Abstract
Purpose
Prostate specific antigen sensitivity increases with lower threshold values but with a corresponding decrease in specificity. Magnetic resonance imaging/ultrasound targeted biopsy detects prostate cancer more efficiently and of higher grade than standard 12-core transrectal ultrasound biopsy but the optimal population for its use is not well defined. We evaluated the performance of magnetic resonance imaging/ultrasound targeted biopsy vs 12-core biopsy across a prostate specific antigen continuum.
Materials and Methods
We reviewed the records of all patients enrolled in a prospective trial who underwent 12-core transrectal ultrasound and magnetic resonance imaging/ultrasound targeted biopsies from August 2007 through February 2014. Patients were stratified by each of 4 prostate specific antigen cutoffs. The greatest Gleason score using either biopsy method was compared in and across groups as well as across the population prostate specific antigen range. Clinically significant prostate cancer was defined as Gleason 7 (4 + 3) or greater. Univariate and multivariate analyses were performed.
Results
A total of 1,003 targeted and 12-core transrectal ultrasound biopsies were performed, of which 564 diagnosed prostate cancer for a 56.2% detection rate. Targeted biopsy led to significantly more upgrading to clinically significant disease compared to 12-core biopsy. This trend increased more with increasing prostate specific antigen, specifically in patients with prostate specific antigen 4 to 10 and greater than 10 ng/ml. Prostate specific antigen 5.2 ng/ml or greater captured 90% of upgrading by targeted biopsy, corresponding to 64% of patients who underwent multiparametric magnetic resonance imaging and subsequent fusion biopsy. Conversely a greater proportion of clinically insignificant disease was detected by 12-core vs targeted biopsy overall. These differences persisted when controlling for potential confounders on multivariate analysis.
Conclusions
Prostate cancer upgrading with targeted biopsy increases with an increasing prostate specific antigen cutoff. Above a prostate specific antigen threshold of 5.2 ng/ml most upgrading to clinically significant disease was achieved by targeted biopsy. In our population this corresponded to potentially sparing biopsy in 36% of patients who underwent multiparametric magnetic resonance imaging. Below this value 12-core biopsy detected more clinically insignificant cancer. Thus, the diagnostic usefulness of targeted biopsy is optimized in patients with prostate specific antigen 5.2 ng/ml or greater.
Keywords: prostate, prostatic neoplasms, diagnostic imaging, prostate-specific antigen, biopsy
Accurate knowledge of pathological grade is vital to PCa management. However, standard template prostate biopsy under grades disease compared to the prostatectomy specimen in about a third of patients, including those previously diagnosed with low risk cancer.1–3 Men with increased total PSA and negative prostate biopsy, and men with PSA 2.5 to 10 ng/ml pose a specific diagnostic dilemma since up to a third of these patients may harbor clinically significant PCa that is missed on initial biopsy or not biopsied due to lack of clinical suspicion.4
Targeted biopsy of the prostate using fusion of MRI and real-time TRUS data may represent a viable solution to this problem. MP-MRI can accurately pinpoint the location of lesions suspicious for tumor, as confirmed on prostatectomy specimens. 5,6 Suspicion scores derived from imaging parameters correlate with improved detection of PCa as well as assessments of clinical risk.7,8 Furthermore, targeted biopsy leads to increased detection of clinically significant PCa, including in men with previously negative biopsies and significantly upgraded disease compared to systematic 12-core random biopsy, which is the current standard of care.9–11 The ability of fusion platforms to document biopsy locations and, therefore, allow for later rebiopsy has spurred interest in deploying targeted biopsy as a tool in the followup of low risk cancer.12,13
Determining which patient populations may be best suited to targeted prostate biopsy as an initial diagnostic strategy may spur its more widespread adoption. The highest Gleason score determined by targeted vs standard template biopsy may more accurately reflect the final pathological grade. Thus, we compared Gleason scores determined by targeted or 12-core biopsy at the same biopsy session across a range of total PSA levels. We further stratified this range into 4 PSA cutoffs selected from values previously reported as possible cancer detection thresholds.14 We then assessed upgrading within and between the cutoff levels to identify subgroups who would benefit most from targeted biopsy.
