Abstract
Objective
Depression among methamphetamine users is more prevalent in females than males, but gender specific treatment options for this comorbidity have not been described. Reduced brain phosphocreatine levels have been shown to be lower in female methamphetamine users compared to males, and, of relevance, studies have demonstrated an association between treatment resistant depression and reduced brain phosphocreatine concentrations. The nutritional supplement creatine monohydrate has been reported to reduce symptoms of depression in female adolescents and adults taking antidepressants, as well as to increase brain phosphocreatine in healthy volunteers. Therefore, the purpose of this pilot study was to investigate creatine monohydrate as a treatment for depression in female methamphetamine users.
Methods
Fourteen females with depression and comorbid methamphetamine dependence were enrolled in an 8 week open label trial of 5 grams of daily creatine monohydrate and of these 14, eleven females completed the study. Depression was measured using the Hamilton Depression Rating Scale (HAMD) and brain phosphocreatine levels were measured using phosphorus magnetic resonance spectroscopy pre- and post-creatine treatment. Secondary outcome measures included anxiety symptoms, measured with the Beck Anxiety Inventory (BAI), as well as methamphetamine use, monitored by twice weekly urine drug screens and self-reported use.
Results
The results of a linear mixed effects repeated measures model showed significantly reduced HAMD and BAI scores as early as week 2 when compared to baseline scores. This improvement was maintained through study completion. Brain phosphocreatine concentrations were higher at the second phosphorus magnetic resonance spectroscopy scan compared to the baseline scan; Mbaseline = 0.223 (SD = 0.013) vs. Mpost-treatment = 0.233 (SD = 0.009), t(9) = 2.905, p < .01, suggesting that creatine increased phosphocreatine levels. Also, a reduction in methamphetamine positive urine drug screens of greater than 50% was observed by week 6. Finally, creatine was well tolerated and adverse events that were related to gastrointestinal symptoms and muscle cramping were determined as possibly related to creatine.
Conclusions
The current study suggests that creatine treatment may be a promising therapeutic approach for females with depression and comorbid methamphetamine dependence.
Clinical Trial Registration
This study is registered on clinicaltrials.gov (NCT01514630).
Keywords: comorbidity, co-occurring, depression, women’s health, substance use disorders, methamphetamine dependence, neuroimaging
Introduction
Depression and methamphetamine use disorders are highly comorbid. In a number of studies, rates of depression among methamphetamine users are consistently documented as greater than 35% (Dyer & Cruickshank, 2005; Kay-Lambkin, Baker, Lee, Jenner, & Lewin, 2011; McKetin, Lubman, Lee, Ross, & Slade, 2011; Sutcliffe et al., 2009). In contrast, according to a survey conducted by the Centers for Disease Control and Prevention (CDC), general population rates for current depression are 9.0%, including 3.4% for major depressive disorder (Centers for Disease Control and Prevention, 2010). Among methamphetamine users and in the general population, females are more likely than males to have depression, but the gender differences in methamphetamine use disorders are substantially higher. For example, findings from the CDC survey noted that 4.0% of females compared to 2.7% of males met criteria for current major depression (Centers for Disease Control and Prevention, 2010), whereas rates as high as 70% in females compared to 30% in males have been documented in methamphetamine users (Glasner-Edwards et al., 2008).
The relationship of methamphetamine use and depression is likely bidirectional, with methamphetamine use contributing to changes in mood and being used as a self-medicating behavior to reduce symptoms of depression (McKetin et al., 2011; Semple, Zians, Strathdee, & Patterson, 2007). Multiple lines of evidence, such as cerebral vascular accidents (Perez, Arsura, & Strategos, 1999; Ohta, Mori, Yoritaka, Okamoto, & Kishida, 2005) as well as anatomic changes like left ventricular dilation (Lord, Shenouda, Mcllwain, Charalampidis, Lucchesi, & Varner, 2010), suggest that neurotoxicity occurs as a consequence of chronic methamphetamine exposure. Also, reductions in neurotransmitters, such as dopamine and serotonin, with methamphetamine use are well documented (McCann, Wong, Yokoi, Villemagne, Dannals, & Ricaurte, 1998; Sekine et al., 2001; Volkow et al., 2001), as well as high rates of neuropsychiatric symptoms, including depression (Zweben et al., 2004; Chen et al., 2015). On the other hand, the self-medication hypothesis of drug use posits that individuals use substances as compensatory behavior to alleviate symptoms of an underlying mental illness, and that the substance an individual comes to rely on is not a random choice (Khantzian, 1985). Considering some of the symptoms of depression: anhedonia, sleep disturbances, weight loss or gain, fatigue, and impaired concentration, it is not unreasonable to surmise that individuals with depression use methamphetamine to alleviate symptoms. Regardless of depression being pre-morbid or a consequence of methamphetamine use, the comorbidity of depression and methamphetamine use confounds treatment outcomes and worsens overall prognosis (Glasner-Edwards, Mooney, Marinelli-Casey, Hillhouse, Ang, & Rawson, 2010). For example, in a 3 year follow up study of adults with methamphetamine dependence, recurrence of methamphetamine use was significantly higher in participants with an Axis I psychiatric diagnosis relative to those without an Axis I psychiatric diagnosis (Glasner-Edwards et al., 2010). Moreover, self-reported depressive symptoms appear to persist up to 12 months after discontinuing methamphetamine use (Rawson et al., 2002), which may increase the risk of relapse (Glasner-Edwards et al., 2010).
There are no clear pharmacological treatment modalities for comorbid depression and methamphetamine use disorders (Hellem, Lundberg, & Renshaw, 2014). In fact, there is limited available data on medication treatment options for methamphetamine dependence and/or withdrawal. For example, investigations of antidepressants as a treatment option for methamphetamine dependence (Colfax et al., 2011; Elkashef et al., 2008; Galloway, Newmeyer, Knapp, Stalcup, & Smith, 1996; Heinzerling et al., 2010; Shoptaw et al., 2008; Shoptaw et al., 2006) or withdrawal (Cruickshank et al., 2008) have generally shown that antidepressants are not an adequate treatment option. Notably, in a randomized controlled trial, adults with methamphetamine dependence (n = 120) assigned to 100mg of the selective serotonin reuptake inhibitor (SSRI) sertraline daily for 12 weeks had no change in self-reported methamphetamine use and had more methamphetamine positive urine drug screens compared to the placebo group (n =90; Shoptaw et al., 2006).
Neuroimaging findings from both depression and methamphetamine use disorder research are associated with changes in brain energy metabolism (Forester et al., 2009; Iosifescu et al., 2008; Kondo et al., 2011; Sung et al., 2013). Phosphorus magnetic resonance spectroscopy is used to measure in vivo brain energy metabolite level changes. Due to its role in adenosine triphosphate production, phosphocreatine is one of the primary neurometabolites of interest in psychiatry neuroimaging research. Mitochondria are responsible for producing adenosine triphosphate, and converging lines of evidence implicate mitochondrial dysfunction in psychiatric disorders, including depression (Rezin, Amboni, Zugno, Quevedo, & Streck, 2008; Shao et al., 2008; Marazziti et al., 2011). Further, substances, such as stimulants, have been shown to induce mitochondrial dysfunction (Yamamoto, Moszczynska, & Gudelsky, 2010; Cunha-Oliveira, Silva, Silva, Moreno, Oliveira, & Santos, 2013). Considering that phosphocreatine plays an essential role in serving as a buffer to maintain constant adenosine triphosphate levels via the creatine kinase reaction, it’s not surprising that phosphocreatine has been reported as altered in both depression (Forester et al., 2009; Iosifescu et al., 2008; Moore, Christensen, Lafer, Fava, & Renshaw, 1997) and methamphetamine use disorders (Sung et al., 2013). For example, when compared to healthy volunteers, elevated phosphocreatine and reduced beta nucleotide triphosphate (β-NTP) concentrations have been documented in adults with depression (Iosifescu et al., 2008; Moore, Christensen, Lafer, Fava, & Renshaw, 1997; Volz et al., 1998). This pattern, which is more common in females than males (Renshaw et al., 2001), suggests that some depressed patients have increased brain energy stores that are not being accessed to support cerebral activity. Further, decreased frontal lobe phosphocreatine in chronic methamphetamine users relative to healthy controls has been documented, and this effect was greater in female methamphetamine users compared to male methamphetamine users (Sung et al., 2013).
Changes in brain metabolism using other neuroimaging methods such as positron emission tomography (PET) have also been reported in methamphetamine users. For example, in a study of 15 detoxified methamphetamine abusers, compared to healthy volunteers, brain metabolism was noted as lower in the thalamus and striatum and higher in the parietal cortex (Volkow et al., 2001), and these findings may indicate brain injury via glial response, as glial cell metabolism is higher than neurons and methamphetamine causes pyramidal glutamatergic neuronal damage (Chang, Alicata, Ernst, & Volkow, 2007). Similarly, PET studies of major depressive disorder have documented alterations in glucose metabolism. In a recent meta-analysis of PET studies of depression, increased glucose metabolism in the right thalamus pulvinar, left culmen of vermis in the anterior lobe cortex and right declive of the posterior lobe and decreased glucose metabolism in the right thalamus, posterior lobe declive and left culmen of vermis was noted (Su, Cai, Dutt, Shi, & Bramon, 2014). Together with the phosphorus magnetic resonance spectroscopy findings in depression, these PET study findings suggest that brain metabolism may be involved in the pathophysiology of depression.
Due to its critical role in bioenergetic metabolism, creatine monohydrate (hereinafter “creatine”) has shown promise in treating a number of disorders. For example, creatine has been reported to be neuroprotective in neurodegenerative disorders such as Huntington’s disease (Adhihetty & Beal, 2008), in addition to improving neurological symptoms in rats with traumatic brain and spinal cord injury (Hausmann, Fouad, Wallimann, & Schwab, 2002; Rabchevsky, Sullivan, Fugaccia, & Scheff, 2003; Sullivan, Geiger, Mattson, & Scheff, 2000). Also, creatine has gender specific antidepressant effects in rats (Allen et al., 2010, 2012). For example, when male and female rats were administered creatine in the diet, the female rats’ depression-like behavior improved, whereas the male rats’ depression-like behavior declined (Allen et al., 2010). Along those same lines of creatine’s gender dependent antidepressant effects, adjunctive oral creatine use has been associated with reduced depressive symptoms in female adolescents (Kondo et al., 2011) and adults (Lyoo et al., 2012) taking an SSRI. Also of relevance, creatine administration has been shown to increase brain phosphocreatine concentrations (Dechent, Pouwels, Wilken, Hanefeld, & Frahm, 1999; Lyoo et al., 2003).
