Abstract
Background
Ketamine, the NMDA glutamate receptor antagonist drug, is increasingly employed as an experimental model of psychosis in healthy volunteers. At sub-anesthetic doses, it safely and reversibly causes delusion-like ideas, amotivation, and perceptual disruptions reminiscent of the aberrant salience experiences that characterize first-episode psychosis. However, auditory verbal hallucinations (AVHs), a hallmark symptom of schizophrenia, have not been reported consistently in healthy volunteers even at high doses of ketamine.
Methods
Here we present data from a set of healthy participants who received moderately dosed, placebo controlled ketamine infusions in the reduced stimulation environment of the magnetic resonance imaging scanner. We highlight the phenomenological experiences of three participants who experienced particularly vivid hallucinations.
Results
Participants in this series reported auditory verbal and musical hallucinations at a ketamine dose that does not induce auditory hallucination outside of the scanner.
Discussion
We interpret the observation of ketamine-induced AVHs in the context of the reduced perceptual environment of the magnetic resonance scanner, and offer an explanation grounded in predictive coding models of perception and psychosis: the brain fills in expected perceptual inputs and it does so more in situations of reduced perceptual input. The reduced perceptual input of the MRI scanner creates a mismatch between top-down perceptual expectations and the heightened bottom-up signals induced by ketamine; such circumstances induce aberrant percepts including musical and auditory verbal hallucinations. We suggest that these circumstances might represent a useful experimental model of AVHs and highlight the impact of ambient sensory stimuli on psychopathology.
Keywords: ketamine, hallucinations, predictive coding, psychosis, computational psychiatry, prediction, psychosis
Introduction
The introduction of phencyclidine (PCP) and its pharmacologic relative, ketamine, as anesthetic agents in the late 1950s and early 1960s was followed in short order by the recognition of their psychotomimetic effects [1]. In the years since, ketamine has been employed as an experimental model of psychosis, reproducing the positive and negative symptoms of schizophrenia transiently, reversibly and safely in healthy non-psychotic volunteers [2]. A noncompetitive N-methyl d-aspartate (NMDA) receptor antagonist, ketamine has been instrumental in establishing a potential role for glutamatergic signaling in psychosis [3, 4]. Only inconsistently present in the constellation of ketamine-induced symptoms, however, are auditory verbal hallucinations (AVHs), a hallmark of schizophrenia described in 60–80% of patients [5, 6]. Here we evaluate descriptions of perceptual abnormalities produced by acute exposure to PCP and ketamine, and present three cases of hallucinatory experiences with ketamine administration in a functional neuroimaging setting. We attempt to explain the inconsistent presence of hallucinations following ketamine within a predictive coding framework.
Table 1 highlights the perceptual abnormalities reported in placebo-controlled investigations of the arylcyclohexamine family of noncompetitive NMDA antagonists, of which ketamine and PCP are members. Notable similarities among the studies include the amplification or sharpening of sensory input [2, 7, 8] and alteration of the spatial and temporal relationships within or among individual sensory streams [1, 2, 9, 10]. Hallucinations are described only intermittently and, in many cases, vaguely enough for the discerning reader to suspect conflation with sensory illusions—that is, distortions or amplifications of stimuli that are actually present (see [8, 11]). Some of the earliest accounts of phencyclidine (PCP) and ketamine responses describe memory-driven dissociative experiences that were rich in sensory detail. Luby and colleagues [1] described so-called “hypnogogic states” produced by PCP:
“The subjects reported feeling as though they were in some specific setting and were able to describe it in detail. While the reports typically had reference to past events, they were expressed as though the experiences were taking place at the moment.”
Table 1.
Characteristics and findings of systematic studies of psychotomimetic properties of PCP and ketamine.
| Authors | Year | Design | Sample | NMDA Antagonist |
Dose | Perceptual Findings |
|---|---|---|---|---|---|---|
| Luby et al.[1] | 1959 | Non-placebo-controlled PCP administration | 9 TD; 9 SZ |
Phencyclidine (PCP) | 0.1mg/kg IV for 12 mins | Perceived limb lengthening; feelings of flying; complete change in setting to past experience; “sensory experiences were overvalued if not eroticized”; mild diminution in auditory and visual acuity |
| Lawes[7] | 1963 | 2×3 dosage group vs. environmental stimulation level | 8 TD | Phencyclidine (PCP) |
Group A (N = 5): 5mg PO Group B (N = 3): 10mg PO |
Amplification or alteration of quality of sounds, sharpening of vision, room expansion; body “lightness”; dose-response relationship observed |
| Oye [60] | 1992 | Double-blind crossover with 4 doses of 2 enantiomers, 2 placebo doses | 6 TD | Ketamine |
(S)-ketamine: 0.05, 0.1,0.15,0.2 mg/kg IV (R)-ketamine: 0.2, 0.4,0.6,0.8 mg/kg IV |
Blurred vision, visual field narrowing; preoccupation with irrelevant environmental stimuli; sound amplification |
| Krystal et al.[2] | 1994 | Double-blind placebo-controlled 2-dose level crossover | 19 TD | Ketamine |
Low dose: 0.1mg/kg IV High dose: 0.5mg/kg IV |
Increased perceived intensity or closeness within a discrete spatial field; no outright hallucinations noted |
| Vollenweider et al. [41] | 1997 | Within-subject placebo controlled counterbalanced | 10 TD | Ketamine | Loading dose of 15mg IV racemic ketamine followed by continuous infusion 0.014–0.02mg/kg/min | Visual illusions as well as complex hallucinations present with (S)-ketamine administration; auditory hyperacusis but no hallucinations noted |
| Bowdle et al.[11] | 1998 | Single-blind placebo-controlled crossover | 10 TD | Ketamine | IV administration set to keep plasma concentration at 0, 50, 100, 150, 200ng/ml in progressive steps | Hallucinations as rated on a visual analogue scale (“I heard voices or sounds that were not real”) differed significantly between ketamine and saline and correlated with plasma concentration; these experiences not described further. |
| Newcomer et al.[10] | 1999 | Double-blind placebo-controlled 3-dose level | 15 TD | Ketamine |
