Abstract
Interleukin-1 family members are central mediators of host defense. Here we show that the novel IL-1 family member, IL-36γ, was expressed during experimental colitis and human inflammatory bowel disease (IBD). In response to dextran sodium sulfate (DSS)-induced damage, germ-free (GF) mice failed to induce IL-36γ, suggesting that gut microbiota are involved in its induction. Surprisingly, IL-36R-deficient (Il1rl2−/−) mice exhibited defective recovery following DSS-induced damage and impaired closure of colonic mucosal biopsy wounds, which coincided with impaired neutrophil accumulation in the wound bed. Failure of Il1rl2−/− mice to recover from DSS-induced damage was associated with a profound reduction in IL-22 expression, particularly by colonic neutrophils. Defective recovery of Il1rl2−/− mice could be rescued an aryl hydrocarbon receptor (AhR) agonist, which was sufficient to restore IL-22 expression and promote full recovery from DSS-induced damage. These findings implicate the IL-36/IL-36R axis in the resolution of intestinal mucosal wounds.
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC), the two major clinical phenotypes of IBD, are associated with dysregulated innate and adaptive immune responses towards gut microbiota (1). Members of the IL-1 family of cytokines are up-regulated in the inflamed mucosa during experimental colitis and human IBD and contribute to intestinal inflammation (2). Interestingly however, mice deficient in IL-1β or IL-18, or components of their processing, are more susceptible to DSS-induced colitis (3–6) and polymorphisms leading to decreased Nlrp3 expression in humans are associated with increased risk for developing CD (7). Thus, IL-1 family members may contribute not only to pro-inflammatory responses, but also resolution of inflammation (2, 8, 9).
Recently, information on the role of IL-36 family members, which are members of the IL-1 superfamily, has begun to emerge (10, 11). However, little data exists about the function of the IL-36/IL-36R axis in the intestine. Here we demonstrate that IL-36γ was induced during intestinal injury/inflammation and that IL-36R influenced neutrophil accumulation, IL-22 production, and repair of intestinal damage following injury. Treatment with an AhR agonist was sufficient to induce intestinal IL-22 expression and promote recovery in Il1rl2−/− mice. Collectively, these findings support a novel contribution of the IL-36/IL-36R axis in repair of damaged intestinal mucosa.
Materials and Methods
Mice
Wild-type (WT) C57BL/6 and Rag1−/− mice were from The Jackson Laboratory. Il1rl2−/− mice were from Amgen. GF mice were maintained as previously described (12). Protocols were approved by the Georgia State University Institutional Animal Care and Use Committee.
Reagents
Antibodies were from eBioscience except for CD45, CD103, CD4 (Becton Dickinson). Dead cells were identified using the Aqua dead cell staining kit (Invitrogen). Murine IL-36γ was from R&D. ELISAs for IL-36γ (antibodies-online.com) and IL-22, CXCL1, and CXCL2 (eBioscience) were performed following the manufacturer’s instructions.
Isolation of colonic lamina propria (cLP) cells and flow cytometry
Isolation of cLP cells, staining, and analyses were performed as described (13). cLP cells were defined as: CD45+IAb+CD11b+F4/80+CD103-macrophages (Mϕ), CD45+IAb+CD11c+F4/80-CD103+ dendritic cells (DC); Lin-CD90+RORgt+NKp46+CD117intCD127+ innate lymphoid cells (ILC), CD45+CD11b+Ly6C+Ly6G+ cells neutrophils (Nϕ).
Microarray analysis
Microarray processing was done by Vanderbilt Microarray Shared Resource using Murine Genome 430 2.0 microarray chips (Affymetrix). GEO accession number is GSE68269 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=olgvmwkanfgzlcd&acc=GSE68269).
Real-time PCR
RNA isolation and first-strand cDNA synthesis were performed as previously described (13). Primers were as previously described (14) except for: mIl1f9 (F, TTGACTTGGACCAGCAGGTGTG; R, GGGTACTTGCATGGGAGGATAG), hIl1f9 (F, GTCTATCAATCAATGTGTAAACC; R, ATCTTCTGCTCTTTTAGCTGCAAT). cDNA from human IBD tissues were obtained in plate arrays (OriGene).
