Abstract
Objective
To evaluate the utility of time to follow commands (TFC) in predicting functional outcome after pediatric traumatic brain injury (TBI), as assessed by an outcome measure sensitive to the range of outcomes observed after pediatric TBI, the Glasgow Outcome Scale – Extended, Pediatrics Revision (GOS-E Peds).
Setting
Pediatric inpatient rehabilitation hospital and associated multidisciplinary brain injury follow-up clinic.
Participants
67 children with moderate-to-severe TBI (mean age at injury 10.9 years, range 3–18 years).
Design
Outcomes were scored retrospectively based on documentation from an outpatient follow-up evaluation one-to-two years post injury (days from injury to follow-up: mean 518, standard deviation 137). Correlations between measures of severity and functional outcome were examined. Hierarchical logistic and linear regression analyses were performed to examine predictors of outcome.
Main Measures
Earliest documented Glasgow Coma Scale (GCS), TFC, post traumatic amnesia (PTA), total duration of impaired consciousness (TFC+PTA), GOS-E Peds.
Results
For the logistic regression, TFC and TFC+PTA were significant predictors of outcome above and beyond GCS. For the linear analysis, PTA was also a significant predictor of functional outcome above and beyond GCS and TFC. The overall models were very comparable, with R2 values ranging from .31 to .36 for prediction of GOS-E Peds.
Conclusion
Above and beyond the influence of GCS, TFC, PTA, and TFC+PTA are important predictors of later outcome after TBI.
Keywords: Traumatic Brain Injury, Child, Adolescent, Glasgow Outcome Scale, Outcome Assessment, Consciousness Disorders, Anterograde Amnesia
INTRODUCTION
Improving the accuracy of long-term outcome prediction following pediatric TBI is essential in order to better inform families about the anticipated trajectory of recovery and long-term residual needs related to injury. Moreover, accurate prediction allows for the identification and implementation of long-term supports within home and school settings. This is particularly important given the wide variability of functional, academic, vocational, and neurocognitive outcomes following pediatric TBI, even within various levels of injury severity.1
Injury severity variables including the Glasgow Coma Scale (GCS) and the Abbreviated Injury Scale (AIS), as well as markers of duration impaired consciousness, such as time to follow commands (TFC) and length of post-traumatic amnesia (PTA), have been found to be useful for predicting short and long term outcome after pediatric TBI.2–5 In particular, total duration of impaired consciousness (TFC+PTA) has been highlighted by many authors as predictive of later functional,1 behavioral,6 neurocognitive,7 and academic outcomes.8
More recently, research has begun to demonstrate the utility of TFC as a stand-alone predictor, suggesting that it may be better or at least equally as predictive as PTA or TFC+PTA.2,3 Among children with moderate-to-severe TBI who received inpatient rehabilitation, TFC accounted for the greatest portion of variance in WeeFIM (Uniform Data System for Medical Rehabilitation, 2006) scores at discharge compared to PTA and TFC+PTA.1 A follow up study by Austin and colleagues found that TFC, PTA, and TFC+PTA were all associated with functional outcome one year following discharge from inpatient rehabilitation as measured by the WeeFIM.3 These authors concluded that given similar predictive performance, TFC was the best predictor because it could be identified earlier in recovery. The authors also noted, however, that at one year following discharge, most children scored well on the motor-driven WeeFIM, resulting in a limited range of outcomes. It is possible that TFC is not as predictive when examining the broader range of outcomes after TBI.
The Glasgow Outcome Scale – Extended, Pediatric Revision (GOS-E Peds) is a recently published extension of the well validated adult version (GOS-E) designed to assess functional outcome across a wide range of settings inside and outside the home.9 Given the limitations of using the WeeFIM to evaluate the full range of longer term outcomes following TBI, the GOS-E Peds may represent a viable alternative. A validation study of the GOS-E Peds on a sample of 159 children and adolescents with mild-to-severe TBI found the GOS-E Peds to be sensitive to injury severity and to reflect change in functioning over time after TBI. Moreover, ratings of the GOS-E Peds are based on change in function compared with pre-injury status while the WeeFIM does not consider pre-injury function.
The purpose of this study was to evaluate the utility of TFC, in the context of other injury severity variables, in predicting functional outcomes after pediatric TBI as measured by the GOS-E Peds one-to-two years post injury. We hypothesized that TFC would be as strong of a predictor as initial GCS score, duration of PTA, and total duration of impaired consciousness (TFC+PTA). As a cut-off value for TFC which predicts outcome has previously been proposed based on WeeFIM outcome, we also explored the clinical utility of this TFC cut-off value for predicting outcome as measured by GOS-E Peds. As this is the first study to investigate the prediction of injury severity variables using the GOS-E Peds as a measure of long-term functional outcome, quantitative and qualitative aspects of GOS-E Peds ratings were also explored.
