Abstract
Background
Loss of protein mass and lower fat-free mass index (FFMI) are associated with longer length of stay, post-surgical complications and other poor outcomes in hospitalized patients Normative data for FFMI of U.S. populations does not exist. This work aims to create a stratified FFMI percentile table for the U.S. population using the large bioelectric impedance analysis data obtained from National Health and Nutrition Examination Surveys (NHANES).
Methods
Fat-free mass (FFM) was calculated from the NHANES III bioelectric impedance analysis and anthropometric data for males and females ages 12 to over 90 years for three race-ethnicities (non-Hispanic white, non-Hispanic black, and Mexican-American). FFM was normalized by subject height to create a FFMI distribution table for the U.S. population. Selected percentiles were obtained by age, sex, and race-ethnicity. Data was collapsed by race-ethnicity before and after removing obese and underweight subjects to create a FFMI decile table for males and females aged 12 and over for the healthy weight U.S. population.
Results
FFMI increased during adolescent growth but stabilized in the early 20s. The FFMI deciles were similar by race-ethnicity and age group remaining relatively stable between ages of 22 and 80 years. The FFMI deciles for males and females were significantly different.
Conclusions
After eliminating the obese and extremely thin, FFMI percentiles remain stable during adult years allowing creation of age- and race/ethnicity-independent decile tables for males and females. These tables allow stratification of individuals for nutrition intervention trials to depict changing nutrition status during medical, surgical and nutritional interventions.
INTRODUCTION
The depletion of the protein compartment by disease or treatment correlates with poor clinical outcomes in hospitalized patients. To identify these patients, investigators use weight loss, serum protein levels, body mass index (BMI), subjective global assessment, anthropometric measurements, fat-free mass (FFM), muscle function, and combinations of these measurements to predict clinical outcome with varied success. However, reproducibility of most predictors is inconsistent and thus research to identify consistent criteria is needed to detect malnutrition and; in particular, alterations in the protein compartment to allow identification and treatment of nutritionally at-risk individuals.
With the advent of bioelectric impedance analysis (BIA), clinical assessment of the protein rich FFM compartment became more widespread due to its low cost, portability, and safety making it ideal for clinical trials. For example, FFM successfully identified cancer patients at risk of post-operative complications. Fritz et al.1 studied 115 patients with gastrointestinal cancer and noted the “average physical characteristics of the patients were within normal range with a wide range of variation.” They reported that while the magnitude of the surgical procedure influenced outcome, a history of weight loss and the deficit relative to “normal values” of FFM adjusted by age and gender identified patients at risk of serious post-operative complications which is similar to our prior work.2 Moreover, patients with a negative FFM trajectory (i.e. weight loss by history) sustained a significantly higher complication rate than patients with a positive FFM trajectory. However, the use of BIA to measure FFM is limited by the wide range of normal values including age, height, and numerous other factors.3
VanItallie et al.4 recommended the use of fat-free mass index (FFMI) in place of FFM alone, in which the measured FFM is indexed by height. These authors argued that FFMI would parallel BMI and thus should be relatively independent of height, sex, and age. They studied FFMI and the FFMI percentiles as predictors of nutritional depletion by comparing the 50th percentile of a healthy population to those experiencing 24 weeks of semi-starvation in the classic Keys study of conscientious objectors.5 Starved subjects exhibited significant decreases in FFMI with advancing weeks of food deprivation, decreasing below the 5th percentile at semi-starvation week 24; refeeding resulted in an increase in both FFMI and FFMI percentile. This study suggests the clinical usefulness of FFMI to identify nutritional depletion. The reference population used by VanItallie et al, however, was small and not nationally representative.
BIA measurement of FFM and FFMI have predicted negative outcomes in groups of Swiss hospital patients using Swiss population norms,6 but similar normative data does not exist for the U.S. population. This study uses anthropomorphic data from the National Health and Nutrition Examination Survey (NHANES)7 to generate normative values of FFM and FFMI for the U.S. population. The expectation is that these data will be useful in subsequent prospective studies of patients using BIA or multi-frequency BIA as a measure of protein rich tissue in pre- and post-intervention trials.
SUBJECTS AND METHODS
The NHANES III data available online at http://www.cdc.gov/nchs/nhanes.htm served as the data set as approved by the NHANES Institutional Review Board. The NHANES provides body measurements, health, and nutritional data for the non-institutionalized U.S. population. NHANES III (1988-1994) survey obtained weight, height, BMI, and anthropometric measurements from over 30,000 individuals from 2 months to 99 years of age including BIA. Approximately 16,000 subjects across all age groups in this population underwent BIA measurements. The NHANES III data set (1988-1994) was selected because it includes a large amount of BIA data for males and females from ages 12 to over 90. BIA was measured in the NHANES III sample population using Valhalla 1990B Bio-Resistance Body Composition Analyzer (Valhalla Scientific, San Diego, CA) at 50 kHz as part of the physician examination, using a random procedure to recruit participants.7
Data from youth and adult demographic files (including age, sex, and race-ethnicity) of subjects aged 12 years and older were combined into one data set. The identification number (SEQN) linked subjects from these files to the examination components that included anthropometric and resistance values (height, weight, BMI, and resistance). We omitted subjects with missing height, weight, BMI or resistance values and subjects with race-ethnicity of “other” from analysis.