Creatine is an organic acid that is common to all vertebrates and is produced endogenously by the liver, kidneys and pancreas (Cooper, Naclerio, Allgrove, & Jimenez, 2012) and is obtained exogenously via an omnivorous diet (Persky & Brazeau, 2001). Some reports suggest that vegetarians have lower concentrations of creatine compared to non-vegetarians (Burke, Chilibeck, Parise, Candow, Mahoney, & Tarnopolsky, 2003; Lukaszuk, Robertson, Arch, & Moyna, 2005) considering that half of the creatine distributed in the body is acquired through diet. Ninety-five percent of creatine is stored in skeletal muscle and the remainder is found in the brain, liver, kidneys and testes (Persky & Brazeau, 2001; Cooper et al., 2012). Creatine plays a vital role in brain energy metabolism. Via the creatine kinase reaction, creatine facilitates production of adenosine triphosphate, the nervous system’s principal energy source (Andres, Ducray, Schlattner, Wallimann, & Widmer, 2008).
Creatine supplementation is widely used among athletes to enhance athletic performance with an estimated use of 51% among 12th grade United States high school football players (Castillo & Cornstock, 2007). Despite this enormous exposure, there is no evidence for creatine-associated harm beyond gastrointestinal upset, muscle cramping and intracellular weight gain (Juhn, O’Kane, & Vinci, 1999; Chrusch, Chilibeck, Chad, Davison, & Burke, 2001; Groeneveld et al., 2003; Bizzarini & De Angelis, 2004; Kendall, Jacquemin, Frost, & Bums, 2005).
On the basis of the aforementioned preliminary findings and the role of creatine in energy metabolism, this pilot study focused on female methamphetamine users diagnosed with depression in an open label study of standalone creatine treatment for comorbid depression and methamphetamine use disorders. Even though this was a pilot study, because we were interested in studying if creatine had an impact on depression and brain bioenergetics in methamphetamine users, the purpose of our study was to test the hypothesis that 8 weeks of creatine administration would be associated with a decrease in depression severity scores and an increase in brain phosphocreatine concentrations. Because co-occurring anxiety is highly prevalent among depressed substance users (Lubman, Allen, Rogers, Cementon, & Bonomo, 2007; Grant et al., 2004) and among depressed individuals in the general population (Hirschfeld, 2001), measures of anxiety were also collected as a secondary outcome. Finally, in addition, methamphetamine use was collected as a secondary outcome.
Methods
Recruitment and Consent
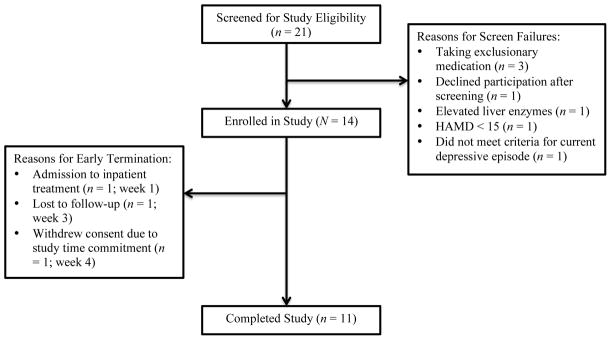
A total of 21 females were screened for study participation and 14 females completed the baseline assessments and were enrolled in this open label study of creatine (see Figure 1). Of these 14 females, 11 completed the study. Females were informed of the study by recruitment flyers and staff referrals from a clinic that provides substance abuse assessment and referrals for adults with substance abuse and/or mental health issues. The inclusion criteria for study entry were: 1) female gender, ages 18 – 64 years inclusive, 2) diagnosis of major depressive disorder with a current major depressive episode as determined by the Structured Clinical Interview for DSM-IV Disorders (SCID-I/P; First et al., 2007), 3) a score of greater than or equal to 15 on the 17-item Hamilton Depression Rating Scale (HAMD; Hamilton, 1960), 4) identification of methamphetamine as primary drug of choice and 5) since depressive symptoms have been reported to persist up to 12 months after methamphetamine discontinuation (Rawson et al., 2002), a SCID-I/P diagnosis of methamphetamine dependence or abuse within the last 12 months. The exclusion criteria included: 1) a SCID-I/P diagnosis of bipolar disorder, 2) known or suspected diagnosis of schizophrenia or schizoaffective disorder, 3) a diagnosis of renal disease, type I or II diabetes mellitus, colitis, diverticulitis or seizure disorder, 4) human immunodeficiency virus (HIV) seropositive status or elevated liver enzymes by lab determination, 5) current treatment with an antidepressant, mood stabilizer or antipsychotic medication, 6) current serious suicide risk identified by the Columbia Suicide Severity Rating Scale (C-SSRS; Posner, 2010), 7) positive urine pregnancy test and 8) contraindication to magnetic resonance scan. Gift card compensation to locations with alcohol and cigarette sale restrictions was offered to participants after each completed visit. If all visits were attended, participants received a total of $290.
Figure 1.
Screening, enrollment and study completion data
Prior to obtaining written informed consent, the study was fully explained to potential participations. The study was conducted in accordance with the Declaration of Helsinki and was approved by the University of Utah Institutional Review Board. The study was conducted under Investigational New Drug number 114,316 issued by the Division of Psychiatry Products of the FDA.
Study Design and Procedures
In this within subjects design, each enrolled participant was treated with Creapure® brand of creatine (Alzchem, LLC, Trostberg, Germany) at a fixed dose of 5 grams by mouth daily for 8 weeks. A dose of 5 grams daily was selected because this dose has been commonly used in clinical research evaluating creatine as a treatment option for depression. There have not been any safety concerns reported using 5 grams of creatine in studies of depression, and changes in depressive symptoms at this dose have been documented (see Roitman, Green, Osher, Karni, & Levine, 2006; Lyoo et al., 2012). After consent was obtained, the principal investigator (P.I.; T.H.) administered the SCID-I/P and HAMD, and if a female met SCID-I/P criteria and scored ≥ 15 on the HAMD, she continued with study procedures. The C-SSRS1, vital signs, concomitant medications, self-reported drug use over the past 48 hours for cigarettes, alcohol, cocaine, methamphetamine, marijuana, heroin and prescription controlled substances, urine drug screen for methamphetamine, opiates, benzodiazepines, marijuana and cocaine, pregnancy testing and attendance in outpatient treatment and/or 12 step programs (e.g., Narcotics or Alcoholics Anonymous) was gathered at each visit and twice weekly from weeks 1 through 8. The HAMD and Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988) were collected weekly throughout the course of the study. A complete blood count, comprehensive metabolic panel, including liver enzymes to assess for active liver disease, and HIV testing were obtained at week −1, and with the exception of HIV testing, they were repeated at week 8 prospectively identify abnormalities associated with creatine administration. Adverse events were collected at each visit starting at week 1.
The principal investigator administered the SCID-I/P and therefore interrater reliability is not a concern for this study. However, since four researchers administered the HAMD on different participants throughout the study, an interrater reliability analysis using the intraclass correlation coefficient was performed to determine consistency among raters. The intraclass correlation coefficient for the HAMD raters was determined to be 0.98. The BAI is a self-reported tool and thus concerns of interrater reliability are not applicable, but other forms of reliability of the BAI in a psychiatric population have been well documented (see De Ayala, Vonderharr-Carlson & Kim, 2005).
Urine samples were collected twice weekly for testing of methamphetamine, opiates, benzodiazepines, marijuana and cocaine in addition to pregnancy testing. Finally, creatine adherence was assessed weekly with the collection of used and unused creatine bottles. If participants missed more than 3 daily doses of creatine, they were withdrawn from the study for protocol noncompliance. Similarly, if participants missed more than 3 consecutive study visits, they were withdrawn from the study for protocol noncompliance. During the informed consent process, participants were informed that they could be withdrawn for protocol noncompliance.
Phosphorus magnetic resonance spectroscopy scans were acquired using a Siemens 3 T MRI scanner (Siemens AG, Munich, Germany) that is approved for clinical use. Three dimensional-chemical shift imaging (3D-CSI) free induction decay pulse sequence was used with TR / TE = 3000 / 2.3 ms, number of average = 16, field of view = 20 cm × 20 cm × 20 cm, acquisition matrix 8 × 8 × 8, size of vector = 1024, voxel dimension = 2.5 cm × 2.5 cm × 2.5 cm, flip angle = 90°, and bandwidth = 2.5 kHz. Advanced Method for Accurate, Robust and Efficient Spectral fitting (AMARES; Vanhamme, van den Boogaart, & Van Huffel, 1997) routine within jMRUI software package (Naressi, et al, 2001) was used for quantitation of phosphorus metabolites of frontal lobe. Phosphocreatine integral values were expressed relative to the total phosphorus signal (Blumberg, et al, 1999).
Measures
The 17-item HAMD (Hamilton, 1960) was used to assess changes in the severity of depression and the BAI (Beck et al., 1988) was used to assess changes in the severity of anxiety. Treatment response for the HAMD (Furukawa et al., 2007) and BAI (Leyer, Ruberg, & Woodruff-Borden, 2006) was defined a priori as a decrease of 50% or more from baseline. Psychometric properties of the HAMD and BAI for this study were not calculated because of the small sample size. Other reports of reliability and validity of these tools, however, have reported acceptable reliability and validity of both the HAMD (Bagby, Ryder, Schuller, & Marshall, 2004) and BAI (De Ayala, Vonderharr-Carlson & Kim, 2005) in depressed patients.
Self-reported use of methamphetamine, cocaine, heroin, marijuana, prescription controlled substances and alcohol over the last 48 hours and a 5 panel urine drug screen for methamphetamine, cocaine, opiates, marijuana and benzodiazepines were collected at weeks −1, 0, and 1, twice weekly during the treatment period and weekly during the follow up phase.