METHODS
The Johns Hopkins University School of Medicine’s Institutional Review Board granted approval for the study. Data were obtained from two sources. Some data were collected prospectively as part of routine clinical care and recorded in a program evaluation database; a separate research database was created from the program evaluation database. Other data points were generated for research based on medical record review.
Participants
We identified one hundred and sixty-four children, ages 3–18 years, who were admitted to a single pediatric inpatient brain injury rehabilitation unit between April 1998 and July 2011 following occurrence of a TBI. Of the 165 children, 72 had a follow-up outpatient evaluation in the associated interdisciplinary brain injury clinic at least 11 months but less than 3 years post-injury and were thus included in the primary analyses. For children with more than one follow-up visit within the specified time frame, review of records was used to identify the follow-up visit that occurred closest to one year post-injury. All 72 children had a moderate-to-severe TBI as defined by a first available GCS score less than or equal to 12 and/or the presence of injury-related intracranial neuroimaging findings. Children were not excluded due to the presence of pre-injury psychiatric diagnoses (e.g., Attention Deficit/Hyperactivity Disorder, depression, or anxiety) or receipt of special education services. No child displayed intellectual disability or significant deficits in daily functional abilities prior to injury. As detailed below, five children were ultimately excluded from all analyses due to remaining in a state of PTA at the time of their follow-up evaluation, yielding 67 children with outcome data. For the 93 children without follow-up data between 11 months and 3 years post-injury, demographic and injury severity information were collected for the purpose of comparing children with and without follow-up data in order to detect sample bias.
Measures
Glasgow Coma Scale (GCS)
The GCS is an assessment measure used to define an individual’s level of consciousness following trauma.10 For the present study, GCS is defined as the earliest available GCS score documented in available medical records. GCS scores were obtained as part of routine clinical care and collected from the program database. Consistent with previous literature, severe TBI was defined as an initial GCS score of 3–8; Moderate TBI was defined as an initial GCS score of 9–12 or 13–15 with intracranial abnormalities identified on neuroimaging.
Time to Follow Commands (TFC)
TFC was collected as part of routine clinical care and is defined as the interval in days from injury until the individual followed simple verbal commands twice in a 24 hour period as documented in acute care medical records (if it occurred prior to rehabilitation admission) or reported by rehabilitation staff. If a patient had achieved command-following by the time of rehabilitation admission but no information about TFC was available via review of medical records, then TFC was determined based on family report. When family report was used, clinicians queried types of commands followed in order to exclude that family report was based on reflexive movements.
Duration of posttraumatic amnesia (PTA)
PTA is defined as the interval in days from TFC to formation of new memories. PTA was evaluated as part of routine clinical care using age-appropriate standardized instruments (Children’s Orientation and Amnesia Test [COAT],11 Galveston Orientation and Amnesia Test [GOAT],12 Memory Orientation and Attention Test,13 or Orientation Log [O-log]).14 The end of PTA was defined by the child achieving two consecutive scores on the standardized instrument within two standard deviations of the mean for age or above the specified cutoff. For children who had achieved command-following yet remained in PTA at the time of their admission to the inpatient rehabilitation facility, a neuropsychologist or neuropsychology trainee (postdoctoral fellow or doctoral intern) evaluated PTA once daily as part of clinical care. For children who were felt to have emerged from PTA at the time of their first evaluation on the inpatient rehabilitation unit, medical record review was conducted with a focus on notes from physiatry, neurology, and/or speech or occupational therapy detailing orientation status. If medical documentation was not revealing, then parent/child report were used to provide the best clinical estimate for PTA emergence. When needed, as part of clinical care parents were asked to describe when their child was both “back to themselves” with regard to resolution of confusion/agitation and when the child was able to lay down new memories related to the current situation. For 15 children who remained in PTA following discharge, resolution from PTA was estimated via a review of medical records as the date of the first follow-up visit in which accurate orientation and current laying down of new memories was reported. If the clinical note included relevant information in the history and/or examination but did not include a clinical impression regarding resolution of PTA then the records were reviewed by all authors to obtain consensus. As previously mentioned, five children remained in a state of PTA at the time of their follow-up evaluation and were excluded from all analyses.
Total duration of impaired consciousness (TFC+PTA)
TFC+PTA was defined as the sum of the durations of TFC and PTA.
