Abstract
Cannabis use has been reported to increase the risk of developing schizophrenia and to worsen symptoms of the illness. Both of these outcomes might be attributable to the disruption by cannabis of the endogenous cannabinoid system's spatiotemporal regulation of the inhibitory circuitry in the prefrontal cortex that is essential for core cognitive processes, such as working memory, which are impaired in schizophrenia. In the healthy brain, the endocannabinoid 2-arachidonylglycerol (2-AG) is 1) synthesized by diacylglycerol lipase in pyramidal neurons; 2) travels retrogradely to nearby inhibitory axon terminals that express the primary cannabinoid receptor CB1R; 3) binds to CB1R which inhibits GABA release from the cholecystokinin-containing population of interneurons; and 4) is metabolized by either monoglyceride lipase, which is located in the inhibitory axon terminal, or by α-β-hydrolase domain 6, which is co-localized presynaptically with diacylglycerol lipase. Investigations of the endogenous cannabinoid system in the prefrontal cortex of subjects with schizophrenia have found evidence of higher metabolism of 2-AG, as well as both greater CB1R receptor binding and lower levels of CB1R mRNA and protein. Current views on the potential pathogenesis of these alterations, including disturbances in the development of the endogenous cannabinoid system, are discussed. In addition, how interactions between these alterations in the endocannabinoid system and those in other inhibitory neurons in the prefrontal cortex in subjects with schizophrenia might increase the liability to adverse outcomes with cannabis use is considered.
Keywords: CB1R, cannabis, cannabinoid, prefrontal cortex, inhibitory, cognitive
Introduction
In recent years, cannabis use has increased in prevalence in parallel with an earlier age of onset of cannabis use during adolescence and a greater content of Δ9-tetrahydrocannabinol, the major psychoactive component of cannabis (1). These trends raise the question of whether the higher rates and earlier onset of use of more potent cannabis may lead to potentially deleterious societal health consequences. One area of potential concern arises from evidence of a relationship between cannabis use and schizophrenia.
Schizophrenia is a serious psychiatric illness associated with high levels of disability and morbidity, elevated rates of suicide, and early mortality (2). The disorder is typically characterized by the triad of 1) psychotic symptoms, such as hallucinations and delusions; 2) negative symptoms, such as alogia and avolition; and 3) impairments in cognitive functions such as working memory and cognitive control. Multiple lines of evidence suggest that cannabis use worsens the course of schizophrenia, and may be a risk factor for development of the illness. First, intravenous administration of Δ9-tetrahydrocannabinol has been reported to transiently worsen or induce psychotic symptoms and cognitive deficits in individuals with or without schizophrenia, respectively (3, 4). Second, a large number of studies have identified cannabis use as a risk factor for developing schizophrenia (1, 5-12). This elevated risk has been reported to remain significant, though may be attenuated, after correcting for confounding variables such as psychotic symptoms preceding cannabis use, familial risk, parental factors, other psychiatric illness, social background, affiliation with deviant peers, socioeconomic status, trauma, baseline IQ, substance use, and level of education (1, 5-12). In addition, this risk is inversely related to the age of onset of cannabis use and directly related to the amount of cannabis use. For example, onset of cannabis use at age 15 relative to age 18 was associated with a higher risk of a psychotic disorder at age 26 (6). Third, cannabis use is associated with an earlier age of onset of schizophrenia (13-15), particularly with higher levels of daily cannabis consumption and when the onset of cannabis use begins prior to age 15 (16, 17). Fourth, individuals with schizophrenia who use cannabis have longer durations of hospital stays and more frequent readmissions (18). However, these lines of evidence do not demonstrate that cannabis use is either necessary or sufficient to develop schizophrenia.
Cannabis use in healthy subjects has been reported to result in deficits in the same cognitive domains that are disturbed in schizophrenia and that have the strongest association with poor functioning and long-term outcomes (19-21). For example, otherwise healthy individuals with prolonged exposure to high levels of cannabis have performance deficits on neuropsychological tests that persist into periods of abstinence and are more severe in individuals who began using cannabis in adolescence (22). A prospective cohort study found a decline in cognitive function that only occurred in adolescent-onset cannabis users and that did not recover with cessation of cannabis use in adulthood (23). Paradoxically, schizophrenia subjects with a prior history of cannabis use have been reported to have higher performance on multiple cognitive tasks relative to schizophrenia subjects without a cannabis use history (24, 25). However, this finding may be explained by the observation that individuals with schizophrenia who have a history of cannabis use have a higher premorbid IQ than those without a history of cannabis use (26). Indeed, higher neurocognitive functioning is also seen in individuals with schizophrenia who abuse other substances (27-29), suggesting that individuals with a more severe course of schizophrenia may lack the cognitive and social capacities to obtain and use illicit substances (27) while a subgroup of schizophrenia subjects with less cognitive impairments may be capable of abusing substances. Alternatively, given the evidence of cannabis use as a risk factor for developing schizophrenia, a subset of individuals at risk for schizophrenia who have relatively normal cognitive functioning may have developed schizophrenia after exposure to cannabis and consequently may have otherwise higher cognitive performance relative to individuals who develop schizophrenia regardless of exposure to cannabis use. However, while epidemiological studies do not present a fully clear picture of the relationship between cannabis use and cognitive functioning in schizophrenia, direct administration of Δ9-tetrahydrocannabinol to individuals with schizophrenia has been reported to transiently worsen cognitive deficits (4).
Experimental studies in animals have provided more direct evidence of a causal relationship between cannabis use and cognitive impairments. For example, administration of Δ9-tetrahydrocannabinol or other CB1R agonists produced cognitive deficits in adolescent, but not adult, rats (30-32). Similarly, regular administration of Δ9-tetrahydrocannabinol to adolescent monkeys altered the trajectory of developmental improvements in spatial working memory (33). These findings suggest that the long-lasting deleterious effects of Δ9-tetrahydrocannabinol on cognitive function may be more prominent when the exposure occurs during periods of neural circuitry development such as adolescence, which is also the time period when the symptoms of schizophrenia become clinically apparent.