Statistical Analysis
For this pilot study, a sample size of 10 was proposed. Assuming a 40% attrition rate, we anticipated enrolling 14 females for a final sample size of 10. Given that this was a pilot study, neither a post hoc nor an a priori power analyses were performed (Leon, Davis, & Kraemer, 2011).
Descriptive statistics are reported in Tables 1 and 2 for baseline demographic variables and in the results section for retention and missed medication doses. Figures and tables are provided for displaying the change in HAMD and BAI scores in addition to the change in brain phosphocreatine concentrations. Analyses for change in HAMD and BAI scores were performed using a linear mixed effects model repeated measures analysis, which is capable of handling missing data and time varying covariates (Mallinckrodt et al., 2003). HAMD and BAI scores were averaged over weeks −1, 0 and 1, and considered as “baseline,” and HAMD and BAI scores were averaged over weeks 9, 10 and 11 and considered as “follow up.” Time was included as a fixed factor and subject was treated as a random factor. Age and weekly treatment program attendance in days were used as covariates. A linear mixed effects repeated measures model was also conducted to evaluate the effect of treatment attendance on methamphetamine use. Time was included as a fixed factor and subject was treated as a random factor. Sidak correction, a method to compensate for multiple comparisons, (West, Welch, & Gałecki, 2006) was used to control for Type I error for all linear mixed effects repeated model analyses. A paired t-test and Wilcoxon Signed Ranks test was conducted to evaluate changes in brain phosphocreatine levels, and an independent samples t-test was used to evaluate the relationship between treatment response and number of days abstinent from methamphetamine. An alpha value of less than 0.05 was considered significant for one tailed tests, and one tailed tests were used considering our hypotheses were directional (e.g., decrease in depressive symptoms as well as an increase in brain phosphocreatine levels with 8 weeks of creatine treatment). The data were analyzed with IBM SPSS Statistics for Mac Version 20.
Table 1.
Baseline demographic characteristics (frequencies)
| Characteristic | n | % |
|---|---|---|
| Race | ||
| Caucasian | 13 | 92.9 |
| American Indian | 1 | 7.1 |
| Ethnicity | ||
| Not Hispanic or Latino | 14 | 100 |
| Marital status | ||
| Married | 2 | 14.3 |
| Divorced | 4 | 28.6 |
| Never married | 8 | 57.1 |
| Education level | ||
| Did not graduate high school | 5 | 35.7 |
| Graduated high school or GED | 3 | 21.4 |
| Part college | 4 | 28.6 |
| Graduated 2 year college | 2 | 14.3 |
| Employment status | ||
| Employed full-time | 2 | 14.3 |
| Employed part-time | 1 | 7.1 |
| Unemployed | 8 | 57.1 |
| Student | 1 | 7.1 |
| Homemaker | 2 | 14.3 |
| Antidepressant treatment history | 9 | 64 |
| DSM-IV diagnoses | ||
| Major Depressive Disorder | 14 | 100 |
| Alcohol use disorder, current | 1 | 7.1 |
| Stimulant use disorder, current | 8 | 57.1 |
| Cannabis use disorder, current | 1 | 7.1 |
| Opioid use disorder, current | 4 | 28.6 |
| Social phobia, current | 1 | 7.1 |
| PTSD, current | 1 | 7.1 |
| Substance induced anxiety, current | 4 | 28.6 |
| Active in treatment program | 9 | 64 |
Table 2.
Baseline demographic characteristics (descriptive statistics)
| Characteristic | Mean | SD | Range |
|---|---|---|---|
| Age in years | 37.4 | 9.9 | 24 – 52 |
| Estimated lifetime use of methamphetamine in grams | 5,662.9 | 9,671.0 | 10.5 – 37,280.0 |
| Abstinence from methamphetamine in days | 52.9 | 94.6 | 0 – 363 |
| Total years of methamphetamine use | 18.9 | 9.4 | 4 – 35 |
| Hamilton Depression Rating Scale score | 16.9 | 3.5 | 11 – 21 |
| Beck Anxiety Inventory score | 18.0 | 11.2 | 4 – 39 |
Results
Participants
Of the 21 females screened for participation, 14 (M = 37.4, SD = 9.9 years of age) were enrolled in the study (see Figure 1). All of the participants met SCID-I/P criteria for major depressive disorder as a primary disorder. The average baseline (weeks −1, 0, 1) HAMD score was 16.9 (SD = 3.5; see Tables 1 and 2), which suggests moderate illness with respect to severity of depression (Furukawa et al., 2007). All participants met SCID-I/P criteria for lifetime methamphetamine dependence and 57% of the sample met criteria for current methamphetamine dependence. Current comorbid substance use disorders included alcohol (7.1%), cannabis (7.1%) and opioid (28.6%), and current comorbid psychiatric disorders included social phobia (7.1%) and post-traumatic stress disorder (7.1%). On average, the participants used an estimated lifetime amount of 5,662.9 (SD = 9,671.0) grams of methamphetamine with a mean of 18.9 (SD = 9.4) years of total use. Concomitant medications reported at baseline included: Enalapril (n = 1), vitamins (n = 1), Estridiol (n = 1), Proventil (n = 1), Aciphex (n = 1), Neurontin (for nerve pain; n = 1), Methadone (n = 1) and Ambien (n = 1).
Outcomes
Depression
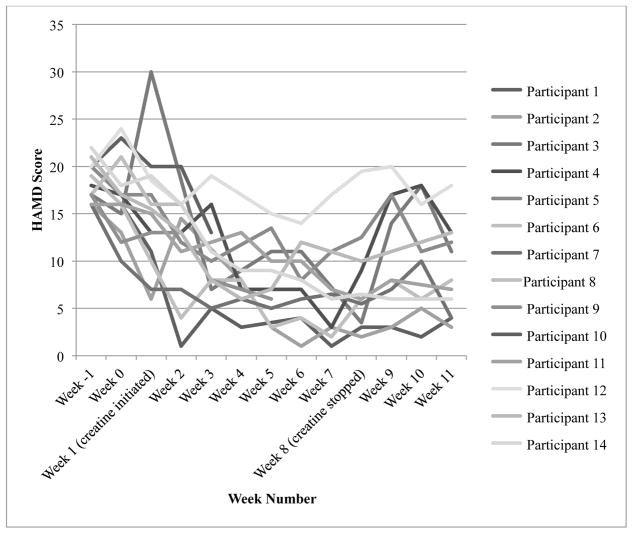
By visual inspection of individual HAMD scores (see Figure 2), it appears that scores generally improved, with the exception of one participant, throughout the course of the treatment period, and there was an increase in two participants’ scores at week 5 and one participant’s score at week 6. Participant 12’s HAMD score did not drop below 14, whereas all other participants scored below 14 by week 4. Further, unlike the majority, participant 1 had a marked and unusual decrease in her HAMD score at week 2 compared to week 1 (week 1 score = 11; week 2 score = 1). Differences in baseline characteristics and other extraneous variables, which are discussed in the Discussion section, might explain these outliers. In addition, the fluctuation in scores at weeks 5 and 6 is discussed in the Discussion section. We evaluated if there was a relationship between treatment response and number of days abstinent from methamphetamone, and these findings were not significant. Finally, change scores are presented in Table 3.
Figure 2.
Change in individual Hamilton Depression Rating Scale scores
HAMD = Hamilton Depression Rating Scale
Treatment response = score of 8.75 or below
Table 3.
Percent change in Hamilton Depression Rating Scale and Beck Anxiety Inventory Scores
| HAMD score | Percent change in score | BAI score | Percent change in score | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Time | N | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Baseline | 14 | 16.86 | 3.40 | -- | -- | 17.95 | 11.20 | -- | -- |
| Week 2 | 12 | 10.71 | 4.75 | −34.90 | 28.77 | 9.23 | 11.25 | −57.79 | 34.83 |
| Week 3 | 11 | 9.81 | 4.45 | −42.31 | 21.67 | 6.08 | 7.34 | −66.58 | 27.20 |
| Week 4 | 9 | 7.33 | 2.74 | −50.56 | 18.48 | 2.39 | 1.32 | −76.53 | 23.90 |
| Week 5 | 10 | 8.35 | 4.12 | −52.34 | 17.41 | 4.65 | 5.21 | −78.57 | 20.75 |
| Week 6 | 11 | 7.73 | 3.88 | −55.35 | 18.15 | 3.05 | 3.72 | −83.91 | 17.31 |
| Week 7 | 9 | 6.28 | 5.07 | −62.60 | 24.42 | 1.44 | 2.79 | −94.85 | 9.18 |
| Week 8 | 11 | 7.36 | 4.59 | −56.06 | 22.71 | 3.95 | 4.98 | −79.46 | 16.54 |
| Follow up | 11 | 9.83 | 5.11 | −43.87 | 26.17 | 4.74 | 5.43 | −76.30 | 23.47 |
With respect to linear mixed effects repeated model assumptions, visual inspection did not reveal any obvious deviations from linearity of the residual plot, homoscedasticity or normality of residuals. The linear mixed effects repeated measures model analyses revealed a statistically significant reduction in mean HAMD scores from baseline as early as week 2 (M = 10.04, SD = 1.19 days on creatine) and maintained through the follow up period (see Figure 3). There was not a significant age by time interaction or weekly treatment program attendance by time interaction and thus the covariates were removed from the model.
Figure 3.
