Abstract
Excessive activation of the cough reflex is a major clinical problem in respiratory diseases. The cough reflex is triggered by activation of nociceptive sensory nerve terminals innervating the airways by noxious stimuli. Oxidative stress is a noxious stimuli associated with inhalation of pollutants and inflammatory airway disease. Here, we discuss recent findings that oxidative stress, in particular downstream of mitochondrial dysfunction, evokes increased electrical activity in airway nociceptive sensory nerves. Mechanisms include activation of transient receptor potential (TRP) channels and protein kinase C. Such mechanisms may contribute to excessive cough reflexes in respiratory diseases.
Keywords: Cough, Nociception, Reactive Oxygen Species, Mitochondria, TRP channel, Protein kinase C
Introduction
The cough reflex serves to protect the airways of mammals from noxious substances/events. Guinea pigs and larger mammals have been shown to cough in response to multiple airway stimuli, including mechanical perturbation, acid, water and irritants [1]. Electrophysiological recordings have demonstrated that the cough reflex is dependent on the activation of sensory afferent nerves innervating the airways, and that these nerves are specifically stimulated by tussive stimuli [2, 3]. Although mice and rats do not cough, they also have similar afferent innervation of the airways.
Despite the fact that the cough reflex is in general protective, chronic cough or cough hypersensitivity is a major clinical issue. It is thought that excessive activity in cough afferents contributes to an excessive cough reflex and there has been considerable effort made in elucidating the underlying mechanisms. In particular we have focused on oxidative stress as a likely contributor to cough hypersensitivity. As we shall discuss in more detail below, the lung is exposed to many oxidative and/or electrophilic agents and, furthermore, the afferents themselves may even be the source of oxidative stress during inflammation.
Afferents involved in cough
The afferent innervation of the airways is almost exclusively derived from vagal sensory nerves [2, 4, 5]. Sensory afferents are heterogeneous with respect to protein expression, structure, conduction velocity and function. At the most basic level, sensory afferents are either (1) low threshold mechanosensors involved in the homeostatic control of breathing or (2) afferents activated by noxious stimuli (termed ‘nociceptors’) involved in defense of the airways [6]. However, further complexity of phenotypes has been described. In particular, in guinea pigs and larger mammals the vagal ganglion is split into two separate structures: the nodose vagal ganglion and the jugular vagal ganglion. The phenotype of a given airway afferent is, in part, dependent its ganglionic origin [5, 7, 8]. In mice the ganglia are fused, but the distinct nodose and jugular phenotypes have been observed within airway afferent populations [9].
Overwhelming electrophysiological and behavioral studies demonstrate that cough can be elicited through two separate but interacting afferent pathways. Firstly, cough can be elicited in anesthetized animals by mechanical punctate stimulation, acid and nonisotonic solutions (e.g. water) applied to the larynx, trachea and main bronchi [3]. These responses are dependent on the activation of nodose Aδ fibers, which are sensitive to punctate force, acid and non-isotonic solutions [2, 3]. Secondly, cough can be elicited in conscious animals by a vast range of irritants and inflammatory mediators, such as capsaicin, bradykinin, acid, acrolein, allyl isothiocyanate, cinnamaldehyde and cigarette smoke [3, 10–16]. These responses are dependent on the activation of jugular C fibers, which are polymodal sensors of noxious stimuli due to their expression of multiple ion channels and receptors (see below). Cough evoked by activation of C fibers is blocked by anesthesia. Nevertheless, C fiber activity increases the sensitivity of Aδ fiber cough under anesthesia due to pathway interactions within the brainstem [17].
Oxidative stress is a noxious stimulus
Oxidative stress is a term that defines an aberrant redox state in which phospholipids, proteins and nucleic acids are oxidized by reactive oxygen species (ROS) causing major cellular dysfunction [18]. Oxidation and autooxidation of phospholipids can also lead to further damage via the actions of electrophilic products of peroxidation and nitration (e.g. alpha-beta-unsaturated carbonyl groups such as 4-hydroxynonenal) [19].
Oxidative stress in the airways can arise from 2 distinct sources: inhalation of electrophilic irritants and endogenous inflammation. Air pollution from multiple sources is replete with oxidative/electrophilic agents such as ozone, acrolein, chlorine, aldehydes and isocyanates. The initial step in endogenous oxidative stress is the formation of the ROS superoxide, which can then converted into hydrogen peroxide and the hydroxyl radical depending on conditions [18]. Endogenous superoxide is mainly derived from NADPH oxidase, xanthine oxidase and inefficiencies in mitochondrial electron transfer within mitochondria. Under resting conditions NADPH oxidase and xanthine oxidase are inactive and only a tiny amount of superoxide is produced from mitochondria. However, with inflammatory conditions superoxide production dramatically increases. In particular, NADPH oxidase is activated in infiltrating leukocytes such as neutrophils and eosinophils as part of phagocytosis. In addition, inflammatory pathways such as TNFα[20], neurotrophins via p75NTR [21], TGFβ[22] and Toll-like Receptors [23] have been shown to increase ROS production from mitochondria. Regardless of the source, oxidative stress is a major threat to airway cellular function and as such oxidative stress can be considered a noxious stimuli.
