SUMMARY
Huntington disease (HD) is caused by a CAG·CTG expansion in the huntingtin (HTT) gene. While most research has focused on the HTT polyGln-expansion protein, we demonstrate that four additional, novel, homopolymeric expansion proteins (polyAla, polySer, polyLeu, and polyCys) accumulate in HD human brains. These sense and antisense repeat-associated non-ATG (RAN) translation proteins accumulate most abundantly in brain regions with neuronal loss, microglial activation and apoptosis, including caudate/putamen, white matter, and, in juvenile-onset cases, also the cerebellum. RAN protein accumulation and aggregation are length dependent, and individual RAN proteins are toxic to neural cells independent of RNA effects. These data suggest RAN proteins contribute to HD and that therapeutic strategies targeting both sense and antisense genes may be required for efficacy in HD patients. This is the first demonstration that RAN proteins are expressed across an expansion located in an open reading frame and suggests RAN translation may also contribute to other poly-glutamine diseases.
INTRODUCTION
Huntington disease (HD) is a progressive neurodegenerative disorder characterized by severe movement, cognitive, and behavioral changes caused by a CAG·CTG expansion in the HTT gene (Orr and Zoghbi, 2007). Upon translation, this expansion mutation results in the production of a mutant huntingtin protein (HTT) with an expanded polyGln repeat tract. HD is one of nine neurologic diseases caused by CAG expansion mutations that encode broadly expressed proteins containing expanded poly-Gln tracts. While there is substantial evidence that mutant HTT and other polyGln expansion proteins are toxic and contribute to disease, the differential vulnerability of specific brain regions is not yet understood. In HD, expansions >40 repeats are fully penetrant, and longer alleles are associated with earlier, sometimes juvenile, onset and increased disease severity. While nearly all research into the molecular mechanisms of HD has focused on the downstream effects of the mutant HTT protein, CUG HD-antisense transcripts (Chung et al., 2011) and CAG microRNAs have also been reported and may play a role in disease (Bañez-Coronel et al., 2012). The discovery of repeat associated non-ATG (RAN) translation (Zu et al., 2011) in spinocerebellar ataxia type 8 (SCA8) raises the possibility that sense and antisense mutant proteins may be expressed from the HD CAG·CTG expansion mutation.
RAN translation has now been reported in several diseases with non-coding expansion mutations (Ash et al., 2013; Mori et al., 2013; Todd et al., 2013; Zu et al., 2011, 2013). It is not yet clear if RAN translation can occur across expansion mutations located in an open reading frame (ORF), such as the polyglutamine diseases. In these cases, RAN could be limited because these repeats are generally shorter than non-coding expansions and because the position of these repeats in conventional ORFs may inhibit RAN translation. To test if RAN translation can occur in a polyglutamine disease, we examined one of the most prevalent polyglutamine disorders, HD.
RESULTS
RAN Proteins Accumulate in Cells Expressing Mutant HTT and in Human HD Brains
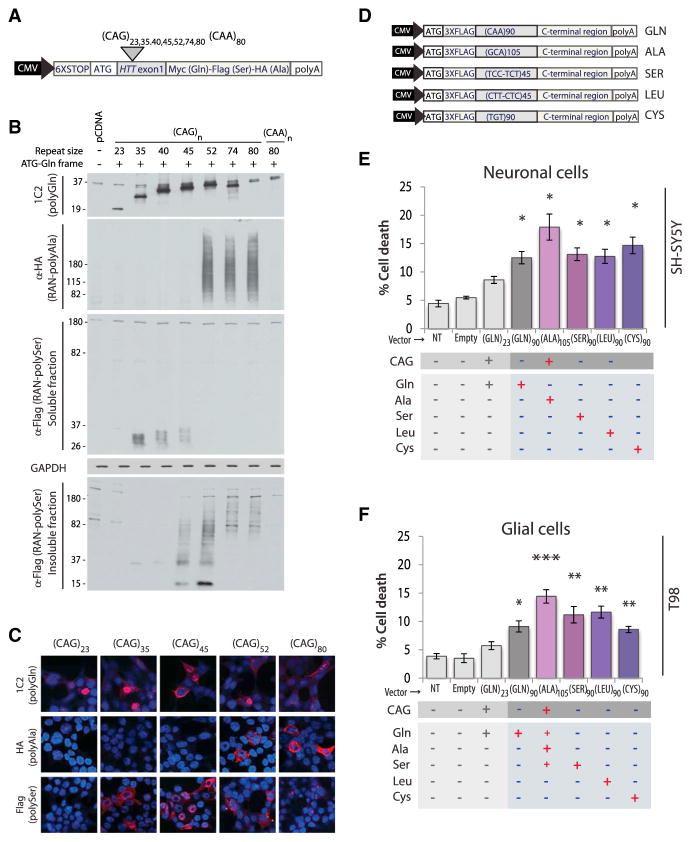
To test if RAN translation can occur across the HTT ORF in transfected cells, we generated a series of minigenes containing HTT exon 1 with expansion mutations (Figure 1A). Immunoblots and immunofluorescence (IF) performed on transiently transfected HEK293T cells demonstrate that RAN translation occurs across a CAGEXP located in the ATG-initiated ORF of an HTT-exon 1 minigene and produces polyAla and polySer RAN proteins. Additionally, constructs lacking the ATG demonstrate that an ATG initiation codon is not required for expression of the polyGln, polyAla, or polySer expansion proteins (Figures 1B and S1Aa). Furthermore, polyAla and polySer are expressed at similar levels with and without an ATG initiation codon in the polyGln frame, indicating that frameshifting from an AUG-initiated polyGln reading frame is not required for polyAla or polySer expression in these minigenes. In contrast, (CAG)23 and non-hairpin-forming (CAA)80 constructs produce ATG-initiated polyGln but not RAN polyAla or polySer proteins (Figures 1B and S1Ab). In summary, these data show that HTT exon-1 (CAG)80 transcripts can express RAN proteins in all three reading frames in transfected cells.
Figure 1. RAN Translation in HD.
(A) CAG and CAA HTT-exon1 minigenes with a 6×STOP codon cassette (two stops in each frame) upstream of HTT exon 1 and C-terminal epitope tags in each of the three reading frames.
(B) Immunoblots of HEK293T cells show polyGln expression from all +ATG-constructs and RAN polyGln, polyAla and polySer proteins from (CAG)80 but not (CAG)23 or non-hairpin-forming (CAA)80 expansions.
