Abstract
Objectives
Bipolar disorders’ (BD) onset before age 18 is a potential marker for a more severe illness course. Adolescence is also a period of significant normative maturation of inhibitory control and reward-relevant decision-making processes, such as decreased delay discounting (i.e., decreased preference for smaller, immediate versus larger, delayed rewards). Adults with BD exhibit elevated delay discounting rates. Very little is known about developmental changes in delay discounting in adolescents with BD, or about associations between inhibitory control and delay discounting in BD. The present study addresses these questions.
Methods
The sample included 78 participants, ages 13 to 23, with BD or without history of mental illness. Group differences and group by age interaction effects on delay discounting (32 BD, 32 controls with valid responses), probability discounting (34 BD, 37 controls) and inhibitory control indices (34 BD, 38 controls) were assessed.
Results
Among healthy controls, less discounting of delayed rewards was associated with older age, whereas adolescents with BD did not show age-related associations. There were no group differences in probability discounting or inhibitory control.
Limitations
The cross-sectional nature of the study cannot fully rule out the less likely interpretation of group differences in cohort effects.
Conclusions
The lack of age-related improvement in delay tolerance in BD suggests disrupted development of executive control processes within reward contexts, which in turn may contribute to understanding more severe course of pediatric onset BD. Longitudinal studies are needed to examine delay discounting in relation to maturation of neural reward systems among adolescents with BD.
Keywords: Bipolar Disorder, Adolescence, Delay Discounting, Reward
Introduction
Two separate constructs are proposed to explain “excessive involvement in pleasurable activities that have a high potential for painful consequences” (p. 362, DSM-IV-TR, 2000), which often accompany manic/hypomanic periods in bipolar disorders (BD). Hypersensitivity of the behavioral approach system (BAS), a system implicated in facilitating approach to rewards in the environment (Depue & Collins, 1999), could explain manic/hypomanic reward-related risk-taking and other bipolar symptoms (Depue & Iacono, 1989; Johnson et al., 2012; Urosevic et al., 2008). Alternatively, manic/hypomanic risk-taking may reflect a general/xref failure to inhibit behavioral responses regardless of the reward context (Swann, 2010). Supporting the latter hypothesis, high levels of trait impulsivity predict prospective first-time onset of mania (Alloy et al., 2012a).
Normative adolescence is also marked by high rates of engaging in reward-related risks, like substance use, risky sexual practices, and reckless driving (Eaton et al., 2006), which are attributed to both normative changes in BAS sensitivity and inhibitory control failures (e.g., Luciana et al., 2012). Empirical studies support normative increases in BAS sensitivity (Ernst et al., 2006; Urosevic et al., 2012; Wahlstrom et al., 2010). High levels of BAS activity during typical adolescence may interact with inefficiencies in prefrontal control (Luciana et al., 2012; Steinberg, 2010) to promote decisions based on immediate versus future goal-attainment. Consistently, there is evidence for greater intolerance for delayed rewards (i.e., delay discounting [DD]) in healthy adolescents and increased tolerance for these delays with maturation (Olson et al., 2007; Steinberg et al., 2009). Moreover, with maturation, healthy adolescents exhibit an increased ability to inhibit prepotent responses outside of reward contexts (Hooper et al., 2004). Compared to healthy adults, healthy adolescents exhibit weaker connectivity between frontal cortical regions involved in cognitive control (e.g., right inferior frontal gyrus) and other brain regions during an inhibitory control task (Hwang et al., 2010).
DD and inhibitory control are related but separate processes. Among healthy adolescents, the general ability to inhibit prepotent responses appears to be unrelated to disadvantageous decision-making within reward contexts (Hooper et al., 2004). Still, DD in particular is associated with inhibitory control in other studies of healthy adolescents (Olson et al., 2007). A recent meta-analysis of functional neuroimaging studies indicates distinct neural networks, but with some overlap in structures, implicated in DD versus response inhibition (Wesley & Bickel, 2014). There are no studies examining whether adolescents with BD are similar to healthy adolescents in their DD behaviors, inhibitory control, or in the age-related maturation of these skills. The present study addresses these questions.
The present study’s findings may be clinically significant for several reasons. Onset of BD in adolescence/childhood predicts poor prognosis—greater rates of comorbidity, suicidality, episode recurrence, and shorter euthymic periods (Lewinsohn et al., 1995; Perlis et al., 2004; Schuroff et al., 2000; Strober et al., 1995). Regardless of onset age, adolescent age predicts poorer functioning compared to younger ages in pediatric BD (Goldstein et al., 2009). Childhood/adolescent onset of BD is also more common than previously believed (Perlis et al., 2004; Van Meter et al., 2011). A recent longitudinal community study of 3,021 individuals found the first onset of mania to peak in the teens. The first onset of hypomania has two peaks—in early childhood and adolescence, and the first onset of major depression is between ages 12 to 25 (Beesdo et al., 2009). Deviations from typical patterns of reward responding and/or behavioral inhibition in adolescence may represent early indicators of BD vulnerability and/or BD course severity.
Little work has examined reward functioning in adolescents with BD, despite growing empirical support for the BAS/reward hypersensitivity model (Alloy et al., 2012a; Alloy et al., 2012b; Alloy et al., 2008; Johnson et al., 2000; Meyer et al., 2001; Nusslock et al., 2007; Salavert et al., 2007) and overall abnormalities in reward processing (Johnson et al., 2005; Murphy et al., 2001; Pizzagalli et al., 2008) in adult BD. There are deficits in learning reward contingencies in reversal learning tasks in studies combining children and adolescents with BD (Dickstein et al., 2004; Dickstein et al., 2010; Gorrindo et al., 2005). However, two studies failed to find deficits when examining decision-making with explicit reward contingencies (Rau et al., 2008), or with variable probabilities of monetary reward (Ernst et al., 2004) in pediatric BD. Additional data are needed to fully understand reward processing abnormalities in adolescents with BD.
DD tasks provide an opportunity to examine a specific aspect of reward processing—the tendency to prefer immediate rewards that stems from an inability/unwillingness to tolerate delayed reward delivery. The preferences for immediate rewards are suboptimal when immediacy comes at the cost of smaller rewards. In healthy adolescents, maturation-related changes in DD appear to be specific, since probability discounting (PD; preference for smaller but certain versus larger but uncertain, probabilistic rewards) does not show maturational changes (Olson et al., 2007; Scheres et al., 2006). PD predicts concurrent rates of externalizing behaviors in normative adolescence, whereas DD is related to executive functioning indices (Olson et al., 2007). Adult studies support psychological distinctions between these two discounting types (Green & Myerson, 2004). The assessment of both PD and DD may yield important insights regarding processes that contribute to BD.
