Abstract
Mycobacterium tuberculosis commonly causes persistent or chronic infection, despite development of antigen-specific CD4 T cell responses. We hypothesized that M. tuberculosis evades elimination by CD4 T cell responses by manipulating MHC class II antigen presentation and CD4 T cell activation, and tested this hypothesis by comparing activation of Ag85B-specific CD4 T cell responses to M. tuberculosis and M. bovis BCG Pasteur in vivo and in vitro. We found that, while M. tuberculosis persists in lungs of immunocompetent mice, M. bovis BCG is cleared, and clearance is T cell dependent. We further discovered that M. tuberculosis-infected macrophages and dendritic cells activate Ag85B-specific CD4 T cells less efficiently and less effectively than do BCG-infected cells, in vivo and in vitro, despite higher production and secretion of Ag85B by M. tuberculosis. During BCG infection, activation of Ag85B-specific CD4 T cells requires fewer infected dendritic cells, and fewer antigen-producing bacteria than during M. tuberculosis infection. When dendritic cells containing equivalent numbers of M. tuberculosis or BCG were transferred to mice, BCG-infected cells activated proliferation of more Ag85B-specific CD4 T cells than did M. tuberculosis-infected cells. Differences in Ag85B-specific CD4 T cell activation were attributable to differential antigen presentation, rather than differential expression of costimulatory or inhibitory molecules. These data indicate that subpoptimal antigen presentation contributes to persistent infection, and that limiting antigen presentation is a virulence property of M. tuberculosis.
Introduction
CD4 T cells are essential for control of infection with Mycobacterium tuberculosis. In humans co-infected with M. tuberculosis and HIV, CD4 T cell deficiency leads to a higher frequency of progression to active tuberculosis (TB), a higher frequency of disseminated extrapulmonary disease, and higher mortality (1–3). Similarly, M. tuberculosis infection of mice deficient in CD4 T cells results in higher bacterial burdens in the lung and other tissues and in shortened survival, compared with infection of immunocompetent mice (4–6). While CD4 T cells are essential for control of infection, T cell responses rarely, if ever, eliminate M. tuberculosis from infected humans (7, 8) or animals (9, 10). Consequently, understanding the mechanisms that limit the efficacy of CD4 T cells in TB is essential to guide rational approaches to improving control of TB, including development of effective vaccines.
Previous studies have revealed evidence that M. tuberculosis subverts CD4 T cell-dependent immunity. For example, priming of antigen-specific CD4 T cells occurs much later after M. tuberculosis infection compared with other infections, and this provides time for the bacterial population to expand markedly prior to appearance of effector T cells in the lungs (11–13). In addition, CD4 effector T cells specific for the immunodominant M. tuberculosis antigen 85B (Ag85B) are activated poorly at the site of infection in the lungs (14), and regulatory T cells dampen the effector CD4 T cell response during infection (15). Furthermore, mycobacteria have been reported to interfere with MHC class II antigen presentation to CD4 T cells in vitro (16–22), although the in vivo significance of this mechanism has not previously been determined. Since direct recognition of M. tuberculosis-infected cells by CD4 T cells is required for optimal control of intracellular infection in vivo (23), the effectiveness of antigen presentation by infected cells may be an important determinant of the outcome of infection.
M. bovis BCG, which has been widely used as a TB vaccine, is less virulent than wild-type M. bovis and M. tuberculosis. Although the genomic differences between M. bovis BCG strains and M. tuberculosis are well characterized (24), and the contribution of the loss of the RD-1/Exs-1 locus to attenuation is well established (25–27), the consequences of its attenuation on host-pathogen interactions have not been studied in depth. Similar to control of infection with M. tuberculosis, CD4 T cells are important for control of M. bovis BCG (hereafter termed BCG) infection in humans (28, 29) and mice (6, 30–32). However, in contrast to the inability of CD4 T cell responses to eliminate M. tuberculosis, CD4 T cell responses to BCG infection are associated with gradual clearance of the bacteria (31, 33). The differential effects of T cell responses on the course of infection with M. tuberculosis and BCG prompted us to hypothesize that, compared with BCG, M. tuberculosis impedes the generation, activation, or action of CD4 T cells. Since M. tuberculosis resides in professional antigen-presenting cells (34), we further hypothesized that M. tuberculosis impedes CD4 T cell activation by acting on antigen-presenting cells. We found that dendritic cells and macrophages infected with BCG are more capable of activating CD4 T cells in vivo and in vitro than are cells infected with virulent M. tuberculosis H37Rv, and found evidence that this is attributable to more effective antigen presentation. These results establish that ineffective antigen presentation is associated with virulence in tuberculosis, and likely contributes to the ability of M. tuberculosis to evade elimination in immunocompetent hosts.
Materials and Methods
Mice
C57BL/6 mice of WT and TCRβ/δ−/− genotypes were either bred in the New York University School of Medicine Skirball animal facility or purchased from Taconic Farms, Inc for aerosol and iintratracheal infection. Mice aged 6–8 weeks were used for infection, and at various time points following infection mice were euthanized and lungs and mediastinal lymph nodes were isolated for CFU enumeration and flow cytometry. P25TCR-Tg CD4 T cells, specific for Ag85B peptide 25 (amino acids 240–254 of the mature protein) were isolated from P25TCR-Tg mice on the C57BL/6 background (11, 35). All mouse experiments were performed in accordance with the NYUSM IACUC.
Bacterial strains and infections
WT M. tuberculosis strain H37Rv and BCG Pasteur were initially acquired from ATCC and the Ag85B deletion mutant (ΔAg85B) H37Rv strain was generated as described previously (11). All bacterial strains were stored at −80°C; bacteria were thawed and cultured to mid-log phase in Middlebrook 7H9 media supplemented with 10% (v/v) ADC enrichment prior to use for aerosol infection of mice or infection of cultured cells. Mice were inoculated with 102 CFU of H37Rv or 5×104 BCG Pasteur using an Inhalation Exposure Unit (Glas-Col). The dose delivered was verified one day following aerosol infection by euthanizing infected mice to isolate and homogenize infected lungs in PBS-Tween-80 (0.5%) for CFU plating on Middlebrook 7H11 medium supplemented with 10%(v/v) ADC enrichment. Infected cells were counted and lysed in PBS-Tween-80 and plated on 7H11 medium to determine multiplicity of infection in BMDC and BMMØ.
