Abstract
Objectives
Policing is considered a high-stress occupation and officers have elevated cardiovascular morbidity and mortality. To investigate a potential connection, we evaluated the association between salivary cortisol response to a high-protein meal challenge and the metabolic syndrome (MetSyn), a subclinical disorder associated with increased cardiovascular risk.
Methods
Cross-sectional data were from the Buffalo Cardio-Metabolic Occupational Police Stress Study (2004–2009). MetSyn was defined as having ≥3 components: abdominal obesity, hypertension, elevated triglycerides, reduced high-density lipoprotein cholesterol, and glucose intolerance. Officers provided five saliva samples for cortisol analysis, one before challenge (high-protein shake) and four at 15-minute intervals thereafter, where the usual response is increase. Regression models were used to examine trends in mean number of MetSyn components across quartiles of area under the curve (AUC) salivary cortisol. Patterns of mean cortisol response were assessed by MetSyn status using repeated-measures analysis of covariance.
Results
Prevalence of MetSyn was 25.7% among 373 officers (74.0% male). The mean count of MetSyn components decreased (1.89, 1.75, 1.55, 1.37; P < 0.01) across increasing quartiles of AUC salivary cortisol. Mean salivary cortisol decreased from baseline (5.55, 4.58, 4.47, 4.79, 4.75 nmol/L) in officers with MetSyn and increased (5.08, 5.82, 5.92, 5.82, 5.60 nmol/L) in their counterparts. The test for interaction between MetSyn status and time of saliva collection was statistically significant (p < 0.001).
Conclusions
Reduced cortisol response to a high-protein meal challenge may be associated with MetSyn. Future longitudinal studies could provide useful evidence for planning intervention studies on cardiovascular risk among police officers.
Keywords: metabolic syndrome, high-protein meal challenge, cortisol, cardiovascular diseases, police
Introduction
Work stress can be reflected in deleterious physical and emotional responses to job requirements (i.e., stressors) that differ from the capabilities, resources, or needs of the worker. In the United States, 40% of workers have reported that their job is “very or extremely stressful” (National Institute for Occupational Safety and Health, 1999). Effort-reward imbalance at work is related to increased cardiovascular risk (Peter et al., 1998; Siegrist et al., 1997; Vrijkotte et al., 1999). Policing is considered to be an inherently stressful occupation. Police officers have higher cardiovascular disease (CVD) morbidity and mortality than expected and have more adverse levels of traditional (e.g., dyslipidemia) and non-traditional (e.g., sudden physical exertion) CVD risk factors compared to the general population (Franke et al., 2002; Gershon et al., 2002; Hartley et al., 2011b; Joseph et al., 2009; Joseph et al., 2010; Marmar et al., 2006; Zimmerman, 2012). Cardiovascular disease accounts for 7% of on-duty fatal events, a problem recognized by the Hometown Heroes Act of 2003 that provides death benefits for fatal heart attack or stroke associated with strenuous or stressful activities performed in the line of duty (Zimmerman, 2012). With 780,000 active-duty police officers in the United States, and an expected increase to 821,000 by 2022 (U.S. Bureau of Labor Statistics), there may be significant potential for public health impact through prevention of work-related illness.
The relationship between stress and CVD may develop in physiologic responses that can occur when an individual is exposed to a stressor and can involve activation of the hypothalamic-pituitary adrenal (HPA) axis. This axis is a major part of the neuroendocrine system that controls the reactions to stress or exposure and regulates many body processes. Functionally, this axis involves a complex set of direct influences and feedback interactions among the hypothalamus, the pituitary gland, and the adrenal glands. Activation of the HPA axis results in a release of the glucocorticoid, cortisol, which can have metabolic consequences among its many effects. Responses to a stressor may also involve activation of the sympathetic nervous system (SNS) which is constantly active at a basic level to maintain homeostasis. The SNS is best known for mediating the neuronal and hormonal stress response commonly known as the fight-or-flight response, and the products of this activation act on the cardiovascular system by resulting in increases in blood pressure and heart rate (Anagnostis et al., 2009; McEwen, 1998). Extended and/or repeated exposure to stressors resulting in dysregulation of the HPA axis and SNS, along with the physiologic processes they control, may be associated with the development of the metabolic syndrome (MetSyn) (Bjorntorp and Rosmond, 2000a; Brunner et al., 2002; Chandola et al., 2006), a clustering of risk factors that may progress to subclinical CVD, and ultimately to clinical presentation of CVD (Gade et al., 2010). For example, constant excessively high levels of cortisol such as those found in Cushing’s disease result in a deposition of abdominal fat, one of the components of MetSyn (Lee et al., 2014). Although the relationship between stress and CVD develops gradually over time, an intermediate assessment of the functioning of the HPA axis, using a metabolic challenge and subclinical metabolic and cardiovascular disease indices, may prove valuable for workers in an occupation more likely to develop CVD.