MATERIALS AND METHODS
Study Population
Patients were enrolled in a prospective, institutional review board approved trial of MRI/US fusion guided prostate biopsy with electromagnetic tracking at NCI (National Cancer Institute), NIH (National Institutes of Health) between August 2007 and February 2014 (ClinicalTrials.gov identifier NCT00102544). All patients provided appropriate written informed consent. Patients underwent digital rectal examination and standardized MP-MRI. Total serum PSA in all patients was assessed before biopsy. MRI/US fusion guided biopsy was then performed in 1,003 men during the study period. For men with multiple biopsy sessions only the initial session was included in analysis.
Image Acquisition and Interpretation
Diagnostic 3.0 Tesla MP-MRI was performed in all patients before biopsy using the Achieva device with a 16-channel SENSE cardiac surface coil positioned over the pelvis (Philips Healthcare, Andover, Massachusetts) and a BPX-30 endorectal coil (Medrad©) as described previously.11 MRI parameters included T1-weighted imaging, triplanar (coronal, sagittal and axial) T2 with diffusion guided weighted imaging and apparent diffusion coefficient mapping, multivoxel 3-dimensional localized spectroscopy and axial 3-dimensional fast field echo dynamic contrast enhanced sequences.
Centralized, blinded independent review and consensus reads of all MP-MRI studies were performed by 2 genitourinary radiologists (BT and PLC) to identify lesions and assign suspicion scores to each using our previously validated MP-MRI sequence suspicion correlation chart.15,16 Briefly, the number of positive sequences on MP-MRI for each lesion determined the score, which was graded as low, moderate or high suspicion. The greatest suspicion score per patient served as the overall suspicion score.
Biopsy Session Protocol
At a single session patients with any lesion suspicious for PCa on MP-MRI underwent standard 12-core TRUS guided, extended sextant biopsy performed by the same operator, who was blinded to the MP-MRI and fusion biopsy of MP-MRI target lesions, using an office based UroNav platform (Invivo, Gainesville, Florida).11 Prebiopsy T2-weighted images were segmented, registered and fused with the real-time TRUS image. Lesions suspicious for PCa were displayed as targets and sampled once in each of the axial and sagittal planes, resulting in 2 cores per target. Needle trajectories were mapped with the real-time electromagnetic tracking incorporated into the biopsy platform (Northern Digital, Waterloo, Ontario, Canada). Median time from initial MP-MRI to the biopsy session was 43 days. Pathological specimens were reviewed by a single genitourinary pathologist (MJM).
Study Design
The highest Gleason score from the 12-core or the targeted biopsy was determined for each patient. In accordance with START (Standards of Reporting for MRI-targeted Biopsy Studies) Working Group recommendations patients were classified with clinically significant high grade disease (4 + 3 or greater) or clinically insignificant lower grade disease (3 + 4 or less).17 This classification was in keeping with our previously reported study based on the prognostic difference of 4 + 3 vs 3 + 4 cancers. 10,18,19 The population of 1,003 men was divided into 4 cohorts based on prebiopsy total PSA, including 0 to less than 2.5, 2.5 to less than 4, 4 to 10 and greater than 10 ng/ml. The detection of clinically significant or clinically insignificant disease by targeted over 12-core biopsy or vice versa was determined in patients within these cutoffs as well as over the entire range of PSA of the population, incorporating the number of patients who underwent MP-MRI at each PSA level. We determined threshold values at or above which clinically significant disease was detected.
Statistical Analysis
Proportions of cases upgraded by 12-core or targeted biopsy across PSA cutoffs were compared using the McNemar test. Potential predictors of upgrading to clinically significant disease were included in a multivariate logistic regression model if significantly associated on univariate analysis. Statistical significance was considered at p <0.05. JMP©, version 11.0 was used for all analyses.