Flow Cytometry
Single cell suspensions from infected lungs and lymph nodes were stained using the following fluorescently-labeled antibodies (Biolegend, BD Pharmingen, or eBioscience): anti-CD3ε APC-Cy7 (145– 2C11), anti-CD4 (L3T4) Pacific Blue, anti-CD45.1 (A20) PerCp or Alexa750, anti-CD69 (H1.2F3) APC, anti-CD44 (IM7) PE, anti-IFN-γ (XMG1.2) PerCp-Cy5.5. MHC class II tetramers I-Ab Ag85B280–294 PE and I-Ab EsxG46–61 PE were obtained from the NIAID Tetramer Core Facility (Emory University, Atlanta, GA). The epitope recognized by the Ag85B-specific tetramers is also termed Ag85B240–254, when the amino acid numbering excludes the 40 amino acid signal peptide. The epitope recognized by the Ag85B tetramers is identical to that of the P25TCR-Tg CD4 T cells used in these studies. Infected BMDC and BMMØ were stained with the following antibodies: anti-CD11c (N418) PerCp, anti-F4/80 (BM8) APC, anti-MHC II IAb (M5/114.15.2) Pacific Blue, anti-CD86 (PO3) Alexa700, anti-CD40 (3/23) PE, anti-PDL-1 (10F.9G2) PE. Flow cytometry was performed using a LSR II (BD Biosciences) at the NYU Flow Cytometry and Cell Sorting core facility. Flow cytometry data was analyzed using FlowJo software.
Western Blotting
Culture filtrates and pellet lysates of mycobacteria were collected from overnight cultures in Saunton’s medium. 0.5 µg of total lysate protein and 2.5 µg of culture filtrate proteins were loaded and run on 10% Ready Gel Tris-HCl gels (BioRad). Proteins were transferred in Tris-Glycine buffer lacking SDS, and blotted for Ag85B (polyclonal rabbit antisera prepared in our laboratory), MPT32 (polyclonal rabbit antisera, BEI NR-13807), and GroEL2 (BEI, clone IT-70).
Bone marrow derived dendritic cell and macrophage cultures
Bone marrow was isolated from WT mice aged 6–8 weeks. To generate BMDC, bone marrow was cultured for 7 days in complete RPMI-10 media supplemented with 12 ng/mL GM-CSF (PeproTech). The media was replaced every 3 days and on the 7th day the floating fraction of cells was collected, magnetically labeled with anti-CD11c (N418) microbeads, and sorted using an AutoMACS (Miltenyi Biotech). The CD11c+ cells were counted and re-plated for infection. BMMØ were differentiated in complete DMEM-10 supplemented with 20% L929 conditioned media. The media was replaced every 3 days for 7 days. On day 7 the adherent fraction of cells were collected by incubation in PBS at 37°C, counted, and re-plated for infection.
Intratracheal transfer of infected BMDC
BMDC were infected at a multiplicity of infection (MOI) of 2 overnight and the following day infected cells were treated with amikacin (20 µg/mL) to kill extracellular bacteria. Cells were then washed with PBS, collected, and counted. 105 infected BMDC were transferred to WT mice, which received 2×106 CFSE-labeled P25TCR-Tg CD4 T cells one day prior to the iintratracheal transfer of infected BMDC. And 60 hours following BMDC transfer, mice were euthanized and lung draining mediastinal lymph nodes were isolated for CFU enumeration and stained for flow cytometric analysis of P25TCR-Tg cell proliferation.
P25TCR-Tg cell Th1 differentiation
CD4 T cells from the lymph nodes of P25TCR-Tg mice were magnetically labeled with CD4 (L3T4) microbeads and sorted using an AutoMACS. CD4+ P25TCR-Tg cells were co-cultured with irradiated splenocytes from C57BL/6 mice in the presence of Th1 skewing cytokines as previously described (14). Frozen stocks of Th1-differentiated P25TCR-Tg cells were stored in liquid nitrogen after differentiation and thawed for co-culture with infected BMDC and BMMØ.
In vitro co-culture of infected cells and Th1 P25TCR-Tg cells
BMDC and BMMØ were infected overnight at an MOI of 2. The following day infected cells were treated with amikacin (20 µg/mL) to kill extracellular bacteria and washed extensively with PBS. The infected cells were then collected, counted, and assessed for viability. Infected cells were re-plated at 2×103, 2×104, and 2×105 cells per well in a 96-well plate and 2×104 Th1-P25TCR-Tg cells were added to each well. Infected cells were also lysed in PBS-Tween-80 and plated at various dilutions to determine multiplicity of infection. Supernatants from these co-cultures were collected and filtered 6 hours later for mouse IFN-γ ELISA (BD Biosciences).
Results
T cells promote resolution of M. bovis BCG but not virulent M. tuberculosis pulmonary infection
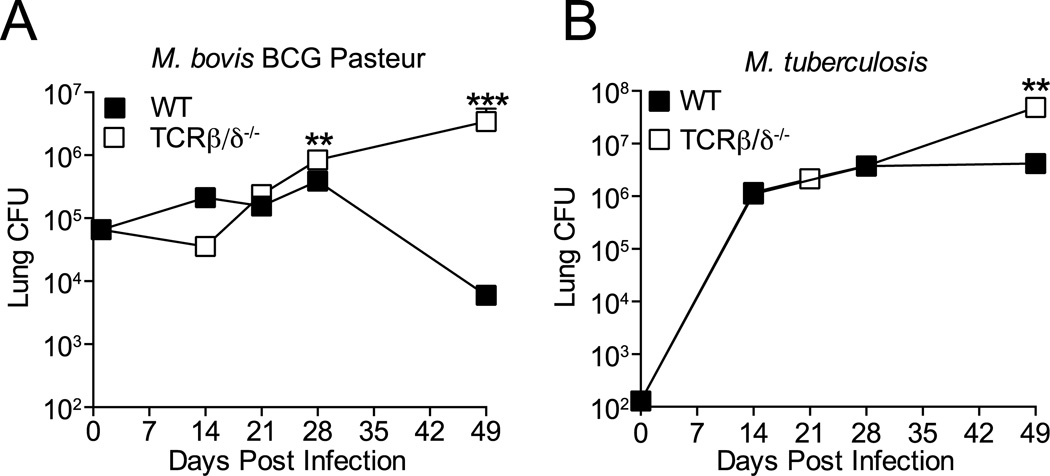
To test the hypothesis that differential T cell activation or effector action contributes to the distinct outcomes of infection with BCG or M. tuberculosis, we first confirmed that T cells are required for clearance of BCG from mice after aerosol infection. Initial studies with aerosol inocula of 102–103 BCG/mouse resulted in clearance of BCG from lungs of wild-type mice within 25 days of infection, with minimal induction of T cell responses, indicating that innate mechanisms are sufficient to clear BCG after these low dose inocula (not shown). Increasing the aerosol inoculum to 5 × 104 BCG/mouse established infection in the lungs where the bacterial burden increased, albeit with a growth curve dissimilar to that of M. tuberculosis, in wild-type (WT) or T cell deficient (TCRβ/δ−/−) C57BL/6 mice during the first 28 days. After 28 days post infection, BCG continued to expand in the lungs of T cell deficient mice, while the bacteria began to be cleared from the lungs of WT mice (Figure 1A). These data indicate aerosol BCG infection with a higher inoculum establishes infection in the lungs that requires T cells for control and clearance. In contrast, the presence of T cells in wild-type mice prevented progressive growth of M. tuberculosis after day 28, but the bacteria were not cleared from the lungs (Figure 1B). Therefore, T cells contribute to resolution of BCG infection, but they are unable to resolve M. tuberculosis infection. These findings support the hypothesis that M. tuberculosis interferes with activation and/or effector action of T cells in vivo and thereby avoids being cleared.