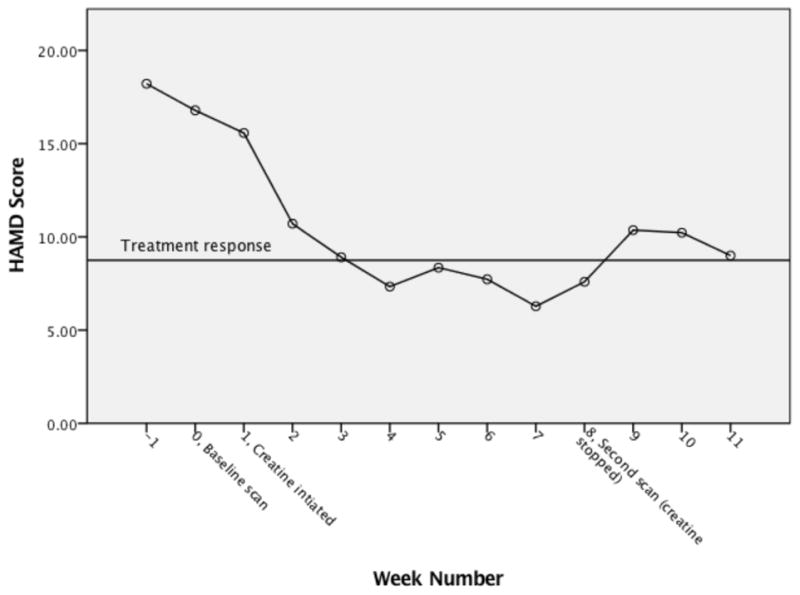
Change in mean Hamilton Depression Rating Scale score
HAMD = Hamilton Depression Rating Scale
Phosphocreatine
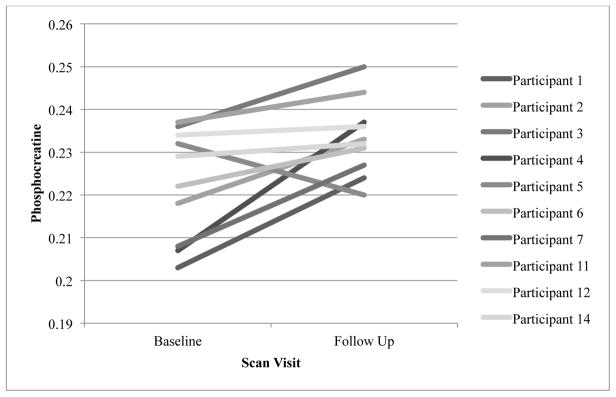
One of the enrolled participants was unable to complete the magnetic resonance spectroscopy scan and therefore there were a total of 10 scan completers. A visual inspection of the individual phosphocreatine levels at the baseline to post treatment scans demonstrate that all but one participant’s phosphocreatine appeared to consistently increase by the second scan. The outlier’s phosphocreatine was decreased at the post treatment scan (0.22 compared to 0.23 at baseline; see Figure 4), and since her serum creatinine increased from 0.71 at baseline to 1.22 at end of treatment, she reported only missing one dose of creatine, returned the exact number of empty creatine bottles that were dispensed and attended 86% of study visits, the uncertainty of measurement (Elster, Schubert, Link, Walzel, Seifert, & Rinneberg, 2005) might explain the decrease in phosphocreatine.
Figure 4.
Individual brain phosphocreatine levels pre and post creatine treatment for completers
A paired t-test was used to evaluate pre and post creatine phosphocreatine values, and the results of the test indicated that after 8 weeks of creatine treatment, mean frontal lobe phosphocreatine values were significantly higher than baseline measures; Mbaseline = 0.223 (SD = 0.013) vs. Mpost treatment = 0.233 (SD = 0.009), t(9) = 2.905, p < .01, 95% CI [0.002, 0.019]. The standardized effect size, d, was 0.92. Because of the small sample size and non-normal distribution of the dependent variable (i.e., phosphocreatine), a Wilcoxon Signed Ranks test was also performed. The results of this test were similar to the results from the paired t-test: median phosphocreatine values increased from the baseline scan; Mdbaseline = 0.226 vs. Mdpost treatment = 0.236 (Z = 2.293, p = .01). The results of both the paired t test and Wilcoxon Signed Ranks suggest that creatine treatment may increase brain phosphocreatine levels.
Anxiety
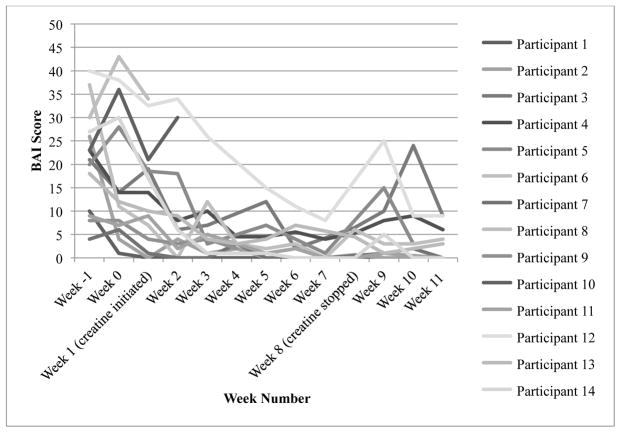
By visual inspection of individual BAI scores (see Figure 5), it appears that scores improved relatively consistently after a slight increase in scores at week 0 for three participations, and one participant’s change in score was steeper than the other participants’ scores. This one outlier had a higher week −1 BAI score (40 compared to a range of 4 – 37), and her score decreased by 9 points from week 3 to week 4, whereas the remaining participants’ scores decreased more gradually beginning at week 0. Differences in baseline characteristics, which are discussed in the Discussion section, might explain this outlier. All of the participants scored below 9.8, which was defined as treatment response, during at least 1 of the study weeks. As a result, we did not evaluate the relationship between BAI treatment response and abstinence from MA. Finally, percentage change scores are presented in Table 3.
Figure 5.
Change in individual Beck Anxiety Inventory scores
BAI = Beck Anxiety Inventory
Treatment response = 9.8
With respect to linear mixed model assumptions, visual inspection did not reveal any obvious deviations from linearity of the residual plot, homoscedasticity or normality of residuals. Significant improvements in anxiety symptoms were found as early as week 2 and maintained through the follow up period (see Figure 6). There was not a significant age by time interaction or weekly treatment program attendance by time interaction and thus the covariates were removed from the model.
Figure 6.
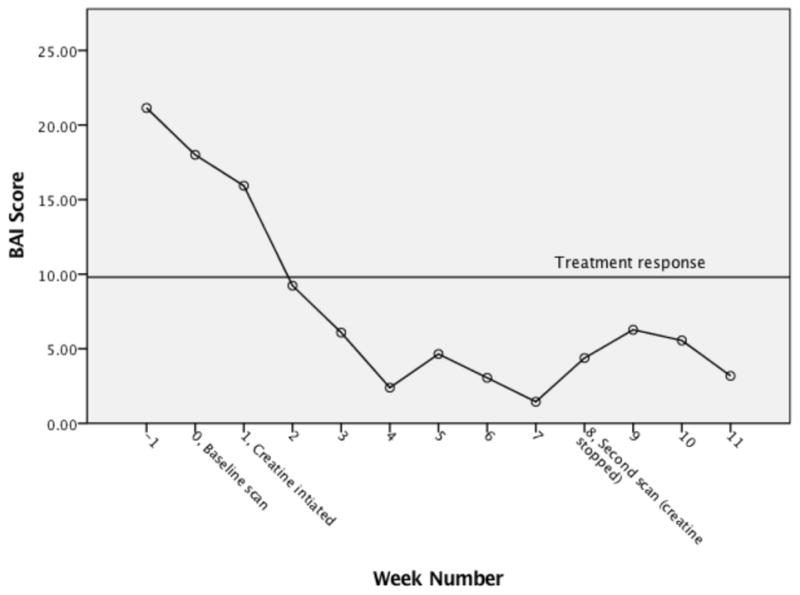
Change in mean Beck Anxiety Inventory scores
BAI = Beck Anxiety Inventory
Methamphetamine use
At baseline, 50% of the urine drug screens were positive for methamphetamine. By week 6, the percentage of urine drug screens positive for methamphetamine was reduced by more than half (21.4%; data not shown). The percentage of urine drug screens positive for methamphetamine remained at 21.4% from week 6 through the follow up period. Self-reported methamphetamine use also reduced throughout the course of the study. At baseline, a mean of 0.26 (SD = 0.02) grams of daily methamphetamine was reported, and by the end of 8 weeks of creatine treatment a mean of 0.13 (SD = 0.03) grams of daily methamphetamine was reported. The results of the linear mixed effects repeated measures model evaluating the effect of treatment attendance on methamphetamine use was not significant.
With respect to other drugs of abuse, predominantly, the participants were mono- methamphetamine users. As shown in Table 1, one participant met criteria for a current cannabis use disorder and opiate use disorder and three participants met criteria for a current opiate use disorder. The former participant was considered lost to follow up in the third week of the treatment period, and her urine drug screens were positive for methamphetamine and opiates 50% of the attended visits and positive for marijuana 100% of the attended visits. Of the three participants who met criteria for concurrent methamphetamine and opiate use disorders, one of them dropped out of the study at week 4, and her methamphetamine and opiate urine screens were positive 100% of the attended visits. The second participant who met criteria for concurrent methamphetamine and opiate use disorders, had no change in methamphetamine use throughout the study with urine drug screens positive for methamphetamine 100% of the visits, and one urine drug screen positive for opiates. Her opiate use disorder, however, was due to methadone, and the urine drug tests utilized for this study did not detect methadone. The third participant who met criteria for concurrent methamphetamine and opiate use disorders had negative urine drug screens for all substances throughout the course of the study.
Retention and Missed Visits
A total of 11 participants (78.6%) completed the study from baseline through follow-up. Three participants dropped out of the study: one participant was admitted to inpatient substance abuse treatment during the first week of creatine treatment, another participant was considered lost to follow-up after missing three consecutive study visits during weeks 3 and 4 of creatine treatment and, finally, the third drop-out was due to the participant withdrawing consent after four weeks of creatine treatment because of the study time commitment (see Figure 1). On average, there were a total of two missed visits per participant.
Medication Adherence, Safety and Tolerability
Medication adherence was monitored by counting returned used and unused vials of creatine. Bottles that were not returned were considered missed doses. Participants took on average 83.3% of dispensed study medication. A paired t-test was used to evaluate pre- and post-creatine serum creatinine values as an indicator of medication adherence considering that creatine is converted to creatinine. We expected to see higher serum creatinine values after 8 weeks of creatine compared to the baseline serum creatinine value. The results of the paired t-test suggested that the 11 participants who completed the 8 weeks of creatine administration increased their mean serum creatinine values from pre-treatment (M = 0.76, SD = 0.09) to post-treatment (M = 0.89, SD = 0.14), t(10) = −4.11, p = .002.