RESULTS
Table 1 lists the demographics of the study cohort and the 4 PSA based subgroups. Median patient age was 62 years (IQR 57–67) and median PSA was 6.7 ng/ml (IQR 4.4–10.7). Of the 1,003 men 564 had a positive 12-core and/or targeted biopsy for an overall 56.2% cancer detection rate. The individual cancer detection rates of 12-core and targeted biopsies did not differ significantly overall or within the PSA cutoffs (fig. 1).
Table 1.
Patient demographics, MP-MRI assigned prostate cancer suspicion scores and fusion guided biopsy findings by PSA cutoff
| PSA Cutoff (ng/ml)
|
|||||
|---|---|---|---|---|---|
| 0–Less Than 2.5 | 2.5–Less Than 4 | 4–10 | Greater Than 10 | Overall | |
| No. pts (%) | 81 (8.1) | 131 (13.1) | 513 (51.1) | 278 (27.7) | 1,003 |
| Mean ± SD age | 59.4 ± 7.4 | 60.6 ± 8.0 | 62.0 ± 7.4 | 63.6 ± 7.1 | 62.1 ± 7.5 |
| Mean ± SD MRI: | |||||
| Prostate vol (ml) | 36 ± 15 | 45 ± 17 | 57 ± 30 | 74 ± 43 | 59 ± 34 |
| No. lesions/pt | 2.4 ± 1.1 | 2.8 ± 1.3 | 2.6 ± 1.3 | 2.8 ± 1.3 | 2.7 ± 1.3 |
| No. anterior lesions/pt | 0.7 ± 1.2 | 1.1 ± 1.5 | 1.3 ± 1.9 | 1.8 ± 2.2 | 1.4 ± 1.9 |
| Mean ± SD No. biopsy cores/pt | 17.0 ± 2.4 | 17.8 ± 2.9 | 17.5 ± 2.8 | 17.7 ± 2.7 | 17.6 ± 2.7 |
| No. prior neg biopsy (%) | 8 (9.9) | 13 (9.9) | 100 (19.5) | 74 (26.6) | 195 (19.4) |
| No. prostate Ca (%)* | 36 (44.4) | 75 (57.3) | 284 (55.4) | 169 (60.8) | 564 |
On targeted or 12-core biopsy.
Figure 1.
Cancer detection rate by PSA cutoffs for standard 12-core vs MRI/US fusion targeted biopsy. There was no significant difference between groups.
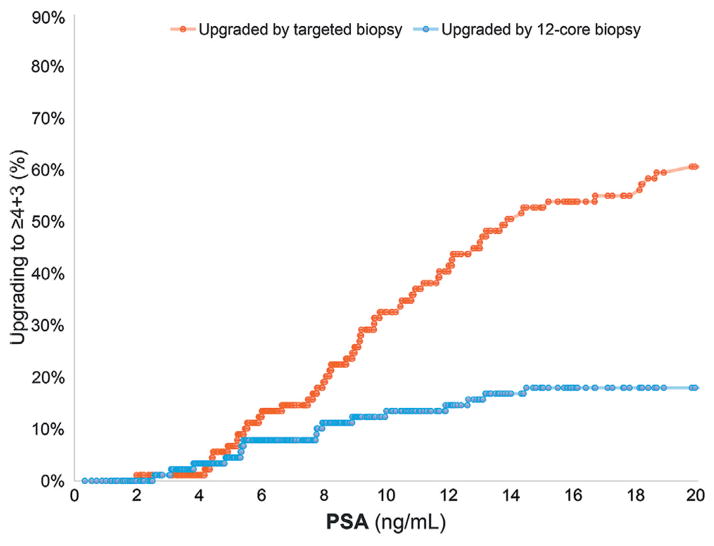
The 2 biopsy modalities were compared in terms of the highest Gleason score detected per patient. Of the 564 men 192 (34.0%) were classified with clinically significant disease (Gleason score 4 + 3 or greater). Across PSA cutoffs targeted biopsy upgraded more cases to clinically significant disease than 12-core biopsy at PSA 4 to 10 and greater than 10 ng/ml (9.9% vs 2.8% and 24.3% vs 4.7%, respectively, fig. 2). There was no significant difference between the proportions of clinically significant cancer detected at PSA less than 4 ng/ml between the 2 biopsy methods. However, greater proportions of clinically insignificant cancer (3 + 4 or less) were detected by 12-core than by targeted biopsy (69.7% vs 57.8% and 40.9% vs 28.5% for PSA 4 to 10 ng/ml and greater than 10 ng/ml, respectively, p <0.01). Table 2 shows biopsy results in all patients across all PSA cutoffs.