BIA utilizes a drop in voltage due to resistance and reactance (impedance) from body tissues to determine impedance and estimate fat-free and fat mass.8 Several available devices measure BIA resistance, however the resultant data lie on different scales. Since the BIA data in NHANES III were generated with a Valhalla device, and the validated prediction equations for U.S. FFM were developed with an RJL device,9 Valhalla resistance measures were converted to RJL resistances using the widely accepted formula of Chumlea et al:10
For males: RJL resistance = 2.5 + 0.98 Valhalla resistance
For females: RJL Res = 9.6 + 0.96 Valhalla resistance.
This corrected resistance value was used to calculate fat-free mass (FFM) with the validated formula:9, 11
For males: FFM = −10.678 + 0.262 weight + 0.652 S2/Res + 0.015 Res
For females: FFM = −9.529 + 0.168 weight + 0.696 S2/Res + 0.016 Res,
where weight = weight in kilograms, S = stature in centimeters, Res = corrected RJL resistance in ohms. We normalized FFM by height to create the fat-free mass index (FFMI): FFM/Ht2 where FFM is in kilograms and Ht in meters. These equations were validated in their development using a multicomponent body-composition model with cross-validation based on densitometry from underwater weighting, total body water from isotope dilution, and total body bone mineral content using dual-energy X-ray absorptiometry.9
STATISTICAL METHODS
Non-parametric quantile regression11, 12 was used to model the 2.5, 5, 10, 25, 50, 75, 90, 95, and 97.5 percentiles as a function of sex, age (in years), and race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American). A cubic B-spline basis was used for age, with knots at 14[2]22, 25, and 30[10]80 years (i.e. from 14 to 22 in increments of 2). Terms were entered additively into the model. Separate models were fitted to unrestricted BMI and to BMI in the [18.5 to 30) range (i.e. greater than or equal to 18.5 and lower than 30). If the sex term was found to be significant, separate models were fitted to each sex. Conversely, if the race-ethnicity term was found to be insignificant, the models were simplified by omitting the term from the model.
To assess the impact of considering the FFMI percentiles constant across age for those 25 and older, the maximum percent change was computed separately for males and females, with BMI unrestricted and restricted. First, empirical FFMI quantiles were obtained for each of the age groups defined by the half-closed intervals [a,b) = {x | a ≤ x < b} resulting from the following cut-points: 12[2]20[5]80. For example, let qi denote the median (50% quantile) for the i-th age group. Second, the percent change between any two such medians qi, qj across age groups was computed as Δ50% ij = 100 × [qi / qj − 1]. Third, the maximum percent change was computed as Δ50% max = max i,≠ j { |Δ50% ij| }, where |x| denotes the absolute value of x. This process was repeated for each percentile. Finally, the maximum percent change over all the percentiles was obtained, as Δ* = max k {.Δkmax}, where k = 2.5, 5, 10, 25, 50, 75, 90, 95, 97.5%.
A table for individual FFMI for each percentile from 1-99 by gender was constructed so that it could be used to quantify a value for individual patients.
All P-values are two-sided; p < 0.05 was used as the criterion for significance. Statistical modeling was done with the quantreg package version 4.0813 in R for Windows version 2.5.1 patched.14
RESULTS
The initial data set includes all subjects aged 12 to over 90 years with values for resistance at 50 kHz, height, weight, and BMI along with age, sex, and race-ethnicity of non-Hispanic white, non-Hispanic black, and Mexican-American, (data not shown). The race-ethnicity distribution of the males and females below age 50 reflects the over-sampling of non-Hispanic black and Mexican-American subjects for NHANES III.7 The male and female subjects older than age 50 with BIA data were predominantly non-Hispanic white.
Evaluation of Gender differences in FFMI
Gender differences were statistically significant for all percentiles (p < 0.001), with women having significantly lower FFMI quantiles than the corresponding ones for males. Due to this difference, males and females were modeled separately. Separate modelling was done in both the BMI unrestricted and restricted analyses.