Loading: 0.27 mg/kg, 0.081 mg/kg, 0.0243 mg/kg IV Maintenance: 0.00225 mg/kg/min, 0.000675 mg/kg/min, and 0.0002025 mg/kg/min |
Visual distortions (e.g., hands perceived as shrunken, “shadow of a body falling” noted in window); no outright hallucinations noted |
| Lahti et al.[40] | 2001 | 2×2 Group by placebo vs. drug crossover | 18 TD; 17 SZ |
Ketamine | 0.3mg/kg IV | Formed auditory hallucinations (e.g., articulated voices) in SZ subjects (despite low levels of positive symptoms outside drug administration), and in a minority of TD subjects; visual and olfactory hallucinations in both groups |
| Gouzoulis et al. | 2005 | Double-blind 2-drug, 2-dose crossover | 15 TD | Ketamine and N,N-dimethyltryptamine (DMT) |
Ketamine: 0.1mg/kg IV (Low) and 0.2mg/kg IV (High) DMT: 0.15mg/kg (Low) and 0.3mg/kg(High) |
Limb lengthening and other somatic distortions; visual hallucinations present in 1 subject on ketamine; auditory distortions but no hallucinations reported. DMT with more pronounced visual and auditory phenomena, and musical hallucinations and whispers occurred in 2 cases; visual hallucinations on DMT were present in all subjects at high dose levels |
| Pamarol-Clotet et al.[8] | 2006 | Experimental case series for phenomenological description | 15 TD | Ketamine | IV administration set to keep plasma concentration at 100ng/ml, then 200ng/ml | Increased sensitivity to noise and increased brightness of colors; loss of visual depth perception; alterations of perceived size, shape, or position of body parts; one participant reported sensing the presence of more people than were visibly present and hearing voices that were unable to be accounted for by those present. |
The authors provide a striking example: a participant who experienced the full sensory array associated with a scene from his childhood:
“He stated that he was in his third-grade classroom, which he described elaborately, including the recognition of an old friend in the classroom.”
This is echoed in a later description of prominent eidetic imagery (but otherwise a lack of outright hallucinations) by Stone and colleagues during ketamine administration in healthy volunteers [12]. Similarly, the earliest descriptions of ketamine’s psychotomimetic effects include the presence of memory-like “frank hallucinations,” although these are not characterized in more detail [13]. Indeed, discerning such dissociative or illusory experiences from ‘true’ hallucinations has posed a practical problem in this literature. Some, like Newcomer and colleagues, solved the dilemma by rating all perceptual abnormalities as hallucinations in standard scales like the Brief Psychiatric Rating Scale (BPRS [14]) while stating separately that these may not represent true hallucinations [10], while others have maintained that true hallucinatory experiences have not arisen in trials of ketamine administration and the perceptual effects of ketamine are better characterized as illusions or sensory distortions [2, 7, 8].
Despite this seeming paucity of reports describing hallucinations in the setting of controlled administration of ketamine, several case reports do describe hallucinations during acute treatment with ketamine and in the hours, days, and weeks following dosing. One such case report describes vivid visual hallucinations associated with recovery from ketamine anesthesia [15] with spontaneous resolution and intact memories for said phenomena (typically referred to as emergence phenomena [13]). A similar emergence pattern was observed in another study [16] meant to examine the rate of psychiatric adverse reactions in patients in a general hospital setting receiving intravenous ketamine at sub-anesthetic doses (mean = 0.19mg/kg/hr) for pain control. Here the authors reported hallucinations in 4 of 50 patients, although they did not elaborate further on the nature of these phenomena. Intriguingly, these hallucinatory emergence phenomena have at times been reported to extend beyond the acute phase of administration. One such case report describes an incident in which an eleven-year-old boy experienced a vivid visual hallucination of his grandmother at his bedside during emergence from ketamine anesthesia but also sporadically for 5 days following initial recovery from anesthesia while lying in bed [17]. In another pediatric case, Meyers and Charles [18] describe the experience of a 3-year-old girl who reported malicious red and green lights in the room 30 minutes after recovery from ketamine anesthesia but continued to report similar phenomena at least one day afterward and even developed fears of traffic lights. In this case, nightmares involving her hallucinatory experience persisted for one year after ketamine anesthesia. Similarly, Fine and Finestone [19] report vivid dreams in 80% of patients given ketamine at anesthetic doses and provide 3 cases of visual hallucinations in these patients, who continued to experience flashback-like visual disturbances in the weeks following administration. The first case is that of a vivid dream of riding a rocket downward, then recurrence of these sensations for 3 weeks afterward, but only when descending a staircase; the second case was similar, with floating/flying sensations noted during recovery from ketamine and a 1-week history of the same sensation afterward; the last case describes vivid dreams of seeing faceless people around the recovery bed followed by one week of experiencing the same visions after lying down in bed. All of these experiences resolved spontaneously after several months.
Despite the well-documented nature of the perceptual disturbances often experienced with ketamine administration, there remains significant discrepancy regarding the presence, characteristics, and environmental settings necessary for the experience of hallucinations induced by NMDA antagonists. Here we present data from participants taking part in a pharmacological functional neuroimaging study [20], highlighting the phenomenological experiences of three participants who experienced particularly vivid hallucinations, and discuss the implications of these findings for the role of predictive coding in hallucinations.
Methods
The present data were drawn from a double-blind, placebo-controlled, randomized, within-subjects design. Subjects completed 3 visits, prior to which they were randomly assigned to pre-treatment with a different dose of a partial allosteric modulator (PAM) of metabotropic glutamate receptors (mGlur, 0mg/placebo, 50mg or 180mg). In the MRI scanner, during saline and ketamine administration, subjects completed a delayed spatial WM task described in detail in a prior publication [21].