Figure 1.
T cells clear M. bovis BCG but not M. tuberculosis infection in the lungs. T cell-deficient (TCRβ/δ−/−) or WT C57BL/6 mice were infected with 5 × 104 BCG or 100 M. tuberculosis H37Rv CFU by aerosol, and live bacteria were quantitated in the lungs for 7 weeks post infection. (A) M. bovis BCG. (B) M. tuberculosis H37Rv. Data are expressed as mean ± SEM, n = 5 mice per time point. Student t test comparing bacterial CFU in WT and TCRβ/δ−/− mice at each time point; *** P=0.0002, ** P =0.0078 The data shown are representative of 2 similar experiments.
Priming of antigen-specific CD4 T cells is initiated earlier and requires fewer antigen-producing bacteria in the local lymph node during BCG compared with M. tuberculosis infection
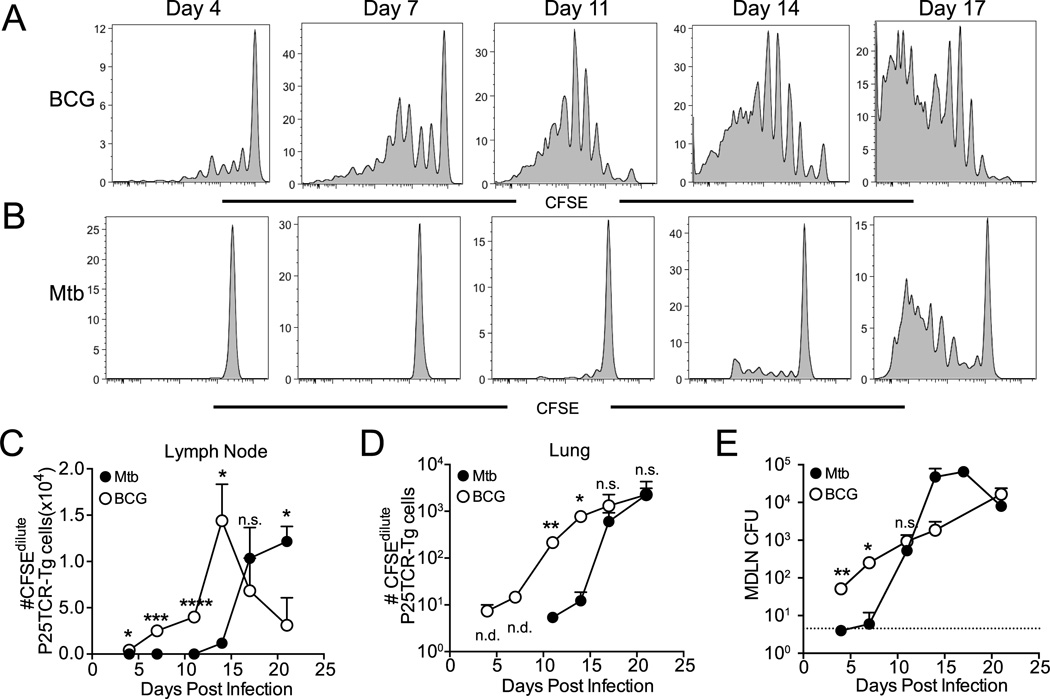
Since CD4 T cells are essential for control of M. tuberculosis in mice (4) and humans (36), and since in vitro studies indicate that M. tuberculosis can inhibit antigen presentation to CD4 T cells (37), we focused our subsequent studies on CD4 T cells. To test the hypothesis that CD4 T cell interactions with antigen-presenting cells differ during BCG or M. tuberculosis infection, we first characterized the earliest encounter of antigen-specific CD4 T cells and antigen presenting cells after infection: priming of T cells in the local lymph node. For these analyses, we used P25TCR-Tg CD4 T cells, which recognize peptide 25 (amino acids 240–254 of the mature protein) of Ag85B (11, 35, 38), which is expressed by both BCG and M. tuberculosis (24). We transferred CFSE labeled, P25TCR-Tg CD4 T cells to wild-type mice one day prior to aerosol infection with either BCG or M. tuberculosis, and assessed in vivo proliferation (as dilution of CFSE) of P25TCR-Tg CD4 T cells from the lung-draining mediastinal lymph node (MDLN) at multiple time intervals. P25TCR-Tg CD4 T cells proliferated as early as 4 days after aerosol infection with BCG (Figures 2A and 2C), while proliferation was not observed before 11 days post infection with M. tuberculosis (Figures 2B and 2C). Consistent with the earlier onset of CFSE dilution as an indication of proliferation, more CFSEdilute P25TCR-Tg cells were present in the MDLN between 4 and 11 days following BCG infection than in lymph nodes of M. tuberculosis-infected mice (Figure 2C). The earlier priming of P25TCR-Tg cells during BCG infection resulted in earlier arrival of CFSEdilute P25TCR-Tg cells in lung (Figure 2D). Consistent with earlier activation of P25TCR-Tg cells in the MDLN, live BCG were transported to the MDLN significantly earlier than M. tuberculosis (Figure 2E). We considered the possibility that the earlier P25TCR-Tg activation and proliferation observed in the MDLN could be due to the higher aerosol inoculum of BCG, and the early presence of a large number of antigen-producing BCG in the MDLN. However, this was not the full explanation, as we also observed that fewer BCG CFU initiated proliferation in the MDLN; approximately 60 BCG colony forming units (CFU) were present in the MDLN at the onset of P25TCR-Tg cell proliferation on day 4, while initial proliferation in response to M. tuberculosis required >1,000 bacteria in the MDLN on day 11 (Figure 2E). These results indicate that earlier priming of antigen-specific CD4 T cells after BCG infection may be due to two factors: earlier appearance of bacteria in the lymph node and priming of antigen-specific CD4 T cells in the presence of fewer bacteria than are required after M. tuberculosis infection.
Figure 2.
Differential kinetics and sensitivity of antigen-specific CD4+ cell priming in M. bovis BCG and M. tuberculosis infection. Proliferation of adoptively transferred CFSE-labeled Ag85B peptide 25-specific TCR transgenic (P25TCR-Tg) CD4+ T cells in the lung-draining lymph node was assayed at selected times after aerosol M. bovis BCG or M. tuberculosis infection. Representative CFSE dilution profiles of P25TCR-Tg CD4+ T cells after aerosol infection with (A) M. bovis BCG or (B) M. tuberculosis. (C) Total number of CFSEdilute P25TCR-Tg cells CD4+ T cells in lung-draining lymph nodes after infection with M. bovis BCG or M. tuberculosis, calculated from frequencies determined by flow cytometry. (D) Quantitation of live M. bovis BCG or M. tuberculosis H37Rv in lung-draining lymph nodes after aerosol infection. The dashed line depicts the limit of detection for viable bacteria (6 cfu/lymph node). Data are expressed as mean ± SEM and are representative of 2 experiments where n = 4 mice per time point for each bacterial infection. Student t test was used to compare BCG and M. tuberculosis samples at each time point: *, P<0.05; **, P<0.01; ***, P<0.005; ****, P<0.0001.