Food intake can be used as a challenge to assess the functioning of the HPA axis. Quigley and Yen (1979) described a novel observation of a midday surge of cortisol levels connected with food intake. The magnitude and time course of the midday peak were markedly attenuated by food deprivation (Quigley and Yen, 1979). Follenius and colleagues evaluated how diurnal status may play a role in food stimulated cortisol secretion. At midday, but not evening, a reproducible, rapidly increasing meal-related peak appeared in fasting and non-fasting subjects. The midday peak was observed in subjects with differing dietary schedules (Follenius et al., 1982). Gibson and colleagues then demonstrated that an increased cortisol response after a protein-rich meal could be measured in saliva and that this response was repeatable (Gibson et al., 1999). Salivary cortisol can offer a non-invasive measurement of stress, is an excellent indicator of the amount of biologically active (e.g., unbound) serum concentration of cortisol (Kirschbaum and Hellhammer, 1994). Thus, salivary cortisol levels in response to various conditions (e.g., time of day) and challenges (e.g., high-protein meal at midday) can provide information about the status and functioning of the HPA axis.
The components of the MetSyn: 1) abdominal obesity, 2) hypertension, 3) elevated triglycerides, 4) reduced high-density lipoprotein cholesterol, and 5) glucose intolerance provide an established method to identify risk for subclinical metabolic and cardiovascular disease (Bjorntorp and Rosmond, 2000a). The connection between cortisol and abdominal obesity has been studied widely. Hypercortisolism and abdominal obesity induced by exposure to stressors are associated with a two to three-fold increase in all-cause mortality risk and reduced life expectancy by several years (Chrousos, 2000). Dysregulation of the HPA axis has been observed with abdominal obesity (Duclos et al., 2005) and elevated waist-to-hip ratio, independent of body mass index (Ljung et al., 2000). Cortisol levels collected during perceived stressful events along with abdominal obesity have been associated with endocrine, metabolic, and hemodynamic complications (Rosmond et al., 1998). In contrast, little evidence was found that the MetSyn or its components were associated with cortisol output or patterns in a population-based study of subclinical CVD (DeSantis et al., 2011). As exposure to frequently repeated or chronic environmental stress challenges is often associated with HPA axis abnormality (Rosmond and Bjorntorp, 2000), our study examined associations of HPA axis function with measures of subclinical metabolic and cardiovascular disease among workers in the high-stress occupation of policing. This was accomplished by evaluation of the association between cortisol response to a high-protein challenge and MetSyn status and also by identification of which MetSyn components are most strongly associated with the cortisol response.
Methods
Subjects were from the Buffalo Cardio-Metabolic Occupational Police Stress (BCOPS) Study, a cross-sectional study conducted during 2004–2009. All 710 police officers in the Buffalo, New York Police Department were invited to participate and 464 (65%) officers accepted. Of the 431 officers who were currently employed, 377 provided all needed salivary cortisol samples, and MetSyn status was known for 414 officers. A total of 373 police officers met both criteria and were included in the analytic sample. All subjects were sworn officers and willingly participated. The study protocol was approved by The State University of New York at Buffalo Institutional Review Board and the National Institute for Occupational Safety and Health Human Subjects Review Board.
Salivary Cortisol
Prior to the clinic visit, officers were asked to refrain from use of stimulants or smoking on the day of the challenge and received a 290 calorie standardized breakfast (protein, 12 grams; carbohydrate, 46 grams; fat, 6 grams) for a non-fasting cortisol sample. A standardized high-protein challenge was delivered in the clinic at midday, a time of day when this challenge provokes a response in the HPA axis similar to that from a stress stimulus (Follenius et al., 1982; Gibson et al., 1999; Quigley and Yen, 1979). The shake consisted of 55 grams of a commercially-available protein powder mixed with carbohydrate powder in milk, corresponding to protein intake equivalent to two tuna sandwiches. The shake contained 710 calories (protein, 65 grams; carbohydrate, 77 grams; fat, 15 grams).
Five saliva samples were collected using a commercial device, a Salivette®, consisting of an absorbent cotton roll, a plastic container with a perforated bottom, and a centrifuge tube that holds the plastic container and roll. A baseline sample was taken prior to ingestion of the high-protein shake, and four additional samples were taken beginning 15 minutes after the shake, and then 3 others at 15-minute intervals thereafter. This approach of sequential sampling over time is more informative than a single measure in assessing how well the HPA axis is functioning because it shows the pattern of the response to the challenge. The usual response is a 2–4 fold increase after a high-protein meal. In comparison, the cortisol response is diminished for an individual experiencing chronic or prolonged stressor exposure (Gibson et al., 1999; Rosmond and Bjorntorp, 1998; Rosmond et al., 1998).
Following collection, samples were stored at 4°C and later shipped to the National Institute for Occupational Safety and Health where they were centrifuged to provide a nonviscous saliva sample. Prepared samples were stored at −20°C until assayed for cortisol by a time-resolved immunoassay with fluorescence detection (DELFIA) (Dressendorfer et al., 1992) at the University of Dresden, Dresden, Germany. For quality control purposes, each batch contained samples of sufficient number to include a low, medium, and high concentration quality control sample on each assay plate to allow assessment of laboratory drift. Also included were 5% blind replicate samples of participants’ saliva for general quality control purposes. Samples with values outside of normal range were re-assayed. These procedures are a modified version of those employed by Gibson and colleagues (Gibson et al., 1999). The protein shake was a substitute for an actual meal for convenience of administration during the officers’ daylong clinic visit that involved several other clinical measurements.