Activation of airway C fibers by oxidative stress and electrophilic irritants
The activation of sensory nerve terminals (i.e. action potential discharge) by irritants is dependent on ion fluxes through specific irritant-sensitive ion channels in the plasma membrane. Extensive efforts have been made to understand the ion channels involved in the activation of airway C fibers by noxious stimuli. For example, the canonical noxious stimulus capsaicin [24], the pungent ingredient in chili peppers, activates airway C fibers through the gating of transient receptor potential (TRP) vanilloid 1 (V1) [25, 26]. TRPV1 expression is largely restricted to nociceptive afferents, and inhibition or genetic ablation of TRPV1 renders nerves insensitive to capsaicin. As such capsaicin-induced cough in conscious guinea pigs is abolished by TRPV1 inhibitors [27, 28].
Another member of the TRP superfamily, TRP ankyrin 1 (A1), is also selectively expressed in nociceptive sensory nerves [29]. TRPA1 was shown to be activated by multiple irritants such as the isothiocyanates, cinnamaldehyde and acrolein and ablation of TRPA1 largely abolished nociceptor activation by these irritants [29–31]. The similarities in electrophilicity, not structure, of TRPA1 activators suggested that covalent modification of TRPA1 thiols was critical for its activation. Evidence for this unusual activation mechanism was demonstrated in studies of mutant channels with select point mutations of N-terminal cysteines that had greatly reduced sensitivity to electrophiles [32, 33].
In the mouse airways, TRPA1 is expressed on the majority of slowly-conducting nociceptive C fibers [34]. These fibers are robustly activated by cinnamaldehyde and allyl isothiocyanate (AITC), and such responses are inhibited by TRPA1 inhibition or genetic ablation [34–36]. Furthermore approximately 30–40% of dissociated vagal neurons are activated by TRPA1 agonists (again abolished in TRPA1 knockout neurons) [34, 35, 37]. In vivo inhalation of AITC evoked TRPA1-dependent reflex bradypnea in mice, identical to the defensive reflexes evoked by capsaicin [38]. In the guinea pig, TRPA1 agonists activate dissociated jugular nociceptive neurons [39] and jugular C fibers innervating the airways [16]. Consistent with the reported role of jugular C fibers in cough reflexes, inhalation of AITC, cinnamaldehyde and acrolein causes cough in conscious guinea pigs in a TRPA1 inhibitor-sensitive manner [12, 13, 16]. TRPA1 agonists, like capsaicin, failed to activate nodose Aδ fibers innervating the guinea pig trachea [16].
Given that TRPA1 activation correlated with electrophilic reactivity rather than structure, we investigated the potential for other electrophiles, present in air pollution or endogenously as part of the inflammatory response, to activate TRPA1 on airway nociceptors.
We focused on the exogenous irritants toluene diisocyanate (TDI, product of the manufacture of polyurethane) and ozone (formed by the reaction of exhaust pollutants in sunlight), both of which are associated with respiratory morbidity including cough, dyspnea and chest tightness [40, 41]. Both TDI and ozone activated human TRPA1 channels when expressed in HEK293 cells [38, 42]. Furthermore, TDI and ozone activated a subset of vagal nociceptive neurons in wild-type mice but not TRPA1 knockout mice. In recordings of airway C fibers, ozone only activated TRPA1-sensitive afferents and these responses were inhibited by ruthenium red (a TRP inhibitor). Like AITC, TDI evoked bradypnea with an associated increase in ‘time of break’ in wild-type mice but not TRPA1 knockout mice.
As mentioned above, inflammation is associated with oxidative stress and the production of numerous electrophiles including 4-hydroxynonenal (4HNE), 4-oxononenal (4ONE), prostaglandin A2, 15-deoxy- Δ12,14-prostaglandin J2 and 9-nitrooeate [43–45]. Consistent with their electrophilicity, all these compounds activated human TRPA1, unlike their nonreactive analogs (e.g. prostaglandins B2 and D2 and oleic acid) [35, 37, 46, 47]. 4ONE also activated human TRPV1, albeit at higher concentrations [35]. 4HNE, prostaglandin A2, 15-deoxy- Δ12,14-prostaglandin J2 and 9-nitrooeate selectively activated dissociated nociceptive neurons, which was abolished by TRPA1 inhibition and/or genetic ablation. 4ONE activated vagal dissociated neurons via a combination of TRPA1 and TRPV1. In recordings of airway C fibers, both 4ONE and 9-nitrooeate caused robust action potential discharge. Other groups identified hydrogen peroxide as an activator of TRPA1 [46, 48], and we have recently shown that 1mM H2O2 selectively activates AITCsensitive airway C fibers, but fails to activate AITC-insensitive fibers [49]. Higher concentrations of H2O2 (120mM) activate bronchopulmonary C-fibers in the rat via a combination of TRPA1 and P2X channels [50].