Subjects
All subjects provided informed consent approved by the Institutional Review Board at Yale University. Nineteen healthy, neurologically and psychiatrically intact right-handed volunteers (10 male) with a mean±SD age of 27.5±6.3 years were recruited from the local community by advertisement. Subjects were screened using an initial telephone interview and subsequent personal interview. Psychiatric or physical illness, head injury, drug or alcohol dependence, and smoking were excluding factors, as were family history of psychiatric illness and alcohol problems.
Infusion Protocol
Subjects were administered racemic ketamine (1 mg/mL, Bedford Laboratories) intravenously via initial bolus (0.23mg/kg to be administered over 1 minute) followed by subsequent continuous infusion of 0.58 mg/kg/hr for 75 minutes. Prior studies from our laboratory using very similar infusion paradigms consistently produced plasma ketamine levels during infusion of approximately 200 ng/ml [22] and reliably and transiently produced positive symptoms, negative symptoms, and cognitive impairments redolent of schizophrenia [23]. This infusion approach produces stable effects across repeated test days on most behavioral outcome measures [24] and does not appear to be associated with persisting negative effects [23].
Clinical Measures
Subjects underwent clinical rating scales before and after infusions: here we focus on the Positive and Negative Syndrome Scale (PANSS [25]), which is designed to assess positive, negative and general psychotic symptoms. It should be noted that, unlike other uses of the PANSS in the setting of ketamine administration (e.g. Newcomer ref), our approach rated perception in the absence of physical stimulus (true hallucinations) on the P3 Hallucinatory Behavior item and did not take into account perceptual distortions or illusions. It should also be noted that these ratings reflected phenomena in any of the sensory systems (auditory, visual, olfactory/gustatory, or somatic) and did not necessarily reflect the presence of auditory hallucinations (although see vignettes below, the hallucinations are primarily auditory, some visual and somatic and rarely olfactory/gustatory).
We also report phenomenological descriptions from three participants who experienced auditory verbal and musical hallucinations while in the MRI scanner on ketamine (rated on the PANSS as a score or 3 or higher). We only considered quantitative and qualitative data from study visits when individuals were pre-treated with 0mg mGlur PAM (thus, “placebo” here refers to the period of saline infusion, which always preceded the period of ketamine infusion). Other clinical and neuroimaging data will be the subject of future reports.
Results
Ketamine-induced symptoms
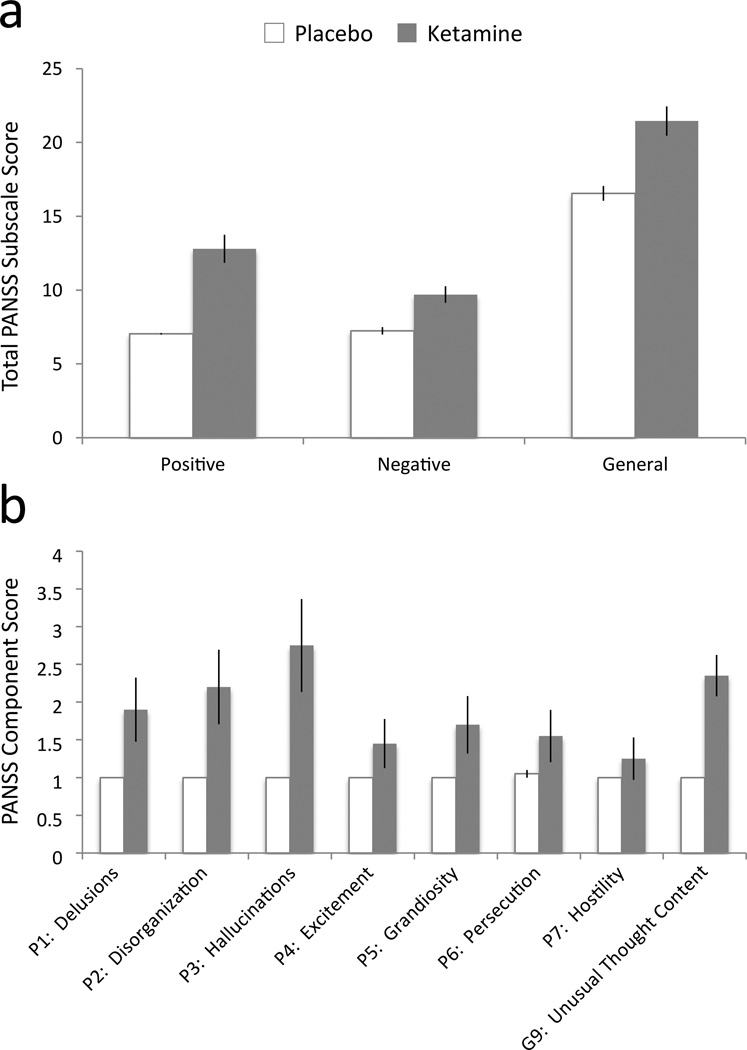
Analysis of symptom scores was performed on the entire sample of 19 participants. Compared to ratings prior to the infusion, ketamine induced positive, negative and general symptoms (see Figure 1). Repeated-measures analysis of variance with drug (placebo/ketamine) and symptom (positive, negative and general) factors revealed a significant main effect of drug (F 1,19= 45.8, p=0.001), symptom (F2,38 =263.2, p=0.0001) and a significant drug by symptom interaction (F1.8,34.5 =6.9, p=0.004, applying Huyhn-Feldt correction for non-sphericity of the covariance matrices). Post-hoc tests revealed that ketamine engendered more positive than negative symptoms (t=2.9, d.f. = 19, p = 0.008) and more general than negative (t=13.3, d.f = 19, p = 0.0001) or positive symptoms (t=13.3, d.f = 19, p = 0.0001). For purposes of the present study, we examined the positive symptoms more closely with a similar analysis: repeated-measures analysis of variance with drug (placebo/ketamine) and symptom dimension (Delusions, Hallucinations, Conceptual Disorganization, Hostility, Persecution, Excitement, Grandiosity, Unusual Thought Content). This revealed a main effect of drug (F1,19 = 36.4, p=0.0001), symptom dimension (F5,92 = 8.4, p=0.009) and a significant drug by symptom dimension interaction (F 1,19= 8.79, p=0.008; applying Huyhn-Feldt correction). Post-hoc tests revealed that hallucinations were the most common symptom, with hallucination scores exceeding those of the other PANSS items (e.g., P3 (Hallucinatory behavior) vs. P1 (Delusions), t=5.01, d.f. = 19, p=0.001).