Creatine appeared to be well tolerated, and none of the participants withdrew due to adverse effects from creatine. Using the medication adverse effect checklist, participants reported a total of 32 adverse effects during the treatment phase of the study (see Table 4). The investigator reviewed each adverse effect, and based on other published clinical trials of creatine (Juhn, O’Kane, & Vinci, 1999; Chrusch, Chilibeck, Chad, Davison, & Burke, 2001; Groeneveld et al., 2003; Kendall, Jacquemin, Frost, & Bums, 2005), gastrointestinal symptoms and muscle cramping were determined as possibly related to study participation. All of the reported adverse effects were mild in severity and resolved without intervention. Adverse events included: cold and flu symptoms (n =10), indigestion (n = 1), flank pain (n = 1), polydipsia (n = 1), headache (n = 3), swelling in hands (n = 4), diarrhea (n = 4), stomach discomfort (n = 3), numbness and tingling in hands (n = 1), muscle cramps (n = 2), blurry vision (n = 1), lightheaded (n = 1) and nausea (n = 1). There were no abnormalities detected on laboratory assessments that were drawn at end of treatment.
Table 4.
Adverse events during the treatment phase by week
| Adverse Event name | Week 1 n (%)** | Week 2 n (%)** | Week 3 n (%)** | Week 4 n (%)** | Week 5 n (%)** | Week 6 n (%)** | Week 7 n (%)** | Week 8 n (%)** | Week 9 n (%)** | Total n (%)** |
|---|---|---|---|---|---|---|---|---|---|---|
| Cold or flu symptoms | 2 (14.3) | 4 (30.7) | -- | -- | 3 (27.2) | 1 (9.1) | -- | -- | -- | 10 (71.4) |
| Indigestion | 1 (7.1) | -- | -- | -- | -- | -- | -- | -- | -- | 1 (7.1) |
| Flank pain | 1 (7.1) | -- | -- | -- | -- | -- | -- | -- | -- | 1 (7.1) |
| Polydipsia | 1 (7.1) | -- | -- | -- | -- | -- | -- | -- | -- | 1 (7.1) |
| Headache | 1 (7.1) | -- | 1 (8.3) | -- | -- | -- | -- | -- | 1 (9.1) | 3 (21.4) |
| Swelling in hands | 1 (7.1) | 1 (7.1) | -- | -- | ||||||
| Diarrhea | -- | 1 (7.1) | 1 (8.3) | 2 (18.2) | -- | 1 (9.1) | 1 (9.1) | -- | -- | 4 (28.6) |
| Stomach discomfort | -- | 1 (7.1) | -- | 1 (9.1) | -- | -- | -- | -- | -- | 4 (28.6) |
| Numbness & tingling in hands | -- | 1 (7.1) | -- | -- | -- | -- | -- | -- | 1 (9.1) | 3 (21.4) |
| Muscle cramps | -- | -- | 1 (8.3) | 1 (9.1) | -- | -- | -- | -- | -- | 1 (7.1) |
| Blurry vision | -- | -- | 1 (8.3) | -- | -- | -- | -- | -- | -- | 2 (14.3) |
| Lightheaded | -- | -- | -- | -- | -- | -- | -- | -- | -- | 1 (7.1) |
| Nausea | -- | -- | -- | -- | -- | 1 (9.1-- | -- | -- | 1 (9.1) | 1 (7.1) |
| Total | 7 (50.0) | 8 (62.4) | 4 (33.3) | 3 (27.3) | 3 (27.3) | 3 (27.3) | 1 (9.1) | 0 | 3 (27.3) | 11 (78.6)§ |
% calculated by participants active in study by week
% calculated by total number enrolled
Total number of participants who experienced an adverse event
Discussion
To our knowledge, the current study is the first open label trial of 5 grams of daily creatine for the treatment of depression in female methamphetamine users. This study demonstrated improvements in depressive symptoms, measured by the HAMD, as early as the second week of creatine treatment and maintained through study completion. Further, anxiety symptoms, measured by the BAI, were also reduced as early as week 2 of creatine treatment and maintained throughout the study. Urine drug screens positive for methamphetamine were reduced from 50% to 21.4% by the sixth week of creatine treatment and were maintained until the end of the study, and 8 weeks of creatine treatment was associated with a mean increase in brain phosphocreatine concentrations. Finally, creatine was well tolerated with few reported adverse effects. In summary, this study shows positive effects from creatine treatment on depression, anxiety, methamphetamine use and brain bioenergetics in female methamphetamine users with comorbid depression.
The finding related to reduced HAMD scores is consistent with other studies of creatine in depressed females. In fact, Lyoo and colleagues (2012) and Kondo and colleagues (2011) noted decreased HAMD and Children’s Depression Rating Scale-Revised (CDRS-R) scores, respectively, as early as the second week of 8 weeks of creatine augmentation. Roitman and colleagues (2006) also noted improvements in HAMD, Clinical Global Improvement (CGI) and Hamilton Anxiety Scale (HAS) scores as early as week 2 in a 4 week trial of adjunctive creatine treatment. Moreover, the reduction in HAMD scores in the present study appears to be a slow progression, which is also consistent with other creatine depression studies. The exact antidepressant mechanism of creatine is not clear, but one possible explanation involves its role in cellular energy metabolism given that accumulated evidence suggests that altered cellular energy metabolism is involved in the pathophysiology of depression (Su et al., 2014). Since creatine plays a critical role in cellular energy homeostasis, treatment with it might improve cellular energy metabolism.
Several lines of evidence suggest mitochondrial dysfunction plays a role in the pathophysiology of depression (Jou, Chiu, & Liu, 2009; Rezin, Amboni, Zugno, Quevedo, & Streck, 2008; Streck et al., 2014) and with methamphetamine neurotoxicity (Yamamoto, Moszczynska, & Gudelsky, 2010; Sung et al., 2013). As a key regulator of energy production, one of the consequences of mitochondrial dysfunction is reduced adenosine triphosphate production (Streck et al., 2014). The role of the creatine-phosphocreatine system is to provide an intracellular buffer against adenosine triphosphate depletion. Creatine treatment may increase brain intracellular phosphocreatine, and since brain energy metabolism has been suggested to play a role in the pathophysiology of depression, this potentially explains why reduced depressive symptoms, thereby reducing methamphetamine use, are associated with oral creatine treatment. Creatine’s involvement as an intracellular buffer against adenosine triphosphate may also explain our finding of increased brain phosphocreatine levels, which is consistent with other creatine neuroimaging studies. Kondo and colleagues (2011) reported a significant increase in phosphocreatine values in participants treated with 8 weeks of daily creatine compared to untreated controls. In addition, Lyoo et al. (2003) showed an increase in brain phosphocreatine concentrations in participants treated with daily creatine for 2 weeks compared to a placebo group.
We initially thought that creatine might have a neuroprotective effect in methamphetamine use disorders considering that some reports have suggested this in neurodegenerative diseases such as Parkinson’s disease (Adhihetty & Beal, 2008), and this neuroprotection could have potentially explained some of our findings. However, the National Institute of Neurological Disorders and Stroke (NINDS) Exploratory Trials of Parkinson Disease (NET-PD) program recently conducted a randomized controlled trial of creatine in Parkinson’s disease, and the study was terminated early after 5 years of 10 grams daily of creatine or placebo because an interim analyses revealed that there were no differences between the groups with respect to the clinical progression of Parkinson’s disease (Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators, 2015). These findings were surprising considering cellular energy production may be impaired in Parkinson’s disease (Adhihetty & Beal, 2008), and creatine plays a role in cellular energy metabolism. The authors noted that the dose of creatine might have been too low as a treatment for slowing the progression of Parkinson’s disease.
There is new evidence suggesting that methamphetamine use increases the risk of Parkinson’s disease. For example, in a recent population-based assessment, methamphetamine users were found to have an approximate three-fold risk of Parkinson’s disease compared to non-methamphetamine users (Curtin, Fleckenstein, Robison, Crookston, Smith & Hanson, 2015). Further, female methamphetamine users were noted to have a higher risk of Parkinson’s disease compared to male methamphetamine users (Curtin et al., 2015). Given these findings, we believe that creatine should continue to be investigated in methamphetamine users with depression even though the NET-PD study did not observe positive effects of creatine in Parkinson’s disease. The findings from the NET-PD study suggest that creatine is not effective at slowing the progression of Parkinson’s disease, but perhaps creatine is useful at reducing the risk of Parkinson’s disease in methamphetamine users, or it is possible that the reduction in mood symptoms that we observed is independent of neuroprotection. More work should be done in this area to investigate the role creatine might play in methamphetamine users long-term.
Although the average depression and anxiety data revealed a reduction in mood symptoms, not all of the participants responded to the creatine treatment. There were two participants that were considered outliers with respect to change in HAMD scores. As described in the results section, one of these two participants did not score below 14 on the HAMD, whereas all other participants scored below 14 by week 4. In addition to SCID-I/P diagnoses of recurrent major depressive disorder and current stimulant use disorder, she also met criteria for a current opioid use disorder and current substance induced anxiety disorder. In fact, she was taking methadone and started a methadone taper during the fourth week of study participation. Tapering from methadone may explain why her HAMD score never dropped below 14, as it is possible that she was experiencing symptoms from the taper that resulted in symptoms of depression. However, since variables that relate to changes in methadone doses were not collected in the present study, this is a speculation.
The other outlying participant had a considerable decrease in her HAMD score at week 2 followed by a stable score of < 5 through study completion. This particular participant’s SCID-I/P diagnoses were similar to the other participants with diagnoses of recurrent major depressive disorder and current stimulant use disorder. In fact, all of her baseline characteristics (e.g., onset of methamphetamine use, total estimated lifetime of methamphetamine used and total number of years using methamphetamine) were similar to the other participants’ baseline characteristics. Perhaps she responded to creatine extremely well in a short period, or perhaps there were extraneous variables that we did not collect that contributed to improvements in her mood.