Figure 2.
Cancer cases upgraded to clinically significant (4 + 3 or greater) disease by standard 12-core vs MRI/US fusion targeted biopsy by PSA cutoff.
Table 2.
Results of standard 12-core and MRI/US fusion targeted biopsies by PSA cutoff
| PSA ng/ml Cutoff (targeted biopsy result) | No. 12-Core Biopsy Result/Total No. (%)
|
||
|---|---|---|---|
| No Ca | Clinically Insignificant Ca | Clinically Significant Ca | |
| 0–Less than 2.5: | |||
| No Ca | 45/81* | 8/36 | (22.2)* 0* |
| Clinically insignificant Ca | 10/36 (27.8)* | 16/36 (44.4)* | 0* |
| Clinically significant Ca | 1/36 (2.8)† | 0* | 1/36 (2.8)* |
| 2.5–Less than 4: | |||
| No Ca | 56/131* | 19/75 (25.3)* | 1/75 (1.3)† |
| Clinically insignificant Ca | 11/75 (14.7)* | 37/75 (49.3)* | 2/75 (2.7)† |
| Clinically significant Ca | 0* | 0* | 5/75 (6.7)* |
| 4–10: | |||
| No Ca | 229/513* | 54/284 (19.0)* | 1/284 (0.4)† |
| Clinically insignificant Ca | 35/284 (12.3)* | 122/284 (43.0)* | 7/284 (2.5)† |
| Clinically significant Ca | 6/284 (2.1)† | 22/284 (7.7)† | 37/284 (13.0)* |
| Greater than 10: | |||
| No Ca | 109/278* | 17/169 (10.1)* | 3/169 (1.8)† |
| Clinically insignificant Ca | 13/169 (7.7)* | 30/169 (17.8)* | 5/169 (3.0)† |
| Clinically significant Ca | 19/169 (11.2)† | 22/169 (13.0)† | 60/169 (35.5)* |
| Overall: | |||
| No Ca | 439/1,003* | 98/564 (17.4)* | 5/564 (0.9)† |
| Clinically insignificant Ca | 69/564 (12.2)* | 205/564 (36.3)* | 14/564 (2.5)† |
| Clinically significant Ca | 26/564 (4.6)† | 44/564 (7.8)† | 103/564 (18.3)* |
Biopsy methods concordant or missed clinically insignificant disease, defined as Gleason score 7 (3 + 4) or less.
Biopsy method under graded or missed clinically significant disease, defined as Gleason score 7 (4 + 3) or greater.
Upgrading to clinically significant disease was assessed across the entire PSA range for the patient population. The cumulative number of cases at each PSA cutoff upgraded to clinically significant disease by each biopsy modality was determined as a proportion of the total number upgraded to clinically significant disease (fig. 3). As PSA increased, an increasing proportion of the upgrades to clinically significant disease were noted in men in whom tumors were upgraded by targeted biopsy. Conversely 12-core biopsy resulted in greater detection of clinically insignificant disease overall.
Figure 3.
Cumulative PSA in men with upgraded clinically significant disease by 12-core or targeted biopsy.
