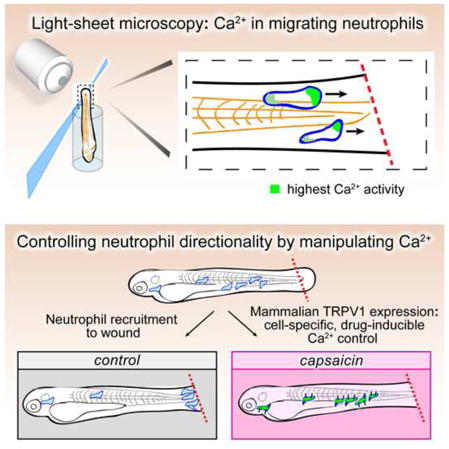
SUMMARY
Calcium signaling has long been associated with key events of immunity, including chemotaxis, phagocytosis, and activation. However, imaging and manipulation of calcium flux in motile immune cells in live animals remains challenging. Using light-sheet microscopy for in vivo calcium imaging in zebrafish, we observe characteristic patterns of calcium flux triggered by distinct events, including phagocytosis of pathogenic bacteria and migration of neutrophils toward inflammatory stimuli. In contrast to findings from ex vivo studies, we observe enriched calcium influx at the leading edge of migrating neutrophils. To directly manipulate calcium dynamics in vivo, we have developed transgenic lines with cell-specific expression of the mammalian TRPV1 channel, enabling ligand-gated, reversible and spatiotemporal control of calcium influx. We find that controlled calcium influx can function to help define the neutrophil’s leading edge. Cell-specific TRPV1 expression may have broad utility for precise control of calcium dynamics in other immune cell types and organisms.
Graphical Abstract
INTRODUCTION
Intracellular calcium concentrations [Ca2+]i are tightly regulated; changes in the frequency and spatial distribution of [Ca2+]i affect multiple aspects of cellular activity. The first neutrophil behavior associated with calcium flux was identified almost 40 years ago (Boucek and Snyderman, 1976). However, subsequent analysis using alternative methods to visualize [Ca2+]i dynamics (Grienberger and Konnerth, 2012; Tsien, 1980) were surprisingly divergent, with reports of enriched calcium at the front edge of migrating neutrophils (Sawyer et al., 1985), whole-cell calcium flux in migrating neutrophils with no calcium gradient (Marks and Maxfield, 1990), and no detectable changes in neutrophil calcium levels during chemotaxis (Laffafian and Hallett, 1995). A low amplitude leading-edge Ca2+ signal has been detected in polarized RAW cells in culture, a model system for macrophage chemotaxis, and this Ca2+ signal was shown to be essential for leading edge stability and ruffling (Evans and Falke, 2007). However, the presence of a leading-edge Ca2+ signal has not been demonstrated for any leukocyte in tissue.
Light-sheet microscopy enables whole-animal recordings of calcium dynamics in multiple cells simultaneously in small, transparent organisms (Keller et al., 2015). Zebrafish larvae are optically transparent and therefore ideal for studying immune-cell behaviors in real-time and in whole animals (Renshaw and Trede, 2012). There is a high degree of genetic and functional conservation between the zebrafish and mammalian innate immune systems (Cambier et al., 2014; Harvie and Huttenlocher, 2015; Henry et al., 2013). Detailed characterizations of zebrafish neutrophils have motivated the establishment of new animal models for both neutrophil dysfunction, such as WHIM syndrome, and bacterial infections, including Pseudomonas aeruginosa (Brannon et al., 2009; Clatworthy et al., 2009; Phennicie et al., 2010; Walters et al., 2010).
Here we carry out high-resolution intravital recordings of calcium signaling in migrating neutrophils in whole, live vertebrates. Analysis of calcium dynamics using light-sheet microscopy reveals calcium enrichment at the leading edge of migrating neutrophils, rather than the classical model of highest calcium levels at the uropod (Wei et al., 2012). We describe a tool to perturb calcium dynamics in a cell-specific and controllable manner that has broad application for other immune cells. Cell-specific expression of a pharmacologically inducible calcium channel enables temporal and spatial regulation of calcium influx in live animals. We find that regulated neutrophil calcium flux is required for neutrophil migration, and that calcium enrichment at the leading edge can help direct polarized migration. These approaches provide a platform for direct in vivo observation and manipulation of calcium dynamics in specific immune cell populations.
RESULTS
In vivo detection of leading-edge calcium enrichment in migrating neutrophils
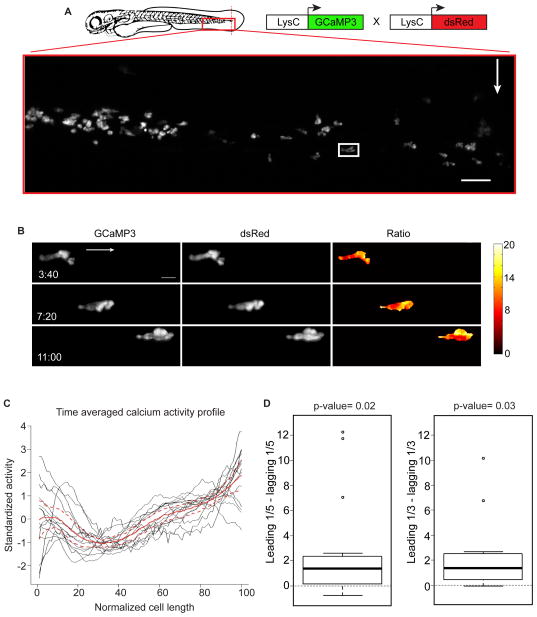
To examine in vivo calcium dynamics during neutrophil recruitment, we coupled light-sheet microscopy with transgenic zebrafish lines expressing the genetically encoded calcium indicator GCaMP3 specifically in neutrophils. We expressed GCaMP3 specifically in neutrophils using the LysC promoter and generated Tg(LysC:GCaMP3) transgenic animals (Hall et al., 2007; Meijer et al., 2008; Oehlers et al., 2015; Tian et al., 2009). We amputated a small segment of the caudal fin, a well-established injury assay for inducing a local inflammatory response and robust neutrophil recruitment (Figure 1A) (Henry et al., 2013). We employed light-sheet fluorescence microscopy to image intracellular calcium with high spatial and temporal resolution in live animals 3 days post-fertilization (Ahrens et al., 2013). To avoid artifacts associated with cell movement, we performed ratiometric imaging in animals that carried both GCaMP3 and a cytoplasmic red fluorescent protein, both driven under the same neutrophil-specific promoter, Tg(LysC:GCaMP3; LysC:dsRed) (Figure 1A).