In vitro stimulation
Bone marrow derived macrophages (1×106 cells/ml) were stimulated with TLR agonists for 12 h.
DSS-induced colitis
Mice were provided 3% (wt/vol) DSS (MP Biomedicals) in the drinking water for 5 d and then switched to normal drinking water for recovery. Daily clinical assessment of DSS-treated animals was performed as described previously (13).
CD4+CD45RBhi-induced colitis
Purified WT CD4+CD45RBhi T cells (4×105) were injected i.p. into Rag1−/− recipients. Colons were harvested when clinical signs of chronic colitis were evident (4 wk).
Helicobacter hepaticus-induced colitis
Helicobater hepaticus (1 X 108 CFU strain 51449; ATCC) was gavaged to mice on 0, 2, 4 d. Mice also received 1mg of anti-IL-10R (BioXCell; 1B1.2) antibody via i.p. injection on 0, 7, 14, 21 d after H. hepaticus infection (15).
Wound healing assays
Mucosal wound healing assays were performed as previously described (16).
In vivo AhR treatment
FICZ (Enzo Life Sciences; 1μg/mouse) was injected i.p. at 3 d of DSS treatment for 5 d.
Histology
Colons were fixed in 10% formalin. Paraffin embedding, sectioning, Hematoxylin/Eosin staining, and slide scanning was performed at the Emory’s WCI-Pathology Core.
Statistical analyses
Statistical analyses were performed using unpaired Student’s t test or one-way ANOVA.
Results and Discussion
IL-36γ is expressed during experimental colitis and human IBD
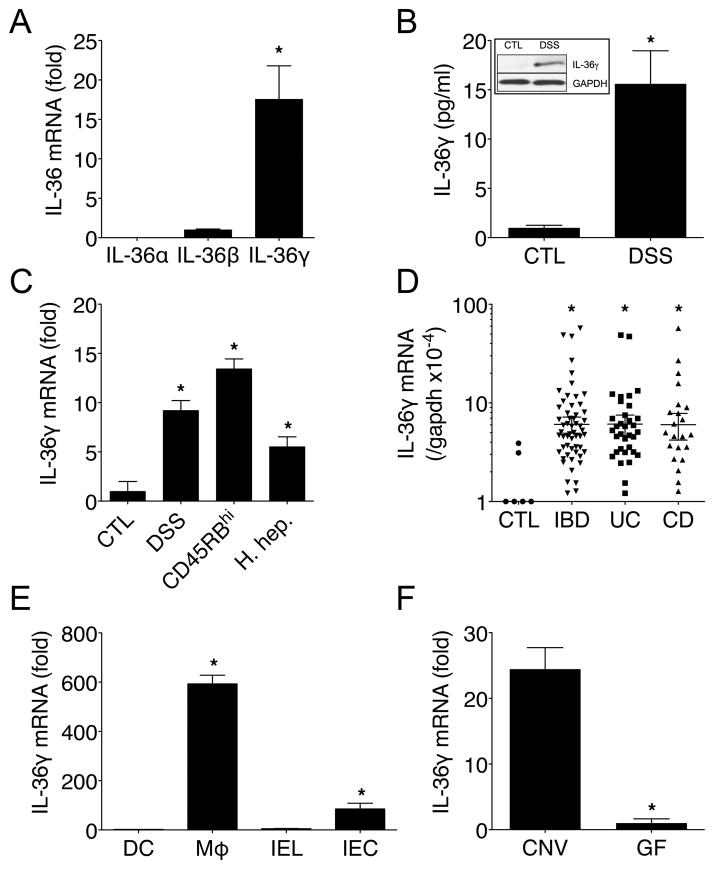
Resident macrophages (Cx3cr1hiLy6C−) are abundant in the healthy cLP, while a distinct subset of Cx3cr1low/intLy6C+ cells infiltrate the colon after DSS treatment ((17) and Supplemental Fig. 1A), which are predominantly inflammatory macrophages (Mϕ). To identify genes that are preferentially expressed by these inflammatory Mϕ, we conducted microarray analysis between FACS-sorted CD45+IAb+CD11b+F4/80+ cLP Mϕ isolated from healthy mice (>80% Cx3cr1hiLy6C− “resident”) or 5 d DSS-treated mice (>50% Cx3cr1lo/intLy6C+ “infiltrating”). Using hierarchical clustering, 820 genes were differentially expressed > 2-fold (log2 scale) between cLP Mϕ isolated from healthy control (CTL) or inflamed (DSS) colon (Supplemental Fig. 1B middle panel). Among these genes, the gene ontology (GO) processes enriched by DAVID showed Immune response as top ranked process. Of the 62 genes in this category, the top 10 (fold change) are shown. Interestingly, the novel IL-1 family member Il1f9 (renamed IL-36γ was the top-ranked gene that was most preferentially expressed by cLP Mϕ isolated from DSS-treated mice (5.4-fold increase log2 scale) while other members of the IL-1 family only showed modest increases or remained unchanged (data not shown). Validation of these observations by qPCR confirmed that IL-36γ mRNA, but not IL-36α or IL-36β, was highly expressed in the inflamed colonic of DSS-treated mice (Fig. 1A), and correspondingly, protein levels were also increased as detected by western blot (WB) and ELISA (Fig. 1B). This increased IL-36γ expression (~9-fold) in the inflamed colon following DSS treatment was recapitulated in additional models of colitis including the CD45RBhi transfer model (~13-fold), and the Helicobacter hepaticus model (15) (~5-fold) (Fig. 1C). Importantly, IL-36γ mRNA expression was also increased in the human colonic mucosa from individuals with IBD when compared to CTL, and no significant differences were noted between UC and CD samples (Fig. 1D).
FIGURE 1.
IL-36γ is expressed during experimental colitis and human IBD. (A) IL-36γ, IL-36γ, and IL-36γ mRNA expression in colons of WT mice treated for 5 d with DSS. (B) ELISA and WB analyses of IL-36γ in colons of WT mice treated as in (A). (C) IL-36γ mRNA expression in colonic tissue from CTL, DSS (5 d), CD45RBhi, and H. hepaticus models of colitis. (D) IL-36γ mRNA expression in CTL, IBD, UC, or CD human mucosa samples. (E) IL-36γ mRNA expression from cLP DC, Mϕ, IEL, and IEC from WT mice treated as in (A). (F) IL-36γ mRNA expression in colons of WT mice housed under CNV or GF conditions and treated as in (A). Data are representative of two or three independent experiments with four or five mice per group. *P < 0.05.
To investigate cellular sources of IL-36γ in the inflamed intestine, IL-36γ mRNA expression was analyzed among cLP DCs, Mϕ, intestinal epithelial lymphocytes (IEL), and intestinal epithelial cell (IEC). cLP Mϕ and IECs expressed ~600-fold and ~85-fold higher levels of IL-36γ, respectively, as compared to DCs or IEL (Fig. 1E). As DSS treatment disrupts the epithelial barrier and exposes immune cells to microbes, the role of the microbiota in IL-36γ induction was explored. Conventionally-housed (CNV) or GF mice were treated with DSS for 5 d and IL-36γ mRNA expression was assessed. As shown in Fig. 1F, colonic tissue from GF mice expressed ~25-fold lower levels of IL-36γ mRNA than CNV mice. Consistent with this observation, stimulation of bone marrow-derived macrophages with LPS or CpG significantly induced IL-36γ mRNA expression (Supplemental Fig. 1C).