Few studies have assessed DD rates in adults vulnerable to or with BD. In a study of young adults at risk for BD, hypomania-proneness was related to a greater discounting of delayed rewards in a two-choice task and greater N100 event-related amplitudes in response to immediate versus delayed rewards (Mason et al., 2012). In another study, adults with BD exhibited greater DD in a paradigm with hypothetical rewards, with discounting rates related to measures of trait impulsivity but not to current bipolar symptoms, psychotropic medications, or executive functioning (Ahn et al., 2011). In another study, adults at high-risk for BD exhibited delay aversion in a gambling task, but no motor response inhibition deficits, compared to controls (Wessa et al., 2015). Still, there are no examinations of whether DD in particular is related to deficits in response inhibition outside of reward contexts in BD.
Based on this past research on adult BD and normative adolescence, 1) we hypothesize that adolescents with BD will exhibit greater DD rates than healthy adolescents and a non-normative lack of age-related declines in DD. 2) In order to determine the specificity of DD abnormalities, we examined group differences and age associations in discounting of probabilistic rewards. Based on data from healthy adolescents (Olson et al., 2007), it is hypothesized that adolescents with BD will exhibit greater PD than healthy adolescents, but neither group will exhibit age-related decreases. 3) In order to determine whether differences in DD and PD are due to general inhibitory failures, we examined group differences and age-related associations with behavioral inhibition using a Go/NoGo task. We also examined relationships between DD, PD, and behavioral inhibition.
Methods
Participants
The present sample includes 78 participants, whose age range reflects the full range of BAS-relevant neurodevelopment (Sowell et al., 1999; Urosevic et al. 2012), recruited into two groups—participants with BD and participants without DSM-IV Axis I disorders. Table 1 provides the sample’s demographic and clinical information. Participants were recruited from the local community (flyers; volunteer participants database) and university-affiliated clinics (referrals, mailings to eligible families). Exclusion criteria were: history of neurological disorders; current major physical conditions; birth complications; history of extended loss of consciousness/severe head injury; IQ < 70; learning disabilities/severe developmental problems; uncorrected vision/hearing problems; learning English after age 5; and left-handedness (Oldfield, 1971), due to issues related to psychophysiological measures.
Table 1.
Demographic and Clinical Characteristics of the Bipolar Disorders (BD) and Control Groups’ Participants
| BD (n = 38) |
Control (n = 40) |
||
|---|---|---|---|
| Age range in years | 13.02 – 23.71 | 13.03 – 23.65 | |
| Age, M (SD) | 17.78 (3.20) | 17.06 (2.75) | |
| Male gender, n (%) | 23 (61%) | 25 (63%) | |
| Caucasian, n (%) | 27 (71%) | 30 (75%) | |
| SES, M (SD) | 46.12 (13.71) | 47.70 (10.66) | |
| IQ, M (SD) | 110.66 (14.44) | 115.88 (12.69) | |
| ISS Activation, M (SD)* | 158.95 (120.98) | 73.81 (64.54) | |
| ISS Depression, M (SD)* | 35.53 (32.44) | 10.00 (22.76) | |
| ISS Well Being | 185.79 (62.20) | 203.25 (54.46) | |
| ISS Perceived Conflict, M (SD)* | 111.18 (84.71) | 56.50 (56.41) | |
| BD I Dx, n (%) | 18 (47%) | -- | |
| BD II Dx, n (%) | 13 (34%) | -- | |
| BD NOS Dx, n (%) | 7 (18%) | -- | |
| BD Age of Onset | 9.61 (4.38) | -- | |
| Number of Lifetime Comorbid Dx, | 2.55 (1.84) | --a | |
| M (SD) | |||
| Any Substance Use Dx, n (%) | 19 (50%) | ||
| Any Anxiety Dx, n (%) | 18 (47%) | ||
| ADHD, n (%) | 10 (26%) | ||
| Enuresis, n (%) | 7 (18%) | ||
| Any Eating Dx, n (%) | 7 (18%) | ||
| ODD/Conduct Dx, n (%) | 6 (16%) | ||
| Current psychotherapy, n (%) | 19 (50%) | ||
| Current psychopharmacology, n (%) | 31 (82%) | -- | |
| Number of psychotropic | 2.26 (1.62) | -- | |
| medications, M (SD) | |||
| Antipsychotics, n (%) | 21 (55%) | -- | |
| Anticonvulsants, n (%) | 11 (29%) | -- | |
| Lithium, n (%) | 8 (21%) | -- | |
| Antidepressants, n (%) | 13 (34%) | -- | |
| ADHD medications, n (%) | 9 (24%) | -- | |
| Antianxiolytics, n (%) | 8 (21%) | -- | |
| Other (e.g., sleep), n (%) | 10 (26%) | -- | |
Note:p < .05.
One control participant had a history of enuresis in full remission.
ADHD = attention deficit/hyperactivity disorder; BD NOS= bipolar disorder not otherwise specified; ISS = Internal State Scale; SES = socio-economic status based on parental education and family income (Hollingshead, 1975). Non-Caucasian participants identified as African American (14.1%), multi-racial (6.4%), Asian-Pacific Islander (2.6%), Native American (2.6%), other (1.3%); additional 2.6% as Hispanic.
DSM-IV Axis I disorders were assessed with the Kiddie-SADS-Present and Lifetime Version (K-SADS-PL; Axelson et al., 2009; Kaufman et al., 1996) semi-structured interview. For minors, one interviewer interviewed a parent and another interviewed the minor. Adult participants (age > 18) provided all information themselves. A clinical psychologist (SU) conducted one interview for each participant and supervised consensus meetings where symptom ratings were derived based on all available information. A pediatric BD assessment expert (EAY) reviewed a subset of BD interviews (40%) for reliability purposes. Inter-rater reliability for K-SADS-PL symptom assessments was excellent (weighted kappa = .87).
In the BD group, participants with Bipolar Disorder NOS diagnoses were included. Each met all hypomanic episode criteria except for duration, along with a history of major depressive episodes, psychiatric hospitalizations, and prescribed mood stabilizers. BD NOS participants met all criteria for Bipolar II Disorder except that their hypomanic periods lasted 1–2 days, i.e., < four days. Individuals with BD NOS do not differ from individuals with more severe BD forms in their BD family history, comorbidity, age of onset, suicidality, illness duration, current symptom severity, or current functional impairment (Axelson et al., 2006; Hafeman et al., 2013). In a longitudinal study, 25% of youth with BD NOS/cyclothymia converted to BD I or BD II during a two-year interval (Birmaher et al., 2006), and 45% converted within five years (Axelson et al., 2011). In another longitudinal study, 56% of young adults with BD NOS/cyclothymia converted to BD I or BD II during a five-year interval (Alloy et al., 2012a). This evidence suggests continuity across bipolar diagnostic categories. Modal length of hypomanic periods is often 2 days (e.g., Angst et al., 2011; Youngstrom, 2009), which suggests that a large number of individuals would fit criteria for BD NOS as defined in the present study. In community studies, the majority of youth with bipolar symptoms fit BD NOS criteria (Lewinsohn et al., 1995), making this a particularly common pediatric BD diagnosis. For all these reasons, the full spectrum of BD was included in the present study. Still, it should be noted that the debate about the exact definitions of pediatric BD is ongoing (Leibenluft et al., 2011).