The increase in HAMD scores at weeks 5 and 6 are not easily explained. One of the two participants who had an increase in her HAMD score at week 5 was actively using methamphetamine with positive methamphetamine urine drug screens at each attended visit, and intoxication from methamphetamine may have influenced her HAMD scores. She also missed more study visits than the other participants, which might also be explained by active methamphetamine use. The other participant who had a week 5 HAMD score increase, however, was not actively using methamphetamine. In fact, 100% of her urine drugs for all substances tested negative throughout the course of the study, and her HAMD score decreased again at week 6, whereas the active methamphetamine user’s HAMD score remained the same at week 6 compared to week 5. The participant who had an increase in her HAMD score at week 6 was also abstinent from all substances including methamphetamine during the entire study, and her HAMD score remained > 10 from week 6 through study completion. Considering the fluctuation in these three participants’ scores without a similar pattern of methamphetamine use, it is difficult to conclude why their scores increased at weeks 5 and 6.
The BAI score outlier was the female tapering from methadone. She presented with high anxiety symptoms (score > 30) for the first 4 weeks of the study (i.e., weeks −1, 0, 1, and 2), and then her score mildly decreased at week 3 (score = 26) and then had a steeper decrease until week 7 when her score began steeply to rise again, whereas the remaining participants’ scores decreased as early as week 0 and continued to progressively decline. She was prescribed methadone for chronic pain, and it is possible that pain symptoms and the anticipation of the upcoming methadone taper, which started during the fourth week of study participation, explain why her anxiety scores were higher in the beginning of the study than the other participants’ scores. Her BAI scores steadily decreased from week 2 to week 7 (score = 8), and then increased each week from week 7 through study completion (final score = 18). However, as previously mentioned, we can only speculate because methadone doses were not collected in the present study.
In this study, a decrease in the number of positive urine drug screens for methamphetamine and self-reported amount of daily methamphetamine use throughout the course of the study was observed. By week 6, the percentage of positive methamphetamine urine drugs screens was less than almost half of the positive drugs screen for methamphetamine that were observed at baseline, and by the end of the treatment period, the amount of self-reported methamphetamine use was reduced by half. In agreement with the self-medication hypothesis of drug use, which suggests that individuals use substances in an effort to cope with mood symptoms (Khantzian, 1985), it is possible that as the severity of depressive and anxiety symptoms were reduced, methamphetamine use was also reduced.
However, it is worth pointing out that the three participants who dropped out of the study were actively using methamphetamine, whereas, nearly half (45.5%) of the study completers maintained abstinence from methamphetamine from study entry through study completion. We did not observe, however, a relationship between methamphetamine use and attendance in a treatment program. Nonetheless, this is the first monotherapy study that reports a decrease in the number of positive methamphetamine urine drug screens. There have been studies that have investigated a medication versus placebo in conjunction with usual care in an outpatient psychiatric clinic setting (e.g., cognitive behavioral therapy [CBT], counseling or contingency management [CM]). For example, Shoptaw and colleagues (2008) evaluated bupropion compared to placebo for methamphetamine dependence in an outpatient treatment clinic where participants also received CBT and CM. While the percent of positive methamphetamine urine drug screens decreased over the course of the 12 week medication period, the difference in percent of positive methamphetamine urine drug screens between the placebo and bupropion groups was not significantly different (Shoptaw et al., 2008). Therefore, it is possible that CBT or CM had an effect on methamphetamine use or that CBT or CM coupled with study participation had an effect on methamphetamine use. Regardless, the results from this study suggest that creatine, without standard of care treatment, might be effective at reducing methamphetamine use.
With respect to safety and tolerability, a dose of 5 grams daily of creatine appeared to be well tolerated with few adverse events related to study participation. As an Investigational New Drug study governed by the FDA, by definition, a change from baseline, regardless of relatedness to study participation, is considered an adverse event (Food and Drug Administration, 2014). In the present study, 10 participants experienced cold or flu-like symptoms, such as rhinorrhea, cough and/or sore throat, during the course of the 8 week treatment period or 3 week follow up period. We did not consider these symptoms as related to study participation, but rather, a common occurrence in adults that was likely related to a virus. Adverse events that we did consider as possibly attributed to study participation included gastrointestinal symptoms and muscle cramping given that other published creatine studies also reported these potential adverse effects (Juhn, O’Kane, & Vinci, 1999; Chrusch, Chilibeck, Chad, Davison, & Burke, 2001; Groeneveld et al., 2003; Kendall, Jacquemin, Frost, & Bums, 2005).
Our study has limitations that merit consideration. First, the lack of a placebo group makes it difficult to know if creatine, as opposed to the Hawthorne effect (McCarney, Warner, Iliffe, van Haselen, Griffin, & Fisher, 2007), played a role in reducing depressive and anxiety symptoms and methamphetamine use. Moreover, both HAMD and BAI scores decreased over weeks 0 and 1 before creatine was initiated. It is possible that the participants’ moods improved from the personal attention provided by the research team. Also, the gift card compensation may have influenced changes in mood, particularly considering that three of the participants were homeless.
Second, the lack of a control group and small sample size raises concerns of external validity, and consequently, inferential statistics should be interpreted cautiously. Future studies with a placebo group and/or a control group that does not receive creatine but is assessed over time are warranted. Third, during the consent process, participants were informed that they could be withdrawn from the study for medication noncompliance (e.g., missing more than 3 consecutive creatine doses), and as a result, it is possible that participants discarded creatine prior to the researchers counting empty bottles rather than taking it daily. However, we found increased serum creatinine levels after 8 weeks of creatine treatment, and since creatine converts to creatinine, this suggests at least partial adherence with creatine treatment. Nonetheless, our adherence tracking process may have been inaccurate given that we considered un-returned bottles as missing. Participants may have lost bottles after taking the creatine rather than not returning them because they did not take the creatine, but we erred on the side of being conservative and counted the bottles as missing. Future studies might consider not withdrawing participants from the study for non-adherence, and using non-adherence as a variable to reduce potential dishonesty regarding compliance. In addition, future studies should develop a more reliable measure of adherence such as drawing serum creatinine values at one or more time points during the treatment phase.
Another limitation to consider is that this study enrolled four (28%) females who were dependent on multiple substances. Since the aim of our study focused on methamphetamine use only, it is possible that concurrent substance use confounded the study results. Further, the directionality of our study results is unclear. For example, we are not certain if creatine reduces depression, which in turn reduces methamphetamine use, or if creatine reduces methamphetamine use, thereby decreasing symptoms of depression. Alternatively, perhaps creatine directly affects depression and methamphetamine use. Future studies might consider creatine as a treatment option for female methamphetamine users without depression to evaluate if it has an effect on methamphetamine use.
This study was limited to females with comorbid depression and methamphetamine dependence because preclinical studies suggest creatine has antidepressant effects in female but not male rodents (Allen et al., 2010). Consequently, gender effects of creatine were not evaluated in this study; and therefore, future studies with male methamphetamine users included are necessary. Moreover, in this study, the HAMD criteria of 15 and above was used even though a score of greater than 15 suggests moderate illness (Furukawa et al., 2007). A score of 15 or greater was selected to capture females with dysthymia in addition to moderate to severe depression since dysthymia is also associated with poor treatment outcomes (Katon et al., 2002). Regardless, none of the enrolled participants’ baseline HAMD scores were less than 16. Finally, this study may have been underpowered.
Conclusions
The present study suggests that creatine, when used as a standalone treatment, may be beneficial for treating symptoms of depression and anxiety, methamphetamine dependence and increasing brain phosphocreatine concentrations in methamphetamine using females. Creatine was well tolerated with minimal adverse events and is an inexpensive, easily attainable product Replication of the current study in a larger sample with depressed male and female methamphetamine users is required. Importantly, though, given that there are not any FDA approved treatments for either methamphetamine dependence or comorbid depression and methamphetamine use disorders, interventions with the potential to reduce mood symptoms, improve brain bioenergetics and reduce methamphetamine use are urgently required.
Acknowledgments
We would like to acknowledge AlzChem AG for supplying Creapure® for this study.
Funding
Funding for this study was provided by NIDA grant 1R36DA036767-01 awarded to Dr. Tracy Hellem and by NIDA grant 1K05DA031247-01 awarded to Dr. Perry Renshaw.
Footnotes
The Division of Psychiatry Products at the FDA requires administration of the C-SSRS for safety.
Disclosures
Dr. Perry Renshaw serves as a consultant to Kyowa Hakko, Tal Medical and Ridge Diagnostics. He has received research support from GlaxoSmithKline and Roche. Dr. Perry Renshaw is an inventor on a patent application that has been assigned to the University of Utah, which describes the use of creatine as a treatment for depressive disorders. All other authors declare that they have no conflicts of interest.
Contributor Information
Tracy L. Hellem, Email: tracy.hellem@hsc.utah.edu.
Young-Hoon Sung, Email: yh.sung@utah.edu.
Xian-Feng Shi, Email: xianfeng.shi@hsc.utah.edu.
Marjorie A. Pett, Email: marge.pett@nurs.utah.edu.
Gwen Latendresse, Email: gwen.latendresse@nurs.utah.edu.
Jubel Morgan, Email: jubel.morgan@hsc.utah.edu.
Rebekah S. Huber, Email: rebekah.huber@utah.edu.
Danielle Kuykendall, Email: mkduyk@gmail.com.
Kelly J. Lundberg, Email: kelly.lundberg@hsc.utah.edu.
Perry F. Renshaw, Email: perry.renshaw@hsc.utah.edu.