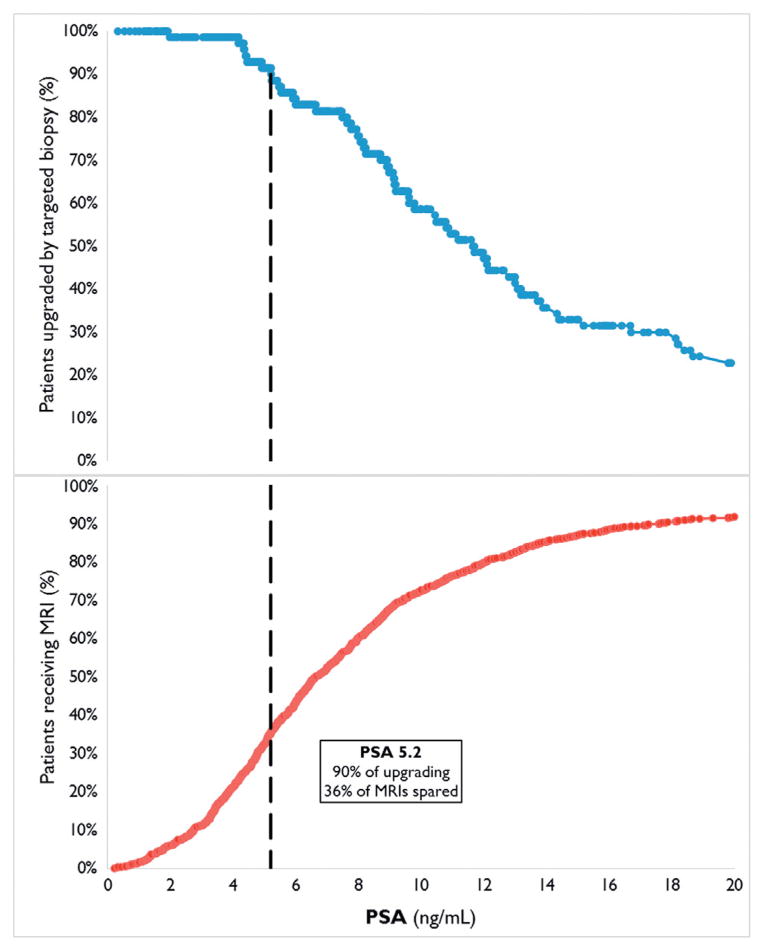
Of the cases upgraded to clinically significant disease by targeted biopsy we determined the proportion with PSA at or above each cutoff value (fig. 4). This was related to the cumulative proportion of PSA values in all men who underwent MP-MRI (fig. 4). In the study population 90% of tumors upgraded to clinically significant disease by targeted biopsy occurred at PSA 5.2 ng/ml or greater. Of all patients who underwent MP-MRI 36% had PSA at or below this threshold value. Accordingly if only men with PSA at or greater than the threshold of 5.2 ng/ml had been imaged by MP-MRI, only 64% of the total cohort would have needed to be imaged to capture 90% of those who would have benefited from the improved detection of clinically significant PCa by MRI/US fusion guided biopsy. Table 3 shows a range of upgrading rates as a function of PSA and the corresponding percent of patients who could potentially have been spared MP-MRI if that threshold value had been used.
Figure 4.
Cumulative PSA in men with upgraded clinically significant disease by targeted biopsy and men who underwent MP-MRI.
Table 3.
Cumulative incidence of patients with disease upgraded by targeted biopsy and those spared MP-MRI by PSA
| % Upgraded | % Spared MP-MRI | PSA (ng/ml) |
|---|---|---|
| 95 | 25 | 4.5 |
| 90 | 36 | 5.2 |
| 85 | 43 | 6.0 |
| 80 | 57 | 7.6 |
| 75 | 61 | 8.2 |
| 70 | 67 | 8.9 |
The performance of targeted biopsy was further assessed in the biopsy naïve vs repeat biopsy setting. In this patient population 196 men had no history of prostate biopsy, of whom 124 were diagnosed with PCa. At PSA 6.5 ng/ml a 90% incidence of upgrading to clinically significant disease by targeted biopsy was detected, corresponding to 64% of patients who underwent MP-MRI. In contrast, 807 men underwent at least 1 previous prostate biopsy before study enrollment, of whom 440 were diagnosed with PCa. At PSA 5.2 ng/ml 90% of upgrading to clinically significant disease by targeted biopsy was detected, corresponding to 36% of patients who underwent MP-MRI.