Figure 1. Ketamine-induced symptoms.
a. Total PANSS subscale scores for all 19 participants tested after placebo (white) and ketamine (gray) administration. b. PANSS component scores for all positive symptom (P1–P7) and one general symptom dimension (G9). Analysis revealed a main effect of drug (F1,19 = 36.4, p=0.0001), symptom dimension (F5,92 = 8.4, p=0.009) and a significant drug by symptom dimension interaction. Post-hoc tests revealed that hallucinations were the most common symptom, with hallucination scores exceeding those of the other PANSS items. Error bars = 1 SEM.
Case Descriptions
Case 1. Subject 23
Hallucinations Component Score (P3) = 5, Total Positive Symptoms Score (Ptot) = 16
This 21-year-old female graduate student in political science volunteered for our research study and passed our screen for safety and appropriateness of participation. She described herself as a soccer fan and had been watching a lot of soccer the day before the infusion (including a penalty shoot-out in the World Cup). One of the authors (PRC) had discussed this shoot-out with her before the infusion. As the infusion began, she felt that the computer screen in front of her turned into a soccer pitch and she could see the players on it. She described how the head coil transformed into a soccer goal. She felt transported to a specific soccer game, the 2005 FIFA Champion’s League Final in Istanbul, Turkey, between Liverpool FC and AC Milan – believing she was there. She could hear the Liverpool fans in the crowd singing “You’ll Never Walk Alone.” She felt irritated because in the first half of the game, Liverpool conceded 3 goals. But she knew what would happen after half-time (Liverpool scored 3 goals to tie the game and won in a penalty shoot-out), and felt superior to the others in the crowd around her because they did not know this and she would not share it with them.
Case 2. Subject 30
Hallucinations Component Score (P3) = 5, Total Positive Symptoms Score (Ptot) = 16
This 21-year-old female graduate student in robotics volunteered and qualified for our research study. When the infusion began and her first blood sample was drawn (for plasma ketamine assay), she became concerned that something had gone awry with the experiment. She thought that she might have died and that her left arm had disappeared. She reported thinking more about life and its purpose and that her philosophy was “somewhere between Dr. Who and the Matrix” (she had been watching Dr. Who the day before). She asked, “What if existence isn’t real? What if everyone is a figment of my imagination?” She described less control over her thoughts and that one of the authors (PRC) had taken control of her thoughts whilst she stood back. At the same time, she described being able to predict the trials of a working memory task before they happened. She felt disconnected from her body and she imagined she was talking whilst in the scanner, receiving the infusion. When told that she hadn’t been talking during the infusion, she reported that her thoughts were being broadcast out loud. She reported hearing the voices of friends and family members talking, reassuring her about her future. She described how the noise of the MRI scanner came to sound like drums and then more complete music, music she had not heard before. Overall she rated her experiences as pleasant and enjoyable.
Case 3. Subject 42
Hallucinations Component Score (P3) = 4, Total Positive Symptoms Score (Ptot) = 17
This 30-year-old male real estate agent from the United Kingdom participated in our study having passed our screening telephone call and in-person interview. He described the ketamine infusion as “like a tidal wave” that made him think more about black holes and cosmology. He described feeling confused and “befuddled,” which made him distrust the study team that was working with him but at the same time feeling that we were his “oldest and best friends.” He described the world becoming “spaghetti-like”—vertical lines pervaded his vision. His inner speech, the thoughts in his head, became very clear at times and dulled at others. Being in the scanner, he saw part of his face reflected in the mirror used to present tasks, and felt he looked distorted, as though he had undergone plastic surgery (“I looked like Joan Rivers”). He felt at times he was being brainwashed (“like in A Clockwork Orange” – the novel by Anthony Burgess and the movie by Stanley Kubrick) and that although he was stationary in the scanner, he was also moving. He described the noise of the scanner turning into music and that the music changed his vision—he heard the Nutcracker Suite and saw the wooden soldiers, he heard Yellow Submarine by the Beatles and saw the animated characters from that movie, and he heard the entirety of the album Diamond Dogs by David Bowie.
Discussion
Despite its reputation as a hallucinogen, sub-anesthetic ketamine does not consistently produce frank hallucinations. We report three cases of placebo-controlled ketamine administration that produced frank musical and auditory verbal hallucinations accompanied by dissociation and a loss of insight. The experiences of these three participants, while discussed in detail here because of the finer points of their phenomenology, are consistent with the larger sample of subjects participating in this paradigm and thus represent a departure from the historically inconsistent inclusion of hallucinations among the symptoms produced by ketamine.
We now attempt to reconcile these disparate reports by considering how hallucinations arise within the information-processing hierarchy of the brain and why the relatively sparse sensory environment of the MRI scanner might be particularly conducive to the genesis of hallucinations under ketamine. A number of recent works (see [26–28]) have posited a Bayesian framework for understanding the relationship between top-down predictive coding and bottom-up inference in perceptual processing. We propose that ketamine perturbs the balance between priors, prediction errors and their precision [29], such that bottom-up noise is enhanced and top-down priors attempt to accommodate the noise [27]. There are rodent [30] and human [31] data that support this notion.
