sociodemographic factors (age, gender, race/ethnicity, region and SES), comorbidities using the SLE risk adjustment index, and baseline number of medications. We also examined models that additionally adjusted for healthcare utilization during the baseline period. We conducted secondary analyses utilizing three categories for the MPR (0– 49%, 50–79% and ≥80%) in multivariable Poisson regression models to determine whether degree of nonadherence behavior was associated with acute care utilization. The objective of these analyses was to investigate whether there was a temporal association between adherence behavior, as measured by the MPR, and acute care utilization, not to infer causality.
In sensitivity analyses, we utilized a six-month period for adherence assessment, rather than the one-year period we used in our primary analysis, with a subsequent one-year period to ascertain acute care utilization. We chose to do this because immunosuppressive medications, particularly for lupus nephritis, may be prescribed as induction therapy for a six-month period and we hoped to differentiate between medication discontinuation and nonadherence.
All analyses were conducted using SAS, Version 9.3 (Cary, NC). Data were obtained from the Centers for Medicare and Medicaid Services through an approved data use agreement. Results are presented in accordance with their policies; cell sizes <11 are suppressed. The Partners Healthcare Institutional Review Board approved this study.
Results
Hydroxychloroquine and Immunosuppressive New User Characteristics
We identified 9,600 patients with prevalent SLE who filled a new prescription for HCQ. Their mean age was 39.8 years (SD 11.4), 9,150 (95.3%) were female, 3,866 (40.3%) were Black, 3,190 (33.2%) were White, and 1,492 (15.5%) were Hispanic, 454 (4.7%) were Asian (Table 1). Among the new HCQ users, 69.2% had low SLE risk-adjustment index scores. The mean number of medications at baseline was 11.9 (SD 8.6). The mean MPR for HCQ use was 47.8% (SD 30.3); 2,048 (21.3%) were adherent with MPR ≥80% and 7,552 (78.7%) were nonadherent with MPR<80%.
Table 1.
Baseline characteristics of SLE new users of hydroxychloroquine (HCQ) and immunosuppressive drugs (IS)
| Characteristics | HCQ New Users N=9600 |
IS New Users N=3829 |
|---|---|---|
| Years of follow-up – Mean (SD) | 4.0 (1.3) | 3.6 (1.2) |
| Age – Mean (SD) | 39.8 (11.4) | 38.7 (11.7) |
| Age group – N (%) | ||
| 18–30 years | 2429 (25.3) | 1146 (29.9) |
| 31–45 years | 4111 (42.8) | 1552 (40.5) |
| 46–64 years | 3060 (31.9) | 1131 (29.5) |
| Gender – N (%) | ||
| Female | 9150 (95.3) | 3638 (95.0) |
| Male | 50 (4.7) | 191 (5.0) |
| Race/Ethnicity – N (%) | ||
| White | 3190 (33.2) | 1127 (29.4) |
| Black | 3866 (40.3) | 1555 (40.6) |
| Hispanic | 1492 (15.5) | 691 (18.1) |
| Asian | 454 (4.7) | 212 (5.5) |
| Native American | 126 (1.3) | 63 (1.7) |
| Other | 472 (4.9) | 181 (4.7) |
| Geographic Region – N (%) | ||
| Midwest | 1718 (17.9) | 704 (18.4) |
| Northeast | 2372 (24.7) | 921 (24.1) |
| South | 3265 (34.0) | 1206 (31.5) |
| West | 2245 (23.4) | 998 (26.1) |
| Socioeconomic Status | ||
| Mean (SD) | 1.2 (1.8) | 1.3 (1.8) |
| Median | 1.1 | 1.2 |
| Lower | 4834 (50.4) | 1922 (50.2) |
| Higher | 4766 (49.7) | 1907 (49.8) |
| SLE Risk Adjustment Index | ||
| Mean (SD) | 1.0 (1.8) | 1.3 (2.1) |
| Low | 6643 (69.2) | 2283 (59.6) |
| High | 2957 (30.8) | 1546 (40.4) |
| Number of Medications – Mean (SD) | 11.9 (8.6) | 13.3 (9.5) |
| Medication Possession Ratio (MPR) | ||
| Mean % (SD) | 47.8 (30.3) | 42.7 (30.4) |
| MPR ≥80% (Adherent) | 2048 (21.3) | 651 (17) |
| MPR <80% (Nonadherent) | 7552 (78.7) | 3178 (83) |
| Baseline Health Care Utilization – Mean (SD) | ||
| Emergency Department Visits | 1.6 (3.5) | 1.8 (3.7) |
| Inpatient Visits | 0.6 (1.5) | 0.78 (1.8) |
| Outpatient Visits | 9.0 (9.0) | 10.3 (9.9) |
There were 3,829 new users of IS drugs with a mean follow-up time of 3.6 years (SD 1.2) (Table 1). The mean age was 38.7 years (SD 11.7), 95% were female, 29.4% were White, and 40.6% were Black. The mean SLE risk-adjustment index was 1.3 (SD 2.1) and the mean number of baseline medications was 13.3 (SD 9.5). The mean MPR was 42.7% (SD 30.4) and 3,178 (83%) were nonadherent.