Evaluation of FFMI with and without BMI restrictions
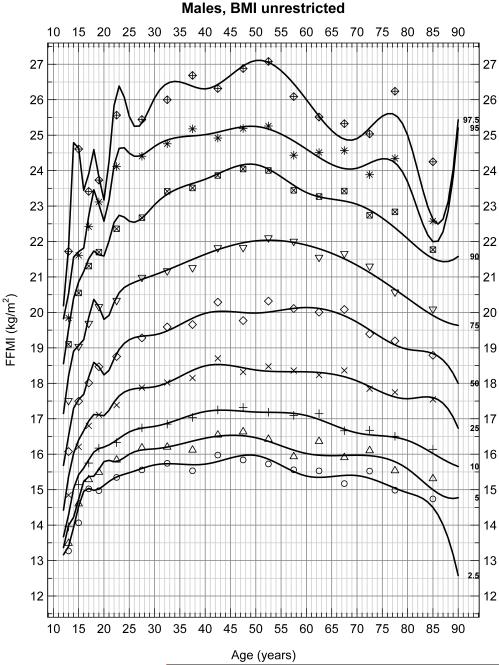
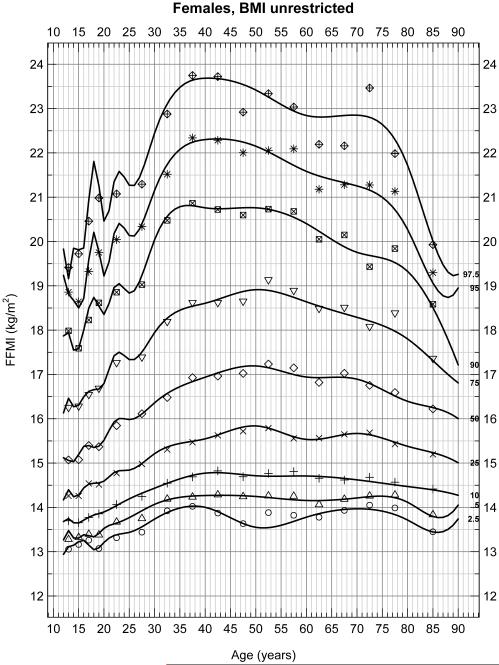
With no restrictions on BMI in males (Figure 1A) and females (Figure 1B), FFMI significantly increased between the ages of 15 and 23 due to adolescent growth. Between 25 and 80 years of age, there were significant increases in FFMI with peaks between 40 and 50 years of age which then decreased to age 80 (p < 0.05). These data include 8,217 males and 8,690 females, for a total of 16,907 subjects. Figures 1A and 1B show the 2.5th, 5th, 10th……97.5th percentiles for FFMI over these age groups and the peak at 40 to 50 years of age is very evident.
Figure 1A.
Selected percentiles (2.5, 5, 10, 25, 50, 75, 90, 95, 97.5) for male FFMI. Continuous line depicts quantile regression spline fits; dots represent the corresponding observed quantiles centered at the following half-closed age group intervals [a,b) = {x | a ≤ x < b} with cut-points at 12[2]20[5]80 years.
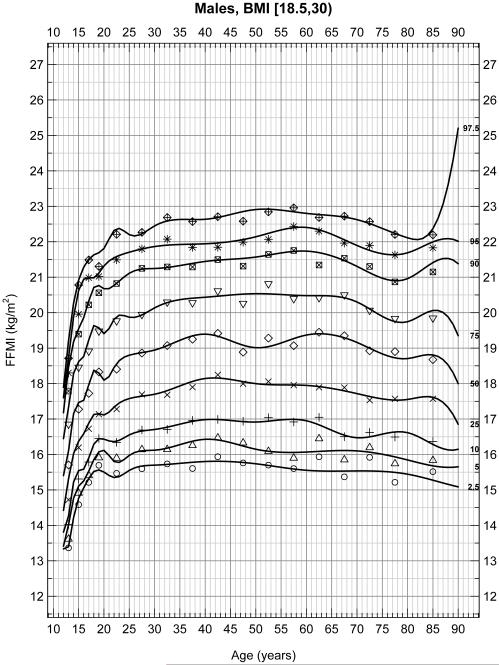
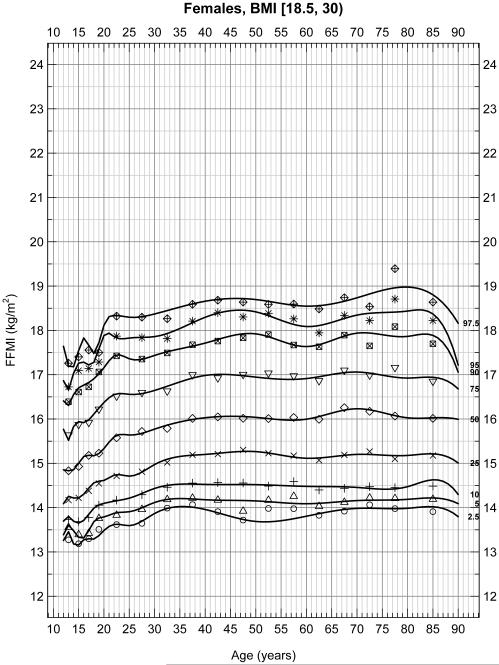
To assess whether extremely thin or obese patients altered these percentiles, reanalysis of data excluded patients with a BMI less than 18.5 or greater than 30 in male (Figure 2A) and female (Figure 2B) subjects. These figures represent 6,495 male and 6,062 female for a total of 12,557 subjects. Again FFMI significantly differed across sex (p < 0.001). In these weight restricted populations, significant increases in FFMI occurred between the ages of 15 and 23 as expected due to growth during the adolescent and early adult years. However, FFMI values remained relatively stable between the ages of 25 and 80 in both males and females in this restricted population flattening the peak between 40 and 50 years of age seen in the unrestricted populations. Although some curvature still exists with a peak at approximately 50 years of age, the maximum percent difference is at most 4.95% and 6.18% at any age point on any percentile line for males and females, respectively, (or about 1 kg/m2) between the ages of 25-80. The average percent differences are 4.23% and 3.68% for males and females, respectively.
