Abstract
Background
Post-injury hyperfibrinolysis (HF), defined as LY30 ≥3% on rapid thrombelastography (rTEG), is associated with high mortality and large utilization of blood products. We observed that some cases of HF are reversible and are associated with patients who respond to hemostatic resuscitation; whereas, other cases of severe HF appear to be associated with these patients’ inevitable demise. We therefore sought to define this unsurvivable subtype of hyperfibrinolysis as a recognizable rTEG tracing pattern.
Methods
We queried our trauma registry for patients who either died or spent at least one day in the ICU, received at least one unit of PRBCs, and had an admission rTEG. Within this group of 572 patients, we identified 42 pairs of non-survivors and survivors who matched on age, sex, injury mechanism and NISS. We inspected the rTEG tracings to ascertain if any pattern was found exclusively within the non-surviving group and applied these findings to the cohort of 572 patients to assess the predictive value for mortality.
Results
Within the matched group 17% of patients developed HF. Within the HF subgroup, a unique rTEG pattern was present in 14 HF patients who died and in none of the survivors. This pattern was a “diamond-shaped” tracing with a short time to maximum amplitude (TMA) of ≤14 min and complete lysis before the LY30 point. Applying these criteria to the 572 unmatched patients, this pattern had a 100% positive predictive value for mortality.
Conclusions
Patients displaying the “black diamond” pattern on their admission rTEG are at higher risk for mortality. Given the volume of blood products and other resources that these patients consume, this TEG pattern may represent an objective criterion to discontinue efforts at hemostatic resuscitation.
Keywords: hyperfibrinolysis, mortality, thrombelastography, trauma induced coagulopathy, hemorrhagic shock
Background
Post-injury hyperfibrinolysis (HF), defined as LY30 ≥3% on rapid thrombelastography (rTEG), is associated with high mortality and large utilization of blood products.(1, 2) While studies such as CRASH-2 and MATTERs have focused much attention on the treatment of HF with antifibrinolytic agents such as tranexamic acid (TXA), it remains unclear whether pharmacological reversal of HF is beneficial.(3-12) Fundamental to answering this question is the resolution of a long standing ambiguity of causality in trauma induced coagulopathy (TIC) and massive hemorrhage (MH). The question is whether patients die from coagulopathic bleeding, with the implication that reversal of this coagulopathy may be life-saving, or whether patients that are in the process of dying from irreversible hemorrhagic shock exhibit their physiologic exhaustion, in part, as an upregulation of fibrinolysis in a last desperate effort to maintain microvascular patency. (13) This conundrum of causality is best summed up as the dilemma: “Are they dying because they are bleeding, or bleeding because they are dying?” (14, 15)
This question is particularly appropriate in patients with TIC, when advanced HF is observed. HF is theoretically treatable using TXA, but severe HF is a strong predictor of mortality and may be a biomarker for irreversible processes leading to inevitable death.(1, 2, 7, 16) Moreover, patients with HF are far more likely to receive a massive transfusion (MT) than other patients of similar severity, and therefore concerns about resource utilization bear significantly on how best to treat these patients, particularly if resource-intensive attempts at hemostatic resuscitation of these patients ultimately prove futile. (1, 2, 7, 15-18)
An alternative school of thought maintains that today's definition of “unsurvivable” merely defines a new clinical challenge, that will evolve into tomorrow's “great save”. From this viewpoint, the presence of biomarkers of highly lethal pathophysiology may in fact represent a rational basis for attempting “heroic” measures to salvage the patient, such as emergent cardiopulmonary bypass, resuscitative thoracotomy, or resuscitative balloon occlusion of the aorta (REBOA). As such, the identification of early, reliable, and objective indicators of high-lethality forms of physiologic decompensation would be of great clinical utility in the setting of trauma resuscitation.
