Abstract
Background
Mitochondrial metabolism is known to be important for T cell activation. However, its involvement in effector T cell differentiation has just begun to gain attention. Importantly, how metabolic pathways are integrated with T cell activation and effector cell differentiation and function remains largely unknown.
Objective
We sought to test our hypothesis that RhoA GTPase orchestrates glycolysis for Th2 cell differentiation and Th2-mediated allergic airway inflammation.
Methods
Conditional RhoA-deficient mice were generated by crossing RhoAflox/flox mice with CD2-Cre transgenic mice. Effects of RhoA on Th2 differentiation were evaluated by in vitro Th2-polarized culture conditions, and in vivo in ovalbumin (OVA)-induced allergic airway inflammation. Cytokines were measured by intracellular staining and ELISA. T cell metabolism was measured by Seahorse XF24 Analyzer and flow cytometry.
Results
Disruption of RhoA inhibited T cell activation and Th2 differentiation in vitro and prevented the development of allergic airway inflammation in vivo, with no effect on Th1 cells. RhoA deficiency in activated T cells led to multiple defects in metabolic pathways such as glycolysis and oxidative phosphorylation. Importantly, RhoA couples glycolysis to Th2 cell differentiation and allergic airway inflammation via regulating IL-4 receptor mRNA expression and Th2-specific signaling events. Finally, inhibition of Rho-associated protein kinase (ROCK), an immediate downstream effector of RhoA, blocked Th2 differentiation and allergic airway inflammation.
Conclusion
RhoA is a key component of the signaling cascades leading to Th2-differentiation and allergic airway inflammation, at least in part, through the control of T cell metabolism and via ROCK pathway.
Keywords: RhoA, T cell metabolism, glycolysis, Th2 differentiation, allergic airway inflammation
Introduction
Mature T cells are maintained in the peripheral lymphoid organs as resting naive cells with low metabolic activity.1 Antigen recognition activates naïve T cells, resulting in a drastic increase of mitochondrial activities (e.g. glycolysis), T cell receptor (TCR) signaling transduction, and subsequent clonal expansion and effector cell differentiationn.1 Naïve CD4+ T cell can differentiate into a variety of effector cell subsets, among which T helper (Th) 1 and 2 are best-studied.2 Th1 and Th2 cells exert their immune functions through secretion of distinct patterns of cytokines: Th1 cells mediate cellular immunity against intracellular pathogens and autoimmunity by producing IFN-γ, whereas Th2 cells play key roles in humoral immunity and allergy by secreting IL-4, IL-5, and IL-13.2–4 Although a role of metabolic pathways in T cell activation is well appreciated, their involvement in effector T cell differentiation has just begun to gain attention.1,5–7 Interestingly, a recent publication reveals that oxidative phosphorylation (OXPHOS) is required for T cell activation, whereas aerobic glycolysis is important for effector acquisition.8 How metabolic pathways are integrated with T cell activation and effector cell differentiation remains largely unknown.
Asthma is a chronic allergic airway inflammation, in which Th2 cells play a fundamental role in its pathogenesis. The molecular mechanisms controlling Th2 differentiation and function, especially in vivo, have been the focus of intensive investigation over years because of their relevance to inflammatory diseases, for which novel and less toxic therapies are urgently needed.9
RhoA is an intracellular signal transducer of the Rho family small GTPases that cycles between an inactive GDP-bound form and an active GTP-bound form under tight regulation.10,11 RhoA has been shown to modulate actin cytoskeleton organization, cell adhesion, migration, proliferation, and survival.12–16 In T cells, RhoA plays a role in T cell polarization, thymocyte adhesion, and thymic egress.17–22 Furthermore, inactivation of RhoA by C3 transferase in transgenic mice caused thymocyte developmental blocks.23,24 However, whether RhoA is important for T cell metabolism, activation and Th differentiation remains unclear.
In this study, by characterizing T cells deficient for RhoA, we demonstrate that RhoA is a key mediator of Th2 differentiation and allergic airway inflammation. We found that ablation of RhoA abrogated OXPHOS and glycolysis in activated T cells. By combining genetic and pharmacological approaches, we show that RhoA-mediated glycolysis orchestrates Th2 cell acquisition and allergic airway inflammation by regulating signals that instructs Th2 cell differentiation.
Methods
Mice
RhoAflox/flox mice were generated as previously described.25,26 The floxed allele contains loxP sites flanking exon 3 of the RhoA allele. To delete RhoA in vivo in T cells, RhoAflox/flox mice were mated with mice expressing Cre recombinase under the control of a CD2 proximal promoter (Jackson Laboratory, Bar Harbor, ME). Mice used for experiments ranged in ages from five to eight weeks. Animals were housed under specific pathogen-free conditions in the animal facility at Cincinnati Children’s Hospital Research Foundation in compliance with the Cincinnati Children’s Hospital Medical Center Animal Care and Use Committee protocols.
Flow cytometry
Cells were incubated with anti-CD16/32 (2.4G2) (BD Bioscience, San Jose, CA) to block FcγR II/III, and then stained with various conjugated antibodies as indicated. BD Cytofix/Cytoperm kit (BD Bioscience) was used for intracellular cytokine staining. BrdU incorporation was assayed by a BrdU Flow kit per manufacturer’s protocol (BD Bioscience). Apoptosis was evaluated with an Annexin-APC Flow kit (BD Bioscience) following the manufacturer’s instructions. Stained cells were analyzed by FACSCalibur or FACSCanto with FACSDiva (BD Bioscience) or FCS Express (De Novo Software, Los Angeles, CA) software.
T cell activation and differentiation
Sorted naive T cells (CD62LhiCD44lo) were used for T cell activation and differentiation. Naïve T cells were activated with plate-bound anti-CD3 (10 µg/ml) plus soluble anti-CD28 (2 µg/ml) (BD Bioscience). For T cell differentiation, CD4+ naïve T cells were differentiated into Th0, Th1, or Th2 cells as previously reported. 9,27,28 The culture supernatants were collected at different times after activation to assess cytokines by ELISA. Where indicated, sodium pyruvate (Gibco, Grand Island, NY), 2-deoxy-D-glucose (2-DG, Sigma-Aldrich, St Louis, MO) or fasudil (Selleck Chemicals, Houston, TX) was added to the culture.
OVA-induced allergic airway inflammation
Allergic airway inflammation was induced as described in our previous reports.9,28 Briefly, mice were immunized i.p. with 50 µg of OVA (Grade V; Sigma-Aldrich) in 100 µl (2 mg) of alum (Imject Alum; Pierce, IL) on day 0 and day 7. On day 14, mice were challenged two times (60 min each delivered 4 h apart) with aerosolized 1% OVA dissolved in PBS by an Omron NE-C25 Nebulizer (Omron Healthcare, Bannockburn, IL). On day 15, mice were challenged one more time. Control animals were challenged with PBS. Where indicated, 2-DG or fasudil was injected i.p. into the mice. Mice were sacrificed 24 h after the last challenge. Bronchoalveolar lavage (BAL) fluid was aspirated and centrifuged and total cells in the pellet were counted by using a hemacytometer. Differential cell counts on >400 cells were performed on cytospins stained with Shandon Kwik-Diff Stain kit (Thermo Scientific, Rockford, IL). The BAL fluid from each mouse was concentrated to 0.5 ml by centrifugation with an Amicon Ultra-4 filter unit (Millipore, Billerica, MA) for determination of cytokines by ELISA. For lung histology, the lower lobe of the right lung was fixed with 4% paraformaldehyde overnight, dehydrated, embedded in paraffin, cut into 4 mm sections, and processed for hematoxylin/eosin (H&E) staining. Lung tissue mRNA was analyzed by Real-time PCR. Serum levels of various OVA-specific antibodies were measured by ELISA with the use of biotinylated goat anti-mouse IgE (BD Bioscience), IgM, IgG1, IgG2a, and streptavidin-HRP (Southern Biotech, Birmingham, AL).
For the adoptive transfer experiments, wild type (WT) mice were injected i.p. with 100 µg of OVA in 2 mg of alum. Seven days later, the mice were sacrificed and splenic CD4+ T cells were isolated and cultured toward Th2 cell differentiation for 5 days, in the presence of irradiated antigen-presenting cells and OVA (50 µg/ml). Th2 cells (5 × 106 per mouse) were then injected i.v. into RhoAflox/floxCD2-Cre mice (hereafter referred to as RhoA−/−). One day later, the recipient mice and their control mice without receiving donor Th2 cells were challenged with aerosolized 1% OVA for 60 min daily for 4 consecutive days. Three days later, the mice were challenged with aerosolized 1% OVA for 60 min daily for another 3 days. The mice were sacrificed 24 h after the last challenge and analyzed for allergic airway inflammation.