Metabolic syndrome
The MetSyn criteria were based on the National Cholesterol Education Program Adult Treatment Panel guidelines with recent modifications from the American Heart Association (AHA) and the National Heart, Lung, and Blood Institute (NHLBI) (Grundy et al., 2005). The five MetSyn components include: 1) elevated waist circumference (≥102 cm in men, ≥88 cm in women), 2) elevated triglycerides (≥150 mg/dL or reported treatment with nicotinic acid or fibrates), 3) reduced high-density lipoprotein cholesterol (HDL-C < 40 mg/dL in men, < 50 mg/dL in women, or reported treatment with nicotinic acid or fibrates), 4) elevated glucose (≥100 mg/dL or reported treatment for diabetes), and 5) elevated blood pressure (BP ≥130/85 mmHg or reported physician-diagnosed hypertension and antihypertensive treatment). An individual was considered to have this syndrome if three or more of the five components were present.
The specific laboratory and clinic procedures were conducted by trained laboratory personnel. The procedures were measurement of waist circumference (abdominal girth at the highest point of the iliac crest and the lowest point of the costal margin in the mid-axillary line), collection of a 12-hour fasting blood sample by a certified phlebotomist, and blood pressure using the average of the second and third of three separate measurements of resting systolic and diastolic blood pressure obtained with a standard sphygmomanometer while in the clinic. Blood parameters were measured by standard laboratory techniques on the Beckman Coulter LX20 clinical chemistry analyzer and included a blood lipid panel for HDL-C and triglycerides and chemistry panels for glucose. Medication use was ascertained through self-report and by inventory of current medications brought to the clinic.
Statistical methods
We used analysis of variance (ANOVA) to characterize the trend in the mean number of MetSyn components across quartiles of the total salivary cortisol response, expressed as area under the curve (AUC) relative to the baseline salivary cortisol measurement (Fekedulegn et al., 2007). Linear regression was used to assess the statistical significance of this relationship. In this analysis, the overall cortisol response was conceptualized as the independent variable. In contrast, we also conceptualized the salivary cortisol response as the dependent variable and used repeated-measures ANOVA to assess the mean salivary cortisol response over time by MetSyn status and by the status of individual MetSyn components. Repeated-measures ANOVA accounted for the lack of independence between the repeated salivary cortisol measurements and modeled the within-subject correlation using an unstructured covariance model. We used this approach to test the null hypothesis that the two time courses were parallel by assessing the significance of the interaction term between the timing of the cortisol sample and MetSyn status. The cortisol concentration data were log-transformed due to its skewed nature and to approximate a normal distribution for the analyses. The reported results are back-transformed values. All models were adjusted for age and sex, except the unadjusted models that estimated the effect of each MetSyn component.
Results
Table 1 presents characteristics of the 373 police officers of which 276 were men and 97 were women. On average, officers were 41 years of age and had 14.5 years of police service. The average number of MetSyn components was 1.6 overall and 25.7% of the officers met the criteria for the MetSyn. The most prevalent component was reduced HDL-C (41.8%) and the least prevalent was elevated fasting glucose (23.1%). Several officer characteristics were identified from the literature as potential confounders and evaluated by their associations with MetSyn and salivary cortisol (Table 2). Based on these associations, the final models were only age and sex-adjusted. Table 3 presents the mean salivary cortisol concentration by number of MetSyn components. The mean salivary cortisol concentration increased following the high-protein shake for officers with fewer than three components, while officers with three or more components experienced a decline in mean salivary cortisol concentration. On average, an increasing number of components showed a more diminished cortisol response.
Table 1.
Demographic, lifestyle, and physiological characteristics
| Characteristicsa,b | Total (N=373) N (%) |
|---|---|
| Male | 276 (74.0) |
| Metabolic syndrome components | |
| Abdominal obesity | 113 (30.3) |
| Elevated blood pressure | 142 (38.1) |
| Elevated fasting glucose | 86 (23.1) |
| Reduced HDL cholesterol | 156 (41.8) |
| Elevated triglyceride | 116 (31.1) |
| Metabolic syndrome | 96 (25.7) |
|
| |
| Mean (S.D.) | |
| Age (years) | 41.0 (7.1) |
| Police service (years) | 14.5 (7.5) |
| Alcohol (drinks/week) | 5.0 (8.0) |
| Body mass index (kg/m2) | 29.0 (4.5) |
| Systolic blood pressure (mmHg) | 120.7 (12.3) |
| Diastolic blood pressure (mmHg) | 77.3 (10.2) |
| Physical activity/week (METS) | 284.2 (46.3) |
| Abdominal height (cm) | 20.7 (3.4) |
| Waist circumference (cm) | 93.8 (14.1) |
| Fasting glucose (mg/dL) | 92.6 (13.3) |
| HDL cholesterol (mg/dL) | 46.3 (14.7) |
| Triglycerides (mg/dL) | 136.7 (130.7) |
| Mean number of components | 1.6 (1.4) |
For categorical variables, the values are numbers and percentages.