Cortical circuitry and cognitive processes in schizophrenia
Understanding the mechanisms by which cannabis may disrupt the cognitive functions that are also impaired in schizophrenia requires knowledge of aspects of the cortical circuitry that supports cognitive processes such as working memory. For example, spatial working memory, which is altered in adolescent monkeys by persistent exposure to Δ9-tetrahydrocannabinol (33), depends on a distributed neural circuit that includes the dorsolateral prefrontal cortex (DLPFC) (34). In the DLPFC, layer 3 pyramidal neurons provide reciprocal excitatory connections to other layer 3 pyramidal neurons that sustain circuitry activity during the delay period of working memory tasks (35). In addition, the subpopulation of cortical inhibitory (GABA) basket neurons that express the calcium-binding protein parvalbumin provide inhibitory input that tunes pyramidal neuron firing (36). The interaction between layer 3 pyramidal neurons and parvalbumin basket neurons generates gamma frequency (30-80 Hz) oscillations whose power scales with the demands of working memory tasks (37, 38). In schizophrenia, DLPFC layer 3 pyramidal neurons and parvalbumin basket neurons are altered. For example, the density of dendritic spines, the postsynaptic targets of excitatory inputs from other pyramidal neurons, are lower on layer 3 pyramidal neurons in schizophrenia (39, 40). Furthermore, deficits in mRNA levels for parvalbumin (41-45) and in parvalbumin protein levels in basket cell axon terminals (46) are present in the DLPFC in schizophrenia. In addition, deficits in mRNA levels for the GABA synthesizing enzyme GAD67 (47-52) are particularly prominent in parvalbumin neurons (41), and GAD67 protein levels are lower in parvalbumin neuron axon terminals (52). Thus, the connectivity among pyramidal neurons and parvalbumin basket neurons appears to be impaired in the DLPFC in schizophrenia and could contribute to the alterations in gamma oscillations that underlie working memory deficits (53, 54).
Active cannabis use may affect cognitive functioning in schizophrenia by further interfering with the regulation of the already dysfunctional interaction between DLPFC pyramidal neurons and parvalbumin basket neurons. For example, in the primate neocortex, the principal receptor for cannabinoids, the cannabinoid 1 receptor (CB1R), is strongly expressed in the DLPFC (55). In the neocortex, CB1Rs are preferentially localized to the axon terminals of GABA-containing basket neurons that contain the neuropeptide cholecystokinin (CCK) and, to a lesser extent, of some pyramidal neurons (56-58). In fact, the density of CB1Rs receptor is much higher in the axon terminals of CCK basket cells than of pyramidal cells (59-61). The inhibitory axon terminals from CCK basket neurons target both pyramidal neurons and parvalbumin neurons (56, 57). Activation of CB1Rs suppresses GABA release from CCK neuron axon terminals onto pyramidal neurons (62), and CCK basket neurons also regulate pyramidal neuron activity (56, 63, 64). Thus, exogenous cannabinoids such as cannabis broadly suppress CCK neuron-mediated inhibition of pyramidal neurons and parvalbumin neurons without the tightly-regulated, spatiotemporal selectivity of endocannabinoids.
The endocannabinoid system
Further exploration of the relationship between cannabis use, cortical circuitry disturbances, and cognitive impairments in schizophrenia also requires knowledge of the endocannabinoid system. The primary endocannabinoid ligands in the brain include anandamide and 2-arachidonylglycerol (2-AG) (65). Anandamide is a partial CB1R agonist and is found at relatively low concentrations in brain (65, 66). In contrast, 2-AG is a full CB1R agonist and is found at much higher concentrations than anandamide in the brain (67).
Physiological and anatomical lines of evidence suggest that 2-AG serves as a retrograde signal from pyramidal neurons to nearby CCK basket neuron axon terminals that contain high concentrations of the CB1R (Figure 1, left panel). In a process known as depolarization-induced suppression of inhibition (DSI), the rapid depolarization of a pyramidal neuron induces short-term depression of GABA release from local CCK neuron axon terminals. Both CB1R and 2-AG are required for DSI. For example, bath application of 2-AG reduces the amplitude of inhibitory postsynaptic currents (IPSCs) (68). Furthermore, 2-AG is synthesized by diacylglycerol lipase (DAGL) (67), which has two isoforms. DAGLα is expressed at much higher levels than DAGLß in adult cortex and is primarily responsible for 2-AG synthesis (69). DAGLα is predominantly localized to pyramidal neurons (58, 70-72), and pharmacological inhibition of DAGL blocks CB1R-mediated DSI (68, 73-75).
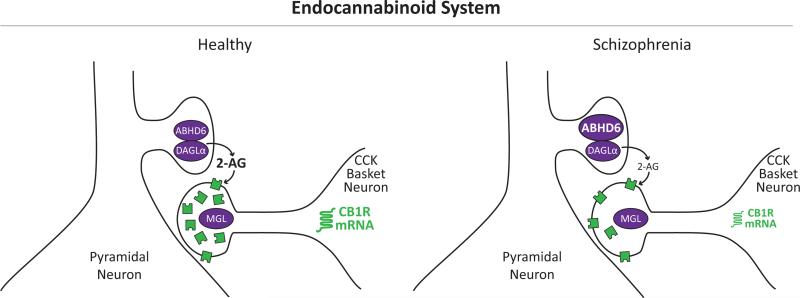
Figure 1. Schematic illustration of the endogenous cannabinoid system in the DLPFC in the healthy state and in schizophrenia.
