Abstract
Tissue engineering hydrogels are primarily cured in situ using ultraviolet (UV) radiation which limits the use of hydrogels as drug or cell carriers. Visible green light activated crosslinking systems are presented as a safe alternative to UV photocrosslinked hydrogels, without compromising material properties such as viscosity and stiffness. The objective of this study was to fabricate and characterize photocrosslinked hydrogels with well-regulated gelation kinetics and mechanical properties for the repair or replacement of soft tissue. An anhydrous methacrylation of hyaluronan (HA) was performed to control the degree of modification (DOM) of HA, verified by 1H-NMR spectroscopy. UV activated crosslinking was compared to visible green light activated crosslinking. While the different photocrosslinking techniques resulted in varied crosslinking times, comparable mechanical properties of UV and green light activated crosslinked hydrogels were achieved using each photocrosslinking method by adjusting time of light exposure. Methacrylated HA (HA-MA) hydrogels of varying molecular weight, DOM and concentration exhibited compressive moduli ranging from 1 kPa to 116 kPa, for UV crosslinking, and 3 kPa to 146 kPa, for green light crosslinking. HA-MA molecular weight and concentration were found to significantly influence moduli values. HA-MA hydrogels did not exhibit any significant cytotoxic affects towards human mesenchymal stem cells. Green light activated crosslinking systems are presented as a viable method to form natural-based hydrogels in situ.
Keywords: Hyaluronan, Injectable, Osteochondral, Visible Light, Hydrogel, methacrylation, gelation time, photocrosslinking
INTRODUCTION
The possibility of transplanting stem cells in vivo to treat diseases and injuries, in a regenerative way, is becoming a reality. While challenges exist in the successful translation of stem cell-based engineered tissues, such as articular cartilage,(1)–(3) stem cell transplantation to aid in tissue regeneration may be a viable option. The necessary requirements of a tissue engineering construct for a long-term successful regenerative approach include physiologically relevant bulk material properties for congruency in mechanical loading and energy dissipation, and the ability to biologically guide tissue regeneration.(4) However, the translation of engineering techniques in situ relies on the improvement of hydrogel implantation. One minimally invasive approach is an injectable system, in which the hydrogel sets or cures in situ to provide mechanical stability and/or serve as a cell and/or drug carrier.(5) Hydrogels are used as minimally invasive injectable scaffolds that fill focal articular cartilage lesions.(6)–(8) Injectable hydrogels offer an alternative to traditional surgical procedures by developing 3-D scaffolds that promote regeneration of cartilage.(5),(9)–(14)
Natural, biocompatible materials, such as extracellular matrix (ECM) proteins and polysaccharides, have been used to explore the effect of substrate stiffness on mesenchymal stem cell (MSC) differentiation and tissue growth.(15–16) Hyaluronan (HA) is a linear, anionic, high molecular weight polysaccharide found in soft tissue and synovial fluid that swells in the presence of water and provides lubrication to articulating surfaces and resistance to compressive loads in vivo. A wide variety of cell types express the archetypal HA receptor CD-44; CD-44-HA interactions are essential for maintaining normal cartilage homeostasis. These cellular interactions are advantageous for promoting wound healing and tissue regeneration.(17)–(19) Integrin-mediated cell-material interactions and substrate stiffness of hydrogels impact MSC differentiation.(20–22) The focus of the current study was to mechanically analyze methacrylated HA (HA-MA) hydrogels undergoing either ultraviolet (UV) or visible green light crosslinking for the purpose of achieving a wide range of elastic moduli values for various tissue engineering applications.
Through careful selection of both the DOM and molecular weight of HA, a range of mechanical properties can be achieved and optimized for the regeneration of desired tissues. Methacrylation of polysaccharides, including HA, have been reported in the literature.(23)–(25) However, most studies have not examined non-aqueous methacrylation reactions, which allow for more precise control over stoichiometric ratios and the DOM, nor do previous studies report on variance of photocrosslinking methods via different light sources and photoinitiator systems. Accurate control of the DOM allows for tunable crosslink densities and mechanical properties, with future direction focused on injectable tissue repair. This work aimed to determine how the source of light-activated covalent crosslinking of HA-based hydrogels affects rheological and mechanical properties for the development of an injectable hydrogel for tissue engineering applications. The goal of this study was to design and fabricate green light activated crosslinking systems are presented as a safe alternative to UV photocrosslinked hydrogels, without compromising material properties such as viscosity and stiffness.
MATERIALS AND METHODS
Synthesis of Methacrylated Hyaluronan (HA-MA)
Sodium HA (Lifecore Biomedical) lyophilized powders of two different molecular weights (Mw = 100 and 700 kDa) were rendered soluble in anhydrous dimethyl sulfoxide (DMSO, 99% anhydrous, Sigma Aldrich) through an ion exchange with hexadecyltrimethylammonium bromide salt (CTAB, Sigma Aldrich). Ion exchange resin (Dowex 50WX8-400) was loaded with CTA+ ions by submersion in a 1–2% (w/v) CTAB ethanol:water solution (1:1 ratio), then mixed with 1% (w/v) HA in deionized water for 24 hours at 40°C. The polymer solution was filtered to remove ion exchange resin, frozen and lyophilized. A 1% (w/v) HA-CTA/DMSO solution was reacted with methacrylic anhydride (MA, Sigma Aldrich) in the presence of a catalyst, 4-(dimethylamino)pyridine (DMAP, Sigma Aldrich), for 24 hours at room temperature. The amount of MA was adjusted to achieve varying DOMs based on molar ratios of the hydroxyl groups (modification sites) per HA repeat unit to MA. The amounts of MA utilized in this study were 1×, 1.5×, and 2× the molar quantity of hydroxyl groups. The resulting solution was hydrolyzed through extensive dialysis and periodic adjustment to pH 8 with 5N sodium hydroxide. The DOM, or ratio of methacrylate groups per repeat unit of HA-MA, was determined using 1H-NMR spectroscopy (Bruker AVANCE III 500 MHz high-field NMR spectrometer). A 1% (w/v) polymer in deuterium oxide solution was analyzed at room temperature, spinning at 20 Hz for 16 scans. The DOM was calculated by taking the ratio of the relative integrations of the methacrylate peaks (6.1, 5.6, and 1.8 ppm), and HA methyl protons (1.9 ppm).(26),(27)
Green Light Crosslinking
HA-MA solutions of different concentrations (2, 3 or 4% (w/v)) and having various molecular weights and DOMs were prepared for visible green light crosslinking. To analyze different combinations of crosslinking reactants, aqueous solutions of the following concentrations were prepared: 1 mM Eosin Y (EY, photosensitizer), 125 mM triethanolamine (TEOA, initiator), 20 mM 1-vinyl-2-pyrrolidinone (VP, catalyst).(26),(28),(29) To determine the most effective photoinitiator system for green light activation, an absorption assay was conducted on solutions containing EY, EY supplemented with TEOA, EY supplemented with VP, and EY supplemented with TEOA and VP. The solution with the highest absorbance at 530 nm was chosen as the photoinitiator system for subsequent tests.