Afferent terminal mitochondria as a potential source of ROS
The peripheral nerve terminals of vagal airway sensory fibers are densely packed with mitochondria [51–53]. Mitochondria can also be observed in axonal terminals of vagal neurons in long-term culture [54]. In order to further understand the functional effect of increased mitochondrial ROS on airway afferent, we have used specific inhibitors of the mitochondrial electron transfer chain. Antimycin A selectively inhibits the Qi site on complex III, resulting in superoxide formation and the subsequent production of numerous ROS, mitochondrial membrane depolarization and a decrease in ATP production [55–58]. Whereas Inhibition of the Qo site on complex III by myxothiazol causes mitochondrial membrane potential depolarization and a decrease in ATP production but only very mild superoxide production [55, 57, 58]. Furthermore, inhibition of Qo substantially reduces subsequent superoxide production evoked by inhibition of the Qi site (antimycin A) [55, 57, 58]. Finally, oligomycin inhibits complex V (also known as ATP synthase) and thus prevents ATP production by the oxidative phosphorylation machinery [59, 60]. Inhibition of complex V also evokes a mild hyperpolarization of the mitochondrial membrane potential but has little effect on superoxide production in most systems.
Surprisingly, ATP production from these terminal mitochondria does not seem to be required for the maintenance of electrical gradients (glycolysis is sufficient). Inhibition of mitochondrial ATP production failed to cause significant reductions in the activity of either peripheral afferents or dissociated vagal neurons of the course of more than 1 hour recordings [36, 49, 61]. Nevertheless, we expect that chronic exposure to these mitochondrial inhibitors may lead to loss of terminal function. Given the density of mitochondria in airway terminals and their close proximity with the transductive machinery of the terminal, we hypothesized that mitochondrial ROS may modulate airway afferent activity.
Mitochondrial ROS activate airway nociceptors via TRP channels
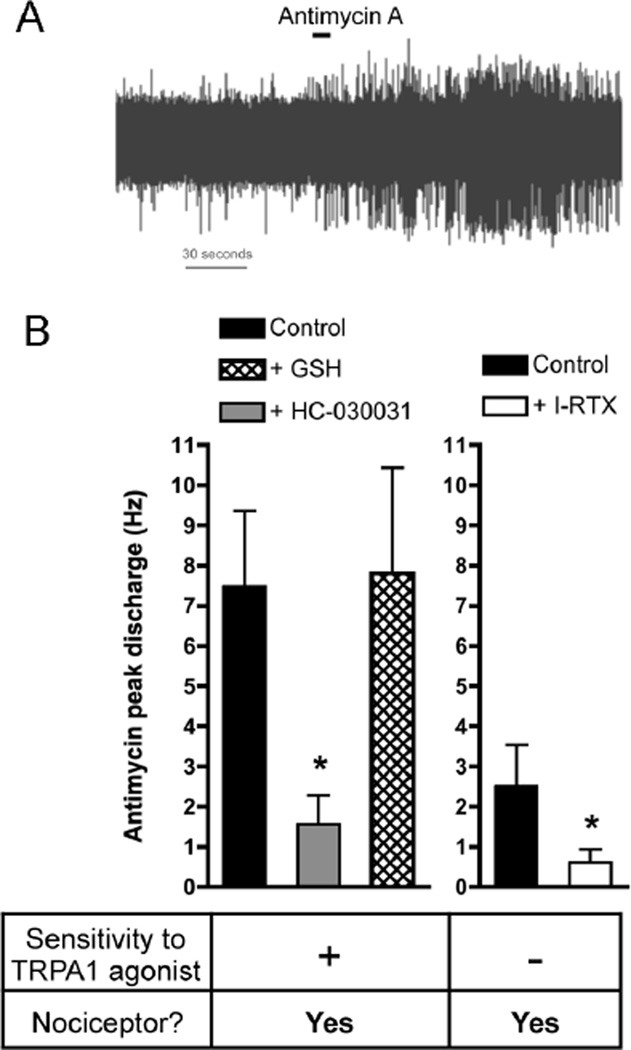
Antimycin A (20 µM), which evokes ROS from mitochondrial complex III, evoked action potential discharge from nociceptive C fiber terminals innervating the mouse airways [36]. Antimycin A-induced nociceptive C fiber activation was significantly greater in nociceptors that expressed TRPA1 compared to nociceptors that did not express TRPA1. Antimycin A failed to activate non-nociceptive fibers. Consistent with the sensitivity of TRPA1 to ROS, the antimycin A-induced action potential discharge was reduced by the TRPA1 antagonist HC-030031 (30 µM), although inhibition of TRPV1 with iodoresiniferatoxin (1 μM) further reduced C-fiber activation. Similar responses were observed in Fura 2AM Ca2+ imaging studies of dissociated mouse vagal neurons. Antimycin A (20 µM) evoked Ca2+ influx in nociceptive neurons that was inhibited by approximately 50% either by genetic ablation of TRPA1 or by HC-030031 (30 µM). The residual Antimycin A-induced Ca2+ responses were sensitive to TRPV1 inhibition. Such data suggested that TRPA1 is the primary mechanism by which mitochondrial ROS activate airway nociceptors, although TRPV1 may also contribute.