Left: The synthesizing enzyme for the endogenous cannabinoid 2-AG, diacylglyercol lipase (DAGLα), is localized to the dendritic spines of pyramidal neurons. In response to repeated depolarization, 2-AG is synthesized, travels retrogradely, and activates the endogenous cannabinoid receptor CB1R (green) in nearby inhibitory axon terminals from cholecystokinin (CCK)-containing basket neurons. 2-AG is then degraded by either monoglyceride lipase (MGL) localized to CCK neuron axon terminals or by the serine hydrolase α-β-hydrolase domain 6 (ABHD6) which is co-localized with DAGLα in the dendritic spines of pyramidal neurons. Right: In schizophrenia, higher mRNA levels for ABHD6 (larger font size) have been reported in the DLPFC. Because ABHD6 is co-localized with DAGL in the dendritic spines of DLPFC pyramidal neurons, higher ABHD6 mRNA levels may lead to higher metabolism of 2-AG directly at the source of 2-AG production, which may in turn lower 2-AG activity at CB1R. In addition, studies have found lower levels of CB1R mRNA (smaller font size) and protein immunoreactivity and higher CB1R receptor binding in the DLPFC of the same schizophrenia subjects. One potential explanation for this apparent discrepancy may be the presence of higher levels of membrane-bound CB1R due to altered trafficking that are accessible to ligand binding, while the total amount of intracellular CB1R is lower.
Once 2-AG has been synthesized and released, it is degraded by monoglyceride lipase (MGL) (76, 77) or by α-β-hydrolase domain 6 (ABHD6; Figure 1, left panel) (78). MGL is localized almost exclusively in presynaptic axon terminals (71, 72, 79). Consistent with the role of 2-AG in suppressing GABA release, inhibitors of MGL prolong the suppression of IPSCs (68, 73, 80), an effect that is reversible by a CB1R antagonist (68, 80). In contrast, inhibitors of fatty acid amide hydrolase (FAAH), which hydrolyzes anandamide but not 2-AG in vivo (76), have no effect on 2-AG-mediated DSI (68, 73, 81). Taken together, these data suggest that MGL is anatomically positioned to inactivate 2-AG at its site of action at CB1R in presynaptic inhibitory axon terminals. In contrast, ABHD6 is co-localized with DAGL in the pyramidal neuron dendritic spines (70, 71, 78) which allow ABHD6 to regulate 2-AG at its site of production.
In concert, these findings raise the question of whether alterations in the endocannabinoid system in schizophrenia may contribute to, or compensate for, the disturbances in DLPFC circuitry described above. Multiple studies have addressed this question by investigating the status of endocannabinoids and CB1R in the DLPFC in schizophrenia, and we review these studies next. However, it is important to note that the endocannabinoid system is also involved in the regulation of fear and anxiety (82-85) and regulates other neurotransmitter systems in other brain regions, such as glutamatergic signaling in the amygdala (86). Consequently, disturbances in endocannabinoid signaling may also be present in other psychiatric disorders and may have consequences on neurotransmitter function in other brain regions; however, the primary focus of this review paper is on endocannabinoid regulation of cortical GABA neuron dysfunction in schizophrenia.
Alterations in the endocannabinoid system in schizophrenia
The status of endocannabinoid signaling in the DLPFC can first be informed by investigating whether the primary endocannabinoids themselves (i.e., anandamide and 2-AG) are altered in schizophrenia. Initial studies focused on levels of these ligands in the cerebrospinal fluid (CSF) in the illness (Table 1). For example, CSF levels of anandamide were reported to be eight-fold higher in a cohort of 47 first-episode, antipsychotic-naive schizophrenia subjects (87) and a cohort of 27 subjects with prodomal psychotic symptoms (88) relative to healthy subjects. Furthermore, a three-fold elevation in blood levels of anandamide were reported in a cohort of 12 subjects in an acute phase of schizophrenia and off antipsychotic medications (89). However, CSF studies have not been able to detect quantifiable levels of 2-AG (90) which is the endocannabinoid present in the highest concentration in the brain (67). Thus, the relationship between levels of endocannabinoids measured in the CSF and peripheral blood and endocannabinoid function in brain parenchyma is not clear. In addition, attempts to quantify 2-AG directly in brain parenchyma in human subjects have also not been successful due to the prominent effect of postmortem delay on 2-AG (91).
Table 1.