We have observed that some cases of HF are reversible by intervention and are associated with patients who respond to hemostatic resuscitation; whereas, some cases of severe HF appear to be associated with these patients’ inevitable demise. We have further observed a wide variety of qualitatively distinct tracing morphologies on the thrombelastogram (TEG) of HF patients. We therefore hypothesized that there is a highly-lethal subtype of HF, and that this subtype would be typified by a recognizable TEG tracing pattern.
Methods
Study Design
We conducted a retrospective cohort study to derive TEG-based diagnostic criteria related to HF and predictive of death, and then applied these criteria to a larger group of patients to validate them. The first (derivation) phase of the study was a nested case control analysis performed on a subset of trauma patients, pairing survivors with non-survivors and matching them according to age, sex, injury mechanism and new injury severity score (NISS). The second (validation) phase of the study was based upon analysis of the entire patient set.
Patients
We queried our trauma registry in a deidentified manner for all patients admitted to our level 1 trauma center from 2009-2014 who met the following criteria: either died or spent at least one day in the ICU, received at least one unit of PRBCs, and had an admission Rapid TEG (R-TEG). Patients who were under the age of 18 or who were transferred from other facilities were excluded. Within this group of 572 patients, we identified 42 pairs of non-survivors and survivors who matched on age, sex, injury mechanism and NISS, for use in the derivation portion of the study. Also included in this prospective database were: survival time, admission R-TEG results, injuries, operative interventions, blood component transfusions and hemostatic pharmaceutical administration; within the first six hours from injury.
Analysis of R-TEG
Admission R-TEG tracings for the initial group of 42 patients pairs were visually inspected to ascertain if any pattern of R-TEG was found exclusively within the non-surviving group. R-TEGs were run in our clinical laboratory (as part of the routine standard of trauma care) on uncitrated samples within 4 minutes of collection and activated with the R-TEG reagent (consisting of tissue factor and kaolin) in accordance with the manufacturer's instructions (Haemonetics LLC, Boston, MA, USA). The standard TEG tracing parameters (activated clotting time [ACT], alpha angle, maximum amplitude [MA] and LY30) related to clot initiation, clotting kinetic, final clot strength and clot lysis, respectively, were reported. Patients were initially classified with respect to the LY30 TEG parameter, which reports the degree of clot lysis at 30 minutes after the attainment of maximum clot strength/TEG amplitude (MA). Patients with an LY30 ≥3% were classified as having HF, in accordance with our previous work (1, 16). Further observationally-derived metrics of various portions of the TEG curve were then analyzed to provide a more detailed description of the TEG tracing shape within this group. TEG tracing features unique to the non-survivor cohort were then applied to the entire group of 572 patients for validation of their prognostic value for death.
Statistical Methods and Matching
Matching was performed with the SAS statistical software package (SAS Institute Inc. , Cary, NC, USA). Matching was conducted with 10 unit wide bins for age and NISS and an exact match for sex and injury mechanism (blunt or penetrating). Other statistical analyses were performed using GraphPad Prism v.6.0f for Mac OSX (GraphPad Software, La Jolla California USA). Groups were compared using a two-tailed Mann-Whitney test. Binary contingency tables were analyzed with the Fishers exact test. Goodness of binary outcome prediction by continuous variables was reported as the area under the receiver operating characteristic curve (AUROC) with a 95% confidence interval (CI).
Results
Derivation Phase (Matched Patients)
42 pairs of non-surviving patients were able to be matched by gender, age and NISS with survivors. There were 35 males (83%) and 11 penetrating injuries (26%) in each group. Median ISS in this nested subset was 49 (IQR 29-59), with a median of 50 (IQR 29-59) in the non-survivor group and 48 (27-57) in the survivors. Median age was 42 years, overall (IQR 27-57), with a median of 42 (IQR 28-58) in the non-survivors and 40 (IQR 26-57) in the survivors.