Based on K-SADS-PL assessments, 20 BD participants (53%) were euthymic (i.e., met DSM-IV mood episode full remission criteria in the last two months), while 9 (24%) met major depressive episode criteria, 6 (16%) met hypomanic episode criteria, and 3 (8%) met manic episode criteria within the last two months. No participants exhibited current psychotic symptoms at the time of interview.
Procedures
Participants and their parents/legal guardians provided written informed consent/assent prior to participation. The protocol was approved by the University of Minnesota’s Institutional Review Board. Participants completed two visits: one including a diagnostic interview, questionnaires, and intelligence testing; and a second including neurobehavioral testing and other assessments. This analysis focuses on age, intelligence (Wechsler, 1999; 2011), Delay/Probability Discounting Tasks (Olson et al., 2007; Richards et al., 1999), and a Response Inhibition Task (Hooper et al., 2004) measures. The latter two tasks were administered using E-Prime software (Psychology Software Tools, Inc.; Sharpsburg, PA).
Measures
Delay/Probability Discounting Tasks (Olson et al., 2007; Richards et al., 1999). Participants were told to choose carefully on each trial and that they would receive the amount of money provided by a randomly chosen response at task completion. In reality, this selection was constrained to immediate pay-off trials for pragmatic reasons. On DD trials, participants chose between a smaller, immediate reward and $10 after a delay (e.g., “Would you rather have $5 now or $10 in 10 days?”). On PD trials, participants chose between a smaller, certain reward and $10 with a variable probability (e.g., “Would you rather have $5 for sure or $10 with a 75% chance?”). Discounting was assessed at delays of 1, 2, 10, 30, 180, and 365 days, and probabilities of 25%, 50%, 75%, 90%, and 95%. DD and PD trials were randomly inter-mixed.
The amounts of smaller rewards, available immediately in DD trials, or with 100% certainty in PD trials, were based on the random adjustment procedure described previously (Richards et al., 1999). This procedure reduces the range of smaller monetary values based on the participant’s prior choices, converging on an indifference point for each delay interval and probability level. An indifference point is a subjective value of delayed or probabilistic $10, which is reflected by the participant’s choices across trials (e.g., based on DD trials’ choices, a participant may discount $10 after 10 days to a value of $9). A mean of 176.82 trials (SD = 30.14) was needed to calculate all indifference points.
For each participant, indifference points were plotted separately against delays versus probabilities. Based on prior studies of adolescents (Olson et al., 2007) using atheoretical models of discounting (Myerson et al., 2001), area under the curve (AUC) scores were calculated separately for delay versus probability discounting. Consistent with prior studies (e.g., Olson et al., 2007), participants with inconsistent responses (i.e., less than two decreases in indifference points and more than one increase as delays, or odds against, increased) were excluded from analyses. This procedure yielded 32 BD and 32 control group participants with valid DD responses and 34 BD and 37 control group participants with valid PD responses. There were no significant group differences in rates of inconsistent responses (ps > .63).
Response Inhibition Task (Hooper et al., 2004). Five blocks with 114 trials each were administered in the following order: 1) Go/NoGo, with instructions to respond to all letters except for X; 2) Target Detection, with instructions to respond only when the letter X appeared; 3) Response Shift, with instructions to press one button if X appeared and another button for any other letter; 4) a repeated Go/NoGo; and 5) a repeated Response Shift. The letter X appeared 25% of the time. When calculating outcome measures, the two Go/NoGo and Response Shift blocks were averaged.
The outcome measure assessing overall ability to discriminate go from no-go trials was d’ (i.e., z-score transformation of hit rate minus z-score transformation of false alarm rate, with adjustments for perfect score performance, see Stanislaw & Todorov, 1999), calculated separately for each condition. Follow-up analyses of significant group differences in d’ examined hit rates (i.e., number of correct go trials divided by total number of go trials) and false alarm rates (i.e., number of failed inhibition/no-go trials divided by the total number of no-go trials). Three participants (1 BD, 2 control) had incomplete data due to technical issues, while three additional BD participants failed to finish the task, yielding a total of 72 participants (34 BD; 38 control) with complete Response Inhibition task data. d’ measures approximated normal distributions, except for Target Detection d’ for which additional non-parametric tests are provided.
Internal State Scale (ISS; Bauer et al., 1991; 2000). The ISS is a self-report measure of current bipolar symptoms with four subscales: Activation, Well-Being, Perceived Conflict, and Depression. ISS Activation and Depression scores show strong convergent correlations with interview-based ratings of current mania and depression, respectively (Bauer et al., 1991), thus we have included them as continuous measures of current symptom severity.
Wechsler’s Abbreviated Scale of Intelligence’s (WASI; Wechsler, 1999; 2011) Vocabulary and Matrix Reasoning subtests were administered to assess general intellectual ability. There was a change part-way through the study from the WASI to WASI-II. In a validity study (Wechsler, 2011), Vocabulary and Matrix Reasoning subscale scores from the WASI and WASI-II were significantly correlated .88 and .71, respectively, suggesting that the two versions assess highly similar constructs.
Statistical Approach
A series of hierarchical regression analyses were conducted separately for DD AUCs, PD AUCs, and each measure of the Response Inhibition task. All analyses included: a mean-centered age predictor and a covariate of estimated full-scale IQ in Step 1; current clinical symptoms, as assessed by ISS Activation and Depression subscales, as covariates in Step 2; a dummy-coded group predictor in Step 3; and the interaction cross-product term for group by mean-centered age in Step 4. Supplemental Tables 1 and 2 present reduced models that do not account for current clinical state.
Significant group differences in DD or PD were followed up by regression analyses including the Go/NoGo block false alarm rate, as the best measure of response inhibition, to examine potential mediation effects.
Results
Preliminary Analyses
Table 1 provides demographic and clinical information and Table 2 behavioral tasks’ descriptive statistics. There were no group differences in demographic characteristics. The BD group exhibited higher current hypomania (ISS Activation Scale, t (76) = 3.91, p < .001) and higher current depression (ISS Depression Scale, t (76) = 4.04, p < .001) than the control group. Table 3 summarizes associations among DD, PD, and the Response Inhibition measures in the control and BD groups separately and for the full sample.
Table 2.
Descriptive Statistics for Delay and Probability Discounting and Response Inhibition Tasks
| BD Group | Control Group | |
|---|---|---|
| Delay and Probability Discounting Task | ||
| DD valid data, n | 32 | 32 |
| DD AUC, M (SD) | 0.43 (0.30) | 0.53 (0.28) |
| PD valid data, n | 34 | 37 |
| PD AUC, M (SD) | 0.42 (0.20) | 0.40 (0.17) |
| Response Inhibition Task | ||
| Valid data, n | 34 | 38 |
| Go/NoGo d’, M (SD) | 1.90 (1.18) | 2.27 (0.87) |
| Response Shift d’, M (SD) | 2.24 (0.88) | 2.30 (0.83) |
| Target Detection d’, M(SD)* | 3.60 (1.06) | 4.17 (0.58) |
Note: p < .05. d’ = measure of accurate performance for Response Inhibition task based on false alarm and hit rates
DD AUC= delay discounting area under the curve; PD AUC = probability discounting area under the curve. The smaller the AUC value, the greater the discounting behavior.