Summary of key findings from studies of endogenous cannabinoid markers in schizophrenia
| Reference | Schizophrenia Cohort [n] | Technique | Finding in Schizophrenia Subjects |
|---|---|---|---|
| Cerebrospinal fluid | |||
| (1) | first-episode, antipsychotic-naive [47] | HPLC/MS | elevated CSF anandamide levels |
| (2) | prodromal psychosis [27] | HPLC/MS | elevated CSF anandamide levels |
| (3) | [10] | HPLC/MS | elevated CSF anandamide levels |
| Blood | |||
| (4) | acute symptoms, off antipsychotics [12] | LC-APCI-MS | elevated blood anandamide levels |
| Imaging studies | |||
| (5) | [9] | PET [11C]-OMAR | elevated CB1R binding in all brain regions, significant in pons |
| Postmortem brain studies | |||
| Endocannabinoid enzymes | |||
| (6) | [42] | qPCR | no change in mRNA levels for endocannabinoid synthesizing (DAGL) and metabolizing (MGL, FAAH) enzymes in PFC area 9 |
| (7) | [42] | qPCR | elevated mRNA levels for 2-AG metabolizing enzyme ABHD6 in PFC area 9 of younger subjects; no changes in antipsychotic- or THC-exposed monkeys |
| CB1R mRNA and protein | |||
| (8) | [23] | in situ hybridization, immunocytochemistry | lower CB1R mRNA and protein levels in PFC area 9; no change in antipsychotic-exposed monkeys |
| (9) | [26] | immunocytochemistry | lower CB1R immunoreactivity in PFC area 46, no change in depression |
| (10) | [37] | qPCR | no change in CB1R mRNA levels in PFC area 46 |
| (11) | [31] | qPCR | no change in CB1R mRNA levels in PFC area 9 |
| CB1R autoradiography | |||
| (12) | [14] | CB1R agonist [3H]-CP55940 | elevated CB1R binding in PFC area 9, but not hippocampus, caudate, or putamen |
| (10) | [37] | [3H]-CP55940 | elevated CB1R binding in PFC area 46 in paranoid subjects |
| (13) | [47] | CB1R inverse agonist [3H]-MePPEP | elevated CB1R binding in PFC areas 9 and 46 |
| (14) | [10] | CB1R antagonist [3H]-SR141716 | elevated CB1R binding in anterior cingulate cortex |
| (15) | [8] | [3H]-CP55940 | elevated CB1R binding in superficial layers of posterior cingulate cortex |
| (16) | [21] | [3H]-OMAR | elevated CB1R binding in PFC area 9 in same schizophrenia subjects with lower CB1R mRNA and protein levels (8) |
Abbreviations: 2-AG: 2-arachidonoylglyercol; ABHD6: α-β-hydrolase domain 6; CB1R: CB1 receptor: CSF: cerebrospinal fluid; DAGL: diacylglyercol lipase; FAAH; fatty acid amide hydrolase; HPLC/MS: high performance liquid chromatography / mass spectrometry; LC-APCI-MS: liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry; MGL: monoglyceride lipase; PET: positron emission tomography; PFC: prefrontal cortex; qPCR: quantitative polymerase chain reaction; THC: tetrahydrocannabinol
The status of endocannabinoid signaling in the DLPFC has been further investigated, though indirectly, by quantifying the synthesizing and metabolizing enzymes for 2-AG (Table 1). In the DLPFC of 42 schizophrenia subjects, mRNA levels for DAGLα, DAGLβ, MGL or FAAH did not differ between schizophrenia and healthy subjects (92) (Figure 1, right panel). However, ABHD6 mRNA levels were elevated in the DLPFC of schizophrenia subjects who were younger (< 40 years old) and had a shorter illness duration (< 15 years of illness) (93). Because of the co-localization of ABHD6 with DAGL in the dendritic spines of DLPFC pyramidal neurons (70, 71, 78), higher ABHD6 mRNA levels may lead to higher metabolism of 2-AG directly at the source of 2-AG production in dendritic spines, which may in turn lead to lower 2-AG levels and binding at CB1R in the earlier stages of the illness (Figure 1, right panel).
Understanding endocannabinoid signaling in the DLPFC in schizophrenia also requires knowledge of the status of the principal receptor, CB1R. Postmortem studies employing receptor autoradiography using radiolabeled CB1R ligands have consistently reported higher CB1R binding in the frontal cortex in schizophrenia (Table 1) (94-98). For example, a study utilizing the CB1R agonist [3H]-CP55940 found higher CB1R binding in DLPFC area 9 in a cohort of 14 schizophrenia subjects relative to healthy subjects (94). Similarly, another study using [3H]-CP55940 found higher levels of CB1R binding in DLPFC area 46 from a cohort of 37 schizophrenia subjects relative to healthy subjects; however, higher CB1R binding was only observed in subjects with paranoid schizophrenia (97). CB1R binding measured using the inverse agonist [3H]-MePPEP was 20% higher in DLPFC area 9 and 46 in a cohort of 47 schizophrenia subjects (98). In the anterior cingulate cortex, binding of the CB1R antagonist [3H]-SR141716 was 64% higher in a cohort of 10 schizophrenia subjects (95). In posterior cingulate cortex, levels of CB1R binding measured using [3H]-CP55940 were 25% higher in the superficial, but not deeper, cortical layers in a cohort of 8 schizophrenia subjects (96). Furthermore, a pilot in vivo PET study utilizing the novel CB1R selective ligand [11C]-OMAR found higher brain CB1R binding in a cohort of nine schizophrenia subjects (99) and also an inverse correlation between CB1R binding and negative symptoms in schizophrenia subjects (100).
While these radiolabeled CB1R ligand binding studies have consistently reported higher levels of CB1R binding in schizophrenia, particularly in the DLPFC, studies of CB1R mRNA and protein levels have provided contrasting results (Table 1). For example, a radioimmunocytochemistry study found lower CB1R protein levels in DLPFC area 9 of the right hemisphere in a cohort of 23 schizophrenia relative to healthy subjects (101). This study also reported lower CB1R protein levels using diaminobenzidine-based immunocytochemistry in DLPFC area 9 of the left hemisphere in a subset (n=12) of the same schizophrenia subjects, suggesting that lower CB1R are present in both hemispheres of the DLPFC in schizophrenia. This finding appears unlikely to be attributable to a loss of CB1R-expressing neurons as the total number of neurons has been reported to be unaltered in the frontal cortex in schizophrenia (102). Furthermore, the density of CB1R-immunopositive neurons was also reported to not differ, at least in the anterior cingulate cortex, of 15 schizophrenia subjects relative to subjects with major depressive disorder, bipolar disorder or control subjects (103), although this study did not attempt to quantify CB1R levels. In contrast, two studies of CB1R mRNA levels by quantitative PCR in DLPFC areas 9 or area 46 found no differences from comparison subjects in cohorts of 31 and 37 schizophrenia subjects, respectively (97, 104). However, a more detailed in situ hybridization study reported an 11% decrease in CB1R mRNA levels that was most prominent in layers 2, 3, 5, and 6 in the same 23 schizophrenia subjects that had lower CB1R protein levels quantified by radioimmunocytochemistry (101). Furthermore, long-term exposure to the typical antipsychotic haloperidol or the atypical antipsychotic olanzapine in monkeys did not affect CB1R mRNA levels quantified by in situ hybridization in the DLPFC (101).