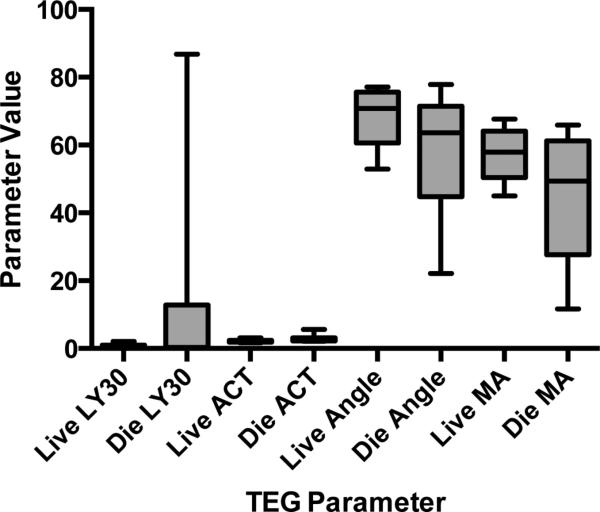
LY30 was not significantly different between the non-survivor and survivor groups (0.25% versus 0.05%, IQR 0-0.95% versus 0-12.75%, p=0.08). ACT was significantly higher (144 seconds, IQR 128-194) and alpha angle and MA were significantly lower in the non-survivor group at 63.6 degrees (IQR 44.8-71.4) and 49.4 mm (IQR 27.6-61.2) versus 121 seconds (IQR 113 -144), 70.8 degrees (IQR 60.6-75.6) and 57.8 mm (IQR 50.4-64.1 mm) for survivors, respectively (p=0.0002, 0.02, and 0.001) (Figure1) . Thus, LY30 was not an effective predictor of mortality with an AUROC of 0.61 (95% CI 0.48-0.72), and ACT, alpha angle and MA were all weak predictors of mortality with AUROCs of 0.73 (95% CI 0.62-0.84), 0.65 (95% 0.53-0.77), and 0.70 (95% CI 0.59-0.82), respectively.
Figure 1. Conventional Thrombelastography (TEG) Parameters in Matched Subset of Survivors and Non-Survivors.
Values of the conventional TEG parameters LY30, ACT, alpha angle and MA overlap greatly when dichotomized between those patients that lived and those that died of their injuries. While elevated LY30 is a known predictor of mortality, LY30 in the non-surviving group of the matched subset was not significantly different from that of survivors.
The only qualitatively unique feature of the TEG tracings within the non-surviving group was fibrinolysis that went to completion (i.e. returned of the TEG tracing to baseline amplitude) by the LY30 point at which the tracings terminated (100% specificity and positive predictive value for death). This pattern was present in 7 (17%) of 42 non-survivors. We describe this characteristic as the time to total lysis (TTL) measured from MA. TTL was a median of 12 minutes (IQR 5-20) in the patients displaying total lysis, and is undefined in patients without complete lysis by termination of the assay (at LY30, by definition). Notably, the time to MA (TMA) was ≤14 minutes in all the tracings with complete lysis, compared to a reference interval of 18.5-22.0 minutes.
Validation Phase (Unmatched Patients)
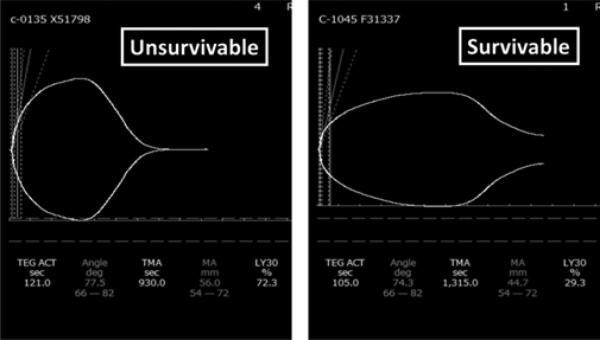
Within the 572 unmatched patients produced by our trauma registry query, 122 (21.3%) died. Overall, in the non-survival cohort, the incidence of HF was 25.4% (31 of 122), compared to 12% (54 of 450) for survivors, with a positive predictive value for death of 36% for HF alone. Applying the one unique TEG tracing characteristic associated with death in the matched subset (TTL before LY30), presence of this pattern had a positive predictive value of 95.2% (20 deaths, 1 survival). Restricting the analysis to those with a TMA <14 and a TTL <30 yielded a positive predictive value of 100% (14 deaths, no survivors). This pattern of steep rise to MA and rapid collapse to baseline, we dubbed the “black diamond” (figure 2).