HCQ nonadherers were younger, with a mean age of 39 (SD 11.3) years compared to the mean age of 42.8 (SD 11.3) years among the adherers (p<0.0001) (Table 2). A greater percentage of nonadherers were Black (p<0.0001), and Hispanic (p<0.0001), and fewer were White (p<0.0001). Nonadherers also had a lower mean SLE-risk adjustment index (p=0.05), and on average, were taking fewer medications at baseline compared to adherers (p<0.0001).
Table 2.
Characteristics of SLE Hydroxychloroquine and Immunosuppressive New Users by Adherers (MPR≥80%) versus Nonadherers (MPR<80%)
| Hydroxychloroquine New Users | Immunosuppressive New Users | |||||
|---|---|---|---|---|---|---|
| MPR≥80% (Adherers) N=2048 |
MPR<80% (Nonadherers) N=7552 |
p-value | MPR≥80% (Adherers) N=651 |
MPR<80% (Nonadherers) N=3178 |
p-value | |
| Age – Mean (SD) | 42.8 (11.3) | 39.0 (11.3) | <0.0001 | 40.3 (11.8) | 38.3 (11.7) | <0.0001 |
| Age group – N (%) | ||||||
| 18–30 years | 346 (16.9) | 2083 (27.6) | <0.0001 | 165 (25.4) | 981 (30.9) | 0.005 |
| 31–45 years | 863 (42.1) | 3248 (43.0) | 0.48 | 255 (39.2) | 1297 (40.8) | 0.43 |
| 46–64 years | 839 (41.1) | 2221 (29.4) | <0.0001 | 231 (35.5) | 900 (28.3) | 0.0003 |
| Gender – N (%) | ||||||
| Female | 1934 (94.4) | 7216 (95.6) | 0.03 | 614 (94.3) | 3024 (95.2) | 0.37 |
| Male | 114 (5.6) | 336 (4.5) | 37 (5.7) | 154 (4.9) | ||
| Race/Ethnicity – N (%) | ||||||
| White | 912 (44.5) | 2278 (30.1) | <0.0001 | 208 (32.0) | 919 (28.9) | 0.12 |
| Black | 620 (30.3) | 3246 (43.0) | <0.0001 | 217 (33.3) | 1338 (42.1) | <0.0001 |
| Hispanic | 240 (11.7) | 1252 (16.6) | <0.0001 | 135 (20.7) | 556 (17.5) | 0.05 |
| Asian | 112 (5.5) | 342 (4.5) | 0.07 | 38 (5.8) | 174 (5.5) | 0.71 |
| Native American | 26 (1.3) | 100 (1.3) | 0.84 | 11 (1.7) | 52 (1.6) | 0.92 |
| Other | 138 (6.7) | 334 (4.4) | <0.0001 | 42 (6.5) | 139 (4.4) | 0.02 |
| Geographic Region – N (%) | ||||||
| Midwest | 295 (14.4) | 1423 (18.8) | <0.0001 | 84 (12.9) | 620 (19.5) | <0.0001 |
| Northeast | 593 (29.0) | 1779 (23.6) | 0.41 | 212 (32.6) | 709 (22.3) | <0.0001 |
| South | 681 (33.3) | 2584 (34.2) | 0.99 | 178 (27.3) | 1028 (32.4) | 0.01 |
| West | 479 (23.4) | 1776 (23.4) | <0.0001 | 177 (27.2) | 821 (25.8) | 0.47 |
| Socioeconomic Status – Mean (SD) | 1.14 (1.86) | 1.25 (1.77) | 0.01 | 1.56 (1.82) | 1.29 (1.80) | 0.0005 |
| SLE Risk Adjustment Index – Mean (SD) | 1.05 (1.91) | 0.96 (1.83) | 0.05 | 1.53 (2.33) | 1.27 (2.05) | 0.004 |
| Medications- Mean (SD) | 14.05 (8.69) | 11.26 (8.52) | <0.0001 | 14.78 (9.07) | 13.03 (9.51) | <0.0001 |
Similarly, IS nonadherers were younger on average than adherers, 40.3 years (SD 11.8) compared to 38.3 years (SD 11.7) (p<0.0001) (Table 2). A greater percentage of nonadherers were Black compared to adherers (p<0.0001). Nonadherers had a lower mean socioeconomic status score (p=0.0005), a lower mean SLE risk-adjustment index (p=0.004) and, on average, fewer baseline medications compared to nonadherers (p<0.0001).
Acute Care Utilization
During the follow-up period, 2,375 (31.5%) HCQ nonadherers had two or more all-cause ED visits (mean number of visits 1.70, SD 3.72), compared to 461 (22.5%) adherers (mean 1.13, SD 2.71) (Table 3). Among IS nonadherers, 1,110 (34.9%) had two or more all-cause ED visits (mean 1.94, SD 3.89) compared to 164 (25.2%) adherers (mean 1.15, SD 2.06). In terms of SLE-related visits, 7.4% of HCQ nonadherers had two or more visits compared to 4.3% of adherers. Among IS users, 9.1% of nonadherers had two or more visits that were SLE-related compared to 6.5% of adherers. In addition, 13.7% of HCQ nonadherers had two or more all-cause hospitalizations compared to 10.4% of adherers. Among IS nonadherers, 17.4% had two or more all-cause hospitalizations (mean 0.84, SD 1.88), compared to 12.8% of adherers (mean 0.52, SD 1.17).