Because almost all studies of CB1R mRNA, protein, and binding levels were not conducted in the same subjects, the reason for the apparent discrepancy in CB1R measures in schizophrenia was not immediately clear. To first address the issue of whether the differences across studies could reflect differences in the cohorts studied, we conducted a receptor binding study using [3H]-OMAR in the same schizophrenia subjects in whom lower CB1R mRNA and protein immunoreactivity levels had been previously reported (Table 1) (105). Schizophrenia subjects with lower CB1R mRNA and protein immunoreactivity levels were found to also have higher levels of [3H]-OMAR binding to CB1R, and [3H]-OMAR receptor binding was negatively correlated with CB1R mRNA levels (105). The surprising combination of lower CB1R mRNA and protein immunoreactivity with higher CB1R receptor binding in the DLPFC of the same schizophrenia subjects suggests at least four different interpretations that can guide further studies that may shed insight into the nature of endocannabinoid system dysfunction in schizophrenia.
First, higher levels of CB1R binding might reflect higher levels of membrane-bound CB1R due to altered trafficking of the receptor (Figure 1, right panel). Indeed, in contrast to most G-protein coupled receptors which are found in the plasma membrane, CB1Rs are largely localized intracellularly (106). Thus, the number of CB1Rs that are accessible to ligand binding at the membrane could be increased in schizophrenia, while the total amount of intracellular CB1R is reduced, as suggested by the findings of lower CB1R mRNA and protein immunoreactivity levels. The recent development of super-resolution microscopy may make it possible to test this hypothesis.
Second, higher levels of CB1R binding might reflect greater receptor affinity. Unfortunately, CB1R affinity has generally not been quantified in schizophrenia. One study reported no differences in CB1R affinity in the DLPFC in schizophrenia but attributed this lack of change in part due to the high levels of assay variance observed in postmortem tissue combined with an insufficient sample size (98). Additional studies that assess CB1R affinity and binding levels in cohorts of schizophrenia subjects that are large enough to detect changes in affinity in the face of high variance are needed to test this interpretation. In addition, although speculative, higher CB1R affinity might be related to higher ABHD6 levels in schizophrenia (93). For example, ABHD6 mRNA and CB1R binding levels were positively correlated in the DLPFC in schizophrenia (93). Because of the co-localization of ABHD6 with DAGL in the dendritic spines of cortical pyramidal neurons (70, 71, 78), greater expression of ABHD6 mRNA could lead to higher metabolism of 2-AG directly at the source of 2-AG production in dendritic spines, which would in turn lower 2-AG activity at CB1Rs (Figure 1, right panel). Lower 2-AG signaling could then lead to a compensatory up-regulation in membrane localization through altered trafficking or higher affinity of the affected CB1R in that location. Direct testing of this hypothesis would require animal models that overexpress ABHD6.
Third, the GABA neuron axon terminals that normally express CB1R might not fully develop in schizophrenia, leading to lower overall levels of CB1R mRNA and protein. For example, the normal increase in CB1R immunoreactivity across adolescence in the middle layers of primate DLPFC (107) might not occur in schizophrenia, consistent with findings that other markers of DLPFC GABA neurons fit a model of arrested developmental trajectories in schizophrenia (108-110). Thus, it is possible that CB1R affinity in the remaining CB1R-containing axon terminals increases in schizophrenia as a compensatory response to maintain homeostasis of endocannabinoid signaling. Although longitudinal developmental in vivo PET imaging studies of CB1R binding in individuals at high risk for schizophrenia are not currently possible, proof-of-concept tests of this hypothesis might be possible in appropriate animal models.
Fourth, higher levels of CB1R binding may reflect higher CB1R levels in excitatory axon terminals (58, 111) while CB1R levels are lower in inhibitory axon terminals. The previous radioimmunocytochemistry study that reported lowered CB1R protein levels in schizophrenia (101) utilized an CB1R antibody against an epitope present on inhibitory, but not excitatory, axon terminals (112), while the [3H]-OMAR ligand binds to all available CB1R. Thus, studies that quantify CB1R levels specifically in pyramidal neurons in schizophrenia are required to further test this interpretation.
Interactions between alterations in endocannabinoid and GABA signaling in schizophrenia
These alterations in endocannabinoid signaling may have important effects on GABA neuron disturbances in the DLPFC in schizophrenia. For example, as described earlier, the combination of higher levels of ABHD6, and consequently higher 2-AG metabolism and lower 2-AG levels, may lead to a compensatory up-regulation of CB1R affinity and/or membrane localization (Figure 1, right panel). This interpretation is supported by the significant correlation between CB1R binding and ABHD6 mRNA in schizophrenia subjects (93), and the net effect of these changes may be to maintain homeostasis of endocannabinoid regulation of GABA signaling. However, the disturbances in GABA neurons could also be upstream to the changes in the endocannabinoid system in schizophrenia. For example, deficits in mRNA levels for the GABA synthesizing enzyme GAD67 are one of the most consistently reported findings in the DLPFC in schizophrenia (47-52). The resulting deficit in GABA synthesis could evoke compensatory changes, such as down-regulation of CB1R expression (levels of GAD67 and CB1R mRNAs are positively correlated in the DLPFC in schizophrenia (101)), in order to preserve GABA signaling strength. The idea that lower GAD67 expression is upstream of lower CB1R expression in schizophrenia is supported by experimental studies. For example, mice with a GAD67 heterozygous null mutation exhibit lower CB1R mRNA levels, without a change in DAGL mRNA levels, in the medial PFC (113). This pattern of findings is similar to that seen in schizophrenia. In contrast, mice with a CB1R heterozygous or homozygous null mutation had no change in GAD67 mRNA levels in medial PFC (113). Finally, although the alterations in the endocannabinoid and GABA systems in the DLPFC in schizophrenia are correlated, it is possible that both change in response to another disturbance in the illness.