Figure 1B.
Selected percentiles (2.5, 5, 10, 25, 50, 75, 90, 95, 97.5) for female FFMI. Continuous line depicts quantile regression spline fits; dots represent the corresponding observed quantiles centered at the following half-closed age group intervals [a,b) = {x | a ≤ x < b} with cut-points at 12[2]20[5]80 years.
Evaluation of FFMI and Race-ethnicity
Unrestricted by BMI
Males
When BMI is unrestricted, FFMI quantiles for Mexican-American males are not significantly different from those of non-Hispanic white males (p ≥ 0.493, for all quantiles). However, non-Hispanic black males have significantly different FFMI quantiles than those of non-Hispanic white males (p < 0.003) except for the median and third quartile (p = 0.236 and p = 0.317, respectively). The quantiles up to the first quartile inclusive (2.5%, 5%, 10%, and 25%) of non-Hispanic black males are lower than those of their non-Hispanic white counterparts, while the direction is reversed for quantiles above the third quartile (90%, 95%, 97.5%).
Females
When BMI is unrestricted, non-Hispanic black females have higher FFMI quantiles than those of their non-Hispanic white peers (p < 0.002) for percentiles above the 5th percentile. Similarly, Mexican-American females have higher quantiles than non-Hispanic white females (p < 0.001) for quantiles up to the 90th percentile
Restricted by BMI
Males
When BMI is restricted (18.5 ≤ BMI < 30), the race effects are significant for non-Hispanic black males up to the third quartile (p < 0.05), while no differences are seen for Mexican-American males for the 10th to 90th percentiles, i.e. significant differences are only seen at the tails (2.5%, 95.0%, and 97.5%). Non-Hispanic white males consistently had significantly higher FFMI than Mexican-American males, which in turn were significantly higher than non-Hispanic black males between the 10th and 50th percentile (p < 0.05). However, the maximum percentage difference ranged between 1.3% and 4.75% which is likely not clinically relevant to warrant differentiation by race/ethnicity.
Females
The restricted FFMI of non-Hispanic black females was significantly greater than non-Hispanic white females between the 50th and 95th percentile, but not below the 50th percentile or at the upper tail. Mexican-American females differ from their non-Hispanic white counterparts from the 10th to 90th percentile (p<0.05), but do not differ at the extremes. The differences varied between 1.3% and a maximum of 3.75% at various percentiles.
Percentile values by gender
Individual data for FFMI restricted by weight for each decile by gender but not ethnicity are provided in Table 1. Data are limited to patients 25 to 69 years of age.
Table 1.
Standard fat-free mass index (FFMI) for males and females age 25-69 years old with body mass index (BMI) 18.5 to 30 for all race-ethnicity groups in the U.S. population. FFMI is in kg/m2. The chart is used as follows: A 40 year old male starts a medical therapy with a FFMI of 19.631 and completes therapy with a FFMI of 18.668 denoting a 20 percentile drop in FFMI (60th to 40th). Outcomes could be stratified by percentile change in FFMI to determine the metabolic expense of the therapy or subsequently the effect of a nutritional intervention on outcomes. The same device would be necessary for both pre- and post-treatment measurements.