Figure 1. Calcium is enriched at the leading edge of migrating neutrophils in vivo.
(A) Illustration of a caudal fin amputation (red dotted line) in a double transgenic larva Tg(LysC:GCaMP3; LysC:dsRed) with the field of view in the red box. White arrow indicates fin amputation and white box highlights the neutrophil in the still frames in (B). Scale bar is 50 μm. (B) Maximum-intensity projections from time-lapse experiments with light-sheet fluorescence microscopy follow a single neutrophil migrating toward wound, displaying image data from each channel (GCaMP3 or dsRed) and resulting ratiometric image (GCaMP3/dsRed). Arrow indicates overall direction of migration. t=0 corresponds to 40 min post wounding. Scale bar is 10μm. (C) Time-averaged calcium activity profile across the length of a migrating neutrophil (normalized relative to direction of migration: 0 is the lagging edge and 100 is the leading edge). Each neutrophil is graphed in black (n=15, from 4 animals) with the mean profile (red line), bound by 95% confidence intervals (dashed red lines). (D) Left box plot shows the difference between the average calcium signal at the leading and lagging edge (one-fifth of the length) for each neutrophil examined in (C). Middle line is the median, the box is the middle 50%, the upper and lower bars indicate data range, with outliers as dots. Calculations were also repeated using the leading and lagging one-third of each cell (right box plot). Hypothesis that the average difference was positive was tested using a one sample t-test. See also Figures S1–S3, and Movie S1.
Ratiometric analyses of neutrophils migrating toward the wound site revealed fluctuations in calcium across the migrating cells through time (Figure 1B, Figure S1, and Movie S1 part 1). In contrast to previous ex vivo observations in neutrophils (Laffafian and Hallett, 1995), but similar to observations in a macrophage model (Evans and Falke, 2007), the zone of highest calcium enrichment was consistently observed at the leading edge of migrating neutrophils in vivo (Figure 1C, 1D and Figure S2). The middle of the cell generally displayed the lowest calcium, and the lagging edge had transient instances of high calcium, but overall showed only slightly elevated calcium levels that did not reach those of the leading edge (Figure 1C, 1D and Figure S2). Stationary cells from these same animals failed to show any consistent subcellular calcium enrichment patterns over time (Figure S3 and Movie S1 part 2). Thus, elevated calcium concentrations at the leading edge are a notable feature of migrating neutrophils in vivo.
Neutrophils exhibit whole-cell calcium flux at wound sites and during phagocytosis
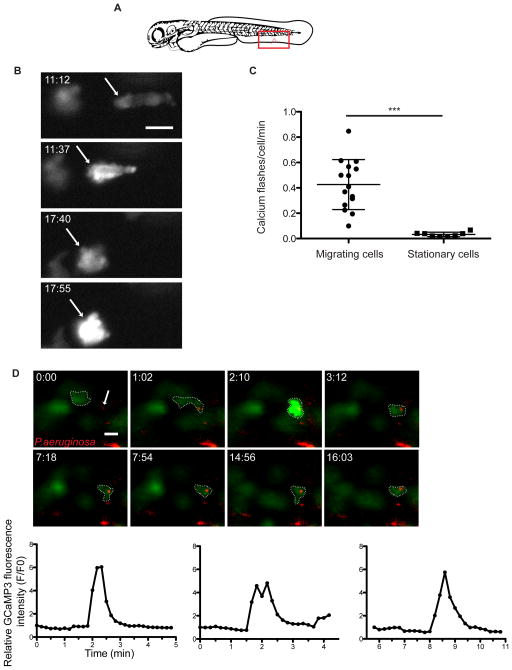
Neutrophils from Tg(LysC:GCaMP3) larvae also exhibited dramatic whole-cell calcium flux once they reached a distance of ~50μm from the wound. To analyze calcium flux during recruitment we carried out ventral fin wounding assays (Figure 2A). Neutrophils migrating toward the wound exhibited whole-cell calcium flux more often as they approached the site of injury compared to nearby neutrophils that were stationary (Figures 2B, 2C and Movie S2). The duration and frequency of whole-cell calcium signals were variable amongst the migrating neutrophils, lasting from approximately 30 seconds to well over one minute.
Figure 2. Neutrophils undergo whole-cell calcium flux at sites of injury and upon phagocytosis of bacteria.
(A) Cartoon of a ventral fin wound (red notch) and field of view during imaging (red square). (B) Fluorescent images from a time-lapse following GCaMP3 expression in a neutrophil migrating toward a ventral fin wound in a Tg(LysC:GCaMP3) larva. t=0 corresponds to 5 min post wounding. Arrow highlights a single neutrophil as it periodically flashes during migration toward a ventral wound. (C) Mean number of whole-cell calcium flashes per cell per min during first hr post wounding in migrating neutrophils and stationary neutrophils. Error bars are mean ± s.d. Mann-Whitney test ***, p<0.0001. (n=15 cells from 5 larvae for migrating cells and n=8 cells from 4 larvae for stationary cells). (D) Still frames from time-lapse capturing calcium flash upon LysC:GCaMP3 neutrophil (green) phagocytosis of P. aeruginosa (red). Dotted line surrounds the phagocytic neutrophil in each frame and the arrow highlights bacteria that will be phagocytosed. t=0 corresponds to 30 min post infection. Scale bars are 10 μm. Graphs quantify the increase in relative fluorescence intensity from GCaMP3 (F/F0) during phagocytosis of PAO1. (Left graph corresponds to the panels above and the center and right graphs correspond to additional examples shown in Movie S2, parts 3 and 4, respectively). See also Movie S2.