(C) Schematic diagram of putative HD-RAN proteins. C-terminal regions used to generate peptide for HD-RAN polyclonal antibodies are underlined.
(D) IHC staining of human autopsy tissue shows positive staining (red) for sense (polyAla and polySer) and antisense (polyCys and polyLeu) RAN proteins in HD ([De]–[Dh]) but not HDL2 ([Di]–[Dl]), control ([Da]–[Dd]) or preimmune ([Dm]–[Dp]) controls. Red, positive staining; blue, nuclear counterstain.
(E) Protein blots showing RAN proteins detected from the insoluble fraction of HD but not control frontal cortex lysates. See also Figures S1A and S1B. HMW, high molecular weight.
Because frameshifting has been previously reported in HD (Davies and Rubinsztein, 2006) and SCA3 (Gaspar et al., 2000; Toulouse et al., 2005), we performed a second series of experiments to test if frame-shifted products, which initiate at the AUG in the glutamine frame, can also produce hybrid polyGln/polyAla and polyGln/polySer proteins. We transfected cells using a modified HD exon 1 minigene with a 5′ V5 tag in the polyGln frame and three C-terminal epitope tags to label proteins translated in each of the three reading frames (Figure S1Ac). Immunoprecipitations using antibodies against the C-terminal tags in the polyAla (α-HA) and polySer (α-Flag) frames followed by immunoblotting using the N-terminal and C-terminal tags shows that the polyAla and polySer proteins do not contain the N-terminal V5 tag in the ATG-initiated polyGln frame (Figure S1Ac).
To test if RAN proteins are expressed in vivo, a panel of polyclonal antibodies against the predicted C-terminal regions available for four of the five possible novel RAN proteins was generated (Figure 1C). The specificities of these antibodies, which recognize the putative polyAla (GCA), polySer (AGC), polyLeu (CUG), and polyCys (UGC) proteins, were demonstrated in transfected cells (Figure S1B). Next, we tested if HD-RAN proteins could be detected by immunohistochemistry (IHC) in the striatum, a prominently affected brain region in HD. We found striking positive staining using all four HD-RAN antibodies: α-Ala-Ct, α-Ser-Ct, α-Leu-Ct, and α-Cys-Ct (Figure 1D; Table 1) in HD but not HDL2 or control samples. No similar staining was seen with the corresponding pre-immune control sera for the various antibodies (Figure 1D). Protein blot analyses of insoluble lysates from human HD frontal cortex provide additional evidence that RAN proteins accumulate in HD but not control brains (Figure 1E). These results show that, in addition to the HTT polyGln expansion protein, the HD CAG·CTG expansion mutation also expresses four novel HD-RAN proteins and that these proteins are expressed from both sense and antisense transcripts.
Table 1.
Summary of RAN Protein Staining in HD Cases and Controls
| Case | Age | Sex/Race | Repeat | PMD | Vonsattel Grade | Striatum | Frontal Cortex | Cerebellum | |
|---|---|---|---|---|---|---|---|---|---|
| CTRL | 1 | 40 | M/W | 6 | − | − | − | ||
| 2 | 56 | F/W | 13 | − | − | − | |||
| 3 | 45 | F/W | 20 | − | − | − | |||
| 4 | 57 | M/W | − | − | − | ||||
| HD | 1 | 47 | M/W | N/A | 30 | 2 | ++ | ++ | ++ |
| 2 | 57 | M/W | N/A | 26 | 2 | ++ | + | ++ | |
| 3 | 60 | F/W | N/A | 24 | 3 | +++ | + | + | |
| 4 | 57 | F/W | N/A | 9.5 | 3 | +++ | + | + | |
| 5 | 52 | M/B | 49 | 24 | 3/4 | +++ | ++ | + | |
| 6 | 41 | M/W | 52 | 8 | 4 | +++ | + | + | |
| 7 | 46 | F/W | N/A | 7 | 4 | +++ | + | + | |
| JHD | 1 | 8 | F/ | >100* | − | +++ | ++ | ++++ | |
| 2 | 8 | M/B | ~100* | − | 3 | ++++ | +++ | ++ | |
| 3 | 27 | M/W | 69 | 22 | 4 | +++ | ++ | ++ | |
| 4 | 23 | F/B | 76 | 14 | 4 | ++++ | ++ | + | |
| 5 | 23 | F/W | 64 | 13 | 4 | +++ | ++ | ++ | |
| HDL2 | 1 | 58 | M/B | 11.5 | 4 | − | − | − | |
| 2 | 41 | F/B | 3 | 3 | − | − | − | ||
| SCA8 | 80 | F | NA | NA | − |
RAN staining:−, negative; +, low-intensity staining; ++, moderate intensity; +++, frequent and intense; ++++, highly frequent positive cells with high intensity staining. CTRL, control; HD, Huntington disease; JHD, juvenile Huntington disease; HDL2, Huntington disease like-2; SCA-8, Spinocerebellar ataxia type 8; N/A, not available;
repeat length estimated from autopsy brains;
M, male; F, female.
RAN Proteins Accumulate in Specific Striatal Regions
To explore areas of the striatum that are predominantly affected in HD (Figure 2A), we performed a series of IHC experiments using the HD-RAN protein antibodies in HD cases with Vonsattel grade II–IV. These experiments show strong HD-RAN protein staining in the caudate and putamen of the striatum, two regions severely affected in HD (Figure 2B, 2D–2G, and S2A–S2D; Table 1). Both nuclear and cytoplasmic RAN protein staining was found in neurons and can appear as diffuse staining or as punctate aggregates. Additional staining was seen in astrocytes and microglia in the caudate and putamen. HD-RAN antibodies also show consistently strong nuclear staining in the white matter bundle regions of the caudate and putamen, a region containing astrocytes and oligodendrocytes (Figure 2B, 2D–2G, and S2A–S2D; Table 1). In contrast, HD-RAN staining is only rarely observed in the internal capsule, a white matter structure containing both ascending and descending axons that separate the caudate nucleus from the putamen, and which is relatively preserved in HD. While the pattern of sense and antisense RAN protein staining across the caudate-putamen is strikingly similar, the pattern of staining using the 1C2 or EM48 antibodies to detect the expanded HTT polyGln protein was distinct. First, although 1C2/EM48 staining of neurons in the caudate and putamen is present and detects previously reported nuclear aggregates, positive cells are much less frequent (Figures 2B, 2C, and S2E). A second notable difference is that polyGln aggregates detected by1C2 or EM48 are not detected in regions with the most intense RAN-protein staining, the white matter bundles of the caudate and putamen (Figures 2B, 2C, and S2E).