In the presence of equivalent numbers of bacteria, BCG-infected cells are superior to M. tuberculosis-infected cells for priming antigen-specific CD4 T cells in vivo
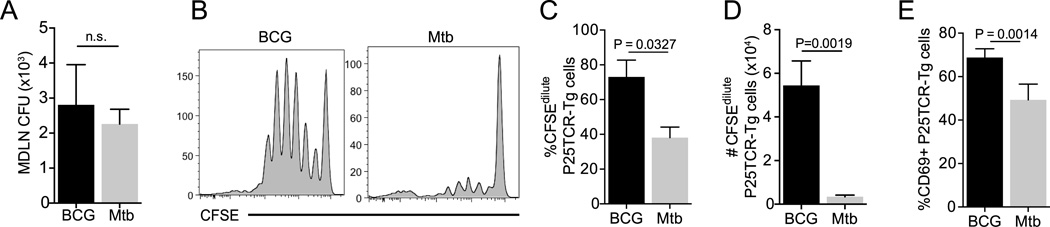
The finding that priming of P25TCR-Tg CD4 T cells after aerosol infection happens in the presence of fewer BCG than M. tuberculosis in the local lymph node suggested that BCG-infected cells may be superior to M. tuberculosis-infected cells in activating CD4 T cells. To compare P25TCR-Tg CD4 T cell responses to equal numbers of BCG and M. tuberculosis in vivo, we used intratracheal transfer of infected bone marrow-derived dendritic cells (BMDC) (41, 42). We transferred 105 BCG- or M. tuberculosis-infected BMDC intratracheally to mice that received CFSE-labeled P25TCR-Tg CD4 T cells one day earlier. P25TCR-Tg cell proliferation in the lung-draining lymph node was quantitated ~60 hours after transfer of the infected BMDC. We found that when equivalent numbers (~2000) of BCG or M. tuberculosis were present in the lung-draining lymph node after transfer of infected BMDC (Figure 3A), BCG was more effective in priming P25TCR-Tg CD4 T cells than was M. tuberculosis (Figure 3B). While ~70% of P25TCR-Tg cells had proliferated in response to BCG, only ~40% of P25TCR-Tg cells had proliferated in response to M. tuberculosis at the same time point (Figure 3C). Consistent with this finding, the total number of CFSEdilute P25TCR-Tg cells present in BCG-infected lymph nodes was 15-fold greater than in M. tuberculosis-infected lymph nodes (Figure 3D). As an additional indication of antigen-specific CD4 T cell priming, the frequency of P25TCR-Tg cells expressing CD69 was higher in response to BCG than M. tuberculosis (Figure 4E). These results support the hypothesis that cells infected with BCG are more efficient than those infected with M. tuberculosis at activating P25TCR-Tg CD4 T cells in vivo.
Figure 3.
Superior in vivo priming of antigen-specific CD4+ T cells by M. bovis BCG compared to M. tuberculosis when the number of bacteria is equivalent. Bone marrow-derived dendritic cells (105/mouse) infected with M. bovis BCG or M. tuberculosis H37Rv (MOI, 2) were administered intratracheally to mice that also received CFSE-labeled P25TCR-Tg CD4+ T cells intravenously. (A) Quantitation of M. bovis BCG or M. tuberculosis CFU in lung-draining lymph nodes 60 h after iintratracheal transfer. (B) Representative CFSE dilution plots from M. bovis BCG- or M. tuberculosis-infected mice. (C) Frequency and (D) total number of CFSEdilute P25TCR-Tg cells in lung-draining lymph nodes. (E) Frequency of P25TCR-Tg CD4+ cells expressing CD69 in M. bovis BCG- or M. tuberculosis-infected mice. Data are expressed as mean ± SEM. Student t test was performed to compare M. tuberculosis- and M. bovis BCG-infected samples.
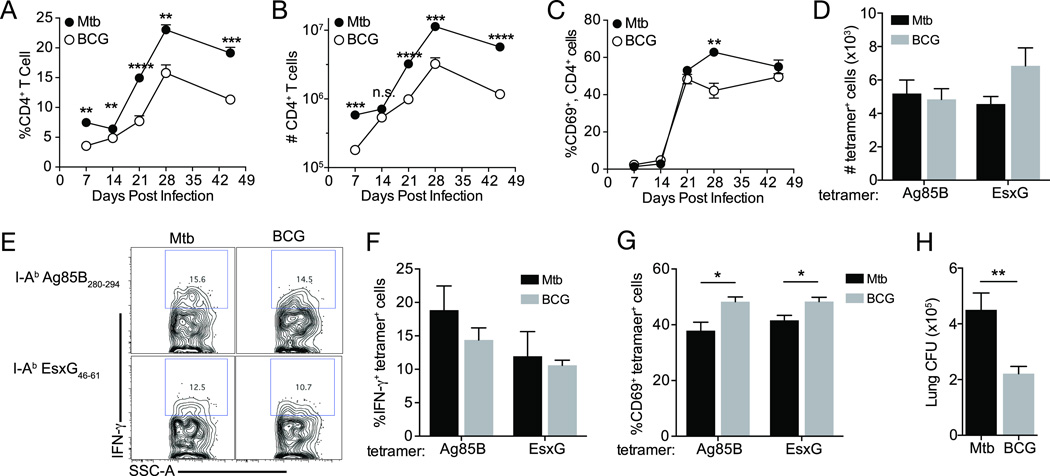
Figure 4.
Fewer BCG than M. tuberculosis are required for equivalent CD4 T cell activation in the lungs. CD4+ T cells were quantitated in lungs of WT mice following aerosol infection. (A) Frequency of CD4+ T cells expressed as the percentage of total lung cells. (B) Total number of lung CD4+ T cells, calculated from cell frequencies indicated in (A). Frequency of CD4+ cells expressing CD69 (C). (D) Total numbers of I-Ab:Ag85B280–294 and I-Ab:EsxG46–61-specific CD4 T cells. (E) Representative flow plots of IFN-γ expression in antigen-specific CD4 T cells. Frequencies of IFN-γ+ (F) and CD69+ (G) antigen-specific cells. (H) Lung CFU 21 days following BCG or M. tuberculosis infection. Data are expressed as mean ± SEM and are representative of 2 experiments where n = 4 mice per time point for each bacterial infection. Student t test was used to compare cell frequencies and cell numbers in M. bovis BCG- versus M. tuberculosis-infected mouse lungs; *, P<0.05; **, P<0.01; ***, P<0.005; ****, P<0.0001
CD4 T cell recruitment and activation in the lungs
We next examined the dynamics and activation of CD4 T cell populations in the lungs following infection with either BCG or M. tuberculosis. The frequency (Figure 4A) and number (Figure 4B) of CD4 T cells increased markedly in the lungs between 14 and 28 days post infection. Notably, both the frequency and number of CD4 T cells were higher in lungs of mice infected with M. tuberculosis than with BCG, indicating that the inability of T cells to clear M. tuberculosis is not due to deficient trafficking of CD4 T cells to the lungs. Despite differences in the total number CD4 T cells, the overall frequencies of lung CD4 T cells expressing CD69 were similar in M. tuberculosis-infected mice compared with BCG-infected mice (Figure 4C), suggesting that the general phenotype and state of activation of CD4 T cells in the lungs does not explain the inability of T cells to clear M. tuberculosis infection. When we used MHC class II tetramers (I-Ab:Ag85B280–294 and I-Ab:EsxG46–61) to characterize activation of antigen-specific CD4 T cells in the lungs 21 days after aerosol infection, we found similar numbers of Ag85B- and EsxG- specific CD4 T cells in mice infected with M. tuberculosis or BCG (Figure 4D). We also found similar frequencies of IFN-γ+ Ag85B- and EsxG-specific CD4 T cells in both groups of mice (Figure4E and 4F), despite lower lung bacterial burdens of BCG compared with M. tuberculosis (Figure 4H). Notably, significantly greater proportions of the Ag85B- and EsxG-specific CD4 T cells expressed CD69 in lungs of BCG-infected mice (Figure 4G). Since these results suggest that CD4 T cell responses are more efficacious in the context of BCG infection than virulent M. tuberculosis infection, we hypothesized that any difference in T cell efficacy is related to differences in interactions of CD4 T cells with infected antigen-presenting cells such as dendritic cells and macrophages.