Figure 2. Unsurvivable versus survivable hyperfibrinolysis.
Both TEG tracings pictured show profound and dangerous hyperfibrinolysis, but the “death diamond” pattern on the left, with a rapid rise to maximum amplitude (MA) in 14 minutes or less, paired with rapid total clot lysis (time to total lysis [TTL] <30 minutes from MA) was universally lethal in this study. The TEG tracing on the right with incomplete lysis by 30 minutes after MA belonged to a patient who survived.
Median blood product utilization in the “black diamond” group (100% mortality, n=14) was 17 units (IQR 13-38) of packed red blood cells (PRBCs) and 6 units (IQR 3-10) of fresh frozen plasma (FFP) with 36% of these patients also requiring platelet and cryoprecipitate transfusion, 43% receiving antifibrinolytic agents and 14% receiving recombinant factor VII concentrate. Within the larger TTL <30 group (95% mortality, n=21) median PRBC consumption was 16 units (IQR 10-42), FFP was 6 units (3-17) with 62% requiring platelets, 43% requiring cryoprecipitate, 33% receiving antifibrinolytics, and 14% receiving factor VII.
In contrast, in all HF patients without complete fibrinolysis (17% mortality, n=64) median PRBC consumption was 4 units (IQR 1-9), FFP was 2 (IQR 0-5), with 38% requiring platelets, 30% requiring cryoprecipitate, 5% receiving antifibrinolytics, and 3% receiving factor VII. In all non-HF patients (19% mortality, n=487) median PRBC utilization was 4 units (IQR 2-8), FFP 2 units (IQR 0-4). See table 1.
Table 1. Summary of Blood Product Utilization in the Unmatched Patient Population (n=572).
Non-survivor and survivor groups are subdivided by thrombelastogram (TEG) pattern. “Black diamond” refers to TEG tracings with a time to maximum amplitude (TMA) ≤ 14 minutes and a time to total lysis (TTL) <30 minutes past MA. Non-black diamond (non-BD) TTL <30 min refers to all other TEG tracing pattern that go to complete clot lysis (i.e. return of the TEG tracing to baseline) by the termination of the test at 30 minutes past MA (at the calculation point for the LY30 parameter). Other hyperfibrinolysis (HF) is all other tracings displaying an LY30 ≥3% but not going to complete lysis within the test period. Non-HF refers to all TEG tracings not displaying HF (i.e. LY30 <3%). PRBC = packed red blood cells; FFP = fresh frozen plasma.
| TEG Pattern | N | PRBCs | FFP | Platelets | Cryoprecipitate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |||
| Non-Survivors | Black Diamond | 14 | 17 | 13--38 | 6 | 10 -- 13 | 1 | 0--3 | 0 | 0--2 |
| Non-BD, TTL <30 | ||||||||||
| Min | 6 | 12 | 9--35 | 5 | 2--15 | 0 | 0--2 | 0 | 0--0 | |
| Other HF | 11 | 6 | 5--24 | 4 | 2--11 | 1 | 0--2 | 2 | 0--2 | |
| Non-HF | 91 | 6 | 2--14 | 4 | 2--8 | 1 | 0--2 | 0 | 0--2 | |
| Survivors | Black Diamond | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Non-BD, TTL <30 | ||||||||||
| Min | 1 | 34 | n/a | 26 | n/a | 0 | n/a | 4 | n/a | |
| Other HF | 53 | 3 | 1--8 | 2 | 0--5 | 0 | 0--0 | 0 | 0--0 | |
| Non-HF | 396 | 3 | 2--7 | 0 | 0--4 | 0 | 0--1 | 0 | 0--0 | |
Median survival time in the 100% mortality “black diamond” group was 2 hours (IQR 1.75-4.75) with two outliers that lived beyond 1 day at 2 days and 4.5 days. The one survivor in the TTL<30 group had a transecting iliac artery injury, which was rapidly controlled.