Table 3.
Associations between Delay Discounting, Probability Discounting, and Response Inhibition Task Performance
| BD Group | |||||
|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 5. | |
| 1. DD AUC | -- | .44* | .40* | .30 | .40* |
| 2. PD AUC | -- | −.18 | −.27 | .01 | |
| 3. d’ No Go Condition | -- | .78** | .69** | ||
| 4. d’ Shift Condition | -- | .66** | |||
| 5. d’ Target Condition | -- | ||||
| Control Group | |||||
| 1. DD AUC | -- | .28 | .25 | .21 | −.18 |
| 2. PD AUC | -- | −.02 | −.05 | .07 | |
| 3. d’ No Go Condition | -- | .65** | .66** | ||
| 4. d’ Shift Condition | -- | .38* | |||
| 5. d’ Target Condition | -- | ||||
| Whole Sample | |||||
| 1. DD AUC | -- | .33* | .37* | .27* | .25 |
| 2. PD AUC | -- | −.13 | −.17 | .00 | |
| 3. d’ NoGo Condition | -- | .71** | .69** | ||
| 4. d’ Shift Condition | -- | .52** | |||
| 5. d’ Target Condition | -- | ||||
Note: p < .05,
p < .001.
AUC, = area under the curve.BD = bipolar r disorders; DD, AUC = delay discounting area under the curve; PD AUC, = probability discounting area under the curve; larger AUC, values indicate less discounting behavior. Only participants with consistent PD or DD data used, resulting in slightly different n’s for each correlation.
BD participants currently taking psychotropic medications versus those who were unmedicated, and those currently in psychotherapy versus those who are not, did not significantly differ on any outcome measures. BD participants with BD I, BD II, versus BD NOS, also did not significantly differ on any outcome measures.
Group and Group by Age Effects on Delay Discounting
Table 4 presents hierarchical regression examining group and group by age interaction effects on DD (Hypothesis 1) for 32 BD and 32 control participants. In the Step 3 model without the interaction term, there was a non-significant trend for BD participants to exhibit smaller DD AUCs (i.e., steeper discounting) compared with control participants.
Table 4.
Hierarchical Regressions Examining Group Differences and Group by Age Interaction Effects on Delay and Probability Discounting
| Model | Adjusted R2 | F | p | Partial r | t | p |
|---|---|---|---|---|---|---|
| Delay Discounting | ||||||
| Step 1 Model | .10 | 4.50 | .015* | |||
| Age, mean-centered | .18 | 1.41 | ns | |||
| IQ | .24 | 1.96 | .055 | |||
| Step 2 Model | .19 | 4.74 | .002* | |||
| ISS Activation | −.30 | −2.42 | .019* | |||
| ISS Depression | .34 | 2.81 | .007* | |||
| Step 3 Model | .22 | 4.53 | .001* | |||
| Dx Group | −.22 | −1.74 | .088 | |||
| Step 4 Model | .26 | 4.73 | .001* | |||
| Age, mean-centered | .29 | 2.26 | .028* | |||
| IQ | .27 | 2.08 | .042* | |||
| ISS Activation | −.31 | −2.43 | .018* | |||
| ISS Depression | .40 | 3.30 | .002* | |||
| Dx Group | −.17 | −1.29 | ns | |||
| Age X Dx group | −.27 | −2.10 | .040* | |||
| Probability Discounting | ||||||
| Step 1 Model | −.03 | 0.03 | ns | |||
| Age, mean-centered | −.01 | −0.11 | ns | |||
| IQ | −.02 | −0.17 | ns | |||
| Step 2 Model | −.05 | 0.14 | ns | |||
| ISS Activation | .07 | 0.55 | ns | |||
| ISS Depression | .01 | 0.09 | ns | |||
| Step 3 Model | −.07 | 0.13 | ns | |||
| Dx Group | .04 | 0.30 | ns | |||
| Step 4/Full Model | −.07 | 0.22 | ns | |||
| Age, mean-centered | .05 | 0.42 | ns | |||
| IQ | .02 | 0.16 | ns | |||
| ISS Activation | .02 | 0.17 | ns | |||
| ISS Depression | .02 | 0.17 | ns | |||
| Dx Group | .06 | 0.48 | ns | |||
| Age X Dx group | −.11 | −0.85 | ns | |||
Note: ns = denotes a p < .10 and not statistically significant; all other p values are provided and denotes statistical significance, p < .05.
The Step 4/final model was significant, with unique effects of IQ and age—older and higher IQ participants discounted less. In addition, more severe current depression was related to lower DD, whereas more severe current hypomania was related to higher DD.
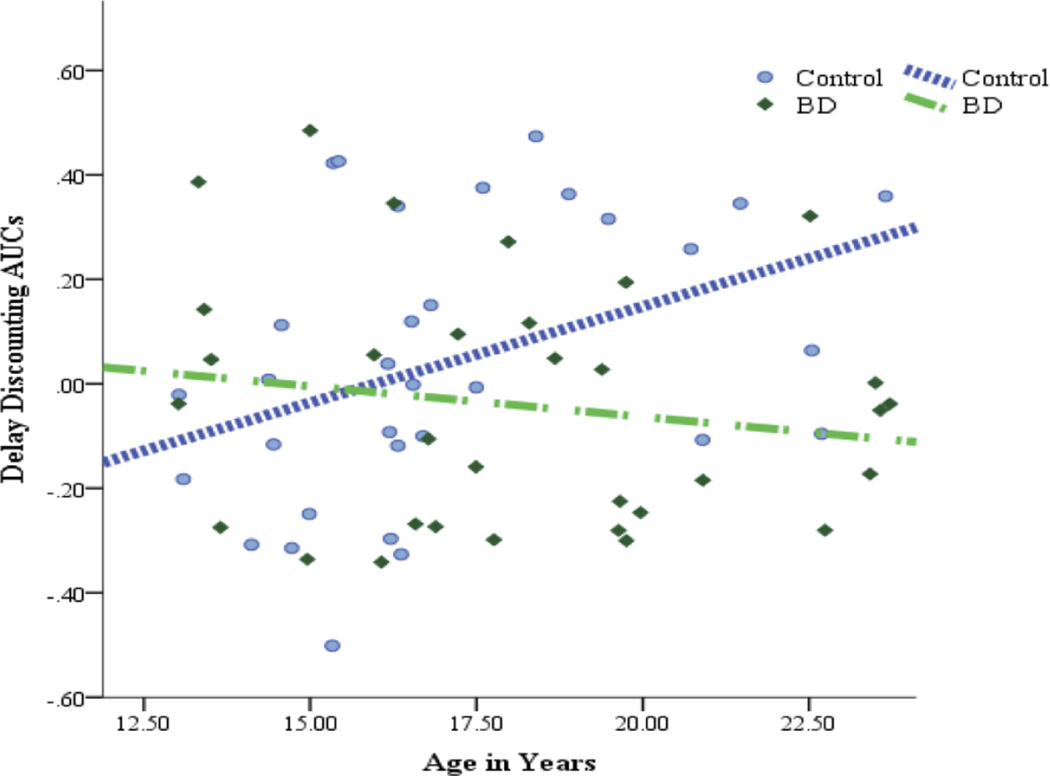
There was a significant group by age interaction. Follow-up partial correlations (see Figure 1), controlling for ISS scales and IQ, yielded a positive association between age and DD AUCs for the control group, partial r = .40, p = .030, with older control participants exhibiting less DD. In the BD group, there was no such relationship, partial r = −.11, p = .568. Repeated analysis with participants aged < 18 still yielded significant age by group interaction effect.