IL-36R contributes to colonic wound healing
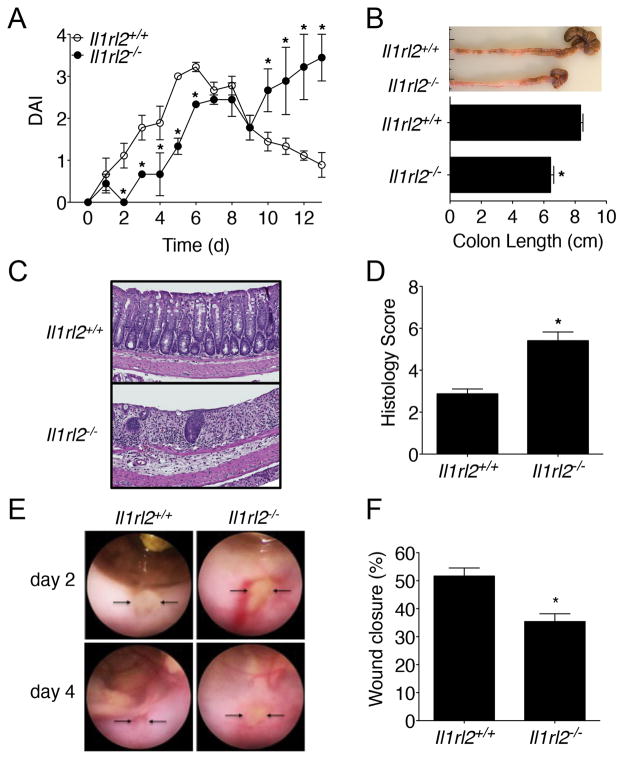
To evaluate the biological functions of IL-36γ in vivo, Il1rl2−/− mice were treated with DSS for 5 d and the severity of colonic inflammation was assessed. Consistent with a pro-inflammatory role for the IL-36 pathway (10), we observed modestly decreased DAI in Il1rl2−/− mice when compared to WT (Il1rl2+/+) mice as early as 1 d of DSS treatment that remained until 5 d (Fig. 2A). To evaluate recovery and repair from acute mucosal injury, DSS was replaced with normal water at 5 d and a progressive increase in the DAI for Il1rl2−/− mice was observed, which eventually matched that of Il1rl2+/+ mice on 7 d (Fig. 2A). While Il1rl2+/+ mice eventually recovered from colitis, the DAI of Il1rl2−/− mice continued to increase until they had to be euthanized due to being moribund and having met humane endpoint criteria. Examination of Il1rl2−/− colons at sacrifice revealed significant shortening compared to Il1rl2+/+ colons (Fig. 2B). Additionally, histological analyses of Il1rl2−/− colons showed significantly enhanced mucosal damage/inflammation as compared to Il1rl2+/+ colons (Fig. 2C, 2D). Since Il1rl2−/− mice failed to recover from DSS-induced colitis, the role of IL-36R in colonic wound recovery was further examined by mechanically generating mucosal wounds using an endoscope equipped with biopsy forceps. Images captured from the wound sites were used to quantify colonic mucosal wound healing at 2 d and 4 d post-injury (Fig. 2E). Analogous to the defective repair observed with acute DSS treatment, Il1rl2−/− mice showed a significant delay in wound healing (35.1% wound closure compared with 51.7% in Il1rl2+/+ mice; Fig. 2F). Interestingly, impaired wound healing in Il1rl2−/− mice correlated with a significant reduction in neutrophil accumulation in the wound bed (Supplemental Fig. 2A, 2B). Since neutrophils can aid in wound repair (18), these cells were examined in the wound beds of Il1rl2+/+ and Il1rl2−/− mice following mucosal biopsy. As shown in Supplementary Fig. 2A, 2B, wound beds of from Il1rl2−/− mice exhibited a significant reduction in neutrophil accumulation as compared to Il1rl2−/− mice. Consistent with these observations, treatment of colonic explants or intestinal epithelial cell cultures with IL-36γ significantly induced expression of the neutrophil chemokines CXCL1 and CXCL2 (Supplemental Fig. 2C, 2D). These data were also supported by evidence showing that intestinal epithelial cells constitutively expressed Il1rl2 mRNA during the steady-state and following treatment of mice with DSS (data not shown). Collectively, these results suggest an important role for IL-36R in promoting wound healing of damaged intestinal mucosa.
FIGURE 2.
IL-36R contributes to colonic wound healing. (A) DAI of Il1rl2+/+ and Il1rl2−/− mice treated for 5 d with DSS followed by normal water for 8 d. (B) Image and length of Il1rl2+/+ and Il1rl2−/− colons from mice treated as in (A) at 13 d. (C) H&E staining and (D) histology scoring of colon sections from Il1rl2+/+ and Il1rl2−/− mice treated as in (A) at 13 d. (E) Endoscopic images at 2 d and 4 d and (F) Quantification at 4 d of wound repair in Il1rl2+/+ and Il1rl2−/− mice after biopsy-induced injury. Arrows indicate the wound bed. Data are representative of three independent experiments with five mice per group. *P < 0.05.