Photo-Rheometry
Viscosity and shear stress, as functions of shear rate, were evaluated on the reaction solutions for the preparation of photocrosslinked HA-MA hydrogel. All measurements were carried out on a rheometer (AR2000, TA Instruments) equipped with a Peltier plate maintained at 25°C and a 40 mm diameter 1°59'47" steel cone geometry. 100 kDa and 700 kDa HA-MA with varying DOMs were mixed with photoinitiator solutions for either UV or green light-activated chemistries to form 3% (w/v) concentrated hydrogel pre-curser solutions. To form solutions for UV-activated photocrosslinking, a 0.05% (w/v) Irgacure D-2959 (Ciba) solution in phosphate buffered saline (PBS) was prepared.(25),(30)–(35) To form solutions for green light crosslinking, a 1 mM EY, 125 mM TEOA, 20 mM VP solution was prepared. (26),(28),(29) Aliquots of 500 μL were placed within a 27 μm gap between the Peltier plate and cone geometry. Viscosity (Pa*s) and shear stress (Pa) of the polymer solutions were determined at varied shear rates, 1–50 (1/s), at 1% radial strain over 2 minutes (n = 3) and analyzed using analytical software (TA Data Analysis).
The gelation of HA-MA hydrogels was examined immediately following shear sweep experiments. Oscillatory time sweeps were conducted at 10% radial strain and 1 Hz during exposure to UV (320 – 390 nm, Uvitron Intelliray 400) or green light (530 nm, custom 20 LED array, 14 kmCd, Victory Rush Electronics) (n = 3). Data collection was initiated upon the start of light exposure. Shear loss (G') and storage (G") moduli were recorded; tan delta (ratio of G" to G') was analyzed using analytical software (TA Data Analysis). HA-MA gelation initiation was identified at the inflection point in the tan delta curve and the terminal gelation time was approximated to occur as G’ plateaus.
Unconfined Compression Testing
Uniaxial unconfined compressive testing was performed on 2, 3 and 4% (w/v) HA-MA hydrogels crosslinked using either UV or green light to elucidate any differences in mechanical properties between the two crosslinking systems (n = 4). The effects of molecular weight and DOM were also investigated. Hydrogel pre-curser solutions were placed in a custom mold consisting of glass microscope slides and 1.6 mm thick Teflon® spacers. Molds were exposed to either UV light for 10 minutes or green light for 20 minutes at room temperature; photocrosslinking times were chosen to ensure that the hydrogels were terminally crosslinked using each method. Crosslinked samples were equilibrated in PBS, pH 7.4, for 24 hours at room temperature (n = 5) prior to testing. Specimens 6 mm in diameter and 1.6 mm in height were stamped using a biopsy punch. Compression testing was performed on a rheometer, equipped with a normal force transducer using an 8 mm parallel plate geometry and an opposing Peltier plate at 25°C. A 10% uniaxial compressive strain was applied at a rate of 10 µm/s. Force (N) and changes in gap height (μm) were obtained using analytical software (TA Universal Analysis) and were subsequently used to calculate elastic strain (ε, %) and stress (σ, kPa). The elastic modulus (E) was calculated as the slope of a linear fit between 4 and 10% compressive strain within the linear-elastic region.
Cytotoxicity Assay
The cytotoxicity of photocrosslinked hydrogels to primary bone-marrow derived human MSCs was assessed spectrophotometrically as a function of mitochondrial activity in living cells using an MTT based assay. Primary bone-marrow derived human MSCs (passage 7) were seeded in treated 48-well tissue culture polystyrene plates at a density of 20,000 cells/well in 100 µL/well of standard MSC growth medium (alpha minimum essential medium, 10% fetal bovine serum, 100 U/mL penicillin, 100 µg/mL streptomycin) and allowed to adhere for 24 hours. MSCs were incubated in the presence of various photocrosslinked HA-MA hydrogels (n = 4) at 37°C and 5% CO2. Hydrogels were fabricated using 100 kDa or 700 kDa HA-MA, 2 or 4% (w/v) polymer concentrations, and high DOMs. Hydrogels were terminally photocrosslinked prior to cell culture during exposure to either UV for 10 minutes or green light for 20 minutes. Hydrogel specimens were 6 mm wide and 2 mm tall. After 24 hours of incubation, medium was removed and cells were rinsed two times in sterile PBS. Mitochondrial activity was analyzed using an MTT-based In Vitro Toxicology Assay Kit (Sigma) following the manufacturer’s protocol with a plate reader (H1 Synergy, BioTek). Briefly, mitochondrial dehydrogenases in viable cells cleave the tetrazolium ring in MTT, yielding formazan crystals which can be dissolved and measured spectrophotometrically. Absorbance values were recorded at 570 nm with background absorbance at 690 nm deducted. Average absorbance values for the experimental samples were compared to positive control values recorded for wells containing media and cells alone.(36)
Statistical Analysis
All experiments were performed in triplicate with results reported as mean ± standard deviation. Statistical analysis was performed with a GLM procedure using Statistical Analysis System software. A fixed effect tri-factorial (concentration, molecular weight, and crosslinking method) model was generated to study the contribution of each factor to HA-MA hydrogels with a range of compressive elastic moduli. The multiple comparisons were performed based on a two-way ANOVA of individual and interaction effects of the three factors. For analyzing cytotoxicity, similar GLM procedures were preformed to obtain one-way ANOVA results. A p < 0.05 was considered significantly different.