Glasgow Outcome Scale – Extended, Pediatrics Revision (GOS-E Peds)
The GOS-E Peds is an 8 item instrument designed to provide a developmentally appropriate measure of outcome following TBI in children.9 This measure assesses functional independence inside and outside of the home, capacity for work/school, participation in social and leisure activities, and family and peer interactions/psychological problems. The 8 categories of outcome are (from highest score of 8 to lowest score of 1): Dead, Vegetative State (unable to follow simple motor commands or communicate), Lower Severe Disability (always needs support in the home), Upper Severe Disability (sometimes needs support inside the home or always needs support outside of the home), Lower Moderate Disability (self-contained school program, unable to participate in social activities, or daily intolerable psychosocial difficulties), Upper Moderate Disability (reduced academic capacity, significant decrease in social/leisure participation, or frequent/weekly psychosocial difficulties), Lower Good Recovery (slightly reduced social/leisure participation, occasional psychosocial difficulties, or any other persisting TBI symptoms), and Upper Good Recovery (no identifiable difficulties related to the injury that affect daily life). As GOS-E Peds ratings for our sample were scored retrospectively based on clinical notes from the selected outpatient follow-up evaluation, our scores were limited to range from 1-to-7.
In order to assure inter-rater reliability in scoring of the GOS-E Peds by retrospective chart review, the authors planned to compare ratings completed by the primary rater (KD) to the consensus rating of two other authors (SS and BS) until a percent agreement of at least 75% was attained. After review of 15 notes, inter-rater reliability was high with quadratic weighted kappa =.87, p<.001. Once this was achieved, every fifth case was co-rated in the same manner to assess for rater drift. For notes in which ratings were discrepant, a consensus rating was obtained via adjudication discussion by all three raters. In an effort to address difficult scoring issues identified during the adjudication discussions, the investigators outlined specific decision-making criteria designed to supplement those provided by the descriptors of category scores provided by the original authors. For example, one criterion developed was that when raters were wavering between two scores, the more impaired rating was chosen. The supplemental criteria are listed in [Appendix A].
Statistical Methods
Descriptive statistics were calculated to examine the demographic, injury, and outcome characteristics of the sample. Characteristics of children with and without GOS-E Peds follow-up ratings were examined via nonparametric Mann-Whitney U tests and chi-square to detect sample bias. Chi-square and independent sample t-tests were used to evaluate the relationship between pre-injury psychiatric diagnosis or receipt of special education services and GOS-E Peds outcomes. Spearman Rho correlations were used to investigate relationships between demographic (age and gender) and injury severity variables (GCS, TFC, PTA, and TFC+PTA) and follow-up GOS-E Peds ratings. A general interpretive guide to correlation coefficient strength is as follows: small (.10), medium (.25), and large (.40).15 Variables found to be correlated with GOS-E Peds were included in regression models examining predictors of outcome.
Due to concerns with non-normality and the bimodal nature of the GOS-E Peds ratings for this cohort, hierarchical binomial logistic regression analyses were run as the primary regression analyses to examine the contribution of the predictors on long-term outcome above and beyond initial injury severity (GCS). For this purpose, GOS-E Peds ratings were dichotomized in a fashion consistent with previous research, with ratings of 1 to 3 (Upper Good Recovery to Upper Moderate Disability) considered a “favorable” outcome and ratings of 4 to 7 (Lower Moderate Disability to Vegetative State) considered “unfavorable”.16 Two separate models were examined, both starting with GCS as the initial predictor variable; the first model examined the additive benefit of TFC and then PTA, and the second model examined the additive benefit of TFC+PTA. Change in variance (Δχ2) values were examined at each step to evaluate the contribution of each predictor variable above and beyond the previously entered variable(s).
While examination of the dichotomous outcome was prioritized due to concerns with the data, reducing the 7 point outcome data to a dichotomous outcome results in loss of sensitivity to the full range of ratings achieved across the ordinal scale. Thus, a secondary analysis was performed using hierarchical linear regression. Again two separate models were examined, using predictor variables as described above for the logistic regression models, and the change in variance (ΔR2) was evaluated to examine the contribution of each predictor.
Previous studies using TFC as a predictor of good versus poor outcome using the WeeFIM have suggested that TFC of greater than 26 days is associated with worse outcome at discharge from inpatient rehabilitation and at one year follow-up.2,3 In an effort to explore the clinical utility of this cut-off value for TFC in predicting GOS-E Peds outcome, the distribution of “favorable” and “unfavorable” outcomes was evaluated with regard to a TFC cut-off value of 26 days.
RESULTS
Description of the Sample
Demographic characteristics of the study sample are presented in Table 1. The 67 children with follow-up data ranged from 3 to 18 years of age at the time of injury. Forty-two were boys (63%), and 47 were Caucasian (70%). Nineteen children (28%) had a pre-injury psychiatric diagnosis or received special education services prior to injury. Most children in this cohort were classified as having a severe injury: 87% (n=58) of the children had a GCS less than or equal to 8 at injury. The average length of total hospital stay (acute + rehabilitation) was 64 days (SD = 56 days). Follow-up data were collected an average of 517 days following injury (SD = 137), with the earliest collection 11 months post injury and the latest collection 2.9 years post injury. Skew and kurtosis values for measures of injury severity and duration of impaired consciousness were remarkable for positive skew (Kolmogorov-Smirnov p < .001).