Cannabis and the endocannabinoid system in schizophrenia
As noted above, individuals with schizophrenia appear to be particularly susceptible to the cognitive-impairing effects of cannabis. For example, under normal physiological conditions, activation of CB1Rs suppresses GABA release only from the CB1R-containing terminals that synapse onto the pyramidal cells whose activity stimulates endocannabinoid signaling. In contrast, cannabis use suppresses GABA release onto pyramidal cells without spatiotemporal selectivity because exogenous cannabinoids affect all CB1R terminals. The adverse consequences of this indiscriminate activation of CB1Rs may be exacerbated in schizophrenia due to the presence of higher membrane-bound levels of CB1R or higher levels of CB1R affinity (94-98, 105).
Furthermore, deficits in GABA synthesis due to lower GAD67 mRNA levels in schizophrenia might be partially compensated for by a reduction in endocannabinoid-mediated suppression of GABA release from CB1R-containing axon terminals due to lower 2-AG signaling. Such a compensation could be driven by greater expression of ABHD6 in the early stages of schizophrenia (93). If this hypothesis is true, then the negative effects of cannabis in schizophrenia may occur because exogenous activation of CB1R by cannabis counteracts the compensatory effect of lower 2-AG signaling, at least in the earlier stages of the illness. Consistent with this interpretation, the deleterious effects of cannabis use appear to be most prominent in younger individuals (10, 13, 14). Interestingly, administration of a CB1R agonist to adolescent but not adult rats impairs the maturation of inhibitory processes in the PFC (114). Taken together, these data suggest the need for preventative strategies directed towards reducing cannabis use in adolescents at risk for schizophrenia, which is especially important given the increasing prevalence of cannabis use, earlier age of onset of cannabis use during adolescence, rising Δ9-tetrahydrocannabinol content of cannabis in recent years, and recent legalization of recreational cannabis use in multiple states (1). Furthermore, the recently announced National Institutes of Health initiative termed Adolescent Brain Cognitive Development will hopefully provide critical insights into the long-term effects of cannabis use on adolescent brain development by prospectively studying the relationship between substance use and cognitive development in thousands of youth.
Acknowledgments
This work was supported by grants from the National Institutes of Health (MH100066 to Dr. Volk; MH043784 and MH051234 to Dr. Lewis).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures. David A. Lewis currently receives investigator-initiated research support from Pfizer and in 2012-2014 served as a consultant in the areas of target identification and validation and new compound development to Autifony, Bristol-Myers Squibb, Concert Pharmaceuticals, and Sunovion. David W. Volk reports no biomedical financial interests or potential conflicts of interest.
References
- 1.Radhakrishnan R, Wilkinson ST, D'Souza DC. Gone to Pot - A Review of the Association between Cannabis and Psychosis. Front Psychiatry. 2014;5:54. doi: 10.3389/fpsyt.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insel TR, Scolnick EM. Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol Psychiatry. 2006;11:11–17. doi: 10.1038/sj.mp.4001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- 4.D'Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Andreasson S, Allebeck P, Engstrom A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2:1483–1486. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- 6.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: Historical cohort study. BMJ. 2002;325:1199–1204. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- 9.Fergusson DM, Poulton R, Smith PF, Boden JM. Cannabis and psychosis. BMJ. 2006;332:172–175. doi: 10.1136/bmj.332.7534.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casadio P, Fernandes C, Murray RM, Di Forti M. Cannabis use in young people: the risk for schizophrenia. Neurosci Biobehav Rev. 2011;35:1779–1787. doi: 10.1016/j.neubiorev.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Andreasson S, Allebeck P. Cannabis, schizophrenia and other non-affective psychoses: 35 years of follow-up of a population-based cohort. Psychol Med. 2012;42:1321–1328. doi: 10.1017/S0033291711002078. [DOI] [PubMed] [Google Scholar]
- 12.Giordano GN, Ohlsson H, Sundquist K, Sundquist J, Kendler KS. The association between cannabis abuse and subsequent schizophrenia: a Swedish national co-relative control study. Psychol Med. 2014:1–8. doi: 10.1017/S0033291714001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compton MT, Kelley ME, Ramsay CE, Pringle M, Goulding SM, Esterberg ML, et al. Association of pre-onset cannabis, alcohol, and tobacco use with age at onset of prodrome and age at onset of psychosis in first-episode patients. Am J Psychiatry. 2009;166:1251–1257. doi: 10.1176/appi.ajp.2009.09030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galvez-Buccollini JA, Proal AC, Tomaselli V, Trachtenberg M, Coconcea C, Chun J, et al. Association between age at onset of psychosis and age at onset of cannabis use in non-affective psychosis. Schizophr Res. 2012;139:157–160. doi: 10.1016/j.schres.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veen ND, Selten JP, van d TI, Feller WG, Hoek HW, Kahn RS. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161:501–506. doi: 10.1176/appi.ajp.161.3.501. [DOI] [PubMed] [Google Scholar]
- 16.Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68:555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- 17.Di Forti M, Sallis H, Allegri F, Trotta A, Ferraro L, Stilo SA, et al. Daily Use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509–1517. doi: 10.1093/schbul/sbt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Andreasson S, Allebeck P. Prognosis of schizophrenia in persons with and without a history of cannabis use. Psychol Med. 2014;44:2513–2521. doi: 10.1017/S0033291714000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 67 Suppl. 2006;9:3–8. [PubMed] [Google Scholar]