For continuous variables the values are means and standard deviations.
Table 2.
Demographic, lifestyle, and physiological characteristics by number of metabolic syndrome components and AUCi in salivary cortisol
| Characteristics | MetSyn Components | Salivary Cortisol AUCi | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % | Mean | S.D. | Mean | S.D. | |
| Age group (years) | ||||||
| < 40 | 158 | 42.4 | 1.35 | 1.32 | 21.66 | 284.89 |
| 40–49 | 167 | 44.8 | 1.72 | 1.46 | 12.86 | 267.51 |
| ≥ 50 | 48 | 12.9 | 2.35 | 1.47 | 43.62 | 323.66 |
| p-valuea | <.0001 | 0.6232 | ||||
| Sex | ||||||
| Male | 276 | 74.0 | 1.92 | 1.44 | 1.28 | 288.39 |
| Female | 97 | 26.0 | 0.85 | 1.10 | 75.36 | 202.50 |
| p-valueb | <.0001 | 0.0201 | ||||
| Race | ||||||
| White | 281 | 76.8 | 1.63 | 1.42 | 20.34 | 272.63 |
| Black | 79 | 21.6 | 1.63 | 1.47 | 17.15 | 276.08 |
| Hispanic | 6 | 1.6 | 1.83 | 1.17 | −46.25 | 81.30 |
| p-valueb | 0.9400 | 0.8371 | ||||
| Education | ||||||
| ≤ High school/GED | 38 | 10.2 | 1.87 | 1.34 | 30.00 | 277.73 |
| College < 4 yrs | 202 | 54.5 | 1.62 | 1.43 | 34.18 | 286.60 |
| College ≥ 4 yrs | 131 | 35.3 | 1.63 | 1.48 | −3.81 | 241.57 |
| p-valuea | 0.3771 | 0.4981 | ||||
| Years of service | ||||||
| 0–9 yrs | 104 | 28.0 | 1.23 | 1.25 | 41.00 | 300.86 |
| 10–14 yrs | 84 | 22.6 | 1.49 | 1.48 | −7.13 | 266.37 |
| 15–19 yrs | 83 | 22.4 | 1.78 | 1.35 | 17.53 | 309.53 |
| ≥ 20 yrs | 100 | 27.0 | 2.11 | 1.53 | 25.87 | 199.95 |
| p-valuea | <.0001 | 0.8646 | ||||
| Smoking status | ||||||
| Current | 62 | 16.8 | 1.55 | 1.47 | 30.35 | 303.27 |
| Former | 83 | 22.5 | 1.76 | 1.57 | 78.53 | 282.95 |
| Never | 224 | 60.7 | 1.65 | 1.39 | −1.39 | 255.39 |
| p-valueb | 0.6785 | 0.0695 | ||||
| Rank | ||||||
| Police officer | 274 | 73.9 | 1.53 | 1.40 | 21.44 | 265.31 |
| Sergeant/Lieutenant | 48 | 12.9 | 1.96 | 1.56 | 13.67 | 303.07 |
| Captain/Detective | 49 | 13.2 | 2.02 | 1.48 | 24.00 | 273.90 |
| p-valueb | 0.6330 | 0.9795 | ||||
| Alcohol drinks/week | ||||||
| 0 | 71 | 19.4 | 1.32 | 1.27 | 64.66 | 258.13 |
| < 1 | 61 | 16.7 | 1.67 | 1.50 | 1.33 | 290.94 |
| 1–6 | 143 | 39.1 | 1.79 | 1.40 | 2.97 | 262.74 |
| ≥ 6 | 91 | 24.9 | 1.58 | 1.51 | 21.84 | 272.77 |
| p-valuea | 0.2091 | 0.3459 | ||||
| Physical activity intensity | ||||||
| 0–1.50 | 93 | 24.9 | 1.78 | 1.50 | −31.47 | 249.38 |
| 1.51–6.50 | 90 | 24.1 | 1.57 | 1.37 | 45.63 | 261.77 |
| 6.51–14.50 | 93 | 24.9 | 1.80 | 1.42 | 20.51 | 279.43 |
| ≥ 14.51 | 97 | 26.0 | 1.43 | 1.46 | 47.18 | 285.43 |
| p-valuea | 0.2112 | 0.0898 | ||||
Test for trend;
ANOVA (test of differences in means); AUCi (area under the curve increase)
Table 3.
Mean salivary cortisol concentration (nmol/L) by number of metabolic syndrome components
| Number of components | N | Baseline | +15 min | +30 min | +45 min | +60 min |
|---|---|---|---|---|---|---|
| 0 | 101 | 4.76 (1.82) | 5.42 (1.84) | 5.47 (1.86) | 5.64 (1.90) | 5.75 (1.86) |
| 1 | 91 | 6.42 (1.95) | 7.10 (1.92) | 7.61 (1.75) | 7.10 (1.92) | 6.62 (1.90) |
| 2 | 85 | 5.21 (2.01) | 6.23 (1.70) | 6.05 (1.75) | 5.99 (1.75) | 5.53 (1.82) |
| 3 | 49 | 6.49 (2.05) | 5.93 (2.18) | 5.58 (2.36) | 5.58 (1.88) | 5.64 (1.72) |
| 4 | 30 | 5.31 (2.03) | 4.53 (1.97) | 4.39 (1.79) | 5.31 (1.68) | 4.95 (1.82) |
| 5 | 17 | 7.32 (1.45) | 4.22 (3.16) | 4.66 (2.69) | 4.95 (2.41) | 5.21 (1.77) |
Standard deviation values in parentheses.