Figure 2. HD-RAN Proteins in Striatum.
(A) Control and HD brain showing striatal sub-regions with summary of staining.
(B) Quantification of IHC-positive cells for α-Gln, α-RAN, and IBA1 staining ± SEM in caudate nucleus.
(C–I) IHC of striatal sub-sections from HD brains using (C) α-Gln antibody (1C2), ([D]–[G]) α-RAN antibodies, (H) α-IBA1 for microglia, and (I) α-active caspase-3 for cell death. (A)–(I): red, positive staining; blue, nuclear counterstain.
(J) Double staining for RAN protein cocktail using mixture of all four anti-RAN antibodies (brown) plus anti-IBA1 antibody to label microglia with quantitation (± SEM) of single-, double-labeled, and IBA1 cells that are in close proximity to RAN-positive cells.
(K) Double staining for RAN protein cocktail using mixture of all anti-RAN antibodies (brown) plus anti-caspase-3 antibody to label apoptotic cells with quantitation of the percent active Caspase-3-positive cells that are also positive for RAN proteins. See also Figure S2. Cau-WMB, caudate white matter bundles; Int-Cap, internal capsule; Put-WMB, putamen white matter bundles.
RAN Proteins Accumulate in Regions with Neuroinflammatory Changes
Neuroinflammatory changes including an increase in microglial cells have been reported in HD and correlate with neuronal loss. To test if neuroinflammatory changes are found in areas that are RAN positive, we stained serial sections of the human autopsy material with the IBA1 antibody that recognizes microglia. RAN-positive regions of the caudate and putamen were also strongly positive for IBA1, and similar to the HD-RAN staining, the most intense IBA1 staining was seen in the RAN-positive white matter bundles (Figures 2B, 2H, and S2F). Next, we performed a double labeling experiment in which RAN-positive cells were labeled with a brown chromogen using a mixture of the α-polyAla-Ct, α-poly-Ser-Ct, α-polyLeu-Ct, and α-polyCys-Ct antibodies, and microglia/macrophages were labeled using the α-IBA1 antibody with a blue chromogen. The IBA1/RAN double labeling experiments show that microglia and RAN-positive cells are distributed in three patterns in the various regions of striatum: (1) RAN-negative microglia, (2) RAN-negative microglia in close proximity to a RAN-positive cell, and (3) RAN-positive microglia (Figure 2J).
RAN Proteins Accumulate in Regions Undergoing Cell Death
Because caspase-3 is a prominent marker of cell death in HD, we also stained serial sections to determine if RAN-positive areas are caspase-3 positive. Caspase-3-positive cells were found in both the caudate and putamen and also within the caudate and putamen white matter bundles, which are positive for HD-RAN proteins but negative for the polyGln HTT protein (Figure S2G). Additional double labeling experiments show 40%–60% of cells positive for active caspase-3 are also positive for one or more RAN proteins in the caudate, caudate white-matter bundles, putamen white matter bundles, and putamen (Figure 2K). These studies show RAN-positive cells are found in regions with markers of cell death.
HD-RAN Proteins in Frontal Cortex and Cerebellum
Since neurodegenerative changes in HD also occur in additional regions of the brain including the frontal cortex and the cerebellum, we tested these regions for RAN protein staining. Neuropathology in the frontal cortex is well established in HD, with cortical degeneration, neuronal loss in all layers, and increased density of large glial cells (Bates et al., 2014). Additionally, variable involvement of the cerebellum has recently been reported in HD (Bates et al., 2014; Rüb et al., 2013).
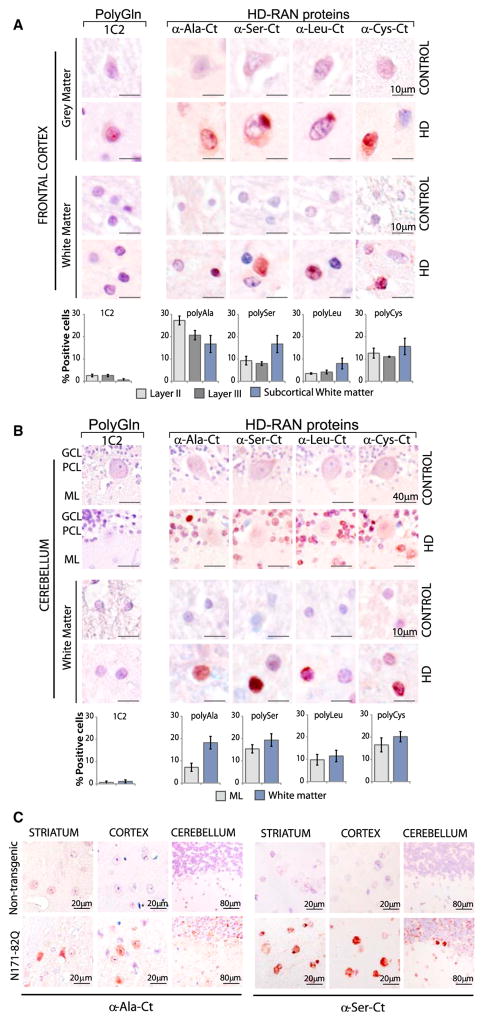
In the frontal cortex, positive staining for the four HD-RAN proteins was found in clustered patches, with the most prominent staining found in cortical layers II and III in HD but not controls. Nuclear staining for HD-Ala, HD-Ser, HD-Leu, and HD-Cys was also found in HD cortical white matter (Figures 3A and S3A–S3D; Table 1). In the cerebellum, HD patients but not controls show RAN-protein staining (Figures 3B and S3A–S3D; Table 1). Staining is found in the molecular and granule cell layers and in areas of Bergmannglial proliferation surrounding the Purkinje cells. Staining of neurons in both the cortex and cerebellum was nuclear, cytoplasmic, or perinuclear and was diffuse or localized to dense aggregates. In contrast, staining in cerebral and cerebellar white matter showed intense nuclear localization.
Figure 3. HD-RAN Proteins in Human Frontal Cortex and Cerebellum and HD Mice.