- 20.Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70:1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- 22.Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabin RA, Zakzanis KK, George TP. The effects of cannabis use on neurocognition in schizophrenia: a meta-analysis. Schizophr Res. 2011;128:111–116. doi: 10.1016/j.schres.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Yucel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM, et al. The impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample. Schizophr Bull. 2012;38:316–330. doi: 10.1093/schbul/sbq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferraro L, Russo M, O'Connor J, Wiffen BD, Falcone MA, Sideli L, et al. Cannabis users have higher premorbid IQ than other patients with first onset psychosis. Schizophr Res. 2013;150:129–135. doi: 10.1016/j.schres.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 27.Joyal CC, Halle P, Lapierre D, Hodgins S. Drug abuse and/or dependence and better neuropsychological performance in patients with schizophrenia. Schizophr Res. 2003;63:297–299. doi: 10.1016/s0920-9964(02)00387-0. [DOI] [PubMed] [Google Scholar]
- 28.McCleery A, Addington J, Addington D. Substance misuse and cognitive functioning in early psychosis: a 2 year follow-up. Schizophr Res. 2006;88:187–191. doi: 10.1016/j.schres.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 29.Potvin S, Joyal CC, Pelletier J, Stip E. Contradictory cognitive capacities among substance-abusing patients with schizophrenia: a meta-analysis. Schizophr Res. 2008;100:242–251. doi: 10.1016/j.schres.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- 31.O'Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol. 2004;18:502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- 32.Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, et al. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33:1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- 33.Verrico CD, Gu H, Peterson ML, Sampson AR, Lewis DA. Repeated delta9-tetrahydrocannabinol exposure in adolescent monkeys: persistent effects selective for spatial working memory. Am J Psychiatry. 2014;171:416–425. doi: 10.1176/appi.ajp.2013.13030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 35.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull. 2012;38:950–957. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 39.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 40.Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65:1006–1014. doi: 10.1016/j.biopsych.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 44.Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, et al. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry. 2012;169:1082–1091. doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volk DW, Edelson JR, Lewis DA. Cortical inhibitory neuron disturbances in schizophrenia: role of the ontogenetic transcription factor Lhx6. Schizophr Bull. 2014;40:1053–1061. doi: 10.1093/schbul/sbu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glausier JR, Fish KN, Lewis DA. Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry. 2014;19:30–36. doi: 10.1038/mp.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney JWE, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 48.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 49.Guidotti A, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 50.Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, et al. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- 51.Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Shannon WC. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatr Res. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: Clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology. 2010;35:2590–2599. doi: 10.1038/npp.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- 56.Karson MA, Tang AH, Milner TA, Alger BE. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci. 2009;29:4140–4154. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience. 2010;169:1651–1661. doi: 10.1016/j.neuroscience.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, et al. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hajos N, Katona I, Naiem SS, Mackie K, Ledent C, Mody I, et al. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 62.Klausberger T, Marton LF, O'Neill J, Huck JH, Dalezios Y, Fuentealba P, et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foldy C, Lee SY, Szabadics J, Neu A, Soltesz I. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci. 2007;10:1128–1130. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- 64.Karson MA, Whittington KC, Alger BE. Cholecystokinin inhibits endocannabinoid-sensitive hippocampal IPSPs and stimulates others. Neuropharmacology. 2008;54:117–128. doi: 10.1016/j.neuropharm.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 2012;35:529–558. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 68.Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci. 2007;27:1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, et al. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ludanyi A, Hu SS, Yamazaki M, Tanimura A, Piomelli D, Watanabe M, et al. Complementary synaptic distribution of enzymes responsible for synthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in the human hippocampus. Neuroscience. 2011;174:50–63. doi: 10.1016/j.neuroscience.2010.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshida T, Uchigashima M, Yamasaki M, Katona I, Yamazaki M, Sakimura K, et al. Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proc Natl Acad Sci U S A. 2011;108:3059–3064. doi: 10.1073/pnas.1012875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szabo B, Urbanski MJ, Bisogno T, Di MV, Mendiguren A, Baer WU, et al. Depolarization-induced retrograde synaptic inhibition in the mouse cerebellar cortex is mediated by 2-arachidonoylglycerol. J Physiol. 2006;577:263–280. doi: 10.1113/jphysiol.2006.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hashimotodani Y, Ohno-Shosaku T, Maejima T, Fukami K, Kano M. Pharmacological evidence for the involvement of diacylglycerol lipase in depolarization-induced endocanabinoid release. Neuropharmacology. 2008;54:58–67. doi: 10.1016/j.neuropharm.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 75.Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 80.Makara JK, Mor M, Fegley D, Szabo SI, Kathuria S, Astarita G, et al. Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci. 2005;8:1139–1141. doi: 10.1038/nn1521. [DOI] [PubMed] [Google Scholar]