Table 4 presents the ANOVA and ANCOVA results. A statistically significant decrease was identified in the mean number of MetSyn components across increasing quartiles of AUC salivary cortisol. The mean number of MetSyn components was 2.03 (S.D. 1.54) for officers in the first quartile of AUC salivary cortisol and 1.35 (S.D. 1.36) for officers in the fourth quartile. The trend differed very little after adjustment for age and sex.
Table 4.
Mean number of metabolic syndrome components by quartiles of AUCi in salivary cortisol (nmol/L)
| Salivary Cortisol AUCi | Unadjusted
|
Age-adjusted
|
Age- and sex-adjusted
|
||||
|---|---|---|---|---|---|---|---|
| N | Mean | S.D. | Mean | S.E. | Mean | S.E. | |
| Q1 | 93 | 2.03 | 1.54 | 2.00 | 0.14 | 1.89 | 0.14 |
| Q2 | 93 | 1.63 | 1.41 | 1.72 | 0.14 | 1.75 | 0.14 |
| Q3 | 92 | 1.54 | 1.37 | 1.50 | 0.14 | 1.55 | 0.14 |
| Q4 | 94 | 1.35 | 1.36 | 1.34 | 0.14 | 1.37 | 0.13 |
| p-value | 0.0007 | 0.0004 | 0.0030 | ||||
Q1: (−1,265.87 to −106.775), Q2: (−106.776 to 19.51), Q3: (19.52 to 143.565), Q4: (143.566 to 1,067.70)
AUCi (area under the curve increase)
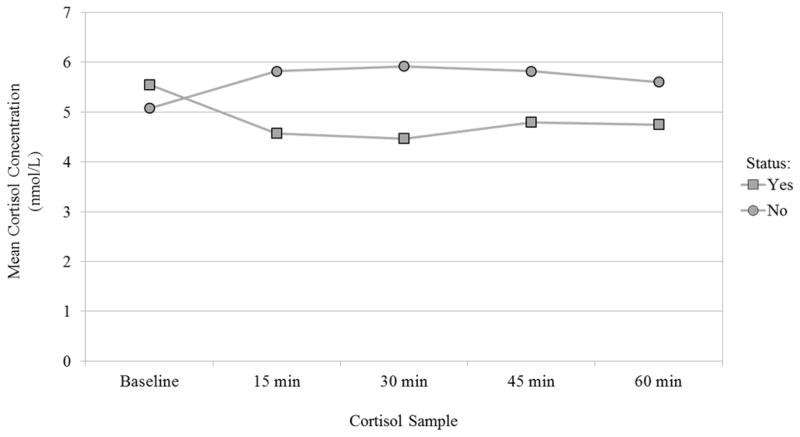
Figure 1 displays results from the repeated-measures ANOVA where we assessed the age and sex-adjusted mean cortisol response following the high-protein shake by MetSyn status. Individuals with the MetSyn had a diminished response following the shake, while individuals without the MetSyn showed an initial increase after the shake followed by a gradual decline. Effect modification between MetSyn status and time since the high-protein shake was statistically significant (p = 0.0002).
Fig. 1.
Adjusted mean salivary cortisol response by metabolic syndrome status (N = 373, p = 0.0002) nmol/L nanomoles per liter
Table 5 presents the age- and sex-adjusted results for the repeated measures ANOVA by MetSyn status and unadjusted results for the status of the individual MetSyn components. Effect modification was statistically significant between time since the high-protein shake and the individual components of abdominal obesity (p = 0.0167) and hypertension (p = 0.0356). Effect modification was not statistically significant between time since the high-protein shake and elevated triglycerides (p = 0.0504), reduced HDL-C (p = 0.0797), or with glucose intolerance (p = 0.5410). Although for elevated triglycerides, there is little difference following the sample at 15 minutes after the shake, but a slight shift appeared in the two response patterns.
Table 5.