Table 3.
Acute care utilization among SLE hydroxychloroquine (HCQ) and immunosuppressive (IS) new users by adherers (MPR≥80%) versus nonadherers (MPR<80%) during follow-up year
| Number of Visits |
Emergency Department Visits |
Hospitalizations | |||
|---|---|---|---|---|---|
| All-cause N (%) |
SLE-related N (%) |
All-cause N (%) |
SLE-related N (%) |
||
| HCQ Adherers N=2048 | 0 | 1188 (58.0) | 1783 (87.1) | 1551 (75.7) | 1674 (81.7) |
| 1 | 399 (19.5) | 177 (8.6) | 284 (13.9) | 227 (11.1) | |
| ≥2 | 461 (22.5) | 88 (4.3) | 213 (10.4) | 147 (7.2) | |
| Mean (SD) | 1.13 (2.71) | 0.21 (0.70) | 0.49 (1.28) | 0.32 (0.92) | |
| HCQ Nonadherers N=7552 | 0 | 3789 (50.1) | 6113 (81.0) | 5378 (71.2) | 5924 (78.4) |
| 1 | 1388 (18.4) | 882 (11.7) | 1143 (15.1) | 952 (12.6) | |
| ≥2 | 2375 (31.5) | 557 (7.4) | 1031 (13.7) | 676 (9.0) | |
| Mean (SD) | 1.70 (3.72) | 0.36 (1.13) | 0.65 (1.61) | 0.42 (1.12) | |
| IS Adherers N=651 | 0 | 367 (56.4) | 551 (84.6) | 471 (72.4) | 514 (79.0) |
| 1 | 120 (18.4) | 58 (8.9) | 97 (14.9) | 88 (13.5) | |
| ≥2 | 164 (25.2) | 42 (6.5) | 83 (12.8) | 49 (7.5) | |
| Mean (SD) | 1.15 (2.06) | 0.25 (0.77) | 0.52 (1.17) | 0.33 (0.77) | |
| IS Nonadherers N=3178 | 0 | 1465 (46.1) | 2465 (77.6) | 2114 (66.5) | 2400 (75.5) |
| 1 | 603 (19.0) | 423 (13.3) | 512 (16.1) | 418 (13.2) | |
| ≥2 | 1110 (34.9) | 290 (9.1) | 552 (17.4) | 360 (11.3) | |
| Mean (SD) | 1.94 (3.89) | 0.46 (1.36) | 0.84 (1.88) | 0.53 (1.36) | |
All p-values <0.05 from Chi-square tests comparing adherers to nonadherers for all-cause and SLE-related emergency department visits and hospitalizations
In our multivariable Poisson regression models, we adjusted for calendar year, age, gender, race/ethnicity, geographic region, socioeconomic status, SLE-risk adjustment index, and baseline number of medications (Table 4). We found statistically significant greater acute care utilization among HCQ and IS nonadherers compared to adherers. Comparing HCQ nonadherers to adherers, the IRR for all-cause ED visits was 1.55 (95% CI 1.43–1.69) and the IRR of SLE-related ED visits was 1.60 (95% CI 1.43–1.80). The IRR of all-cause hospitalizations for HCQ nonadherers compared to adherers was 1.37 (95% CI 1.25–1.50) and for SLE-related hospitalizations, 1.30 (95% CI 1.18–1.44). For IS nonadherers compared to adherers, the IRR of all-cause ED visits was 1.64 (95% CI 1.42–1.89), and of SLE-related was 1.69 (95% CI 1.38–−2.05). In terms of hospitalizations, the IRR for all-cause hospitalizations was 1.67 (95% CI 1.41–1.96) and SLE-related was 1.60 (95% CI 1.34–1.91), comparing IS nonadherers to adherers.
Table 4.
Adjusted incidence rate ratios (IRR) of acute care utilization (emergency department (ED) visits and hospitalizations), comparing nonadherers (MPR<80%) to adherers (MPR ≥80%)
| All-cause | SLE-related | ||||
|---|---|---|---|---|---|
| IRR* | 95% CI | IRR* | 95% CI | ||
| ED Visits | HCQ | 1.55 | 1.43–1.69 | 1.60 | 1.43–1.80 |
| IS | 1.64 | 1.42–1.89 | 1.69 | 1.38–2.05 | |
| Hospitalizations | HCQ | 1.37 | 1.25–1.50 | 1.30 | 1.18–1.44 |
| IS | 1.67 | 1.41–1.96 | 1.60 | 1.34–1.91 | |
Incidence Rate Ratios (IRR) compare nonadherers (medication possession ratio, MPR<80%) to adherers (MPR≥80%) with 95% confidence intervals. Multivariable Poisson regression models adjusted for calendar year, age, gender, race/ethnicity, geographic region, socioeconomic status, SLE risk-adjustment index and baseline number of medications
For both HCQ and IS users, we additionally adjusted for health care utilization (ED visits, hospitalizations and outpatient visits) during the baseline period and similarly found statistically significant greater acute care utilization among nonadherers versus adherers with IRRs in line with our aforementioned results.