Figure 1.
Associations between Age and Delay Discounting in Adolescents with Bipolar Disorders and Healthy Adolescents. After controlling for IQ and ISS scales, healthy adolescents exhibit greater preference for larger but delayed rewards versus smaller but immediate rewards with older age, whereas adolescents with bipolar disorders did not. Depicted DD values reflect unstandardized residuals after regressing effects of IQ and ISS on these areas under the curve scores.
After controlling for age and IQ, two analyses comparing the BD group participants without (n = 14) versus those with lifetime SUD comorbidity (n = 18) and BD group participants without (n = 23) versus those with ADHD comorbidity (n = 9) found no significant differences.
Group and Group by Age Effects on Probability Discounting
When examining group and group by age interaction effects on PD AUCs (Hypothesis 2) for 34 BD and 37 control participants, there were no significant effects (Table 4).
Group and Group by Age Effects on Response Inhibition Task Performance
There were 34 BD and 38 control participants with complete Response Inhibition data in all analyses.
Go/NoGo Condition. In a hierarchical regression examining effects on d’ in the Go/NoGo condition (Hypothesis 3; see Supplemental Table 3), the Step 4/final model was significant, R2 = .53, F (6, 65) = 12.21, p < .001. There were significant effects of IQ, partial r = .47, t = 4.34, p < .001, and age, partial r = .31, t = 2.59, p = .012, with older and higher IQ participants being better at discriminating among cues during response inhibition. There were no significant group or group by age interaction effects.
Response Shift Condition. When examining effects on d’ in the Response Shift condition, the final model was significant, R2 = .42, F (6, 65) = 7.83, p < .001. There were significant effects of IQ, partial r = .33, t = 2.82, p = .010, and age, partial r = .26, t = 2.13, p = .037. Older participants and those with higher IQs were better at accurately switching between responses. There were no significant group or group by age interaction effects.
Target Detection Condition. When examining d’ in the Target Detection, the final model was significant, R2 = .40, F (6, 65) = 7.22, p < .001 with significant effects of IQ, partial r = .28, t = 2.33, p = .023. As above, participants with higher IQs performed better in their vigilance skills.
In the Step 3 model without the interaction term, there was a significant group effect on d’, change R2 = .08, change F (1, 66) = 8.10, p = .006, with the BD group performing worse than controls. Since some participants had perfect performance, Target Detection d’ measures were skewed. Non-parametric Mann-Whitney-U tests also yielded significant group differences in d’ with the BD group performing worse than controls, U = 437.50, p = .017, just as the parametric tests did. In follow-up analyses, the BD group exhibited significantly, change R2 = .055, change F (1, 66) = 4.76, p = .033, lower hit rates than controls (Mean = 0.92, Median = .98, SD = 0.12, and Mean = 0.96, Median = .98, SD = 0.04, respectively) and significantly, change R2 = .075, change F (1, 66) = 6.85, p = .011, higher false alarm rates than controls (Mean = 0.05, Median = 0.02, SD = 0.08, and Mean = 0.02, Median = .01, SD = 0.02, respectively).
In the Step 4/final model of the d’ analysis, there was a significant group by age interaction, change R2 = .053, change F (1, 65) = 5.79, p = .019 (Supplemental Figure 1). Follow-up hierarchical regression with the outcome variables of hit rates and false alarm rates yielded a significant group by age interaction only for hit rates, change R2 = .088, change F (1, 65) = 8.51, p = .005. In the BD group, after controlling for IQ and current symptoms, there was a positive association between age and hit rates, partial r = .41, p = .022. There was no such association for the control group, partial r = .05, p = .763.
After controlling for age and IQ, analyses comparing the BD group participants without (n = 15) versus those with lifetime SUD comorbidity (n = 19) and the BD group participants without (n = 25) versus those with ADHD comorbidity (n = 9) found no differences on Target Detection performance.
Does Response Inhibition Mediate Group Effects on Delay Discounting?
There was a significant association between Go/NoGo false alarm rate (i.e., response inhibition index) and DD AUC for the BD group, r = −.40, p = .029, but not for the control group, r = −.26, p = .171, and an inverse association with age for BD, r = −.57, p = .001, and control groups, r = −.42, p = .008. A hierarchical regression model with the Go/NoGo false alarm rate as an added predictor, along with age, IQ, and ISS scales, still yielded a significant group effect on DD, change R2 = .061, change F (1, 53) = 4.85, p = .032. The BD group exhibited steeper DD than controls. However, the group by age interaction effect was no longer significant.
In summary, there was a significant group by age interaction on DD with a moderate effect size (Cohen’s d = 0.56). There were no group effects on PD or response inhibition (i.e., Go/NoGo condition). Furthermore, response inhibition did not fully explain BD-specific abnormalities in DD.
Discussion
The present study examines two reward-relevant decision-making processes, i.e., discounting of delayed rewards and discounting of uncertain rewards, and response inhibition irrespective of reward, in adolescents with BD versus healthy adolescents. A strength of the study is that the participants’ ages encompass the full range of neurodevelopment to best capture changes in prefrontal cortical and striatal regions that underpin reward processing (Lebel & Beaulieu, 2011; Luna et al., 2013). Older healthy adolescents showed better ability to tolerate increasing delays for rewards, consistent with prior studies of normative adolescent development (Olson et al., 2007). No such positive correlation with chronological age was observed among adolescents with BD. These findings were unique to temporal discounting. There were no such effects on PD. Thus, age associations between groups on DD cannot be attributed to differences in overall risk-taking, since PD directly assesses responses to risk. The lack of significant differences in PD is consistent with prior work on pediatric BD (Ernst et al., 2004). To understand this pattern of findings, it is necessary to consider specific processes, and their neural correlates, assessed by the temporal discounting paradigm.
Neural substrates of DD are not fully understood. Some neuroimaging studies exploring neural substrates for DD implicate two separate neural systems: a system including ventral striatum and medial orbitofrontal cortex (OFC), for processing of immediate rewards, and a system including parietal regions, dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), and lateral OFC, for processing of delayed rewards (McClure et al., 2004). Other studies have posited a larger number of DD-relevant neural networks and more complex interconnection between them (Li et al., 2013). Still, some DD-relevant brain structures overlap with those implicated in the BAS dysregulation model of BD (Depue & Iacono, 1989).