Metabolic Assays
Naïve T cells were stimulated with or without plate-bound ant-CD3 and soluble anti-CD28 overnight, or induced for Th1 or Th2 differentiation under polarized condition. For measurement of oxygen consumption rate (OCR), an indicator of OXPHOS, cells were resuspended in XF assay medium (pH 7.4) containing 2 mM GlutaMax, 1 mM sodium pyruvate and 25 mM glucose, plated onto Seahorse Bioscience XF24 cell culture plates pre-coated with Cell Tak (BD Bioscience), and incubated without CO2 at 37°C. Respiration was measured using the Seahorse XF24 Analyzer (Seahorse Biosciences, North Billerica, MA). For measurement of extracellular acidification rate (ECAR), an indicator of aerobic glycolysis, cells were resuspended in assay medium (pH 7.4) (Sigma-Aldrich) containing 2 mM L-Glutamin and 2.5 mM Glucose, plated onto XF24 cell culture plates and incubated as for OCR. ECAR was measured using the Seahorse XF24 Analyzer in the presence of the glycolysis substrate glucose (10 mM).29,30 For measurements of ROS, mitochondrion numbers and mitochondrial membrane potential, cells stained for CD4 and CD8 were incubated with 5 µM DCFDA, 100 nM Mitotracker Green and 50 nM DilC-5 (Invitrogen, Carlsbad, CA), respectively, accordingly to the manufacturer’s protocols. The cells were then analyzed by flow cytometry.30 For ATP assay, cells were lyzed with a cytosol extraction buffer (Biovision, Milpitas, CA) and homogenized, then ATP was determined by an ATP Determination Kit (Molecular Probes, Eugene, OR), according to the manufacturer’s protocol.
Results
RhoA is important for T cell homeostasis
To determine the physiological function of RhoA in T cells, we generated a mouse strain bearing a conditional deletion of RhoA in T cell lineage by cross-breeding RhoAflox/flox mice with CD2-Cre transgenic mice. The deletion of RhoA in T cells was confirmed by Western blot of thymocytes and splenic T cells from RhoA−/− mice (Fig E1, A and data not shown). There was a marked reduction in both the percentages and absolute numbers of CD4+ and CD8+ T cells from RhoA-deficient mice versus their WT littermates (Fig E1, B). We next analyzed naive and memory-phenotype T cells in WT and RhoA-deficient mice by staining freshly prepared splenocytes with CD44/CD62L antibodies for FACS analysis. We found that both the percentages and absolute numbers of CD4+ and CD8+ naive cells (CD44loCD62Lhi) were drastically reduced in RhoA-deficient mice (Fig E1, C). Whereas the percentages of CD4+ and CD8+ effector memory (TEM, CD44hiCD62Llo) and central memory (TCM, CD44hiCD62Lhi) cells were increased or statistically unchanged, the absolute numbers of effector memory and central memory T cells were reduced or unchanged in RhoA KO mice (Fig E1, C), due to a significant reduction in total T cells. These results suggest that RhoA plays a critical role in maintaining T cell homeostasis.
CD2-Cre could mediate gene deletion in B cell lineage. Indeed, while CD11b+, CD11c+ or NK1.1+ cells did not differ significantly in RhoA-deficient mice from control WT littermates, B220+ B cells were dramatically reduced in the mutant mice (Fig E1, D). To examine if the impaired T cell homeostasis in the absence of RhoA is intrinsic to T cell lineage, we achieved T cell-specific deletion of RhoA by crossing RhoAflox/flox mice with Lck-Cre transgenic mice. We found that RhoAflox/floxLck-Cre mice showed similar changes as RhoAflox/floxCD2-Cre mice in the percentages and numbers of T cells (data not shown). These data suggest that RhoA plays a cell-intrinsic role in T cells.
RhoA coordinates mitochondrial function and T cell activation
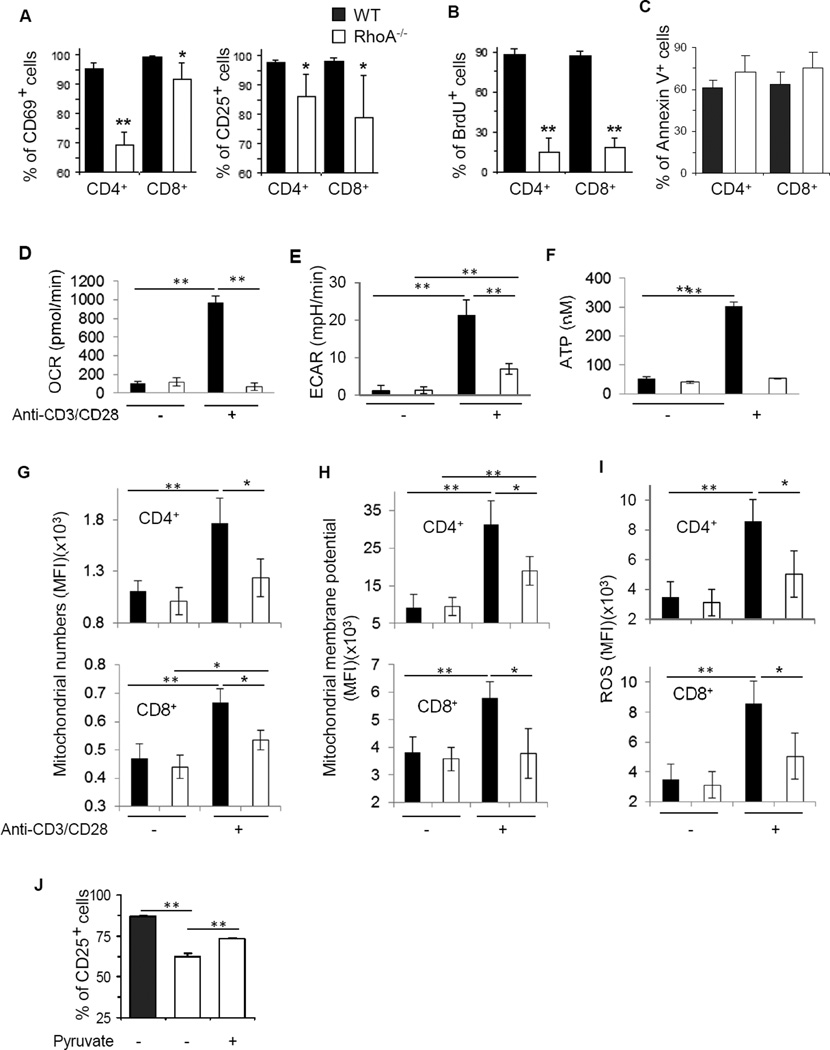
We next examined whether RhoA deficiency affected T cell activation. When stimulated with anti-CD3/CD28 in vitro for 2 days, over 90% of WT naïve CD4+ and CD8+ T cells were activated and expressed activation markers CD69 and CD25. However, the activation-induced upregulation of CD69 and CD25 was significantly inhibited in RhoA-deficient T cells (Fig 1, A). Consistent with the diminished T cell activation, RhoA-deficient T cells were impaired in proliferation, as evidenced by a 3- to 4-fold reduction in BrdU incorporation compared to WT cells (Fig 1, B). In contrast, Rho deficiency did not affect T cell survival upon activation (Fig 1, C).
FIG 1. RhoA deficiency impairs T cell activation and mitochondrial metabolism.
A and B, RhoA deficiency impairs T cell activation (A) and proliferation (B). Naïve T cells were stimulated with anti-CD3/CD28 for 2 days. BrdU (10 µM) was added at the last 20 h. The surface expression of T cell activation markers CD69 and CD25 (A) and BrdU staining (B) in CD4+ and CD8+ cells was analyzed by flow cytometry. C, RhoA deficiency has no effect on cell apoptosis. Cell apoptosis was detected by Annexin V staining and flow cytometry. D–I, RhoA deficiency impairs mitochondrial metabolism in activated T cells. Naïve T cells were stimulated with or without anti-CD3/CD28 overnight, followed by analysis of oxygen consumption rate (OCR) (D), extracellular acidification rate (ECAR) (E), ATP production (F), Mitochondria numbers (G), mitochondrial membrane potential (H), and ROS (I). J, Pyruvate partially rescues RhoA deficiency-induced defect in T cell activation. Naïve CD4+ T cells were stimulated with anti-CD3/CD28 for 2 days, in the presence or absence of pyruvate (2 mM). The surface expression of CD25 was analyzed by flow cytometry. n=4–8 mice per group. Results are representative of two (D–J) or three (A–C) independent experiments. Error bars represent SD. *P < .05, **P < .01.
One hallmark of T cell activation is elevated metabolic activities.1 Because RhoA deficiency caused defects in T cell activation, we hypothesized that depletion of RhoA may affect mitochondrial function in the course of T cell activation. To substantiate this hypothesis, we measured OCR/OXPHOS and ECAR/glycolysis in WT and RhoA−/− T cells with or without anit-CD3/CD28 stimulation. As expected, anti-CD3/CD28 stimulation led to an increased OCR in WT T cells. However, this increase was abolished in RhoA−/− T cells (Fig 1, D). Similarly, RhoA−/− T cells exhibited a compromised ECAR compared to WT T cells, in response to anti-CD3/CD28 stimulation (Fig 1, E). As a result, a markedly reduced ATP production was detected in RhoA−/− T cells (Fig 1, F). We next measured mitochondrion mass and mitochondrial membrane potential and found that RhoA deficiency caused a decrease in mitochondrion contents and mitochondrial membrane potential in activated CD4+ as well as CD8+ T cells (Fig 1, G and H). In line with these defects, metabolic byproducts reactive oxygen species (ROS) were inhibited in RhoA−/− T cells (Fig 1, I). Importantly, when we added pyruvate, the final product of glycolysis, into culture medium of RhoA−/− T cells to bypass the intermediate steps of glycolysis, mimicking restoration of glycolysis in RhoA−/− T cells,31 we found that this direct supplementation of glycolysis-derived pyruvate partially rescued RhoA deficiency-induced defects in T cell activation (Fig 1, J). Together with the recent finding that mitochondrial metabolism is important for T cell activation,32,33 our data suggest that mitochondrial function contributes to RhoA-mediated T cell activation and that RhoA orchestrates metabolic programs for T cell activation.