Mean salivary cortisol response (nmol/L) by metabolic syndrome and component status
| Baseline | +15 min | +30 min | +45 min | +60 min | p-valueb | |
|---|---|---|---|---|---|---|
|
|
||||||
| Metabolic syndromea | ||||||
| Yes | 5.55 (1.04) | 4.58 (1.04) | 4.47 (1.04) | 4.79 (1.04) | 4.75 (1.04) | 0.0002 |
| No | 5.08 (1.07) | 5.82 (1.07) | 5.92 (1.07) | 5.82 (1.07) | 5.60 (1.07) | |
| Abdominal obesity | ||||||
| Yes | 6.05 (1.07) | 5.39 (1.07) | 5.34 (1.06) | 5.43 (1.06) | 5.31 (1.06) | 0.0167 |
| No | 5.42 (1.04) | 6.13 (1.04) | 6.22 (1.04) | 6.22 (1.04) | 6.01 (1.04) | |
| Elevated blood pressure | ||||||
| Yes | 5.91 (1.06) | 5.64 (1.06) | 5.48 (1.06) | 5.77 (1.06) | 5.76 (1.05) | 0.0356 |
| No | 5.42 (1.05) | 6.06 (1.05) | 6.24 (1.04) | 6.09 (1.04) | 5.81 (1.04) | |
| Elevated triglycerides | ||||||
| Yes | 6.30 (1.06) | 5.77 (1.07) | 5.75 (1.06) | 6.06 (1.06) | 5.77 (1.06) | 0.0504 |
| No | 5.31 (1.04) | 5.96 (1.04) | 6.02 (1.04) | 5.93 (1.04) | 5.80 (1.04) | |
| Reduced HDL cholesterol | ||||||
| Yes | 6.14 (1.06) | 5.90 (1.06) | 5.85 (1.06) | 5.96 (1.05) | 5.63 (1.05) | 0.0797 |
| No | 5.25 (1.05) | 5.89 (1.05) | 6.00 (1.05) | 5.98 (1.04) | 5.91 (1.04) | |
| Glucose intolerance | ||||||
| Yes | 5.24 (1.08) | 5.08 (1.08) | 5.25 (1.07) | 5.37 (1.07) | 5.02 (1.07) | 0.5410 |
| No | 5.72 (1.04) | 6.17 (1.04) | 6.16 (1.04) | 6.16 (1.04) | 6.04 (1.04) | |
Age and sex-adjusted model. Models for individual components are unadjusted. Standard error values in parentheses.
The P-value is for the interaction term between the timing of the cortisol sample and metabolic syndrome or component status.
Discussion
This study demonstrates a link between HPA axis functioning and the MetSyn in subjects who are members of an occupational group that is subjected to a variety of stressors and has an increased risk of developing CVD (Gade et al., 2010; Zimmerman, 2012). Subjects from this cohort have previously been shown to have more subclinical CVD, increased levels of atherosclerosis (Joseph et al., 2009) and decreased endothelial function, as compared with a similarly aged civilian population sample from the same geographical region (Joseph et al., 2010). After the protein shake challenge, HPA axis functioning was assessed by the total salivary cortisol response above the baseline measurement using area under the curve (Fekedulegn et al., 2007), and as a cortisol response over time across the individual cortisol measurements. Associations between these responses and MetSyn and several of its components were statistically significant and in the expected direction (Bjorntorp and Rosmond, 2000a; Brunner et al., 2002; Chandola et al., 2006). Overall, the total salivary cortisol response was inversely associated with an increasing number of MetSyn components present, an indicator of the severity of subclinical disease. The cortisol response over time was associated with MetSyn and subsequently with two of the five MetSyn components (abdominal obesity and hypertension) individually. Individuals with the MetSyn had a diminished response following the protein shake, while individuals without the MetSyn had a more typical response. The early cortisol response to the high-protein challenge in these models resembles the results of Gibson and colleagues (Gibson et al., 1999) and earlier studies involving other types of food challenges (Follenius et al., 1982; Quigley and Yen, 1979). Our results differ from those of the Multi-Ethnic Study of Atherosclerosis (MESA) that found little evidence that the MetSyn or its components were associated with cortisol output or patterns in a group of subjects without clinical diabetes (DeSantis et al., 2011). These contrasting results may be because subjects were from a population-based study who may not experience the repeated or chronic environmental stress challenges often associated with HPA axis abnormality (Rosmond and Bjorntorp, 2000). Likely most important, the cortisol awakening response, cortisol decline across the waking day, and total cortisol output were used in the MESA study (DeSantis et al., 2011) as opposed to a high-protein challenge.
Among the individual MetSyn components, irregularities in cortisol levels have been linked with abdominal obesity most often (Bjorntorp and Rosmond, 2000b; Chrousos, 2000; Duclos et al., 2005; Ljung et al., 2000; Rosmond et al., 1998). Given that the cortisol response can reflect dysregulation in the HPA axis, an association would be expected with both abdominal obesity and fasting glucose as cortisol acts to increase the supply of blood glucose for tissues during exposure to stressors (Rosmond et al., 1998). Among the associations between the cortisol response across time and the individual components, abdominal obesity was most strongly associated with the cortisol response in our study. These results agree with a related study of variability in cortisol among these subjects in which visceral adiposity, as measured by lean trunk mass index, was consistently associated with within-subjects cortisol variability, but not with the within-subjects cortisol mean (Sharp et al., 2013).
Although fasting glucose was not associated with the cortisol response across time, subjects with glucose intolerance had a slightly more suppressed response overall compared with subjects without glucose intolerance. The lack of significant effect modification by glucose intolerance status may be due in part to the small percentage of officers having glucose intolerance, but sensitivity analyses using tertiles or quartiles of fasting glucose did not indicate significant effect modification either. The lack of a significant result may be a consequence of studying active duty police officers. Type 2 diabetes is less prevalent among police officers than in the general population and more similar after retirement (Zimmerman, 2012), perhaps glucose intolerance follows a similar pattern. It was the least prevalent MetSyn component among these officers.