(A and B) IHC staining of control and HD gray and white matter of (A) frontal cortex and (B) cerebellum using α-RAN and α-Gln (1C2) antibodies show punctate nuclear and cytoplasmic staining with α-polyAla, α-polySer, α-polyLeu, and α-polyCys. GCL, granule cell layer; PCL, Purkinje-cell layer; ML, molecular layer. Staining of the cortex and cerebellum in adult-onset HD cases is variable. IHC images and quantification of percent positive cells represent typical positive regions.
(C) IHC staining of indicated brain regions in N171-82Q and control mice using the α-polyAla, α-polySer. Red, positive staining; blue, nuclear counterstain. See also Figures S3A–S3E.
In summary, while RAN protein staining in the cortex and cerebellum is easily detected, staining in the striatum was consistently more intense. IHC using the 1C2 antibody to detect expanded polyGln protein also showed nuclear staining in the neuronal layers of the frontal cortex (Figures 3A and S3E), but no staining was evident in the white matter regions of the frontal cortex or the cerebellum (Figures 3B and S3E).
HD-RAN Proteins in N171-82Q HD Mice
Next, we tested if HD-RAN proteins can be expressed in a mouse model of HD. We chose the N171-82Q HD mice (Schilling et al., 1999) for analysis because the transgene contains both a RAN-competent CAG expansion mutation and human flanking sequences that allow detection with the C-terminal polySer and polyAla antibodies that we developed. Figure 3C shows that, similar to human HD autopsy tissue, HD-polyAla and HD-polySer are also detected in this mouse model by IHC (Figure 3C). It is not known at this time if antisense transcripts or antisense RAN proteins are expressed in these mice. Our findings of sense RAN proteins in these mice provide proof-of-principle evidence that RAN proteins can be expressed in mouse models of HD and that mice may be a useful tool for understanding the impact of HD-RAN proteins on disease.
In summary, RAN proteins accumulate in two additional affected brain regions, the frontal cortex and cerebellum. Additionally, prominent RAN-positive staining was observed in the absence of detectable polyGln staining in white matter regions. This latter observation suggests that RAN proteins play a role in white matter abnormalities previously described in HD, such as gliosis and white matter changes detected by DTI/MRI imaging (Bohanna et al., 2011; Fennema-Notestine et al., 2004; Paulsen et al., 2010; Reading et al., 2005).
RAN-Protein Accumulation and Aggregation Are Length Dependent
Longer CAG expansions are associated with earlier onset and increased severity in HD and other polyGln expansion disorders (Ross et al., 1999). To investigate the effects of repeat length on HD-RAN proteins, we developed a series of HTT-exon-1 minigenes with repeats ranging in length from 23 to 80 CAGs (Figure 4A). Transient transfections were performed and cell lysates examined for the accumulation of ATG-initiated polyGln and HD-RAN polyAla and polySer proteins. As expected, ATG-initiated polyGln proteins are expressed and accumulate at all repeat lengths (23–80 repeats) (Figures 4B, 4C, and S4A). In contrast, RAN-protein accumulation and aggregation is length dependent. For example, protein blotting and IF show HD-polyAla RAN proteins accumulate in cells expressing CAG repeats ≥52 but not at repeats ≤45. In the polySer frame, RAN protein accumulation is evident at ≥35 repeats, and as the repeats get longer, RAN-poly-Ser shifts from being in the soluble (35–40 repeats) to the insoluble fraction (45–80 repeats) by protein blot (Figure 4B). Similarly, in transfected cells, polySer staining changes from a diffuse pattern (35–40 repeats) to a progressively more punctate, almost pin-like staining pattern forming granular cytoplasmic inclusions (45–80 repeats) (Figures 4C and S4A). In summary, the selective accumulation of RAN polyAla and punctate polySer proteins in this in vitro system occurs at repeat lengths typically associated with early-onset and juvenile cases of HD.
Figure 4. Length-Dependent RAN Protein Expression, Aggregation, and Toxicity.
(A) CAG and CAA HTT-exon1 minigenes.
(B and C) (B) Immunoblots and (C) IF of HEK293T cells after transfection with indicated constructs.
(D) Transfected minigenes (top) containing non-hairpin-forming alternative codons in the repeat region for polyGln90 (CAA), polySer90 (TCC-TCT), polyLeu90 (CTT-CTC), and polyCys90 (TGT) constructs. Because non-haipin forming codon substitutions encoding polyAla were not available, polyAla was expressed by a +ATG-GCA construct using a slightly longer repeat tract of 105 repeats.
(E and F) LDH assays of SH-SY5Y and T98 cells expressing polyGln and individual HD-RAN proteins 42 hr post-transfection. Values equal percent of cell death ± SEM (n = 5; *p < 0.05, **p < 0.01, and ***p < 0.001). See also Figures S4A–S4F.
HD-RAN Proteins Decrease Cell Survival In Vitro
The discovery of HD-RAN proteins in caspase-3-positive brain regions strongly suggests that RAN proteins are toxic. To examine the effect of each individual RAN protein independently of RNA-mediated effects, we generated polyGln, polySer, polyLeu, and polyCys minigenes using alternative codons to prevent RNA hairpin formation and RAN translation (Zu et al., 2011). Because non-hairpin forming codons are not available for polyAla, we increased polyAla expression relative to polyGln and polySer by including an ATG-initiation codon in the polyAla reading frame. ATG-initiated polyAla was generated with a GCA expansion (Figure 4D). All the minigenes expressed proteins with the repeat expansion and the complete C-terminal region for each protein.
SH-SY5Y neuronal and T98 glial cells transfected with these constructs showed a significant increase in cell death compared to cells expressing a short repeat. HD-RAN polySer, polyLeu, and polyCys proteins are equally or more toxic than polyGln alone. The transfection with the polyAla construct, which expresses high polyAla levels, but also low levels of polyGln and polySer, dramatically increased cell death (Figures 4E, 4F, and S4C). In contrast, toxic effects of these proteins were less robust or not found in transfected HEK293T cells at 18, 24, or 42 hr post-transfection (Figures S4B, S4C, and S4F), suggesting that the vulnerability to HD-RAN proteins is cell type specific. Taken together, these data demonstrate that the expression of individual polySer, poly-Leu, and polyCys HD-RAN proteins using non-hairpin-forming alternative codons increases cell death in neuronal and glial cells.