Calcium signaling has also been implicated in phagocytosis and ensuing downstream responses (Nunes and Demaurex, 2010). We infected Tg(LysC:GCaMP3) larvae with fluorescently labeled Pseudomonas aeruginosa, and performed high-speed time-lapse imaging to capture individual phagocytosis events (Brannon et al., 2009). We found that larval neutrophils consistently exhibited an increase in intracellular calcium (lasting ~20–30 s) upon phagocytosis of P. aeruginosa (Figure 2D and Movie S2 parts 2–4). These studies highlight the complex nature of calcium signaling in vivo, as we observed distinct calcium signaling patterns for each neutrophil activity examined: leading edge enrichment of calcium during migration, long whole-cell pulses of calcium influx at close proximity to a wound site, and relatively shorter whole-cell calcium pulses upon phagocytosis of P. aeruginosa.
Pharmacological control of calcium dynamics in zebrafish neutrophils via expression of a mammalian ion channel
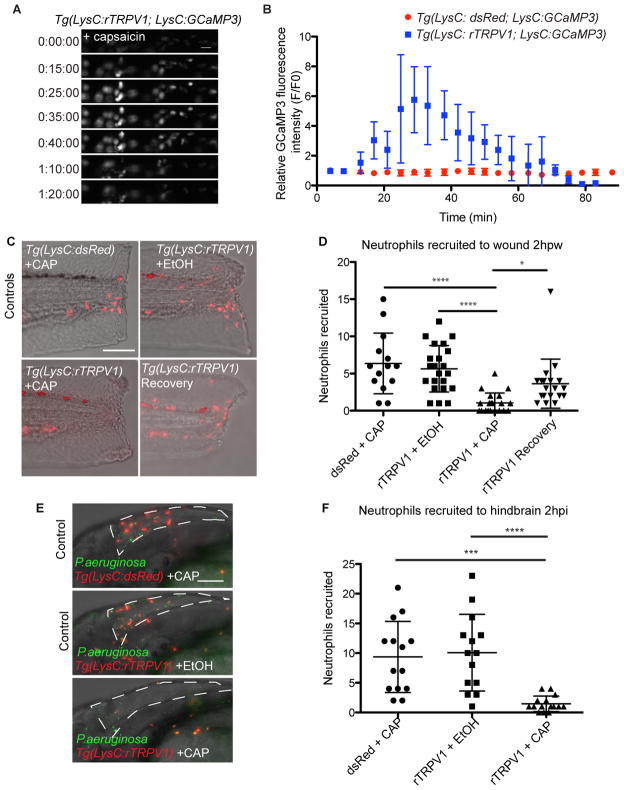
To assess the functional relevance of these dynamics we developed a generalizable method for directly manipulating calcium in immune cells in vivo. The cation channel TRPV1 is highly permeable to calcium, widely studied for its role in nociception, and was first identified based on its gating by capsaicin (Caterina et al., 1997). Administration of capsaicin to transgenic C. elegans expressing mammalian TRPV1 in specific sensory neurons can mediate neuronal depolarization (Tobin et al., 2002). We used the zebrafish LysC promoter to drive rat TRPV1 (rTRPV1) specifically in neutrophils, creating the stable line Tg(LysC:rTRPV1-tdTomato). The critical amino acid residues required to confer capsaicin sensitivity to mammalian TRPV1 are not conserved in zebrafish, and wildtype zebrafish exhibit no behavioral response to capsaicin treatment (Caterina et al., 1997; Graham et al., 2013; Jordt and Julius, 2002). We found that intracellular calcium flux was specifically induced in neutrophils from Tg(LysC:rTRPV1; LysC:GCaMP3) larvae treated with capsaicin, but was absent if treated with vehicle, or in control larvae lacking the rTRPV1 channel and exposed to capsaicin (Figures 3A, 3B, Movie S3, and data not shown). Prolonged capsaicin treatment resulted in sustained calcium entry in rTRPV1 positive neutrophils for up to 60 minutes (Figures 3A, 3B, and Movie S3 part 2).
Figure 3. Regulated calcium flux is required for neutrophil recruitment.
Time-lapse experiment shows no change in GCaMP3 fluorescence in neutrophils from control Tg(LysC:dsRed; LysC:GCaMP3) larva after addition of capsaicin, and increased GCaMP3 fluorescence in neutrophils from Tg(LysC:rTRPV1; LysC:GCaMP3) larva. (A) Selected still frames show GCaMP fluorescence in Tg(LysC:rTRPV1; LysC:GCaMP3) larva during extended exposure to capsaicin. t=0 corresponds to 5 min after addition of capsaicin. Scale bar is 20 μm. (B) Quantification of the relative GCaMP fluorescence intensity (F/F0) during extended capsaicin treatment (n=5 neutrophils for each larva). Error bars are mean ± s.d. See also Movie S3. (C–F) Tg(LysC:rTRPV1; LysC:GCaMP3) or Tg(LysC:dsRed; LysC:GCaMP3) larvae were wounded (C, D) or infected with P. aeruginosa in the hindbrain (E, F) and briefly pulsed with capsaicin (CAP) or soaked in ethanol (EtOH) for 2 hr before neutrophil recruitment was quantified. Recovery Tg(LysC:rTRPV1; LysC:GCaMP3) larvae were pulsed with CAP before wounding, followed by EtOH soak for 2 hr to assess neutrophil recovery after capsaicin treatment (C, D). (C) Representative maximum-intensity projections show red-fluorescent neutrophils merged with brightfield at 2hr post wounding. (E) Representative maximum-intensity projections show red-fluorescent neutrophils and green-fluorescent P. aeruginosa at 2hr post infection within the hindbrain ventricle outlined by white dashed line. Scale bars are 100μm. (D, F) Graphs display the number of neutrophils recruited for each larva. Error bars are mean ± s.d. Kruskal-Wallis test followed by Dunn’s Multiple Comparison test: ****, adjusted p<0.0001, ***, adjusted p=0.0002, and *, adjusted p=0.014. Each experiment was carried out at least twice.