RhoA controls Th2 but not Th1 cell differentiation
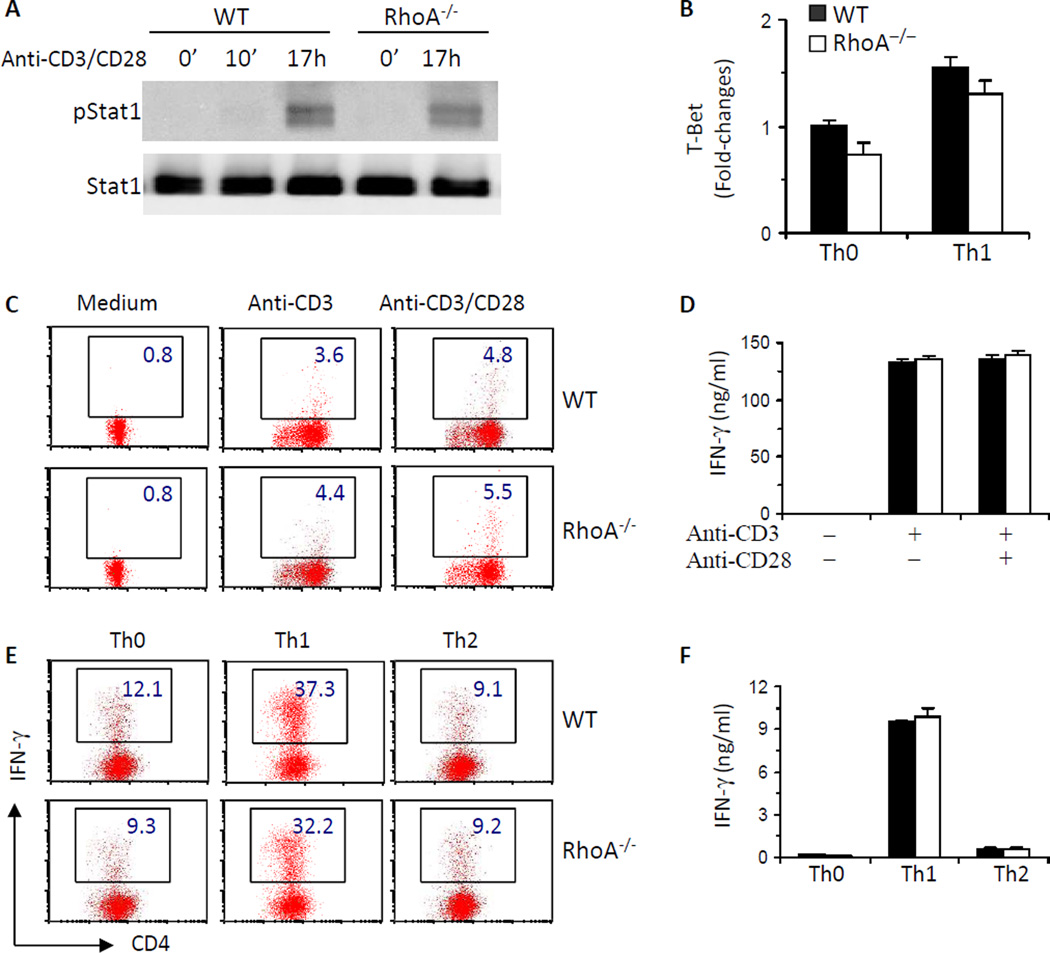
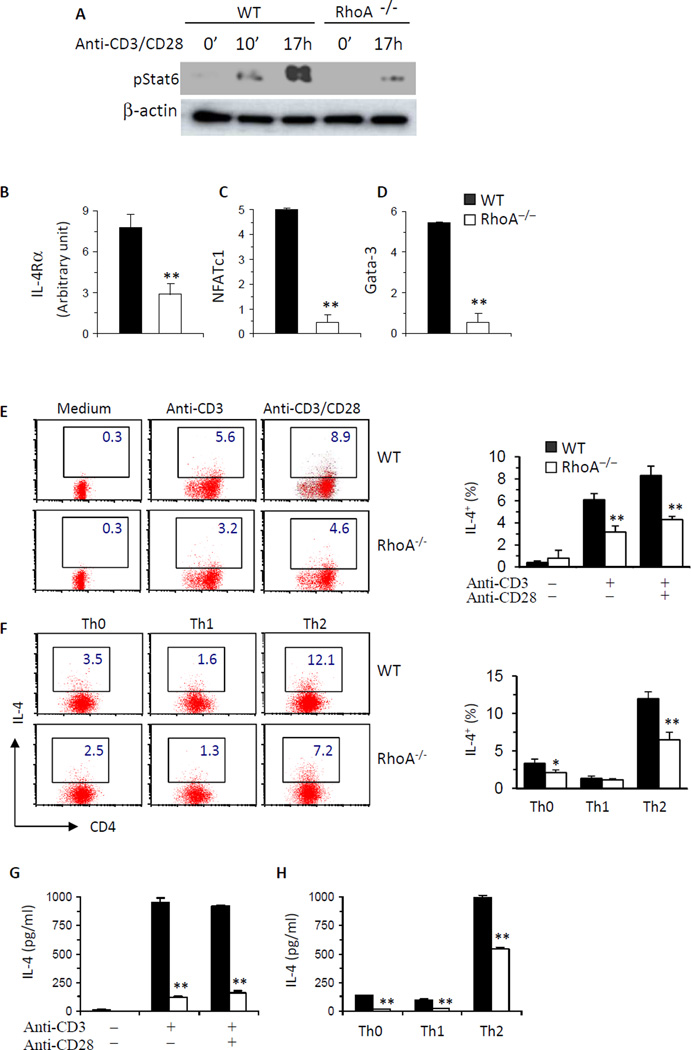
RhoA deficiency causes defects in T cell activation and proliferation, raising a possibility that subsequent effector cell differentiation may be altered in the absence of RhoA. However, RhoA−/−T cells appeared to retain their ability to differentiate into Th1 cells. Phosphorylated Stat1 and the expression of T-Bet, two key Th1 transcriptional factors, remained unchanged in RhoA−/− T cells compared to WT counterparts (Fig 2, A and B). Accordingly, the frequency of IFN-γ-producing cells and IFN-γ secretion were similar between RhoA−/− and WT T cells, under both neutral and polarized differentiation conditions (Fig 2, C-F). In contrast to its lack of effect on Th1 acquisition, disruption of RhoA led to an apparent blockade in Th2 differentiation. Phosphorylated Stat6 and mRNA levels of IL-4 receptor (IL-4R), nuclear factor of activated T cells c1 (NFATc1) and GATA3, all of which have been reported to specify and/or potentiate Th2 differentiation,34 were dramatically reduced in RhoA-deficient activated T cells or Th2 cells (Fig 3, A–D). As a result, IL-4-producing T cells and IL-4 secretion were significantly decreased in the absence of RhoA as detected by FACS intracellular staining (Fig 3, E and F) or ELISA (Fig 3, G and H), respectively. Together, these results reveal that RhoA is essential for Th2 but not Th1 cell differentiation.
FIG 2. RhoA deficiency has no effect on Th1 cell differentiation.
A, RhoA deficiency has no effect on Stat1 activation. CD4+ naïve T cells were cultured with anti-CD3/CD28 for 0~17 h. Phosphorylated (p) and total Stat1 were examined by immunoblot. B, RhoA deficiency has no effect on T-bet expression. CD4+ naïve T cells were cultured under Th0 or Th1 conditions for 4 days and restimulated with PMA plus ionomycin for 5 h. T-Bet mRNA was analyzed by realtime PCR. C–F, RhoA deficiency has no effect on IFN-γ production. CD4+ naïve T cells were cultured with or without anti-CD3/CD28 for 2 days (C and D) or differentiated under Th0, Th1-, or Th2-skewed conditions for 4 days and restimulated with PMA plus ionomycin for 5 h (E and F). BD GolgiPlug™ was added at the last 2 h. The cells were collected for surface staining of CD4 and intracellular staining of IFN-γ. Percentages of IFN-γ +CD4+ T cells are shown in representative dot plots (C and E). Supernatants were collected from other sets of cultures without BD GolgiPlug™ for ELISA assays to detect IFN- γ secretion (D and F). CD4+ naïve T cells were pooled from 5–8 mice. Results are representative of three independent experiments. Error bars represent SD of triplicates.
FIG 3. RhoA deficiency impairs Th2 cell differentiation.