RESULTS
HA-MA Synthesis and Characterization
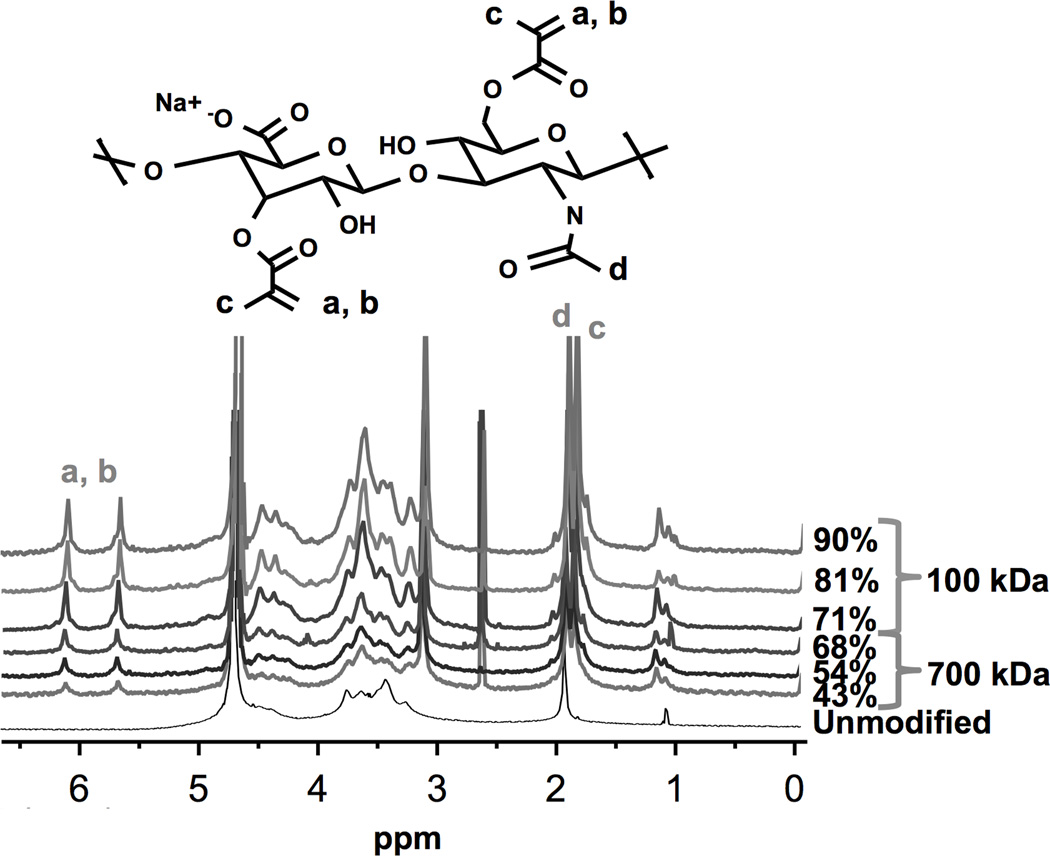
An anhydrous methacrylation of HA resulted in a functionalized polymer with a controllable DOM. Relative integrations of methacrylate groups, as identified in the chemical structure and 1H-NMR spectra (Figure 1), resulted in varied DOM for both 100 kDa and 700 kDa HA. The various DOMs achieved were 71% (low), 81% (medium), and 90% (high) for 100 kDa HA-MA, and 43% (low), 54% (medium), and 68% (high) for 700 kDa HA-MA. Higher DOMs were achieved for the lower molecular weight HA. The CTA+ groups were removed and replaced with Na+ as confirmed through HA-MA dissolution in aqueous based solutions (i.e., DI water and PBS) and 1H-NMR spectra.
Figure 1.
Chemical structure schematic of methacrylated hyaluronan (HA-MA) repeat unit (top) and 1H-NMR spectra of various HA-MA polymers. Varying degrees of modification (DOM) were achieved through controlled stoichiometric ratios of HA and methacrylic anhydride. The DOM was calculated by taking the ratio of relative integrations of methacrylate peaks (6.1, 5.6, or 1.8 ppm), and HA methyl protons (1.9 ppm). Methacrylate protons at a, b, c and HA’s methyl proton at d are identified in the chemical structure and associated 1H-NMR spectra peaks.