Additionally, quantitative approaches to ketamine’s effects on sensory processing have produced results that match qualitative reports of sensory amplification. Plourde and colleagues [32] determined that, unlike most general anesthetics, ketamine produces an increase in auditory event-related gamma-band oscillations in patients administered anesthetic doses. Similar increases in gamma-band oscillations have also been demonstrated in somatosensory evoked potentials in subjects administered sub-anesthetic ketamine[13]. In the context of predicted normal ambient input, top-down priors can be mobilized, resulting in delusion-like interpretations of aberrant salience, but not hallucinations [27] (Figure 2, left panels). However, in the reduced perceptual environment of the MRI scanner (Figure 2, right panels), perceptual expectations are already being violated, which triggers prediction error responses that are exacerbated by ketamine. A more robust top-down response is mounted (as a result of the sensory isolation) and hallucinations result. We have proposed that this enhanced top-down response is mediated by the slow neuromodulators dopamine and acetylcholine [27], which have been shown to mediate attentional gain on incoming sensory information [33] and encode salience of environmental stimuli [34].
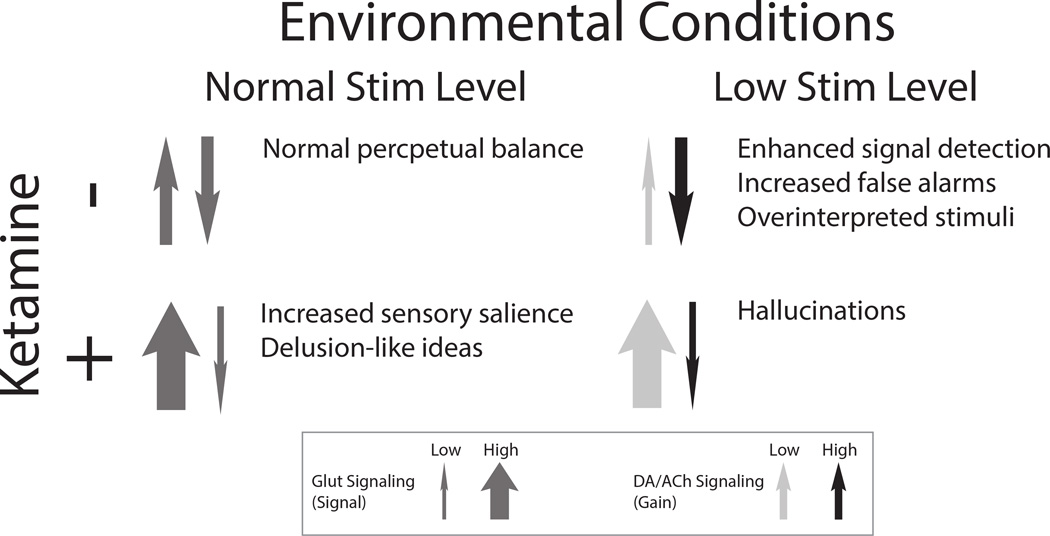
Figure 2. Influence of environmental conditions and ketamine administration on perceptual processing.
In the absence of ketamine and under everyday environmental conditions (top left panel), a balanced equilibrium between bottom-up (upward arrow) and top-down (downward arrow) processing in perceptual inference. If environmental information is minimized, resulting in decreased signal (via bottom-up AMPA-dependent glutamate signaling; top right panel, narrowed upward arrow), improved perceptual performance is achieved by increasing the (neuromodulator-mediated) gain on top-down priors in perceptual inference (darkened downward arrow). In the presence of ketamine (bottom panels), which blocks NMDA-mediated top-down signaling and boosts AMPA-mediated bottom-up signaling (bottom left panel; narrow downward arrow, widened upward arrow), increased sensory salience is observed and delusion-like ideas result. However, in the setting of minimized environmental stimulation, the (normally adaptive) increased gain on top-down priors seen in this setting combines with increased bottom-up prediction errors to produce experience-related hallucinations.
Efforts in computational modeling of perception have had some success using simple neural network models to help explain the occurrence of hallucinations in cases of sensory deprivation like Charles- Bonnet Syndrome [28], in which deafferentation of lower levels of the visual processing hierarchy gives rise to homeostatic mechanisms that increase excitability of the network and result in spontaneous activation of complex perceptual representations in deep association layers. This is plausible given the model’s performance in rapid deafferentation and the rapid effects of homeostatic mechanisms meant to keep neural firing within its optimal dynamic range [28]. Similar phenomena have been described in the auditory system. Indeed, most investigations of musical hallucinations have focused on their tendency to arise in the context of deafferentation of the central auditory processing hierarchy via sensory deprivation or, most commonly, progressive sensorineural hearing loss [35–37]. However, recent work has investigated musical hallucinations occurring in the setting of cochlear implantation [38, 39]. In the most recent study only 7 of the 18 patients reporting musical hallucinations had such experiences before cochlear implantation [38]. The remainder (61%) developed the phenomenon following the operation. Of these 7, many reported that cochlear implant placement had made the musical hallucinations less prominent but 2 reported that they became louder with implantation [38]. We speculate that implantation introduces new sensory inputs that have yet to be properly accommodated by top-down priors and, during that process, musical hallucinations arise as attempts to accommodate the new inputs in a system accustomed to the absence of bottom-up information. If true, this process would predict the observation of hallucinations in ketamine under conditions of sparse sensory input as described here.