A, RhoA deficiency inhibits Stat6 activation. CD4+ naïve T cells were cultured with anti-CD3/CD28 for 0~17 h. Phosphorylated (p) and total Stat6 were examined by immunoblot. B–D, RhoA deficiency suppresses IL-4Ra, NFATc1 and GATA3 expression. CD4+ naïve T cells were cultured under Th2 conditions for 24 h (B), or for 4 days and restimulated with PMA and ionomycin for 5 h (C and D). mRNA levels of IL-4Rα (B), NFATc1 (C) and GATA3 (D) were analyzed by real-time PCR. E–H, RhoA deficiency inhibits IL-4 production. CD4+ naïve T cells were cultured with or without anti-CD3/CD28 for 2 days (E and G) or differentiated under Th0, Th1-, or Th2-skewed conditions for 4 days and restimulated with PMA plus ionomycin for 5 h (F and H). BD GolgiPlug™ was added at the last 2 h. The cells were collected for surface staining of CD4 and intracellular staining of IL-4 (E and F). Percentages of IL-4+CD4+ T cells are shown in representative dot plots and mean percentages in histogram (E and F). Supernatants were collected from other sets of cultures without BD GolgiPlug™ for ELISA assays to detect IL-4 secretion (G and H). CD4+ naïve T cells were pooled from 5–8 mice. Results are representative of two (B) or three (A, C–H) independent experiments. Error bars represent SD of triplicates. **P < .05, **P < .01.
To begin to understand why RhoA selectively regulates Th2, but not Th1, cell differentiation, we examined mammalian target of rapamycin (mTOR) pathway in RhoA−/− and WT T cells. mTOR, present in two cellular complexes: mTOR complex 1 (mTORC1) and mTORC2, is a serine/threonine kinase and a key signaling regulator in mammalian cells.35 In T cells, recently published literatures suggest that mTORC1 is important for Th1 cell differentiation, whereas mTORC2 is critical for Th2 differentiation.36 Interestingly, we found that RhoA deficiency dampened mTORC2 activity, as evidenced by a significantly reduced activation of Akt and PKCθ, two important signaling mediators of mTORC2,37 In contrast, RhoA deficiency appeared not to affect mTORC1 activity, because RhoA−/− and WT T cells showed comparable levels of active S6K and 4E–BP, two immediate downstream effectors of mTORC1 (Fig E2).37 These data suggest that RhoA selectively promotes mTORC2, but not mTORC1, activation, which may explain why RhoA regulates Th2, but not Th1, cell differentiation.
RhoA is necessary for OVA-induced allergic airway inflammation
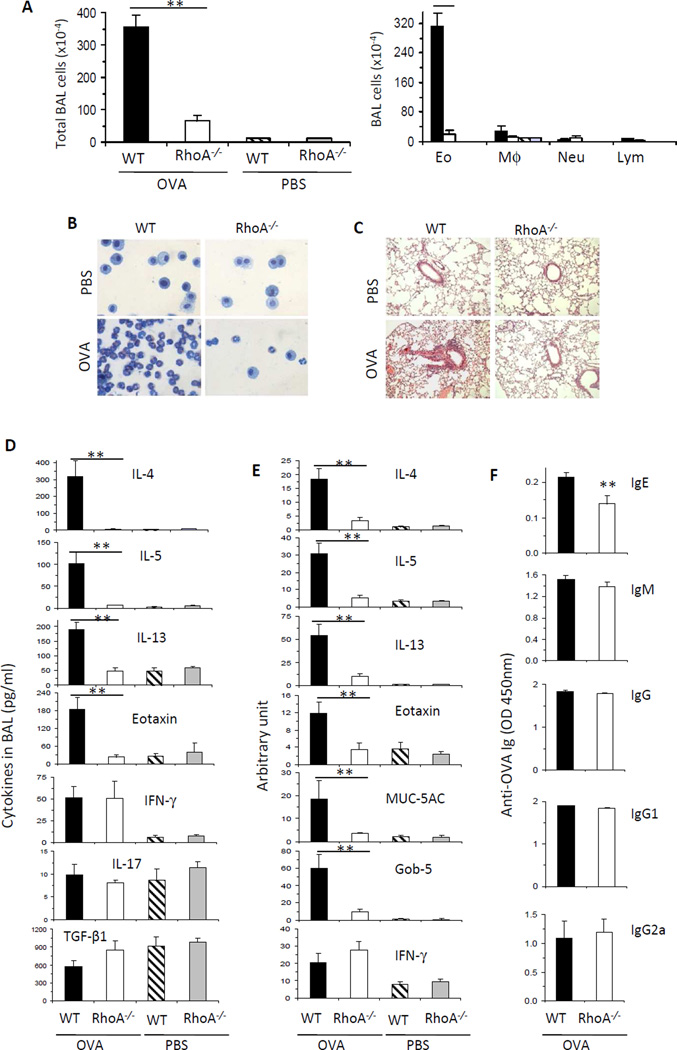
Since RhoA regulates Th2 cell differentiation, we investigated whether RhoA was necessary for an optimal Th2-mediated inflammatory response in vivo in a classical OVA-induced model of allergic airway inflammation/asthma. Total numbers of BAL cells in WT mice challenged with OVA had over 100-fold increases as compared to PBS-challenged mice (Fig 4, A). Eosinophils accounted for most of this increase in inflammatory cells (Fig 4, A and B). However, RhoA deficiency led to a dramatic reduction in total numbers of BAL cells, particularly owing to markedly decreased eosinophils. As revealed by lung histological analysis, WT mice showed massive perivascular and peribronchial infiltration of inflammatory cells upon OVA challenge, whereas this inflammatory response was markedly attenuated in RhoA−/− mice (Fig 4, C). We reasoned that the inhibitory effect of RhoA loss on allergic airway inflammation was attributable to reduced Th2 cytokine secretion from RhoA-deficient mice as demonstrated by in vitro experiments. Consistent with this notion, IL-4, IL-5, and IL-13 in BAL fluids were significantly less in RhoA-deficient mice than that in WT littermates in response to OVA challenge, whereas Th1 cytokine IFN-γ, Th17 cytokine IL-17 and Treg cytokine TGF-β1 were comparable between the two genotypes (Fig 4, D). The mRNA levels of these cytokines in lung followed similar patterns as protein levels in BAL (Fig 4, E). Moreover, several non-T cell-derived mediators of allergic airway inflammation including Eotaxin, MUC-5AC and Gob-59,38 were highly reduced in RhoA−/− mice compared to that in WT mice upon OVA challenge (Fig 4, D and E).
FIG 4. RhoA deficiency suppresses OVA-induced allergic airway inflammation.
WT and RhoA−/− mice were immunized i.p. with OVA and then challenged with aerosolized OVA or PBS as control. Mice were sacrificed 24 h after the last challenge. A, Quantification of total cells (left), eosinophils (Eo), macrophages (Mφ), neutrophils (Neu), and lymphocytes (Lym) (right) in BAL fluids. B and C, Representative Kwik-Diff staining for BAL cytospins (B) and H&E staining of lung tissue sections (C). D, Cytokine levels in BAL fluids determined by ELISA. E, mRNA levels of IL-4, IL-5, IL-13, Eotaxin, MUC-5AC, Gob-5 and IFN-γ in lung tissue determined by real-time PCR. Data are normalized to an 18S reference and expressed as arbitrary units. F, OVA-specific IgE, IgM, IgG, IgG1 and IgG2a levels in sera of mice immunized and challenged with OVA. Similar levels of OVA-specific Ig subclasses were detected from OVA-immunized and PBS-challenged control groups and not shown. Results are representative of two independent experiments. Error bars represent SE of 8 mice. **P < .05, **P < .01.
Apart from cellular responses in airway, humoral systemic responses to OVA are also important for asthma development.9,39 We found that OVA-specific IgE in sera was markedly reduced in the absence of RhoA, whereas OVA-specific IgM, total IgG, IgG1 and IgG2a were similar between RhoA−/− and WT mice (Fig 4, F). Collectively, RhoA deficiency resulted in a significant inhibition of Th2-mediated allergic airway inflammation, suggesting that RhoA critically contributes to asthma development.