Secondary Analyses
In secondary analyses, we divided HCQ and IS nonadherers into two categories, MPR 0–49% and MPR 50–79%, and compared each group to adherers (MPR≥80%) in adjusted Poisson models (Table 5). We found the highest IRRs for all-cause and SLE-related ED visits and hospitalizations both for HCQ and IS users, comparing those with the poorest adherence (MPR 0–49%) to adherers (MPR ≥80%). The IRRs were incrementally lower but still statistically significant for moderate nonadherers (MPR 50–79%) with the exception of the HCQ group for which the IRR of SLE-related hospitalizations was comparable to adherers.
Table 5.
Secondary analysis of the incidence rate ratio (IRR) of emergency department (ED) visits and hospitalizations for hydroxychloroquine (HCQ) and immunosuppressive (IS) nonadherers at two levels (MPR 0–49% and 50–79%) compared to adherers (MPR ≥80%)
| ED Visits IRR* (95% CI) |
Hospitalizations IRR* (95% CI) |
||||
|---|---|---|---|---|---|
| All-Cause | SLE-related | All-Cause | SLE-related | ||
| HCQ | MPR 0–49% | 1.71 (1.57–1.86) | 1.75 (1.56–1.97) | 1.50 (1.36–1.65) | 1.43 (1.29–1.58) |
| MPR 50–79% | 1.26 (1.14–1.39) | 1.31 (1.15–1.50) | 1.13 (1.01–1.26) | 1.07 0.95–1.21) | |
| IS | MPR 0–49% | 1.42 (1.26–1.59) | 1.71 (1.40–2.09) | 1.75 (1.48–2.07) | 1.65 (1.38–1.98) |
| MPR 50–79% | 1.18 (1.04–1.34) | 1.62 (1.30–2.03) | 1.47 (1.21–1.77) | 1.48 (1.20–1.81) | |
Incidence Rate Ratios (IRR) compare each nonadherence category (MPR 0–49% and MPR 50–79%) to adherers (MPR ≥80%) with 95% confidence intervals. Poisson regression models adjusted for calendar year, age, gender, race/ethnicity, geographic region, socioeconomic status, SLE risk-adjustment index and baseline number of medications.
Sensitivity Analysis
We conducted a sensitivity analysis using a six-month period, instead of the one-year period in our primary analysis, to assess adherence (as measured by MPR) and the subsequent one-year to evaluate acute care utilization (Supplemental Table 1). In our fully adjusted model, we found IRRs in line with our primary analysis with statistically significantly greater acute care utilization among nonadherers compared to adherers.
Discussion
In this study, we demonstrated that nonadherence to hydroxychloroquine and to immunosuppressive medications among individuals with SLE was associated with significantly higher acute care utilization in the subsequent year. Nonadherent SLE patients who were new users of HCQ had more than a 55% greater incidence rate of ED visits, and nearly 40% increased rate of hospitalizations compared to adherent patients. New users of IS drugs who were nonadherent also had nearly 65% greater incidence of ED visits and nearly 70% greater incidence of hospitalizations compared to adherent patients. We also demonstrated that individuals with the poorest level of adherence (MPR 0–49%) had the highest rates of acute care utilization, and for all categories except HCQ SLE-related hospitalizations, moderate nonadherence (MPR 50–79%) was also associated with statistically significant increases in utilization.