BCG-infected cells activate Th1 P25TCR-Tg effector CD4 T cells more efficiently than M. tuberculosis-infected cells in vitro
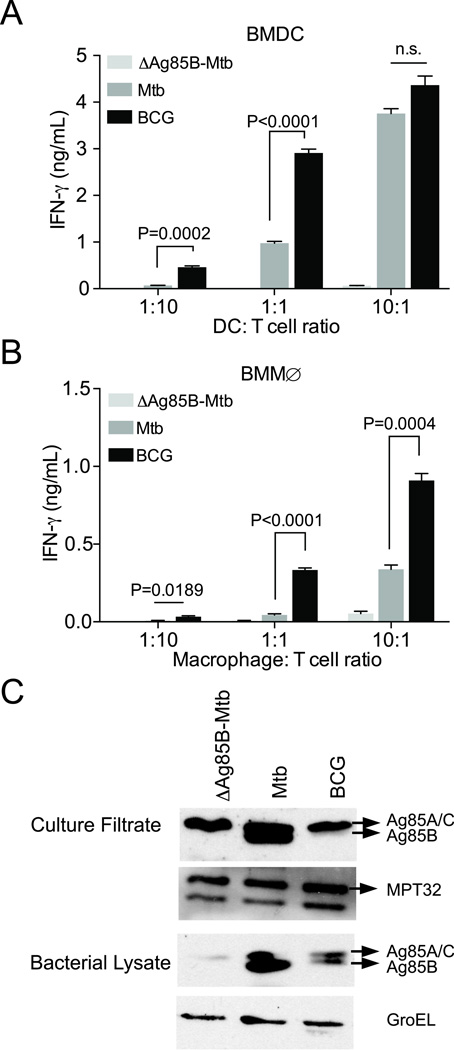
To better understand the difference in activation of antigen-specific CD4 T cells by BCG compared with M. tuberculosis, we investigated activation of Th1 differentiated P25TCR-Tg CD4 effector T cells in vitro. At ratios of 1:10 or 1:1 BMDC to T cell, P25TCR-Tg Th1 effector T cells consistently secreted significantly more IFN-γ in response to BCG-infected BMDC than to M. tuberculosis-infected BMDC (Figure 5A). Similarly, P25TCR-Tg effector cells secreted more IFN-γ in response to BCG-infected bone marrow-derived macrophages (BMMØ) when compared with M. tuberculosis-infected macrophages at all APC: T cell ratios examined (Figure 5B). BCG-infected cells were consistently superior to M. tuberculosis-infected cells as activators of P25TCR-Tg T cells when the MOI was titrated (data not shown). Thus, consistent with the in vivo proliferation results, BCG-infected bone marrow-derived DC and macrophages are superior to M. tuberculosis-infected cells for activating Th1 P25TCR-Tg CD4 effector cells in vitro.
Figure 5.
Antigen-specific CD4 T cell activation by M. bovis BCG-infected cells is more efficient than by M. tuberculosis-infected cells (A) Comparison of activation (assayed as IFNγ secretion) of Th1-polarized P25TCR-Tg CD4 T cells in response to M. bovis BCG- or M. tuberculosis-infected BMDC. Ratios of infected BMDC: T cells were varied (1:10, 1:1, 10:1). (B) T cell activation assayed as in (A), using infected bone marrow-derived macrophages instead of DC. (C) M. bovis BCG Pasteur expresses lower quantities of Ag85B than does M. tuberculosis H37Rv. Ag85B immunoblot of culture filtrates and bacterial pellets of M. tuberculosis H37Rv:ΔAg85B, M. tuberculosis H37Rv, and M. bovis BCG Pasteur. αMPT32 blot serves as loading control for culture filtrate. αGroEL is shown as a loading control for pellet lysates. Data are expressed are mean ± SEM of triplicate wells, and are representative of 3 independent experiments. Student t test was performed to compare M. tuberculosis- and M. bovis BCG-infected samples.*** P<0.0003
One potential explanation for the difference in P25TCR-Tg CD4 T cell responses to BCG and M. tuberculosis is that BCG could express greater quantities of Ag85B. To assess this possibility, we compared Ag85B protein expression and secretion by BCG and M. tuberculosis by immunoblotting bacterial lysates and culture filtrates. This revealed that, contrary to expectation, BCG expresses lesser quantities of Ag85B than does M. tuberculosis H37Rv in both bacterial lysates and culture filtrates (Figure 5C). Therefore, despite expressing lower quantities of Ag85B, BCG is able to activate P25TCR-Tg CD4 T cells more effectively than is M. tuberculosis. These data indicate that the same antigen is presented more efficiently by BCG-infected cells than by M. tuberculosis-infected cells.