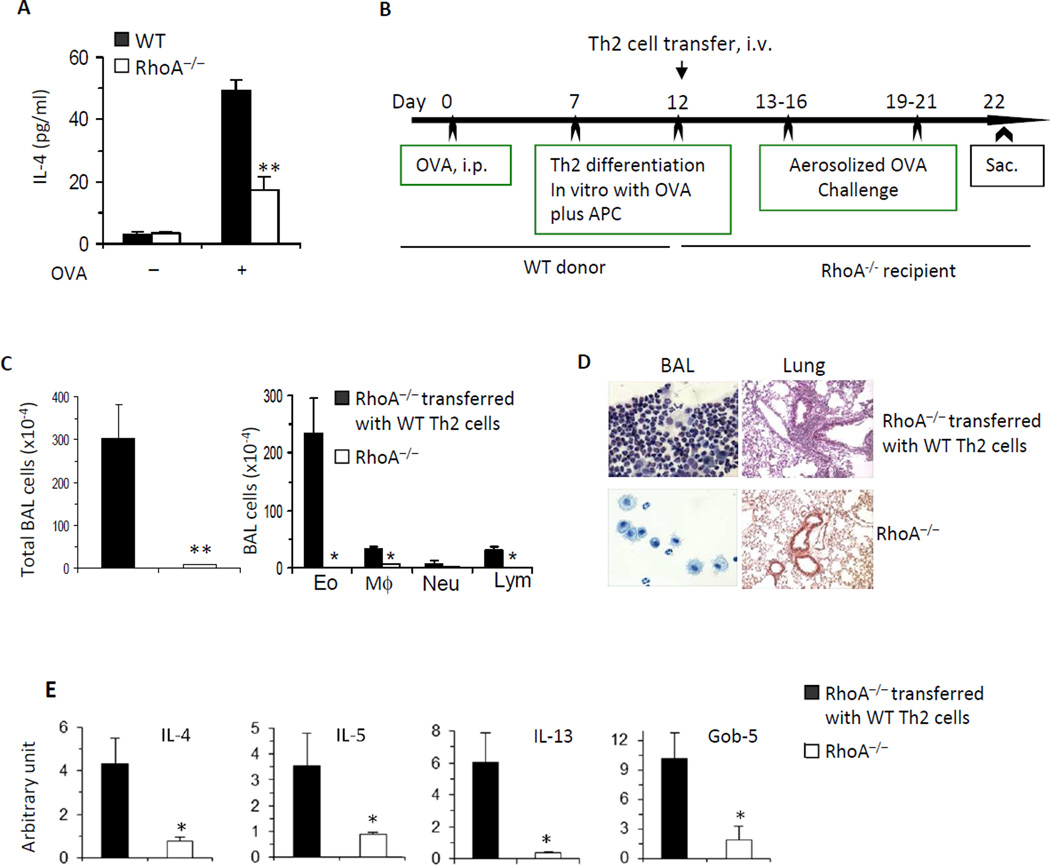
The results from the asthma model are consistent with a crucial role of RhoA in Th2 cell differentiation. To show direct evidence that RhoA modulates Th2 differentiation in vivo, we isolated spleen cells from OVA-immunized WT and RhoA−/− mice, cultured the cells in the presence or absence of OVA for 3 days, and then detected IL-4 in the culture supernatants. We found that IL-4 secretion from RhoA−/− cells was significantly reduced compared to that from WT cells (Fig 5, A). We then carried out adoptive T cell transfer experiments to see if in vitro-generated OVA-specific WT Th2 cells can restore allergic airway inflammation in RhoA−/− mice. As described in Methods, WT mice were injected i.p. with OVA to induce Th2 cells and splenic CD4+ T cells from the immunized mice were then cultured under Th2 cell differentiation condition, in the presence of OVA. These Th2 cells were injected i.v. into RhoA−/− mice, whereas some control RhoA−/− mice did not receive cells. The recipient mice and their control mice were then challenged with aerosolized OVA (Fig 5, B). As shown in Fig 5, C-E, there was no significant inflammation in the control RhoA−/− mice. However, inflammatory response was evident in the RhoA−/− mice transferred with OVA-specific WT Th2 cells, as evidenced by markedly increased inflammatory cells (Fig 5, C), particularly eosinophils in BAL (Fig 5, C and D) and lung (Fig 5, D), and Th2 cytokines and Gob-5 in lung tissue (Fig 5, E). Thus, transfer of OVA-specific WT Th2 cells appears to restore allergic airway inflammation in RhoA−/− mice. Together, these data confirm that RhoA regulates allergic airway inflammation through its effect on Th2 cell differentiation.
FIG 5. The inhibitory effect of RhoA deficiency on allergic airway inflammation is caused by suppression of Th2 cell differentiation.
A, OVA-induced Th2 cell differentiation is impaired in the absence of RhoA. Spleen cells from OVA-immunized WT and RhoA−/− mice were cultured in the presence or absence of OVA (100 µg/ml) for 3 days. IL-4 in the culture supernatants was detected by ELISA. B–E, Adoptively transferred WT Th2 cells can trigger allergic airway inflammation in RhoA−/− mice. WT Th2 cells were generated by culturing splenic CD4+ T cells from OVA-immunized WT mice with OVA plus irradiated APC. The cells were then injected into RhoA−/− mice. The mice were challenged with aerosolized OVA and sacrificed for analysis of allergic airway inflammation (B). Total BAL cells and differential cell counts (C), representative Kwik-Diff staining for BAL cytospins (D) and H&E staining of lung tissue sections (D), and mRNA expression of cytokines and Gob-5 in lung tissues (E) are shown. Error bars represent SE of 4 mice. **P < .01.
Hyperactivation of RhoA is sufficient to promote Th2 cell differentiation and Th2-mediated allergic airway inflammation
By genetic deletion of RhoA, we have shown that RhoA is necessary for Th2 cell differentiation and Th2-mediated allergic airway inflammation. Alliteratively, it would be of great interest to determine if RhoA gain-of-function is sufficient to promote Th2 differentiation and allergic airway inflammation. To address this, we generated RhoA gain-of-function chimeric mice by transplantation of embryonic day 14.5 fetal liver cells deficient for p190-B Rho GTPase activating protein (p190-B RhoGAP; hereafter referred to as RhoGAP), a negative regulator of RhoA activity, into syngeneic BoyJ mice.40 By immunoblotting, we confirmed that RhoA was hyperactivated in CD4+ T cells from RhoGAP−/− chimeric mice compared to that from chimeric mice transplanted with WT fetal liver cells, as reflected by increased activation of RhoA downstream signaling mediators LIMK1/2 and MLC2 (Fig E3, A). Similar to RhoA deficiency, RhoA gain-of-function/RhoGAP deficiency had no effect on Th1 cell differentiation (Fig E3, B). In contrast, Th2 cell differentiation was enhanced upon RhoGAP deletion (Fig E3, C). In accordance with this, OVA-induced allergic airway inflammation was more severe in RhoGAP−/−chimeric mice compared to WT chimeric mice, as evidenced by significantly increased inflammatory cells (Fig E3, D), particularly neutrophils in BAL (Fig E3, D and E) and lung (Fig E3, F), and Th2 cytokines in BAL (Fig E3, G). Taken together, these data suggest that RhoA gain-of-function is sufficient to promote Th2 differentiation and allergic airway inflammation.
RhoA connects glycolysis to Th2 cell differentiation and allergic airway inflammation
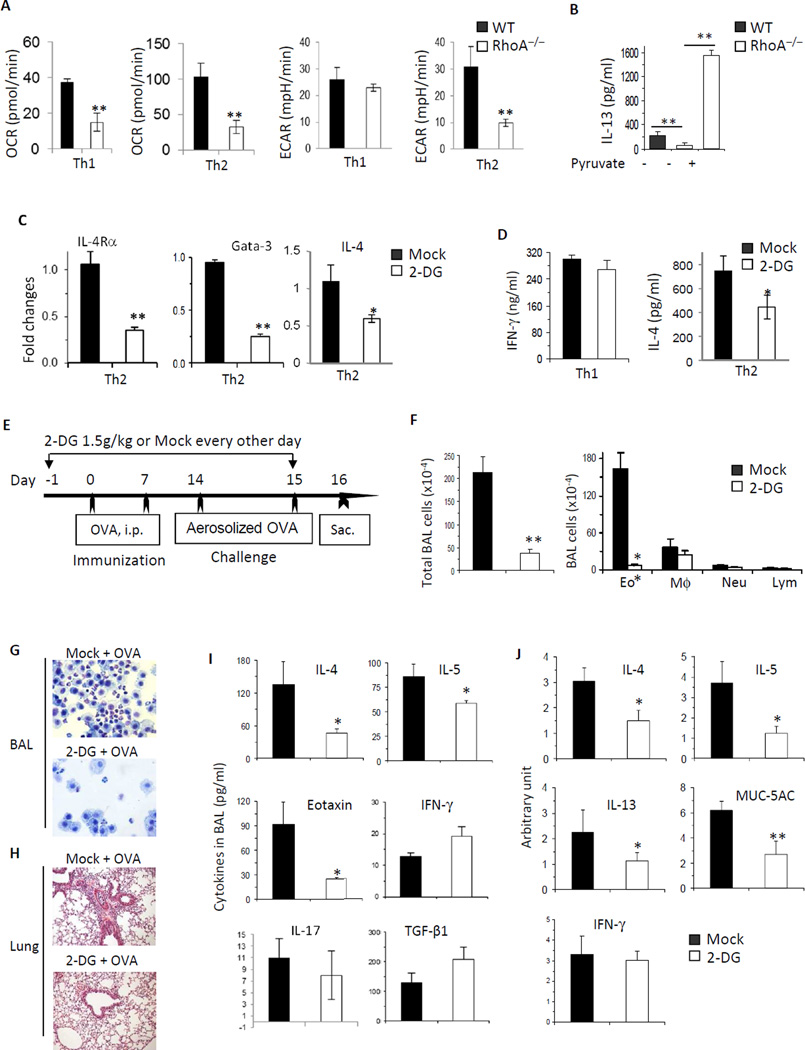
RhoA deficiency diminished OXPHOS and glycolysis in activated T cells, impaired Th2 but not Th1 cell differentiation and prevented the development of allergic airway inflammation. To investigate this in more detail, we examined OXPHOS and glycolysis in RhoA−/− T cells under Th1/Th2 differentiation condition. It seemed that OXPHOS was attenuated in both RhoA−/− Th1 and RhoA−/− Th2 cells (Fig 6, A). In contrast, glycolysis was not changed in RhoA−/− Th1 cells, but was decreased in RhoA−/− Th2 cells compared to WT counterparts (Fig 6, A). Because glycolysis, but not OXPHOS, in RhoA−/− Th1 and RhoA−/− Th2 cells was correlative with their differentiation phenotypes, we examined if control of glycolysis is important for RhoA to regulate Th2 cell differentiation. To this end, we added glycolysis-derived pyruvate into culture medium of RhoA−/− Th2 cells to bypass the intermediate steps of glycolysis, mimicking restoration of glycolysis in RhoA−/− Th2 cells.31 We found that the supplementation of pyruvate rescued RhoA deficiency-induced defects in Th2 cell differentiation (Fig 6, B). These data suggest that RhoA regulates Th2 cell differentiation through governing glycolysis.