Discussion
Patients presenting with the hallmark “black diamond” pattern of early MA and rapid total lysis on their TEG tracing are nearly certain to die of their injuries despite the most vigorous resuscitative efforts. Comprising only 2.4% of the patients in this study, patients with the full “black diamond” pattern of TMA ≤14 minutes and TTL <30 minutes, which was 100% predictive of death, consumed an average of nearly four times as many units of blood products (approximately 36 in total) as surviving patients, despite generally surviving less than 4 hours from the time of injury. Interestingly, some “black diamond” patients (four of 14) died later than 12 hours after injury (up to almost 5 days in one case). Clearly these patients did not merely exsanguinate, and this fact argues that the “black diamond” TEG pattern is a biomarker for an irreversible pathophysiology that inevitably results in death.
Patients with less rapid attainment of MA, but still with the pattern of TTL<30 minutes represent a group which, in certain instances, may be salvageable. In addition to the one such patient detected in this study, we uncovered three other similar cases of survival, that were excluded from this current study: two who were excluded as transfers from other institutions, and one who was enrolled in another active study, after this database query. These survivors shared a similar mechanism of penetrating cardiac or large vessel injury, which was rapidly controlled in the emergency department.
As there is proven benefit for a small number of patients of rapid renormalization of blood pressure (in controllable cardiovascular injuries) the utility of highly aggressive resuscitation efforts in a other “black diamond” patients may be inferred. While the “black diamond” may, in most cases, be an indicator of an eventual inevitable death, that fact is not synonymous with futility. Our findings suggest that the “black diamond” TEG pattern is associated with dismal outcomes, but we regard this as a spur toward earlier and more aggressive hemostatic resuscitation. The presence of “black diamond” physiology may in fact represent an objective criterion for the initiation of “heroic” measures to salvage the patient, such as emergent cardiopulmonary bypass, resuscitative thoracotomy, or REBOA.
While the authors favor an aggressive approach to these patients, given the massive consumption of resources by patients presenting with the “black diamond” pattern, there may be situations where futility of resuscitative efforts should be considered. For example: in mass casualty scenarios, battlefield injuries or in other austere environments, detection of this TEG pattern may warrant re-triage from the critical to expectant category, particularly if the patient does not have an isolated and controllable cardiovascular injury.
While it is premature to recommend (on the basis of a single study) that resuscitative efforts should be discontinued based upon the results of a single hemostatic assay, this concept is analogous to the now accepted restriction of resuscitative thoracotomy to patients whose injury pattern predicts at least some hope of success. Therefore, in controlled situations where a single patient can be the focus of the full resources and staff of a dedicated trauma center, the approach to patients presenting with the “black diamond” TEG pattern should be extremely aggressive, given the extreme threat to life that this TEG pattern heralds. It could, however, be reasonably argued that if mechanical control of bleeding has been achieved, and the patient has received a large volume of blood products, and the TEG pattern has failed to improve that the patient may be deteriorating into a state of irreversible physiologic exhaustion of which failure of their hemostatic system is an easily detectable manifestation.
Thus, in order to develop firm clinical guidelines, the response to hemostatic resuscitation of patients exhibiting these extremely morbid TEG patterns, is in urgent need of prospective study. Moreover, studies of very early intervention with hemostatic resuscitative agents are needed to help define the temporal point in relation to injury at which the “black diamond” truly becomes irreversible.