Limited antigen availability on M. tuberculosis infected cells contributes to poor activation of antigen-specific CD4 T cell
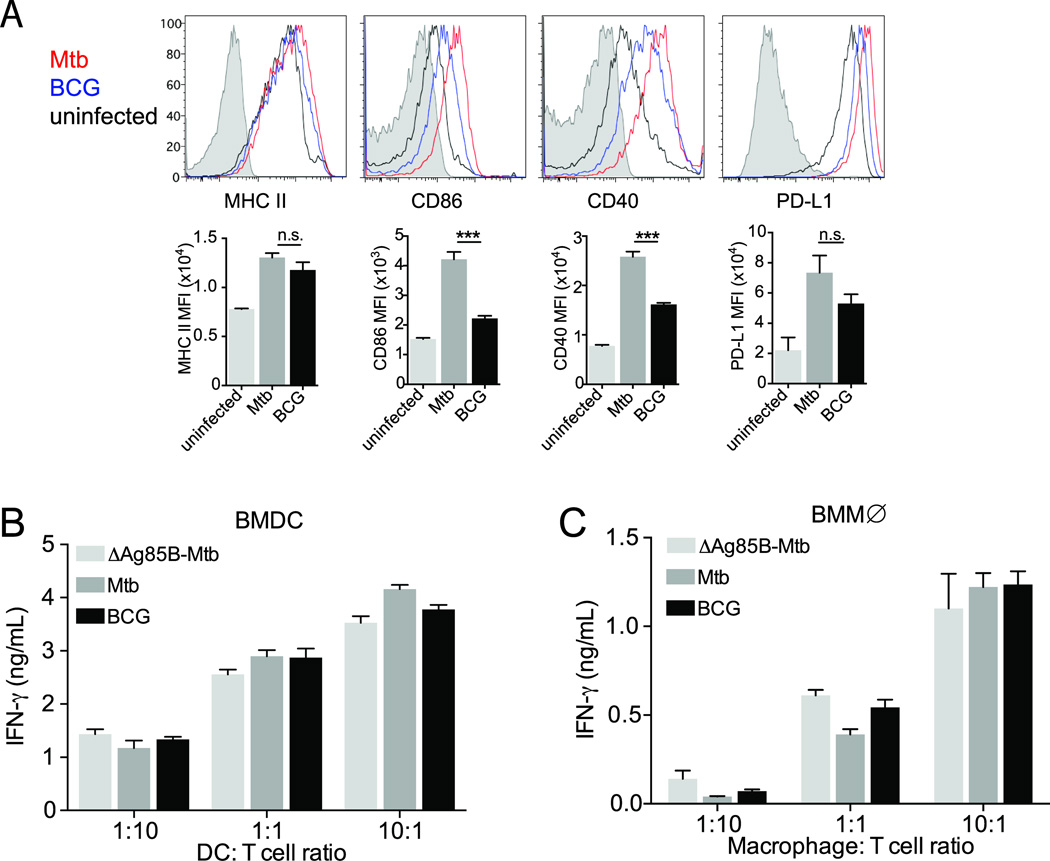
Since differences in efficiency or effectiveness of T cell activation can be a function of differential antigen presentation and/or differential co-stimulatory or inhibitory signaling, we investigated the possibility that BCG-infected cells express higher levels of MHC class II and/or co-stimulatory molecules to explain their superior T cell stimulating activity. We found that BCG and M. tuberculosis-infected BMDC expressed similar levels of MHC-II on the cell surface (Figure 6A), and that the co-stimulatory molecules CD86 and CD40 and the inhibitory molecule PD-L1 were expressed at higher levels on M. tuberculosis-infected cells than on BCG-infected cells (Figure 6A). Moreover, the difference in activation of P25TCR-Tg CD4 effector cells was abrogated when we pulsed BCG- or M. tuberculosis-infected BMDC (Figure 6B) or BMMØ (Figure 6C) with a saturating concentration of peptide 25 (the epitope recognized by P25TCR-Tg cells). Together, these results indicate that differential co-stimulatory or inhibitory molecule expression on BCG and M. tuberculosis infected cells does not account for the differences in T cell activation observed. Instead, these results indicate that less antigen is presented by M. tuberculosis-infected cells than by BCG-infected cells.
Figure 6.
Limited antigen availability on M. tuberculosis infected cells contributes to poor activation of antigen-specific CD4 T cell. (A) Comparison of surface MHC II, CD86, CD40, and PDL-1 expression on uninfected (black), M. bovis BCG-infected (blue), and M. tuberculosis-infected (red) BMDC, isotype control (shaded histogram). (B) IFNγ secretion assayed as in (B) after BMDC were pulsed with 1 µM peptide 25 and co-cultured with Th1-polarized P25TCR-Tg cells. (C) IFNγ secretion after infected macrophages were pulsed with 1µM peptide 25 and co-cultured with Th1-P25TCR-Tg cells. Data are expressed are mean ± SEM of triplicate wells, and are representative of 3 independent experiments. Student t test was performed to compare M. tuberculosis- and M. bovis BCG-infected samples.*** P<0.0003
Discussion
The major finding reported here is that, compared with an attenuated mycobacterial strain (M. bovis BCG), a virulent strain of M. tuberculosis (H37Rv) activates antigen-specific CD4 T cells poorly in vitro and in vivo, and this differential T cell activation is associated with clearance of BCG and persistence of M. tuberculosis. We found that BCG-infected cells are more efficient and more efficacious than M. tuberculosis-infected cells for activating Ag85B-specific CD4 T cells, despite production of larger quantities of Ag85B by M. tuberculosis. The superior ability of BCG-infected cells to activate Ag85B-specific CD4 T cells was manifest by a lower number of BCG-infected cells needed to initiate proliferation of CD4 T cells after aerosol infection, indicating that fewer antigen-producing BCG are needed for sufficient antigen presentation. When the number of bacteria and the number of infected dendritic cells were adjusted to be equivalent and transferred intratracheally to naive mice, BCG-infected cells activated a larger proportion of adoptively-transferred CD4 T cells than when M. tuberculosis-infected cells were transferred. The experimental evidence supports the conclusion that superior CD4 T cell activation is due to superior antigen presentation, as the difference in T cell activation could not be explained by differential expression of co-stimulatory or inhibitory molecules on the infected cells, and epitope peptide complementation abrogated the difference between BCG- and M. tuberculosis-infected cells. Importantly, superior antigen presentation and CD4 T cell activation are tied to clearance of M. bovis BCG from the lungs. These findings support previously published evidence that M. tuberculosis interferes with MHC class II antigen presentation, and provide novel evidence that this mechanism operates in vivo and is associated with persistent infection with M. tuberculosis.
Studies of diverse pathogens including Salmonella (43), HIV (44), and Leishmania (45) have revealed that strains with higher virulence can inhibit or evade antigen processing and presentation. These examples underscore the importance of antigen presentation in the overall pathology of infectious disease, where attenuated pathogens are associated with superior CD4 T cell activation and superior control of infection. Similarly, mycobacteria have been reported capable of limiting global MHC II antigen processing or presentation to CD4 T cells (16, 46–48). Several studies have shown that various mycobacteria can impact the presentation of exogenous antigen by macrophages (18, 19, 49). In addition, live mycobacteria have been found to limit the presentation of endogenous bacterial antigens to CD4 T cells (16, 20, 50). Mycobacterial strains differing in virulence (H37Rv, H37Ra, Erdman, and BCG) have been used in various studies to demonstrate that members of the M. tuberculosis complex are capable of limiting antigen presentation. One prior study has directly compared mycobacteria of differing virulence to determine their relative ability to present MHC-II restricted mycobacterial antigen (20); in this study BCG and M. tuberculosis showed limited T cell activation when compared with avirulent H37Ra, suggesting that the ability to present antigen to CD4 T cells can further stratify the virulence of mycobacteria. Our findings with BCG and M. tuberculosis reinforce the evidence that virulence in mycobacteria is associated with poor activation of antigen-specific CD4 T cells, and extend the findings of previous studies by revealing that poor activation of CD4 T cells by virulent M. tuberculosis also occurs in vivo. The importance of the efficacy of antigen presentation by M. tuberculosis-infected cells is emphasized by our earlier finding that direct recognition of infected cells by CD4 T cells is critical for control of intracellular M. tuberculosis in vivo (23). The in vitro observations we report here and those of earlier in vivo work (14) demonstrate that providing exogenous epitope peptide can enhance CD4 P25TCR-Tg cell activation, indicating that suboptimal abundance of peptide:MHC complexes, a result of limited antigen presentation, is an important determinant of CD4 T cell activation in the context of TB.