FIG 6. RhoA connects glycolysis to Th2 cell differentiation and allergic airway inflammation.
A, RhoA deficiency impairs OXPHOS in both Th1 and Th2 cells, but glycolysis in Th2 but not Th1 cells. WT and RhoA−/− CD4+ naïve T cells were cultured under Th1- or Th2-skewed conditions for 4 days and restimulated with PMA plus ionomycin for 5 h, followed by measurement of OCR and ECAR. B, Pyruvate rescues RhoA deficiency-induced defect in Th2 cell differentiation. WT and RhoA−/− CD4+ naïve T cells were differentiated under Th2 conditions for 3 days, in the presence or absence of pyruvate (2 mM). IL-13 production was analyzed by ELISA. C, Inhibition of glycolysis impairs IL-4Rα, Gata-3, and IL-4 expression. WT CD4+ naïve T cells were cultured under Th2 conditions in the presence of PBS (Mock) or 2-DG (0.3 mM) for 24 h. mRNA levels of IL-4Rα, Gata-3, and IL-4 were analyzed by real-time PCR. D, Inhibition of glycolysis impairs IL-4 secretion. WT CD4+ naïve T cells were cultured under Th1 or Th2 conditions in the presence of Mock or 2-DG (0.3 mM). IFN-γ and IL-4 secretion were analyzed by ELISA. E–J, Inhibition of glycolysis impairs allergic airway inflammation. WT mice were injected i.p with 2-DG (1.5 g/kg) or PBS (Mock), starting 1 day before OVA immunization until 1 day before sacrifice as indicated (E). Total BAL cells and differential cell counts (F), representative Kwik-Diff staining for BAL cytospins (G) and H&E staining of lung tissue sections (H), levels of cytokines and eotaxin in BAL fluids (I), and/or mRNA expression of cytokines and MUC-5AC in lung tissues (J) are shown. Results are representative of two (A–D) independent experiments. Error bars represent SD of 4–10 mice (A–D) or SE of 4 mice (F, I and J). *P < .05, **P < .01.
We then treated WT CD4+ T cells with 2-DG, a well-recognized inhibitor of glycolysis, and examined if blockade of glycolysis has a direct effect on Th2 differentiation. As shown in Fig 6, C and D, while 2-DG had no effect on IFN-γ production in Th1 cells (Fig 6, D), it inhibited IL-4R and GATA3 mRNA expression in Th2 cells (Fig 6, C), leading to a reduction in IL-4 mRNA content and IL-4 secretion (Fig 6, C and D). Our data suggest that RhoA links glucose metabolism to Th2 cell commitment through regulating IL-4R expression and Th2-specific signaling transduction. Intriguingly, when 2-DG was administered into mouse model of Th2-mediated allergic airway inflammation (Fig 6, E), it significantly reduced infiltrating inflammatory cells (Fig 6, F), particularly eosinophils in BAL (Fig 6, F and G) and lung (Fig 6, H). Furthermore, 2-DG treatment markedly reduced Th2 cytokines, Eotaxin and MUC-5AC in BAL (Fig 6, I), and/or their mRNA levels in lung tissue (Fig 6, J), whereas Th1 cytokine IFN-γ, Th17 cytokine IL-17 and Treg cytokine TGF-β1 were unchanged (Fig 6, I and J). Taken together, these results suggest that RhoA-dependent glycolysis impinges upon Th2 cell differentiation in vitro and allergic airway inflammation in vivo.
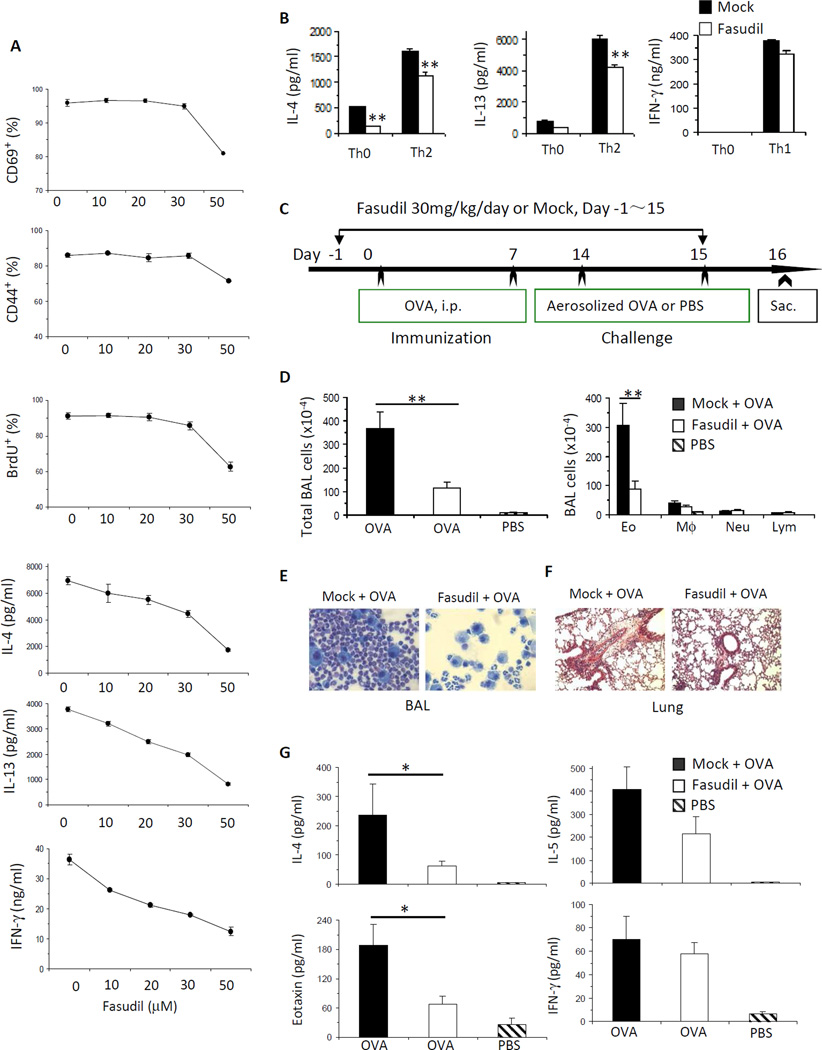
ROCK acts downstream of RhoA to mediate T cell activation and Th2 cell differentiation and allergic airway inflammation
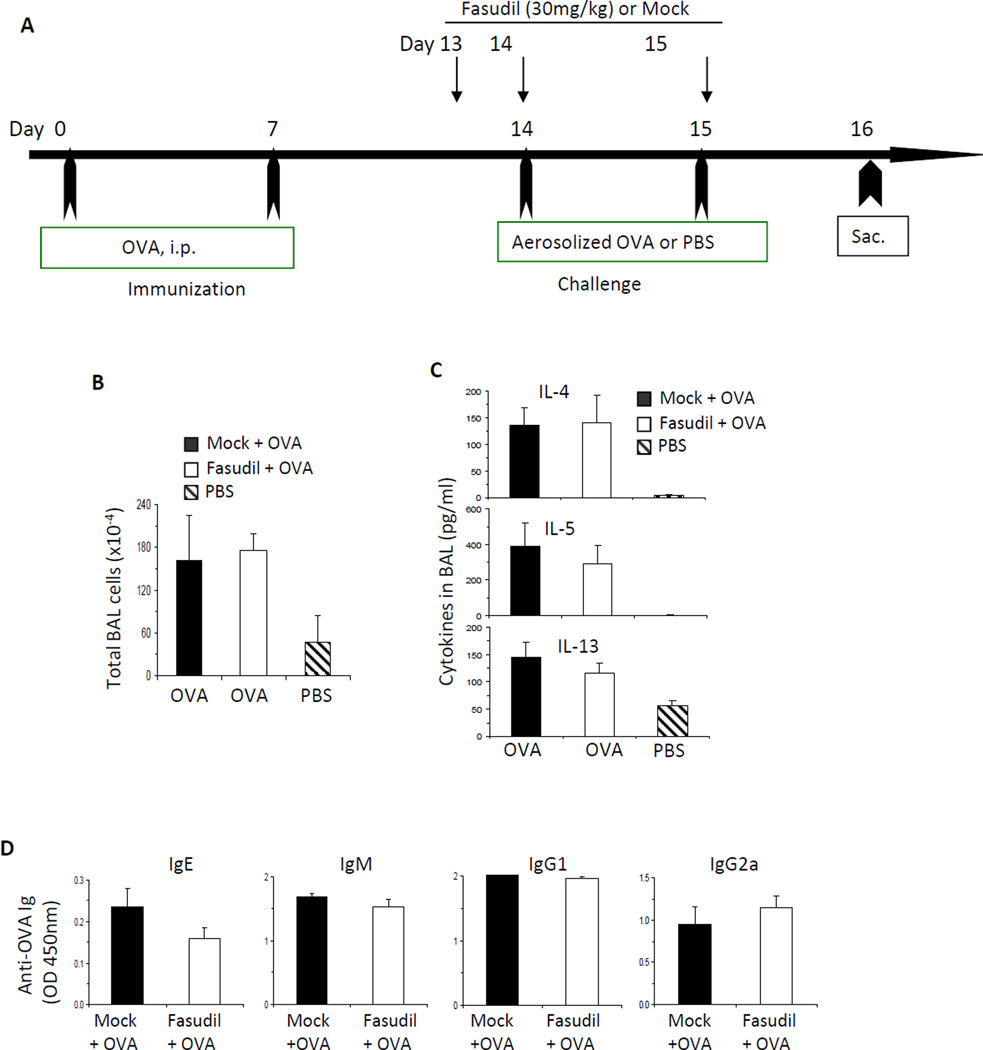
Our data reveal that RhoA plays a key role in T cell activation, Th2 cell differentiation and Th2-mediated allergic airway inflammation. To begin to understand RhoA downstream signaling pathways in these processes, we investigated ROCK, an immediate effector of RhoA,41 by using ROCK selective inhibitor fasudil. To this end, WT naive CD4+ T cells were stimulated with anti-CD3/CD28 in the presence or absence of different dosages of fasudil. We found that fasudil inhibited T cell activation and proliferation at 50 µM, as evidenced by a marked decrease in the expression of T cell activation markers, CD69 and CD44, and in BrdU incorporation (Fig 7, A). Under the neutral culture condition, fasudil effectively blocked the production of Th2 cytokines, IL-4 and IL-13, in a dose-dependent manner (Fig 7, A). When WT cells were cultured under Th2-polarized condition, fasudil (50 µM) also inhibited IL-4 and IL-13 secretion (Fig 7, B). In contrast, when WT cells were cultured under Th1-polarized condition, fasudil (50 µM) did not affect IFN-γ production (Fig 7, B). We noticed that fasudil inhibited IFN-γ production when WT cells were cultured under neutral condition (Fig 7, A), which is inconsistent with that observed in RhoA−/− cell. This could be an off-target effect of fasudil. Alternatively, ROCK may regulate IFN-γ production independent of RhoA in activated T cells. We then examined the effect of fasudil on allergic airway inflammation in vivo. We started to treat WT mice with fasudil one day before OVA immunization and continued treatment every day until one day before sacrifice of the mice (Fig 7, C). Mice treated with fasudil showed a marked reduction in infiltrating inflammatory cells (Fig 7, D), mainly reflective of eosinophils in BAL (Fig 7, D and E) and lung (Fig 7, F), and drastically reduced IL-4 and eotaxin in BAL, compared to mice without treatment with fasudil but immunized and challenged with OVA (Fig 7, G). INF-g was similar between the two groups (Fig 7, G). These data demonstrate that fasudil treatment can recapitulate the phenotypes of RhoA-deficient cells/mice, indicating that ROCK pathway acts downstream of RhoA in T cell activation, Th2 cell acquisition and function. Interestingly, when we injected fasudil after OVA immunization but before OVA challenge (Fig 8, A), we could not detect any inhibition in inflammatory cell infiltration (Fig 8, B), Th2 cytokine secretion in BAL (Fig 8, C), and OVA-specific antibody production (Fig 8, D). These results suggest that RhoA/ROCK pathway regulates Th2 inflammatory immune responses by affecting T cell activation and Th2 differentiation but not inflammatory cell recruitment into lung, and that targeting RhoA/ROCK pathway preferentially serves as a prophylactic versus therapeutic regimen.