While this is the first longitudinal study to investigate the relationship between adherence and acute care utilization in SLE patients, our findings are in line with prior cross-sectional studies. One study demonstrated that SLE patients who self-reported difficulty with adherence were 45% more likely to visit the ED.(10) Although this study did not find a difference in hospitalizations between adherent and nonadherent patients, both adherence and health care utilization were self-reported measures. In addition, the prior study was cross-sectional and therefore a temporal relationship could not be investigated. It is also plausible, however, that there is a stronger relationship between adherence and ED visits that may or may not result in hospitalization, compared to planned and direct hospitalizations, for which other factors may dominate. However, one prior small study of 180 patients with SLE who visited an ED over the course of eight months, did find an association between poor adherence and increased hospitalization.(21) In other chronic diseases including diabetes and cardiovascular disease, nonadherence has been associated with increased acute care utilization, specifically increased number of hospitalizations, and with greater net health care costs and higher mortality.(36–41)
In this low-income high-risk cohort of Medicaid beneficiaries with SLE, we found extremely poor adherence overall; only 21% of HCQ new users and 17% of IS new users were adherent to their medications (MPR≥80%). While it is challenging to compare rates of adherence across SLE studies because many different measures and adherence thresholds are used, our study confirms prior findings that adherence to SLE medications overall is very poor.(8–10, 42, 43) While it was beyond the scope of this study to examine predictors of nonadherence in this population, we did note that disproportionately greater percentages of HCQ and IS nonadherers compared to adherers were young (aged 18–30 years) or Black. Prior studies have similarly shown that young age and Black race were associated with increased rates of SLE medication nonadherence.(9, 11)
Interestingly, in our unadjusted analyses, we found that both HCQ and IS adherers had, on average, more baseline medications and higher SLE-specific risk adjustment indices compared to nonadherers, suggesting that SLE patients with more severe disease and more comorbidities were more adherent in this cohort. The literature to date presents conflicting findings regarding these relationships. One study by Daleboudt and colleagues demonstrated no association between nonadherence and numbers of comorbidities, extent of organ involvement and number of medications.(9) Other studies have shown that increased number of medications and more comorbidities may be associated with poorer adherence; however these studies were cross-sectional in nature and therefore subject to reverse causation.(8, 10, 11) We hypothesize that in this cohort, patients with more severe disease may have greater incentive to adhere to their medications in order to alleviate symptoms (e.g. joint pain, rash, edema) or may be more motivated to prevent complications (e.g. skin scarring, renal damage). Prior studies in other chronic diseases have similarly shown that individuals with a higher perceived risk of disease-related complications and with symptomatic disease were more likely to adhere to their medications compared to those with asymptomatic disease.(44, 45) Qualitative studies among SLE patients should be considered to further investigate this issue.
There are several noteworthy strengths to this study. First, this study was conducted in a large, nationwide population of racially and ethnically diverse Medicaid beneficiaries with SLE, shown to have a high burden of disease, and an increased risk of adverse outcomes.(25, 46) Prior studies note that patients with frequent ED visits and hospitalizations generate a disproportionate share of health care costs.(47) Particularly among Medicaid beneficiaries, significant efforts are underway to identify, target and improve care for the highest cost patients.(48) Interventions to improve medication adherence may provide an opportunity to decrease health care costs for this vulnerable population. Second, this is the first adherence study in SLE patients to use a new medication user design, as opposed to a prevalent user design. Prevalent user designs are subject to healthy survivor bias; patients must have “survived” and adhered during the initial, often critical time when a medication is started in order to be included, which overestimates adherence.(49) Third, this is a longitudinal study, which allowed us to examine whether there was temporal relationship between adherence and the outcomes of interest. Prior studies in SLE that investigated this question have been cross-sectional, and thus subject to reverse causation. In addition, separate periods were used to assess baseline covariates, adherence and outcomes, to limit over-adjustment by factors that may lie on the causal pathway. Fourth, in addition to a dichotomized MPR cutoff of 80 percent, we examined levels of nonadherence and demonstrated a dose-response relationship between degree of nonadherence and increased acute care utilization.
There are limitations to this study. We used administrative claims data for these analyses, which lack clinical information regarding disease activity or duration. We therefore cannot assess whether medications were initiated or discontinued because of SLE flares, adverse reactions or ineffectiveness. In addition, there may be misclassification of SLE cases. However, we used a conservative definition of ≥3 ICD-9 codes to increase specificity and to exclude individuals who were seen once for “rule-out” SLE and once in follow-up. This definition was used previously to examine the prevalence and incidence of SLE in the Medicaid population and yielded results in line with prior studies.(24, 50) Further, medication adherence is a complex behavior that is challenging to measure. While the MPR is considered to be among the best measures, it may not be able to accurately predict long-term use patterns or differentiate between patients who stop medication entirely versus those with a gap in use.(51) In addition, adherence may not be a static behavior and may have changed after the period in which it was assessed during which outcomes were measured. We also restricted our analyses to oral immunosuppressives and therefore our findings may not be reflective of the relationship between intravenous immunosuppressive adherence and acute care utilization.
Finally, there is the possibility of residual confounding from a healthy adherer effect. This refers to the phenomenon whereby adherence may be associated with other healthy behaviors and thus patients who are more likely to adhere may be at lower risk for adverse outcomes for these same reasons.(52) Prior studies suggest that patients who adhere to one medication for a chronic disease are more likely to adhere to other therapies as well and may be more likely to obtain cancer screening and vaccinations.(53, 54) Residual confounding from a healthy adherer effect may overestimate the preventive value of adherence behavior itself and is a potential limitation of this study.(55) However, in our study population, on average, adherers had higher SLE-specific risk adjustment indices and more baseline medications compared to nonadherers suggesting that in terms of comorbidities, they were not healthier overall. In addition, a large study of post-myocardial infarction patients specifically examined the degree to which the healthy adherer effect played a role in the relationship between medication adherence and outcomes and did not find significant evidence to support this.(19)
We acknowledge that there are multiple measures used to assess both medication adherence and persistence in the literature to date. Two measures, the MPR and the proportion of days covered (PDC) are among the most widely used particularly in administrative data. The MPR has been used in multiple Medicaid database studies.(20, 30) In one study among Medicaid beneficiaries, both the MPR and the PDC had the highest predictive validity for hospitalization episodes, one of the main outcomes in our study.(56) The definition we used for MPR in the context of a new user design and a 365-day adherence assessment period beginning on the date of first prescription yields a value almost identical to an interval-based approach for PDC calculation.(57) While adherence is a complex behavior the full extent of which is challenging to capture regardless of the method, we feel that the measure of adherence utilized here accurately reflects days covered by medication prescription refills during the study period.