A recent study evaluated P25TCR-Tg cell activation after intradermal injection of BCG in mice. Consistent with our findings, that study found that BCG activation in the draining lymph node occurs early following intradermal infection (39). Based on our results, we propose that poor antigen presentation by M. tuberculosis infected cells is a significant factor that limits the efficacy of CD4 T cell responses and allows persistence of the bacteria. If M. tuberculosis-infected cells present other mycobacterial antigens as poorly as they do Ag85B, it is unclear that vaccine strategies that only induce larger numbers of antigen-specific T cells will have greater efficacy than the vaccines developed to date. Our findings imply that further studies of a wide spectrum of TB antigens, as well as studies to understand the virulence mechanisms that limit antigen presentation, will be necessary to improve immunity to M. tuberculosis and contribute to overcoming the global problem of TB.
Acknowledgements
We thank the NYU Flow Cytometry and Sorting core facility for their expertise and technical assistance. We thank Smita Srivastava, Ph.D. for assisting with intratracheal infection of mice and her thoughtful discussion of these data. We acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of MHC II tetramers. We also thank members of the Ernst lab for their thoughts and suggestions for this work.
Footnotes
Supported by funding from NIH grants R01 AI084041 (JDE) and F31 AI94957 (PG).
References
- 1.Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, Walker AT, Friedland GH. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 2.Chaisson RE, Schecter GF, Theuer CP, Rutherford GW, Echenberg DF, Hopewell PC. Tuberculosis in patients with the acquired immunodeficiency syndrome. Clinical features, response to therapy, and survival. Am Rev Respir Dis. 1987;136:570–574. doi: 10.1164/ajrccm/136.3.570. [DOI] [PubMed] [Google Scholar]
- 3.Perlman DC, el-Sadr WM, Nelson ET, Matts JP, Telzak EE, Salomon N, Chirgwin K, Hafner R. Variation of chest radiographic patterns in pulmonary tuberculosis by degree of human immunodeficiency virus-related immunosuppression. The Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA). The AIDS Clinical Trials Group (ACTG) Clin Infect Dis. 1997;25:242–246. doi: 10.1086/514546. [DOI] [PubMed] [Google Scholar]
- 4.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 2001:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladel CH, Blum C, Dreher A, Reifenberg K, Kaufmann SH. Protective role of gamma/delta T cells and alpha/beta T cells in tuberculosis. Eur. J. Immunol. 1995:2877–2881. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- 6.Flory CM, Hubbard RD, Collins FM. Effects of in vivo T lymphocyte subset depletion on mycobacterial infections in mice. J Leukoc Biol. 1992;51:225–229. doi: 10.1002/jlb.51.3.225. [DOI] [PubMed] [Google Scholar]
- 7.Robertson HE. The Persistence of Tuberculous Infections. The American journal of pathology. 1933;9:711–718. 711. [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman WH, Baggenstoss AH. The residual infectivity of the primary complex of tuberculosis. The American journal of pathology. 1938;14:473–490. 473. [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn PL, North RJ. Persistent infection with virulent but not avirulent Mycobacterium tuberculosis in the lungs of mice causes progressive pathology. Journal of medical microbiology. 1996;45:103–109. doi: 10.1099/00222615-45-2-103. [DOI] [PubMed] [Google Scholar]
- 10.Rhoades ER, Frank AA, Orme IM. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tubercle and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 11.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. Journal of Experimental Medicine. 2008:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiley WW, Calayag MD, Wittmer ST, Huntington JL, Pearl JE, Fountain JJ, Martino CA, Roberts AD, Cooper AM, Winslow GM, Woodland DL. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10961–10966. doi: 10.1073/pnas.0801496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. The Journal of experimental medicine. 2008;205:2359–2368. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bold TD, Banaei N, Wolf AJ, Ernst JD. Suboptimal Activation of Antigen-Specific CD4+ Effector Cells Enables Persistence of M. tuberculosis In Vivo. PLoS Pathog. 2011:e1002063. doi: 10.1371/journal.ppat.1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. Journal of Experimental Medicine. 2010:1409–1420. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pancholi P, Mirza A, Bhardwaj N, Steinman RM. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science. 1993:984–986. doi: 10.1126/science.8098550. [DOI] [PubMed] [Google Scholar]
- 17.Hmama Z, Gabathuler R, Jefferies WA, de Jong G, Reiner NE. Attenuation of HLA-DR expression by mononuclear phagocytes infected with Mycobacterium tuberculosis is related to intracellular sequestration of immature class II heterodimers. Journal of immunology. 1998;161:4882–4893. [PubMed] [Google Scholar]
- 18.Fulton SA, Reba SM, Pai RK, Pennini M, Torres M, Harding CV, Boom WH. Inhibition of Major Histocompatibility Complex II Expression and Antigen Processing in Murine Alveolar Macrophages by Mycobacterium bovis BCG and the 19-Kilodalton Mycobacterial Lipoprotein. Infection and immunity. 2004:2101–2110. doi: 10.1128/IAI.72.4.2101-2110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noss EH, Harding CV, Boom WH. Mycobacterium tuberculosis Inhibits MHC Class II Antigen Processing in Murine Bone Marrow Macrophages. Cellular immunology. 2000:63–74. doi: 10.1006/cimm.2000.1633. [DOI] [PubMed] [Google Scholar]
- 20.Singh CR, Moulton RA, Armitige LY, Bidani A, Snuggs M, Dhandayuthapani S, Hunter RL, Jagannath C. Processing and presentation of a mycobacterial antigen 85B epitope by murine macrophages is dependent on the phagosomal acquisition of vacuolar proton ATPase and in situ activation of cathepsin D. J. Immunol. 2006:3250–3259. doi: 10.4049/jimmunol.177.5.3250. [DOI] [PubMed] [Google Scholar]