Green Light Crosslinking
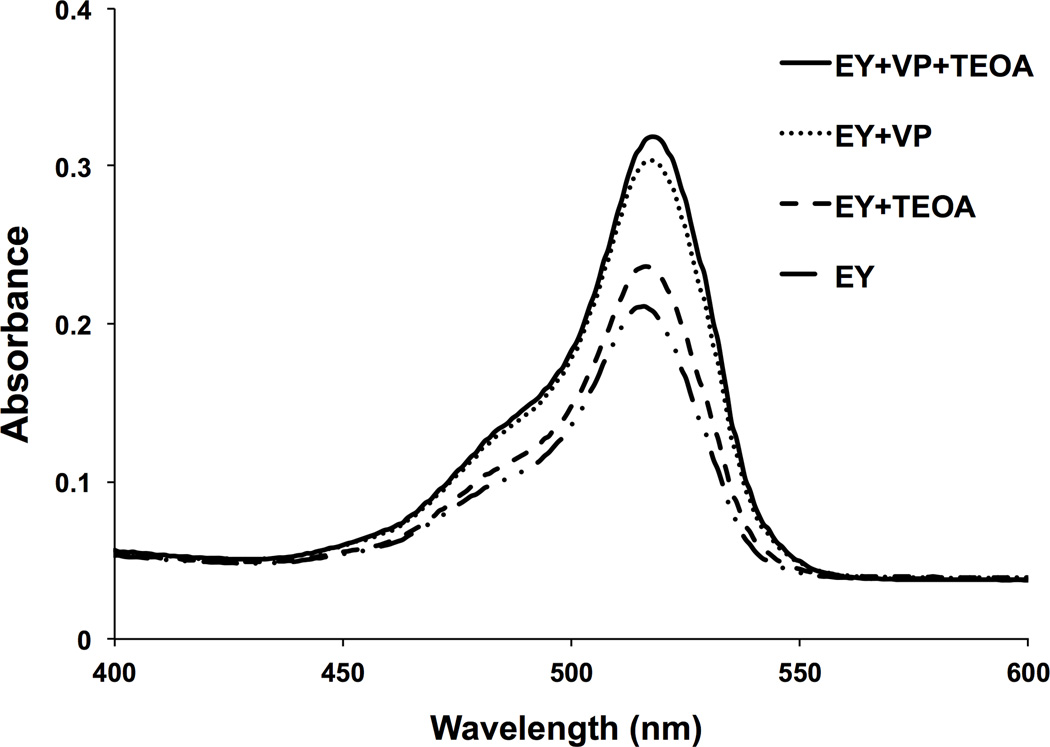
The photoinitiator system for green light crosslinking and the effects of including an initiator and a catalyst were investigated for optimum absorbance upon light exposure. An absorption assay, performed on a microplate reader, was utilized to quantify light absorbance of various photoinitiator solutions (Figure 2). The photoinitiator solution comprising 1 mM EY, 125 mM TEOA, 20 mM VP exhibited the highest absorbance at 530 nm and was thus used for green light crosslinked HA-MA hydrogels.
Figure 2.
Visible light absorption of green light-activated crosslinking reactants was quantitatively determined using an aqueous-based absorbance assay. Reactants were placed in DI water at the following concentrations: 1 mM Eosin Y (EY, photosensitizer), 125 mM triethanolamine (TEOA, initiator), 20 mM 1-vinyl-2-pyrrolidinone (VP, catalyst). Using a microplate reader, absorbance at 530 nm was recorded for solutions containing EY (dashed and dotted line), EY supplemented with TEOA (dashed line), EY supplemented with VP (dotted line), and EY supplemented with TEOA and VP (solid line).
Shear Strain Sweep Measurements
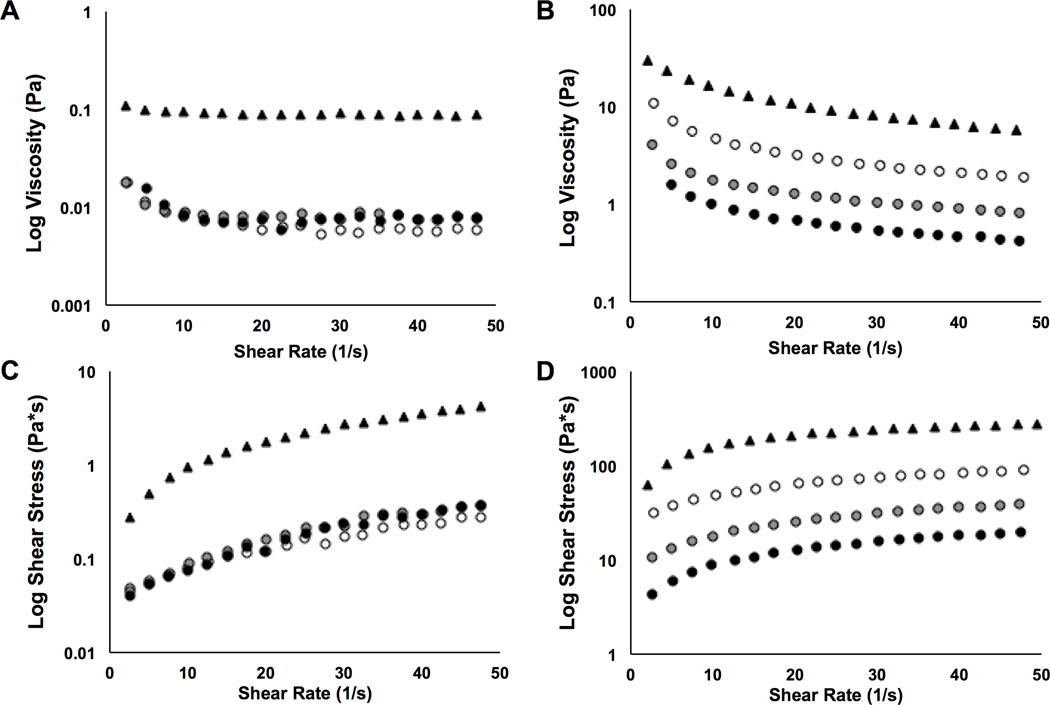
Viscosity and shear rate values for various HA-MA hydrogel pre-curser solutions varied; the effects of shear rate on viscosity and shear stress values of 3% HA and HA-MA solutions are shown in Figure 3. Viscosity values for 100 kDa HA-based solutions were lower compared the 700 kDa HA-based solutions. Solution viscosities decreased exponentially with increasing shear for all solutions tested (Figure 3A,B). The HA-MA solutions exhibited lower viscosity values compared to the HA controls. At a shear rate of 10 (1/s) and 1% displacements, all three of the 100 kDa HA-MA solutions (DOM = low, medium, high) exhibited an 11-fold decrease in viscosity values compared to the non-modified HA control (Figure 3A), while the 700 kDa HA-MA solutions exhibited 3, 9, and 16-fold decreases in viscosity values for low, medium, and high DOM compared to the HA control (Figure 3B). For the experimental groups, viscosity decreased with increasing DOM.
Figure 3.
Viscosity and shear stress as a function of shear rate are presented for HA-MA hydrogel pre-curser solutions. 100 kDa or 700 kDa HA-MA, with varying DOMs, were mixed with photoinitiators in DI water; 3% (w/v) HA-MA solutions were tested at 1% radial strain and 10 Hz. Viscosity values are shown for (A) 100 kDa and (B) 700 kDa HA-MA solutions (n = 3); shear stress values are shown for (C) 100 kDa and (D) 700 kDa HA-MA solutions (n = 3). Legend: HA control – solid triangle; HA-MA low DOM – white circles; HA-MA med DOM – grey circles; HA-MA high DOM – black circles.