Targeted perturbation of calcium flux inhibits neutrophil recruitment
We next developed a pulsed capsaicin delivery protocol that would maximize calcium influx while minimizing any potential cell death from prolonged depolarization (see Experimental Procedures). We amputated the caudal fin to induce neutrophil recruitment, and then pulsed with capsaicin or vehicle. Two hr post-wounding (2 hpw), neutrophils in the control groups (Tg(LysC:dsRed) larvae pulsed with capsaicin or Tg(LysC:rTRPV1) treated with vehicle) were recruited normally to the wound (Figures 3C and 3D). However, neutrophils in the Tg(LysC:rTRPV1) larvae pulsed with capsaicin failed to reach the wound site (Figures 3C and 3D), suggesting that disrupted calcium flux is sufficient to perturb recruitment. We also pretreated Tg(LysC:rTRPV1) larvae with capsaicin pulses before amputation, and then followed the amputation with a 2 hr treatment in ethanol vehicle. Neutrophils from these pretreated animals exhibited partial to full recovery, showing that the suppression of neutrophil recruitment after capsaicin treatment is reversible (Figures 3C and 3D).
Neutrophil recruitment is associated with the immune response to bacterial infection with P. aeruginosa (Deng et al., 2013; Yang et al., 2012). We assessed whether the neutrophil-specific rTRPV1 could modulate neutrophil recruitment to sites of infection. We infected Tg(LysC:rTRPV1) animals with P. aeruginosa in the hindbrain ventricle, an epithelial-lined compartment normally devoid of leukocytes, and quantified the number of neutrophils recruited 2 hr post infection (2 hpi) (Figures 3E and 3F). Neutrophil recruitment to the P. aeruginosa-filled hindbrain was normal in both control groups, but severely impaired in capsaicin-pulsed Tg(LysC:rTRPV1) larvae (Figures 3E and 3F). Thus, in both sterile inflammation and bacterial infection, excess calcium flux through capsaicin-gated rTRPV1 prevents normal neutrophil recruitment.
Generation of a capsaicin gradient drives directed neutrophil migration
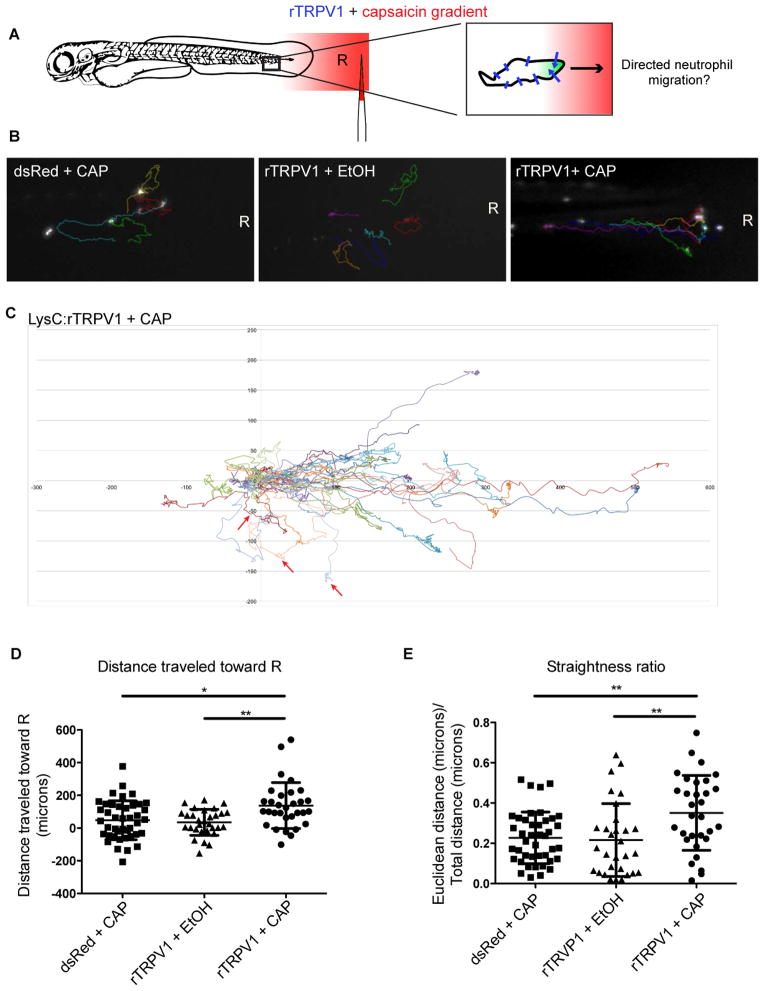
We next tested whether the observed calcium enrichment at the leading edge of neutrophils could instruct neutrophil movement in vivo. We sought to generate an intracellular calcium gradient by producing a directional capsaicin gradient, based on potential bias for channel gating on the side of the cell that first experiences an effective concentration of ligand (Figure 4A). We embedded Tg(LysC:rTRPV1) larvae in agarose and injected capsaicin proximal to the caudal fin. We observed sequential caudal to rostral fluorescence increases in GCaMP3-expressing neutrophils along the tail of Tg(LysC:rTRPV1; LysC:GCaMP3) larvae, suggesting that we could generate a gradient in the context of embedded larvae (Movie S4 part 1). However, the rapid neutrophil depolarization we observed with the high-dose gradient was incompatible with the slower time-frame of endogenous neutrophil migration. Thus, we developed a lower-dose capsaicin gradient, to attempt to experimentally induce leading-edge calcium enrichment in the absence of endogenous stimuli (Figure 4A).
Figure 4. A capsaicin gradient directs rTRPV1-neutrophil motility.