| Centile | Male FFMI |
Female FFMI |
Centile | Male FFMI |
Female FFMI |
Centile | Male FFMI |
Female FFMI |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1% | 15.278 | 13.544 | 34% | 18.369 | 15.425 | 67% | 19.936 | 16.536 |
|
| ||||||||
| 2% | 15.560 | 13.776 | 35% | 18.411 | 15.461 | 68% | 19.991 | 16.573 |
|
| ||||||||
| 3% | 15.751 | 13.944 | 36% | 18.459 | 15.493 | 69% | 20.039 | 16.602 |
|
| ||||||||
| 4% | 15.978 | 14.032 | 37% | 18.506 | 15.523 | 70% | 20.095 | 16.633 |
|
| ||||||||
| 5% | 16.168 | 14.123 | 38% | 18.558 | 15.558 | 71% | 20.165 | 16.685 |
|
| ||||||||
| 6% | 16.357 | 14.202 | 39% | 18.612 | 15.589 | 72% | 20.213 | 16.719 |
|
| ||||||||
| 7% | 16.465 | 14.267 | 40% | 18.668 | 15.618 | 73% | 20.267 | 16.763 |
|
| ||||||||
| 8% | 16.581 | 14.327 | 41% | 18.706 | 15.644 | 74% | 20.319 | 16.809 |
|
| ||||||||
| 9% | 16.692 | 14.393 | 42% | 18.769 | 15.675 | 75% | 20.378 | 16.868 |
|
| ||||||||
| 10% | 16.807 | 14.444 | 43% | 18.809 | 15.703 | 76% | 20.434 | 16.917 |
|
| ||||||||
| 11% | 16.924 | 14.509 | 44% | 18.866 | 15.739 | 77% | 20.486 | 16.958 |
|
| ||||||||
| 12% | 17.008 | 14.561 | 45% | 18.902 | 15.786 | 78% | 20.537 | 17.005 |
|
| ||||||||
| 13% | 17.116 | 14.596 | 46% | 18.945 | 15.819 | 79% | 20.599 | 17.062 |
|
| ||||||||
| 14% | 17.183 | 14.643 | 47% | 18.997 | 15.851 | 80% | 20.652 | 17.113 |
|
| ||||||||
| 15% | 17.241 | 14.688 | 48% | 19.059 | 15.883 | 81% | 20.719 | 17.161 |
|
| ||||||||
| 16% | 17.315 | 14.741 | 49% | 19.107 | 15.924 | 82% | 20.775 | 17.211 |
|
| ||||||||
| 17% | 17.378 | 14.779 | 50% | 19.160 | 15.962 | 83% | 20.834 | 17.259 |
|
| ||||||||
| 18% | 17.428 | 14.817 | 51% | 19.204 | 15.991 | 84% | 20.905 | 17.321 |
|
| ||||||||
| 19% | 17.496 | 14.867 | 52% | 19.254 | 16.017 | 85% | 20.966 | 17.367 |
|
| ||||||||
| 20% | 17.568 | 14.908 | 53% | 19.302 | 16.046 | 86% | 21.044 | 17.427 |
|
| ||||||||
| 21% | 17.638 | 14.950 | 54% | 19.346 | 16.087 | 87% | 21.139 | 17.477 |
|
| ||||||||
| 22% | 17.689 | 15.003 | 55% | 19.386 | 16.126 | 88% | 21.232 | 17.545 |
|
| ||||||||
| 23% | 17.773 | 15.036 | 56% | 19.425 | 16.164 | 89% | 21.324 | 17.600 |
|
| ||||||||
| 24% | 17.830 | 15.073 | 57% | 19.478 | 16.195 | 90% | 21.428 | 17.663 |
|
| ||||||||
| 25% | 17.891 | 15.110 | 58% | 19.530 | 16.224 | 91% | 21.537 | 17.733 |
|
| ||||||||
| 26% | 17.954 | 15.138 | 59% | 19.579 | 16.255 | 92% | 21.629 | 17.812 |
|
| ||||||||
| 27% | 17.992 | 15.175 | 60% | 19.631 | 16.287 | 93% | 21.740 | 17.918 |
|
| ||||||||
| 28% | 18.046 | 15.210 | 61% | 19.669 | 16.328 | 94% | 21.865 | 18.049 |
|
| ||||||||
| 29% | 18.114 | 15.250 | 62% | 19.722 | 16.367 | 95% | 22.013 | 18.162 |
|
| ||||||||
| 30% | 18.168 | 15.289 | 63% | 19.762 | 16.405 | 96% | 22.285 | 18.337 |
|
| ||||||||
| 31% | 18.224 | 15.316 | 64% | 19.799 | 16.430 | 97% | 22.522 | 18.512 |
|
| ||||||||
| 32% | 18.277 | 15.356 | 65% | 19.847 | 16.463 | 98% | 22.826 | 18.680 |
|
| ||||||||
| 33% | 18.322 | 15.394 | 66% | 19.894 | 16.498 | 99% | 23.492 | 19.017 |
|
| ||||||||
DISCUSSION
Preexisting malnutrition of surgical patients is associated with increased complication rates,15-20 higher mortality and morbidity,21-25 increased hospital length of stay,6, 26, 27 and increased healthcare costs.25, 26, 28 Unfortunately, pre-operative identification of these patients is complicated due to 1) a general shift to outpatient evaluation with admission on the day of surgery,29 and 2) the inability to cost-effectively identify alterations in nutritional status and existing deficiencies in lean tissue mass. This work defines a template against which measurement of FFM with BIA allows stratification of an individual patient’s values when compared with the general U.S. population. It also allows for systematic quantification of changes in a patient’s FFM and FFMI associated with age, surgical procedure or development of complications.
BIA utilizes physical properties of the body to measure drops of voltage due to the impedance from tissues; this technique is portable, low-cost, and low-risk. Single frequency BIA at 50 kHz can estimate total body water based on extra-cellular water as well as FFM. FFMI allows the FFM to be indexed by height.