References
- Adhihetty PJ, Beal MF. Creatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseases. Neuromolecular Medicine. 2008;10(4):275–290. doi: 10.1007/s12017-008-8053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PJ, D’Anci KE, Kanarek RB, Renshaw PF. Chronic creatine supplementation alters depression-like behavior in rodents in a sex-dependent manner. Neuropsychopharmacology. 2010;35(2):534–546. doi: 10.1038/npp.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain Research Bulletin. 2008;76(4):329–343. doi: 10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: Has the gold standard become a lead weight? American Journal of Psychiatry. 2004;161:2163–2177. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bizzarini E, De Angelis L. Is the use of oral creatine supplementation safe? Journal of Sports Medicine and Physical Fitness. 2004;44(4):411–416. [PubMed] [Google Scholar]
- Blumberg RM, Taylor DL, Yue X, Aguan K, McKenzie J, Cady EB, Weiner CP, Mehmet H, Edwards AD. Increased nitric oxide synthesis is not involved in delayed cerebral energy failure following focal hypoxic-ischemic injury to the developing brain. Pediatric Research. 1999;46:224–231. doi: 10.1203/00006450-199908000-00016. [DOI] [PubMed] [Google Scholar]
- Brown EL, Snow RJ, Wright CR, Cho Y, Wallace MA, Kralli A, Russell AP. PGC-1α and PGC-1β increase CrT expression and creatine uptake in myotubes via ERRα. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2014 doi: 10.1016/j.bbamcr.2014.08.010. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Brown ES, Gabrielson B. A randomized, double-blind, placebo-controlled trial of citicoline for bipolar and unipolar depression and methamphetamine dependence. Journal of Affective Disorders. 2012;143:257–260. doi: 10.1016/j.jad.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Burke DG, Chilibeck PD, Parise G, Candow DG, Mahoney D, Tarnopolsky M. Effect of creatine and weight training on muscle creatine and performance in vegetarians. Medicine & Science in Sports & Exercise. 2003;35(11):1946–1955. doi: 10.1249/01.MSS.0000093614.17517.79. [DOI] [PubMed] [Google Scholar]
- Castillo EM, Cornstock RD. Prevalance of use of performance-enhancing substances among United States adolescents. Pediatric Clinics of North America. 2007;54(4):663–675. ix–x. doi: 10.1016/j.pcl.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Current depression among adults -- United States, 2006 and 2008. Morbidity and Mortality Weekly Report. 2010 Oct 1;59(38):1229–1235. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5938a2.htm. [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chen CK, Lin SK, Chen YC, Huang MC, Chen TT, Ree SC, Wang LJ. Persistence of psychotic symptoms as an indicator of cognitive impairment in methamphetamine users. Drug and Alcohol Dependence. 2015;148:158–164. doi: 10.1016/j.drugalcdep.2014.12.035. [DOI] [PubMed] [Google Scholar]
- Chrusch MJ, Chilibeck PD, Chad KE, Davison KS, Burke DG. Creatine supplementation combined with resistance training in older men. Medicine & Science in Sports & Exercise. 2001;33(12):2111–2117. doi: 10.1097/00005768-200112000-00021. [DOI] [PubMed] [Google Scholar]
- Colfax GN, Santos GM, Das M, Santos DM, Matheson T, Gasper J, Vittinghoff E. Mirtazapine to reduce methamphetamine use: A randomized controlled trial. Archives of General Psychiatry. 2011;68(11):1168–1175. doi: 10.1001/archgenpsychiatry.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Naclerio F, Allgrove J, Jimenez A. Creatine supplementation with specific view to exercise/sports performance: An update. Journal of the International Society of Sports Nutrition. 2012;9(1):33. doi: 10.1189/1550-2783-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank CC, Montebello ME, Dyer KR, Quigley A, Blaszczyk J, Tomkins S, Shand D. A placebo-controlled trial of mirtazapine for the management of methamphetamine withdrawal. Drug and Alcohol Review. 2008;27:326–333. doi: 10.1080/09595230801935672. [DOI] [PubMed] [Google Scholar]
- Cunha-Oliveira T, Silva L, Silva AM, Moreno AJ, Oliveira CR, Santos MS. Mitochondrial complex I dysfunction induced by cocaine and cocaine plus morphine in brain and liver mitochondria. Toxicology Letters. 2013;219:298–306. doi: 10.1016/j.toxlet.2013.03.025. [DOI] [PubMed] [Google Scholar]
- Curtin K, Fleckenstein AE, Robison RJ, Crookston MJ, Smith KR, Hanson GR. Methamphetamine/amphetamine abuse and risk of Parkinson’s diease in Utah: A population-based assessment. Drug and Alcohol Dependence. 2015;146:30–38. doi: 10.1016/j.drugalcdep.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ayala RJ. Assessing the reliability of the Beck Anxiety Inventory scores. Educational and Psychological Measurement. 2005;65(5):742–756. doi: 10.1177/0013164405278557. [DOI] [Google Scholar]
- Dechent P, Pouwels PJ, Wilken B, Hanefeld F, Frahm J. Increase of total creatine in human brain after oral supplementation of creatine-monohydrate. American Journal of Physiology. 1999;277(3 Pt 2):R698–704. doi: 10.1152/ajpregu.1999.277.3.R698. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Liu B. Gender differences in methamphetamine use and responses: A review. Gender Medicine. 2008;5(1):24–35. doi: 10.1016/s1550-8579(08)80005-8. [DOI] [PubMed] [Google Scholar]
- Dyer KR, Cruickshank CC. Depression and other psychological health problems among methamphetamine dependent patients in treatment: Implications for assessment and treatment outcome. Australian Psychologist. 2005;40(2):96–108. doi: 10.1080/00050060500094647. [DOI] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Elster C, Schubert F, Link A, Walzel M, Seifert F, Rinneberg H. Quantitative magnetic resonance spectroscopy: Semi-parametric modeling and determination of uncertainties. Magnetic Resonance Medicine. 2005;53(6):1288–1296. doi: 10.1002/mrm.20500. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR axis I disorders - patient edition (with psychotic screen) (SCID-I/P (W/PSYCHOTIC SCREEN), 1/2007 revision) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 2007. [Google Scholar]
- Food and Drug Administration. 2014;312 21 C.F.R. §. [Google Scholar]
- Forester BP, Harper DG, Jensen JE, Ravichandran C, Jordan B, Renshaw PF, Cohen BM. 31Phosphorus magnetic resonance spectroscopy study of tissue specific changes in high energy phosphates before and after sertraline treatment of geriatric depression. International Journal of Geriatric Psychiatry. 2009;24(8):788–797. doi: 10.1002/gps.2230. [DOI] [PubMed] [Google Scholar]
- Furukawa TA, Akechi T, Azuma H, Okuyama T, Higuchi T. Evidence-based guidelines for intepretation of the Hamilton Rating Scale for Depression. Journal of Clinical Psychopharmacology. 2007;27(5):531–533. doi: 10.1097/JCP.0b013e31814f30b1. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Newmeyer J, Knapp T, Stalcup SA, Smith D. A controlled trial of imipramine for the treatment of methamphetamine dependence. Journal of Substance Abuse Treatment. 1996;13(6):493–497. doi: 10.1016/s0740-5472(96)00154-7. [DOI] [PubMed] [Google Scholar]
- Glasner-Edwards S, Mooney LJ, Marinelll-Casey P, Hillhouse M, Ang A, Rawson R. Anxiety disorders among methamphetamine dependent adults: association with post-treatment functioning. American Journal of Addiction. 2010;19(5):385–390. doi: 10.1111/j.1521-0391.2010.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner-Edwards S, Mooney LJ, Marinelll-Casey P, Hillhouse M, Ang A, Rawson R, Authors MTPC. Identifying methamphetamine users at risk for major depressive disorder: Findings from the Methamphetamine Treatment Project at three-year follow-up. The Journal on Addictions. 2008;17:99–102. doi: 10.1080/10550490701861110. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou P, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalance and co-occurrence of substance use disorders and independence mood and anxiety disorders. Results from the National Epidemologic Survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61(8):807–816. doi: 10.001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Groeneveld JG, Veldink JH, van der Tweel I, Kalmijn S, Beijer C, Wokke, van den Berg LH. A randomized sequential trial of creatine in amyotrophic lateral scleoris. Annals of Neurology. 2003;53(4):437–445. doi: 10.1002/ana.10554. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann ON, Fouad K, Wallimann T, Schwab ME. Protective effects of oral creatine supplementation on spinal cord injury in rats. Spinal Cord. 2002;40(9):449–456. doi: 10.1038/sj.sc.3101330. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson AN, Kim S, Cederblom L, Moe A, Ling W, Shoptaw S. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2010;109(1–3):20–29. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellem TL, Lundberg KJ, Renshaw PF. A Review of Treatment Options for Co-Occurring Methamphetamine Use Disorders and Depression. Journal of Addictions Nursing. 2014;26(1):14–23. doi: 10.1097/JAN.000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang YC. Treatment outcomes among women and men methamphetamine abusers in California. Journal of Substance Abuse Treatment. 2005;28(1):77–85. doi: 10.1016/j.jsat.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Iosifescu DV, Bolo NR, Nierenberg AA, Jensen JE, Fava M, Renshaw PF. Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biological Psychiatry. 2008;63(12):1127–1134. doi: 10.1016/j.biopsych.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Jou SH, Chiu NY, Liu CS. Mitochondrial dysfunction and psychiatric disorders. Chang Gung Medical Journal. 2009;32(4):370–379. [PubMed] [Google Scholar]
- Juhn MS, O’Kane JW, Vinci DM. Oral creatine supplementation in male collegiate athletes: A survey of dosing habits and side effects. Journal of the American Dietic Association. 1999;99(5):593–595. doi: 10.1016/s0002-8223(99)00145-5. [DOI] [PubMed] [Google Scholar]
- Katon W, Russo J, Frank E, Barrett J, Williams JW, Oxman T, Cornell J. Predictors of nonresponse to treatment in primary care patients with dysthymia. General Hospital Psychiatry. 2002;24:20–27. doi: 10.1016/s0163-8343(01)00171-2. [DOI] [PubMed] [Google Scholar]
- Kay-Lambkin FJ, Baker AL, Lee NM, Jenner L, Lewin TJ. The influence of depression on treatment for methamphetamine use. Medical Journal of Australia. 2011;195(3):S38–43. doi: 10.5694/j.1326-5377.2011.tb03264.x. [DOI] [PubMed] [Google Scholar]