(A) Injecting capsaicin (red) into surrounding agarose generates an intracellular calcium gradient (green) in neutrophils expressing rTRPV1 channels (blue lines). R indicates the reference point, defined as the initial point source of the gradient. Three experimental groups were imaged: Tg(LysC:dsRed; LysC:GCaMP3) larvae in a capsaicin gradient (dsRed+CAP), Tg(LysC:rTRPV1; LysC:GCaMP3) larvae in an ethanol gradient (rTRPV1+EtOH), or capsaicin gradient (rTRPV1+CAP). (B) Last frames from Movie S4 show the tracks of individual neutrophils from each experimental group (quantified in D, E) relative to the reference point R at the focal point of the gradient. (C) (x,y) coordinates for each neutrophil at every frame tracked within the Tg(LysC:rTRPV1) larvae exposed to a capsaicin gradient visually illustrate the directed motion toward the source. The track for each cell was normalized such that the starting (x,y) position was (0,0) for every cell. All units are μm. One larva from the rTRPV1 + CAP group was assessed after exposure to a ventrally-centered gradient along the bottom of the animal and the three tracked cells from that animal are highlighted with red arrows. (D, E) Graphs show the final distance traveled toward the reference point (D), and straightness ratio (E) for tracked neutrophils. Error bars are mean ± s.d. (D) Kruskal-Wallis test followed by Dunn’s multiple comparison test: *, adjusted p=0.012 and **, adjusted p value= 0.0075. (E) One-way ANOVA followed Tukey’s multiple comparisons test: **, adjusted p value= 0.0052 for dsRed+ CAP versus rTRPV1+CAP and **, adjusted p value= 0.005 for rTRPV1+ EtOH versus rTRPV1+CAP. For each group: n≥30 cells from ≥10 larvae. See also Figure S4 and Movie S4.
The lower-dose capsaicin gradient had no effect on neutrophil migration in either control group: Tg(LysC:dsRed) plus a capsaicin gradient or Tg(LysC: rTRPV1) plus an ethanol gradient (Figure 4B, Figure S4, and Movie S4). However, upon exposure to a capsaicin gradient, neutrophils expressing the rTRPV1 transgene migrated in a more directed trajectory toward the source (Figures 4B–4E and Movie S4), in the absence of any other stimulus. In comparison to controls, neutrophils in Tg(LysC:rTRPV1) animals traveled greater distances along straighter paths toward the highest point source of capsaicin (Figures 4D, 4E, and Movie S4 parts 2–4). rTRPV1-neutrophils exhibited greater directionality toward the capsaicin gradient, but their mean velocities and total distances traveled were similar to those quantified from neutrophils in the control groups (Figures S4D and S4E). The similar velocities for random and directed neutrophil migration are consistent with previous recordings during baseline motility in interstitial tissue compared with chemotaxis toward a wound site (Yoo et al., 2010; Yoo et al., 2011). Importantly, when we moved the point source of the capsaicin to the ventral side of the larva, migrating neutrophils were redirected toward the new source, illustrating that the capsaicin gradient itself may help to define the direction of migration of rTRPV1-expressing neutrophils, even in the absence of any other stimuli (Figure S4C and Movie S4 part 5).
Calcium channels influence directed neutrophil motility
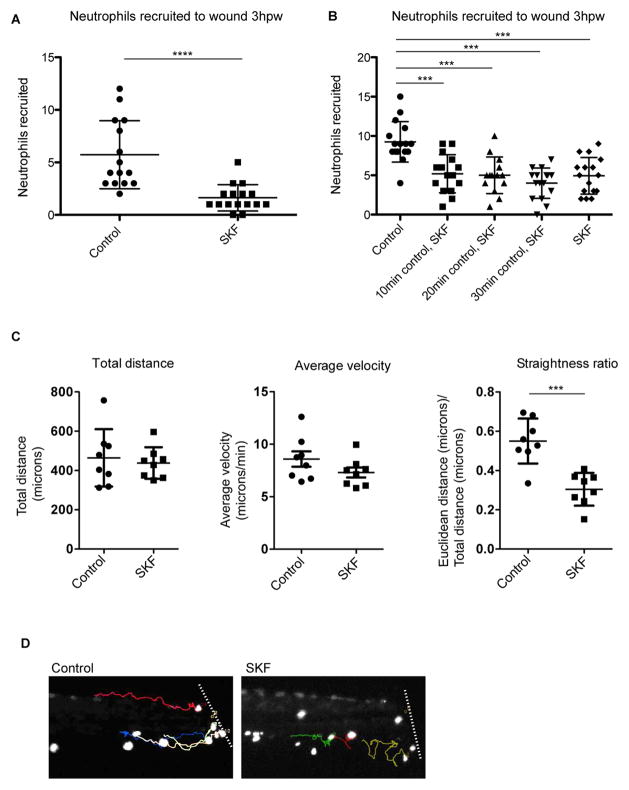
We next asked, via a reciprocal loss-of-function approach, whether calcium influx is required for chemotaxis in vivo. The calcium channel antagonist SKF 96365 displays activity against multiple calcium channels, including TRP channels and voltage-gated calcium channels (Harteneck et al., 2011; Merritt et al., 1990). Application of 20μM SKF 96365 to whole animals after caudal fin amputations compromised neutrophil recruitment (Figure 5A). We next modified these experiments, allowing a 30-minute window after injury for chemotactic gradients to be established before applying the calcium channel antagonist, a time-frame in which neutrophils have already begun to migrate toward the wound site ((Niethammer et al., 2009) and Movie S5). Neutrophils from larvae treated at these later timepoints were also compromised for recruitment, suggesting acute effects of the antagonist on neutrophil directional motility (Figure 5B).
Figure 5. Inhibition of calcium channels disrupts neutrophil directionality during recruitment.
(A–D) After caudal fin amputation, Tg(LysC:GFP) or Tg(LysC:dsRed) larvae were immediately treated with vehicle alone (control) or 20μM SKF 96365 (SKF), followed by quantification of neutrophil recruitment 3 hr post wounding (A, B). (A) Error bars are mean ± s.d. Mann-Whitney test **** p<0.0001 (B) Groups of larvae were placed in control treatments for 10, 20, or 30 minutes before replacement of media with SKF for the remainder. Error bars are mean ± s.d. One-way ANOVA followed by Tukey’s multiple comparisons test ***, p<0.001. Each experiment was carried out at least twice. (C, D) Immediately after wounding, larvae were mounted in agarose, immersed with either control or SKF, and then imaged with time-lapse microscopy. (C) For the neutrophils tracked, graphs show their total distance traveled, average velocity, and straightness ratio. Total distance and straightness ratio error bars are mean ± s.d. and average velocity error bars are mean ± s.e.m. t-test ***, p=0.0002. For each group: n=8 cells from 2–3 larvae. (D) Final frames from Movie S5 show the tracks of individual neutrophils (quantified in C) near the amputated fin (approximated by the white line). See also Movie S5.