Table 1.
Patient Characteristics (n = 67)
| Patient Characteristic | Mean | Median | SD | Range |
|---|---|---|---|---|
| Age at Injury (years) | 10.9 | 11.6 | 4 | 3.3–18.2 |
| Initial GCS (n=64) | 5.7 | 6 | 3 | 3–14 |
| TFC (days) | 15.5 | 9 | 18 | 0–102 |
| Duration of PTA (days) | 32.2 | 13 | 53 | 0–331 |
| TFC+PTA (days) | 47.6 | 21 | 65 | 3–368 |
| Length of Stay (acute care plus acute inpatient rehabilitation, in days) | 64.1 | 52 | 56 | 10–261 |
| Injury onset to GOS-E Peds (days) | 517.8 | 474 | 137 | 335–1061 |
| GOSE-Peds | 3.9 | 3 | 1 | 1–7 |
Note. GCS = Glasgow Coma Scale, TFC = Time to Follow Commands, PTA = Post-traumatic Amnesia, TFC+PTA = Total Duration of Impaired Consciousness, GOSE-Peds = Glasgow Outcome Scale – Extended, Pediatric Revision
Of the children without follow-up data (n=93), 63 were boys (68%) and 36 were Caucasian (39%). The injuries in this cohort were mostly categorized as severe (78%), and average length of hospital stay was 53 days. Children with follow-up data were more likely to be Caucasian and demonstrated significantly longer durations of time to follow commands; there was no significant difference between groups with respect to age at injury, sex, or length of stay.
GOS-E Peds Ratings and Reliability
Frequency data for GOS-E Peds scores at follow-up are provided in Figure 1. Skew and Kurtosis were suggestive of a normal distribution; however, qualitative review of the data suggested a bi-modal distribution with most children receiving ratings of 3 or 5. Only 5 children received a rating of 4.
Figure 1. Frequency Data for GOS-E Peds Scores (n = 67).
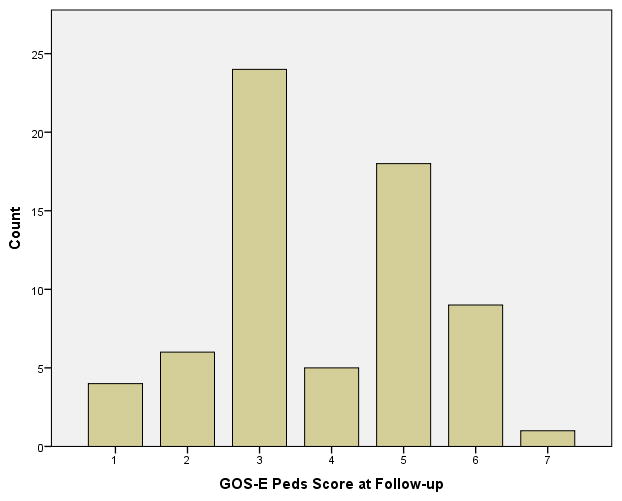
GOS-E Peds = Glasgow Outcome Scale – Extended, Pediatric Revision
Associations among Demographic Variables, Injury Severity, and Outcome
There was no difference between children with or without pre-injury psychiatric diagnosis or special education services with regard to GOS-E Peds dichotomous outcome (favorable vs unfavorable) or ordinal GOS-E Peds ratings. The results of bivariate correlation analyses examining the relationship between demographic, injury severity variables, and GOS-E Peds ratings are presented in Table 2. GCS, TFC, PTA, and TFC+PTA were significantly correlated with GOS-E Peds. Age was also significantly correlated with PTA, with older age at the time of injury related to significantly longer duration of PTA.
Table 2.