Overall, in this study we demonstrated significantly increased acute care utilization among SLE patients who were shown to be nonadherent to either HCQ or IS medications compared to those who were adherent. Patients with the poorest level of adherence had the highest rates of utilization. Further studies are needed to understand whether interventions that improve adherence will reduce acute care utilization and improve outcomes particularly for the most vulnerable groups. In addition, given the higher costs of some SLE medications, and the burden of multiple essential medications and copayments, additional research is needed to understand whether improving adherence results in net cost saving for the patient and for the health care system, especially for a high-risk, low-income population.
Supplementary Material
Significance and Innovation.
We demonstrated that adherence to hydroxychloroquine and immunosuppressive medications was poor among U.S. Medicaid beneficiaries with systemic lupus erythematosus (SLE), a racially/ethnically diverse, low-income population at high risk for adverse health outcomes.
Patients who were nonadherent had significantly greater all-cause and SLE-related emergency department visits and hospitalizations even after adjusting for sociodemographic factors and comorbidities.
This increased acute care utilization among nonadherers suggests that there may be an opportunity to intervene to reduce avoidable morbidity and health care costs by improving adherence behavior.
Acknowledgments
Funding
This study was funded by the Lupus Foundation Career Development Award (Dr. Feldman), by K23AR060259 (Dr. Yazdany), and by NIH R01 AR057327 and K24066109 (Dr. Costenbader). The funding sources played no role in the study design, acquisition, analysis or interpretation of data or presentation of results.
Financial Disclosures
Dr. Feldman is supported by the Lupus Foundation of America Career Development Award and receives research support from Pfizer Pharmaceuticals. Dr. Costenbader received funding from NIH R01 AR057327 and K24066109. Dr. Yazdany receives funding from K23AR060259. Mr. Guan has no disclosures. Dr. Solomon receives funding from NIH AR-K2405598. He receives salary support from unrelated research grants to Brigham and Women's Hospital from Pfizer, Lilly, AstraZeneca, CORRONA and Amgen. He also serves in unpaid roles on unrelated trials run by Pfizer and Bristol Myers Squibb. He receives royalties on unrelated work from UpToDate.
Footnotes
Author Contributions
All authors were involved in drafting this manuscript or revising it critically for important intellectual content and all authors approved the version submitted. Dr. Feldman and Dr. Costenbader had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design: Feldman, Yazdany, Guan, Solomon, Costenbader
Acquisition of data: Feldman, Yazdany, Costenbader
Analysis and interpretation of data: Feldman, Yazdany, Guan, Solomon, Costenbader
References
- 1.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 2.Marcum ZA, Sevick MA, Handler SM. Medication nonadherence: a diagnosable and treatable medical condition. JAMA. 2013;309(20):2105–2106. doi: 10.1001/jama.2013.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. N Engl J Med. 1991;324(3):150–154. doi: 10.1056/NEJM199101173240303. [DOI] [PubMed] [Google Scholar]
- 4.Houssiau FA, D'Cruz D, Sangle S, Remy P, Vasconcelos C, Petrovic R, et al. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis. 2010;69(12):2083–2089. doi: 10.1136/ard.2010.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54(8):2550–2557. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 6.Marengo MF, Waimann CA, de Achaval S, Zhang H, Garcia-Gonzalez A, Richardson MN, et al. Measuring therapeutic adherence in systemic lupus erythematosus with electronic monitoring. Lupus. 2012;21(11):1158–1165. doi: 10.1177/0961203312447868. [DOI] [PubMed] [Google Scholar]
- 7.Koneru S, Kocharla L, Higgins GC, Ware A, Passo MH, Farhey YD, et al. Adherence to medications in systemic lupus erythematosus. J Clin Rheumatol. 2008;14(4):195–201. doi: 10.1097/RHU.0b013e31817a242a. [DOI] [PubMed] [Google Scholar]
- 8.Koneru S, Shishov M, Ware A, Farhey Y, Mongey AB, Graham TB, et al. Effectively measuring adherence to medications for systemic lupus erythematosus in a clinical setting. Arthritis Rheum. 2007;57(6):1000–1006. doi: 10.1002/art.22898. [DOI] [PubMed] [Google Scholar]
- 9.Daleboudt GM, Broadbent E, McQueen F, Kaptein AA. Intentional and unintentional treatment nonadherence in patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2011;63(3):342–350. doi: 10.1002/acr.20411. [DOI] [PubMed] [Google Scholar]
- 10.Julian LJ, Yelin E, Yazdany J, Panopalis P, Trupin L, Criswell LA, et al. Depression, medication adherence, and service utilization in systemic lupus erythematosus. Arthritis Rheum. 2009;61(2):240–246. doi: 10.1002/art.24236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosley-Williams A, Lumley MA, Gillis M, Leisen J, Guice D. Barriers to treatment adherence among African American and white women with systemic lupus erythematosus. Arthritis Rheum. 2002;47(6):630–638. doi: 10.1002/art.10790. [DOI] [PubMed] [Google Scholar]