Finally, it is worth considering the importance of the “black diamond” patients as a distinct subcategory of TIC, for the purposes of studying the utility of antifibrinolytic agents. If the specific reversal of HF is intrinsically beneficial, then the “black diamond” population would be the most likely to show a signal for improvement of survival, and given the overwhelming likelihood of their eventual demise, the “black diamond” population is unlikely to be harmed by this intervention. However, it may well be, that in the more general case of HF, that tPA elaboration is a natural consequence of severe hemorrhagic shock and is, in fact, a protective adaptive response aimed at maintaining the patency of the microcirculation and reducing shunting in low flow states.(19-21) If this is the case, the use of antifibrinolytics may be deleterious, and may only serve to obscure an important biomarker (systemic HF) for microcirculatory failure and tissue hypoperfusion. In this manner, antifibrinolytics may deprive the trauma surgeon of a useful readout of the effectiveness of their resuscitative efforts (i.e. the reversal of systemic HF by return of microcirculatory flow), in much the same way that wanton use of acetaminophen may confound an attempt to determine and treat the root cause of a fever, by normalizing the patient's temperature without treating the underlying cause. Comparison of the outcomes of antifibrinolytic therapy in the “black diamond” population versus the more general population with HF may offer important insights into the mechanism of action and utility of antifibrinolytics in TIC.
Acknowledgements
The authors wish to thank our clinical research assistants: Sarah Ammons, Andrea Emard, and Raymond Shepherd-Singh. Research reported in this publication was supported in part by the US Army Medical Research Acquisition Act of the Department of Defense under Contract Award Number W81XWH1220028 and the National Institute of General Medical Sciences and National Heart, Lung, and Blood Institutes of the National Institutes of Health under Award Numbers P50GM049222, T32GM008315 and UMHL120877 and we receive research support from Haemonetics LLC and TEM GmbH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and the Department of Defense or any of our industry sponsors.
Footnotes
Conflict of Interest Statement: We receive research support from Haemonetics LLC and TEM GmbH, but have no financial interests in these companies. Drs. Chapman, E.E. Moore, H.B. Moore and Gonzalez report their patent on “Identification of Novel Disease States Using Viscoelastic Analysis in the Presence of a Thrombolytic Agent”. Drs. Chapman and E.E. Moore report their patent on “Methodologies and Reagents for Detecting Fibrinolysis and Hyperfibrinolysis”. Studies referenced herein are regulated by the Colorado Multiple Institutional Review Board and are performed in a HIPAA compliant manner under a waiver of informed consent (COMIRB protocol #s: 10-0477 and 13-3087).
Author Contribution:
M.P.C., E.E.M., H.B.M., E.G., A.P.M., A.S., A.B., C.C.C., and A.S. designed the study. J.G.C. , C.D.F. and A.G. managed the study and databases and collected and processed data. M.P.C., A.S., E.E.M., H.B.M., A.P.M. and E.G. prepared and edited the manuscript.