Bivariate correlations between demographic variables and injury severity variables and GOSE-Peds Scores (n=67) at Follow-up Chart Review
| Age | Gender | GCS | TFC | PTA | TFC+PTA | |
|---|---|---|---|---|---|---|
| Age | -- | -- | -- | -- | -- | -- |
| Gender | .18 | -- | -- | -- | -- | -- |
| GCS | .04 | −.09 | -- | -- | -- | -- |
| TFC | .11 | .10 | −.51** | -- | -- | -- |
| PTA | .28* | −.02 | −.33** | .60** | -- | -- |
| TFC+PTA | .24 | .02 | −.42** | .84** | .90** | -- |
| GOSE-Peds | −.10 | .01 | −.46** | .46** | .34** | .42** |
Note. GCS = Glasgow Coma Scale, TFC = Time to Follow Commands, PTA = Post-traumatic Amnesia, TFC+PTA = Total Duration of Impaired Consciousness, GOSE-Peds = Glasgow Outcome Scale – Extended, Pediatric Revision
p < 0.05;
p < 0.01
Regression Models
The results of hierarchical binomial logistic regression analyses predicting “favorable” and “unfavorable” outcome as rated on the GOS-E Peds are presented in Table 3. Because age and gender were not correlated with GOS-E Peds ratings they were not included in the regression analyses. As GCS was correlated with GOS-E Peds it was included in each analysis in order to account for initial injury severity. For the first model, TFC significantly contributed to the predictive model above and beyond GCS, and there was a trend toward PTA adding additional predictive power above and beyond GCS and TFC. For the second model, TFC+PTA significantly contributed to the overall predictive power of the model above and beyond the influence of GCS. Comparison of R2 and odds ratio values indicated that the two models yielded the same predictive power for the dichotomous GOS-E Peds outcome (Nagelkerke R2 = .31).
Table 3.
Hierarchical Regression Analyses
| Dichotomous Outcome | Continuous Outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Δχ2 | Nagelkerke R2 for each step | Odds Ratio for final model | Standard Error for final model | ΔR2 | Standardized β for each step | Standardized β for final model | VIF for final model | |
| Model 1 | ||||||||
| GCS | 6.80** | .13 | .87 | .13 | .16** | −.40 | −.21 | 1.21 |
| TFC | 6.91** | .26 | 1.02 | .03 | .13** | .40 | .20 | 1.62 |
| PTA | 3.04^ | .31 | 1.03 | .02 | .09** | .36 | .36 | 1.47 |
| Overall Model | χ2 = 16.75*, Nagelkerke R2 = .31 | Adjusted R2= .35 | ||||||
| Model 2 | ||||||||
| GCS | 6.80** | .13 | .87 | .12 | .16** | −.40 | −.22 | 1.14 |
| TFC+PTA | 9.95** | .31 | 1.03 | .01 | .22*** | .50 | .50 | 1.14 |
| Overall Model | χ2 = 16.74***, Nagelkerke R2=.31 | Adjusted R2= .36 | ||||||
p<.10;
p<.05;
p<.01;
p<.001;
VIF=Variance Inflation Factor
Results of the hierarchical multiple linear regression analyses investigating these relationships with GOS-E Peds rated as an ordinal measure revealed that for the first model, TFC significantly contributed above and beyond the influence of GCS, and then PTA made a significant contribution to outcome above and beyond GCS and TFC. For the second model, TFC+PTA again provided additional predictive power beyond GCS. The two models yielded very similar overall predictive power (R2=.35–.36).
Predictive Utility of TFC Ranges
In this sample, 92% (11/12) of the children with TFC greater than 26 days had poor outcomes (GOS-E Peds ≤ 4). The one child with TFC greater than 26 days rated as having a favorable outcome had a GOS-E Peds rating of 3 (Upper Moderate Disability). For children with TFC 26 days or less, 40% (22/55) were rated as having had an unfavorable outcome. Sensitivity and specificity values for TFC greater than 26 days predictive of an unfavorable outcome were 33% and 97%, respectively.
DISCUSSION
The primary purpose of this study was to evaluate the utility of TFC, in the context of other injury severity variables, in predicting functional outcomes after pediatric TBI as measured by the GOS-E Peds one-to-two years post injury. Our findings demonstrate that GCS, TFC, PTA, and TFC+PTA were all significantly correlated with GOS-E Peds scores one-to-two years after injury. In the hierarchical logistic regression model, TFC and TFC+PTA both added statistically significant predictive power above and beyond GCS; there was a trend toward an additive benefit of PTA above and beyond GCS and TFC though it did not reach statistical significance. In contrast, linear regression analyses indicated that, in addition to the additive nature of TFC and TFC+PTA above and beyond GCS, PTA also provided significant predictive power above and beyond GCS and TFC.
The discrepancy between these findings, suggesting an additive benefit of PTA above and beyond that of GCS and TFC, and those previously identified based on outcome assessments using the WeeFIM2,3 is likely due to the expanded range of outcomes captured by the GOS-E Peds in comparison to the WeeFIM. This is supported by the increased sensitivity of the ordinal outcome versus the dichotomous outcome to the predictive nature of PTA beyond that of TFC. Moreover, within the current sample, there was a subset of children who demonstrated a relatively short duration of TFC followed by a protracted period of impaired consciousness. It is possible that for these children, TFC becomes less useful than the predictive utility of the total duration of impaired consciousness.