We investigated the direct effect of mitochondrial ROS on TRPA1 and TRPV1 function in HEK293 cells [36]. Antimycin A induced concentration-dependent activation of both human TRPA1 and human TRPV1 but failed to activate non-transfected cells. TRPA1 was more sensitive to mitochondrial ROS. Antimycin A-induced TRPA1 activation was prevented by pretreatment with myxothiazol, confirming the role of ROS produced from complex III. Furthermore a combination of tempol (superoxide dismutase mimetic) and MnTMPyP (super oxide dismutase and catalase mimetic) reduced antimycin A-induced TRPA1 activation by 75%. ROS such as H2O2 could activate TRPA1 either directly [46] or indirectly through their well characterized production of electrophilic lipid peroxidation products such as 4HNE [35]. Reducing agents such as dithiothreitol can prevent/reverse ROS-induced oxidation of cysteines, but have no effect on the Michael reaction underlying covalent modification of cysteines by electrophiles [46]. We found that dithiothreitol effectively reduced Antimycin A-induced TRPA1 activation but had no effect on AITC-induced TRPA1 activation, suggesting that mitochondrial ROS themselves directly activated TRPA1.
Mitochondrial ROS increase airway nociceptor excitability via PKC
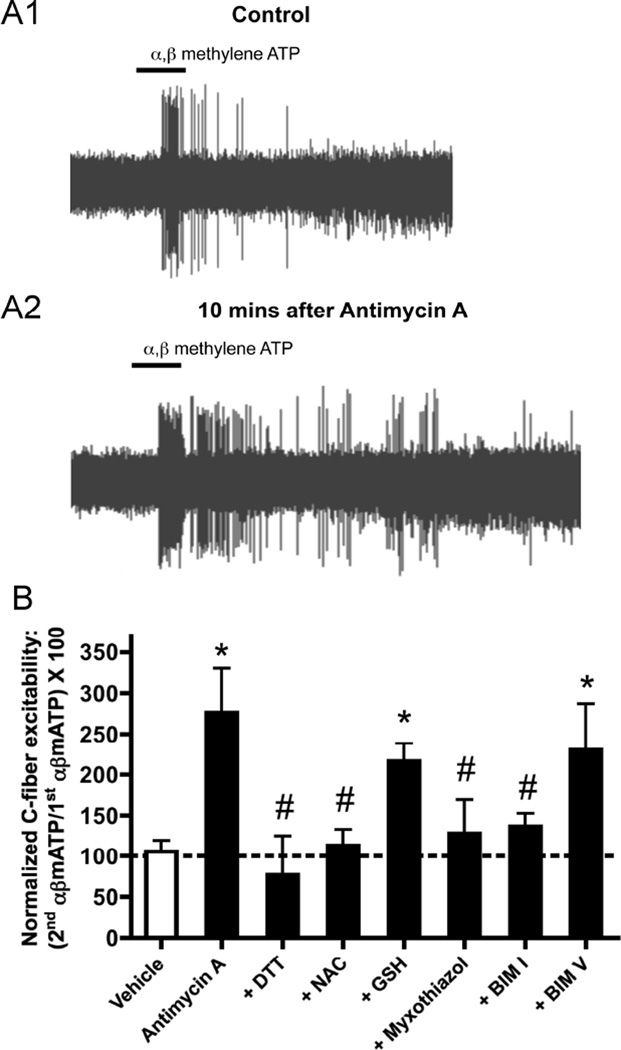
Antimycin A (20 µM) had a profound effect on the excitability of nociceptive C fibers innervating the mouse airways [49]. Antimycin A decreased the threshold sensitivity for mechanical punctate stimulation by 50% and increased the action potential firing elicited by a P2X2/3 agonist (α, β methylene ATP, 30 µM) to 270% of control. Antimycin A had no effect on the excitability of non-nociceptive airway afferents. Antimycin A-induced nociceptor hyperexcitability was independent of TRPA1 expression and was not reduced by either TRPV1 inhibition (iodoresiniferatoxin, 1 µM) or in TRPV1 knockout afferents. Neither myxothiazol nor oligomycin induced nociceptor hyperexcitability, indicating that antimycin A-induced responses were dependent on mitochondrial ROS production and not dependent on the inhibition of mitochondrial ATP production. Consistent with this, H2O2 (1mM) also increased nociceptor excitability (to 207% of control). Antimycin A-induced hyperexcitability was inhibited by dithiothreitol (membrane-permeable reducing agent) and by N-acetyl cysteine (membrane-permeable antioxidant) but not by glutathione (membrane-impermeable antioxidant), suggesting the involvement of intraneuronal ROS. Furthermore, antimycin A-induced hyperexcitability was prevented by myxothiazol pretreatment.