- Kendall RW, Jacquemin G, Frost R, Bums SP. Creatine supplementation for weak muscles in persons with chronic tetraplegia: A randomized double-blind placebo-controlled crossover trial. Journal of Spinal Cord Medicine. 2005;28(3):208–213. doi: 10.1080/10790268.2005.11753814. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. American Journal of Psychiatry. 1985;142(11):1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Kondo DG, Sung YH, Hellem TL, Fiedler KK, Shi X, Jeong EK, Renshaw PF. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: a 31-phosphorus magnetic resonance spectroscopy study. Journal of Affective Disorders. 2011;135(1–3):354–361. doi: 10.1016/j.jad.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. Journal of Psychiatric Research. 2011;45(5):626–629. doi: 10.1016/j.psychires.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Allen NB, Rogers N, Cementon E, Bonomo Y. The impact of co-occurring mood and anxiety disorders among substance-abusing youth. Journal of Affective Disorders. 2007;103:105–112. doi: 10.1016/j.jad.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Lord KC, Shenouda SK, Mcllwain E, Charalampidis D, Lucchesi PA, Varner KJ. Oxidative stress contributes to methamphetamine-induced left ventricular dysfunction. Cardiovascular Research. 2010;87(1):111–118. doi: 10.1093/cvr/cvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszuk JM, Robertson RJ, Arch JE, Moyna NM. Effect of a defined lacto-ovo-vegetarian diet and oral creatine monohydrate supplementation on plasma creatine concentration. Journal of Strength and Conditioning Research. 2005;19(4):735–740. doi: 10.1519/R-16224.1. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Kong SW, Sung SM, Hirashima F, Parrow A, Hennen J, Renshaw PF. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Research. 2003;123(2):87–100. doi: 10.1016/s0925-4927(03)00046-5. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Yoon S, Kim TS, Hwang J, Kim JE, Won W, Renshaw PF. A randomized, double-blind placebo-controlled trial of oral creatine monohyrdate augmentation for enhanced response to a selective serotonin reuptake inhibitor in women with major depressive disorder. American Journal of Psychiatry. 2012;169:937–945. doi: 10.1176/appi.ajp.2012.12010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallinckrodt CH, Sanger TM, Dubé S, DeBrota DJ, Molenberghs G, Carroll RJ, Tollefson GD. Assessing and interpreting treatment effects in longitudinal clinical trials with missing data. Biological Psychiatry. 2003;53(8):754–760. doi: 10.1016/s0006-3223(02)01867-x. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Baroni S, Picchetti M, Landis P, Silvestri S, Vatteroni E, Dell’Osso Catena. Mitochondrial alterations and neuropsychiatric disorders. Current Medicinal Chemistry. 2011;18:4715–4721. doi: 10.2174/092986711797379221. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: Evidence from positron emission tomography studies with [C-11] WIN-35, 428. Journal of Neuroscience. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh J, Mancino MJ, Feldman Z, Chopra MP, Gentry WB, Cargile C, Oliveto A. Open-label pilot study of modafinil for methamphetamine dependence. Journal of Clinical Psychopharmacology. 2009;29(5):488–491. doi: 10.1097/JCP.0b013e3181b591e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R, Lubman DI, Lee NM, Ross JE, Slade TN. Major depression among methamphetamine users entering drug treatment programs. Medical Journal of Australia. 2011;195(3):S51–S55. doi: 10.5694/j.1326-5377.2011.tb03266.x. [DOI] [PubMed] [Google Scholar]
- Moore CM, Christensen JD, Lafer B, Fava M, Renshaw PF. Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: A phosphorus-31 magnetic resonance spectroscopy study. American Journal of Psychiatry. 1997;154:116–118. doi: 10.1176/ajp.154.1.116. [DOI] [PubMed] [Google Scholar]
- Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. Magma. 2001;12(2–3):141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- Persky AM, Brazeau GA. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharamacological Reviews. 2001;53:161–176. [PubMed] [Google Scholar]
- Ohta K, Mori M, Yoritaka A, Okamoto K, Kishida S. Delayed ischemic stroke associated with methamphetamine use. The Journal of Emergency Medicine. 2004;26(2):165–167. doi: 10.1016/j.jemermed.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Perez JA, Asura EL, Strategos S. Methamphetamine-related strokes: Four cases. The Journal of Emergency Medicine. 1999;17(3):469–471. doi: 10.1016/s0736-4679(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Posner K. Columbia-Suicide Severity Rating Scale (C-SSRS) Columbia University Medical Center; New York: Center for Suicide Risk Assessment; 2010. [Google Scholar]
- Rabchevsky AG, Sullivan PG, Fugaccia I, Scheff SW. Creatine diet supplement for spinal cord injury: influences on functional recovery and tissue sparing in rats. Journal of Neurotrauma. 2003;20(7):659–669. doi: 10.1089/089771503322144572. [DOI] [PubMed] [Google Scholar]
- Renshaw PF, Parow AM, Hirashima F, Ke Y, Moore CM, Frederick B, Cohen BM. Multinuclear magnetic resonance spectroscopy studies of brain purines in major depression. American Journal of Psychiatry. 2001;158:2048–2055. doi: 10.1176/appi.ajp.158.12.2048. [DOI] [PubMed] [Google Scholar]
- Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial dysfunction and psychiatric disorders. Neurochemical Resonance. 2008;34(6):1021–1029. doi: 10.1007/s11064-008-9865-8. [DOI] [PubMed] [Google Scholar]
- Roitman S, Green T, Osher Y, Karni N, Levine J. Creatine monohydrate in resistant depression: A preliminary study. Bipolar Disorders. 2007;9:754–758. doi: 10.1111/j.1399-5618.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- Salo R, Flower K, Kielstein A, Leamon MH, Nordahl TE, Galloway GP. Psychiatric comborbidity in methamphetamine dependence. Psychiatry Research. 2011;186(2–3):356–361. doi: 10.1016/j.psychres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Minabe Y, Ouchi Y, Takei N, Iyo M, Nakamura K, Mori N. Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. The American Journal of Psychiatry. 2003;160(9):1699–1701. doi: 10.1176/appi.ajp.160.9.1699. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Mori N. Methamphetamine-related psychiatric symtpoms and reduced brain dopamine transporters studied with PET. American Journal of Psychiatry. 2001;158(8):1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Zians J, Strathdee SA, Patterson TL. Psychosocial and behavioral correlates of depressed mood among female methamphetamine users. Journal of Psychoactive Drugs, Suppl. 2007;4:353–366. doi: 10.1080/02791072.2007.10399897. [DOI] [PubMed] [Google Scholar]
- Shao L, Martin MV, Watson SJ, Schatzberg A, Akil H, Myers RM, Vawter MP. Mitochondrial involvement in psychiatric disorders. Annals of Internal Medicine. 2008;40(4):281–295. doi: 10.1080/07853890801923753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, Ling W. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2008;96(3):222–232. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Huber A, Peck J, Yang X, Liu J, Jeff D, Ling W. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2006;85(1):12–18. doi: 10.1016/j.drugalcdep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willet JB. Applied longitudinal data analysis: Modeling change and even occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- Smith J, Dahm DL. Creatine use among a select population of high school athletes. Mayo Clinic Proceedings. 2000;75(12):1257–1263. doi: 10.4065/75.12.1257. http://dx.doi.org/10.4065/75.12.1257. [DOI] [PubMed] [Google Scholar]
- Steingard RJ, Yurgelun-Todd DA, Hennen J, Moore JC, Moore CM, Vakili K, Renshaw PF. Increased orbitofrontal cortex levels of choline in depressed adolescents as detected by in vivo proton magnetic resonance spectroscopy. Biological Psychiatry. 2000;48(11):1053–1061. doi: 10.1016/s0006-3223(00)00942-2. [DOI] [PubMed] [Google Scholar]
- Streck EL, Gonçalves CL, Furlanetto CB, Scaini G, Dal-Pizzol F, Quevedo J. Mitochondria and the central nervous system: searching for a pathophysiological basis of psychiatric disorders. Revista Brasileira de Psiquiatria. 2014;36(2):156–167. doi: 10.1590/1516-4446-2013-1224. [DOI] [PubMed] [Google Scholar]
- Su L, Cai Y, Xu Y, Dutt A, Shi S, Bramon E. Cerebral metabolism in major depressive disorder: A voxel-based meta-analysis of positron emission tomography studies. BMC Psychiatry. 2014;14(1):321. doi: 10.1186/s12888-014-0321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Geiger JD, Mattson MP, Scheff SW. Dietary supplement creatine protects against traumatic brain injury. Annals of Neurology. 2000;48(5):723–729. [PubMed] [Google Scholar]
- Sung YH, Yurgelun-Todd DA, Shi XF, Kondo DG, Lundberg KJ, McGlade EC, Renshaw PF. Decreased frontal lobe phosphocreatine levels in methamphetamine users. Drug and Alcohol Dependence. 2013;129(1–2):102–109. doi: 10.1016/j.drugalcdep.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe CG, German D, Sirirojn B, Latkin C, Aramrattana A, Sherman SG, Celentano DD. Patterns of methamphetamine use and symptoms of depression among young adults in northern Thailand. Drug and Alcohol Dependence. 2009;101(3):146–151. doi: 10.1016/j.drugalcdep.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Angoa Perez M, Francescutti-Verbeem DM, Shah MM, Kuhn DM. The role of endogenous serotonin in methamphetamine-induced neurotoxicity to dopamine nerve endings of the striatum. Journal of Neurochemistry. 2010;115(3):595–605. doi: 10.1111/j.1471-4159.2010.06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhamme L, van den Boogart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. Journal of Magnetic Resonance. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Miller EN. Association of dopamine transporter reduction with psychomotor impairement in methamphetamine abusers. American Journal of Psychiatry. 2001;158(3):377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, Logan J. Higher cortical and lower subcortical metabolism in detoxified methamphetamine users. American Journal of Psychiatry. 2001;158(3):383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Volz HP, Rzanny R, Riehemann S, May S, Hegewald H, Preussler B, Sauer H. 31P magnetic resonance spectroscopy in the frontal lobe of major depressed patients. European Archives of Psychiatry Clinical Neuroscience. 1998;248(6):289–295. doi: 10.1007/s004060050052. [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, Gałecki AT. Linear mixed models: A practical guide using statistical software. New York: Chapman & Hall/CRC; 2006. [Google Scholar]
- Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators. Effect of creatine monohydrate on clinical progression in patients with Parkinson Disease: A randomized controlled trial. Journal of American Medical Association. 2015;313(6):584–593. doi: 10.1001/jama.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Annals of the New York Academy of Sciences. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweben JE, Cohen BM, Christian D, Galloway GP, Salindari M, Parent D, Iguchi M. Psychiatric symptoms in methamphetamine users. The American Journal on Addictions. 2004;13:181–190. doi: 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]