Previous research has indicated that a TFC cut-off of greater than 26 days may be clinically useful in identifying those children at greatest risk for poor outcome.2,3 This finding is also supported by the current work; with almost all of the children with TFC > 26 days having GOS-E Peds scores of 4 or higher. It is important to note, however, that only 18% of the sample had TFC > 26 days and there was significantly variability in outcome for children with TFC < 26 days. Thus, while a TFC cut-off of greater than 26 days appears to be a clinically useful predictor of unfavorable long-term outcome, the reverse is not true; TFC of 26 days or less does not necessarily result in a favorable outcome.
This study also provides the opportunity to examine the GOS-E Peds as an outcome measure. Qualitative review of GOS-E Peds scores suggests that it is reliable and sensitive to the range of cognitive and behavioral changes after TBI. For example, while only two of the children in the Austin et al. study fell into the poor outcome group at 1 year follow-up based on the WeeFIM,3 a much larger proportion of children were identified as demonstrating an unfavorable outcome in this similar sample (including overlap of 12 of the same patients examined by Austin et al.) using GOS-E-Peds (n=33, 40%). Likewise, even within the favorable outcome there is a distribution, with most children falling into the 3 category, showing that there are still considerable effects of the injury. Only a very small number of children (n=4) were rated within the 1 category, which indicates that that they had returned to their baseline level of functioning.
This study also demonstrates that retrospective rating of the GOS-E Peds can be completed reliably, in a way that appears consistent with the variability in outcomes identified in children who require inpatient rehabilitation after TBI. The use of additional rating rules, as provided in the Appendix, was found to be useful for achieving reliability among raters.
One notable finding regarding GOS-E Peds outcome scores was the high frequency of ratings of 3 and 5, with only 7% (4/72) of children receiving a rating of 4. At an item level, a rating of 4 is equivalent to placement in a self-contained school program, an inability to participate in social activities, or daily intolerable psychosocial difficulties. The raters found that children who met one of these criteria frequently also required more help within the home environment than would be expected for their age, resulting in meeting criteria for the level of need indicated by a score of a 5; thus there was a high percentage of scores of a 5 versus low number of scores of a 4. This finding also likely stems from the fact that the GOS-E Peds was created as a downward extension of the adult version, the GOS-E. As such, not all of the categories may apply as distinctively to a pediatric population. On the GOS-E, a rating of 4 is indicative of an individual only capable of working in a sheltered workshop, non-competitive job, or is unable to work. Educational law warrants that children are provided environmental supports in order to be placed in the least restricted environment (Individuals with Disability Education Act, Public Law 101–476). Moreover, school reintegration/placement and capacity to engage socially are likely to be highly influenced by a variety of environmental factors (e.g., availability of non-public special education classrooms, family socioeconomic status (SES), governmental aid and supports). Given that these broader, environmental level factors may explain the generally low occurrence of children requiring a self-contained or isolated placement in our cohort, the distribution of GOS-E Peds scores should be closely examined in future work using this measure in order to identify whether the bimodal distribution is a consistent finding.
Together, these data continue to support the importance of capturing TFC, PTA and TFC+PTA as clinically relevant milestones in a child’s recovery from TBI, as each of these variables is a significant predictor of short- and long-term outcome. Moreover, given that TFC is obtained earlier in the course of recovery than PTA and TFC+PTA and provides significant predictive power for later outcomes, TFC should be considered an especially important injury severity variable, as it has the potential to guide patient care and future treatment and discharge planning earlier in the course of recovery. Although TFC may not be readily available upon a patient’s admission to inpatient rehabilitation, particularly as it often occurs during the acute care stay, in our experience TFC can often be obtained via medical record review, either through direct reports, or through descriptions of the patients functioning and presentation during acute care procedures. Moreover, although potentially less reliable, we have also found that family members are often able to provide useful information regarding the timing of milestones during the patient’s recovery. While the current data suggest that PTA and TFC+PTA provide a small amount of additional predictive power beyond TFC for predicting outcome as assessed by GOS-E Peds, prior work 11,17 suggests that PTA may be particularly important for predicting specific cognitive outcomes.
Limitations of the current study include a retrospective research design and limited information on other variables known to influence outcome following TBI, including demographic data such as socioeconomic status18 and information on family functioning (e.g., 19–21). The children in the follow-up study were more likely to be Caucasian than in the comparison group, which limits conclusions that can be generalized to multiple ethnicities. Finally, the sample only included children who were seen for follow-up evaluations in our own brain injury follow-up clinic between 11 months and 3 years after injury, therefore generalization to the larger population of children who experience moderate-to-severe TBI may be limited. For example, the children who were not seen in our follow-up clinic during this time frame had less severe injuries (shorter TFC). It is highly possible the child’s lack of return to the clinic was influenced by the family’s perception that there were no follow-up needs necessitating return to the specialty brain injury clinic. Thus, loss of these children to follow-up is assumed to have resulted in a shift toward capturing children with worse outcomes after injury. Similarly, the sample only included children who were admitted to an inpatient rehabilitation facility, again creating a bias toward children with more severe injuries and worse outcomes.