HD-RAN Proteins Accumulate in Severely Affected Cerebellar Regions in Juvenile HD
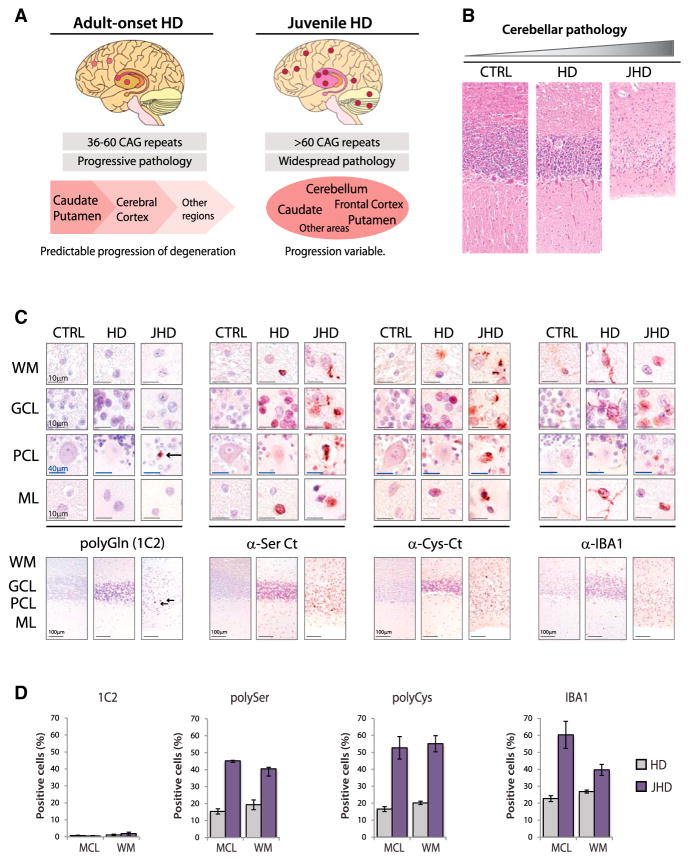
In adult-onset HD, there is a predictable disease progression that first, and most severely, affects the caudate/putamen followed by the frontal cortex and other brain regions. In contrast, disease progression in juvenile-onset cases shows a more widespread pathology, with some patients showing severe involvement of the caudate and putamen while others show relative sparing of this region but severe atrophy of the cortex or cerebellum (Figure 5A) (Bates et al., 2014). Given that RAN protein expression increases with repeat length, we tested if RAN protein staining is more prominent in juvenile HD cases and if severely affected regions show increased RAN protein accumulation. To explore this question, we analyzed autopsy tissue from five juvenile-onset cases. All five cases showed increased frequency and more intense staining for all four RAN proteins in striatum, frontal cortex, and cerebellum (Table 1). Two of these cases, which came to autopsy at 8 years of age with repeats greater than 100, showed marked cerebellar atrophy with abundant RAN protein staining in all layers of the cerebellum (Figures 5B, 5C, and S5A; Tables 1 and 2).
Figure 5. Increased RAN Protein Staining in Juvenile HD.
(A) Schematic diagram summarizing features of adult-onset and juvenile HD pathology.
(B) H&E staining in control, adult-onset, and juvenile-onset HD cases with cerebellar atrophy.
(C) α-polyGln, α-polySer, α-polyCys and α-IBA1staining in cerebellar layers.
(D) Quantitation of IHC-positive cells with 1C2 (polyGln) and α-polySer-Ct, α-polyCys-Ct, and α-IBA1 antibodies. WM, white matter; GCL, granule cell layer; PCL, Purkinje-cell layer; ML, molecular layer. Red, positive staining; blue, nuclear counterstain. Staining of cerebellum in adult-onset HD cases is variable. IHC images and quantification of percent positive cells represent typical positive regions. See also Figures S5A and S5B.
Table 2.
HD-RAN Protein Staining and Cerebellar Atrophy in Juvenile HD
| Cases JHD | Age | Repeat | PMD | CBL Atrophy | RAN |
|---|---|---|---|---|---|
| 1 | 8 | >100* | − | ++++ | ++++ |
| 2 | 8 | ~100* | − | ++ | ++ |
| 3 | 27 | 69 | 22 | + | ++ |
| 4 | 23 | 76 | 14 | + | + |
| 5 | 23 | 64 | 13 | + | ++ |
RAN staining: +, low-intensity staining; ++, moderate intensity; +++, frequent and intense; ++++, highly frequent positive cells with high intensity staining. JHD, juvenile HD.
repeat length estimated from autopsy brains.
In the most severely affected juvenile HD case (JHD-1), the cerebellum showed more intense RAN polySer and polyCys staining compared to adult HD (Table 1; Figure S5A) with JHD showing ubiquitous, densely reactive, often punctate nuclear or perinuclear staining in regions throughout the cerebellum. Positive regions included molecular layer interneurons, Bergmann glia and basket cells surrounding the Purkinje cells, the granular layer and subcortical and deep white matter regions including regions surrounding the dentate nuclei (Figure 5C, S5A, and S5B). Additionally, RAN protein staining in the white matter surrounding the dentate nucleus was found in regions of gliosis, with prominent α-polyCys staining of astrocytes and microglia-like cells (Figures S5A and S5B). A second juvenile case (JHD-2), also with cerebellar atrophy, showed polySer- and polyCys-positive staining in similar regions as well as in the remaining Purkinje cells (Figure S5A). In contrast, polyglutamine staining in the cerebellum was rare in all cases, with no positive staining detected in the adult-onset cases or JHD-2 using the 1C2 or EM48 antibodies (Figures 5C and S5A), and few positive cells per 20× field in JHD-1 detected with 1C2 but not EM48 (Figure S5A).
Similar to the results obtained in the adult-onset HD striatum (Figure 2), IBA1 staining of juvenile HD cerebella showed abundant microglial-like cells in regions strongly positive for HD-RAN polySer and polyCys. Microglia/monocyte staining mostly displayed a ramified morphology in the adult and less affected juvenile cerebellum. In contrast, IBA1-positive cells were more abundant in the severely affected juvenile cerebellum and displayed active, round amoeboid morphology, with a similar distribution pattern to that observed for α-polySer and α-polyCys staining (Figures 5C and S5A). These results show high levels of RAN protein accumulation in severely affected regions with minimal polyGln staining.