IL-36R contributes to DSS-induced IL-22 production
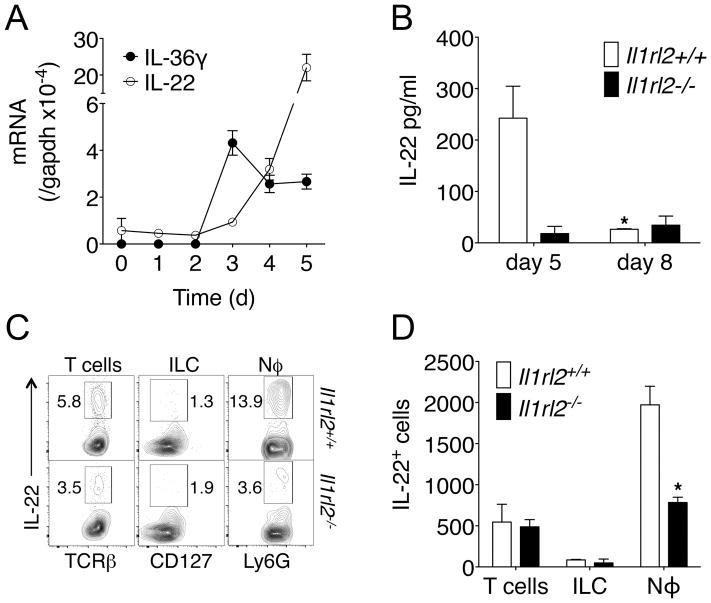
IL-22 is a barrier-protective cytokine that stimulates epithelial proliferation and restitution, the secretion of antimicrobial peptides, and protection from intestinal inflammation (19). Since Il1rl2−/− mice displayed impaired recovery from DSS-induced damage, the role of IL-36R in IL-22-mediated intestinal barrier protection was investigated. Interestingly, upon DSS treatment IL-36γ expression preceded that of IL-22 with IL-36γ peaking on 3 d followed by IL-22 beginning on 4 d and peaking on 5 d (Fig. 3A). These data suggested that IL-36γ may regulate IL-22 production following DSS-induced damage. Therefore, IL-22 expression in the colon of Il1rl2+/+ and Il1rl2−/− mice following DSS treatment was quantitated. After 5 d of DSS treatment, Il1rl2−/− mice had significantly reduced IL-22 protein expression when compared to Il1rl2+/+ mice (Fig. 3B). IL-22 protein expression in Il1rl2+/+ colons returned to baseline by 8 d as these mice repaired intestinal damage, while Il1rl2−/− mice failed to induce IL-22 at 8 d as intestinal damaged progressed. Interestingly, robust IL-22 expression in Il1rl2+/+ colons following DSS was abolished in GF mice, similar to IL-36γ expression (data not shown). These data demonstrate that the IL-36 pathway is involved in DSS-induced IL-22 expression.
FIGURE 3.
IL-36R contributes to DSS-induced IL-22 production. (A) Time course of IL-36γ and IL-22 mRNA expression in colons of WT mice treated for 5 d with DSS. (B) ELISA of IL-22 in colons of Il1rl2+/+ and Il1rl2−/− mice treated for 5 d or 8 d with DSS. (C) FACS plots and (D) absolute cell numbers of IL-22-producing colonic T cells, ILCs and Nϕ in the colon of Il1rl2+/+ or Il1rl2−/− mice treated as in (A). Data are representative of two or three independent experiments with four or five mice per group. *P < 0.05.
In the DSS model, IL-22-producing neutrophils provide a major contribution to the resolution of colonic injury (14). Thus, we explored whether there may be defects in IL-22-producing neutrophils following DSS treatment in the absence of IL-36R. Indeed, a significant decrease in IL-22 production by neutrophils from Il1rl2−/− mice was observed when compared to Il1rl2+/+ mice for both frequency (Fig. 3C) and absolute cell number (Fig. 3D), while T cells and ILC did not exhibit significant differences. These data are consistent with the observations of Zindl et al (14), showing that IL-22-producing neutrophils are important contributors to resolution of DSS-induced colonic damage and suggest that IL-36R is involved in the differentiation of this important cell type. Of note, very low levels of IL-22 were detected in Il1rl2+/+ and Il1rl2−/− wound beds following mucosal biopsy (data not shown), suggesting that the extent and type of injury dictates whether the IL-36 pathway may predominantly control neutrophil recruitment and/or differentiation into IL-22 producers.