The limitations of the current study suggest areas for future research. Replication of these findings using a prospective cohort, basing GOS-E Peds ratings on an interview format would likely be beneficial. Given the importance of accurately predicting outcome as early in the recovery process as possible, additional early predictors (e.g., emergence from a minimally conscious state), as well as dynamic indicators of acute recovery (e.g., trajectory of cognitive and/or motoric recovery throughout the acute care or early inpatient rehabilitation hospital stay) should be investigated. Furthermore, use of post-resuscitation GCS rather than earliest available GCS may improve the prognostic ability of the GCS variable. The current sample may be been too small to capture a relationship between age and the variables examined; in larger, future studies, consideration should be given to analyses that stratify the patient population by age. Lastly, more nuanced measures of functional recovery (e.g., language, cognitive, motor, and behavioral) should be explored in relation to injury severity predictors with the goal of furthering specificity of prognostic capabilities.
In conclusion, the current results suggest that TFC, PTA, and TFC+PTA are important predictors of global functional outcomes 1–2 years after pediatric TBI. TFC has particular importance for predicting outcome earlier in the rehabilitation course and for the consistency of the finding of poorer outcomes in children with TFC beyond 26 days. While identified later in the clinical course, PTA and TFC+PTA show a small amount of additional predictive power for subsequent global outcome as measured by GOS-E Peds.
Acknowledgments
Source of Funding: Dr. Suskauer received a grant (K23HD061611) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
APPENDIX A. Suggestions for Scoring
Scoring Principals
If you are vacillating between two potential scores, error on the side of greater impairment
If there is a possibility that something is problematic, rate it as though it is.
If there is a possibility that a given behavior/problem is associated with the child’s brain injury, rate it as though it is
It is okay to extrapolate from difficulties reported outside of the home that there may be difficulties within the home.
“Older children” as specified on the GOS-E Peds rating form should refer to children 12 years of age and older.
Specific Scoring Clarifications
Vegetative State (Rating of 7)
The patient must present with clear and consistent ability to utter a single word or follow a command. If it is not clear and consistent then a classification of Vegetative State would likely be most appropriate.
Lower Severe Disability (Rating of 6)
A child would meet criteria for Lower Severe Disability (rating of 6) if they present with physical impairment, Intellectual Disability, or Emotional/behavioral outbursts that require a 1:1 at school.
Upper Severe Disability (Rating of 5)
If a child clearly needs a checklist or specific interventions aimed to regulate behavior, the child is likely to be at least partially dependent on others within the home, and to meet classification for Upper Severe Disability.
If the child has a 1:1 aide at school, or there is evidence for a greater need for supervision strongly consider that the child may not be independent in the home or in the community and would meet criteria for Upper Severe Disability.
Lower Moderate Disability (Rating of 4)
If the child requires a specialized non-public school placement or is placed full time in a self-contained classroom, consider a rating of Lower Moderate Disability.
Upper Moderate Disability (Rating of 3)
If the school is providing or anticipating the provision of modifications, the child should be rated as though he/she is NOT functioning in school at his/her original capacity
The initiation of a new medication in an attempt to enhance cognitive functioning (e.g., stimulant) would suggest reduced school capacity
Homework related difficulties are coded on the school (not independence in home) item.
Lower Good Recovery (Rating of 2)
If a child is cleared to “gradual return to activities” with caregiver monitoring, a coding of Lower Good Recovery would likely be appropriate if no additional concerns are reported.
Footnotes
Conflicts of Interest: For the remaining authors none were declared.