Potential predictors of upgrading to clinically significant disease by targeted biopsy were assessed by univariate analysis. The number of biopsy cores per patient, presence of anterior lesions, prior negative biopsy, MP-MRI suspicion score and PSA cutoff were significantly associated with upgrading by targeted biopsy. After incorporation into a multivariate logistic regression model the PSA cutoff remained a significant predictor of upgrading after adjusting for the potentially confounding variables (table 4).
Table 4.
Univariate and multivariate analyses of parameters predicting Gleason score upgrading to clinically significant disease by targeted biopsy over 12-core biopsy
| OR (95% CI) | p Value | |
|---|---|---|
| Univariate: | ||
| PSA cutoff/increment | 3.3 (2.2–5.2) | <0.0001 |
| MRI anterior lesions (yes/no) | 3.3 (1.9–5.8) | <0.0001 |
| MRI suspicion score/suspicion increase | 1.9 (1.1–3.3) | 0.02 |
| Prior neg biopsies (yes/no) | 1.9 (1.0–3.5) | 0.04 |
| No. total biopsy cores/core | 1.1 (1.0–1.2) | 0.02 |
| Age/10 yrs* | 1.4 (0.98–1.9) | 0.06 |
| MRI prostate vol/10 ml* | 1.1 (0.85–1.0) | 0.3 |
| Multivariate: | ||
| PSA cutoff/increment | 3.4 (2.1–6.0) | <0.0001 |
| MRI anterior lesions (yes/no) | 2.6 (1.4–5.1) | 0.003 |
| MRI suspicion score/suspicion increase | 1.1 (0.61–2.1) | 0.7 |
| Prior neg biopsies (yes/no) | 1.5 (0.62–2.5) | 0.3 |
| No. total biopsy cores/core | 1.1 (0.93–1.2) | 0.4 |
Not predictors on multivariate analysis.
DISCUSSION
While no single PSA value predicts a zero risk of PCa, the decision to perform prostate biopsy is often based on PSA level.20 Several cutoffs have been proposed but they show poor sensitivity or specificity. For instance, at a PSA cutoff of 4.1 ng/ml 75% of men with biopsy detectable cancer and almost 60% with aggressive disease are missed by standard template biopsy.14 MP-MRI has a high negative predictive value for cancer at PSA 2.5 to 10 ng/ml but the performance of MRI/US fusion guided biopsy to discriminate clinically indolent from aggressive disease across a PSA range is unknown. 21 Identifying values above which the detection of clinically significant disease is maximal may be useful to avoid potentially unnecessary and costly MP-MRI and targeted biopsy. In this study patients who underwent targeted plus 12-core TRUS biopsies were stratified by PSA to determine a threshold for performing MP-MRI and fusion biopsy.
Targeted biopsy resulted in more upgrading to Gleason score 4 + 3 or greater with PSA greater than 4 ng/ml while most cancers missed or under graded by targeted biopsy were Gleason score 3 + 4 or less by 12-core biopsy. The PSA cutoff value was a significant predictor of upgrading to clinically significant disease by targeted biopsy. It remained a significant predictor after controlling for variables that differed between cutoffs, including increasing prostate volume and the number of anterior lesions on MP-MRI. In our analysis of the detection of clinically significant disease across the entire PSA range of the cohort we found that 90% of all upgrading to clinically significant disease by targeted biopsy over 12-core biopsy was captured by performing imaging and targeted biopsy in men with PSA above a 5.2 ng/ml cutoff. At this cutoff 361 men, representing 36% of the total cohort, would have been spared targeted biopsy or possibly even re-imaging.