Both FFM and FFMI have been shown to correlate with clinical outcomes. Pichard et al.6 studied patients admitted to a Swiss Hospital for medical and surgical indications and evaluated their BMI to determine FFM and FFMI at admission. The ranges of FFM and FFMI were determined in a population of healthy adults and grouped by BMI. A low FFMI was determined to be less than 17.4 kg/m2 in men and less than 15.0 kg/m2 in women with normal ranges of 17.5 to 19.7 kg/m2 for men and 15.1 to 16.6 kg/m2 for women. The authors found that length of stay was significantly higher in patients with a low FFMI (8.7 ± 21.0 days for low FFMI, 4.3 ± 7.2 days for high FFMI, p > 0.0001). The same authors included patients admitted to a Berlin Hospital and found a similar correlation between increased length of stay and decreased FFMI.30
Unfortunately, data from these European studies include a uniform race-ethnicity and weights that are not representative of the U.S. population. Schutz et al.31 postulated that the FFM regression equations generated within one population might not be applicable to another population, which Kyle et al.32 confirmed by demonstrating discrepancies in FFM between BIA equations generated specifically from the Swiss population and the U.S. population NHANES III cohort evaluated here. Since this study evaluates U.S. specific data from a large nationally representative data set, we chose to use the FFM regression equations developed from this exact data by Chumlea et al.9, 10 to generate American age- and race-ethnicity-independent FFMI values, precluding use of an equation that was developed for a different population.
In expectation of using this technique in subsequent prospective studies, we analyzed a large population of data from the NHANES III database to produce a template for individual patient comparison. We limited our analysis to patients with BIA and simultaneous height and weight since Pichard and Schutz et al.6, 31 showed the importance of normalizing FFM data by height. We reached several conclusions in our analysis. First, FFMI differs for males and for females; therefore, a percentile range reflecting the U.S. population should be presented by gender. Secondly, extremes in the BMI reflecting obesity (BMI >30) or extreme asthenia (BMI <18.5) should be analyzed separately from the general population. Thirdly, FFMI increases dramatically in adolescence through young adulthood. However, between 25 years of age and 80 years of age, the FFMI remains relatively stable by percentile. Alterations which occur beyond this age are probably secondary to the decreasing size of the study population. Although some statistical variations occur, these appear of relatively insignificant magnitude. Fourthly, between the ages of 25 and 80, while there are differences in FFMI at a given percentile between non-Hispanic whites, non-Hispanic blacks, and Mexican-Americans, these variations also appear clinically insignificant. Thus, separate percentile tables and figures do not need to be generated based on race-ethnicity.
These conclusions are consistent with other American-specific published work. Chumlea et al.10 described the FFM, total body water, percent body fat, and total body fat the same NHANES III population (ages 12 – 80 years). They concluded that FFM was greater in males than females and increased with age in adolescents and found that FFM differed by race-ethnicity (males: non-Hispanic white > non-Hispanic black > Mexican-American; females: non-Hispanic black > non-Hispanic white > Mexican-American). Forbes noted that the FFM of both genders increased during the adolescent years but remained stable over the age of 20.33 The authors of these studies did not index their values to height, however.
Our data differed in several regards to other trials. Gallagher34 and Kyle32 noted decreases in FFM with increasing age while we did not detect a significant decrease in FFMI, possibly due to decreased height associated with advancing age. The NHANES III anthropometric reference data (Advance Data No. 347) reveals an average height decrease of 5.4 cm for males and 6.6 cm for females between the age groups of 20-29 years and 75 years and older. A 10% decrease in FFM across these two age groups with different average heights would result in only a small decrease in FFMI, as we observed, rather than the pronounced decreases found by Kyle and Gallagher. This reasoning may also explain why Obisesan35 documented a decrease in FFM with aging in non-Hispanic whites and blacks using NHANES III data but found FFMI did not vary much with aging (20.64 kg/m2 for 40-49 year old white males and 19.93 kg/m2 for >80 year old males). Thus, FFMI may be useful in the older age groups to determine how an individual’s lean tissue compares to the U.S. population, while FFM does not. It is also possible, however, that with regard to identifying patients who are at greater risk for post-surgical complications, that FFMI may mask a low part of the at risk population despite making it easier to adjust for differences in BMI for height.
The clinical implications of these observations are important. First, in recruiting patients into randomized prospective studies, patients within the appropriate BMI ranges can add accurate determination of their FFMI for appropriate stratification between the 2.5th and 97.5th percentiles. This stratification will allow investigation of how altered protein mass (measured through its surrogate FFMI) affects outcome. Secondly, this technique can allow examination of the physiologic cost (loss of protein mass) of specific medical interventions and the physiologic cost of associated complications. As an example, we recently examined the effect of radiation and chemotherapy as a treatment for esophageal carcinoma. Patients that lost weight with treatment dropped their FFMI from 19.8±1.1 to 18.4±1.4, representing more than an 18% percentile decrease in FFMI when compared to the U.S. population. Hopefully, just as a height/age figures for young children allow prediction of the final height of that individual in adulthood, the FFMI scale can be used as a means to identify, stratify, and then treat pre-existing malnutrition.