FICZ induces IL-22 and resolution of colonic damage in Il1rl2−/− mice
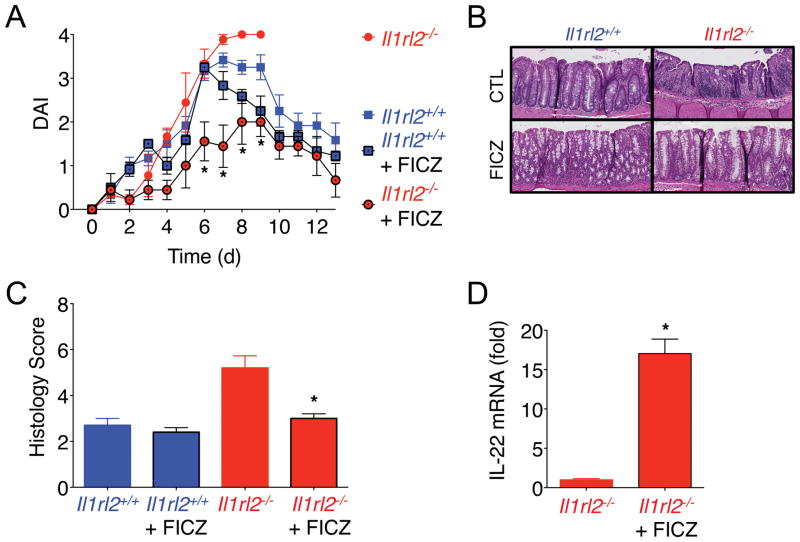
Since IL-22-producing neutrophils were significantly reduced in colons of DSS-treated Il1rl2−/− mice (Fig. 3C/D), we next investigated whether boosting IL-22 production from other cellular sources in vivo could complement this defect. The AhR pathway has been shown to induce IL-22 production from CD4+ T cells and ILC3s in the intestine (20, 21), therefore, we treated Il1rl2−/− mice with the AhR agonist 6-formylindolo(3, 2-b) carbazole (FICZ) during the course of DSS treatment beginning at 3 d. While Il1rl2−/− mice failed to recover from DSS-induced intestinal mucosal damage and had to be euthanized consistent with Fig. 2A and 2B, FICZ treated Il1rl2−/− mice showed a significant reduction in DAI (Fig. 4A) and histological damage (Fig. 4B, 4C), and were able to fully recover from intestinal damage similar to Il1rl2+/+ mice or Il1rl2+/+ mice treated with FICZ. Importantly, FICZ treatment enhanced IL-22 mRNA expression in Il1rl2−/− mice during DSS-induced damage (Fig. 4D), however, we do not exclude the possibility that FICZ mediated protective effects independent of IL-22 induction.
FIGURE 4.
FICZ induces IL-22 and resolution of colonic damage in Il1rl2−/− mice. (A) DAI of Il1rl2+/+ (blue) and Il1rl2−/− (red) mice treated for 5 d with DSS followed by normal water for 8 days, in the presence (black line) or absence (no line) of FICZ. (B) H&E staining and (C) histology scoring of colon sections of Il1rl2+/+ and Il1rl2−/− mice treated as in (A). (D) IL-22 mRNA expression in colons from DSS-treated Il1rl2−/− mice in the presence or absence of FICZ (5 d). Data are representative of three independent experiments with five mice per group. *P < 0.05.