As a clustering of risk factors for CVD, the MetSyn was associated with the cortisol response across time, but the individual components of elevated triglycerides and reduced HDL-C were not. These two components could be working through the autonomic nervous system pathway; and while related to the other components as a syndrome, they were not individually related to the cortisol response. Hartley and colleagues (2011a) investigated sex differences in the associations between the perceived stress of police work, as measured by self-report using the Spielberger Police Stress Survey, and MetSyn in these subjects. Associations were identified with MetSyn, and specifically with abdominal obesity and reduced HDL-C, among female officers only (Hartley et al., 2011a; Spielberger et al., 1981). There are some similarities with our results, but sex differences in self-reporting of perceived stress may account for the lack of significant findings among male officers.
Among the strengths of this study are that a standardized laboratory protocol was used to gather information to assess salivary cortisol and MetSyn among the police officers (Bjorntorp and Rosmond, 2000a; Rosmond et al., 1998). Salivary cortisol is a physiologic measure of HPA axis function and a more quantitative marker of the response to a stressor or challenge than self-reported measures of stress. And, assessing the response to a high-protein challenge is fairly unique and use of a shake may be a more accessible protein source than a traditional meal. Even though salivary cortisol is a non-invasive method of assessing the functioning of the HPA axis and the values reflect biologically active cortisol, the relationship between salivary cortisol levels and the MetSyn following a specific food-related challenge has received little attention compared to those of more traditional challenges such as a dexamethasone-suppression test (de Kloet et al., 2006; Gibson et al., 1999; Kirschbaum and Hellhammer, 1994).
Limitations of the study include the cross-sectional design that prevents assessment of a causal relationship. Lifestyle factors (alcohol intake, smoking, and physical activity) were self-reported. Selection bias may be present through exclusion of officers who did not provide complete cortisol samples or for whom MetSyn status was unknown. As compared to the 373 officers in the sample, the officers who were excluded had a higher average age, years of police service, abdominal height, fasting glucose, systolic and diastolic blood pressure, and alcohol consumption per week, and a lower percentage of never smokers. Exclusion of these officers may have biased the results toward the null and decreased associations. Use of a healthy, working population prevents generalization of results to the general population. Also, there are mixed results as to whether the percent of dietary calories from protein could be associated with the cortisol response to a high-protein challenge (Gibson et al., 1999). The percent of dietary calories from protein, as derived from food frequency questionnaire responses, was examined as a potential confounder in a post-hoc analysis and did not influence our original results.
Conclusion
A reduced cortisol response to a high-protein challenge is associated with MetSyn and several of its components in Buffalo police officers. In the context of preventing subclinical CVD in police officers, these laboratory-based results redirect attention to development of activities that effectively reduce the physiological and psychological responses to stressor exposure and the prevalence of the various MetSyn components. A longitudinal study of these officers is underway that could provide additional useful evidence for the design of intervention studies on cardiovascular risk among police officers. In the meantime, general interventions to reduce exposure and the response to workplace stressors as well as the prevalence of MetSyn may benefit these police officers.
Acknowledgments
Source of funding:
This work was supported by National Institute for Occupational Safety and Health contract number 200-2003-01580.
Footnotes
Conflicts of interest: No conflicts of interest are declared.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Literature cited
- Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab. 2009;94:2692–2701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. The metabolic syndrome--a neuroendocrine disorder? Br J Nutr. 2000a;83(Suppl 1):S49–57. doi: 10.1017/s0007114500000957. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Neuroendocrine abnormalities in visceral obesity. Int J Obes Relat Metab Disord. 2000b;24(Suppl 2):S80–85. doi: 10.1038/sj.ijo.0801285. [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, Shipley MJ, Kumari M, Andrew R, Seckl JR, et al. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002;106:2659–2665. doi: 10.1161/01.cir.0000038364.26310.bd. [DOI] [PubMed] [Google Scholar]
- Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332:521–524A. doi: 10.1136/bmj.38693.435301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S50–55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, DiezRoux AV, Hajat A, Golden SH, Jenny NS, Sanchez BN, Shea S, Seeman TE. Associations of salivary cortisol levels with metabolic syndrome and its components: the Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab. 2011;96:3483–3492. doi: 10.1210/jc.2011-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Duclos M, Marquez Pereira P, Barat P, Gatta B, Roger P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes Res. 2005;13:1157–1166. doi: 10.1038/oby.2005.137. [DOI] [PubMed] [Google Scholar]