We recognize that the current BIA technology may be superior to the BIA technology used in the NHANES III study. However, the principles in this data analysis should remain constant despite these improvements. Our conclusions from this analysis are: 1) The range of FFMI should exclude extremes in BMI (malnutrition or obesity) to represent true normal distribution of FFMI without the influence of extreme weights; 2) FFMI differs for males and females and a percentile range reflecting the U.S. population should be presented by gender; 3) FFMI (adjusted for extremes of BMI) are similar for both genders of non-Hispanic white, non-Hispanic black, and Mexican-Americans so the FFMI of these race-ethnicities can be combined by gender; 4) The FFMI percentiles can be collapsed into the following age groups: a) 12- 20 every 2 years, b) 20-25 years c) 25-80 years and d) greater than 80 years. From these data, FFMI appears stable across the age groups most likely to be entered into adult clinical trials, thus allowing interpretation of the FFMI (and its changes) measured in each patient.
CLINICAL RELEVANCY STATEMENT.
Fat-free mass index is a useful tool to identify nutritional depletion and predict negative outcomes in hospitalized patients when normative data is available. However, no normative data currently exist for the U.S. population. This study generates normative data for the U.S. population as stratified by sex that is independent of race-ethnicity and age from 25-80 years old. As a result of this work, bioelectric impedance analysis data can be used in prospective studies of patients as a measure of protein rich tissue in pre- and post-intervention trials using the patient as their own control.
Figure 2A.
Selected percentiles (2.5, 5, 10, 25, 50, 75, 90, 95, 97.5) for male FFMI, BMI restricted (18.5 ≤ BMI < 30). Continuous line depicts quantile regression spline fits; dots represent the corresponding observed quantiles centered at the following half-closed age group intervals [a,b) = {x | a ≤ x < b} with cut-points at 12[2]20[5]80 years.
Figure 2B.
Selected percentiles (2.5, 5, 10, 25, 50, 75, 90, 95, 97.5) for female FFMI, BMI restricted (18.5 ≤ BMI < 30). Continuous line depicts quantile regression spline fits; dots represent the corresponding observed quantiles centered at the following half-closed age group intervals [a,b) = {x | a ≤ x < b} with cut-points at 12[2]20[5]80 years.
Acknowledgments
Financial disclosure: The project described was supported in part (RAB) by the Surgical Oncology Research Training Program [grant number T32CA090217].
Footnotes
The authors had no conflicts of interest to report.
The contents of this article do not represent the views of the Department of Veterans Affairs or the United States government. This material is the result of work supported with the resources and use of facilities at the William S. Middleton Memorial Veterans Hospital, Madison, WI.
REFERENCES
- 1.Fritz T, Höllwarth I, Romaschow M, Schlag P. The predictive role of bioelectrical impedance analysis (BIA) in postoperative complications of cancer patients. Eur J Surg Oncol. 1990;16:326–331. [PubMed] [Google Scholar]
- 2.Kudsk KA, Tolley EA, DeWitt RC, et al. Preoperative albumin and surgical site identify surgical risk for major postoperative complications. JPEN J Parenter Enteral Nutr. 2003;27:1–9. doi: 10.1177/014860710302700101. [DOI] [PubMed] [Google Scholar]
- 3.Ellis KJ, Bell SJ, Chertow GM, et al. Bioelectrical impedance methods in clinical research: a follow-up to the NIH Technology Assessment Conference. Nutrition. 1999;15:874–880. doi: 10.1016/s0899-9007(99)00147-1. [DOI] [PubMed] [Google Scholar]
- 4.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body's fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52:953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 5.Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL. The Biology of Human Starvation. University of Minnesota Press; Minneapolis: 1950. [Google Scholar]
- 6.Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P. Nutritional assessment: lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr. 2004;79:613–618. doi: 10.1093/ajcn/79.4.613. [DOI] [PubMed] [Google Scholar]
- 7.National Center for Health Statistics (U.S.) NCHS CD-ROM Series 11 no 1. U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; Hyattsville, Md: 1997. National Health and Nutrition Examination Survey III, 1988-94. SETS 1.22a; rev. Oct. 1997. ed. [Google Scholar]
- 8.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004;23:1226–1243. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Sun SS, Chumlea WC, Heymsfield SB, et al. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am J Clin Nutr. 2003;77:331–340. doi: 10.1093/ajcn/77.2.331. [DOI] [PubMed] [Google Scholar]
- 10.Chumlea WC, Guo SS, Kuczmarski RJ, et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–1609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]
- 11.Koenker R. Quantile regression. Cambridge University Press; Cambridge: 2005. [Google Scholar]
- 12.Wei Y, Pere A, Koenker R, He X. Quantile regression methods for reference growth charts. Stat Med. 2006;25:1369–1382. doi: 10.1002/sim.2271. [DOI] [PubMed] [Google Scholar]