Collectively, these data implicate the IL-36/IL-36R axis in the repair of intestinal mucosal wounds. While the IL-36 pathway plays a pathogenic role in chronic psoriatic disorders (10), it is beneficial during acute intestinal damage. Neutrophils may play a central role in IL-36-mediated resolution of intestinal damage by phagocytosing and killing bacteria and/or producing IL-22, and other pro-resolving factors (22), that contribute to epithelial barrier repair and control of inflammation. Future insight into the functions of the IL-36 pathway during acute and chronic mucosal inflammation may contribute to the development of novel therapeutic strategies aimed at manipulating this cytokine axis.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health DK097256 (T.L.D.), DK72564, DK79392, DK61739 (C.A.P.), DK055679, DK059888 (A.N.) and Crohn’s and Colitis Foundation of America (O.M.-C. and B.C.).
Abbreviations
- CD
Crohn’s disease
- cLP
colonic lamina propria
- CTL
healthy control
- CNV
conventionally-housed
- DAI
disease activity index
- DC
dendritic cell
- DSS
dextran sodium sulfate
- FICZ
6-formylindolo (3, 2-b) carbazole
- GF
germ-free
- GO
gene ontology
- IBD
inflammatory bowel disease
- IEC
intestinal epithelial cell
- ILC
innate lymphoid cells
- LI
large intestine
- Mϕ
macrophages
- Nϕ
neutrophils
- UC
ulcerative colitis
- WB
western blot
- WT
wild-type
References
- 1.Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamias G, Corridoni D, Pizarro TT, Cominelli F. New insights into the dichotomous role of innate cytokines in gut homeostasis and inflammation. Cytokine. 2012;59:451–459. doi: 10.1016/j.cyto.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takagi H, Kanai T, Okazawa A, Kishi Y, Sato T, Takaishi H, Inoue N, Ogata H, Iwao Y, Hoshino K, Takeda K, Akira S, Watanabe M, Ishii H, Hibi T. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol. 2003;38:837–844. doi: 10.1080/00365520310004047. [DOI] [PubMed] [Google Scholar]
- 4.Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N, Vallance BA, Saleh M. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bersudsky M, Luski L, Fishman D, White RM, Ziv-Sokolovskaya N, Dotan S, Rider P, Kaplanov I, Aychek T, Dinarello CA, Apte RN, Voronov E. Non-redundant properties of IL-1alpha and IL-1beta during acute colon inflammation in mice. Gut. 2014;63:598–609. doi: 10.1136/gutjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- 7.Villani AC, Lemire M, Fortin G, Louis E, Silverberg MS, Collette C, Baba N, Libioulle C, Belaiche J, Bitton A, Gaudet D, Cohen A, Langelier D, Fortin PR, Wither JE, Sarfati M, Rutgeerts P, Rioux JD, Vermeire S, Hudson TJ, Franchimont D. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat Genet. 2009;41:71–76. doi: 10.1038/ng285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello CA. Overview of the interleukin-1 family of ligands and receptors. Semin Immunol. 2013;25:389–393. doi: 10.1016/j.smim.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 9.McNamee EN, Masterson JC, Jedlicka P, McManus M, Grenz A, Collins CB, Nold MF, Nold-Petry C, Bufler P, Dinarello CA, Rivera-Nieves J. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A. 2011;108:16711–16716. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabay C, Towne JE. Regulation and function of interleukin-36 cytokines in homeostasis and pathological conditions. J Leukoc Biol. 2015;97:645–652. doi: 10.1189/jlb.3RI1014-495R. [DOI] [PubMed] [Google Scholar]
- 11.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A, Parkos CA, Denning TL. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest. 2011;121:4787–4795. doi: 10.1172/JCI59150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zindl CL, Lai JF, Lee YK, Maynard CL, Harbour SN, Ouyang W, Chaplin DD, Weaver CT. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci U S A. 2013;110:12768–12773. doi: 10.1073/pnas.1300318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J, Hilgarth RS, Kundu K, Murthy N, Kusters D, Reutelingsperger C, Perretti M, Parkos CA, Neish AS, Nusrat A. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leoni G, Neumann PA, Sumagin R, Denning TL, Nusrat A. Wound repair: role of immune-epithelial interactions. Mucosal Immunol. 2015;8:959–968. doi: 10.1038/mi.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizoguchi A. Healing of intestinal inflammation by IL-22. Inflamm Bowel Dis. 2012;18:1777–1784. doi: 10.1002/ibd.22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, MacDonald TT, Pallone F, Monteleone G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–248. 248 e231. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.