To determine how limiting calcium influx acutely affects neutrophil behavior in vivo, we carried out detailed time-lapse microscopy and tracking in fin-amputated Tg(LysC:GFP) larvae. In a result consistent with the capsaicin gradient experiments, neutrophils in SKF 96365-treated larvae showed normal motility, traveling similar distances at similar velocities as control neutrophils (Figure 5C and Movie S5). However, administration of the calcium channel antagonist resulted in compromised directionality, with most neutrophils in treated animals exhibiting wandering, undirected patterns of migration (Figure 5C, 5D, and Movie S5).
DISCUSSION
Direct recording of intracellular calcium concentrations [Ca2+]i in migrating neutrophils was previously limited to ex vivo analyses (Boucek and Snyderman, 1976; Laffafian and Hallett, 1995; Marks and Maxfield, 1990; Sawyer et al., 1985), and observations have ranged from transient whole-cell flux, to leading-edge calcium enrichment, to no change in calcium flux at all (Laffafian and Hallett, 1995; Marks and Maxfield, 1990; Sawyer et al., 1985).
Using light-sheet microscopy and ratiometric imaging, we found enrichment of calcium at the leading edge of migrating neutrophils. This description is different from the high-resolution studies on calcium flux patterns in cultured chemotactic endothelial cells and fibroblasts, where there is high [Ca2+]i at the lagging edge, and a low calcium concentration at the leading edge punctuated with periodic sparks of localized [Ca2+]i (Tsai et al., 2014; Wei et al., 2009). In vivo analysis of neutrophils also showed moderately increased [Ca2+]i at the lagging edge, but the highest calcium signal was found at the leading edge. One unique feature of the neutrophils studied here was their fast pace. In vivo, chemotactic neutrophils moved at velocities up to 70-times greater than those recorded in the cited cell culture studies with other cell types.
The leading edge has been molecularly defined by a signaling cascade that translates an extracellular chemokine gradient into cytoskeletal rearrangements that promote movement (Falke and Ziemba, 2014). Many factors enriched at the leading edge of migrating cells also have direct or indirect associations with calcium, including phosphoinositide 3-kinases (PI3Ks) and PKCα (Ching et al., 2001; Evans and Falke, 2007; Hsu et al., 2000; Vossebeld et al., 1997). PI3K activity has been localized to the leading edge of mouse neutrophils in cell culture and in zebrafish neutrophils in vivo, while PKCα is enriched at the leading edge of polarized murine macrophages (Evans and Falke, 2007; Nishio et al., 2007; Yoo et al., 2010). In macrophages, it has been hypothesized that a leading-edge calcium signal plays a role in directional control of cell migration (Evans and Falke, 2007; Falke and Ziemba, 2014).
The role of calcium signaling in immune cell phagocytosis and post-phagocytic vacuole trafficking has also generated conflicting results depending on the model system employed (Dewitt and Hallett, 2002; Jaconi et al., 1990; Lew et al., 1985; Marks and Maxfield, 1990; Murata et al., 1987; Nunes and Demaurex, 2010; Sawyer et al., 1985). We have been able to directly image these dynamics in vivo. The transient rise in [Ca2+]i as P. aeruginosa is phagocytosed is similar to that observed for cultured human neutrophils phagocytosing opsonized antigens (Marks and Maxfield, 1990; Murata et al., 1987). The establishment of an in vivo model system for studying calcium dynamics during neutrophil phagocytosis should motivate greater understanding of how receptor-mediated phagocytosis of bacterial pathogens in animal models compares with cell culture models of opsonized antigens. These methods allow in vivo analysis of calcium flux in single immune cells for hours at high frame rates.
Although many regulators of calcium are used broadly in cell culture, they are largely incompatible with administration in a live organism. Cell-specific expression of TRPV1 provides a generalizable in vivo method to manipulate calcium dynamics in targeted immune cell subtypes. The rTRPV1-capsaicin system is pharmacologically inducible, titratable and relatively sensitive to small variations, allowing for in vivo manipulation of subcellular calcium. By applying a low-dose capsaicin gradient across immobilized zebrafish larvae, neutrophils expressing the capsaicin-activated channel rTRPV1 were stimulated to move in the direction of the gradient. Asymmetric influx of calcium may be a key event in cell migration and may facilitate definition of the leading edge of some chemotactic cells. Additionally, we used a pharmacological loss-of-function approach to demonstrate an acute requirement for calcium channels in defining neutrophil directionality during an endogenous wounding response. Both the gain-of-function and loss-of-function manipulations of calcium influx resulted in reciprocal changes in directionality, but overall velocity remained largely unaffected, suggesting that leading-edge calcium may be an important component of a neutrophil compass (Falke and Ziemba, 2014).
In summary, we have found that, in vivo, migrating neutrophils exhibit enriched calcium flux at the leading edge and that this pattern of intracellular calcium helps define the direction of movement. In addition, we describe a generalizable method for manipulating calcium directly in specific immune cells in vivo. The rTRPV1-capsaicin system requires no additional co-factors or prior knowledge of the functional endogenous calcium channels, thereby making it widely applicable as a method of interrogating the role of calcium signaling in many different cell types in whole animals.
Experimental Procedures
Zebrafish strains
All zebrafish husbandry and experimental protocols were performed in compliance with policies approved by the Duke University Institutional Animal Care and Use Committee. The transgenic lines Tg(LysC:GCaMP3xt1) and Tg(LysC:rTRPV1-tdTomatoxt4), were made by injecting transposase mRNA and Tol2 containing DNA constructs into single cell embryos with additional details provided in Supplemental Experimental Procedures. Tg(LysC:dsRednz50) and Tg(LysC:GFPnz117) have been described elsewhere (Hall et al., 2007).