Analogous to the viscosity results, the 700 kDa HA-based solutions exhibited higher shear stress values compared to the 100 kDa HA-based solutions (Figure 3C,D). The 100 kDa HA-based solutions displayed a linear increase in shear stress with increasing shear rate (Figure 3C); however, shear stress increased exponentially with increasing shear rate for all 700 kDa HA-based solutions tested (Figure 3D).
Gelation Behavior of HA-MA
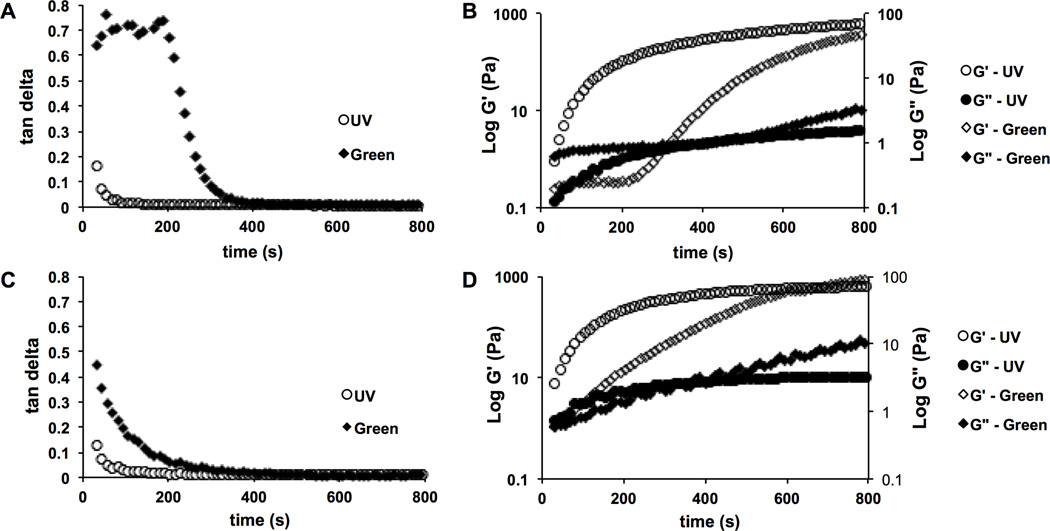
HA-MA solutions of different concentrations, in the presence of photoinitiators, were successfully crosslinked and formed into hydrogels upon exposure to either UV or green light. The gelation times of HA-MA solutions activated by either UV or green light were approximated from oscillatory time sweeps. Tan delta was plotted versus time (Figure 4A,C) and the onset of gelation was determined to be the point of inflection where the slope of the tan delta curve became negative. Due to this clear inflection, the tan delta is a clearer indication of the initiation of gelation, whereas the G’ is more indicative of terminal crosslinking as it plateaus (Figure 4B,D). Due to air exposure and warming to 37°C, dehydration was observed in polymer solutions after 800 seconds causing high variability and unreliable data (data not shown) and thus approximations for terminal gelation times for both crosslinking systems were made as follows. For the UV crosslinked hydrogels, G’ appears to plateau at approximately 600 seconds. For green light crosslinked gels, tan delta exhibits delayed gelation initiation as compared to UV and G’ fails to plateau by 800 seconds indicating that gelation had not terminated by this point. The extended gelation time for green light crosslinked gels can be attributed to the lower intensity of the LEDs used as compared to the high intensity lamp used to produce UV light. Terminal crosslinking densities were approximated to be achieved after 10 and 20 minutes for UV and green light crosslinking, respectively. Thus, these exposure times were used to form hydrogels for mechanical testing.
Figure 4.
The effect of photocrosslinking light source on gelation kinetics and hydrogel formation was analyzed using 3% (w/v) solutions of HA-MA with high degrees of modification (DOMs). Oscillatory time sweep experiments were conducted at 10% radial strain and 1 Hz. Tan delta values for (A) 100 kDa and (C) 700 kDa are plotted alongside corresponding storage (G’) and loss (G”) shear moduli values for (B) 100 kDa and (D) 700 kDa. Solutions were exposed to either UV (320–390 nm, Uvitron Intelliray 400) or green light (530 nm, custom 20 LED array, 14 kmCd, Victory Rush Electronics) to activate photocrosslinking (n = 3).
Unconfined Compression Testing
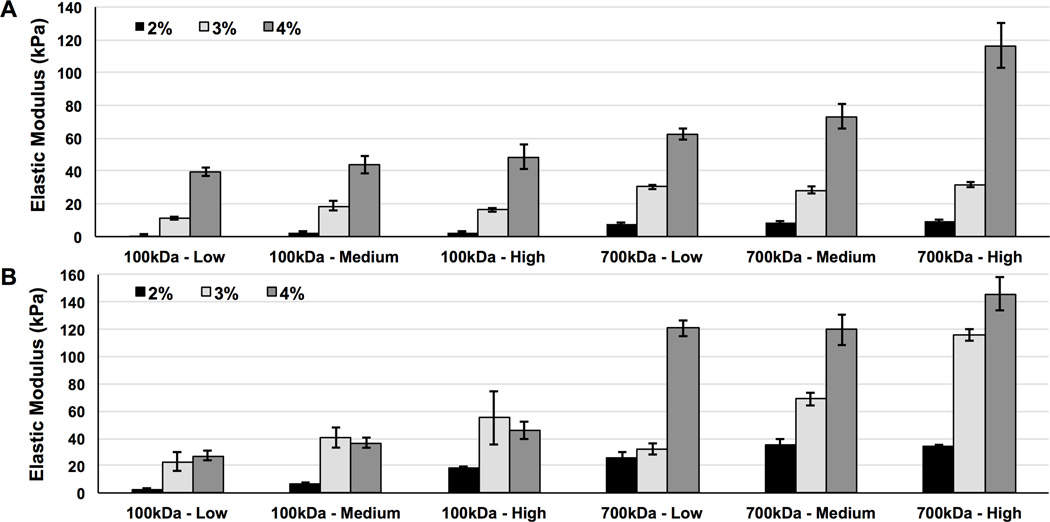
Uniaxial unconfined compression tests were conducted on UV and green light crosslinked HA-MA hydrogels. The UV crosslinked hydrogels exhibited elastic moduli values ranging from 1 kPa to 116 kPa (Figure 5A), while the green light crosslinked hydrogels exhibited elastic moduli values ranging from 3 kPa to 146 kPa (Figure 5B). The high molecular weight hydrogels (700 kDa HA) exhibited higher elastic moduli values compared to the low molecular weight hydrogels (100 kDa HA) for both photocrosslinking systems. The elastic moduli of photocrosslinked HA-MA hydrogels demonstrated significant correlations with molecular weight, DOM, and polymer concentration after performing a factorial analysis of the compressive data (p ≤ 0.003).