DISCUSSION
Our understanding of HD has been built on studying the effects of the AUG-initiated polyGln expansion protein. In this report, we provide evidence that additional expansion proteins, polyAla, poly-Ser, polyLeu, and polyCys, are expressed; accumulate in HD patient brains; and contribute to disease pathology. First, HD-RAN proteins show abundant positive staining in regions of the brain most affected by HD, including the caudate/putamen and, in juvenile-onset cases, also the cerebellum. Second, regions with intense RAN-positive staining show pathologic features of HD, including activated caspase-3 staining, activated microglia, and gliosis. Third, prominent RAN-positive staining was observed in the absence of detectable polyGln (Herndon et al., 2009) staining in several white matter regions, suggesting RAN proteins play a role in HD white matter abnormalities (Bohanna et al., 2011; Fennema-Notestine et al., 2004; Paulsen et al., 2010; Reading et al., 2005). Fourth, in vitro studies indicate that HD-RAN proteins are toxic to neural cells. Fifth, increased polyAla accumulation and aggregation of polySer RAN proteins occurs in cells expressing longer repeats >52, which are typically associated with earlier disease onset and more severe juvenile HD. Additionally, the accumulation of RAN polySer at 35, but not 23 repeats, is worth considering as a possible trigger of disease, since 35 repeats is near the threshold at which HD can manifest. Finally, increased HD-RAN protein staining is found in highly affected juvenile-onset cerebellar tissue.
It was previously suggested that the CAG expansions can undergo frameshifting that results in the production of fusion proteins that start with an AUG-initiation codon in the glutamine frame and shift to alternative reading frames to produce hybrid polyGln/polyAla or polyGln/polySer proteins (Davies and Rubinsztein, 2006; Gaspar et al., 2000; Stochmanski et al., 2012; Toulouse et al., 2005). Here we demonstrate that neither frame-shifting nor an AUG initiation codon is required for the expression of polyAla or polySer and that both sense and antisense HD-RAN proteins are abundantly expressed in affected regions of HD autopsy brains. (Figure 1 and S1Ac). While the detection of HD-RAN proteins with C-terminal antibodies demonstrates that these proteins include C-terminal flanking sequences, future studies will be important to characterize their specific initiation and termination sites. Additionally, it will be important to determine the stability and aggregation properties of these proteins as well as the mechanisms of protein clearance.
The discovery that RAN proteins are expressed and accumulate at sites of HD pathology provides new insights that may explain the selective vulnerability of the caudate/putamen in adult-onset cases and the variability of affected regions of the brain in juvenile-onset HD. First, RAN proteins are frequently found in cells expressing active-caspase-3, including in white-matter-bundle regions that show RAN but not HTT-polyGln accumulation. Additionally, the observation that 40%–50% of IBA1 cells are also RAN-positive suggests microglia/macrophages are recruited to RAN-positive regions and engulf RAN proteins or RAN-positive cells. Alternatively, it is possible that a subset of microglia/macrophages express RAN proteins. Taken together, these results raise the possibility that HD-RAN proteins trigger neuroinflammatory changes (Björkqvist et al., 2008, 2009; Myers et al., 1991; Pavese et al., 2006; Tai et al., 2007) and cell death in HD.
As recently described, cerebellar atrophy has been reported in both adult and juvenile HD cases (Nance et al., 1999; Rüb et al., 2013; Seneca et al., 2004) and may contribute to balance, speech, and gait problems found in HD patients (Koller and Trimble, 1985; Margolis and Ross, 2003; Rüb et al., 2013). The preferential accumulation of HD-RAN versus HD-polyGln proteins in the cerebellum (Figures 3, 5, and S5; Table 1) in both adult and juvenile cases suggests the possibility that RAN proteins trigger the neurodegeneration seen in juvenile cases (Table 2) and may also cause functional problems in adults.
It is striking that RAN but not polyGln proteins accumulate in white matter regions of the striatum, frontal cortex, and cerebellum. These data suggest several possibilities that may explain the region-specific accumulation of HD-RAN proteins: (1) the shorter RAN proteins may be more aggregate prone and easier to detect than HTT-polyGln, (2) degradation pathways may less efficiently target RAN proteins leading to their preferential accumulation, and (3) RAN translation may be more efficient in specific cell types. Additional research will be required to determine if the accumulation of RAN proteins in specific white matter regions underlies the early white matter abnormalities seen by diffusion tensor imaging in HD patients (Rosas et al., 2006; Rüb et al., 2013) and cause early signs of the disease.
While additional work will be required to sort out the contributions of HD-RAN proteins in disease, two mouse models that show HD-like phenotypes were developed using a mixed CAG/CAA repeat encoding a polyGln tract (Gray et al., 2008; O’Brien et al., 2015). While these animals express a polyGln expansion across the CAG/CAA repeat, the CAG/CAA mixed repeat is also predicted to form a complex hairpin-containing structure that may also produce two novel di-peptide (Thr/Ala and Asn/Ser) RAN proteins, which may contribute to the phenotypes found in these models. Additional animal models that allow direct comparisons of the effects of polyGln, individual RAN proteins and sense and antisense RNAs will be required to fully understand how each of these CAG·CTG expansion mutation products contributes to disease.
Although additional work will be needed to understand the individual and combined effects of HD-RAN proteins, it is possible that one or more of these proteins is highly toxic and that strategies to target or increase turnover of selected proteins may mitigate disease (Cleary and Ranum, 2014). It may also be possible to downregulate RAN translation without inhibiting canonical AUG-initiated translation and that decreasing overall RAN protein load will have beneficial effects (Cleary and Ranum, 2014). Alternatively, targeting the HTT sense transcript using antisense oligonucleotides has been described as a promising therapeutic approach for HD (Hu et al., 2012; Kordasiewicz et al., 2012). While preclinical strategies to target the HD sense RNAs, as well as other therapeutic approaches (Lee et al., 2013; Menalled and Brunner, 2014; Switonski et al., 2012), are promising, it will be important to determine which of these mouse models accurately reproduce the sense and antisense expression seen in humans and to correlate these findings with the recovery seen in each of the models studied.