Time-lapse imaging
Tg(LysC:GCaMP3) larvae were imaged at 2 days post fertilization (dpf) for bacterial infection experiments and all other transgenic larvae were imaged at 3 dpf. For microscopy, larvae were anesthetized in 0.016% Tricaine (MS-222) and immobilized in 1% low melting point agarose and imaged with a SiMView microscope (light-sheet microscopy) or a Zeiss axio observer Z1, or Nikon TE-2000U. Additional experimental details are provided in the Supplemental Experimental Procedures.
Infections/bacterial preparations
Pseudomonas aeruginosa (PAO1) carrying a constitutively expressed mCherry or GFP plasmid was grown in LB media supplemented with carbenicillin (200μg/ml) as previously described in (Brannon et al., 2009) and detailed in the Supplemental Experimental Procedures.
Quantitation of calcium flashes and ratiometric analyses
Light-sheet microscopy images were analyzed using maximum projections with background fluorescence subtracted. Details of ratiometric analyses are supplied in the Supplemental Experimental Procedures. Whole-cell calcium flashes were quantified by manually scoring cells that displayed a notable increase in green fluorescence during the time-lapse session.
Capsaicin and SKF 96365 treatments
The final working capsaicin (Sigma) concentration was 20μM in 1.4% ethanol. Capsaicin gradient experiments were carried out by injecting 100μM capsaicin into the agarose, adjacent to the mounted larvae followed by time-lapse microscopy. 10mM SKF 96365 (Cayman Chemical) stock was made freshly in DMSO for each experiment. The final concentration was 20μM SKF 96365 in 0.5% DMSO. See Supplemental Experimental Procedures for additional details.
Supplementary Material
Acknowledgments
We thank Misha Ahrens, Sam Johnson, Wolfgang Liedtke and Chao-Tsung Yang for helpful discussions and technical advice and support, Jörg Grandl, Wolfgang Liedtke, Sam Moskowitz, and John Rawls for reagents and constructs, Jason Comparetto for assistance with video compilations, and Mark Cronan, Allison Rosenberg, and Jörn Coers for helpful discussions and critical comments on the manuscript. This work was supported by the Howard Hughes Medical Institute Janelia Visitor Program, a National Science Foundation Graduate Research Fellowship (M.A.M.), the Duke University Center for AIDS Research, an NIH funded program (5P30 AI064518), and a Mallinckrodt Scholar Award, a Searle Scholar Award, a Vallee Foundation Young Investigator Award, and an NIH Director’s New Innovator Award 1DP2-OD008614 (D.M.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahrens MB, Orger MB, Robson DN, Li JM, Keller PJ. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat Methods. 2013;10:413–420. doi: 10.1038/nmeth.2434. [DOI] [PubMed] [Google Scholar]
- Boucek MM, Snyderman R. Calcium influx requirement for human neutrophil chemotaxis: inhibition by lanthanum chloride. Science. 1976;193:905–907. doi: 10.1126/science.948752. [DOI] [PubMed] [Google Scholar]
- Brannon MK, Davis JM, Mathias JR, Hall CJ, Emerson JC, Crosier PS, Huttenlocher A, Ramakrishnan L, Moskowitz SM. Pseudomonas aeruginosa Type III secretion system interacts with phagocytes to modulate systemic infection of zebrafish embryos. Cellular microbiology. 2009;11:755–768. doi: 10.1111/j.1462-5822.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier CJ, Takaki KK, Larson RP, Hernandez RE, Tobin DM, Urdahl KB, Cosma CL, Ramakrishnan L. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505:218–222. doi: 10.1038/nature12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Ching TT, Hsu AL, Johnson AJ, Chen CS. Phosphoinositide 3-kinase facilitates antigen-stimulated Ca(2+) influx in RBL-2H3 mast cells via a phosphatidylinositol 3,4,5-trisphosphate-sensitive Ca(2+) entry mechanism. The Journal of biological chemistry. 2001;276:14814–14820. doi: 10.1074/jbc.M009851200. [DOI] [PubMed] [Google Scholar]
- Clatworthy AE, Lee JS, Leibman M, Kostun Z, Davidson AJ, Hung DT. Pseudomonas aeruginosa infection of zebrafish involves both host and pathogen determinants. Infection and immunity. 2009;77:1293–1303. doi: 10.1128/IAI.01181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Sarris M, Bennin DA, Green JM, Herbomel P, Huttenlocher A. Localized bacterial infection induces systemic activation of neutrophils through Cxcr2 signaling in zebrafish. Journal of leukocyte biology. 2013;93:761–769. doi: 10.1189/jlb.1012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt S, Hallett MB. Cytosolic free Ca(2+) changes and calpain activation are required for beta integrin-accelerated phagocytosis by human neutrophils. The Journal of cell biology. 2002;159:181–189. doi: 10.1083/jcb.200206089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JH, Falke JJ. Ca2+ influx is an essential component of the positive-feedback loop that maintains leading-edge structure and activity in macrophages. Proc Natl Acad Sci U S A. 2007;104:16176–16181. doi: 10.1073/pnas.0707719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke JJ, Ziemba BP. Interplay between phosphoinositide lipids and calcium signals at the leading edge of chemotaxing ameboid cells. Chemistry and physics of lipids. 2014;182:73–79. doi: 10.1016/j.chemphyslip.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DM, Huang L, Robinson KR, Messerli MA. Epidermal keratinocyte polarity and motility require Ca(2)(+) influx through TRPV1. Journal of cell science. 2013;126:4602–4613. doi: 10.1242/jcs.122192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73:862–885. doi: 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Hall C, Flores MV, Storm T, Crosier K, Crosier P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC developmental biology. 2007;7:42. doi: 10.1186/1471-213X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harteneck C, Klose C, Krautwurst D. Synthetic modulators of TRP channel activity. Advances in experimental medicine and biology. 2011;704:87–106. doi: 10.1007/978-94-007-0265-3_4. [DOI] [PubMed] [Google Scholar]