References
- 1.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, Stringham JR, Sauaia A, Silliman CC, Banerjee A. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013 Dec;75(6):961–7. doi: 10.1097/TA.0b013e3182aa9c9f. discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schöchl H, Wade CE, Holcomb JB, Matijevic N. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012 Aug;73(2):365–70. doi: 10.1097/TA.0b013e31825c1234. discussion 70. [DOI] [PubMed] [Google Scholar]
- 3.CRASH-2 collaborators. Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011 Mar 26;377(9771):1096–101. 101, e1–2. doi: 10.1016/S0140-6736(11)60278-X. [DOI] [PubMed] [Google Scholar]
- 4.CRASH-2 trial collaborators. Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010 Jul 3;376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 5.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Archives of surgery. 2012 Feb;147(2):113–9. doi: 10.1001/archsurg.2011.287. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, Pittet JF. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. 2012 Feb;255(2):379–85. doi: 10.1097/SLA.0b013e318235d9e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez E, Moore EE, Moore HB, Chapman MP, Silliman CC, Banerjee A. Trauma-Induced Coagulopathy: An Institution's 35 Year Perspective on Practice and Research. Scandinavian journal of surgery : SJS : official organ for the Finnish Surgical Society and the Scandinavian Surgical Society. 2014 Apr 30;103(2):89–103. doi: 10.1177/1457496914531927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, et al. The coagulopathy of trauma: a review of mechanisms. The J Trauma. 2008 Oct;65(4):748–54. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 9.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007 Feb;62(2):307–10. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins DH, Rappold JF, Badloe JF, Berséus O, Blackbourne L, Brohi KH, Butler FK, Cap AP, Cohen MJ, Davenport R, et al. Trauma hemostasis and oxygenation research position paper on remote damage control resuscitation: definitions, current practice, and knowledge gaps. Shock. 2014 May;41(Suppl 1):3–12. doi: 10.1097/SHK.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashuk JL, Moore EE, Sawyer M, Wohlauer M, Pezold M, Barnett C, Biffl WL, Burlew CC, Johnson JL, Sauaia A. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann Surg. 2010 Sep;252(3):434–42. doi: 10.1097/SLA.0b013e3181f09191. discussion 43-4. [DOI] [PubMed] [Google Scholar]
- 12.Kutcher ME, Cripps MW, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Redick BJ, Nelson MF, Cohen MJ. Criteria for empiric treatment of hyperfibrinolysis after trauma. J Trauma Acute Care Surg. 2012 Jul;73(1):87–93. doi: 10.1097/TA.0b013e3182598c70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardaway RM. The significance of coagulative and thrombotic changes after haemorrhage and injury. J Clin Pathol Suppl (R Coll Pathol) 1970;4:110–20. doi: 10.1136/jcp.s3-4.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morton AP, Moore EE, Wohlauer MV, Lo K, Silliman CC, Burlew CC, Banerjee A. Revisiting early postinjury mortality: Are they bleeding because they are dying or dying because they are bleeding? J Surg Res. 2013 Jan;179(1):5–9. doi: 10.1016/j.jss.2012.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackbourne LH, Baer DG, Cestero RF, Inaba K, Rasmussen TE. Exsanguination shock: the next frontier in prevention of battlefield mortality. The Journal of trauma. 2011 Jul;71(1 Suppl):S1–3. doi: 10.1097/TA.0b013e3182211286. [DOI] [PubMed] [Google Scholar]
- 16.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014 Dec;77(6):811–7. doi: 10.1097/TA.0000000000000341. discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen MJ. Towards hemostatic resuscitation: the changing understanding of acute traumatic biology, massive bleeding, and damage-control resuscitation. Surg Clin North Am. 2012 Aug;92(4):877–91. viii. doi: 10.1016/j.suc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Johansson PI, Sørensen AM, Larsen CF, Windeløv NA, Stensballe J, Perner A, Rasmussen LS, Ostrowski SR. Low hemorrhage-related mortality in trauma patients in a Level I trauma center employing transfusion packages and early thromboelastography-directed hemostatic resuscitation with plasma and platelets. Transfusion. 2013 Dec;53(12):3088–99. doi: 10.1111/trf.12214. [DOI] [PubMed] [Google Scholar]
- 19.Gando S, Tedo I, Kubota M. Posttrauma coagulation and fibrinolysis. Crit Care Med. 1992 May;20(5):594–600. doi: 10.1097/00003246-199205000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Hardaway RM. The role of intravascular clotting in the etiology of shock. Ann Surg. 1962 Mar;155:325–38. doi: 10.1097/00000658-196203000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardaway RM, Brune WH, Geever EF, Burns JW, Mock HP. Studies on the role of intravascular coagulation in irreversible hemorrhagic shock. Annals of surgery. 1962 Feb;155:241–50. doi: 10.1097/00000658-196200000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]