Figure 5.
Uni-axial unconfined compression experiments were conducted on photocrosslinked HA-MA hydrogels. The effect of polymer concentration (w/v), molecular weight, degree of modification (DOM), and light source were evaluated. (A) UV (10 minute terminal crosslinking time) and (B) green light (20 minute terminal crosslinking time) crosslinked HA-MA hydrogels consisted of low, medium, and or DOM and HA-MA concentrations of 2, 3 or 4% (w/v). Results are shown as average ± standard deviation, n = 5.
Cytotoxicity Assay
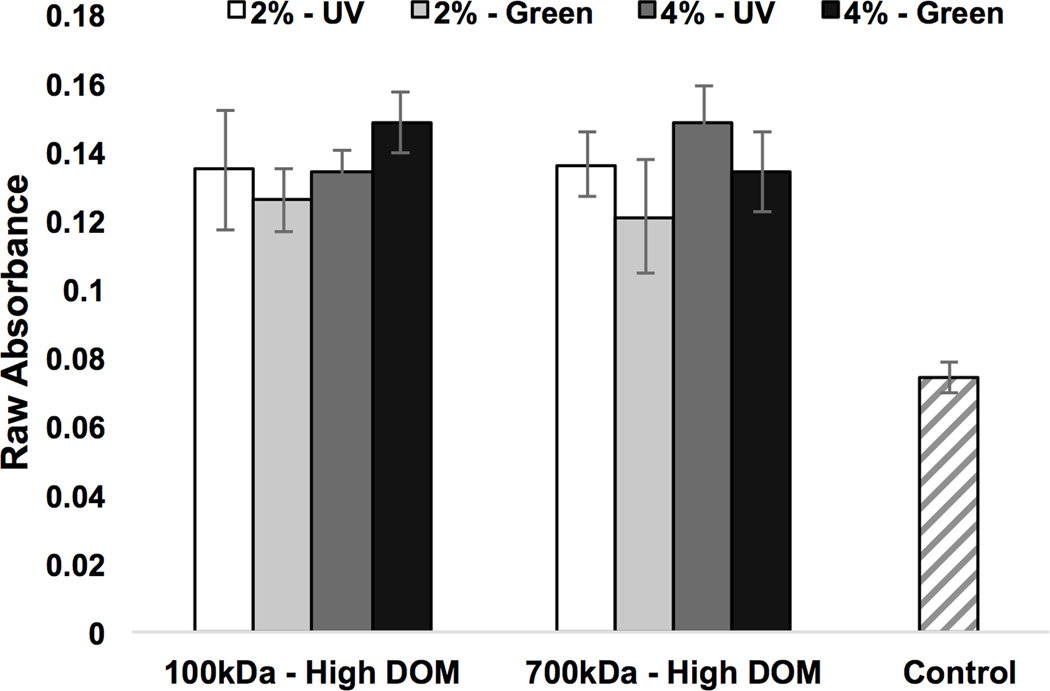
Primary human MSCs were cultured with photocrosslinked HA-MA hydrogels for 24 hours in standard MSC culture medium. Hydrogels varied by crosslinking method, molecular weight, and HA-MA concentration. Measurements were made using a MTT assay to quantify mitochondrial activity and HA-MA experimental hydrogel groups were compared to a positive control consisting of medium and cells alone (see Figure 6). Elevated absorbance corresponds to increased mitochondrial activity and viability; the HA-MA hydrogel scaffolds were found not to be cytotoxic as compared to a positive control. Increased cell mitochondrial activity was found in all four of the tested experimental groups.
Figure 6.
The cytotoxicity of photocrosslinked HA-MA hydrogels was evaluated after 24 hours of culture with primary human MSCs in standard MSC culture medium. Using a MTT assay to quantify mitochondrial activity, average absorbance values for each hydrogel experimental group were recorded and compared to control wells containing medium and MSCs alone. HA-MA with a high DOM was used. Hydrogels varied by crosslinking method (UV or green light), molecular weight (100 kDa or 700 kDa), and HA-MA concentration (2 or 4%, w/v). Elevated absorbance values indicate increased mitochondrial activity in all experimental groups.
DISCUSSION
The implementation of injectable materials curable in situ relies on hydrogel systems that are tunable and easily manipulated. Photocrosslinking systems require light exposure of a polymer solution to induce covalent crosslinking between neighboring chains. Depending on the light source and the polymer/solution conditions, the hydrogel network can vary dramatically resulting in different mechanical properties. In addition to controlling the mechanical properties of the hydrogel, ease of use is an essential requirement of the end user, with low viscosities and short gelation times preferred. Furthermore, careful selection of the light source may eliminate unwanted side effects, such as cell death, and drug and material degradation. Injectable material systems can be accompanied by a laparoscopic lens and LEDs to photocrosslink materials in situ.(5)
The methacrylation chemistry utilized in this study allowed for the controlled modification of HA and thus a broad range of DOMs was achieved. The 100 kDa HA was found to react more efficiently and increased DOMs were achieved compared to the 700 kDa HA-MA hydrogels under the same reaction and crosslinking conditions. This increase in reaction efficiency may be attributed to decreased solution viscosities in the reaction vessel, as a result of fewer intramolecular and intermolecular interaction between shorter polymer chains. Furthermore, shear strain sweeps revealed that a higher DOM resulted in decreased viscosity (Figure 3A). This is attributed to degradation of the polymer chains during the ion exchange process and the use of sodium hydroxide. Decreased viscosity may also be attributed to reduced physical interactions between adjacent HA-MA chains as a result of the conversion of hydroxyl groups to methacrylate groups. In addition, the higher molecular weight HA-based solutions, including the HA-MA experimental groups, exhibited higher viscosities compared to the lower molecular weight polymer. The trends that were seen in the viscosity data were also revealed in the shear stress data, which was expected since the two values are mathematically related. Indeed the viscosity and shear stress values complement the mechanical data collected during compression testing.