There are no studies examining neural correlates of DD in BD. In a study using a different reward paradigm, adolescents with BD exhibited greater medial OFC activation during reward anticipation compared to healthy adolescents (Singh et al., 2013). During normative adolescence, developmental changes in ventromedial PFC and ventral striatum (Christakou et al., 2011), as well as in white matter organization in DLPFC and OFC (Olson et al., 2009), may play a role in age-related decreases in DD. Future neuroimaging studies will need to examine which DD neural correlates (e.g., medial OFC, ventral striatum, DLPFC, parietal cortical areas) are altered in adolescent BD. Moreover, understanding deviations in the maturation of DD networks is needed in adolescent BD.
Another important determination is whether increased DD in BD is driven by current clinical states. In the present study, current hypomanic/manic symptoms’ severity was associated with greater DD, whereas current depressive symptoms’ severity was associated with lesser DD. Still, the group differences in associations of DD with age were observed after controlling for current-state effects. These findings suggest that developmental abnormalities in DD represent features of the illness that may be independent of current state fluctuations. This is consistent with others studies reporting current-state independent abnormalities in reward responses (Di Nicola et al., 2013).
In a non-reward measure of inhibitory control, group differences emerged only during Target Detection performance. Adolescents with BD exhibited higher false alarms (i.e., failure to correctly inhibit responses) and lower hit rates (i.e., failures to correctly respond) compared to healthy adolescents. Unlike healthy adolescents, adolescents with BD exhibited improvement in hit rates with increasing age. However, most healthy adolescents were performing close to ceiling levels, limiting their ability to show age-related improvements. With this caveat in mind, the group differences in the Target Detection condition may reflect failures to maintain the current response set, which is consistent with other studies of pediatric BD (Joseph et al., 2008).
Importantly, there were no group differences in the ability to inhibit prepotent behavioral responses, as assessed by the Go/NoGo condition. This is inconsistent with some prior findings of response inhibition deficits in BD (Larson et al., 2005). However, a meta-analysis of response inhibition fMRI studies found no evidence for deficits in response inhibition during euthymia, despite the presence of hypoactivation of the right frontal gyrus involved in inhibitory control (Hajek et al., 2013). The same meta-analyses found response inhibition deficits during acute mania (Hajek et al., 2013). Since the present study statistically modeled the effects of current bipolar symptoms and had very few acutely manic participants, this may explain the overall lack of group differences. Finally, the present pattern of associations between DD and inhibitory control indices indicate that, while inhibitory control may contribute to some maturational deviations, it does not fully explain steeper DD in adolescent BD. Intriguingly, longitudinal epidemiological studies find decreases in rates of mania with increasing age (Cicero et al., 2009), consistent with the age-related maturation of these neural systems. Additional developmental studies are needed to further examine these associations.
Limitations
The present study is not without limitations. The cross-sectional study design prohibits untangling group differences in developmental trajectories from potential group differences in cohort effects. Although such cohort by diagnostic group interaction effects seem unlikely, future longitudinal studies of DD abnormalities in adolescent BD are needed. For ethical reasons, participants were not asked to refrain from their prescribed psychotropic medications. However, medicated versus non-medicated adolescents with BD did not differ on outcome measures of interest. Participants in the BD group were not excluded based on presence of lifetime SUD, ADHD, or other comorbidities. Given the extremely high comorbidity rates in BD (Goodwin & Jamison, 2007), and especially high comorbidity in pediatric onset BD (Kowatch et al., 2005), the current study’s permissive inclusion criteria likely resulted in a more clinically representative sample. Importantly, when analyses were repeated excluding participants with specific comorbidities (e.g., ADHD), the overall pattern of results was unchanged. The present sample is comparable to other studies of DD (e.g., Ahn et al., 2011) and of cognitive functioning in pediatric BD (e.g., Dickstein et al., 2010). However, it is not sufficiently large to fully examine the current state effects or potential differences among specific bipolar diagnostic categories. The present sample and cross-sectional study design also preclude determining the exact age of deviation from normative development in DD. Future longitudinal studies with larger samples will need to address these limitations. Finally, the present study only examined motor response inhibition and studies examining other types of disinhibition in BD are needed.
Summary
The present findings suggest that BD during adolescence is characterized by abnormal maturation of DD; adolescents with BD, unlike healthy adolescents, discount delayed rewards at a higher rate and fail to show expected improvements with older age. Importantly, these tendencies are not readily attributable to risk-taking tendencies, which did not vary between groups, nor are they attributable to deficits in motor control. Whether other forms of inhibition (e.g., at higher cognitive levels) account for this patterning is a question for future research. Future longitudinal studies might also assess how these DD tendencies are impacted by neurodevelopmental deviations in the reward and executive control systems. Future studies will need to determine the age at which DD development in BD starts to deviate from normative development, which may provide important clinical, age-sensitive tools for identifying adolescents at need of intervention. Future studies could also examine whether behavioral training in tolerating delayed reward delivery within experimental paradigms could lead to more controlled behavior in real-world settings and improved illness course for adolescents with BD.
Supplementary Material
Highlights.
We examined delay discounting in adolescents with and without bipolar disorders. With older age, healthy adolescents discounted delayed rewards less.
Adolescents with bipolar disorders did not show this age-related improvement.
There were no group differences in probability discounting or inhibitory control.
This suggests disrupted development of specific executive control processes in BD.
Acknowledgements
The authors would like to thank the University of Minnesota’s Center for Neurobehavioral Development for resources and support of the presented research; Elizabeth Olson, Ph.D., and Steve Malone, Ph.D., for their creation of the task scripts; and research participants and their families for participation in this research. Data collection, analysis and Snežana Urošević’s work on the manuscript was supported by National Institute of Mental Health Grants T32 MH 017069 and K01 093621. The funding agency did not influence study design, analyses, manuscript preparation, or publication process. Eric Youngstrom, Ph.D., has consulted with Pearson, Western Psychological Services, Otsuka, and Lundbeck about psychological assessment.
Abbreviations
- BAS
behavioral approach system
- BD
bipolar disorders
- DD
delay discounting
- DLPFC
dorsolateral prefrontal cortex
- OFC
orbitofrontal cortex
- PD
probability discounting
- PFC
prefrontal cortex
- VLPFC
ventrolateral prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors have no conflicts of interest to disclose.