References
- 1.Babikian T, Asarnow R. Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropsychology. 2009 May;23(3):283–296. doi: 10.1037/a0015268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suskauer SJ, Slomine BS, Inscore AB, Lewelt AJ, Kirk JW, Salorio CF. Injury severity variables as predictors of WeeFIM scores in pediatric TBI: Time to follow commands is best. Journal of pediatric rehabilitation medicine. 2009;2(4):297–307. [PMC free article] [PubMed] [Google Scholar]
- 3.Austin CA, Slomine BS, Dematt EJ, Salorio CF, Suskauer SJ. Time to follow commands remains the most useful injury severity variable for predicting WeeFIM(R) scores 1 year after paediatric TBI. Brain injury : [BI] 2013;27(9):1056–1062. doi: 10.3109/02699052.2013.794964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heather NL, Derraik JG, Beca J, et al. Glasgow Coma Scale and outcomes after structural traumatic head injury in early childhood. PloS one. 2013;8(12):e82245. doi: 10.1371/journal.pone.0082245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruijs MB, Gabreels FJ, Keyser A. The relation between neurological trauma parameters and long-term outcome in children with closed head injury. European journal of pediatrics. 1993 Oct;152(10):844–847. doi: 10.1007/BF02073384. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz L, Taylor HG, Drotar D, Yeates KO, Wade SL, Stancin T. Long-term behavior problems following pediatric traumatic brain injury: prevalence, predictors, and correlates. Journal of pediatric psychology. 2003 Jun;28(4):251–263. doi: 10.1093/jpepsy/jsg013. [DOI] [PubMed] [Google Scholar]
- 7.Massagli TL, Jaffe KM, Fay GC, Polissar NL, Liao S, Rivara JB. Neurobehavioral sequelae of severe pediatric traumatic brain injury: a cohort study. Archives of physical medicine and rehabilitation. 1996 Mar;77(3):223–231. doi: 10.1016/s0003-9993(96)90102-1. [DOI] [PubMed] [Google Scholar]
- 8.Ewing-Cobbs L, Prasad MR, Kramer L, et al. Late intellectual and academic outcomes following traumatic brain injury sustained during early childhood. Journal of neurosurgery. 2006 Oct;105(4 Suppl):287–296. doi: 10.3171/ped.2006.105.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beers SR, Wisniewski SR, Garcia-Filion P, et al. Validity of a pediatric version of the Glasgow Outcome Scale-Extended. Journal of neurotrauma. 2012 Apr 10;29(6):1126–1139. doi: 10.1089/neu.2011.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974 Jul 13;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 11.Ewing-Cobbs L, Levin HS, Fletcher JM, Miner ME, Eisenberg HM. The Children’s Orientation and Amnesia Test: relationship to severity of acute head injury and to recovery of memory. Neurosurgery. 1990 Nov;27(5):683–691. discussion 691. [PubMed] [Google Scholar]
- 12.Levin HS, O’Donnell VM, Grossman RG. The Galveston Orientation and Amnesia Test. A practical scale to assess cognition after head injury. The Journal of nervous and mental disease. 1979 Nov;167(11):675–684. doi: 10.1097/00005053-197911000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Howard M. Interdisciplinary Neurobehavioral Management. Calgary: Association for Rehabilitation of the Brain Injured; 1986. Memory, orientation, and attention test. [Google Scholar]
- 14.Jackson WT, Novack TA, Dowler RN. Effective serial measurement of cognitive orientation in rehabilitation: the Orientation Log. Archives of physical medicine and rehabilitation. 1998 Jun;79(6):718–720. doi: 10.1016/s0003-9993(98)90051-x. [DOI] [PubMed] [Google Scholar]
- 15.Cohen J. A power primer. Psychological bulletin. 1992 Jul;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 16.Andelic N, Bautz-Holter E, Ronning P, et al. Does an early onset and continuous chain of rehabilitation improve the long-term functional outcome of patients with severe traumatic brain injury? Journal of neurotrauma. 2012 Jan 1;29(1):66–74. doi: 10.1089/neu.2011.1811. [DOI] [PubMed] [Google Scholar]
- 17.McDonald CM, Jaffe KM, Fay GC, et al. Comparison of indices of traumatic brain injury severity as predictors of neurobehavioral outcome in children. Archives of physical medicine and rehabilitation. 1994 Mar;75(3):328–337. doi: 10.1016/0003-9993(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 18.Heffernan DS, Vera RM, Monaghan SF, et al. Impact of socioethnic factors on outcomes following traumatic brain injury. The Journal of trauma. 2011 Mar;70(3):527–534. doi: 10.1097/TA.0b013e31820d0ed7. [DOI] [PubMed] [Google Scholar]
- 19.Yeates KO, Swift E, Taylor HG, et al. Short- and long-term social outcomes following pediatric traumatic brain injury. Journal of the International Neuropsychological Society : JINS. 2004 May;10(3):412–426. doi: 10.1017/S1355617704103093. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy ML, MacKenzie EJ, Durbin DR, et al. Health-related quality of life during the first year after traumatic brain injury. Archives of pediatrics & adolescent medicine. 2006 Mar;160(3):252–260. doi: 10.1001/archpedi.160.3.252. [DOI] [PubMed] [Google Scholar]
- 21.Anderson V, Godfrey C, Rosenfeld JV, Catroppa C. Predictors of cognitive function and recovery 10 years after traumatic brain injury in young children. Pediatrics. 2012 Feb;129(2):e254–261. doi: 10.1542/peds.2011-0311. [DOI] [PubMed] [Google Scholar]