- 13.Koenker R. quantreg: Quantile Regression. 2007 R package version 4.08. [Google Scholar]
- 14.R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2007. [Google Scholar]
- 15.Schiesser M, Kirchhoff P, Müller MK, Schäfer M, Clavien PA. The correlation of nutrition risk index, nutrition risk score, and bioimpedance analysis with postoperative complications in patients undergoing gastrointestinal surgery. Surgery. 2009;145:519–526. doi: 10.1016/j.surg.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Fry DE. Fifty ways to cause surgical site infections. Surg Infect. 2011;12:497–500. doi: 10.1089/sur.2011.091. [DOI] [PubMed] [Google Scholar]
- 17.Cerantola Y, Valerio M, Hubner M, Iglesias K, Vaucher L, Jichlinski P. Are patients at nutritional risk more prone to complications after major urological surgery? J Urol. 2013;190:2126–2132. doi: 10.1016/j.juro.2013.06.111. [DOI] [PubMed] [Google Scholar]
- 18.Bagan P, Berna P, De Dominicis F, et al. Nutritional status and postoperative outcome after pneumonectomy for lung cancer. Ann Thorac Surg. 2013;95:392–396. doi: 10.1016/j.athoracsur.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Shahin ES, Meijers JM, Schols JM, Tannen A, Halfens RJ, Dassen T. The relationship between malnutrition parameters and pressure ulcers in hospitals and nursing homes. Nutrition. 2010;26:886–889. doi: 10.1016/j.nut.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Culebras JM. Malnutrition in the twenty-first century: an epidemic affecting surgical outcome. Surg Infect. 2013;14:237–243. doi: 10.1089/sur.2013.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yosry A, Omran D, Said M, Fouad W, Fekry O. Impact of nutritional status of Egyptian patients with end-stage liver disease on their outcomes after living donor liver transplantation. J Dig Dis. 2014;15:321–326. doi: 10.1111/1751-2980.12141. [DOI] [PubMed] [Google Scholar]
- 22.Gray RT, O'donnell ME, Scott RD, McGuigan JA, Mainie I. Impact of nutritional factors on survival in patients with inoperable oesophageal cancer undergoing self-expanding metal stent insertion. Eur J Gastroenterol Hepatol. 2011;23:455–460. doi: 10.1097/MEG.0b013e3283469761. [DOI] [PubMed] [Google Scholar]
- 23.Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113–117. doi: 10.1016/j.nut.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Chang SH, Tsai YJ, Chou HH, et al. Clinical predictors of long-term outcomes in patients with critical limb ischemia who have undergone endovascular therapy. Angiology. 2014;65:315–322. doi: 10.1177/0003319713515544. [DOI] [PubMed] [Google Scholar]
- 25.Tangvik RJ, Tell GS, Eisman JA, et al. The nutritional strategy: four questions predict morbidity, mortality and health care costs. Clin Nutr. 2014;33:634–641. doi: 10.1016/j.clnu.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Álvarez-Hernández J, Planas Vila M, León-Sanz M, et al. Prevalence and costs of malnutrition in hospitalized patients; the PREDyCES Study. Nutr Hosp. 2012;27:1049–1059. doi: 10.3305/nh.2012.27.4.5986. [DOI] [PubMed] [Google Scholar]
- 27.de Luis DA, Izaola O, Cuellar L, et al. Nutritional assessment: predictive variables at hospital admission related with length of stay. Ann Nutr Metab. 2006;50:394–398. doi: 10.1159/000094362. [DOI] [PubMed] [Google Scholar]
- 28.Barker LA, Gray C, Wilson L, Thomson BN, Shedda S, Crowe TC. Preoperative immunonutrition and its effect on postoperative outcomes in well-nourished and malnourished gastrointestinal surgery patients: a randomised controlled trial. Eur J Clin Nutr. 2013;67:802–807. doi: 10.1038/ejcn.2013.117. [DOI] [PubMed] [Google Scholar]
- 29.Kudsk KA, Reddy SK, Sacks GS, Lai HC. Joint Commission for Accreditation of Health Care Organizations guidelines: too late to intervene for nutritionally at-risk surgical patients. JPEN J Parenter Enteral Nutr. 2003;27:288–290. doi: 10.1177/0148607103027004288. [DOI] [PubMed] [Google Scholar]
- 30.Kyle UG, Pirlich M, Lochs H, Schuetz T, Pichard C. Increased length of hospital stay in underweight and overweight patients at hospital admission: a controlled population study. Clin Nutr. 2005;24:133–142. doi: 10.1016/j.clnu.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Schutz Y, Kyle UG, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18-98 y. Int J Obes Relat Metab Disord. 2002;26:953–960. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- 32.Kyle UG, Genton L, Lukaski HC, et al. Comparison of fat-free mass and body fat in Swiss and American adults. Nutrition. 2005;21:161–169. doi: 10.1016/j.nut.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Forbes GB. Human body composition : growth, aging, nutrition, and activity. Springer-Verlag; New York: 1987. [Google Scholar]
- 34.Gallagher D, Visser M, De Meersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83:229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 35.Obisesan TO, Aliyu MH, Bond V, Adams RG, Akomolafe A, Rotimi CN. Ethnic and age-related fat free mass loss in older Americans: the Third National Health and Nutrition Examination Survey (NHANES III) BMC Public Health. 2005;5:41. doi: 10.1186/1471-2458-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]