Together these data suggest that MRI/US fusion guided prostate biopsy may not be necessary in patients with total PSA less than 5.2 ng/ml because clinically significant disease was unlikely to be detected at lower levels. Above this threshold in tandem with increasing PSA targeted biopsy diagnosed clinically significant high grade disease at a much higher rate than conventional template biopsies. However, the 5.2 ng/ml cutoff is an estimate of the optimal threshold. PSA cutoffs in the range of 4.5 to 8.9 ng/ml would have avoided MP-MRI in 25% to 67% of the men in our population. Notably most additional cancers detected by 12-core TRUS biopsy vs targeted biopsy consisted largely of clinically insignificant disease. This detection of potentially indolent disease was greater than that of targeted biopsy overall.
In a minority of patients 12-core biopsy detected clinically significant disease in those in whom targeted biopsy missed or under graded cancer. It was unclear whether this was due to missed lesions on MP-MRI or to biopsy technique vs an error in image registration or tracking.10,22 Additional studies are necessary to evaluate whether targeted biopsy is most useful as an adjunctive strategy vs a replacement modality for 12-core biopsy. Prospective elucidation of the performance of 12-core vs targeted biopsy specifically in patients with PSA greater than 4 ng/ml is needed to determine disease upgrading.
In this study we evaluated all patients referred to our institution for targeted biopsy, including men with previously negative biopsy and increased PSA. Consequently our patient population may have been enriched in regard to overall cancer detection compared to the community, in addition to the incidence of anterior lesions, which may be under sampled by 12-core biopsy.23,24 Although on multivariate analysis we controlled for anterior lesions, whether patients had prior negative biopsies and the total number of biopsy cores, it is possible that the upgrading rates may reflect a higher risk population. Consequently the PSA cutoffs that we determined may not be universal due to selection bias. In particular thresholds for upgrading differed between the biopsy naïve and repeat biopsy subsets, suggesting that the role of targeted biopsy as an initial diagnostic strategy may remain to be elucidated.
A limitation of our analysis was that the final prostatectomy pathology was unavailable in most patients and, thus, verifying the actual disease burden was not possible. Comparisons of Gleason score between the 2 biopsy modalities studied may not have fully measured the significance of disease since core length may also be a valuable parameter, although the interpretation of core length in targeted biopsies is still controversial.17 Finally, patients without lesions visualized on MP-MRI were not included in study. It is conceivable that some men may have harbored PCa. However, we are not currently able to determine which cases would have been upgraded to clinically significant disease by 12-core biopsy.
CONCLUSIONS
Above a PSA threshold of 5.2 ng/ml most upgrading to clinically significant disease was achieved by targeted over 12-core biopsy. If this threshold had been implemented in the NCI population, 36% of men could have avoided MP-MRI because there was no significant benefit. Below this value 12-core and targeted biopsies detected similar rates of clinically significant disease, although 12-core biopsy detected more clinically insignificant cancer. Other thresholds within a PSA range of between 4 and 10 ng/ml resulted in different pairings of sensitivity for clinically significant disease and rates of men who avoided MP-MRI. Using an optimized threshold, targeted biopsy and by extension MP-MRI may not be necessary below PSA 5.2 ng/ml.
Acknowledgments
Supported by the National Institutes of Health Intramural Research Program, National Cancer Institute, Center for Cancer Research and the National Institutes of Health Medical Research Scholars Program (Pfizer, The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Mr. and Mrs. Joel S. Marcus, the Howard Hughes Medical Institute and other private donors).
Abbreviations and Acronyms
- MP-MRI
multiparametric magnetic resonance imaging
- MRI
magnetic resonance imaging
- PCa
prostate cancer
- PSA
prostate specific antigen
- TRUS
transrectal US
- US
ultrasound
Footnotes
Study received institutional review board approval.