- Harvie EA, Huttenlocher A. Neutrophils in host defense: new insights from zebrafish. Journal of leukocyte biology. 2015 doi: 10.1189/jlb.4MR1114-524R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KM, Loynes CA, Whyte MK, Renshaw SA. Zebrafish as a model for the study of neutrophil biology. Journal of leukocyte biology. 2013;94:633–642. doi: 10.1189/jlb.1112594. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Ching TT, Sen G, Wang DS, Bondada S, Authi KS, Chen CS. Novel function of phosphoinositide 3-kinase in T cell Ca2+ signaling. A phosphatidylinositol 3,4,5-trisphosphate-mediated Ca2+ entry mechanism. The Journal of biological chemistry. 2000;275:16242–16250. doi: 10.1074/jbc.M002077200. [DOI] [PubMed] [Google Scholar]
- Jaconi ME, Lew DP, Carpentier JL, Magnusson KE, Sjogren M, Stendahl O. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. The Journal of cell biology. 1990;110:1555–1564. doi: 10.1083/jcb.110.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- Keller PJ, Ahrens MB, Freeman J. Light-sheet imaging for systems neuroscience. Nat Methods. 2015;12:27–29. doi: 10.1038/nmeth.3214. [DOI] [PubMed] [Google Scholar]
- Laffafian I, Hallett MB. Does cytosolic free Ca2+ signal neutrophil chemotaxis in response to formylated chemotactic peptide? Journal of cell science. 1995;108(Pt 10):3199–3205. doi: 10.1242/jcs.108.10.3199. [DOI] [PubMed] [Google Scholar]
- Lew DP, Andersson T, Hed J, Di Virgilio F, Pozzan T, Stendahl O. Ca2+-dependent and Ca2+-independent phagocytosis in human neutrophils. Nature. 1985;315:509–511. doi: 10.1038/315509a0. [DOI] [PubMed] [Google Scholar]
- Marks PW, Maxfield FR. Transient increases in cytosolic free calcium appear to be required for the migration of adherent human neutrophils. The Journal of cell biology. 1990;110:43–52. doi: 10.1083/jcb.110.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer AH, van der Sar AM, Cunha C, Lamers GE, Laplante MA, Kikuta H, Bitter W, Becker TS, Spaink HP. Identification and real-time imaging of a myc-expressing neutrophil population involved in inflammation and mycobacterial granuloma formation in zebrafish. Developmental and comparative immunology. 2008;32:36–49. doi: 10.1016/j.dci.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Merritt JE, Armstrong WP, Benham CD, Hallam TJ, Jacob R, Jaxa-Chamiec A, Leigh BK, McCarthy SA, Moores KE, Rink TJ. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. The Biochemical journal. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Sullivan JA, Sawyer DW, Mandell GL. Influence of type and opsonization of ingested particle on intracellular free calcium distribution and superoxide production by human neutrophils. Infection and immunity. 1987;55:1784–1791. doi: 10.1128/iai.55.8.1784-1791.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio M, Watanabe K, Sasaki J, Taya C, Takasuga S, Iizuka R, Balla T, Yamazaki M, Watanabe H, Itoh R, et al. Control of cell polarity and motility by the PtdIns(3,4,5)P3 phosphatase SHIP1. Nature cell biology. 2007;9:36–44. doi: 10.1038/ncb1515. [DOI] [PubMed] [Google Scholar]
- Nunes P, Demaurex N. The role of calcium signaling in phagocytosis. Journal of leukocyte biology. 2010;88:57–68. doi: 10.1189/jlb.0110028. [DOI] [PubMed] [Google Scholar]
- Oehlers SH, Cronan MR, Scott NR, Thomas MI, Okuda KS, Walton EM, Beerman RW, Crosier PS, Tobin DM. Interception of host angiogenic signalling limits mycobacterial growth. Nature. 2015;517:612–615. doi: 10.1038/nature13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phennicie RT, Sullivan MJ, Singer JT, Yoder JA, Kim CH. Specific resistance to Pseudomonas aeruginosa infection in zebrafish is mediated by the cystic fibrosis transmembrane conductance regulator. Infection and immunity. 2010;78:4542–4550. doi: 10.1128/IAI.00302-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw SA, Trede NS. A model 450 million years in the making: zebrafish and vertebrate immunity. Disease models & mechanisms. 2012;5:38–47. doi: 10.1242/dmm.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer DW, Sullivan JA, Mandell GL. Intracellular free calcium localization in neutrophils during phagocytosis. Science. 1985;230:663–666. doi: 10.1126/science.4048951. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq A, Bargmann C. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Tsai FC, Seki A, Yang HW, Hayer A, Carrasco S, Malmersjo S, Meyer T. A polarized Ca2+, diacylglycerol and STIM1 signalling system regulates directed cell migration. Nature cell biology. 2014;16:133–144. doi: 10.1038/ncb2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Vossebeld PJ, Homburg CH, Schweizer RC, Ibarrola I, Kessler J, Koenderman L, Roos D, Verhoeven AJ. Tyrosine phosphorylation-dependent activation of phosphatidylinositide 3-kinase occurs upstream of Ca2+-signalling induced by Fcgamma receptor cross-linking in human neutrophils. The Biochemical journal. 1997;323(Pt 1):87–94. doi: 10.1042/bj3230087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A. Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood. 2010;116:2803–2811. doi: 10.1182/blood-2010-03-276972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H. Calcium flickers steer cell migration. Nature. 2009;457:901–905. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Wang X, Zheng M, Cheng H. Calcium gradients underlying cell migration. Current opinion in cell biology. 2012;24:254–261. doi: 10.1016/j.ceb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Yang CT, Cambier CJ, Davis JM, Hall CJ, Crosier PS, Ramakrishnan L. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell host & microbe. 2012;12:301–312. doi: 10.1016/j.chom.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Developmental cell. 2010;18:226–236. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480:109–112. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.