With this framework in mind we may return to the case reports reviewed in the Introduction and in Table 1. It is notable that most reports of true hallucinatory phenomena follow emergence from higherdose ketamine anesthesia (as reviewed in Introduction). In those studies employing placebo-controlled sub-anesthetic ketamine it is often unclear whether reports and ratings of hallucinations were in fact illusions (i.e., distortions of stimuli that were actually present [10]). However, among the 10 studies of controlled ketamine administration listed, we may identify 4 that reported the occurrence of true hallucinations in any sensory modality [9, 11, 40, 41]. The first of these studies [41] took place in a PET scanner and reported complex visual (but not auditory) hallucinations; given the relatively quiet and visually open nature of PET scans when compared with MRI scanners, this observation may be consistent with the model proposed here. Lahti and colleagues [40] administered ketamine to individuals with a diagnosis of schizophrenia and to typically developing controls. Crucially, while controls were noted not to experience true hallucinations, patients were noted to experience well-formed hallucinations (such as voices), a fact that may be related to altered dopaminergic signaling in these individuals. Similar conclusions on the role of neuromodulators in hallucinations may be drawn from Gouzoulis and colleagues’ [9] investigation of the psychological impact of S-ketamine and the serotonergic hallucinogen N,N,-dimethyltryptamine (DMT), which highlights the relative prevalence of visual hallucinations and pervasiveness of auditory hallucinations during DMT administration but only one occurrence of visual hallucination (cartoons running across a computer screen) during S-ketamine administration at the highest dose tested. Unfortunately, a washout period was included and no effect of order was observed, although given the model proposed here one may speculate that the prevalence of hallucinations under ketamine would increase even in subclinical plasma concentrations of DMT. Lastly, Bowdle and colleagues [11] conducted a single-blind placebo-controlled crossover study with controlled plasma concentrations of ketamine and noted that plasma concentration of ketamine correlated significantly with a visual analogue scale rating on the item, “I heard voices or sounds that were not real” but findings were not elaborated upon beyond this and no details are available in the manuscript detailing the environment in which participants produced these ratings. Similarly scanty detail regarding the environmental stimulus level is made available in those studies definitively noting the absence of true hallucinations in the presence of ketamine.
The data analyzed here are derived from only one of several studies in which ketamine was administered during fMRI data acquisition. Unfortunately, the majority of these [42–49] do not specifically address perceptual abnormalities in subjects, providing only general PANSS scores. Other studies, similar to those noted above, do report an increased rate of auditory perceptual abnormalities under ketamine but do not differentiate between frank hallucinations and sensory distortions or illusions [50, 51]. Two studies have specifically commented on the occurrence of hallucinations in an fMRI setting [52, 53], noting an increased rate of perceptual abnormalities during ketamine administration, but both separately describing an absence of frank hallucinations in subjects. It is notable that in both studies the ketamine infusion rate (target plasma concentration of 100ng/ml [52] and continuous infusion of 0.25mg/kg/hr) was markedly lower than that used here (0.58mg/kg/hr), and may represent a dose-related manipulation of the system we propose: as NMDA blockade/AMPA potentiation increases with dose, so do the prediction error signals propagated up the hierarchy, further increasing the need for top-down influence to explain them and resulting in hallucinations. However, further studies will be required to fully test the effect of ketamine dose upon hallucination propensity under conditions of relative sensory deprivation.
We note that the observations made here pertain to acute intoxication with ketamine. Less is known about chronic exposure. Lim and colleagues[54] describe a case series of two patients with chronic exposure to ketamine averaging 4–5 months, who report enhanced sensation of environmental stimuli (e.g., taste, smells, music) observed in many acute studies but also report the occurrence of true auditory, visual, and tactile hallucinations with chronic use. Chronic exposure leads to tolerance to sub-anesthetic ketamine [55]. In prior work we speculated that acute and chronic ketamine differ in their impact on processes of learning and inference [29]. That is, acute ketamine is transient and reversible. As such, it impacts sensory inferences and, whilst on board, vitiates perception. However, as it is metabolized, its synaptic effects subside and it does not have long-lasting effects on learning (i.e., it does not engender long term changes in perceptual priors). However, chronic ketamine abuse appears not only to engage homeostatic changes in glutamate signaling, it also alters slower monoaminergic and cholinergic function [56, 57]. These neuromodulators appear to code the gain on perceptual and learning prediction errors—i.e., the volatility or expected uncertainty of those parameters. Longer-term changes in those parameters may broaden the perceptual possibility space and induce what Hoffman has referred to as a “listening attitude”: broadly, once a threshold of hallucinatory experiences has been surpassed, the patient “expects” to hallucinate (although not necessarily consciously). We believe this process is dopaminedriven and secondary to prolonged aberrant glutamate and GABA signaling [27, 29]. D2 dopamine antagonists do not reverse the acute effects of ketamine [58]. However, consistent with our hypothesis, D2 antagonism does seem to ameliorate symptoms in ketamine abuse [59].
In summary, we report qualitative and quantitative data that suggest ketamine administration in the MRI scanner induces auditory hallucinations at a dose that would not induce such symptoms outside of the scanner. We believe that this observation may have bearing on our use of ketamine to model psychotic symptoms and our understanding of those symptoms in terms of the balance between top-down and bottom-up signaling in the Bayesian brain.
Acknowledgements
A.R. Powers was supported by the Integrated Mentored Patient-Oriented Research Training (IMPORT) in Psychiatry grant (5R25MH071584-07) as well as the Clinical Neuroscience Research Training in Psychiatry grant (5T32MH19961-14) from the NIMH. Additional support was provided by the Yale Detre Fellowship for Translational Neuroscience. Data from the original study were obtained with the support of the Wellcome Trust and the Bernard Wolfe Health Neuroscience fund. The study was conducted at the Wellcome Trust Clinical Research Facility and the Wolfson Brain Imaging Centre (Addenbrooke’s Hospital, Cambridge) and within the Behavioral and Clinical Neuroscience Institute supported by the Medical Research Council and the Wellcome Trust. P.R. Corlett was supported by the Connecticut State Department of Mental Health and Addiction Services. P.R. Corlett was funded by an IMHRO / Janssen Rising Star Translational Research Award and CTSA Grant Number UL1 TR000142 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. P.R. Corlett consults for Otsuka Pharmaceuticals.