References
- Ahn W, Rass O, Fridberg DJ, Bishara AJ, Forsyth JK, Breier A, Busemeyer JR, Hetrick WP, Bolbecker AR, O’Donnell BF. Temporal discounting of rewards in patients with bipolar disorder and schizophrenia. J Abnorm Psychol. 2011;120:911–921. doi: 10.1037/a0023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Hughes M, Iacoviello B, Urošević S, Nusslock R, Whitehouse WG, Hogan ME. Behavioral approach system (BAS) and behavioral inhibition system (BIS) sensitivities and bipolar spectrum disorders: Prospective prediction of bipolar mood episodes. Bipolar Disord. 2008;10:310–322. doi: 10.1111/j.1399-5618.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Urošević S, Abramson LY, Jager-Hyman S, Nusslock R, Whitehouse WG, Hogan M. Progression along the bipolar spectrum: A longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. J Abnorm Psychol. 2012a;121:16–27. doi: 10.1037/a0023973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, Grant DA, Jager-Hyman S, Molz A, Choi JY, Harmon-Jones E, Abramson LY. High behavioral approach system (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: A prospective behavioral high-risk design. J Abnorm Psychol. 2012;121:339–351. doi: 10.1037/a0025877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association; 2000. Text Revised. [Google Scholar]
- Angst J, Azorin JM, Bowden CL, Perugi G, Vieta E, Gamma A, Young AH. Prevalence and characteristics of undiagnosed bipolar disorders in patients with a major depressive episode: the BRIDGE study. Arch Gen Psychiatry. 2011;68:791–798. doi: 10.1001/archgenpsychiatry.2011.87. [DOI] [PubMed] [Google Scholar]
- Axelson DA, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Bridge J, Keller M. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher B, Zelazny J, Kaufman J, Gill MK. Working Draft. Pittsburgh, PA: University of Pittsburgh School of Medicine, Western Psychiatric Institute and Clinics; 2009. Kiddie-SADS-Present and Lifetime (K-SADS-PL)-2009. Available from: http://www.wpic.pitt.edu/research/AssessmentTools/ChildAdolescent/ksads-pl_2009_working_draft_full.pdf. [Google Scholar]
- Axelson DA, Birmaher B, Strober MA, Goldstein BI, Ha W, Gill MK, Goldstein TR, Yen S, Hower H, Hunt JI, Liao F, Iyengar S, Dickstein D, Kim E, Ryan ND, Frankel E, Keller MB. Course of subthreshold bipolar disorder in youth: diagnostic progression from bipolar disorder not otherwise specified. J Am Acad Child Adolesc Psychiatry. 2011;50:1001–1016. doi: 10.1016/j.jaac.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer MS, Crits-Christoph P, Ball WA, Dewees E, McAllister T, Alahi P, Cacciola J, Whybrow PC. Independent assessment of manic and depressive symptoms by self-rating: Scale characteristics and implications for the study of mania. Arch Gen Psychiatry. 1991;48:807–812. doi: 10.1001/archpsyc.1991.01810330031005. [DOI] [PubMed] [Google Scholar]
- Bauer MS, Vojta C, Kinosian B, Altshuler L, Glick H. The Internal State Scale: Replication of its discriminating abilities in a multisite, public sector sample. Bipolar Disord. 2000;2:340–346. doi: 10.1034/j.1399-5618.2000.020409.x. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Hofler M, Leibenluft E, Lieb R, Bauer M, Pfennig A. Mood episodes and mood disorders: Patterns of incidence and conversion in the first three decades of life. Bipolar Disord. 2009;11:637–649. doi: 10.1111/j.1399-5618.2009.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Keller M. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. Neuroimage. 2011;54:1344–1354. doi: 10.1016/j.neuroimage.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Cicero DC, Epler AJ, Sher KJ. Are there developmentally limited forms of bipolar disorder? J Abnorm Psychol. 2009;118:431–447. doi: 10.1037/a0015919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annu Rev Psychol. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Finger EC, Brotman MA, Rich BA, Pine DS, Blair JR, Leibenluft E. Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychol Med. 2010;40:1089–1100. doi: 10.1017/S0033291709991462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Treland JE, Snow J, McClure EB, Mehta MS, Towbin KE, Pine DS, Leibenluft E. Neuropsychological performance in pediatric bipolar disorder. Biol Psychiatry. 2004;55:32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- Di Nicola M, De Risio L, Battaglia C, Camardese G, Tedeschi D, Mazza M, Martinotti G, Pozzi G, Niolu C, Di Giannantonio M, Siracusano A, Janiri L. Reduced hedonic capacity in euthymic bipolar subjects: A trait-like feature? J Affect Disord. 2013;147:446–450. doi: 10.1016/j.jad.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Ross J, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D, Shanklin S, Lim C, Grunbaum JA, Wechsler H. Youth Risk Behavioral Surveillance—United States, 2005. Surveillance Summaries, 55/SS-5, 1–108. 2006 Available from: http://www.cdc.gov/mmwr/pdf/ss/ss5505.pdf. [PubMed]
- Ernst M, Dickstein DP, Munson S, Eshel N, Pradella A, Jazbec S, Pine DS, Leibenluft E. Reward-related processes in pediatric bipolar disorder: A pilot study. J Affect Disord. 2004;82S:89–101. doi: 10.1016/j.jad.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein TR, Birmaher B, Axelson D, Goldstein BI, Gill MK, Esposito-Smythers C, Ryan ND, Strober MA, Hunt J, Keller M. Psychosocial functioning among bipolar youth. J Affect Disord. 2009;114:174–183. doi: 10.1016/j.jad.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-depressive illness: Bipolar disorders and recurrent depression. 2nd ed. New York: Oxford University Press; 2007. [Google Scholar]
- Gorrindo T, Blair RJR, Budhani S, Dickstein DP, Pine DS, Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am J Psychiatry. 2005;162:1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman D, Axelson D, Demeter C, Findling RL, Fristad MA, Kowatch RA, Yongstrom EA, Horwitz SM, Arnold LE, Frazier TW, Ryan N, Gill MK, Hauser-Harrington JC, Depew J, Rowles BM, Birmaher B. Phenomenology of bipolar disorder not otherwise specified in youth: A comparison of clinical characteristics across the spectrum of manic symptoms. Bipolar Disord. 2013;15:240–252. doi: 10.1111/bdi.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek T, Alda M, Hajek E, Ivanoff J. Functional neuroanatomy of response inhibition in bipolar disorders—Combined voxel based and cognitive performance meta-analysis. J Psychiatr Res. 2013;47:1955–1966. doi: 10.1016/j.jpsychires.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. A four factor index of social status. Unpublished manuscript. New Haven, CT: Yale University; 1975. Available from: http://www.yale.edu/sociology/yjs/yjs_fall_2011.pdf. [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents’ performance on the Iowa gambling task: Implications for the development of decision making and ventromedial prefrontal cortex. Dev Psychol. 2004;40:1148–1158. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Hwang K, Velanova K, Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: A functional magnetic resonance imaging effective connectivity study. J Neuroci. 2010;30:15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Edge MD, Holmes MK, Carver CS. The behavioral activation system and mania. Annu Rev Clin Psychol. 2012;8:243–267. doi: 10.1146/annurev-clinpsy-032511-143148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Ruggero C, Carver CS. Cognitive, behavioral and affective responses to reward: Links with hypomanic symptoms. J Soc Clin Psychol. 2005;24:894–906. [Google Scholar]