- 12.Petri M, Perez-Gutthann S, Longenecker JC, Hochberg M. Morbidity of systemic lupus erythematosus: role of race and socioeconomic status. Am J Med. 1991;91(4):345–353. doi: 10.1016/0002-9343(91)90151-m. [DOI] [PubMed] [Google Scholar]
- 13.Adler M, Chambers S, Edwards C, Neild G, Isenberg D. An assessment of renal failure in an SLE cohort with special reference to ethnicity, over a 25-year period. Rheumatology (Oxford) 2006;45(9):1144–1147. doi: 10.1093/rheumatology/kel039. [DOI] [PubMed] [Google Scholar]
- 14.Sutcliffe N, Clarke AE, Taylor R, Frost C, Isenberg DA. Total costs and predictors of costs in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2001;40(1):37–47. doi: 10.1093/rheumatology/40.1.37. [DOI] [PubMed] [Google Scholar]
- 15.Li T, Carls GS, Panopalis P, Wang S, Gibson TB, Goetzel RZ. Long-term medical costs and resource utilization in systemic lupus erythematosus and lupus nephritis: a five-year analysis of a large medicaid population. Arthritis Rheum. 2009;61(6):755–763. doi: 10.1002/art.24545. [DOI] [PubMed] [Google Scholar]
- 16.Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum. 2007;56(6):2092–2094. doi: 10.1002/art.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panopalis P, Yazdany J, Gillis JZ, Julian L, Trupin L, Hersh AO, et al. Health care costs and costs associated with changes in work productivity among persons with systemic lupus erythematosus. Arthritis Rheum. 2008;59(12):1788–1795. doi: 10.1002/art.24063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yazdany J, Marafino BJ, Dean ML, Bardach NS, Duseja R, Ward MM, et al. Thirty-day hospital readmissions in systemic lupus erythematosus: predictors and hospital- and state-level variation. Arthritis Rheumatol. 2014;66(10):2828–2836. doi: 10.1002/art.38768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 20.Esposito D, Bagchi AD, Verdier JM, Bencio DS, Kim MS. Medicaid beneficiaries with congestive heart failure: association of medication adherence with healthcare use and costs. Am J Manag Care. 2009;15(7):437–445. [PubMed] [Google Scholar]
- 21.Rojas-Serrano J, Cardiel MH. Lupus patients in an emergency unit. Causes of consultation, hospitalization and outcome. A cohort study. Lupus. 2000;9(8):601–606. doi: 10.1191/096120300678828785. [DOI] [PubMed] [Google Scholar]
- 22.The Henry J. Kaiser Foundation. Medicaid: A Primer- Key Information on the Nation's Health Coverage Program for Low-Income People. [cited 2015;];Medicaid. 2013 Available from: http://kfforg/medicaid/issue-brief/medicaid-a-primer/ [Google Scholar]
- 23.A Profile of Older Americans. [cited 2014 November 1, 2014];2012 Available from: http://www.aoa.gov/Aging_Statistics/Profile/2012/docs/2012profile.pdf.
- 24.Hiraki LT, Feldman CH, Liu J, Alarcon GS, Fischer MA, Winkelmayer WC, et al. Prevalence, incidence, and demographics of systemic lupus erythematosus and lupus nephritis from 2000 to 2004 among children in the US Medicaid beneficiary population. Arthritis Rheum. 2012;64(8):2669–2676. doi: 10.1002/art.34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 2013;65(3):753–763. doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon DH, Lunt M, Schneeweiss S. The risk of infection associated with tumor necrosis factor alpha antagonists: making sense of epidemiologic evidence. Arthritis Rheum. 2008;58(4):919–928. doi: 10.1002/art.23396. [DOI] [PubMed] [Google Scholar]
- 27.Li P, Blum MA, Von Feldt J, Hennessy S, Doshi JA. Adherence, discontinuation, and switching of biologic therapies in medicaid enrollees with rheumatoid arthritis. Value Health. 2010;13(6):805–812. doi: 10.1111/j.1524-4733.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 28.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7–8):1280–1288. doi: 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 29.Blandford L, Dans PE, Ober JD, Wheelock C. Analyzing variations in medication compliance related to individual drug, drug class and prescribing physician. J Manag Care Pharm. 1999;5:47–51. [Google Scholar]
- 30.Shenolikar RA, Balkrishnan R, Camacho FT, Whitmire JT, Anderson RT. Race and medication adherence in Medicaid enrollees with type-2 diabetes. J Natl Med Assoc. 2006;98(7):1071–1077. [PMC free article] [PubMed] [Google Scholar]
- 31.Svarstad BL, Shireman TI, Sweeney JK. Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatr Serv. 2001;52(6):805–811. doi: 10.1176/appi.ps.52.6.805. [DOI] [PubMed] [Google Scholar]
- 32.Gilmer TP, Dolder CR, Lacro JP, Folsom DP, Lindamer L, Garcia P, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692–699. doi: 10.1176/appi.ajp.161.4.692. [DOI] [PubMed] [Google Scholar]