Photo-rheometry experiments revealed that the reaction kinetics for the two crosslinking methods are different. The initiation of gelation, or the gel point, was identified as the inflection point of the tan delta curve, and this value varied for each of the polymer formulations tested (Figure 4). As shown in Figure 4, the initiation of gelation for UV and green light crosslinking were different and so were the times reached as tan delta approached zero, which indicates the rate of increase of G’, the storage modulus, compared to G”, the loss modulus. For both molecular weights investigated, the green light crosslinked hydrogels took approximately twice as long as the UV crosslinked hydrogels for the tan delta to approach zero, indicating that green light required twice as much time to crosslink and for the storage modulus to equilibrate (i.e., to cease increasing). Indeed, the hydrogels are considered to be terminally crosslinked when the storage modulus, G’, no longer increases with light exposure time. The UV crosslinked hydrogels demonstrated terminal crosslinking at approximately 10 minutes, however the green light crosslinked hydrogels exhibited increasing G’ values after 10 minutes. Limitations of the material and the testing equipment resulted in fluctuating data (noise) after 13 minutes. Thus, combing the photo-rheometry, it was decided to use 10 minutes for UV crosslinked hydrogels and 20 minutes for the green light crosslinked hydrogels. Gelation times are dependent on light intensity; therefore, these times may be adjusted depending on light source and distance. Indeed, future work will also focus on optimizing the reaction kinetics of the visible light crosslinking system through chemical modifications.
The unconfined compressive elastic moduli results of the HA-MA hydrogels demonstrated that the method of photocrosslinking did not significantly impact mechanical properties, as long as the terminal degree of crosslinking was reached, which was approximated from photo-rheometry data. As shown in the literature, the molecular weight and solution concentration had more of an influence on the compressive stiffness; increasing elastic moduli were achieved with higher concentrations and higher molecular weights of HA.(37)
Indeed, the results show that the two photocrosslinking methods result in hydrogels with comparable mechanical properties; thus, visible green light it is a safe alternative to UV without comprising material performance. It is hypothesized that the green light crosslinking system may be better suited for curing in situ. All of the hydrogel formulations tested for toxicity to primary human MSCs displayed enhanced mitochondrial activity, further demonstrating terminal crosslinking and the lack of toxicity due to residual photoinitiators and or methacrylation byproducts. While the crosslinking time for the HA-MA hydrogels exposed to green light is rather long at 20 minutes, the elastic moduli achieved are relatively high for low concentration systems.(38) The use of high molecular weight HA decreases crosslinking efficiency, but results in a stiffer and tougher network hydrogel.
Hydrogel crosslink density has also been shown to influence hypertrophic differentiation of MSCs.(39) It is desirable to achieve moderate stiffness values while maintaining moderate to low crosslink densities. With the use of a relatively low viscosity HA-MA solution, the material can be injected and cured in situ to form a hydrogel using a non-invasive laparoscopic procedure. The use of visible light will enable a safer approach for the use of injectable hyaluronan hydrogel systems.
CONCLUSIONS
In this study, an anhydrous HA methacrylation was performed to control the DOM of HA, thus controlling the mechanical properties of photocrosslinked HA-MA hydrogels. UV and green light activated crosslinking systems resulted in varied gelation reaction kinetics; however, terminally crosslinked hydrogels exhibited comparable mechanical properties after exposure to UV or green light. The terminal crosslinking densities were achieved after 10 and 20 minutes for UV and green light crosslinking, respectively. Thus, these exposure times were used to form hydrogels for mechanical testing. The hydrogel elastic moduli demonstrated significant correlations with molecular weight, DOM, and polymer concentration. In addition to presenting green light as a viable crosslinking methodology for natural-based materials, the compressive elastic moduli of visible light crosslinked HA-MA hydrogels reported here expand upon values in the literature, providing a more mimetic moduli range for biomedical applications.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Meredith Koch and Tianxin Miao for assistance with data analysis, Dr. Genette McGrew at the NMR facility, and the UVM Microscopy Imaging Center. Funding was provided by NIH Grant T32 HL076122, and by the UVM College of Engineering and Mathematical Sciences as well as the College of Medicine.
Footnotes
Lastly, the authors have no conflicts of interest in this work and no benefit of any kind will be received either directly or indirectly by the authors.