- Johnson SL, Sandrow D, Meyer B, Winters R, Miller I, Solomon D, Keitner G. Increases in manic symptoms after life events involving goal attainment. J Abnorm Psychol. 2000;109:721–727. doi: 10.1037//0021-843x.109.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph MF, Frazier TW, Youngstrom EA, Soares JC. A quantitative and qualitative review of neurocognitive performance in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2008;18:595–605. doi: 10.1089/cap.2008.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Kiddie-SADS-Present and Lifetime (K-SADS-PL) Version 1.0. Pittsburgh, PA: University of Pittsburgh School of Medicine, Western Psychiatric Institute and Clinics; 1996. Available from: http://www.wpic.pitt.edu/research/AssessmentTools/ChildAdolescent/ksads-pl.pdf. [Google Scholar]
- Kowatch RA, Youngstrom EA, Danielyan A, Findling RL. Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disord. 2005;7:483–496. doi: 10.1111/j.1399-5618.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Larson ER, Shear PK, Krikorian R, Weigge J, Strakowski SM. Working memory and inhibitory control among manic and euthymic patients with bipolar disorder. J Int Neuropsychol Soc. 2005;11:163–172. doi: 10.1017/s1355617705050228. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011;168:129–142. doi: 10.1176/appi.ajp.2010.10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Klein DN, Seeley JR. Bipolar disorders in a community sample of older adolescents: Prevalence, phenomenology, comorbidity, and course. J Am Acad Child Adolesc Psychiatry. 1995;34:454–463. [PubMed] [Google Scholar]
- Li N, Ma N, Liu Y, He X-S, Sun D-L, Fu X-M, Zhang X, Han S, Zhang D-R. Resting-state functional connectivity predicts impulsivity in economic decision-making. J Neurosci. 2013;33:4886–4895. doi: 10.1523/JNEUROSCI.1342-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Walhstrom D, Porter JN, Collins PF. Dopaminergic modulation of incentive motivation in adolescence: Age-related changes in signaling, individual differences, and implications for the development of self-regulation. Dev Psychol. 2012;48:844–861. doi: 10.1037/a0027432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Paulsen DJ, Padmanabhan A, Geier C. The teenage brain: Cognitive control and motivation. Curr Dir Psychol Sci. 2013;22:94–100. doi: 10.1177/0963721413478416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason L, O’Sullivan N, Blackburn M, Bentall R, El-Deredy W. I want it now! Neural correlates of hypersensitivity to immediate reward in hypomania. Biol Psychiatry. 2012;71:530–537. doi: 10.1016/j.biopsych.2011.10.008. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. J Psychopathol Behav Assess. 2001;23:133–143. doi: 10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES, Sahakian BJ. Decision-making cognition in mania and depression. Psychol Med. 2001;31:679–693. doi: 10.1017/s0033291701003804. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Abramson LY, Harmon-Jones E, Alloy LB, Hogan ME. A goal-striving life event and the onset of bipolar episodes: Perspective from the Behavioral Approach System (BAS) dysregulation theory. J Abnorm Psychol. 2007;116:105–115. doi: 10.1037/0021-843X.116.1.105. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: A diffusion tensor imaging study. J Cogn Neurosci. 2009;21:1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EA, Hooper CJ, Collins P, Luciana M. Adolescents’ performance on delay and probability discounting tasks: Contributions of age, intelligence, executive functioning, and self-reported externalizing behaviors. Pers Individ Diff. 2007;43:1886–1897. doi: 10.1016/j.paid.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, Bowden CL, Sachs GS, Nierenberg AA. Long-Term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, Perlis RH. Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biol Psychiatry. 2008;64:162–168. doi: 10.1016/j.biopsych.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau G, Blair KS, Berghorst L, Knopf L, Skup M, Luckebaugh DA, Pine DS, Blair RJ, Leibenluft E. Processing of differentially valued rewards and punishments in youths with bipolar disorder or severe mood dysregulation. J Child Adolesc Psychopharmacol. 2008;18:185–196. doi: 10.1089/cap.2007.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: Effect of alcohol. J Exp Anal Behav. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salavert J, Caseras X, Torrubia R, Furest S, Arranz B, Duenas R, San L. The functioning of the Behavioral Activation and Inhibition Systems in bipolar I euthymic patients and its influence in subsequent episodes over an 18-month period. Pers Individ Diff. 2007;42:1323–1331. [Google Scholar]
- Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, Castellanos FX. Temporal and probabilistic discounting of rewards in children and adolescents: Effects of age and ADHD symptoms. Neuropsychologia. 2006;44:2092–2103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Schurhoff F, Bellivier F, Jouvent R, Mouren-Simeoni M, Bouvard M, Allilaire J, Leboyer M. Early and late onset bipolar disorders: Two different forms of manic-depressive illness? J Affect Disord. 2000;58:215–221. doi: 10.1016/s0165-0327(99)00111-1. [DOI] [PubMed] [Google Scholar]
- Singh MK, Chang KD, Kelley RG, Cui X, Sherdell L, Howe ME, Gotlib IH, Reiss AL. Reward processing in adolescents with bipolar I disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:68–83. doi: 10.1016/j.jaac.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A behavioral scientist looks at the science of adolescent brain development. Brain Cogn. 2010;72:160–164. doi: 10.1016/j.bandc.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffan E, Banich M. Age differences in future orientation and delay discounting. Child Dev. 2009;80:28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Strober M, Schmidt-Lackner S, Freeman R, Bower S, Lampert C, DeAntonio M. Recovery and relapse in adolescents with bipolar affective illness: A five-year naturalistic, prospective follow-up. J Am Acad Child Adolesc Psychiatry. 1995;34:724–731. doi: 10.1097/00004583-199506000-00012. [DOI] [PubMed] [Google Scholar]
- Swann AC. Mechanisms of impulsivity in bipolar disorder and related illness. Epidemiol Psichiatr Soc. 2010;19:120–130. [PubMed] [Google Scholar]
- Urošević S, Abramson LY, Harmon-Jones E, Alloy LB. Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: Review of theory and evidence. Clin Psychol Rev. 2008;28:1188–1205. doi: 10.1016/j.cpr.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S, Collins P, Muetzel R, Lim KO, Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Dev Psychol. 2012;48:1488–1500. doi: 10.1037/a0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meter A, Moreira AL, Youngstrom EA. Meta-analysis of epidemiological studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72:1250–1256. doi: 10.4088/JCP.10m06290. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: Behavioral implications and issues in assessment. Brain Cogn. 2010;72:146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Bloomington, MN: Pearson; 1999. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Second Edition (WASI-II) Bloomington, MN: Pearson; 2011. [Google Scholar]
- Wesley MJ, Bickel WK. Remember the Future II: Meta-analyses and functional overlap of working memory and delay discounting. Biol Psychiatry. 2014;75:435–448. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Kollman B, Linke J, Schonfelder S, Kanske P. Increased impulsivity as a vulnerability marker for bipolar disorder: Evidence from self-report and experimental measures in two high-risk populations. J Affect Disord. 2015;178:18–24. doi: 10.1016/j.jad.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Youngstrom EA. Definitional issues in bipolar disorder across the life cycle. Clin Psychol-Sci Pr. 2009;16:140–160. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.