- 81.Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- 82.Hermanson DJ, Hartley ND, Gamble-George J, Brown N, Shonesy BC, Kingsley PJ, et al. Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nat Neurosci. 2013;16:1291–1298. doi: 10.1038/nn.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bluett RJ, Gamble-George JC, Hermanson DJ, Hartley ND, Marnett LJ, Patel S. Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Transl Psychiatry. 2014;4:e408. doi: 10.1038/tp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown JA, Horvath S, Garbett KA, Schmidt MJ, Everheart M, Gellert L, et al. The role of cannabinoid 1 receptor expressing interneurons in behavior. Neurobiol Dis. 2014;63:210–221. doi: 10.1016/j.nbd.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shonesy BC, Bluett RJ, Ramikie TS, Baldi R, Hermanson DJ, Kingsley PJ, et al. Genetic disruption of 2-arachidonoylglycerol synthesis reveals a key role for endocannabinoid signaling in anxiety modulation. Cell Rep. 2014;9:1644–1653. doi: 10.1016/j.celrep.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramikie TS, Nyilas R, Bluett RJ, Gamble-George JC, Hartley ND, Mackie K, et al. Multiple mechanistically distinct modes of endocannabinoid mobilization at central amygdala glutamatergic synapses. Neuron. 2014;81:1111–1125. doi: 10.1016/j.neuron.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, et al. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29:2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- 88.Koethe D, Giuffrida A, Schreiber D, Hellmich M, Schultze-Lutter F, Ruhrmann S, et al. Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. The Br J Psychiatry. 2009;194:371–372. doi: 10.1192/bjp.bp.108.053843. [DOI] [PubMed] [Google Scholar]
- 89.De Marchi N, De Petrocellis L, Orlando P, Daniele F, Fezza F, Di Marzo V. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2003;2:5. doi: 10.1186/1476-511X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D. Elevated endogenous cannabinoids in schizophrenia. NeuroReport. 1999;10:1665–1669. doi: 10.1097/00001756-199906030-00008. [DOI] [PubMed] [Google Scholar]
- 91.Palkovits M, Harvey-White J, Liu J, Kovacs ZS, Bobest M, Lovas G, et al. Regional distribution and effects of postmortal delay on endocannabinoid content of the human brain. Neuroscience. 2008;152:1032–1039. doi: 10.1016/j.neuroscience.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Volk DW, Eggan SM, Lewis DA. Alterations in metabotropic glutamate receptor 1alpha and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia. Am J Psychiatry. 2010;167:1489–1498. doi: 10.1176/appi.ajp.2010.10030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Volk DW, Siegel BI, Verrico CD, Lewis DA. Endocannabinoid metabolism in the prefrontal cortex in schizophrenia. Schizophr Res. 2013;147:53–57. doi: 10.1016/j.schres.2013.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience. 2001;103:9–15. doi: 10.1016/s0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- 95.Zavitsanou K, Garrick T, Huang XF. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:355–360. doi: 10.1016/j.pnpbp.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 96.Newell KA, Deng C, Huang XF. Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp Brain Res. 2006;172:556–560. doi: 10.1007/s00221-006-0503-x. [DOI] [PubMed] [Google Scholar]
- 97.Dalton VS, Long LE, Weickert CS, Zavitsanou K. Paranoid schizophrenia is characterized by increased CB1 receptor binding in the dorsolateral prefrontal cortex. Neuropsychopharmacology. 2011;36:1620–1630. doi: 10.1038/npp.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jenko KJ, Hirvonen J, Henter ID, Anderson KB, Zoghbi SS, Hyde TM, et al. Binding of a tritiated inverse agonist to cannabinoid CB1 receptors is increased in patients with schizophrenia. Schizophr Res. 2012;141:185–188. doi: 10.1016/j.schres.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wong DF, Kuwabara H, Horti AG, Raymont V, Brasic J, Guevara M, et al. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. NeuroImage. 2010;52:1505–1513. doi: 10.1016/j.neuroimage.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wong DF, Kuwabara H, Horti AG, Brasic JR, Raymont V, Nandi A, et al. Cannabinoid receptor subtype 1 (CB1) distribution correlates with neuropsychiatric ratings. Society of Biological Psychiatry Abstract. 2012 [Google Scholar]
- 101.Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thune JJ, Uylings HBM, Pakkenberg B. No deficit in total number of neurons in the prefrontal cortex in schizophrenics. J Psychiatr Res. 2001;35:15–21. doi: 10.1016/s0022-3956(00)00043-1. [DOI] [PubMed] [Google Scholar]
- 103.Koethe D, Llenos IC, Dulay JR, Hoyer C, Torrey EF, Leweke FM, et al. Expression of CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J Neural Transm. 2007;114:1055–1063. doi: 10.1007/s00702-007-0660-5. [DOI] [PubMed] [Google Scholar]
- 104.Uriguen L, Garcia-Fuster MJ, Callado LF, Morentin B, La Harpe R, Casado V, et al. Immunodensity and mRNA expression of A2A adenosine, D2 dopamine, and CB1 cannabinoid receptors in postmortem frontal cortex of subjects with schizophrenia: effect of antipsychotic treatment. Psychopharmacology (Berl) 2009;206:313–324. doi: 10.1007/s00213-009-1608-2. [DOI] [PubMed] [Google Scholar]
- 105.Volk DW, Eggan SM, Horti AG, Wong DF, Lewis DA. Reciprocal alterations in cortical cannabinoid receptor 1 binding relative to protein immunoreactivity and transcript levels in schizophrenia. Schizophr Res. 2014;159:124–129. doi: 10.1016/j.schres.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rozenfeld R. Type I cannabinoid receptor trafficking: all roads lead to lysosome. Traffic. 2011;12:12–18. doi: 10.1111/j.1600-0854.2010.01130.x. [DOI] [PubMed] [Google Scholar]
- 107.Eggan SM, Mizoguchi Y, Stoyak SR, Lewis DA. Development of cannabinoid 1 receptor protein and messenger RNA in monkey dorsolateral prefrontal cortex. Cereb Cortex. 2010;20:1164–1174. doi: 10.1093/cercor/bhp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoftman GD, Lewis DA. Postnatal developmental trajectories of neural circuits in the primate prefrontal cortex: identifying sensitive periods for vulnerability to schizophrenia. Schizophr Bull. 2011;37:493–503. doi: 10.1093/schbul/sbr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoftman GD, Volk DW, Bazmi HH, Li S, Sampson AR, Lewis DA. Altered cortical expression of GABA-related genes in schizophrenia: Illness progression vs developmental disturbance. Schizophr Bull. 2013;41:180–191. doi: 10.1093/schbul/sbt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, et al. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eggan SM, Stoyak SR, Verrico CD, Lewis DA. Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: Comparison of schizophrenia and major depressive disorder. Neuropsychopharmacology. 2010;35:2060–2071. doi: 10.1038/npp.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eggan SM, Lazarus MS, Stoyak SR, Volk DW, Glausier JR, Huang ZJ, et al. Cortical glutamic Acid decarboxylase 67 deficiency results in lower cannabinoid 1 receptor messenger RNA expression: implications for schizophrenia. Biol Psychiatry. 2012;71:114–119. doi: 10.1016/j.biopsych.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cass DK, Flores-Barrera E, Thomases DR, Vital WF, Caballero A, Tseng KY. CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Mol Psychiatry. 2014;19:536–543. doi: 10.1038/mp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]