FIG 7. ROCK acts downstream of RhoA to mediate T cell activation and Th2 cell differentiation and allergic airway inflammation.
A and B, Inhibition of ROCK by fasudil impairs T cell activation, proliferation and Th2, but not Th1, cell differentiation. CD4+ naïve T cells pooled from 8 WT mice were stimulated with anti-CD3/CD28 for 2 days with or without fasudil (0~50 mM) (A), or differentiated under Th0, Th1 or Th2 conditions for 4 days and restimulated with PMA plus ionomycin for 5 h, in the presence of PBS (Mock) or fasudil (50 µM) throughout the culture (B). BrdU (10 µM) was added at the last 20 h (A). Cells were harvested for CD69, CD44, or BrdU staining and analyzed by flow cytometry (A). Cytokines in culture supernatants were determined by ELISA (A and B). C–G, Inhibition of ROCK by fasudil impairs allergic airway inflammation. WT mice were injected i.p. daily with fasudil (30 mg/kg) or PBS (Mock), starting 1 day before OVA immunization until 1 day before sacrifice (C). Total BAL cells and differential cell counts (D), representative Kwik-Diff staining for BAL cytospins (E), H&E staining of lung tissue sections (F), cytokine and eotaxin levels in BAL fluids (G) are shown. Results are representative of two independent experiments. Error bars represent SD of triplicates (A and B) or SE of 7–8 mice (D and G). *P < .05, **P < .01.
FIG 8. Administration of ROCK inhibitor fasudil after immunization has no effect on allergic airway inflammation.
A, WT mice were immunized i.p. with OVA in alum on day 0 and day 7. On day 14 and 15, mice were challenged with aerosolized OVA or PBS. Fasudil (30 mg/kg) or PBS was injected i.p. at day 13, 14, and 15. Mice were sacrificed 24 h after the last challenge. B–D, Total BAL cells (B), cytokines in BAL fluid (C), and serum OVA-specific antibodies (D) are shown (n=4–5 mice per group). Results are representative of two independent experiments. Error bars represent SE.
Discussion
RhoA is known to regulate cytoskeleton-related events in T cells such as polarization, adhesion, and migration.17–19,21 However, whether RhoA is involved in T cell activation and Th effector cell differentiation remains unknown. By the use of mice bearing conditional RhoA deficiency in T cells, we have found that RhoA is crucial for T cell activation and Th2 cell differentiation in vitro and Th2-dominated allergic airway inflammation in vivo. Noting that RhoA−/− mice had reduced numbers of T cells as well as B cells, the phenotypes of allergic airway inflammation in vivo in RhoA−/− mice might be complicated by a potential influence from altered T and B cell homeostasis. However, we think this is unlikely because of four folds. First, RhoA−/− T cells or fasudil-treated T cells showed an impaired activation phenotype when equal numbers of WT and RhoA−/− naïve T cells or of fasudil-treated and fasudil-untreated WT naïve T cells were used for induction of T cell activation. Thus, the role of RhoA pathway in T cell activation is not likely secondary to its regulation of T cell homeostasis. Second, while RhoA deficiency or fasudil treatment impaired T cell activation, they did not affect subsequent Th1 cell differentiation in vitro and in vivo in mouse model of Th2-mediated allergic airway inflammation, suggesting that the role of RhoA pathway in Th2 cell differentiation is independent of T cell activation. Thirdly, RhoA regulation of Th2 cell differentiation is intrinsic to T cell lineage because T cell-specific deletion of RhoA by Lck-Cre also inhibited Th2 cell differentiation (data not shown). Finally, IgG and IgM production from B cells did not seem to be affected by RhoA deficiency in allergic airway inflammation model, suggesting that the decreased IgE level in the absence of RhoA is not due to impaired B cell homeostasis but to impaired Th2 cell differentiation.
Activation of T cells induces glycolysis and OXPHOS.8 We show that both of the metabolic processes are regulated by RhoA. As a result, RhoA deficiency led to a considerably reduced ATP production in activated T cells. Together with an impaired mitochondrial biogenesis in the absence of RhoA, these data suggest that RhoA is required by activated T cells for fulfilling the bioenergetics and biosynthetic demands of proliferation and differentiation. This may explain why RhoA−/− T cells were impaired in proliferation and Th2 differentiation. In support of this notion, supplementation of glycolysis-derived pyruvate rescued T cell activation and Th2 cell differentiation in RhoA−/− cells. Furthermore, blockade of glycolysis by 2-DG inhibited Th2-specifying signaling and Th2 acquisition. Of note, it has been shown that 1 mM of 2-DG does not suppress T cell proliferation.5 Thus, the lower dosage of 2-DG used in this study (0.3 mM) should have minimal inhibitory effect on cell proliferation, suggesting a specific role of glycolysis in Th2 cell differentiation. Finally, inhibition of glycolysis prevented Th2 cell-mediated development of allergic airway inflammation, implicating that RhoA-dependent glycolysis pathway could serve as a potential therapeutic target in treating asthma.