REFERENCES
- 1.Anderson JA, Little D, Toth AP, Moorman CT, III, Tucker BS, Ciccotti MG, Guilak F. Stem Cell Therapies for Knee Cartilage Repair The Current Status of Preclinical and Clinical Studies. The American Journal of Sports Medicine. American Orthopaedic Society for Sports Medicine. 2013 doi: 10.1177/0363546513508744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee J, Responte D, Cissell D. Clinical translation of stem cells: insight for cartilage therapies. Critical reviews in Biotechnology. 2013 doi: 10.3109/07388551.2013.823596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman CM, Curtin C, Barry FP, O'Flatharta C, Murphy JM. Mesenchymal stem cells and osteoarthritis: remedy or accomplice? Hum Gene Ther. 2010 doi: 10.1089/hum.2010.138. [DOI] [PubMed] [Google Scholar]
- 4.Huang AH, Farrell MJ, Mauck RL. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. Journal of Biomechanics. 2010 doi: 10.1016/j.jbiomech.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Rodrigues J, Tomás H. Injectable and biodegradable hydrogels: gelation , biodegradation and biomedical applications. Chemical Society Reviews. Royal Society of Chemistry. 2012 doi: 10.1039/c1cs15203c. [DOI] [PubMed] [Google Scholar]
- 6.Evans CH, Palmer GD, Pascher A, Porter R, Kwong FN, Gouze E, Gouze J-N, Liu F, Steinert A, Betz O, Betz V, Vrahas M, Ghivizzani SC. Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue Eng. 2007 doi: 10.1089/ten.2006.0302. [DOI] [PubMed] [Google Scholar]
- 7.Wennink J, Feijen J, Dijkstra PJ. Injectable Hydrogels for Articular Cartilage Tissue Engineering. 2013 [Google Scholar]
- 8.Burdick JAJ. Injectable gels for tissue/organ repair. Biomed Mater. 2012 doi: 10.1088/1748-6041/7/2/020201. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Chen H, Li P, Diao H, Zhu S, Dong L, Wang R, Guo T, Zhao J, Zhang J. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials. 2011 doi: 10.1016/j.biomaterials.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 10.O'Shea T, Miao X. Bilayered scaffolds for osteochondral tissue engineering. Tissue Engineering Part B: Reviews. 2008 doi: 10.1089/ten.teb.2008.0327. [DOI] [PubMed] [Google Scholar]
- 11.Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential Maturation and Structure–Function Relationships in Mesenchymal Stem Cell- and Chondrocyte-Seeded Hydrogels. Tissue Engineering Part A. 2009 doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang P, et al. Engineering Orthopedic Tissue Interfaces. Tissue Engineering Part B:Reviews. 2009 doi: 10.1089/ten.teb.2008.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin I, Miot S, Barbero A, Jakob M, Wendt D. Osteochondral tissue engineering. Journal of Biomechanics. 2007 doi: 10.1016/j.jbiomech.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Mano JF, et al. Osteochondral defects: present situation and tissue engineering approaches. Journal of tissue engineering and Regenerative Medicine. 2007 doi: 10.1002/term.37. [DOI] [PubMed] [Google Scholar]
- 15.Autissier A, Le Visage C, Pouzet C, Chaubet F, Letourneur D. Fabrication of porous polysaccharide-based scaffolds using a combined freeze-drying/cross-linking process. Acta Biomaterialia. 2010 doi: 10.1016/j.actbio.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Levett PA, Melchels FPW, Schrobback K, Hutmacher DW, Malda J, Klein TJ. Chondrocyte redifferentiation and construct mechanical property development in single-component photocrosslinkable hydrogels. J Biomed Mater Res. 2013 doi: 10.1002/jbm.a.34924. [DOI] [PubMed] [Google Scholar]
- 17.Almond A. Hyaluronan. Cell Mol Life Sci. 2007 doi: 10.1007/s00018-007-7032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lapcik L, De Smedt S, Demeester J, Chabrecek P. Hyaluronan: preparation, structure, properties, and applications. Chemical reviews. 1998 doi: 10.1021/cr941199z. [DOI] [PubMed] [Google Scholar]
- 19.Fraser J, Laurent T, Laurent U. Hyaluronan: its nature, distribution, functions and turnover. Journal of internal medicine. 1997 doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 20.Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, Li S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials. 2011 doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010 doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chopra A, Murray ME, Byfield FJ, Mendez MG, Halleluyan R, Restle DJ, Aroush DR-B, Galie PA, Pogoda K, Bucki R, Marcinkiewicz C, Prestwich GD, Zarembinski TI, Chen CS, Puré E, Kresh JY, Janmey PA. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2013.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smeds KA, Pfister-Serres A, Miki D, Dastgheib K, Inoue M, Hatchell DL, Grinstaff MW. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res. 2001 doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, Schmidt CE. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010 doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 25.Leach JB, Bivens KA, Patrick CW, Schmidt CE. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol Bioeng. 2003 doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 26.Smeds KA, Grinstaff MW. Photocrosslinkable polysaccharides forin situ hydrogel formation. J Biomed Mater Res. 2000 doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 27.Oudshoorn MHM, Rissmann R, Bouwstra JA, Hennink WE. Synthesis of methacrylated hyaluronic acid with tailored degree of substitution. Polymer. 2007 [Google Scholar]
- 28.Nettles DL, Vail TP, Morgan MT, Grinstaff MW. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. 2004 doi: 10.1023/b:abme.0000017552.65260.94. [DOI] [PubMed] [Google Scholar]
- 29.Park YD, Tirelli N, Hubbell JA. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003 doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 30.Burdick JAJ, Chung CC, Jia XX, Randolph MAM, Langer RR. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005 doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leach JB, Bivens KA, Collins CN, Schmidt CE. Development of photocrosslinkable hyaluronic acid-polyethylene glycol-peptide composite hydrogels for soft tissue engineering. J Biomed Mater Res. 2004 doi: 10.1002/jbm.a.30063. [DOI] [PubMed] [Google Scholar]
- 32.Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid. Biomaterials. 2011 doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bencherif S, Srinivasan A, Horkay F, Hollinger J, et al. Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials. 2008 doi: 10.1016/j.biomaterials.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 34.Jeon O, Bouhadir KH, Mansour JM, Alsberg E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 35.Jeon O, Alsberg E. Photofunctionalization of alginate hydrogels to promote adhesion and proliferation of human mesenchymal stem cells. Tissue Eng Part A. 2013 doi: 10.1089/ten.tea.2012.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983 doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 37.Erickson IE, Huang AH, Sengupta S, Kestle S, Burdick JA, Mauck RL. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis and Cartilage. 2009 doi: 10.1016/j.joca.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu A, Gwon K, Kim M, Tae G, Kornfield JA. Visible-light-initiated thiol-acrylate photopolymerization of heparin-based hydrogels. Biomacromolecules. doi: 10.1021/bm501543a. [DOI] [PubMed] [Google Scholar]
- 39.Bian L, Hou C, Tous E, Rai R, Mauck RL, Burdick JA. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2012.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]