Previous studies of vagal nociceptors have shown that protein kinase C (PKC) is a powerful regulator of excitability [62–66]. Interestingly, many PKC isoforms can be activated by cellular oxidants (e.g. superoxide and H2O2) independently of either Ca2+ or diacylglycerol [67–69]. We therefore hypothesized that mitochondrial ROS induce airway nociceptor hyperexcitability via activation of protein kinase C. Consistent with this hypothesis antimycin A-induced hyperexcitability was reduced by approximately 80% by the PKC inhibitor bisindolylmaleimide (BIM) I, but was unaffected by the inactive analog BIM V [49]. Under resting conditions PKC is a cytosolic protein, which will translocate to the plasma membrane once activated. Using an antibody that binds all PKC isoforms, we found that antimycin A caused PKC translocation to the plasma membrane of dissociated vagal neurons. Antimycin A-induced PKC translocation was abolished by the pretreatment of the neurons with a combination of tempol and MnTMPyP, indicating a critical role of mitochondrial ROS in these responses. Furthermore, H2O2 also evoked PKC translocation.
At present, there are two major gaps in our understanding of mitochondrial ROS-induced hyperexcitability. Firstly, we have yet to determine which PKC isoform is involved. There are 15 isoforms of PKC and, based upon our preliminary RT-PCR studies, almost all are present in the vagal ganglia. Secondly, we do not yet understand how PKC activation leads to hyperexcitability. Activated PKC isoforms are capable of modifying neuronal excitability via the phosphorylation of multiple cellular targets such as ligand-gated ion channels, voltage-gated ion channels, leak channels and transporters. Nevertheless, it is important to note that non-nociceptive airway afferents were unaffected by antimycin A, and thus the putative mechanism must be selectively expressed only in the nociceptive population.
Conclusions
Oxidative stress, either in the form of ROS or electrophilic lipid product of peroxidation, causes significant increases in the activity of nociceptive neurons. Oxidative stress causes the activation of nociceptors via TRPA1 and increases the excitability of nociceptors via PKC. Nerve terminals of afferents innervating the airways are densely packed with mitochondria. ATP production from nerve terminal mitochondria is not required for continued electrical excitability. Given that multiple inflammatory pathways have been shown to stimulate mitochondrial ROS production we hypothesize that sensory terminal mitochondria function as an integrated transduction mechanism that converts inflammatory signaling into intraneuronal ROS signaling capable of potent modulation of electrical activity. Presently, our studies have only used direct modulators of the mitochondria electron transfer chain. Further studies are needed to determine the role of mitochondrial ROS signaling in aberrant airway nociceptor function in inflammation. It is possible that these pathways contribute to chronic cough and cough hypersensitivity in airway disease.
Fig. 1. Antimycin A activates nociceptive bronchopulmonary C-fibers via TRPA1 and TRPV1.
A, representative trace showing action potential discharge to antimycin A (20 µM, blocked line denotes 10s application) in an individual TRPA1-expressing bronchopulmonary C-fiber. B, Mean ± SEM peak action potential discharge from individual bronchopulmonary C-fibers in response to antimycin A, grouped according to their response to selective TRPA1 agonists (data not shown). Data in all columns only includes nociceptive wild-type, defined by conduction velocity and sensitivity to capsaicin (1 µM) (data not shown). Left, responses in control wild-type TRPA1-expressing fibers (black column) are compared with responses in the presence of 30 µM HC-030031 (gray column) and 1 mM GSH (hatched column). Right, responses in control wild-type fibers not expressing TRPA1 (black column) are compared with responses in the presence of 1 µM I-RTX. * Significant reduction compared to control (p<0.05). Adapted from [36].
Fig. 2. Antimycin A increases nociceptive bronchopulmonary C-fibers excitability.