RhoA selectively regulates Th2 but not Th1 cell differentiation. The exact mechanism for this selectivity remains unknown. Nonetheless, we think it might be due to three folds. First, RhoA selectively regulates the activation or expression of Th2 transcriptional factors (e.g. Stat6, NFATc1, Gata-3), with no effect on Th1 transcriptional factors. Second, RhoA regulates glycolysis in Th2 but not Th1 cells. Thirdly, noting that mTORC2 is important for Th2 cell differentiation, whereas mTORC1 is essential for Th1 cell differentiation, RhoA regulates mTORC2 but not mTORC1 activation.
In summary, the present study reveals that RhoA/ROCK pathway is a key component of the signaling cascades leading to T cell activation, Th2 cell differentiation and allergic airway inflammation. Importantly, RhoA signaling integrates mitochondrial metabolism to T cell activation and Th2 cell differentiation and function.
Supplementary Material
Key Messages.
RhoA deficiency inhibits Th2 cell differentiation and experimental asthma
RhoA deficiency impairs glycolysis in activated T cells and Th2 cells, and blockade of glycolysis inhibits Th2 differentiation and experimental asthma
RhoA-dependent metabolic pathway may be explored as a potent therapeutic target in treating allergic airway inflammation.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (GM 108661 to F.G.)(HL090676 to M.D.F), Cincinnati Cancer Center Just-In-Time Core Subsidy Program (to F.G.), Center for Clinical and Translational Science and Training of University of Cincinnati Academic Health Center (to F.G.), National Natural Science Foundation of China (81373116 to J. Q.Y) and Jiangsu Provincial Key Laboratory of Parasite Molecular Biology (to J.Q.Y).
Abbreviations Used
- 2-DG
2-Deoxy-D-glucose
- BAL
Bronchoalveolar lavage
- ECAR
Extracellular acidification rate
- ELISA
enzyme-linked immunosorbent assay
- H&E
hematoxylin/eosin
- WT
Wild-type
- KO
Knock-out
- OCR
Oxygen consumption rate
- OVA
Ovalbumin
- OXPHOS
Oxidative phosphorylation
- ROCK
Rho-associated protein kinase
- ROS
Reactive oxygen species
- TCR
T cell receptor
- Th
T helper
- p190-B RhoGAP
p190-B Rho GTPase activating protein
- mTOR
mammalian target of rapamycin
- mTORC1
mTOR complex 1
- mTORC2
mTOR complex 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang K, Shrestha S, Zeng H, Karmaus PW, Neale G, Vogel P, et al. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity. 2013;39:1043–1056. doi: 10.1016/j.immuni.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123:326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen H, Kelly A, Lee T, Lavender P. Control of cytokine gene transcription in Th1 and Th2 cells. Clin Exp Allergy. 2008;38:1422–1431. doi: 10.1111/j.1365-2222.2008.03067.x. [DOI] [PubMed] [Google Scholar]
- 4.Nakayama T, Yamashita M. The TCR-mediated signaling pathways that control the direction of helper T cell differentiation. Semin Immunol. 2010;22:303–309. doi: 10.1016/j.smim.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerriets VA, Rathmell JC. Metabolic pathways in T cell fate and function. Trends Immunol. 2012;33:168–173. doi: 10.1016/j.it.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O’Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang JQ, Leitges M, Duran A, Diaz-Meco MT, Moscat J. Loss of PKC lambda/iota impairs Th2 establishment and allergic airway inflammation in vivo. Proc Natl Acad Sci U S A. 2009;106:1099–1104. doi: 10.1073/pnas.0805907106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 11.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 12.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 13.Lin R, Cerione RA, Manor D. Specific contributions of the small GTPases Rho, Rac, and Cdc42 to Dbl transformation. J Biol Chem. 1999;274:23633–23641. doi: 10.1074/jbc.274.33.23633. [DOI] [PubMed] [Google Scholar]
- 14.Guo F, Zheng Y. Involvement of Rho family GTPases in p19Arf- and p53-mediated proliferation of primary mouse embryonic fibroblasts. Mol Cell Biol. 2004;24:1426–1438. doi: 10.1128/MCB.24.3.1426-1438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zohn IM, Campbell SL, Khosravi-Far R, Rossman KL, Der CJ. Rho family proteins and Ras transformation: the RHOad less traveled gets congested. Oncogene. 1998;17:1415–1438. doi: 10.1038/sj.onc.1202181. [DOI] [PubMed] [Google Scholar]
- 16.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 17.Rougerie P, Largeteau Q, Megrelis L, Carrette F, Lejeune T, Toffali L, et al. Fam65b is a new transcriptional target of FOXO1 that regulates RhoA signaling for T lymphocyte migration. J Immunol. 2013;190:748–755. doi: 10.4049/jimmunol.1201174. [DOI] [PubMed] [Google Scholar]
- 18.del Pozo MA, Vicente-Manzanares M, Tejedor R, Serrador JM, Sanchez-Madrid F. Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur J Immunol. 1999;29:3609–3620. doi: 10.1002/(SICI)1521-4141(199911)29:11<3609::AID-IMMU3609>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Heasman SJ, Carlin LM, Cox S, Ng T, Ridley AJ. Coordinated RhoA signaling at the leading edge and uropod is required for T cell transendothelial migration. J Cell Biol. 2010;190:553–563. doi: 10.1083/jcb.201002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vielkind S, Gallagher-Gambarelli M, Gomez M, Hinton HJ, Cantrell DA. Integrin regulation by RhoA in thymocytes. J Immunol. 2005;175:350–357. doi: 10.4049/jimmunol.175.1.350. [DOI] [PubMed] [Google Scholar]
- 21.Mou F, Praskova M, Xia F, Van Buren D, Hock H, Avruch J, et al. The Mst1 and Mst2 kinases control activation of rho family GTPases and thymic egress of mature thymocytes. J Exp Med. 2012;209:741–759. doi: 10.1084/jem.20111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corre I, Gomez M, Vielkind S, Cantrell DA. Analysis of thymocyte development reveals that the GTPase RhoA is a positive regulator of T cell receptor responses in vivo. J Exp Med. 2001;194:903–914. doi: 10.1084/jem.194.7.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henning SW, Galandrini R, Hall A, Cantrell DA. The GTPase Rho has a critical regulatory role in thymus development. Embo J. 1997;16:2397–2407. doi: 10.1093/emboj/16.9.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galandrini R, Henning SW, Cantrell DA. Different functions of the GTPase Rho in prothymocytes and late pre-T cells. Immunity. 1997;7:163–174. doi: 10.1016/s1074-7613(00)80519-1. [DOI] [PubMed] [Google Scholar]
- 25.Katayama K, Melendez J, Baumann JM, Leslie JR, Chauhan BK, Nemkul N, et al. Loss of RhoA in neural progenitor cells causes the disruption of adherens junctions and hyperproliferation. Proc Natl Acad Sci U S A. 2011;108:7607–7612. doi: 10.1073/pnas.1101347108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melendez J, Stengel K, Zhou X, Chauhan BK, Debidda M, Andreassen P, et al. RhoA GTPase is dispensable for actomyosin regulation but is essential for mitosis in primary mouse embryonic fibroblasts. J Biol Chem. 2011;286:15132–15137. doi: 10.1074/jbc.C111.229336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin P, Villares R, Rodriguez-Mascarenhas S, Zaballos A, Leitges M, Kovac J, et al. Control of T helper 2 cell function and allergic airway inflammation by PKC{zeta} Proc Natl Acad Sci U S A. 2005;102:9866–9871. doi: 10.1073/pnas.0501202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JQ, Liu H, Diaz-Meco MT, Moscat J. NBR1 is a new PB1 signalling adapter in Th2 differentiation and allergic airway inflammation in vivo. EMBO J. 2010;29:3421–3433. doi: 10.1038/emboj.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dott W, Mistry P, Wright J, Cain K, Herbert KE. Modulation of mitochondrial bioenergetics in a skeletal muscle cell line model of mitochondrial toxicity. Redox Biol. 2014;2:224–233. doi: 10.1016/j.redox.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurumurthy S, Xie SZ, Alagesan B, Kim J, Yusuf RZ, Saez B, et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature. 2010;468:659–663. doi: 10.1038/nature09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrabi SA, Umanah GK, Chang C, Stevens DA, Karuppagounder SS, Gagne JP, et al. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc Natl Acad Sci U S A. 2014;111:10209–10214. doi: 10.1073/pnas.1405158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kidani Y, Elsaesser H, Hock MB, Vergnes L, Williams KJ, Argus JP, et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat Immunol. 2013;14:489–499. doi: 10.1038/ni.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:44589. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts DJ, Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015;22:248–257. doi: 10.1038/cdd.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem. 2010;285:14071–14077. doi: 10.1074/jbc.R109.094003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Busse PJ, Zhang TF, Srivastava K, Lin BP, Schofield B, Sealfon SC, et al. Chronic exposure to TNF-alpha increases airway mucus gene expression in vivo. J Allergy Clin Immunol. 2005;116:1256–1263. doi: 10.1016/j.jaci.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 39.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003;111:291–297. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H, Eleswarapu S, Geiger H, Szczur K, Daria D, Zheng Y, et al. Loss of the Rho GTPase activating protein p190-B enhances hematopoietic stem cell engraftment potential. Blood. 2009;114:3557–3566. doi: 10.1182/blood-2009-02-205815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348 Pt 2:241–255. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.