References
- 1.Cohen MS, Hanley RS, Kurteva T, et al. Comparing the Gleason prostate biopsy and Gleason prostatectomy grading system: the Lahey Clinic Medical Center experience and an international meta-analysis. Eur Urol. 2008;54:371. doi: 10.1016/j.eururo.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 2.Conti SL, Dall’Era M, Fradet V, et al. Pathological outcomes of candidates for active surveillance of prostate cancer. J Urol. 2009;181:1628. doi: 10.1016/j.juro.2008.11.107. [DOI] [PubMed] [Google Scholar]
- 3.Tosoian JJ, Johnbull E, Trock BJ, et al. Pathological outcomes in men with low risk and very low risk prostate cancer: implications on the practice of active surveillance. J Urol. 2013;190:1218. doi: 10.1016/j.juro.2013.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawachi MH, Bahnson RR, Barry M, et al. NCCN clinical practice guidelines in oncology: prostate cancer early detection. J Natl Compr Canc Netw. 2010;8:240. doi: 10.6004/jnccn.2010.0016. [DOI] [PubMed] [Google Scholar]
- 5.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186:1818. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonn GA, Margolis DJ, Marks LS. Target detection: magnetic resonance imaging-ultrasound fusion-guided prostate biopsy. Urol Oncol. 2014;32:903. doi: 10.1016/j.urolonc.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rastinehad AR, Baccala AA, Jr, Chung PH, et al. D’Amico risk stratification correlates with degree of suspicion of prostate cancer on multiparametric magnetic resonance imaging. J Urol. 2011;185:815. doi: 10.1016/j.juro.2010.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol. 2013;190:1721. doi: 10.1016/j.juro.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64:713. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamatakis L, Siddiqui MM, Nix JW, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013;119:3359. doi: 10.1002/cncr.28216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logan JK, Rais-Bahrami S, Turkbey B, et al. Current status of magnetic resonance imaging (MRI) and ultrasonography fusion software platforms for guidance of prostate biopsies. BJU Int. 2013 doi: 10.1111/bju.12593. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroder FH, Roobol MJ. Defining the optimal prostate-specific antigen threshold for the diagnosis of prostate cancer. Curr Opin Urol. 2009;19:227. doi: 10.1097/MOU.0b013e328329a2d0. [DOI] [PubMed] [Google Scholar]
- 15.Turkbey B, Mani H, Aras O, et al. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology. 2013;268:144. doi: 10.1148/radiol.13121325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rastinehad AR, Turkbey B, Salami SS, et al. Improving detection of clinically significant prostate cancer: magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. J Urol. 2014;191:1749. doi: 10.1016/j.juro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol. 2013;64:544. doi: 10.1016/j.eururo.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 18.Stark JR, Perner S, Stampfer MJ, et al. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3? J Clin Oncol. 2009;27:3459. doi: 10.1200/JCO.2008.20.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makarov DV, Sanderson H, Partin AW, et al. Gleason score 7 prostate cancer on needle biopsy: is the prognostic difference in Gleason scores 4 + 3 and 3 + 4 independent of the number of involved cores? J Urol. 2002;167:2440. [PubMed] [Google Scholar]
- 20.Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Canc Inst. 2006;98:529. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 21.Girometti R, Bazzocchi M, Como G, et al. Negative predictive value for cancer in patients with “gray-zone” PSA level and prior negative biopsy: preliminary results with multiparametric 3.0 Tesla. MR J Magn Reson Imaging. 2012;36:943. doi: 10.1002/jmri.23703. [DOI] [PubMed] [Google Scholar]
- 22.Xu S, Kruecker J, Turkbey B, et al. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg. 2008;13:255. doi: 10.1080/10929080802364645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rais-Bahrami S, Siddiqui MM, Vourganti S, et al. Diagnostic value of biparametric MRI as an adjunct to PSA-based detection of prostate cancer in men without prior biopsies. BJU Int. 2014 doi: 10.1111/bju.12639. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volkin D, Turkbey B, Hoang AN, et al. Multi-parametric MRI and subsequent MR/ultrasound fusion-guided biopsy increase the detection of anteriorly located prostate cancers. BJU Int. 2014 doi: 10.1111/bju.12670. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]