References
- 1.Luby ED, et al. Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry. 1959;81(3):363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- 2.Krystal JH, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 3.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 4.Javitt DC, Freedman R. Sensory Processing Dysfunction in the Personal Experience and Neuronal Machinery of Schizophrenia. Am J Psychiatry. 2015;172(1):17–31. doi: 10.1176/appi.ajp.2014.13121691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slade PD, Bentall RP. The Johns Hopkins series in contemporary medicine and public health. Baltimore: Johns Hopkins University Press; 1988. Sensory deception : a scientific analysis of hallucination; p. 275. x. [Google Scholar]
- 6.Wing JK, Cooper JE, Sartorius N. Measurement and classification of psychiatric symptoms; an instruction manual for the PSE and Catego Program. London, New York: Cambridge University Press; 1974. p. 233. x. [Google Scholar]
- 7.Lawes TGG. Schizophrenia, `Sernyl' and Sensory Deprivation. The British Journal of Psychiatry. 1963;109(459):243–250. [Google Scholar]
- 8.Pomarol-Clotet E, et al. Psychological effects of ketamine in healthy volunteers. Phenomenological study. Br J Psychiatry. 2006;189:173–179. doi: 10.1192/bjp.bp.105.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gouzoulis-Mayfrank E, et al. Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry. 2005;38(6):301–311. doi: 10.1055/s-2005-916185. [DOI] [PubMed] [Google Scholar]
- 10.Newcomer JW, et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20(2):106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 11.Bowdle TA, et al. Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations. Anesthesiology. 1998;88(1):82–88. doi: 10.1097/00000542-199801000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Stone JM, Pilowsky LS. Psychopathological consequences of ketamine. Br J Psychiatry. 2006;189:565–566. doi: 10.1192/bjp.189.6.565b. [DOI] [PubMed] [Google Scholar]
- 13.Domino EF, Chodoff P, Corssen G. Pharmacologic Effects of Ci-581, a New Dissociative Anesthetic, in Man. Clin Pharmacol Ther. 1965;6:279–291. doi: 10.1002/cpt196563279. [DOI] [PubMed] [Google Scholar]
- 14.Overall JE. The Brief Psychiatric Rating Scale in psychopharmacology research. In: Olivier-Martin PPR, editor. Psychological measurements in psychopharmacology. S. Karger: Oxford, England: 1974. p. 267. [Google Scholar]
- 15.Shaw IH, Moffett SP. Ketamine and video nasties. Anaesthesia. 1990;45(5):422. doi: 10.1111/j.1365-2044.1990.tb14815.x. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen KG. Psychiatric side effects of ketamine in hospitalized medical patients administered subanesthetic doses for pain control. Acta Neuropsychiatr. 2014;26(4):230–233. doi: 10.1017/neu.2013.61. [DOI] [PubMed] [Google Scholar]
- 17.Perel A, Davidson JT. Recurrent hallucinations following ketamine. Anaesthesia. 1976;31(8):1081–1083. doi: 10.1111/j.1365-2044.1976.tb11948.x. [DOI] [PubMed] [Google Scholar]
- 18.Meyers EF, Charles P. Prolonged adverse reactions to ketamine in children. Anesthesiology. 1978;49(1):39–40. doi: 10.1097/00000542-197807000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Fine J, Finestone SC. Sensory disturbances following ketamine anesthesia: recurrent hallucinations. Anesth Analg. 1973;52(3):428–430. [PubMed] [Google Scholar]
- 20.Corlett PR, et al. Ketamine effects on memory reconsolidation favor a learning model of delusions. PLoS One. 2013;8(6):e65088. doi: 10.1371/journal.pone.0065088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anticevic A, et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(41):16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Driesen NR, et al. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 2013;18(11):1199–1204. doi: 10.1038/mp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry EB, Jr, et al. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology. 2007;192(2):253–260. doi: 10.1007/s00213-007-0706-2. [DOI] [PubMed] [Google Scholar]
- 24.Krystal JH, et al. Dissociation of ketamine effects on rule acquisition and rule implementation: possible relevance to NMDA receptor contributions to executive cognitive functions. Biol Psychiatry. 2000;47(2):137–143. doi: 10.1016/s0006-3223(99)00097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 26.Friston KJ. Hallucinations and perceptual inference. Behavioral and Brain Sciences. 2005;28(6):764-+. [Google Scholar]
- 27.Corlett PR, Frith CD, Fletcher PC. From drugs to deprivation: a Bayesian framework for understanding models of psychosis. Psychopharmacology (Berl) 2009;206(4):515–530. doi: 10.1007/s00213-009-1561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reichert DP, Series P, Storkey AJ. Charles Bonnet syndrome: evidence for a generative model in the cortex? PLoS Comput Biol. 2013;9(7):e1003134. doi: 10.1371/journal.pcbi.1003134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corlett PR, et al. Glutamatergic model psychoses: prediction error, learning, and inference. Neuropsychopharmacology. 2011;36(1):294–315. doi: 10.1038/npp.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran RJ, et al. Losing control under ketamine: suppressed cortico-hippocampal drive following acute ketamine in rats. Neuropsychopharmacology. 2015;40(2):268–277. doi: 10.1038/npp.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt A, et al. Mismatch negativity encoding of prediction errors predicts S-ketamine-induced cognitive impairments. Neuropsychopharmacology. 2012;37(4):865–875. doi: 10.1038/npp.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plourde G, Baribeau J, Bonhomme V. Ketamine increases the amplitude of the 40-Hz auditory steady-state response in humans. Br J Anaesth. 1997;78(5):524–529. doi: 10.1093/bja/78.5.524. [DOI] [PubMed] [Google Scholar]