In summary, the accumulation of both sense and antisense RAN proteins in human autopsy brains and their toxicity suggests strategies targeting both transcripts may be required to effectively treat disease in humans. Additionally, HD-RAN proteins may prove to be useful biomarkers to predict disease onset and progression and responses to future therapeutic treatments. Finally, the discovery that RAN translation can occur when an expansion mutation is located in an ORF raises the possibility that RAN proteins also contribute to eight other CAG polyglutamine expansion disorders.
Supplementary Material
Highlights.
RAN translation occurs across canonical ORF
Sense and antisense RAN proteins accumulate in Huntington brains
HD-RAN proteins are toxic to cells
HD-RAN protein accumulation and aggregation is CAG length dependent
Acknowledgments
We thank Dr. M. Swanson for helpful suggestions and discussions, Drs. E. Marti and X. Estivill for the generous gift of HTT exon1 plasmids, and Dr. J. Streit for helpful technical suggestions in detecting microglia. This work was funded by CHDI Foundation, Inc., a grant from the W.F. Keck Foundation and start-up funding from the University of Florida (L.P.W.R.); a Sara Borrell contract from the Spanish Ministry of Health, ISCIII (M.B.-C.); a University of Florida Alumni Fellowship (F.A.); and an NIH Training Grant (NS082168) (B.A.P.) and the John Hopkins University Alzheimer’s Disease Research Center (NIH P50AG05146) (C.A.R., R.L.M., and J.C.T.). M.B.-C., T.Z., and L.P.W.R. have patents pending on RAN translation.
Footnotes
Supplemental Information includes five figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2015.10.038.
AUTHOR CONTRIBUTIONS
Author contributions: M.B.-C., T.Z., and L.P.W.R. designed research; M.B.-C., F.A., A.D.T., T.Z., B.A.P., S.K.T., and O.P. performed research; O.P., D.R.B., C.A.R., R.L.M., A.T.Y., and J.C.T. contributed new reagents/analytic tools/samples; M.B.-C., F.A., T.Z., A.T.Y., J.C.T., and L.P.W.R. analyzed data; and M.B.-C. and L.P.W.R. wrote the paper with input from all authors.
No other authors have conflicts.
References
- Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW, 3rd, Rademakers R, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bañez-Coronel M, Porta S, Kagerbauer B, Mateu-Huertas E, Pantano L, Ferrer I, Guzmán M, Estivill X, Martí E. A pathogenic mechanism in Huntington’s disease involves small CAG-repeated RNAs with neurotoxic activity. PLoS Genet. 2012;8:e1002481. doi: 10.1371/journal.pgen.1002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates G, Tabrizi SJ, Jones L, editors. Huntington’s Disease. 4. Oxford University Press; 2014. [Google Scholar]
- Björkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, Raibon E, Lee RV, Benn CL, Soulet D, et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. J Exp Med. 2008;205:1869–1877. doi: 10.1084/jem.20080178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkqvist M, Wild EJ, Tabrizi SJ. Harnessing immune alterations in neurodegenerative diseases. Neuron. 2009;64:21–24. doi: 10.1016/j.neuron.2009.09.034. [DOI] [PubMed] [Google Scholar]
- Bohanna I, Georgiou-Karistianis N, Sritharan A, Asadi H, Johnston L, Churchyard A, Egan G. Diffusion tensor imaging in Huntington’s disease reveals distinct patterns of white matter degeneration associated with motor and cognitive deficits. Brain Imaging Behav. 2011;5:171–180. doi: 10.1007/s11682-011-9121-8. [DOI] [PubMed] [Google Scholar]
- Chung DW, Rudnicki DD, Yu L, Margolis RL. A natural anti-sense transcript at the Huntington’s disease repeat locus regulates HTT expression. Hum Mol Genet. 2011;20:3467–3477. doi: 10.1093/hmg/ddr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JD, Ranum LP. Repeat associated non-ATG (RAN) translation: new starts in microsatellite expansion disorders. Curr Opin Genet Dev. 2014;26:6–15. doi: 10.1016/j.gde.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JE, Rubinsztein DC. Polyalanine and polyserine frame-shift products in Huntington’s disease. J Med Genet. 2006;43:893–896. doi: 10.1136/jmg.2006.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C, Archibald SL, Jacobson MW, Corey-Bloom J, Paulsen JS, Peavy GM, Gamst AC, Hamilton JM, Salmon DP, Jernigan TL. In vivo evidence of cerebellar atrophy and cerebral white matter loss in Huntington disease. Neurology. 2004;63:989–995. doi: 10.1212/01.wnl.0000138434.68093.67. [DOI] [PubMed] [Google Scholar]
- Gaspar C, Jannatipour M, Dion P, Laganière J, Sequeiros J, Brais B, Rouleau GA. CAG tract of MJD-1 may be prone to frameshifts causing polyalanine accumulation. Hum Mol Genet. 2000;9:1957–1966. doi: 10.1093/hmg/9.13.1957. [DOI] [PubMed] [Google Scholar]
- Gray M, Shirasaki DI, Cepeda C, André VM, Wilburn B, Lu XH, Tao J, Yamazaki I, Li SH, Sun YE, et al. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci. 2008;28:6182–6195. doi: 10.1523/JNEUROSCI.0857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon ES, Hladik CL, Shang P, Burns DK, Raisanen J, White CL., 3rd Neuroanatomic profile of polyglutamine immunoreactivity in Huntington disease brains. J Neuropathol Exp Neurol. 2009;68:250–261. doi: 10.1097/NEN.0b013e318198d320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Liu J, Yu D, Chu Y, Corey DR. Mechanism of allele-selective inhibition of huntingtin expression by duplex RNAs that target CAG repeats: function through the RNAi pathway. Nucleic Acids Res. 2012;40:11270–11280. doi: 10.1093/nar/gks907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller WC, Trimble J. The gait abnormality of Huntington’s disease. Neurology. 1985;35:1450–1454. doi: 10.1212/wnl.35.10.1450. [DOI] [PubMed] [Google Scholar]
- Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, McAlonis MM, Pytel KA, Artates JW, Weiss A, Cheng SH, Shihabuddin LS, et al. Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuron. 2012;74:1031–1044. doi: 10.1016/j.neuron.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Cantle JP, Yang XW. Genetic manipulations of mutant huntingtin in mice: new insights into Huntington’s disease pathogenesis. FEBS J. 2013;280:4382–4394. doi: 10.1111/febs.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis RL, Ross CA. Diagnosis of Huntington disease. Clin Chem. 2003;49:1726–1732. doi: 10.1373/49.10.1726. [DOI] [PubMed] [Google Scholar]
- Menalled L, Brunner D. Animal models of Huntington’s disease for translation to the clinic: best practices. Mov Disord. 2014;29:1375–1390. doi: 10.1002/mds.26006. [DOI] [PubMed] [Google Scholar]
- Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, Schmid B, Kretzschmar HA, Cruts M, Van Broeckhoven C, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339:1335–1338. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- Myers RH, Vonsattel JP, Paskevich PA, Kiely DK, Stevens TJ, Cupples LA, Richardson EP, Jr, Bird ED. Decreased neuronal and increased oligodendroglial densities in Huntington’s disease caudate nucleus. J Neuropathol Exp Neurol. 1991;50:729–742. doi: 10.1097/00005072-199111000-00005. [DOI] [PubMed] [Google Scholar]
- Nance MA, Mathias-Hagen V, Breningstall G, Wick MJ, McGlennen RC. Analysis of a very large trinucleotide repeat in a patient with juvenile Huntington’s disease. Neurology. 1999;52:392–394. doi: 10.1212/wnl.52.2.392. [DOI] [PubMed] [Google Scholar]
- O’Brien R, DeGiacomo F, Holcomb J, Bonner A, Ring KL, Zhang N, Zafar K, Weiss A, Lager B, Schilling B, et al. Integration-independent Transgenic Huntington Disease Fragment Mouse Models Reveal Distinct Phenotypes and Life Span in Vivo. J Biol Chem. 2015;290:19287–19306. doi: 10.1074/jbc.M114.623561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Nopoulos PC, Aylward E, Ross CA, Johnson H, Magnotta VA, Juhl A, Pierson RK, Mills J, Langbehn D, Nance M PREDICT-HD Investigators and Coordinators of the Huntington’s Study Group (HSG) Striatal and white matter predictors of estimated diagnosis for Huntington disease. Brain Res Bull. 2010;82:201–207. doi: 10.1016/j.brainresbull.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavese N, Gerhard A, Tai YF, Ho AK, Turkheimer F, Barker RA, Brooks DJ, Piccini P. Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology. 2006;66:1638–1643. doi: 10.1212/01.wnl.0000222734.56412.17. [DOI] [PubMed] [Google Scholar]
- Reading SA, Yassa MA, Bakker A, Dziorny AC, Gourley LM, Yallapragada V, Rosenblatt A, Margolis RL, Aylward EH, Brandt J, et al. Regional white matter change in pre-symptomatic Huntington’s disease: a diffusion tensor imaging study. Psychiatry Res. 2005;140:55–62. doi: 10.1016/j.pscychresns.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, Salat DH. Diffusion tensor imaging in presymptomatic and early Huntington’s disease: Selective white matter pathology and its relationship to clinical measures. Mov Disord. 2006;21:1317–1325. doi: 10.1002/mds.20979. [DOI] [PubMed] [Google Scholar]
- Ross CA, Wood JD, Schilling G, Peters MF, Nucifora FC, Jr, Cooper JK, Sharp AH, Margolis RL, Borchelt DR. Polyglutamine pathogenesis. Philos Trans R Soc Lond B Biol Sci. 1999;354:1005–1011. doi: 10.1098/rstb.1999.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüb U, Hoche F, Brunt ER, Heinsen H, Seidel K, Del Turco D, Paulson HL, Bohl J, von Gall C, Vonsattel JP, et al. Degeneration of the cerebellum in Huntington’s disease (HD): possible relevance for the clinical picture and potential gateway to pathological mechanisms of the disease process. Brain Pathol. 2013;23:165–177. doi: 10.1111/j.1750-3639.2012.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling G, Becher MW, Sharp AH, Jinnah HA, Duan K, Kotzuk JA, Slunt HH, Ratovitski T, Cooper JK, Jenkins NA, et al. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum Mol Genet. 1999;8:397–407. doi: 10.1093/hmg/8.3.397. [DOI] [PubMed] [Google Scholar]
- Seneca S, Fagnart D, Keymolen K, Lissens W, Hasaerts D, Debulpaep S, Desprechins B, Liebaers I, De Meirleir L. Early onset Huntington disease: a neuronal degeneration syndrome. Eur J Pediatr. 2004;163:717–721. doi: 10.1007/s00431-004-1537-3. [DOI] [PubMed] [Google Scholar]
- Stochmanski SJ, Therrien M, Laganière J, Rochefort D, Laurent S, Karemera L, Gaudet R, Vyboh K, Van Meyel DJ, Di Cristo G, et al. Expanded ATXN3 frameshifting events are toxic in Drosophila and mammalian neuron models. Hum Mol Genet. 2012;21:2211–2218. doi: 10.1093/hmg/dds036. [DOI] [PubMed] [Google Scholar]
- Switonski PM, Szlachcic WJ, Gabka A, Krzyzosiak WJ, Figiel M. Mouse models of polyglutamine diseases in therapeutic approaches: review and data table. Part II Mol Neurobiol. 2012;46:430–466. doi: 10.1007/s12035-012-8316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YF, Pavese N, Gerhard A, Tabrizi SJ, Barker RA, Brooks DJ, Piccini P. Imaging microglial activation in Huntington’s disease. Brain Res Bull. 2007;72:148–151. doi: 10.1016/j.brainresbull.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Todd PK, Oh SY, Krans A, He F, Sellier C, Frazer M, Renoux AJ, Chen KC, Scaglione KM, Basrur V, et al. CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron. 2013;78:440–455. doi: 10.1016/j.neuron.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulouse A, Au-Yeung F, Gaspar C, Roussel J, Dion P, Rouleau GA. Ribosomal frameshifting on MJD-1 transcripts with long CAG tracts. Hum Mol Genet. 2005;14:2649–2660. doi: 10.1093/hmg/ddi299. [DOI] [PubMed] [Google Scholar]
- Zu T, Gibbens B, Doty NS, Gomes-Pereira M, Huguet A, Stone MD, Margolis J, Peterson M, Markowski TW, Ingram MA, et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci USA. 2011;108:260–265. doi: 10.1073/pnas.1013343108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, Liu Y, Bañez-Coronel M, Reid T, Pletnikova O, Lewis J, Miller TM, Harms MB, Falchook AE, Subramony SH, et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci USA. 2013;110:E4968–E4977. doi: 10.1073/pnas.1315438110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.