A, representative traces of action potential discharge evoked by 10s challenge with α,β mATP (P2X2/3 agonist, 30 µM) in a nociceptive bronchopulmonary Cfiber before (control, A1) and 10 minutes after treatment with antimycin A (20 µM, A2). B, mean ± SEM response to 2nd application of α, β mATP (30 µM) normalized to response to 1st application of α, β mATP prior to either vehicle (white bar) or antimycin A (20 µM, black bars) in nociceptive C-fibers. The roles of ROS and PKC in hyperexcitability to α, β mATP were determined using pretreatment with DTT (1mM), NAC (1mM), GSH (1mM), myxothiazol (500 nM), BIM I (1 µM) or BIM V (1 µM). * Significant increase in 2nd α, β mATP-induced responses after antimycin A compared to vehicle (p<0.05). # Significant reduction in antimycin A-induced hyperexcitability to α, β mATP (p<0.05). Adapted from [49].
Acknowledgements
This work was supported by the National Heart, Lung & Blood Institute (R01HL119802, Bethesda, USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Canning BJ, Chou YL. Cough sensors. I. Physiological and pharmacological properties of the afferent nerves regulating cough. Handb Exp Pharmacol. 2009:23–47. doi: 10.1007/978-3-540-79842-2_2. [DOI] [PubMed] [Google Scholar]
- 2.Ricco MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol. 1996;496(Pt 2):521–530. doi: 10.1113/jphysiol.1996.sp021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557:543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kollarik M, Dinh QT, Fischer A, Undem BJ. Capsaicin-sensitive and -insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol. 2003;551:869–879. doi: 10.1113/jphysiol.2003.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr MJ, Undem BJ. Bronchopulmonary afferent nerves. Respirology. 2003;8:291–301. doi: 10.1046/j.1440-1843.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- 7.Kwong K, Kollarik M, Nassenstein C, Ru F, Undem BJ. P2X2 Receptors Differentiate Placodal vs Neural Crest C-fiber Phenotypes Innervating Guinea Pig Lungs and Esophagus. Am J Physiol Lung Cell Mol Physiol. 2008;295:L858–L865. doi: 10.1152/ajplung.90360.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lieu T, Kollarik M, Myers AC, Undem BJ. Neurotrophin and GDNF family ligand receptor expression in vagal sensory nerve subtypes innervating the adult guinea pig respiratory tract. Am J Physiol Lung Cell Mol Physiol. 2011;300:L790–L798. doi: 10.1152/ajplung.00449.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nassenstein C, Taylor-Clark TE, Myers AC, Ru F, Nandigama R, Bettner W, et al. Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J Physiol. 2010;588:4769–4783. doi: 10.1113/jphysiol.2010.195339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsberg K, Karlsson JA, Theodorsson E, Lundberg JM, Persson CG. Cough and bronchoconstriction mediated by capsaicin-sensitive sensory neurons in the guinea-pig. Pulm Pharmacol. 1988;1:33–39. doi: 10.1016/0952-0600(88)90008-7. [DOI] [PubMed] [Google Scholar]
- 11.Laude EA, Higgins KS, Morice AH. A comparative study of the effects of citric acid, capsaicin and resiniferatoxin on the cough challenge in guinea-pig and man. Pulm Pharmacol. 1993;6:171–175. doi: 10.1006/pulp.1993.1023. [DOI] [PubMed] [Google Scholar]
- 12.Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, et al. TRPA1 Agonists Evoke Coughing in Guinea-pig and Human Volunteers. Am J Respir Crit Care Med. 2009;180:1042–1047. doi: 10.1164/rccm.200905-0665OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andre E, Gatti R, Trevisani M, Preti D, Baraldi PG, Patacchini R, et al. Transient receptor potential ankyrin receptor 1 is a novel target for pro-tussive agents. Br J Pharmacol. 2009;158:1621–1628. doi: 10.1111/j.1476-5381.2009.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkatasamy R, McKenzie A, Page CP, Walker MJ, Spina D. Use of within-group designs to test anti-tussive drugs in conscious guinea-pigs. J Pharmacol Toxicol Methods. 2010;61:157–162. doi: 10.1016/j.vascn.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Kanezaki M, Ebihara S, Gui P, Ebihara T, Kohzuki M. Effect of cigarette smoking on cough reflex induced by TRPV1 and TRPA1 stimulations. Respir Med. 2012;106:406–412. doi: 10.1016/j.rmed.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Brozmanova M, Mazurova L, Ru F, Tatar M, Kollarik M. Comparison of TRPA1- versus TRPV1-mediated cough in guinea pigs. Eur J Pharmacol. 2012;689:211–218. doi: 10.1016/j.ejphar.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol. 2005;569:559–573. doi: 10.1113/jphysiol.2005.093153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karihtala P, Soini Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. Apmis. 2007;115:81–103. doi: 10.1111/j.1600-0463.2007.apm_514.x. [DOI] [PubMed] [Google Scholar]