- 21.Simeone R, Bobard A, Lippmann J, Bitter W, Majlessi L, Brosch R, Enninga J. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog. 2012:e1002507. doi: 10.1371/journal.ppat.1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sendide K, Deghmane AE, Reyrat JM, Talal A, Hmama Z. Mycobacterium bovis BCG urease attenuates major histocompatibility complex class II trafficking to the macrophage cell surface. Infection and immunity. 2004;72:4200–4209. doi: 10.1128/IAI.72.7.4200-4209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava S, Ernst JD. The Journal of Immunology. American Association of Immunologists; 2013. Cutting edge: Direct recognition of infected cells by CD4 T cells is required for control of intracellular Mycobacterium tuberculosis in vivo; pp. 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Copin R, Coscolla M, Efstathiadis E, Gagneux S, Ernst JD. Impact of in vitro evolution on antigenic diversity of Mycobacterium bovis bacillus Calmette-Guerin (BCG) Vaccine. 2014;32:5998–6004. doi: 10.1016/j.vaccine.2014.07.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, Sherman DR. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guerin attenuation. The Journal of infectious diseases. 2003;187:117–123. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Molecular microbiology. 2002;46:709–717. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 27.Sherman DR, Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Smith S. Mycobacterium tuberculosis H37Rv: Delta RD1 is more virulent than M. bovis bacille Calmette-Guerin in long-term murine infection. The Journal of infectious diseases. 2004;190:123–126. doi: 10.1086/421472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hesseling AC, Marais BJ, Gie RP, Schaaf HS, Fine PE, Godfrey-Faussett P, Beyers N. The risk of disseminated Bacille Calmette-Guerin (BCG) disease in HIV-infected children. Vaccine. 2007;25:14–18. doi: 10.1016/j.vaccine.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Miles DJ, Gadama L, Gumbi A, Nyalo F, Makanani B, Heyderman RS. Human immunodeficiency virus (HIV) infection during pregnancy induces CD4 T-cell differentiation and modulates responses to Bacille Calmette-Guerin (BCG) vaccine in HIV-uninfected infants. Immunology. 2010;129:446–454. doi: 10.1111/j.1365-2567.2009.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ladel CH, Hess J, Daugelat S, Mombaerts P, Tonegawa S, Kaufmann SH. Contribution of alpha/beta and gamma/delta T lymphocytes to immunity against Mycobacterium bovis bacillus Calmette Guérin: studies with T cell receptor-deficient mutant mice. Eur. J. Immunol. 1995:838–846. doi: 10.1002/eji.1830250331. [DOI] [PubMed] [Google Scholar]
- 31.Ladel CH, Daugelat S, Kaufmann SH. Immune response to Mycobacterium bovis bacille Calmette Guérin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur. J. Immunol. 1995:377–384. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- 32.Feng CG, Britton WJ. CD4+ and CD8+ T cells mediate adoptive immunity to aerosol infection of Mycobacterium bovis bacillus Calmette-Guérin. The Journal of infectious diseases. 2000:1846–1849. doi: 10.1086/315466. [DOI] [PubMed] [Google Scholar]
- 33.Fulton SA, Martin TD, Redline RW, Henry Boom W. Pulmonary immune responses during primary mycobacterium bovis- Calmette-Guerin bacillus infection in C57Bl/6 mice. Am J Respir Cell Mol Biol. 2000:333–343. doi: 10.1165/ajrcmb.22.3.3776. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava S, Ernst JD, Desvignes L. Beyond macrophages: the diversity of mononuclear cells in tuberculosis. Immunological reviews. 2014;262:179–192. doi: 10.1111/imr.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamura T, Ariga H, Kinashi T, Uehara S, Kikuchi T, Nakada M, Tokunaga T, Xu W, Kariyone A, Saito T, Kitamura T, Maxwell G, Takaki S, Takatsu K. The role of antigenic peptide in CD4+ T helper phenotype development in a T cell receptor transgenic model. International immunology. 2004;16:1691–1699. doi: 10.1093/intimm/dxh170. [DOI] [PubMed] [Google Scholar]
- 36.Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev. 2011;24:351–376. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Philips JA, Ernst JD. Tuberculosis pathogenesis and immunity. Annu Rev Pathol. 2012;7:353–384. doi: 10.1146/annurev-pathol-011811-132458. [DOI] [PubMed] [Google Scholar]
- 38.Wolf AJ, Linas B, Trevejo-Nuñez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 2007:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 39.Bollampalli VP, Harumi Yamashiro L, Feng X, Bierschenk D, Gao Y, Blom H, Henriques-Normark B, Nylen S, Rothfuchs AG. BCG Skin Infection Triggers IL-1R-MyD88-Dependent Migration of EpCAMlow CD11bhigh Skin Dendritic cells to Draining Lymph Node During CD4+ T-Cell Priming. PLoS Pathog. 2015;11:e1005206. doi: 10.1371/journal.ppat.1005206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. The Journal of experimental medicine. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivastava S, Ernst JD. Cell-to-cell transfer of M. tuberculosis antigens optimizes CD4 T cell priming. Cell Host and Microbe. 2014:741–752. doi: 10.1016/j.chom.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhatt K, Hickman SP, Salgame P. Cutting edge: a new approach to modeling early lung immunity in murine tuberculosis. Journal of immunology. 2004;172:2748–2751. doi: 10.4049/jimmunol.172.5.2748. [DOI] [PubMed] [Google Scholar]
- 43.Bueno SM, Gonzalez PA, Carreno LJ, Tobar JA, Mora GC, Pereda CJ, Salazar-Onfray F, Kalergis AM. The capacity of Salmonella to survive inside dendritic cells and prevent antigen presentation to T cells is host specific. Immunology. 2008;124:522–533. doi: 10.1111/j.1365-2567.2008.02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stumptner-Cuvelette P, Morchoisne S, Dugast M, Le Gall S, Raposo G, Schwartz O, Benaroch P. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12144–12149. doi: 10.1073/pnas.221256498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kima PE, Soong L, Chicharro C, Ruddle NH, McMahon-Pratt D. Leishmania-infected macrophages sequester endogenously synthesized parasite antigens from presentation to CD4+ T cells. Eur J Immunol. 1996;26:3163–3169. doi: 10.1002/eji.1830261249. [DOI] [PubMed] [Google Scholar]
- 46.Torres M, Ramachandra L, Rojas RE, Bobadilla K, Thomas J, Canaday DH, Harding CV, Boom WH. Role of phagosomes and major histocompatibility complex class II (MHC-II) compartment in MHC-II antigen processing of Mycobacterium tuberculosis in human macrophages. Infection and immunity. 2006;74:1621–1630. doi: 10.1128/IAI.74.3.1621-1630.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gercken J, Pryjma J, Ernst M, Flad HD. Defective antigen presentation by Mycobacterium tuberculosis-infected monocytes. Infection and immunity. 1994:3472–3478. doi: 10.1128/iai.62.8.3472-3478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hava DL, van der Wel N, Cohen N, Dascher CC, Houben D, Leon L, Agarwal S, Sugita M, van Zon M, Kent SC, Shams H, Peters PJ, Brenner MB. Evasion of peptide, but not lipid antigen presentation, through pathogen-induced dendritic cell maturation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11281–11286. doi: 10.1073/pnas.0804681105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noss EH, Pai RK, Sellati TJ, Radolf JD, Belisle J, Golenbock DT, Boom WH, Harding CV. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. Journal of immunology. 2001;167:910–918. doi: 10.4049/jimmunol.167.2.910. [DOI] [PubMed] [Google Scholar]
- 50.Ramachandra L, Noss E, Boom WH, Harding CV. Processing of Mycobacterium tuberculosis antigen 85B involves intraphagosomal formation of peptide-major histocompatibility complex II complexes and is inhibited by live bacilli that decrease phagosome maturation. The Journal of experimental medicine. 2001;194:1421–1432. doi: 10.1084/jem.194.10.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]