- 33.Chronic Conditions Data Warehouse. Medicaid Enrollment by Race, 1999–2007. [cited; Available from: http://www.ccwdata.org/summary-statistics/demographics/index.htm. [Google Scholar]
- 34.Ward MM. Medical insurance, socioeconomic status, and age of onset of endstage renal disease in patients with lupus nephritis. J Rheumatol. 2007;34(10):2024–2027. [PubMed] [Google Scholar]
- 35.Ward MM. Development and testing of a systemic lupus-specific risk adjustment index for in-hospital mortality. J Rheumatol. 2000;27(6):1408–1413. [PubMed] [Google Scholar]
- 36.Hope CJ, Wu J, Tu W, Young J, Murray MD. Association of medication adherence, knowledge, and skills with emergency department visits by adults 50 years or older with congestive heart failure. Am J Health Syst Pharm. 2004;61(19):2043–2049. doi: 10.1093/ajhp/61.19.2043. [DOI] [PubMed] [Google Scholar]
- 37.Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 38.Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166(17):1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 39.Spertus JA, Kettelkamp R, Vance C, Decker C, Jones PG, Rumsfeld JS, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113(24):2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]
- 40.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 41.Dragomir A, Cote R, Roy L, Blais L, Lalonde L, Berard A, et al. Impact of adherence to antihypertensive agents on clinical outcomes and hospitalization costs. Med Care. 2010;48(5):418–425. doi: 10.1097/MLR.0b013e3181d567bd. [DOI] [PubMed] [Google Scholar]
- 42.Chambers S, Raine R, Rahman A, Hagley K, De Ceulaer K, Isenberg D. Factors influencing adherence to medications in a group of patients with systemic lupus erythematosus in Jamaica. Lupus. 2008;17(8):761–769. doi: 10.1177/0961203308089404. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira-Santos M, Verani JF, Klumb EM, Albuquerque EM. Evaluation of adherence to drug treatment in patients with systemic lupus erythematosus in Brazil. Lupus. 2011;20(3):320–329. doi: 10.1177/0961203310383736. [DOI] [PubMed] [Google Scholar]
- 44.Sewitch MJ, Abrahamowicz M, Barkun A, Bitton A, Wild GE, Cohen A, et al. Patient nonadherence to medication in inflammatory bowel disease. Am J Gastroenterol. 2003;98(7):1535–1544. doi: 10.1111/j.1572-0241.2003.07522.x. [DOI] [PubMed] [Google Scholar]
- 45.Gao X, Nau DP, Rosenbluth SA, Scott V, Woodward C. The relationship of disease severity, health beliefs and medication adherence among HIV patients. AIDS Care. 2000;12(4):387–398. doi: 10.1080/09540120050123783. [DOI] [PubMed] [Google Scholar]
- 46.Gomez-Puerta JA, Barbhaiya M, Guan H, Feldman CH, Alarcon GS, Costenbader KH. Racial/Ethnic variation in all-cause mortality among United States medicaid recipients with systemic lupus erythematosus: a Hispanic and asian paradox. Arthritis Rheumatol. 2015;67(3):752–760. doi: 10.1002/art.38981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raven MC, Billings JC, Goldfrank LR, Manheimer ED, Gourevitch MN. Medicaid patients at high risk for frequent hospital admission: real-time identification and remediable risks. J Urban Health. 2009;86(2):230–241. doi: 10.1007/s11524-008-9336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Billings J, Mijanovich T. Improving the management of care for high-cost Medicaid patients. Health Aff (Millwood) 2007;26(6):1643–1654. doi: 10.1377/hlthaff.26.6.1643. [DOI] [PubMed] [Google Scholar]
- 49.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 50.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among U.S. adults with medicaid coverage, 2000–2004. Arthritis Rheum. 2012 doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franklin JM, Shrank WH, Pakes J, Sanfelix-Gimeno G, Matlin OS, Brennan TA, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med Care. 2013;51(9):789–796. doi: 10.1097/MLR.0b013e3182984c1f. [DOI] [PubMed] [Google Scholar]
- 52.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 53.Curtis JR, Xi J, Westfall AO, Cheng H, Lyles K, Saag KG, et al. Improving the prediction of medication compliance: the example of bisphosphonates for osteoporosis. Med Care. 2009;47(3):334–341. doi: 10.1097/MLR.0b013e31818afa1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brookhart MA, Patrick AR, Dormuth C, Avorn J, Shrank W, Cadarette SM, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166(3):348–354. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 55.Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26(5):546–550. doi: 10.1007/s11606-010-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. An empirical basis for standardizing adherence measures derived from administrative claims data among diabetic patients. Med Care. 2008;46(11):1125–1133. doi: 10.1097/MLR.0b013e31817924d2. [DOI] [PubMed] [Google Scholar]
- 57.Choudhry NK, Shrank WH, Levin RL, Lee JL, Jan SA, Brookhart MA, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009;15(7):457–464. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