- Fekedulegn D, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, Miller DB. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med. 2007;69:651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- Follenius M, Brandenberger G, Hietter B. Diurnal cortisol peaks and their relationships to meals. J Clin Endocrinol Metab. 1982;55:757–761. doi: 10.1210/jcem-55-4-757. [DOI] [PubMed] [Google Scholar]
- Franke WD, Ramey SL, Shelley MC. Relationship between cardiovascular disease morbidity, risk factors, and stress in a law enforcement cohort. J Occup Env Med. 2002;44:1182–1189. doi: 10.1097/00043764-200212000-00014. [DOI] [PubMed] [Google Scholar]
- Gade W, Schmit J, Collins M, Gade J. Beyond obesity: the diagnosis and pathophysiology of metabolic syndrome. Clin Lab Sci. 2010;23:51–61. [PubMed] [Google Scholar]
- Gershon RR, Lin S, Li X. Work stress in aging police officers. J Occup Env Med. 2002;44:160–167. doi: 10.1097/00043764-200202000-00011. [DOI] [PubMed] [Google Scholar]
- Gibson EL, Checkley S, Papadopoulos A, Poon L, Daley S, Wardle J. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosom Med. 1999;61:214–224. doi: 10.1097/00006842-199903000-00014. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Hartley TA, Burchfiel CM, Fekedulegn D, Andrew ME, Knox SS, Violanti JM. Association between police officer stress and the metabolic syndrome in the BCOPS Study cohort. Int J Emerg Ment Health. 2011a;13:243–256. [PMC free article] [PubMed] [Google Scholar]
- Hartley TA, Burchfiel CM, Fekedulegn D, Andrew ME, Violanti JM. Health disparities in police officers: Comparisons to the U.S. general population. Int J Emerg Ment Health. 2011b;13:211–220. [PMC free article] [PubMed] [Google Scholar]
- Joseph PN, Violanti JM, Donahue R, Andrew ME, Trevisan M, Burchfiel CM, Dorn J. Police work and subclinical atherosclerosis. J Occup Env Med. 2009;51:700–707. doi: 10.1097/jom.0b013e3181a02252. [DOI] [PubMed] [Google Scholar]
- Joseph PN, Violanti JM, Donahue R, Andrew ME, Trevisan M, Burchfiel CM, Dorn J. Endothelial function, a biomarker of subclinical cardiovascular disease, in urban police officers. J Occup Env Med. 2010;52:1004–1008. doi: 10.1097/JOM.0b013e3181f4385c. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta. 2014;1842:473–481. doi: 10.1016/j.bbadis.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung T, Holm G, Friberg P, Andersson B, Bengtsson BA, Svensson J, Dallman M, McEwen B, Bjorntorp P. The activity of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system in relation to waist/hip circumference ratio in men. Obes Res. 2000;8:487–495. doi: 10.1038/oby.2000.61. [DOI] [PubMed] [Google Scholar]
- Marmar CR, McCaslin SE, Metzler TJ, Best S, Weiss DS, Fagan J, Liberman A, Pole N, Otte C, Yehuda R, et al. Predictors of posttraumatic stress in police and other first responders. Ann N Y Acad Sci. 2006;1071:1–18. doi: 10.1196/annals.1364.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health; US Department of Health and Human Services, Centers for Disease Control and Prevention, editor Stress…at work. 1999. [Google Scholar]
- Peter R, Alfredsson L, Hammar N, Siegrist J, Theorell T, Westerholm P. High effort, low reward, and cardiovascular risk factors in employed Swedish men and women: baseline results from the WOLF Study. J Epidemiol Community Health. 1998;52:540–547. doi: 10.1136/jech.52.9.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley ME, Yen SS. A mid-day surge in cortisol levels. J Clin Endocrinol Metab. 1979;49:945–947. doi: 10.1210/jcem-49-6-945. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Bjorntorp P. Endocrine and metabolic aberrations in men with abdominal obesity in relation to anxio-depressive infirmity. Metabolism. 1998;47:1187–1193. doi: 10.1016/s0026-0495(98)90321-3. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000;247:188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Dallman MF, Bjorntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83:1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- Sharp DS, Andrew ME, Fekedulegn DB, Burchfiel CM, Violanti JM, Wactawski-Wende J, Miller DB. The cortisol response in policemen: intraindividual variation, not concentration level, predicts truncal obesity. Am J Hum Biol. 2013;25:499–507. doi: 10.1002/ajhb.22397. [DOI] [PubMed] [Google Scholar]
- Siegrist J, Peter R, Cremer P, Seidel D. Chronic work stress is associated with atherogenic lipids and elevated fibrinogen in middle-aged men. J Intern Med. 1997;242:149–156. doi: 10.1046/j.1365-2796.1997.00167.x. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Westberry L, Grier K, Greenfield G. Human Resources Institute Monograph Series Three, No 6. Tampa, FL: University of South Florida, College of Social and Behavioral Sciences; 1981. The Police Stress Survey: sources of stress in law enforcement. [Google Scholar]
- U.S. Bureau of Labor Statistics. Occupational outlook handbook, police and detectives. 2012 [Google Scholar]
- Vrijkotte TG, van Doornen LJ, de Geus EJ. Work stress and metabolic and hemostatic risk factors. Psychosom Med. 1999;61:796–805. doi: 10.1097/00006842-199911000-00012. [DOI] [PubMed] [Google Scholar]
- Zimmerman FH. Cardiovascular disease and risk factors in law enforcement personnel: a comprehensive review. Cardiol Rev. 2012;20:159–166. doi: 10.1097/CRD.0b013e318248d631. [DOI] [PubMed] [Google Scholar]