- 19.Blair IA. Endogenous glutathione adducts. Curr Drug Metab. 2006;7:853–872. doi: 10.2174/138920006779010601. [DOI] [PubMed] [Google Scholar]
- 20.Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol. 2001;24:762–768. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- 21.Pehar M, Vargas MR, Robinson KM, Cassina P, Diaz-Amarilla PJ, Hagen TM, et al. Mitochondrial superoxide production and nuclear factor erythroid 2-related factor 2 activation in p75 neurotrophin receptor-induced motor neuron apoptosis. J Neurosci. 2007;27:7777–7785. doi: 10.1523/JNEUROSCI.0823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michaeloudes C, Sukkar MB, Khorasani NM, Bhavsar PK, Chung KF. TGF-{beta} regulates Nox4, MnSOD and catalase expression and IL-6 release in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2010 doi: 10.1152/ajplung.00134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 25.Lee LY, Lundberg JM. Capsazepine abolishes pulmonary chemoreflexes induced by capsaicin in anesthetized rats. J Appl Physiol. 1994;76:1848–1855. doi: 10.1152/jappl.1994.76.5.1848. [DOI] [PubMed] [Google Scholar]
- 26.Kollarik M, Undem BJ. Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild-type and TRPV1−/− mice. J Physiol. 2004;555:115–123. doi: 10.1113/jphysiol.2003.054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lalloo UG, Fox AJ, Belvisi MG, Chung KF, Barnes PJ. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol. (1985) 1995;79:1082–1087. doi: 10.1152/jappl.1995.79.4.1082. [DOI] [PubMed] [Google Scholar]
- 28.Trevisani M, Milan A, Gatti R, Zanasi A, Harrison S, Fontana G, et al. Antitussive activity of iodo-resiniferatoxin in guinea pigs. Thorax. 2004;59:769–772. doi: 10.1136/thx.2003.012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 30.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 31.Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, et al. An ion channel essential for sensing chemical damage. J Neurosci. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 34.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, et al. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol. 2008;586:1595–1604. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, et al. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol. 2008;586:3447–3459. doi: 10.1113/jphysiol.2008.153585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nesuashvili L, Hadley SH, Bahia PK, Taylor-Clark TE. Sensory nerve terminal mitochondrial dysfunction activates airway sensory nerves via transient receptor potential (TRP) channels. Mol Pharmacol. 2013;83:1007–1019. doi: 10.1124/mol.112.084319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor-Clark TE, Ghatta S, Bettner W, Undem BJ. Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol. 2009;75:820–829. doi: 10.1124/mol.108.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor-Clark TE, Kiros F, Carr MJ, McAlexander MA. Transient receptor potential ankyrin 1 mediates toluene diisocyanate-evoked respiratory irritation. Am J Respir Cell Mol Biol. 2009;40:756–762. doi: 10.1165/rcmb.2008-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butcher BT, Salvaggio JE, Weill H, Ziskind MM. Toluene diisocyanate (TDI) pulmonary disease: immunologic and inhalation challenge studies. J Allergy Clin Immunol. 1976;58:89–100. doi: 10.1016/0091-6749(76)90110-x. [DOI] [PubMed] [Google Scholar]
- 41.Mudway IS, Kelly FJ. Ozone and the lung: a sensitive issue. Mol Aspects Med. 2000;21:1–48. doi: 10.1016/s0098-2997(00)00003-0. [DOI] [PubMed] [Google Scholar]
- 42.Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol. 2010;588:423–433. doi: 10.1113/jphysiol.2009.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4- hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 44.Stamatakis K, Perez-Sala D. Prostanoids with cyclopentenone structure as tools for the characterization of electrophilic lipid-protein interactomes. Ann N Y Acad Sci. 2006;1091:548–570. doi: 10.1196/annals.1378.096. [DOI] [PubMed] [Google Scholar]
- 45.Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d'Ischia M. Nitro-fatty acid formation and signaling. J Biol Chem. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor-Clark TE, Undem BJ, Macglashan DW, Jr, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-Induced Activation of Nociceptive Neurons via Direct Interaction with Transient Receptor Potential A1 (TRPA1) Mol Pharmacol. 2008;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi N, Mizuno Y, Kozai D, Yamamoto S, Kiyonaka S, Shibata T, et al. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels (Austin) 2008;2:287–298. doi: 10.4161/chan.2.4.6745. [DOI] [PubMed] [Google Scholar]