- 33.Herrero JL, et al. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454(7208):1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 35.Berrios GE. Musical hallucinations. A historical and clinical study. Br J Psychiatry. 1990;156:188–194. doi: 10.1192/bjp.156.2.188. [DOI] [PubMed] [Google Scholar]
- 36.Aziz VM, Asaad M. Musical hallucination in acquired and pre-lingual deafness. Int Psychogeriatr. 2011;23(6):1015–1017. doi: 10.1017/S1041610211000809. [DOI] [PubMed] [Google Scholar]
- 37.Mahendran R. The psychopathology of musical hallucinations. Singapore Med J. 2007;48(2):e68–e70. [PubMed] [Google Scholar]
- 38.Low WK, et al. Musical ear syndrome in adult cochlear implant patients. J Laryngol Otol. 2013;127(9):854–858. doi: 10.1017/S0022215113001758. [DOI] [PubMed] [Google Scholar]
- 39.Auffarth IS, Kropp S. Musical hallucination in a patient after cochlear implantation. J Neuropsychiatry Clin Neurosci. 2009;21(2):230–231. doi: 10.1176/jnp.2009.21.2.230. [DOI] [PubMed] [Google Scholar]
- 40.Lahti AC, et al. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- 41.Vollenweider FX, et al. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) Eur Neuropsychopharmacol. 1997;7(1):25–38. doi: 10.1016/s0924-977x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- 42.Musso F, et al. Ketamine effects on brain function--simultaneous fMRI/EEG during a visual oddball task. Neuroimage. 2011;58(2):508–525. doi: 10.1016/j.neuroimage.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 43.Honey GD, et al. Ketamine disrupts frontal and hippocampal contribution to encoding and retrieval of episodic memory: an fMRI study. Cereb Cortex. 2005;15(6):749–759. doi: 10.1093/cercor/bhh176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corlett PR, et al. Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: linking cognition, brain activity, and psychosis. Arch Gen Psychiatry. 2006;63(6):611–621. doi: 10.1001/archpsyc.63.6.611. [DOI] [PubMed] [Google Scholar]
- 45.Fu CH, et al. Effects of ketamine on prefrontal and striatal regions in an overt verbal fluency task: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183(1):92–102. doi: 10.1007/s00213-005-0154-9. [DOI] [PubMed] [Google Scholar]
- 46.Daumann J, et al. Neuronal correlates of visual and auditory alertness in the DMT and ketamine model of psychosis. J Psychopharmacol. 2010;24(10):1515–1524. doi: 10.1177/0269881109103227. [DOI] [PubMed] [Google Scholar]
- 47.Abel KM, et al. Ketamine alters neural processing of facial emotion recognition in healthy men: an fMRI study. Neuroreport. 2003;14(3):387–391. doi: 10.1097/00001756-200303030-00018. [DOI] [PubMed] [Google Scholar]
- 48.Abel KM, et al. Ketamine and fMRI BOLD signal: distinguishing between effects mediated by change in blood flow versus change in cognitive state. Hum Brain Mapp. 2003;18(2):135–145. doi: 10.1002/hbm.10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagels A, et al. Effects of ketamine-induced psychopathological symptoms on continuous overt rhyme fluency. Eur Arch Psychiatry Clin Neurosci. 2012;262(5):403–414. doi: 10.1007/s00406-011-0281-8. [DOI] [PubMed] [Google Scholar]
- 50.Northoff G, et al. NMDA hypofunction in the posterior cingulate as a model for schizophrenia: an exploratory ketamine administration study in fMRI. Schizophr Res. 2005;72(2–3):235–248. doi: 10.1016/j.schres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Daumann J, et al. Pharmacological modulation of the neural basis underlying inhibition of return (IOR) in the human 5-HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berl) 2008;200(4):573–583. doi: 10.1007/s00213-008-1237-1. [DOI] [PubMed] [Google Scholar]
- 52.Honey GD, et al. Individual differences in psychotic effects of ketamine are predicted by brain function measured under placebo. J Neurosci. 2008;28(25):6295–6303. doi: 10.1523/JNEUROSCI.0910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deakin JF, et al. Glutamate and the neural basis of the subjective effects of ketamine: a pharmacomagnetic resonance imaging study. Arch Gen Psychiatry. 2008;65(2):154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- 54.Lim DK. Ketamine associated psychedelic effects and dependence. Singapore Med J. 2003;44(1):31–34. [PubMed] [Google Scholar]
- 55.Pouget P, et al. Rapid development of tolerance to sub-anaesthetic dose of ketamine: an oculomotor study in macaque monkeys. Psychopharmacology (Berl) 2010;209(4):313–318. doi: 10.1007/s00213-010-1797-8. [DOI] [PubMed] [Google Scholar]
- 56.Nelson CL, et al. Effects of acute and repeated systemic administration of ketamine on prefrontal acetylcholine release and sustained attention performance in rats. Psychopharmacology (Berl) 2002;161(2):168–179. doi: 10.1007/s00213-002-1004-7. [DOI] [PubMed] [Google Scholar]
- 57.Morgan CJ, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction. 2010;105(1):121–133. doi: 10.1111/j.1360-0443.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 58.Krystal JH, et al. Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology (Berl) 1999;145(2):193–204. doi: 10.1007/s002130051049. [DOI] [PubMed] [Google Scholar]
- 59.Giannini AJ, Underwood NA, Condon M. Acute ketamine intoxication treated by haloperidol: a preliminary study. Am J Ther. 2000;7(6):389–391. doi: 10.1097/00045391-200007060-00008. [DOI] [PubMed] [Google Scholar]
- 60.Oye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: evidence for a role of N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1992;260(3):1209–1213. [PubMed] [Google Scholar]