- 49.Hadley SH, Bahia PK, Taylor-Clark TE. Sensory Nerve Terminal Mitochondrial Dysfunction Induces Hyperexcitability in Airway Nociceptors via Protein Kinase C. Mol Pharmacol. 2014;85:839–848. doi: 10.1124/mol.113.091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin YJ, Hsu HH, Ruan T, Kou YR. Mediator mechanisms involved in TRPV1, TRPA1 and P2X receptor-mediated sensory transduction of pulmonary ROS by vagal lung Cfibers in rats. Respir Physiol Neurobiol. 2013;189:1–9. doi: 10.1016/j.resp.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Hung KS, Hertweck MS, Hardy JD, Loosli CG. Innervation of pulmonary alveoli of the mouse lung: an electron microscopic study. Am J Anat. 1972;135:477–495. doi: 10.1002/aja.1001350404. [DOI] [PubMed] [Google Scholar]
- 52.Hung KS, Hertweck MS, Hardy JD, Loosli CG. Ultrastructure of nerves and associated cells in bronchiolar epithelium of the mouse lung. J Ultrastruct Res. 1973;43:426–437. doi: 10.1016/s0022-5320(73)90019-1. [DOI] [PubMed] [Google Scholar]
- 53.von During M, Andres KH. Structure and functional anatomy of visceroreceptors in the mammalian respiratory system. Prog Brain Res. 1988;74:139–154. doi: 10.1016/s0079-6123(08)63008-3. [DOI] [PubMed] [Google Scholar]
- 54.Hooper JS, Hadley SH, Mathews A, Taylor-Clark TE. Store-operated calcium entry in vagal sensory nerves is independent of Orai channels. Brain Res. 2013;1503:7–15. doi: 10.1016/j.brainres.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gyulkhandanyan AV, Pennefather PS. Shift in the localization of sites of hydrogen peroxide production in brain mitochondria by mitochondrial stress. J Neurochem. 2004;90:405–421. doi: 10.1111/j.1471-4159.2004.02489.x. [DOI] [PubMed] [Google Scholar]
- 56.Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 58.Tretter L, Takacs K, Hegedus V, Adam-Vizi V. Characteristics of alpha-glycerophosphate-evoked H2O2 generation in brain mitochondria. J Neurochem. 2007;100:650–663. doi: 10.1111/j.1471-4159.2006.04223.x. [DOI] [PubMed] [Google Scholar]
- 59.Hool LC, Corry B. Redox control of calcium channels: from mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2007;9:409–435. doi: 10.1089/ars.2006.1446. [DOI] [PubMed] [Google Scholar]
- 60.Sipos I, Tretter L, Adam-Vizi V. Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals. J Neurochem. 2003;84:112–118. doi: 10.1046/j.1471-4159.2003.01513.x. [DOI] [PubMed] [Google Scholar]
- 61.Gover TD, Moreira TH, Kao JP, Weinreich D. Calcium regulation in individual peripheral sensory nerve terminals of the rat. J Physiol. 2007;578:481–490. doi: 10.1113/jphysiol.2006.119008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gu Q, Lee LY. Hypersensitivity of pulmonary chemosensitive neurons induced by activation of protease-activated receptor-2 in rats. J Physiol. 2006;574:867–876. doi: 10.1113/jphysiol.2006.110312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gu Q, Lee LY. Effect of protease-activated receptor 2 activation on single TRPV1 channel activities in rat vagal pulmonary sensory neurons. Exp Physiol. 2009;94:928–936. doi: 10.1113/expphysiol.2009.047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsumoto S, Yoshida S, Ikeda M, Tanimoto T, Saiki C, Takeda M, et al. Effect of 8-bromo-cAMP on the tetrodotoxin-resistant sodium (Nav 1.8) current in small-diameter nodose ganglion neurons. Neuropharmacology. 2007;52:904–924. doi: 10.1016/j.neuropharm.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 65.Kagaya M, Lamb J, Robbins J, Page CP, Spina D. Characterization of the anandamide induced depolarization of guinea-pig isolated vagus nerve. Br J Pharmacol. 2002;137:39–48. doi: 10.1038/sj.bjp.0704840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ikeda M, Yoshida S, Kadoi J, Nakano Y, Mastumoto S. The effect of PKC activity on the TTX-R sodium currents from rat nodose ganglion neurons. Life Sci. 2005;78:47–53. doi: 10.1016/j.lfs.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 67.Cosentino-Gomes D, Rocco-Machado N, Meyer-Fernandes JR. Cell Signaling through Protein Kinase C Oxidation and Activation. Int J Mol Sci. 2012;13:10697–10721. doi: 10.3390/ijms130910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gopalakrishna R, Anderson WB. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci U S A. 1989;86:6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knapp LT, Klann E. Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J Biol Chem. 2000;275:24136–24145. doi: 10.1074/jbc.M002043200. [DOI] [PubMed] [Google Scholar]